Introduction
Glioma is one of the most prevalent tumors of the
central nervous system, with an age-adjusted (<78 years old)
annual incidence of 7.3 cases/100,000 individuals (1,2). It is
increasing in incidence by ~1.2% each year (3). Notably, although treatment regimens
comprising a combination of surgery and chemoradiotherapy are used
to treat glioma (4,5), the prognosis of patients with
malignant glioma remains poor. The complete surgical removal of
glioma is challenging as the tumor grows within the brain and often
infiltrates the surrounding normal tissue (6,7).
Targeted therapy has become an important therapeutic method for the
treatment of glioma (8,9). Various targeted drug delivery systems,
including liposomes and nano-micelles, have been used clinically or
in clinical trials (10,11). However, it is important to develop
more effective drugs and delivery systems to improve the treatment
of glioma and its outcomes.
Certain anesthetics have been found to have
antitumor activities (12,13), with effects on the apoptosis,
proliferation, motility and drug resistance of various cancer
cells. For example, one study demonstrated that clinically relevant
concentrations of bupivacaine induced the apoptosis of sarcoma
cells in vitro and suppressed their proliferation (14), and another study revealed that
dibucaine had a pro-apoptotic effect on HL-60 promyelocytic
leukemia cells (15). However, few
studies have focused on the potential antitumor effects of
lidocaine in glioma treatment.
The vascular-corrected concentration of drugs in the
brain can be increased by using a higher drug dose; however, this
may cause serious side effects and severe toxicity. Therefore, the
development of effective systems to deliver anesthetics, such as
lidocaine, into the brain is a topic of great interest. The
targeted delivery of drugs through the blood-brain barrier (BBB)
into the brain may not only increase treatment efficacy, but also
enable the dose of drug administered to be reduced. Previous
studies have focused on increasing the penetration of the BBB to
improve glioma-targeting ability using specific ligands (16,17).
Folic acid (FA) is a most promising ligand for targeting the folate
receptor (FR), which is overexpressed on the cell surface of
multiple types of cancer cells, including U87 and MDA-MB-231 cells
(18,19). Additionally, the expression of the
FR on normal cells is limited, which make it an attractive focus
for efficient glioma-targeting. In addition, the FR is also highly
expressed by cerebral capillary endothelial cells (19). Therefore, FA may have superior
ability to cross the BBB and efficiently target glioma.
To verify the hypothesized ability of FA to cross
the BBB and investigate the antitumor mechanisms of lidocaine, an
innovative lidocaine-loaded liposome modified with FA (Lid-FA-Lip)
was developed in the present study. This modified liposome was
designed based on the assumption that FA will markedly increase the
ability of the liposome to penetrate the BBB and thereby improve
the antitumor effect. Whether Lid-FA-Lip can suppress the motility
of glioma cells and stimulate apoptosis was also investigated. In
addition, the antitumor effects of Lid-FA-Lip on the tumor growth
of glioma cells in mice and the contribution of PI3K/AKT pathway
suppression to the underlying mechanism were analyzed.
Materials and methods
Chemistry Synthesis of cholesterol
tosylate (compound 2)
A solution of cholesterol (compound 1; 1.00 g, 2.59
mmol) in pyridine (5 ml) was prepared, and a solution of
p-toluenesulfonyl chloride (0.74 g, 3.88 mmol) in pyridine
(5 ml) was added. After stirring the mixture for 10 h at 50˚C, the
solvent was removed and the residue was re-dissolved with ethyl
acetate (20 ml). The solution was washed with 1 mol/l HCl and
saturated NaCl. The solvent was then removed to yield compound 2
(1.19 g, 85%), which was used for the next step without further
purification. Melting point, 127-129˚C. High-resolution mass
spectrometry (HRMS): Electrospray ionization (ESI+)
calculated for C34H52O3S Na
[M+Na]+, 563.3535; found, 563.3532. Elemental analysis:
Calculated, C, 75.51; H, 9.69; S, 5.93; found, C, 75.43; H, 9.78;
S, 6.11.
Synthesis of octaethylene glycol
monocholesteryl ether (compound 3)
To a solution of compound 2 (1.00 g, 1.85 mmol) in
dioxane (15 ml) was added octaethylene glycol (6.85 g, 18.49 mmol).
The reaction mixture was refluxed for 20 h. The solvent was then
removed and the residue was re-dissolved with dichloromethane
(CH2Cl2; 30 ml). The solution was washed with
saturated NaCl. After removing the CH2Cl2,
the residue was purified by chromatography to give compound 3 (0.66
g, 48%) as colorless oil. HRMS: (ESI+) calculated for
C43H78O9Na [M+Na]+,
761.5544; found, 761.5540. Elemental analysis: Calculated, C,
69.88; H, 10.64; found, C, 69.76; H, 10.79.
Synthesis of FA-modified ligand
(compound 4)
To a solution of FA (0.50 g, 1.13 mmol) and compound
3 (0.42 g, 0.57 mmol) in dimethyl sulfoxide (DMSO; 10 ml) was added
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC;
0.11 g, 0.57 mmol) and N-hydroxysuccinimide (NHS; 65 mg, 0.57
mmol). The resulting mixture was stirred at room temperature for 24
h. The by-product was removed by filtration. The product was
precipitated from the filtrate by the addition of diethyl ether. A
yellow solid was obtained, which was purified by flash
chromatography to yield the FA-modified ligand (compound 4; 0.20 g,
30%). HRMS: (ESI+) calculated for
C62H95N7O14Na
[M+Na]+, 1,161.6937; found, 1,161.6930. Elemental
analysis: Calculated, C, 64.06; H, 8.24; N, 8.43; found C, 64.18;
H, 8.33; N, 8.29.
Preparation and characterization of
liposomes
Lidocaine-loaded liposomes were prepared using the
thin-film hydration method (20,21).
The ratio of the components was optimized as follows: i)
Conventional liposome (Lip), soybean phosphatidylcholine (SPC;
Shanghai Taiwei Pharmaceutical Co., Ltd.)/cholesterol in a molar
ratio of 62:33; ii) FA-modified liposome (FA-Lip),
SPC/cholesterol/compound 4 in a molar ratio of 62:33:3. In brief,
all the lipid materials were dissolved in a mixed solvent
comprising CH2Cl2 and methanol (2:1 v/v), and
the solution was then warmed on a rotary evaporator at 37˚C to
remove the solvent and form a thin film. After drying in a vacuum
for 10 h, the film was hydrated with PBS (pH 7.4) at 20˚C for 30
min and sonicated intermittently at 80 W for 80 sec to obtain the
liposomes. An appropriate amount of lidocaine (weight ratio of
procaine/lipid, 1/30) or
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein
(CFPE; final concentration, 20 µg/ml) was added to the solution of
lipid before removing the solvent to prepare lidocaine-loaded
liposomes (Lid-Lip and Lid-FA-Lip) or CFPE-labeled liposomes
(CFPE-Lip and CFPE-FA-Lip).
