Introduction
Postoperative cognitive dysfunction (POCD) is a
common clinical syndrome in elderly patients following surgery
(1,2) which negatively affects quality of life
and is associated with high mortality (3,4). The
pathogenesis of POCD is not fully understood, but has been
determined to involve neuroinflammation, oxidative stress,
autophagy disorder, impaired synaptic function and a lack of
neuro-nutritional support (5).
Several studies in animals have found that neuroinflammation might
be the crucial factor in POCD (6-8).
Currently, the treatment of POCD is inconclusive, therefore, the
modifiable factors of POCD should be determined and preventive
strategies must be formulated.
Dexmedetomidine (DEX) is an effective α2-adrenergic
receptor agonist (9). DEX has been
widely reported in ischemic-reperfusion models, exhibiting
resistance in free radicals and cell apoptosis (10,11).
Previous studies have demonstrated that DEX can reduce inflammation
and has neuroprotective effects, thereby improving postoperative
cognitive dysfunctions (12,13).
Further research confirmed that the use of DEX during carotid
endarterectomy can reduce the incidence of POCD after surgery short
term, which is associated with the inhibition of the inflammatory
response and an increase in the expression of brain-derived
neurotrophic factor (14). However,
the specific mechanism by which DEX improves POCD remains
unclear.
Neuronal nitric oxide synthase (nNOS) is a
constitutive neuronal enzyme that is important in regulating
central nervous system functions (15). Previous reports have confirmed that
inhibition of nNOS can impair learning and memory (16). In addition, DEX can serve a
protective role in brain injury by inhibiting nNOS-nitric oxide
signaling (17). Treatment with DEX
can also alleviate traumatic brain injury and promote cognitive and
motor recovery after brain injury (18). Thus, to determine the association
between DEX and nNOS in POCD, NG-nitro-L-arginine methyl ester
(L-NAME), a nonspecific NOS inhibitor, was used in the present
study. The carotid artery of aged rats was exposed to mimic POCD
and the expression of relevant indicators following surgery was
investigated, with or without L-NAME treatment.
Materials and methods
Animals
50 Male Sprague Dawley rats (weight, 500-650 g; age,
20 months old) were purchased from Charles River Laboratories and
housed in groups under controlled environmental conditions. All
animals were grouped in a 12 h light/dark cycle in a room with
controlled temperature and humidity (22±1˚C and 50-60%) and fed
water and food ad libitum. All experiments were approved by
the Animal Experiment Center of the Institute of Radiation Medicine
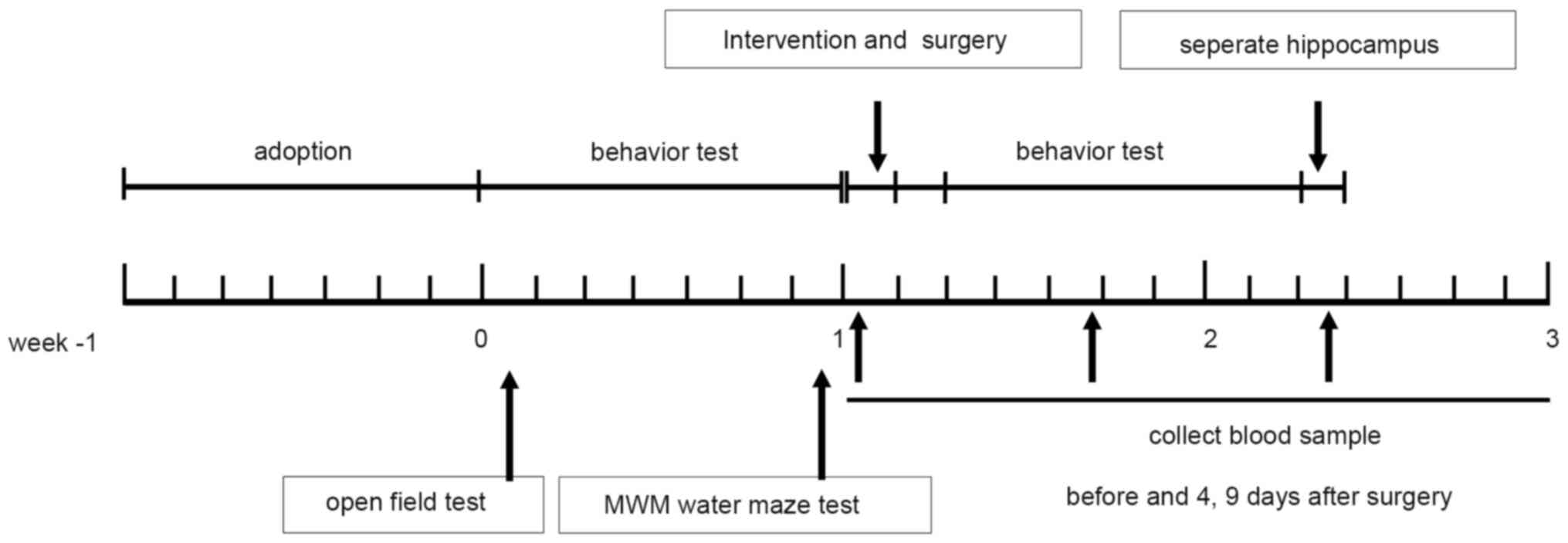
of the Chinese Academy of Medical Sciences.Study design. Rats were
randomly divided into 5 groups: i) Sham; ii) surgery; ii) surgery +
L-NAME; iv) surgery + DEX; and v) surgery + DEX + L-NAME, with 10
rats per group. At 30 min prior to surgery, rats in each group
received the following treatment: i) Rats in the sham group did not
receive any treatment; ii) rats in the surgery + L-NAME group were
injected intraperitoneally with 25 mg/kg L-NAME; iii) rats in the
surgery + DEX group were injected intraperitoneally with 12 µg/kg
DEX; iv) rats in the surgery + L-NAME group were injected with 25
mg/kg L-NAME; and v) rats in the surgery + DEX + L-NAME group were
injected intraperitoneally with 12 µg/kg DEX and 25 mg/kg L-NAME.
The open field test (OFT) was performed on days 8 and 16. The
Morris water maze (MWM) training was conducted on days 9-13 and
days 17-21, and the MWM test was conducted on days 14 and 2 ml tail
vein blood was collected both prior to surgery and on day 4 and 9
following surgery. Surgical methods were based on previous reports
(5). Firstly, following
anesthetization by intraperitoneal injection of 3% sodium
pentobarbital (50 mg/kg), a 1.5 cm opening was cut at the midline
of the neck, opening the soft tissue on the trachea. Subsequently,
a 1 cm long part of the right common carotid artery was removed and
separated from the adjacent tissue. The skin was sutured and
surgery was conducted in a sterile environment. Once tail vein
blood (2 ml) had been collected 9 days post surgery, the rats were
anesthetized with 3% sodium pentobarbital (50 mg/kg) and treated
with cardiac perfusion. Rats were euthanized by intraperitoneal
injection with excessive sodium pentobarbital (200 mg/kg) and the
vena cava blood and the hippocampus tissue were collected for
ELISA, western blotting, terminal dexynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) staining and
immunohistochemical staining (Fig.