The entrapment efficiency [EE (%)] and drug loading
efficiency [DL (%)] of lidocaine by the liposomes were determined
using high performance liquid chromatography (HPLC). The detection
was performed on an Agilent 1200 HPLC System with an Ultimate
LP-C18 column (4.6x250 mm, 5 µm) (both from Agilent Technologies,
Inc.) at 25˚C. The mobile phase comprised water and methanol (70:30
v/v) with a flow rate of 1.0 ml/min. A 10-µl aliquot of the
lidocaine-containing sample was injected and the detection
wavelength was 210 nm. The EE (%) and DL (%) were calculated
according to the following equations: EE (%)=weight of encapsulated
lidocaine/total weight of lidocaine, and DL (%)=weight of
encapsulated lidocaine/total weight of liposome. In addition, the
size and ζ potential of Lid-Lip and Lid-FA-Lip were detected using
a Malvern Zeta sizer Nano ZS90 (Malvern Panalytical).
Release by Lid-Lip and Lid-FA-Lip in
vitro
The release behavior of lidocaine from the Lid-Lip
and Lid-FA-Lip liposomes was assessed using dialysis. Briefly, the
lidocaine-loaded liposomes were put into a dialysis bag (molecular
weight cut-off, 8,000-14,000 Da) with 50 ml PBS containing 0.1%
Tween-80 (v/v) as release medium. The bags were shaken at 37˚C with
gentle oscillation (45 rpm). At various time points between 0 and
48 h, an 0.1-ml sample was extracted and replaced with fresh
medium. The release behavior from free lidocaine was also analyzed
as a control. Subsequently, the amount of lidocaine was detected by
the aforementioned HPLC method.
Stability of Lid-Lip and Lid-FA-Lip in
vitro
The stability of the Lid-Lip and Lid-FA-Lip
liposomes was determined by the measurement of turbidity variations
in the presence of fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.). This comprised mixing each liposome formulation
with an equal volume of FBS, and then continuously shaking (50 rpm)
at 37˚C. The transmittance was measured between 0 and 48 h using a
microplate reader (Bio-Rad model 550; Bio-Rad Laboratories, Inc.)
at a wavelength of 750 nm. The transmittance in PBS was defined as
100%.
Hemolysis assays
A female BALB/c mouse (22 g; 4 weeks old; weight,
18-22 g) was supplied by Beijing Vital River Laboratory Animal
Technology Co., Ltd. The mouse was kept in a 20˚C environment with
40-60% humidity and a 12-h cycle of day and night. Free food and
water were provided during the feeding process, and clean and
hygienic feeding conditions were maintained. The mouse was
anesthetized by the intraperitoneal injection of pentobarbital
sodium at a dose of 50 mg/kg. Fresh blood (0.1 ml) was collected
from the orbit, and centrifuged (4˚C) at 2,000 x g for 5 min. The
supernatant was discarded and the precipitated red blood cells were
washed with PBS until the supernatant was colorless. The cells were
then re-suspended in PBS to a concentration of 2% (w/v).
Afterwards, Lid-Lip and Lid-FA-Lip were diluted with PBS to provide
liposome samples with lipid concentrations of 10, 25, 50, 100, 200
and 400 nM. Subsequently, each liposome sample (0.4 ml) was mixed
with red blood cell suspension (0.1 ml) and the mixture was
incubated at 37˚C for 2 h with gentle shaking (50 rpm). After
centrifuging (4˚C) at 6,000 x g for 10 min, the absorbance of the
supernatant at 540 nm (A540) was measured using a
microplate reader. The hemolysis rate of each sample was calculated
as follows: Hemolysis (%)=(A540 sample-A540
negative)/(A540 positive-A540 negative) x100.
The absorbances of PBS and Triton X-100 mixed with cell solution
were defined as 0 and 100%, respectively.
Cell culture
Brain capillary endothelial cells (BCECs) and the
U87 glioblastoma of unknown origin cell line were obtained from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS and 1% penicillin/streptomycin (HyClone; Cytiva).
MTT assay
Cytotoxicity was analyzed using a standard MTT-based
colorimetric assay. In brief, BCECs and U87 cells were cultured in
DMEM supplemented with 10% FBS at 37˚C in a humidified incubator
containing 5% CO2. The cells were seeded into 96-well
plates at a density of ~5x103 cells/well and cultured
for 24 h. Fresh medium containing lidocaine, Lid-Lip or Lid-FA-Lip
was applied to the cells with lidocaine concentrations ranging from
0.01-10 mM. The cells were incubated for another 24 h at 37˚C, and
then MTT (5 mg/ml) was added to each well with further culturing
for another 4 h at 37˚C. After this, the cells were lysed using 200
µl DMSO and the absorbance at 490 nm (A490) was measured
using a microplate reader. The percentages of surviving cells were
calculated using the following equation: Survival
(%)=[(A490 treated cells)/(A490 control
cells)] x100.
Transendothelial migration in a BBB
model
Millicell Hanging Cell Culture Inserts (Corning,
Inc.) were used to build an in vitro BBB model as previously
described (22). In brief, BCECs
were maintained in DMEM with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and seeded on 6-well plates at a density of
1x106 cells/well and incubated for 7 days at 37˚C. The
transendothelial electric resistance (TEER) of the BBB model was
measured using a Millicell ERS volt-ohm meter (EMD Millipore). Only
BCEC monolayers with TEER values >200 Ω were used for further
experiments. Additionally, the U87 cells were plated on another
6-well plate (1x106 cells/well). The cell culture
inserts with BCEC monolayers were transferred to the plates
containing the U87 cells and the cells were co-cultured for another
24 h. Subsequently, the CFPE-labeled liposomes (CFPE-Lip and
CFPE-FA-Lip; final concentration of CFPE, 2 µg/ml) were added to
the cell culture inserts (donor chamber) of the BBB model and
incubated at 37˚C for 4 h. The BCECs and U87 cells were then each
washed with cold PBS three times, trypsinized and finally
resuspended in 0.5 ml PBS. The fluorescent intensity of the two
types of cells was measured using a flow cytometer (Cytomics FC500;
Beckman Coulter, Inc.) and the flow cytometer's integral analysis
software (FlowJo 10; Becton, Dickinson and Company).
Wound healing assays
A wound healing assay was performed to evaluate the
effects of the liposomal formulations on cell migration. U87 cells
at a confluence of 100% and treated with Lid-Lip or Lid-FA-Lip (1
mM lidocaine; 37˚C; 24 h) were wounded by scraping with a 10-µl
pipette tip and washed twice. The cells were then cultured with
serum-free DMEM to stimulate wound healing. Photographic images
were captured by an Olympus light microscope before culture and at
the 24-h time point to evaluate the migration of the U87 cells. The
images were then analyzed using ImageJ 8.0 software (National
Institutes of Health).
Cell apoptosis assays
Cell apoptosis was evaluated using Annexin V-FITC
and propidium iodide (PI) staining (Sangon Biotech Co., Ltd.). U87
cells were treated with Lid-Lip or Lid-FA-Lip for 48 h (0-10 mM
lidocaine; 37˚C), then centrifuged (200 x g) at 4˚C for 3 min and
washed with PBS. Subsequently, the cells were re-suspended in 100
µl binding buffer with 5 µl Annexin V-FITC and incubated at room
temperature for 10 min. Then, 5 µl PI solution was added and the
cells were incubated for another 5 min at room temperature. The
proportion of apoptotic cells was analyzed using a flow cytometer
(Cytomics FC500; Beckman Coulter, Inc.) and the flow cytometer's
integral analysis software.
Immunoblot assay
Following treatment of U87 cells with Lid-FA-Lip (1
mM lidocaine, 37˚C for 24 h), total protein was extracted with RIPA
buffer (Beyotime Institute of Biotechnology) and protein
determination was performed using the BCA method. Protein samples
(10 µg loaded per lane) were separated on 10% SDS-PAGE and
transferred onto PVDF membranes (250 mA; 2 h), which were then
blocked with 5% milk in TBS with 0.05% Tween-20 at 25˚C for 2 h.
The PVDF membranes were subsequently treated with primary
antibodies targeting the following proteins (all from Abcam): Bcl-2
(ab32124; dilution, 1:500), matrix metalloproteinase 2 (MMP2;
ab92536; dilution, 1:500), Ki67 (ab92742; dilution, 1:1,000),
phosphorylated (p-)PI3Kp85 (phospho Y607; ab182651; dilution,
1:1,000), PI3Kp85 (ab135253; dilution, 1:500), p-AKT (phospho T308;
ab38449; dilution, 1:1,000), AKT (ab18785; dilution, 1:1,000) and
GAPDH (ab8245; dilution, 1:3,000) at room temperature for 2 h. The
membranes were then incubated with anti-rabbit (cat. no. ab6271;
Abcam) or anti-mouse (cat. no. ab6728; Abcam) HRP-conjugated
secondary antibodies (1:5,000 dilution) at room temperature for 1
h. Signals were then visualized using an ECL kit (Novex™ ECL
Chemiluminescent Substrate Reagent kit; Thermo Fisher Scientific,
Inc.) and visualized by ImageJ 8.0 software (National Institutes of
Health).
Antitumor activity in vivo
All experiments, including the extraction of blood
from a mouse for the hemolysis assay, were approved by the Ethics
Committee of the Second Hospital of Tianjin Medical University. A
total of 18 female BALB/c nude mice (4 weeks old; weight, 18-22 g)
were supplied by Beijing Vital River Laboratory Animal Technology
Co., Ltd. The mice were provided with food and water ad
libitum, and were kept under specific pathogen-free conditions
at 20˚C and 60% humidity with a 12-h alternating light/dark cycle.
Adequate humanitarian care was given, and no mice died during the
experiment. For the tumor growth assay, ~5x105 U87 cells
were mixed with Matrigel (BD Biosciences) at a ratio of 2:1, and
subcutaneously implanted into the abdominal region of the mice for
tumor induction over 7 days. To detect the in vivo antitumor
effects of treatment on the glioma xenografts, the mice were
divided into three groups (n=6/group). Lidocaine, Lid-Lip and
Lid-FA-Lip were administered at a dose of 10 mg/kg (calculated as
lidocaine) via intravenous injection every 3 days. After another 12
days, mice were sacrificed by cervical dislocation and the lack of
heartbeat was confirmed to validate death. Three mice from each
group were sacrificed at the 7-day time point and after 12 days of
treatment. The tumors were then isolated from the mice and the
weights of the tumors were measured. Tumor volume was also
calculated as follows: Tumor volume (mm3)=tumor length
(mm) x tumor width (mm)2/2.
Statistical analysis
All data are presented as the mean ± standard
deviation. The statistical analyses were conducted using GraphPad
Prism 6.0 (GraphPad Software, Inc.). Unpaired Student's t-test was
used for statistical comparisons between two groups. Statistical
comparisons among multiple groups were performed via one-way
analysis of variance followed by Tukey's post hoc tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
Chemistry
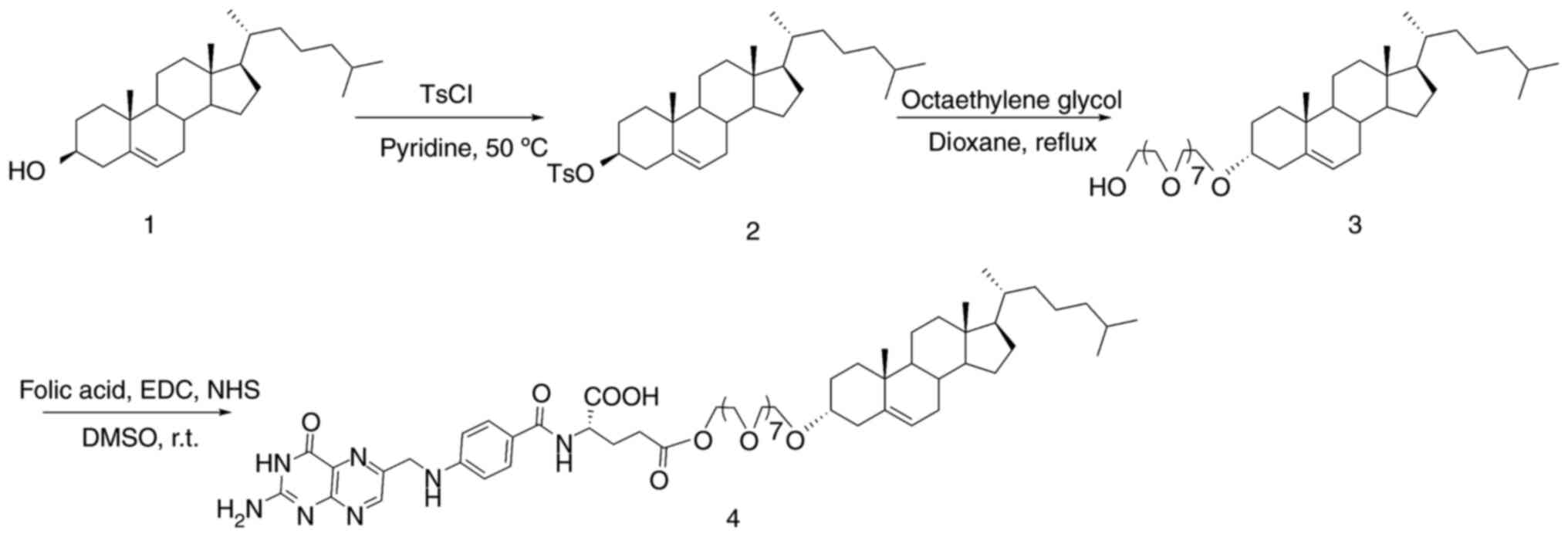
The synthetic pathway of the FA-modified ligand used
in the present study is outlined in Fig. 1. Briefly, cholesterol was esterified
with p-toluenesulfonyl chloride in pyridine to yield
cholesteryl tosylate as an intermediate, followed by etherification
with octaethylene glycol to obtain octaethylene glycol
monocholesteryl ether. Subsequent esterification with FA in the
presence of EDC and NHS yielded the FA-modified ligand.
Characterization of liposomes
For lidocaine-loaded liposomes to cross the BBB and
target glioma, they should have an appropriate size and uniform
distribution. The mean diameters and polydispersity index (PDI) of
the liposomes prepared in the current study were detected using
dynamic light scattering, and the results indicated that these
liposomes had a suitable size (~110 nm) and PDI (<0.2).