1).
OFT
Animal locomotor activity was monitored for 10 min
using an OFT. The test was conducted on days 8 and 16. A wooden box
(100x100x45 cm) was divided into 16 squares and the rats were
placed in the center of the box. Each rat was placed in the corner
to acclimate for 5 min, and then placed into a new square. While
the rat crossed the square, a vertical lattice counting and
horizontal lattice counting was used to monitor and record
movements over a 5 min period. After each rat was tested, the
wooden box was cleaned with 75% ethanol and wiped dry with cotton
balls to minimize the effect of odor on subsequent experiments.
MWM test
The MWM test was used to assess spatial learning,
memory and cognitive flexibility in each group. The maze consisted
of orientation, navigation and spatial probe tests. A swimming pool
with a diameter of 122 cm and a depth of 35 cm was divided into
four equal parts. The water in the swimming pool, with a depth of
17 cm, was heated to 22˚C prior to the experiment. A platform (10
cm2) was invisibly located in the center of the target
quadrant. During the experiment, the hidden platform was placed in
1 of the quadrants 1.5 cm below the water surface. Specific methods
were based on a previous report (19).
TUNEL staining
Hippocampus tissue were fixed with 4%
paraformaldehyde for 24 h at room temperature (RT), dehydrated
using graded ethanol, embedded in paraffin and sliced at a
thickness of 5 µm. Apoptotic cell death in hippocampus tissue was
detected by utilizing a TUNEL kit (cat. no. 11684795910; Roche
Diagnostics) according to the manufacturer's instructions. Slices
were stained using the solution included in the TUNEL kit at RT for
1 h, and then stained using Hematoxylin at RT for ~3 min. After
sealing with neutral gum sections were imaged and captured using
light microscopy (magnification, x40). Nine fields of view were
observed in each group and analyzed using 1.0 ImageJ software
(National Institutes of Health).
Western blot analysis
At 4 days after surgery, proteins were extracted
from hippocampus tissue using RIPA lysis buffer (Applygen
Technologies, Inc.) and a mixture of protease inhibitors and
phosphatase inhibitors (Pierce; Thermo Fisher Scientific, Inc.).
Extracted protein was measured using a BCA kit (Nanjing Jiancheng
Bioengineering Institute) and mixed with 5X loading buffer. Samples
(40 µg/lane) were separated using a 12% (w/v) gradient SDS gel and
transferred to PVDF membranes. After blocking with 5% skimmed milk
at RT for 90 min, the blots were incubated with the following
primary antibodies at 4˚C overnight: Bax (1:2,000; cat.
no. ab32503; Abcam), Bcl-2 (1:2,000; cat. no. ab196495; Abcam),
cleaved caspase-3 (1:1,500; cat. no. ab32042; Abcam), cleaved
caspase-9 (1:2,000; cat. no. ab2324; Abcam) and GAPDH (1:1,000;
cat. no. ab199553; Abcam). The membranes were then incubated with
goat anti-rabbit HRP conjugated secondary antibodies [1:10,000;
cat. no. 70-GAR0072; Multi Sciences (Lianke) Biotech Co., Ltd.] at
RT and washed with TBST (Tris-HCl buffer and 1% Tween). Bands were
then detected using an ECL kit (Bio-Rad Laboratories, Inc.) and
quantified using Image Lab 3.0 software (Bio-Rad).
ELISA
Blood was collected prior to and at 4, 9 days after
surgery and centrifuged at 4,000 x g for 10 min at 4˚C
to prepare the serum. Concentrations of TNF-α (cat. no.
CSB-E11987r; Cusabio Technology), IL-6 (cat. no. CSB-E04640r;
Cusabio Technology), IL-1β (cat. no. CSB-E08055r; Cusabio
Technology) and IL-10 (cat. no. CSB-E04595r; Cusabio Technology) in
serum were determined using ELISA kits following the manufacturer's
instructions.
NOS activity detection in hippocampus
The expression of NOS in the hippocampus was detected according to
a previous report (20)
After protein extraction that was performed as
described above, NOS activity was measured using the NO
Fluoro-metric Assay kit (Nanjing Jiancheng Bioengineering
Institute) in accordance with the manufacturer's protocol. NOS
activity was then detected by measuring absorbance at 550 nm and
calculated using the standard curve.
Immunohistochemical staining
Hippocampus tissue was pretreated according to the
protocol described in the TUNEL staining paragraph.
Immunohistochemical staining was performed using de-paraffinized
sections. Briefly, 5 µm-sections were preheated in an oven,
de-paraffinized by xylene and rehydrated via graded ethanol. The
sections were then incubated with anti-nNOS (1:200; cat. no.
ab5586; Abcam) primary antibodies at RT for 90 min followed by
HRP-conjugated goat anti-rabbit secondary antibodies (1:5,000, cat.
no. ab205718; Abcam) incubation at 37˚C for 90 min. A
DAB kit (cat. no. ZLI-9018; OriGene Technologies, Inc.) was used to
visualize the sections with a microscope. Nuclei were stained using
hematoxylin at RT for 3 min and the images were captured using a
light microscope (magnification, x20).
Flow cytometry
Flow cytometry was used to detect blood T lymphocyte
subsets (CytomicsFC500; Beckman Coulter, Inc.). Blood samples with
the heparin anticoagulant were collected at various times according
to the experimental design. Specimens were prepared using FACS
lysing solution (BD Biosciences) at RT for 10 min. Cells were then
permeabilized and fixed with cytofix/Cytoperm Plus solution (BD
Biosciences) at RT for 20 min. Cells were labeled with the
following monoclonal antibodies conjugated with different
fluorescent dyes at 4˚C for 30 min: Anti-CD3-PE (5
µl/105 cell; cat. no. 15-0038-42; Invitrogen; Thermo
Fisher Scientific, Inc.), anti-CD4-APC (5 µl/105 cell;
cat. no. 17-0049-42; Invitrogen; Thermo Fisher Scientific, Inc.)
and anti-CD8-FITC (0.1 µg/1x106 cells; cat. no.
MA5-17604; Invitrogen; Thermo Fisher Scientific, Inc.). Finally
cells were analyzed using a FACS CANTO™ II flow cytometer
(Becton-Dickinson) and data were analyzed using Flowjo 7.6.1
software (Tree Star, Inc.).
Statistical analysis
All experiments were repeated three times and the
final data were expressed as the mean ± SD. Statistically
significant differences were analyzed using one-way ANOVA analysis
and Tukey multiple comparison tests. A value of P<0.05 was
considered statistically significant. All statistical analyses were
conducted using GraphPad Prism 7.0 (GraphPad Software, Inc.).
Results
Comparison of the behavioral
differences of rats in each group
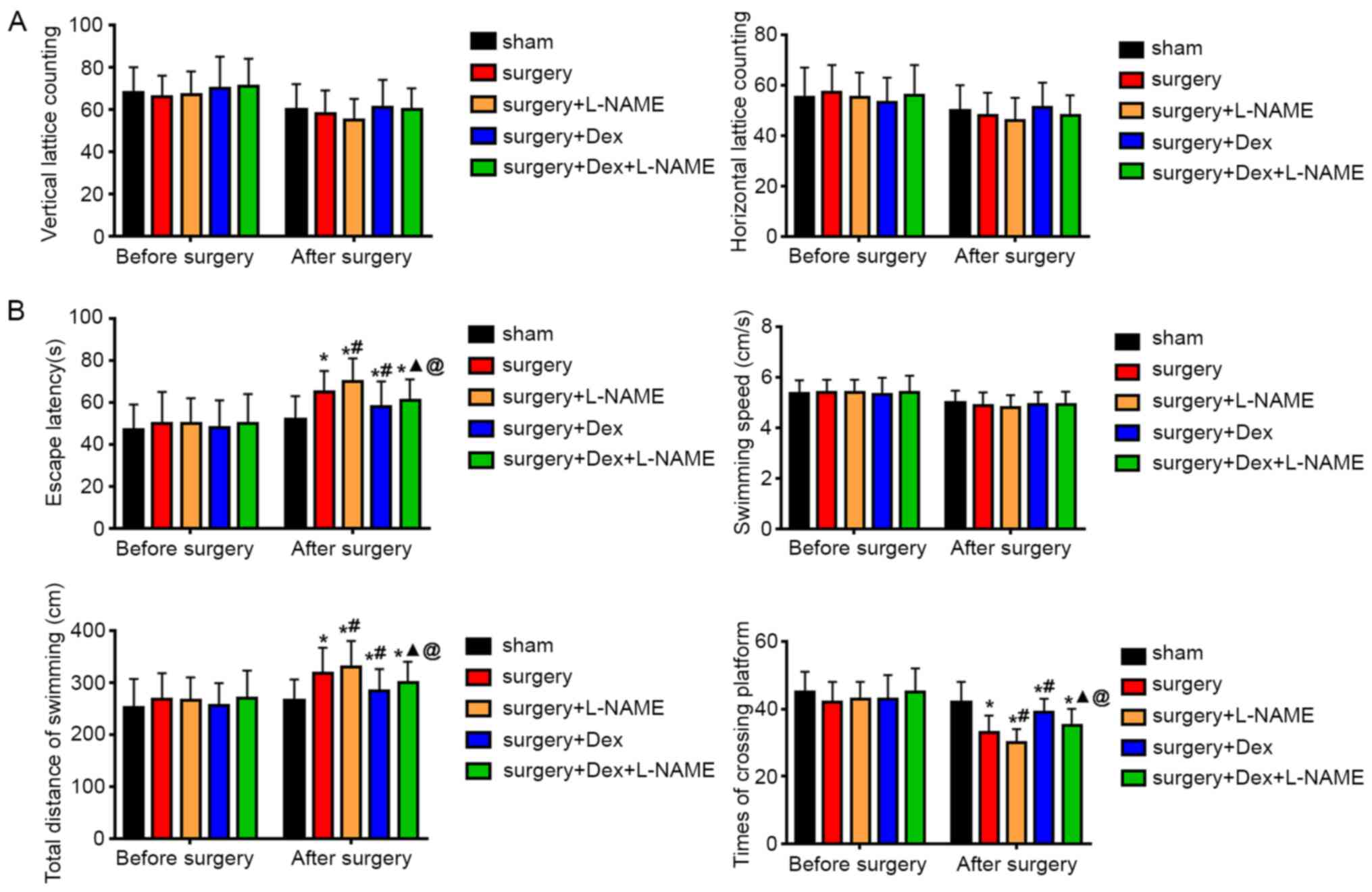
The locomotor activity of rats was initially
measured using the OFT. After evaluating both horizontal lattice
counts and vertical lattice counts, the results demonstrated that
there was no significant difference between the rats in each group
(Fig. 2A), indicating that the
observed cognitive deficits of the OFT test did not affect
locomotor capacity. The MWM test was performed to investigate
spatial learning, memory and cognitive flexibility in each group of
rats. Cognitive and behavioral tests in the surgery group revealed
an impaired exploratory behavior as indicated by significant
increases in the escape latency, total distance of swimming and
decrease in the times of platform crossing, when compared with the
corresponding group prior to surgery (Fig. 2B). Regarding escape latency and
total distance of swimming, the surgery + DEX group demonstrated
significant decreases when compared with the surgery group, while
demonstrating significantly increased times in crossing platforms.
Compared with the surgery + DEX and surgery + L-NAME groups, the
escape latency and total swimming distance of the surgery + DEX +
L-NAME group were significantly increased. In addition, the escape
latency and total swimming distance in the surgery + DEX + L-NAME
group were increased compared with the surgery + DEX group. The
results of swimming speed indicated that there was no statistical
difference between the groups. However, the time of crossing
platform in the surgery + DEX + L-NAME group was significantly
increased compared with the surgery + L-NAME group, but was
remarkably decreased compared with the surgery + DEX group. The
results indicated that cognitive impairment did not affect the
locomotor activity of rats and that DEX improved the cognitive
impairment of rats caused by surgery.