Furthermore, the data presented in Table I also show that the EE (%) of the
two liposomes was >88% and the DL (%) was ~2.8%. Additionally,
the weak negative ζ potential of Lid-FA-Lip suggests that its
absorption by the reticuloendothelial system and effect on the
immune response should be minimal (18).
 | Table ICharacterization of Lid-Lip and
Lid-FA-Lip (n=3). |
Table I
Characterization of Lid-Lip and
Lid-FA-Lip (n=3).
| Liposomes | Size (nm) | PDI | EE (%) | DL (%) | ζ potential
(mV) |
|---|
| Lid-Lip | 108.97±5.6 | 0.189±0.043 | 88.51±4.03 | 2.75±0.17 | -5.31±0.28 |
| Lid-FA-Lip | 112.35±9.4 | 0.196±0.069 | 89.76±6.98 | 2.83±0.49 | -8.65±0.77 |
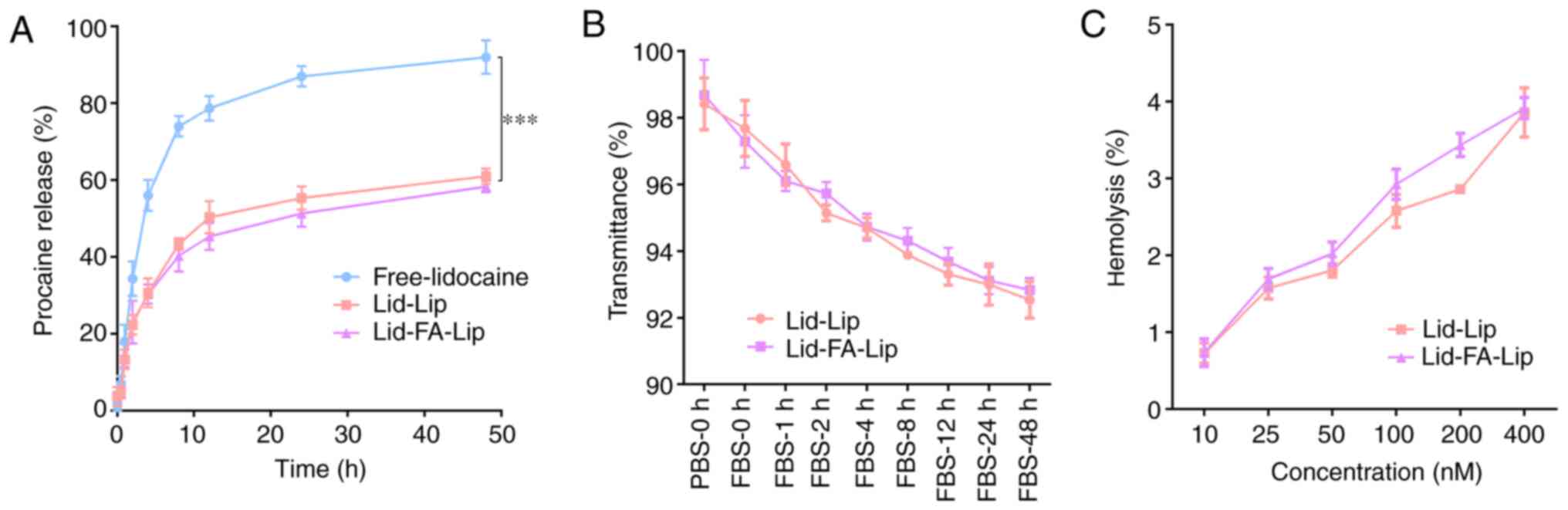
The release behavior study of the liposomes was
investigated in vitro. The results revealed that the free
lidocaine exhibited a fast-release characteristic, with nearly 80%
of the lidocaine being released in 10 h (Fig. 2A). By contrast, the lidocaine-loaded
liposomes released ~40% of the loaded lidocaine within 10 h, and
the release rate slowed subsequently. Fig. 2A also demonstrates that neither of
the two liposome formulations exhibited an initial burst release
profile.
The stability of liposomes under physiological
conditions is important for their in vivo application.
Therefore, the transmittances of the Lid-Lip and Lid-FA-Lip
liposome formulations were evaluated over a 48-h time period. The
transmittance values remained at >92%, with had no marked
differences between the formulations during the 48-h culture upon
FBS treatment (Fig. 2B). The
results suggest that the stability of Lid-Lip and Lid-FA-Lip is
adequate, and supports further investigation in vivo. The
hemocompatibility of Lid-Lip and Lid-FA-Lip was evaluated by
hemolysis assays, and the results showed that the two types of
liposome induced no marked increase in the release of hemoglobin
when the phospholipid concentrations were raised to 400 nM
(Fig. 2C), which implies good
biosafety.
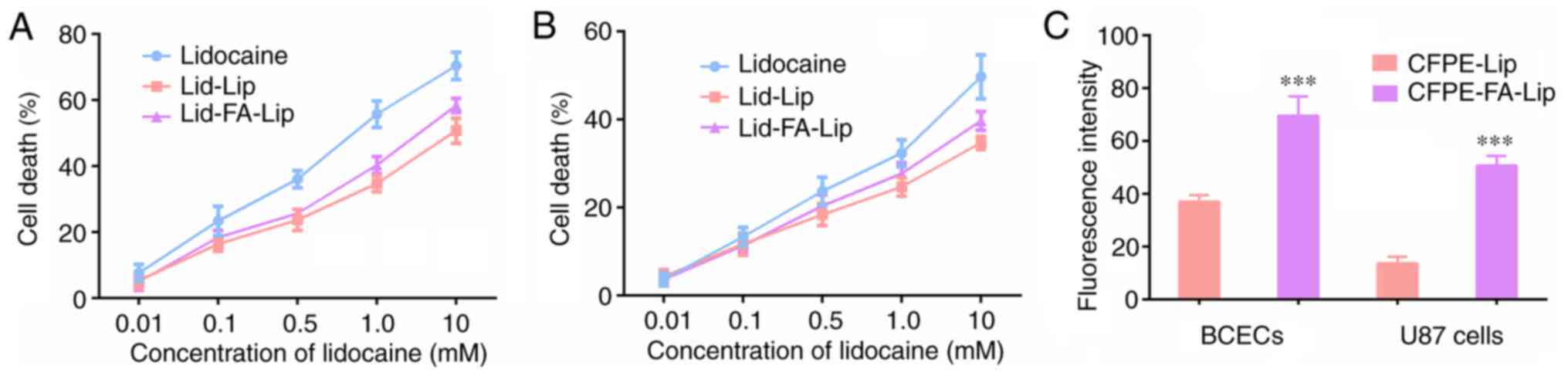
MTT assay
The cytotoxicity of different liposome formulations
to BCECs and U87 cells were evaluated using an MTT assay. As shown
in Fig. 3A, free lidocaine showed a
higher cytotoxic effect than lidocaine-loaded liposomes. This is
likely because the free drug was transported into the cells
directly by passive diffusion, while the liposomes had to undergo a
drug-release process. In addition, Lid-FA-Lip exhibited a slightly
stronger antiproliferative effect than Lid-Lip, which may be due to
the modification by FA enhancing cellular uptake. The inhibitory
effect of the lidocaine formulations on BCEC growth was also
evaluated (Fig. 3B), and the
results demonstrated that the formulations had reduced cytotoxic
effects on the survival of endothelial cells, which indicated the
antitumor specificity of lidocaine.
Transendothelial migration in a BBB
model
BCECs were used to establish a BBB model in
vitro. BCECs on the Transwell membrane from the donor chamber
and U87 cells from the acceptor chamber were collected, and the
fluorescence intensity was measured using a flow cytometer. The
results indicated that the BCEC monolayer on the Transwell membrane
took up and internalized the liposomes (Figs. 3C and S1). In addition, the fluorescence
intensity of the U87 cells in the model in which the BCECs were
treated with the CFPE-FA-Lip formulation was significantly
increased compared with that in which the BCECs were treated with
the CFPE-Lip formulation, which indicates that after penetrating
the BCEC monolayer, CFPE-FA-Lip was transported across the BCEC
monolayer and delivered into the U87 cells more effectively than
CFPE-Lip. These results suggest that the transport of liposomes
across the simulated BBB was enhanced when they were modified with
FA.