Comparison of apoptosis in hippocampus
tissue
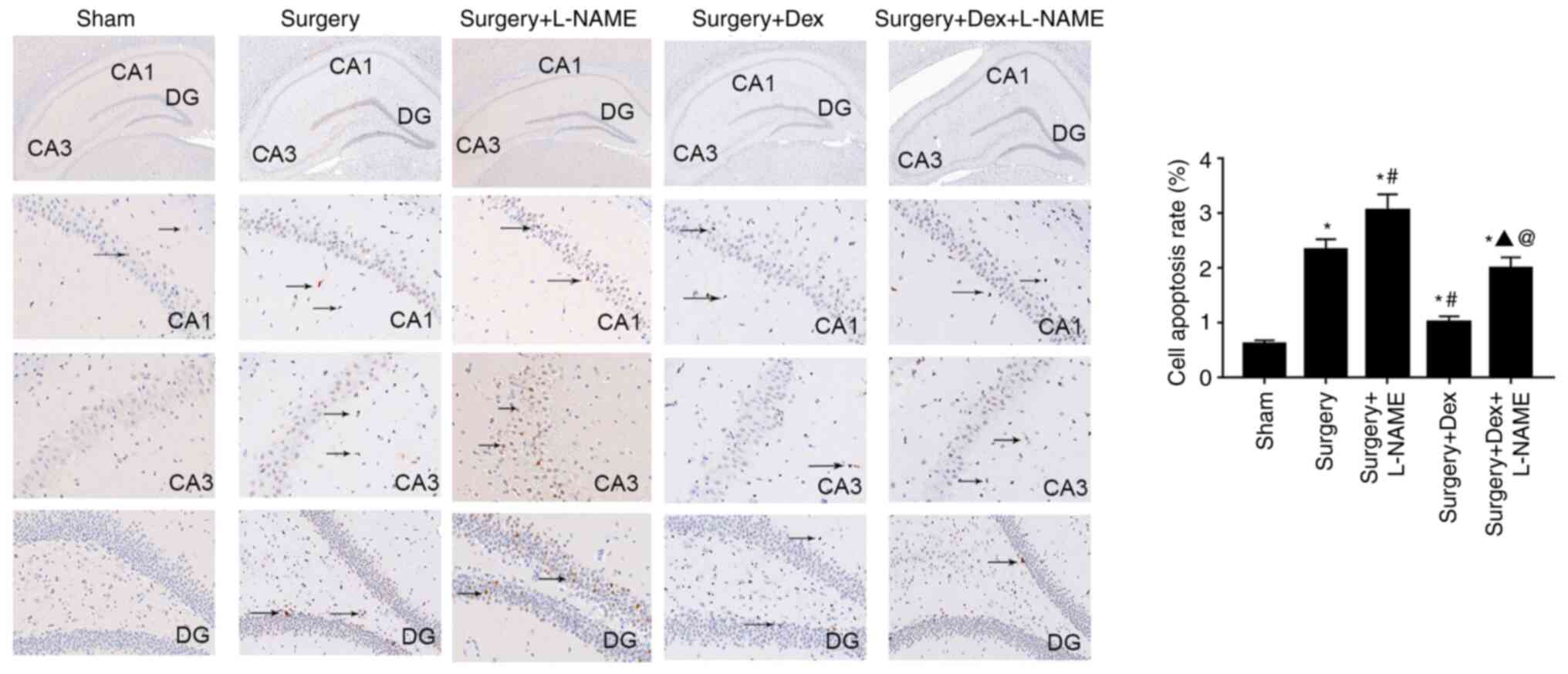
The results of TUNEL staining are indicated in
Fig. 3. Significant neuronal
apoptosis was identified in the hippocampus of the sham group when
compared with the hippocampus of operated rats. CA1, CA3 and DG are
the dentate gyrus that comprise the main hippocampus. There was
ameliorated apoptosis in the hippocampus tissue when the rats were
treated with DEX or DEX + L-NAME following surgery. Moreover, rats
in the surgery + DEX group presented a significantly decreased cell
apoptosis rate compared with the surgery + DEX + L-NAME and the
surgery + L-NAME groups. To further evaluate cell apoptosis in
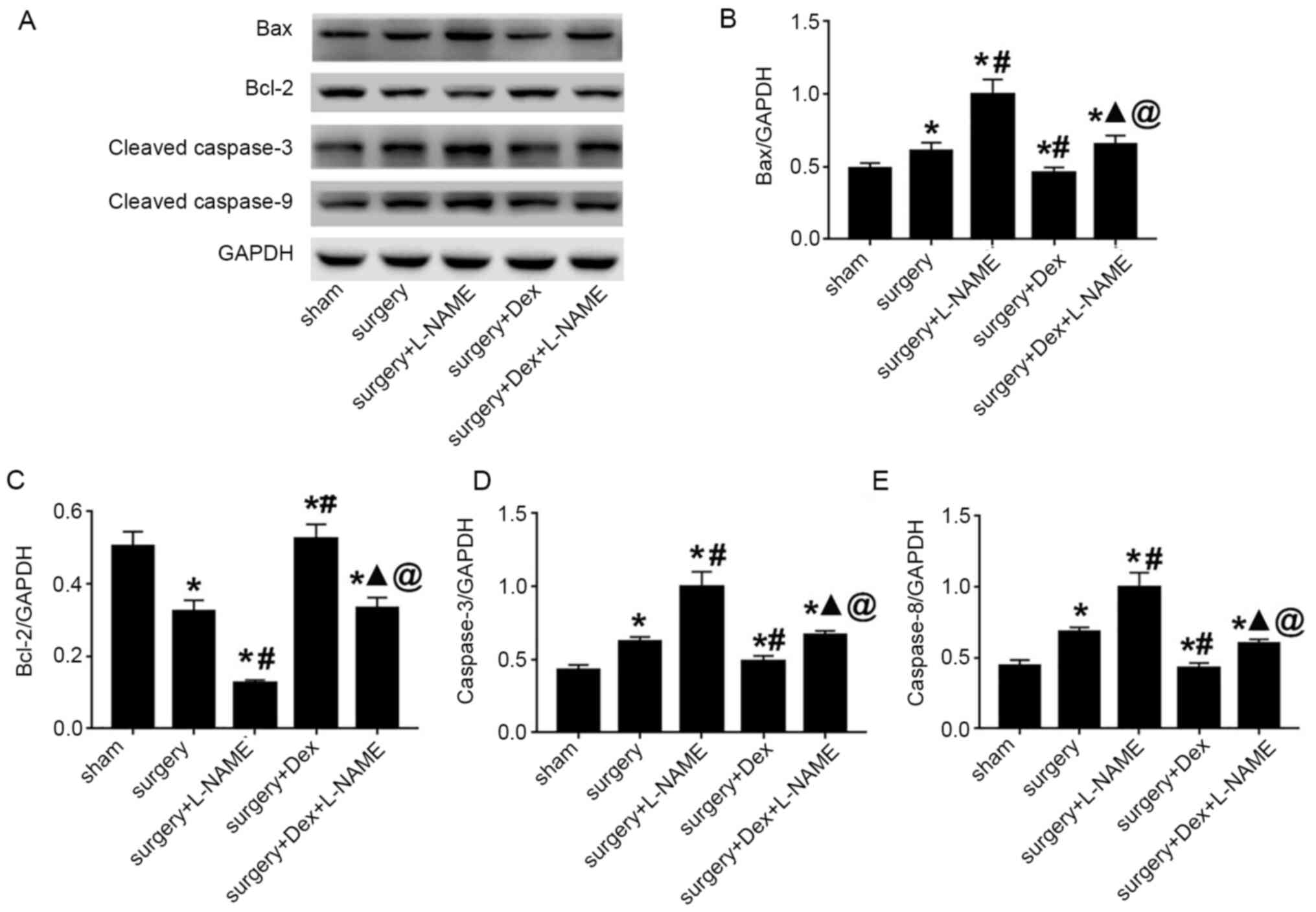
hippocampus tissue, apoptosis-associated proteins were detected by
western blotting (Fig. 4A). The
results indicated that the expression levels of Bax, cleaved
caspase-3 and cleaved caspase-9 of the surgery group were
significantly increased compared with the sham group. Compared with
surgery group, rats treated with DEX and surgery significantly
attenuated the upregulation of Bax, cleaved caspase-3 and cleaved
caspase-9 levels. The surgery + DEX + L-NAME group demonstrated the
inhibition in the expression changes of Bax, cleaved caspase-3 and
cleaved caspase-9, when compared with the surgery + L-NAME group.
However, Bax, cleaved caspase-3 and cleaved caspase-9 expression in
the surgery + DEX + L-NAME group exceeded that of the surgery + DEX
group (Fig. 4B, D and E).
In addition, the surgery + DEX group demonstrated an upregulated
expression of Bcl-2 (Fig. 4C).
Together, these findings indicated that DEX significantly
attenuated apoptosis induced by surgery.
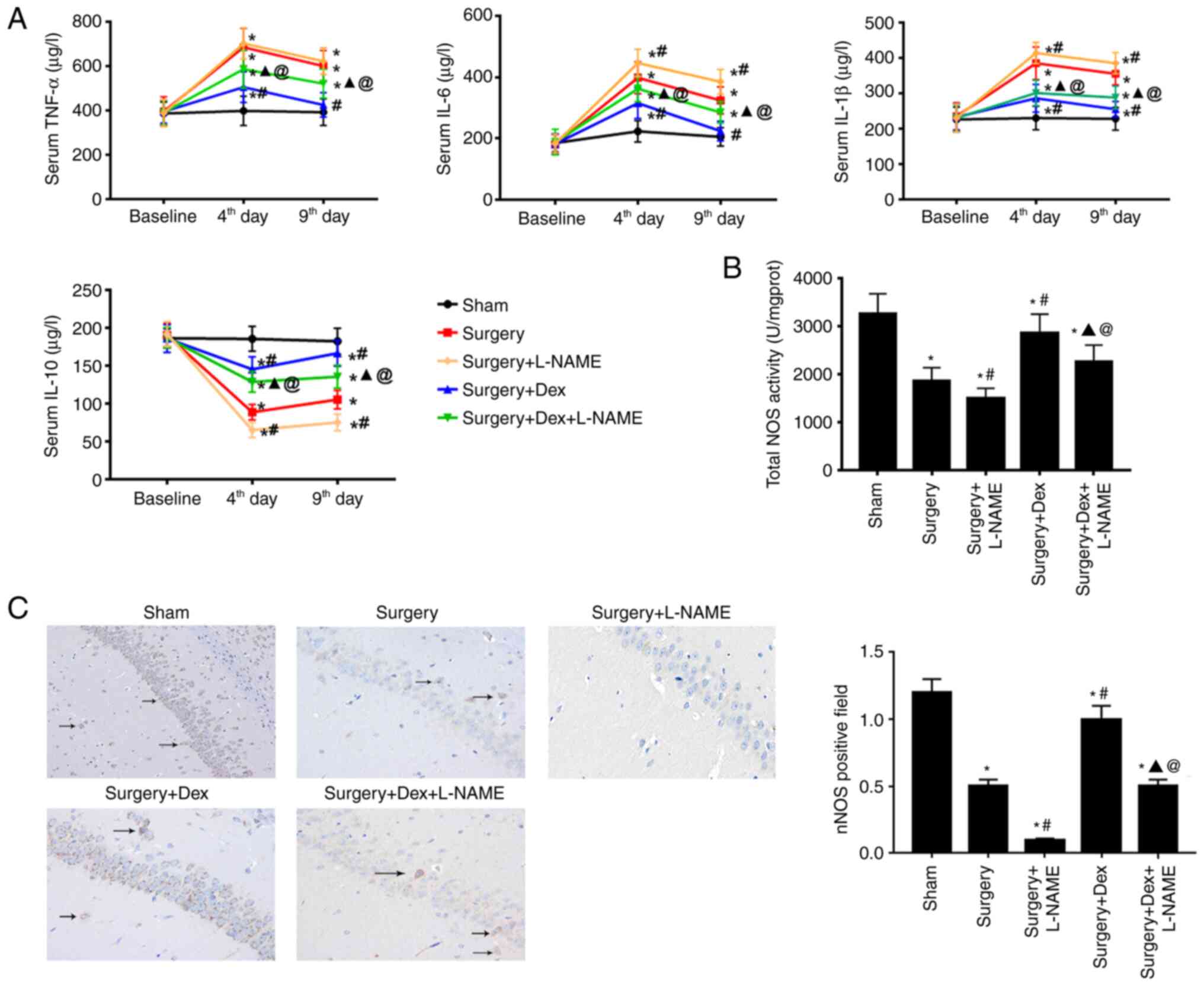
Increase of inflammatory
cytokines
Inflammatory cytokine plasma concentration levels
indicate an apparent systemic inflammatory response, which serves
critical roles in cognitive function (21). The release of proinflammatory
cytokines TNF-α, IL-6 and IL-1β, and anti-inflammatory cytokines
IL-10 were therefore measured at 4 and 9 days after surgery. As
demonstrated in Fig. 5A, all
surgery groups had higher systemic inflammatory cytokine levels
than those of the sham group at 4 days after surgery. Compared with
the surgery + DEX group, the levels of proinflammatory and
anti-inflammatory cytokines in the surgery + DEX + L-NAME group
were statistically significant. Furthermore, compared with the
surgery + L-NAME group, the surgery + DEX + L-NAME group
demonstrated a decreased tendency in the expression of
proinflammatory cytokines and an increased trend in the expression
of anti-inflammatory cytokines. Furthermore, the expressions of
proinflammatory cytokines and anti-inflammatory cytokines in the
surgery + DEX group on days 4 and 9 after surgery were close to the
baseline.