Wound closure assay
A wound closure assay was performed to investigate
the effects of Lid-Lip and Lid-FA-Lip on U87 cell migration. The
results reveal that Lid-FA-Lip significantly suppressed the
migration of U87 cells compared with that of the control cells
(Fig. 4A and B), and indicate the strong suppressive
effect of Lid-FA-Lip on glioma cell motility.
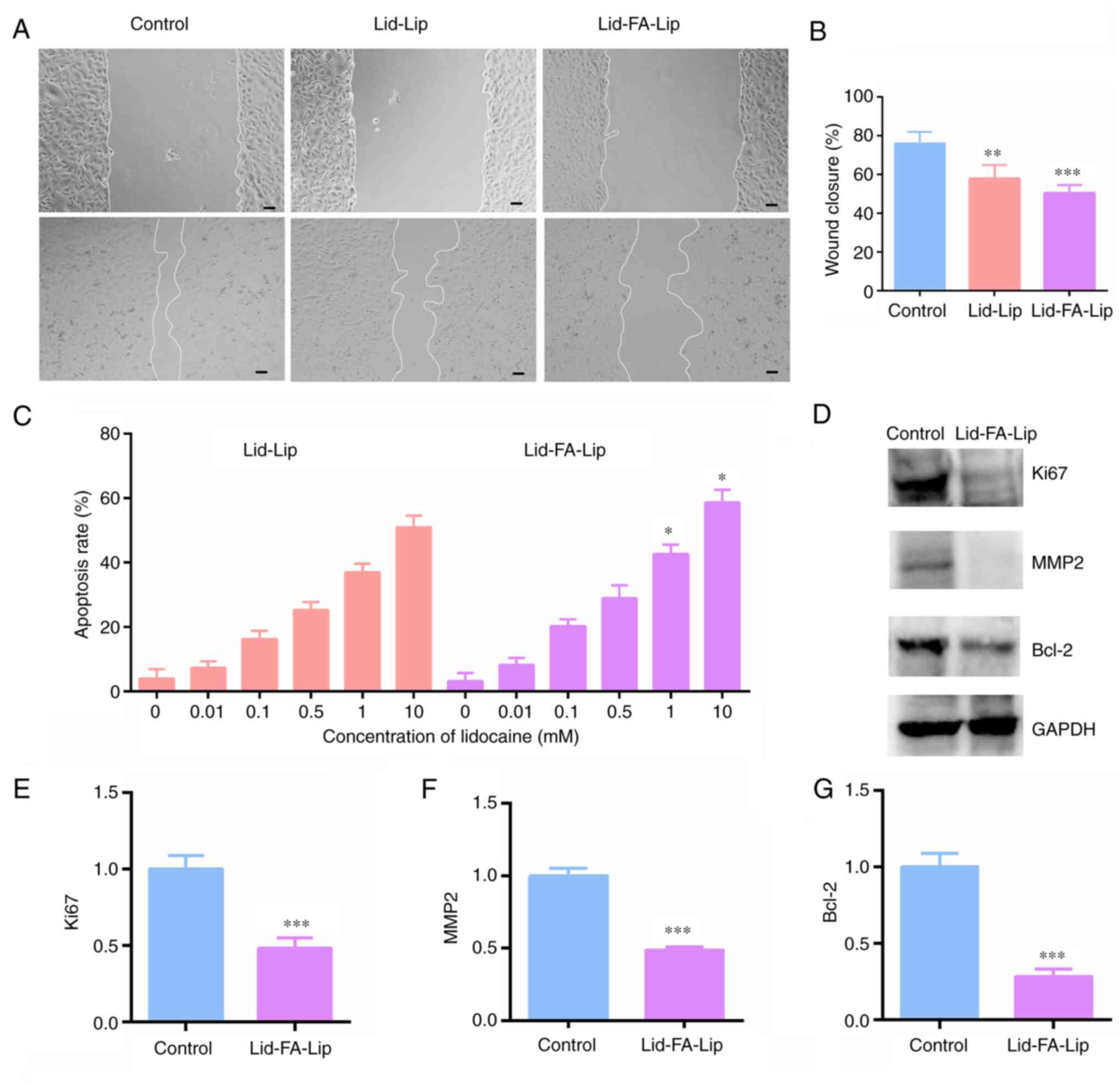 | Figure 4Lidocaine liposome modified with FA
inhibits the migration of glioma cells and stimulates cell
apoptosis. (A) Wound closure assay was performed to evaluate the
migration of U87 cells after treatment with lidocaine-loaded
liposomes. Scale bar, 5 µm. (B) Quantitative analysis of the wound
closure assay. (C) Flow cytometry assays of Annexin V-FITC and PI
staining were performed to evaluate the apoptosis of U87 cells, and
a comparison of quantified apoptosis rates among different groups
is shown. (D) Representative immunoblots showing the expression of
Ki67, MMP2 and Bcl-2 in U87 cells following treatment with
Lid-FA-Lip. (E-G) Results for the quantitative analysis of (E)
Ki67, (F) MMP2 and (G) Bcl-2 expression by western blotting.
Results are presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01,
***P<0.001. FA, folic acid; Lid-Lip, conventional
lidocaine-carrying liposome; Lid-FA-Lip, FA-modified
lidocaine-carrying liposome; MMP2, matrix metalloproteinase 2. |
Cell apoptosis detection assays
Subsequently, the effects of Lid-Lip and Lid-FA-Lip
on the apoptosis of U87 cells were analyzed using flow cytometry.
Notably, the results demonstrated that Lid-FA-Lip stimulated cell
apoptosis in a concentration-dependent manner (Figs. 4C and S2) and can significantly stimulate U87
cell apoptosis.
Western blot analyses demonstrated that Lid-FA-Lip
significantly decreased the expression levels of Ki67, a cell
proliferation marker (Fig. 4D and
E), MMP2, a cell migration marker
(Fig. 4D and F) and Bcl-2, a cell apoptosis marker
(Fig. 4D and G) compared with those in control
cells.
Lid-FA-Lip exhibits antitumor activity
in glioma by targeting the PI3K/AKT pathway
The aforementioned results indicate that Lid-FA-Lip
is able to suppress the proliferation and migration of glioma cells
and stimulate cell apoptosis in vitro. The PI3K/AKT pathway
is known as a target of lidocaine (23); therefore, the effects of Lid-FA-Lip
on this pathway were evaluated. Western blot assays demonstrated
that the phosphorylation levels of PI3Kp85 were significantly
decreased following treatment with Lid-FA-Lip compared with the
control (Fig. 5A and B). Additionally, Lid-FA-Lip treatment
significantly decreased AKT phosphorylation levels compared with
those of the untreated control (Fig.
5A and C). These results
suggest that the antitumor activity of Lid-FA-Lip in glioma is
mediated via targeting the PI3K/AKT pathway.