Comparison of NOS and nNOS in
rats
After evaluating the total NOS activity in
hippocampus tissue, a significant decline in the surgery group was
detected when compared with the sham group (Fig. 5B). The surgery + DEX and surgery +
DEX + L-NAME groups were significantly lower than the sham group
(Fig. 5B) and DEX treatment
significantly increased the total NOS activity, as evidenced by the
higher total NOS activity in the surgery + DEX group compared with
the surgery group. Fig. 5C
indicated that nNOS-positive neurons were widely distributed in the
sham group tissues. After surgery, the nNOS-positive neurons
indicated a significant reduction; the same was also exhibited in
the surgery + L-NAME group compared with the sham group. Compared
with the surgery group, post-surgery rats treated with DEX
exhibited an increase in the nNOS-positive neurons. However, the
surgery + DEX + L-NAME group demonstrated a significantly decreased
nNOS level compared with the surgery + DEX group, which was still
higher that of the surgery + L-NAME group (Fig. 5C).
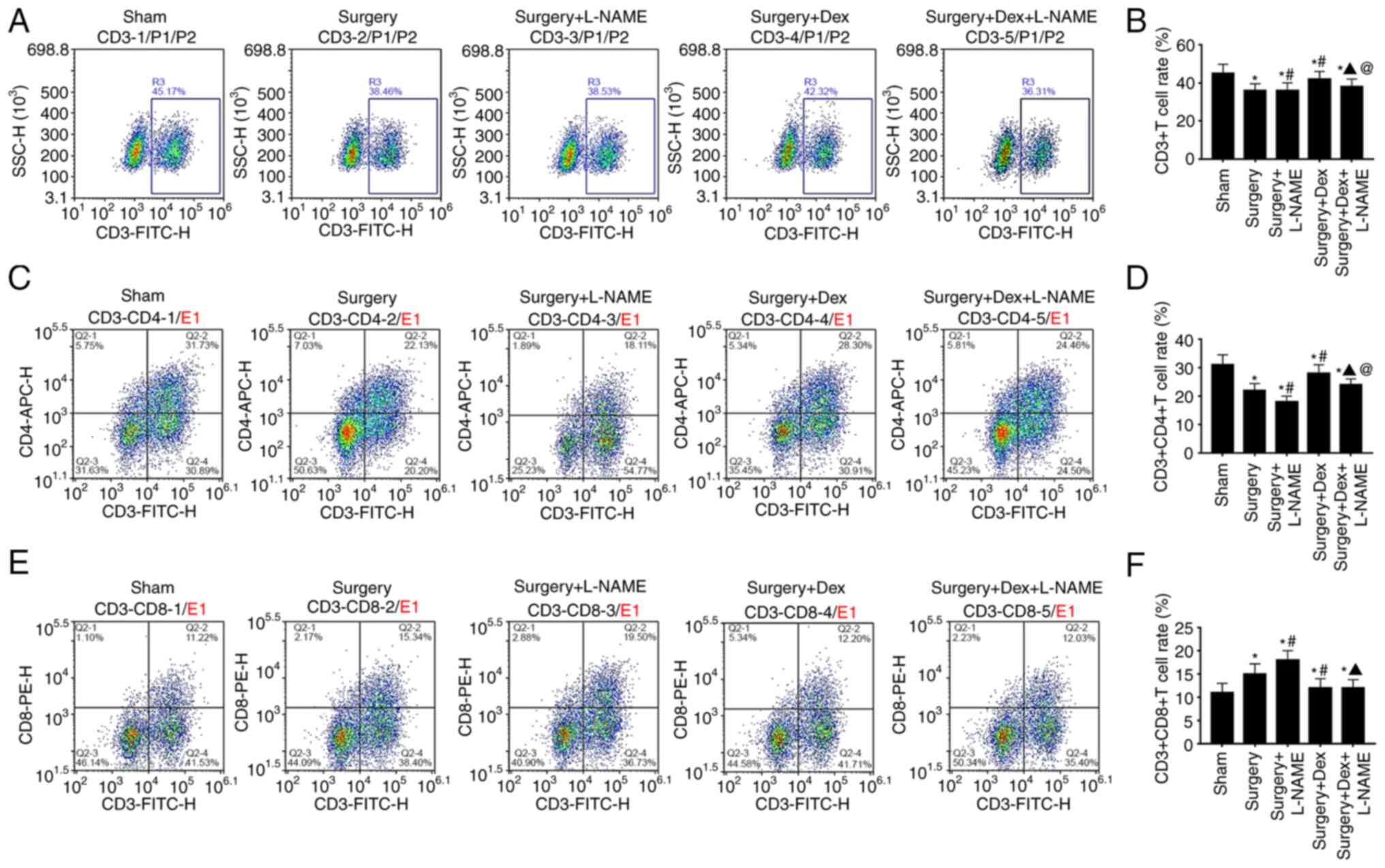
Comparison of the T lymphocyte
subsets
Among the T lymphocyte subsets, CD3+
samples included all mature T cells in the periphery, which
represented the overall immune level of T lymphocyte subsets
(22). Analyses of the T lymphocyte
subsets demonstrated that the CD3+ T cell and
CD3+/CD4+ T cell prevalence of the surgery
group was lower than that of the sham group. After treatment with
DEX, the CD3+ T cell prevalence and
CD3+CD4+ T cell percentage were significantly
inverted, as demonstrated by the higher CD3+ T level and
CD3+CD4+ T cell percentage in the surgery +
DEX group when compared with the surgery group, while surgery + DEX
+ L-NAME group also indicated higher figures of CD3+ T
level and CD3+CD4+ T cell percentage compared
with the surgery + L-NAME group (Fig.
6A-D). Regarding the CD3+CD8+ T cell
percentage, the surgery group had a significant increase in all
groups except the sham group (Fig.
6E and F). In addition, the
surgery + DEX + L-NAME group demonstrated a decreased tendency when
compared with the surgery + L-NAME group.
Discussion
According to previous reports, there is a close
association between neuro-inflammation and cognitive dysfunction
caused by surgery (23-25).
In the present study, cognitive function was assessed using two
methods: The OFT, which has been used to detect the locomotor
activity of rats (26); and the MWM
test, which is a classical method to evaluate spatial learning and
memory (27). The results of the
present study demonstrated that surgery had no effect on the
locomotor activity of rats. Compared with the sham group, the
escape latency of the surgery group was prolonged and the times of
crossing the platform were reduced, indicating that surgery caused
cognitive impairment in aged rats and that a successful POCD model
had been established. After rats were treated with L-NAME, their
learning and memory functions were impaired. To hypothesize, this
may be associated with the suppression of nNOS expression. However,
after treatment with DEX or DEX + L-NAME, cognitive impairment was
ameliorated indicating that treatment with DEX partially improved
cognitive impairment induced by surgery. Previous reports have also
indicated that DEX protects the cognitive impairment of surgery
(13,28). The present study confirmed that the
use of L-NAME, an NOS inhibitor, impaired brain learning and memory
function, similar to previous studies (29-31).