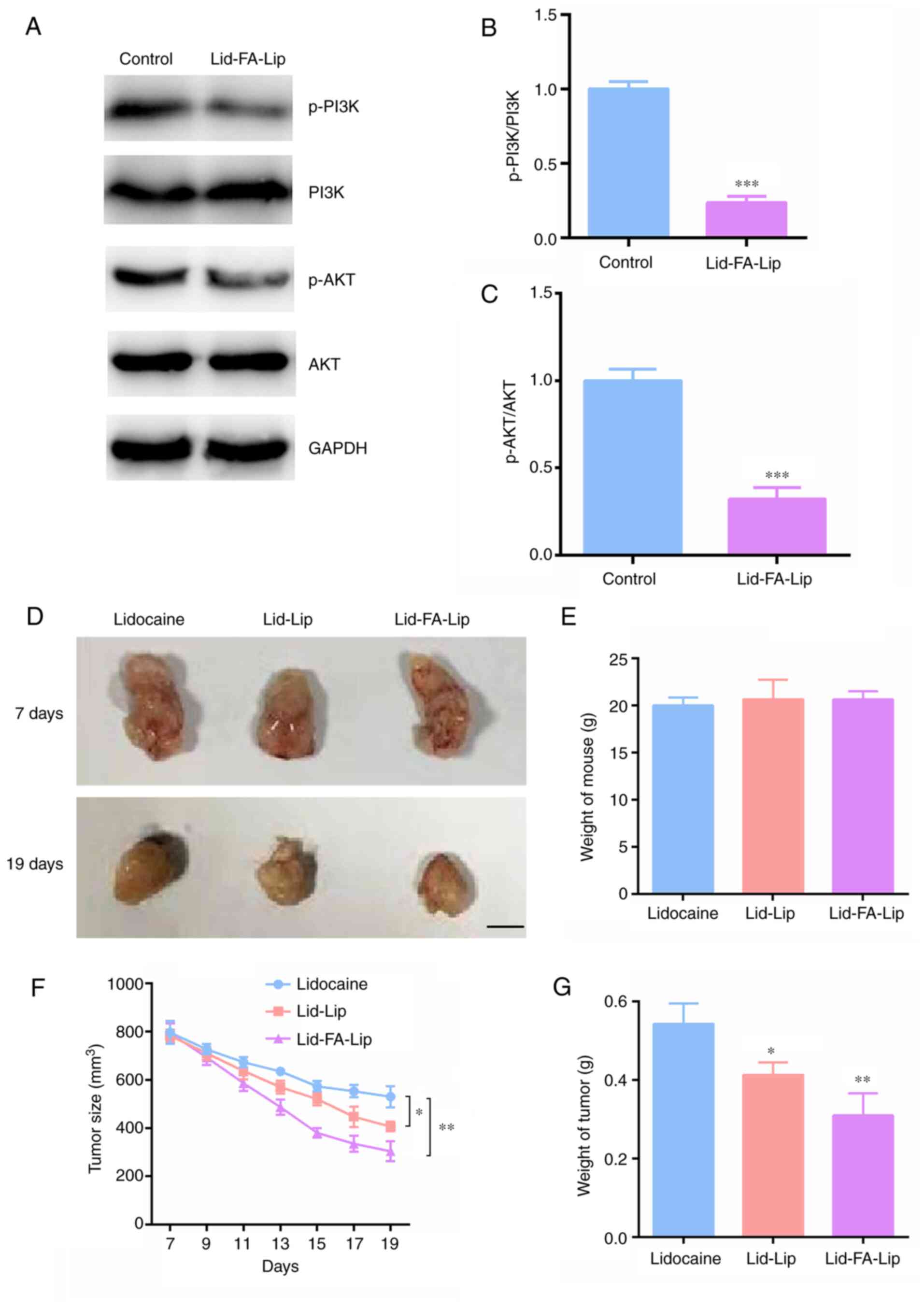 | Figure 5Lidocaine liposome modified with FA
inhibits the tumor growth of glioma cells via the PI3K/AKT pathway.
(A) Representative immunoblots showing p-PI3K, PI3K, p-AKT and AKT
levels in U87 cells following treatment with Lid-FA-Lip.
Quantitative analysis of the (B) p-PI3K/PI3K and (C) p-AKT/AKT
ratios. (D) Representative photographs of tumors formed by U87
cells, isolated from nude mice following the treatment with
different lidocaine formulations (n=6/group). The upper images are
the photographs of the tumors at 7 days, and the lower images show
the tumors at 19 days. Scale bar, 5 mm. (E) Weights of the mice
were measured. (F) Tumor growth curves for the control and the
liposomal treatment groups are shown. (G) Tumor weights were
measured and compared. Results are presented as the mean ± standard
deviation. *P<0.05, **P<0.01,
***P<0.001. FA, folic acid; Lid-Lip, conventional
lidocaine-carrying liposome; Lid-FA-Lip, FA-modified
lidocaine-carrying liposome; p-, phosphorylated. |
The antitumor activity of Lid-FA-Lip was also
validated in vivo. The findings of the xenograft experiment
confirm the effectiveness of this type of targeted therapy. Smaller
tumors were observed in mice from the Lid-FA-Lip treatment group
compared with the Lid-Lip and control groups. Representative images
of tumors from each group are shown in Fig. 5D. The results confirm that, although
there was no significant change in the weight of the mice (Fig. 5E), the tumor volume (Fig. 5F) and tumor weight (Fig. 5G) of the mice treated with
Lid-FA-Lip was significantly reduced. This demonstrates the
antitumor effects of Lid-FA-Lip.
Discussion
Glioma is a craniocerebral malignancy affecting
glial cells (1), and accounts for
~60% of all intracranial tumors (24). Although the BBB results in glioma
having an extremely low rate of metastasis it also hinders the
treatment of glioma using therapeutic drugs. Therefore, the
development of novel targeted drug delivery systems for glioma is
necessary.
Lidocaine is widely used as a local anesthetic. In
addition to having anesthetic effects, lidocaine has also been
reported to have inhibitory effects on tumors (25-27).
A previous study demonstrated that lidocaine can inhibit vascular
endothelial growth factor-A-induced angiogenesis, and therefore may
be able to suppress cancer progression (28). Additionally, lidocaine has been
reported to inhibit the proliferation and invasion of
hepatocellular carcinoma cells by inhibiting the PI3K/Akt pathway
(23). Similarly, another study
demonstrated that the inhibitory effect of lidocaine on lung cancer
cell proliferation is mediated via PI3K/AKT and EGFR pathways
(29). These studies, together with
the findings of the present study, indicate the potential role of
lidocaine in tumor therapy.
In the present study, an FA-cholesterol derivative
was designed and synthesized, which was characterized by HMRS and
elemental analysis to confirm its structure. A lidocaine-loaded
liposomal drug delivery system modified with this ligand was
prepared. Analysis of the characteristics of the liposome in
vitro suggested that it had a suitable size and PDI, which
should contribute to the achievement of passive targeting via
enhanced permeability and retention effects. In addition, the slow
release behavior exhibited in the drug release assay demonstrates
that Lid-FA-Lip significantly improved the release behavior of
lidocaine with a sustained release effect. The hemolysis rate was
<5%, which is regarded as nontoxic (20). The results of all the
characterization assays indicated that the liposomes were suitable
for evaluation in further in vivo and in vitro
experiments.
The present study demonstrated that Lid-FA-Lip had
greater ability to penetrate the BBB in a cell model and improve
cellular uptake compared with Lid-Lip, suggesting that the
transport capacity of the liposomes was enhanced by modification
with FA. The results also revealed that Lid-FA-Lip was able to
inhibit cell migration and induce cell apoptosis. Using wound
closure assays, it was observed that Lid-FA-Lip suppressed the
wound healing of glioma cells, with a reduction in MMP2 expression
compared with that in the control group. Though flow cytometry and
western blotting assays, it was noted that Lid-FA-Lip treatment
significantly stimulated the apoptosis of glioma cells, with a
reduction in the expression levels of Bcl-2, an anti-apoptotic
biomarker. Tumor angiogenesis can affect the prognosis of patients
with tumors. Endothelial cells are important in the formation of
new blood vessels that feed the tumor with nutrients. Notably, the
present confirmed that Lid-FA-Lip had superior antitumor effects
mediated via targeting the PI3K/AKT pathway and thereby suppressed
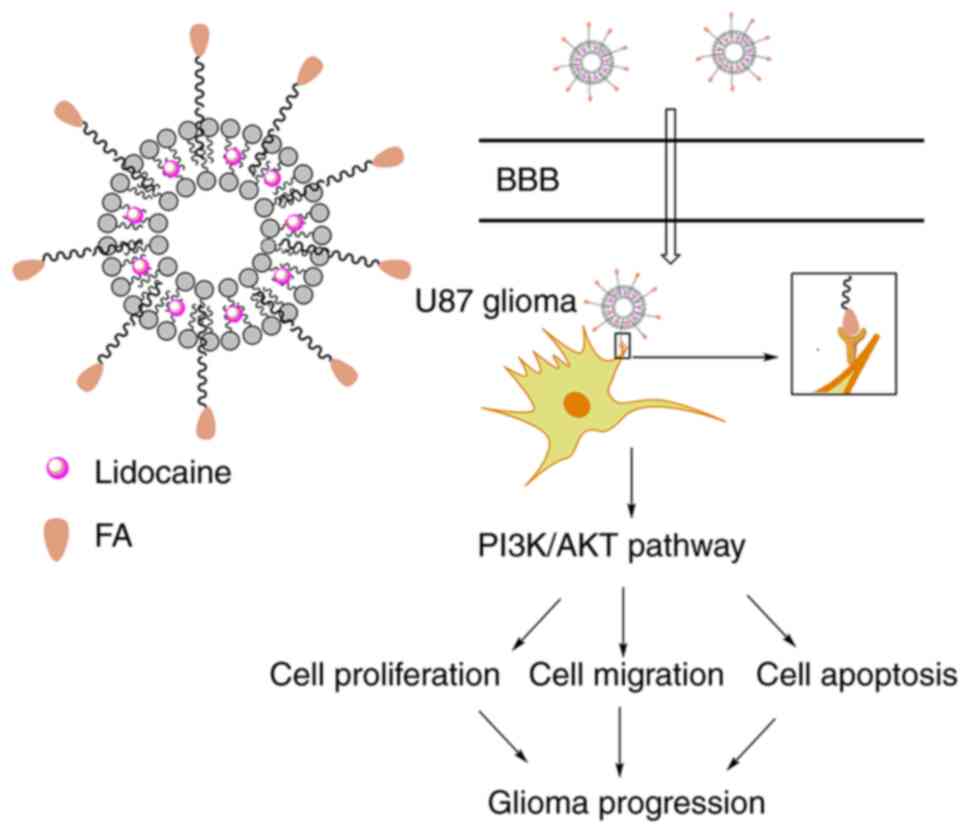
tumor growth. The scheme in Fig. 6
illustrates the suggested mechanism. The PI3K/AKT pathway affects
multiple cellular processes, including cell proliferation,
migration, invasion and apoptosis (30,31),
and is involved in the progression of various cancers (32). A number of proteins promote the
progression and metastasis of cancers, including lung cancer and
pancreatic cancer, via the PI3K/AKT pathway, and drugs targeting
the PI3K/AKT pathway have been used in the clinic or in clinical
trials (33). The PI3K/AKT axis is
also widely known to be involved the regulation of glioma
progression (29). This pathway
affects the proliferation, migration and apoptosis of glioma cells
(29), and the results of the
present study indicate that Lid-FA-Lip exerts antitumor effects via
this pathway; Lid-FA-Lip reduced PI3K and AKT phosphorylation
levels and therefore inhibits this pathway.
In summary, the present study aimed to develop an
effective drug delivery system for the targeting of glioma and to
explore the antitumor activity and mechanism of lidocaine delivered
using this system. A novel FA-cholesterol derivative was designed
and synthesized as a liposomal ligand. The results revealed that FA
increased the ability of the liposome to penetrate an in
vitro model of the BBB and improved cellular uptake. In
addition, Lid-FA-Lip exhibited significant inhibitory effects on
cell motility and induced the apoptosis of glioma cells. It was
further confirmed that Lid-FA-Lip exerted antitumor effects by
targeting the PI3K/AKT pathway and inhibited tumor growth in
vitro. Therefore, it may be concluded that FA is an effective
ligand for transporting lidocaine through the BBB and treating
glioma, and the antitumor effects of Lid-FA-Lip are mediated via
targeting the PI3K/AKT pathway.
Supplementary Material
Representative flow cytometry plots
for the uptake of CFPE-labeled liposomes by BCECs and U87 cells.
BCECs, brain capillary endothelial cells; CFPE-Lip, conventional
CFPE-carrying liposome; CFPE-FA-Lip, FA-modified CFPE-carrying
liposome; CFPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein;
FA, folic acid.
Representative flow cytometry plots
for the stimulation of the apoptosis of U87 cells by Lid-Lip and
Lid-FA-Lip at a range of lidocaine concentrations. Lid-Lip,
conventional lidocaine-carrying liposome; Lid-FA-Lip, FA-modified
lidocaine-carrying liposome; FA, folic acid.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the 2019 Middle-aged and
Young Training Fund Project of the Anesthesiology Branch of Tianjin
Medical Association (grant no. TJMZJJ-2019-03) and the Youth Fund
of the Second Hospital of Tianjin Medical University (grant no.
2018ydey14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, XY, BL and CY carried out the molecular biology
experiments and drafted the manuscript. DL, XY, JS, MY and HW
participated in the design of the study and performed the
statistical analysis. DL, XY and YL conceived of the study,
participated in its design and coordination and helped to draft the
manuscript. DL and YL confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of the Second Hospital of Tianjin
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimou J and Kelly J: The biological and
clinical basis for early referral of low grade glioma patients to a
surgical neuro-oncologist. J Clin Neurosci. 78:20–29.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu M and Wang L: Prognostic significance
of preoperative serum albumin, albumin-to-globulin ratio, and
prognostic nutritional index for patients with glioma: A
meta-analysis. Medicine (Baltimore). 99(e20927)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang J, Yu J, Tu L, Huang N, Li H and Luo
Y: Isocitrate dehydrogenase mutations in glioma: From basic
discovery to therapeutics development. Front Oncol.