Neuronal apoptosis is an important cause of POCD.
Previous studies have demonstrated that DEX attenuates neuronal
apoptosis caused by isoflurane in newborn mice and reduces the
occurrence of POCD (32,33). TUNEL staining and western blotting
were therefore conducted in the present study to determine the
expression of apoptosis proteins in the hippocampus. The results
demonstrated that the surgery + DEX group had a reduced apoptosis
level in the hippocampus and reduced cognitive impairment compared
with the sham group. The results further confirmed that surgical
trauma leading to postoperative learning and memory dysfunction in
aged rats was associated with neuronal damage and neuronal
apoptosis in the hippocampus. Administration of DEX also reduces
apoptosis in the hippocampus and reduces cognitive impairment.
Increased proinflammatory cytokines in the
hippocampus may be a reason for cognitive decline following surgery
(34-36).
The release of inflammatory cytokines in the hippocampus may
interfere with cognitive function. Normally, surgery activates the
body's immune response, thereby releasing inflammatory factors. In
the present study, proinflammatory cytokines in the hippocampus
significantly increased on the 4th day after surgery, but returned
to baseline after 1 week. The expression of proinflammatory
cytokines and anti-inflammatory cytokines were also determined. The
results demonstrated that the proinflammatory cytokines of the
surgery group were increased, suggesting that surgery caused the
inflammatory response. At 9 days after surgery, proinflammatory
cytokines were decreased and anti-inflammatory cytokines were
increased in the DEX and DEX + L-NAME groups.
nNOS is a constitutive neuronal enzyme that is
important in regulating central nervous system function (20). A previous study demonstrated that
decreased expression of nNOS in the hippocampus of POCD rats was
closely associated with cognitive impairment (37). When the expression of nNOS was
measured in the hippocampus in the present study, DEX was found to
upregulate the expression of nNOS. However, the DEX + L-NAME group
had a lower nNOS expression. Previous studies have indicated that
inhibiting the expression of nNOS damages learning and memory
functions (16). It is therefore
hypothesized that DEX improves neuroinflammation and cognitive
decline by promoting the expression of nNOS and this beneficial
effect is reversed by L-NAME.
Finally, the efficiency and specificity of T
lymphocytes were monitored by flow cytometry analyses. The immune
response is the main cause of inflammation in the central nervous
system. CD3+CD4+ T cells can assist other
related cells to participate in the immune response. Additionally,
CD3+CD8+ T cells are immunosuppressive and
suppress the function of other immune cells (38). Previous reports have demonstrated
that immune cells, especially T cells, have an important role in
maintaining brain function, including psychological response,
spatial learning and memory functions (39,40).
In the present study, T helper lymphocyte cells in the DEX group
were higher than those of the DEX + L-NAME group, suggesting that
DEX improved the immune function of rats following surgery by
promoting the expression of nNOS.
In the present study, rats were intraperitoneally
injected with 12 µg/kg DEX 30 min before surgery. This specific
amount was used due to DEX playing a calming and analgesic function
in the central nervous system, with pre-treatment alleviating
postoperative cognitive dysfunction by inhibiting neuron excitation
in aged rats (12). Thus, DEX has
the potential to inhibit pathogenesis in the occurrence of
cognitive dysfunction. In previous studies, DEX exerted both
preconditioning and postconditioning effects against ischemic
injury (41,42) and the current study further
determined the neuroprotective effect of DEX in preconditioning.
However, the present research has several limitations. For example,
experimental observations only lasted for 9 days, multiple
administrations of DEX were not performed and higher doses of DEX
were not administered. Therefore, further experiments are required
for the application of this study in clinical therapy.
In summary, DEX played a neuroprotective role by
promoting the expression of nNOS, thus inhibiting the systemic
inflammatory response. This ensured a stable number of T lymphocyte
subsets, thereby reducing neuronal apoptosis and reducing the
occurrence of postoperative neuroinflammation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS conceived and supervised the study. MW was
responsible for acquisition of data, analysis and interpretation of
data, carried out the experiments and wrote the manuscript. LS and
MW confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols followed the requirements of the
Animal Experiment Center of the Institute of Radiation Medicine of
the Chinese Academy of Medical Sciences.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kotekar N, Kuruvilla CS and Murthy V:
Post-operative cognitive dysfunction in the elderly: A prospective
clinical study. Indian J Anaesth. 58:263–268. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Silbert B, Evered L and Scott DA:
Cognitive decline in the elderly: Is anaesthesia implicated?
Baillieres Best Pract Res Clin Anaesthesiol. 25:379–393.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Monk TG, Weldon BC, Garvan CW, Dede DE,
van der Aa MT, Heilman KM and Gravenstein JS: Predictors of
cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 108:18–30. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS: ISPOCD Group. Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hua M and Min J: Postoperative cognitive
dysfunction and the protective effects of enriched environment: A
systematic review. Neurodegener Dis. 20:113–122. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang W, Zhang XY, Feng ZG, Wang DX, Zhang
H, Sui B, Zhang YY, Zhao WX, Fu Q, Xu ZP, et al: Overexpression of
phosphodiesterase-4 subtypes involved in surgery-induced
neuroinflammation and cognitive dysfunction in mice. Brain Res
Bull. 130:274–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin F, Shan W, Zheng Y, Pan L and Zuo Z:
Toll-like receptor 2 activation and up-regulation by high mobility
group box-1 contribute to post-operative neuroinflammation and
cognitive dysfunction in mice. J Neurochem: Apr 19, 2021 (Epub
ahead of print).
|
|
8
|
Liu Q, Sun YM, Huang H, Chen C, Wan J, Ma
LH, Sun YY, Miao HH and Wu YQ: Sirtuin 3 protects against
anesthesia/surgery-induced cognitive decline in aged mice by
suppressing hippocampal neuroinflammation. J Neuroinflammation.
18(41)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yuki K: The immunomodulatory mechanism of
dexmedetomidine. Int Immunopharmacol. 97(107709)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu Y, Li S, Liu J, Wen Q, Yu J, Yu L and
Xie K: Role of JNK signaling pathway in dexmedetomidine
post-conditioning-induced reduction of the inflammatory response
and autophagy effect of focal cerebral ischemia reperfusion injury
in rats. Inflammation. 42:2181–2191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhai M, Liu C, Li Y, Zhang P, Yu Z, Zhu H,
Zhang L, Zhang Q and Wang J and Wang J: Dexmedetomidine inhibits
neuronal apoptosis by inducing Sigma-1 receptor signaling in
cerebral ischemia-reperfusion injury. Aging (Albany NY).
11:9556–9568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiong B, Shi Q and Fang H: Dexmedetomidine
alleviates postoperative cognitive dysfunction by inhibiting neuron
excitation in aged rats. Am J Transl Res. 8:70–80. 2016.PubMed/NCBI
|
|
13
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ge Y, Li Q, Nie Y, Gao J, Luo K, Fang X
and Wang C: Dexmedetomidine improves cognition after carotid
endarterectomy by inhibiting cerebral inflammation and enhancing
brain-derived neurotrophic factor expression. J Int Med Res.
47:2471–2482. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kelley JB, Balda MA, Anderson KL and
Itzhak Y: Impairments in fear conditioning in mice lacking the nNOS
gene. Learn Mem. 16:371–378. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou L and Zhu DY: Neuronal nitric oxide
synthase: Structure, subcellular localization, regulation, and
clinical implications. Nitric Oxide. 20:223–230. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiong B, Shi QQ and Miao CH:
Dexmedetomidine renders a brain protection on hippocampal formation
through inhibition of nNOS-NO signalling in endotoxin-induced shock
rats. Brain Inj. 28:1003–1008. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao Z, Ren Y, Jiang H and Huang Y:
Dexmedetomidine inhibits the PSD95-NMDA receptor interaction to
promote functional recovery following traumatic brain injury. Exp
Ther Med. 21(4)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chi MM, Lowry CV and Lowry OH: An improved
enzymatic cycle for nicotinamide-adenine dinucleotide phosphate.
Anal Biochem. 89:119–129. 1978.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu T, Liu J, Li XR, Yu Y, Luo X, Zheng X,
Cheng Y, Yu PQ and Liu Y: The mTOR/NF-κB pathway mediates
neuroinflammation and synaptic plasticity in diabetic
encephalopathy. Mol Neurobiol: Apr 15, 2021 (Epub ahead of
print).
|
|
22
|
Zhai S, Xu M, Li Q, Guo K, Chen H, Kong MG
and Xia Y: Successful treatment of vitiligo with cold atmospheric
plasma-activated hydrogel. J Invest Dermatol: May 21, 2021 (Epub
ahead of print).
|
|
23
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin D, Cao L, Wang Z, Li J, Washington JM
and Zuo Z: Lidocaine attenuates cognitive impairment after
isoflurane anesthesia in old rats. Behav Brain Res. 228:319–327.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cao L, Li L, Lin D and Zuo Z: Isoflurane
induces learning impairment that is mediated by interleukin 1β in
rodents. PLoS One. 7(e51431)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Amin SN, Hassan SS, Khashaba AS, Youakim
MF, Latif NS, Rashed LA and Yassa HD: Hippocampal and Cerebellar
Changes in Acute Restraint Stress and the Impact of Pretreatment
with Ceftriaxone. Brain Sci. 10(193)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Barnhart CD, Yang D and Lein PJ: Using the
Morris water maze to assess spatial learning and memory in weanling
mice. PLoS One. 10(e0124521)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang WX, Wu Q, Liang SS, Zhang XK, Hu Q,
Chen QH, Huang HJ, Xu L and Lou FQ: Dexmedetomidine promotes the
recovery of neurogenesis in aged mouse with postoperative cognitive
dysfunction. Neurosci Lett. 677:110–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lai AY, Joo IL, Trivedi AU, Dorr A, Hill
ME, Stefanovic B and McLaurin J: Cerebrovascular damage after
midlife transient hypertension in non-transgenic and Alzheimer's
disease rats. Brain Res. 1758(147369)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bingor A, Haham T, Thornton C, Stern-Bach
Y and Yaka R: Zeta inhibitory peptide attenuates learning and
memory by inducing NO-mediated downregulation of AMPA receptors.
Nat Commun. 11(3688)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ijomone OK, Shallie PD and Naicker T:
Oligodendrocytes death induced sensorimotor and cognitive deficit
in N-nitro-L-arginine methyl rat model of pre-eclampsia. Neurochem
Res. 45:902–914. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Engelhard K, Werner C, Eberspächer E,
Bachl M, Blobner M, Hildt E, Hutzler P and Kochs E: The effect of
the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate
antagonist S(+)-ketamine on the expression of apoptosis-regulating
proteins after incomplete cerebral ischemia and reperfusion in
rats. Anesth Analg. 96:524–531. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sato K, Kimura T, Nishikawa T, Tobe Y and
Masaki Y: Neuroprotective effects of a combination of
dexmedetomidine and hypothermia after incomplete cerebral ischemia
in rats. Acta Anaesthesiol Scand. 54:377–382. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS,
et al: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang J, Tan H, Jiang W and Zuo Z: The
choice of general anesthetics may not affect neuroinflammation and
impairment of learning and memory after surgery in elderly rats. J
Neuroimmune Pharmacol. 10:179–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang J, Jiang W and Zuo Z: Pyrrolidine
dithiocarbamate attenuates surgery-induced neuroinflammation and
cognitive dysfunction possibly via inhibition of nuclear factor κB.
Neuroscience. 261:1–10. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yan XB, Ouyang W, Li G and Duan KM:
Involvement of neuronal nitric oxide synthase in cognitive
impairment in isoflurane-treated rats. Neurosci Lett. 506:240–244.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheng DH, Liu Y and Wang L: antitumor
effects of ethanol extract from ventilago leiocarpa benth on
sarcoma 180 tumor-bearing mice and possible immune mechanism. Chin
J Integr Med: Jan 30, 2021 (Epub ahead of print).
|
|
39
|
Kipnis J, Gadani S and Derecki NC:
Pro-cognitive properties of T cells. Nat Rev Immunol. 12:663–669.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Brynskikh A, Warren T, Zhu J and Kipnis J:
Adaptive immunity affects learning behavior in mice. Brain Behav
Immun. 22:861–869. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Deng Y, Cai L, Wang F, Huang J, Wang H, Li
L and Lv H: Upregulated microRNA-381-5p strengthens the effect of
dexmedetomidine preconditioning to protect against myocardial
ischemia-reperfusion injury in mouse models by inhibiting CHI3L1.
Int Immunopharmacol. 92(107326)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li Y, Qu M, Xing F, Li H, Cheng D, Xing N
and Zhang W: The protective mechanism of dexmedetomidine in
regulating Atg14L-Beclin1-Vps34 complex against myocardial
ischemia-reperfusion injury. J Cardiovasc Transl Res: Apr 29, 2021
(Epub ahead of print).
|