9(506)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang X, Zhao L, Zhai G, Ji J and Liu A:
Erratum: Multifunctional polyethylene glycol (PEG)-Poly
(Lactic-Co-Glycolic Acid) (PLGA)-based nanoparticles loading
doxorubicin and tetrahydrocurcumin for combined chemoradiotherapy
of glioma. Med Sci Monit. 26(e926333)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Finck T, Gempt J, Krieg SM, Meyer B,
Zimmer C, Wiestler B, Kirschke JS and Sollmann N: Assessment of the
extent of resection in surgery of high-grade glioma-evaluation of
black blood sequences for intraoperative magnetic resonance imaging
at 3 tesla. Cancers (Basel). 12(1580)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Opoku-Darko M, Lang ST, Artindale J,
Cairncross JG, Sevick RJ and Kelly JJP: Surgical management of
incidentally discovered diffusely infiltrating low-grade glioma. J
Neurosurg. 129:19–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pisapia DJ: The updated World Health
Organization glioma classification: Cellular and molecular origins
of adult infiltrating gliomas. Arch Pathol Lab Med. 141:1633–1645.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
de Blank P, Fouladi M and Huse JT:
Molecular markers and targeted therapy in pediatric low-grade
glioma. J Neurooncol. 150:5–15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yan C, Wang J, Yang Y, Ma W and Chen X:
Molecular biomarker-guided anti-angiogenic targeted therapy for
malignant glioma. J Cell Mol Med. 23:4876–4882. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li J, Chai Z, Lu J, Xie C, Ran D, Wang S,
Zhou J and Lu W: αvβ3-targeted liposomal drug
delivery system with attenuated immunogenicity enabled by linear
pentapeptide for glioma therapy. J Control Release. 322:542–554.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He Y, Wu C, Duan J, Miao J, Ren H and Liu
J: Anti-Glioma effect with targeting therapy using folate modified
nano-micelles delivery curcumin. J Biomed Nanotechnol. 16:1–13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ye L, Zhang Y, Chen YJ and Liu Q:
Anti-tumor effects of lidocaine on human gastric cancer cells in
vitro. Bratisl Lek Listy. 120:212–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li YC, Wang Y, Li DD, Zhang Y, Zhao TC and
Li CF: Procaine is a specific DNA methylation inhibitor with
anti-tumor effect for human gastric cancer. J Cell Biochem.
119:2440–2449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zuckerman LM, Frames WL, Mirshahidi HR,
Williams NL, Shields TG, Otoukesh S and Mirshahidi S:
Antiproliferative effect of bupivacaine on patient-derived sarcoma
cells. Mol Clin Oncol. 13(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Unami A, Shinohara Y, Ichikawa T and Baba
Y: Biochemical and microarray analyses of bupivacaine-induced
apoptosis. J Toxicol Sci. 28:77–94. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao Y, Zhang L, Peng Y, Yue QM, Hai L,
Guo L, Wang QT and Wu Y: GLUT1-mediated
venlafaxine-thiamine disulfide system-glucose conjugates with
‘lock-in’ function for central nervous system delivery. Chem Biol
Drug Des. 91:707–716. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsou YH, Zhang XQ, Zhu H, Syed S and Xu X:
Drug Delivery to the brain across the blood-brain barrier using
nanomaterials. Small. 13(e1701921)2017.PubMed/NCBI View Article : Google Scholar : Erratum in: Small
14, e1801588, 2018.
|
|
18
|
Yang Y, Zhao Z, Xie C and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228(104882)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akal ZU, Alpsoy L and Baykal A:
Superparamagnetic iron oxide conjugated with folic acid and
carboxylated quercetin for chemotherapy applications. Ceram Int.
42:9065–9072. 2016.
|
|
20
|
Zhao Z, Chen C, Xie C and Zhao Y: Design,
synthesis and evaluation of liposomes modified with dendritic
aspartic acid for bone-specific targeting. Chem Phys Lipids.
226(104832)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao Z, Zhao Y, Xie C, Chen C, Lin D, Wang
S, Lin D, Cui X, Guo Z and Zhou J: Dual-active targeting liposomes
drug delivery system for bone metastatic breast cancer: Synthesis
and biological evaluation. Chem Phys Lipids.
223(104785)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Su Z, Xing L, Chen Y, Xu Y, Yang F, Zhang
C, Ping Q and Xiao Y: Lactoferrin-Modified Poly(ethylene
glycol)-Grafted BSA nanoparticles as a dual-targeting carrier for
treating brain gliomas. Mol Pharm. 11:1823–1834. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Y, Jia J, Jin W, Cao J, Fu T, Ma D
and Zhang Y: Lidocaine inhibits the proliferation and invasion of
hepatocellular carcinoma by downregulating USP14 induced PI3K/Akt
pathway. Pathol Res Pract. 216(152963)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Habimana-Griffin L, Ye D, Carpenter J,
Prior J, Sudlow G, Marsala L, Mixdorf M, Rubin J, Chen H and
Achilefu S: Intracranial glioma xenograft model rapidly
reestablishes blood-brain barrier integrity for longitudinal
imaging of tumor progression using fluorescence molecular
tomography and contrast agents. J Biomed Opt. 25:1–13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Du J, Zhang L, Ma H, Wang Y and Wang P:
Lidocaine suppresses cell proliferation and aerobic glycolysis by
regulating circHOMER1/miR-138-5p/HEY1 axis in colorectal cancer.
Cancer Manag Res. 12:5009–5022. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Siekmann W, Tina E, Von Sydow AK and Gupta
A: Effect of lidocaine and ropivacaine on primary (SW480) and
metastatic (SW620) colon cancer cell lines. Oncol Lett. 18:395–401.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou D, Wang L, Cui Q, Iftikhar R, Xia Y
and Xu P: Repositioning lidocaine as an anticancer drug: The role
beyond anesthesia. Front Cell Dev Biol. 8(565)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suzuki S, Mori A, Fukui A, Ema Y and
Nishiwaki K: Lidocaine inhibits vascular endothelial growth
factor-A-induced angiogenesis. J Anesth. 34:857–864.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun H and Sun Y: Lidocaine inhibits
proliferation and metastasis of lung cancer cell via regulation of
miR-539/EGFR axis. Artif Cells Nanomed Biotechnol. 47:2866–2874.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cierniak S, Koktysz R, Jesiotr M,
Gasowska-Bodnar A and Bodnar L: Expression of the PI3K/AKT/mTOR
pathway as a prognostic factor in patients with advanced high grade
serous ovarian carcinoma treated with neoadjuvant chemotherapy. Eur
J Gynaecol Oncol. 40:744–751. 2019.
|
|
31
|
Zuo X, Li L and Sun L: Plantamajoside
inhibits hypoxia-induced migration and invasion of human cervical
cancer cells through the NF-κB and PI3K/akt pathways. J Recept
Signal Transduct Res. 41:339–348. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xiao Y, Deng T and Wang D: Davanone
terpenoid inhibits cisplatin-resistant acute myeloid leukemia
cancer cell growth by inducing caspase-dependent apoptosis, loss of
mitochondrial membrane potential, inhibition of cell migration and
invasion and targeting PI3K/AKT/MAPK signalling pathway. J BUON.
25:1607–1613. 2020.PubMed/NCBI
|
|
33
|
Liu Z, Mo H, Sun L, Wang L, Chen T, Yao B,
Liu R, Niu Y, Tu K, Xu Q and Yang N: Long noncoding RNA
PICSAR/miR-588/EIF6 axis regulates tumorigenesis of hepatocellular
carcinoma by activating PI3K/AKT/mTOR signaling pathway. Cancer
Sci. 111:4118–4128. 2020.PubMed/NCBI View Article : Google Scholar
|