Introduction
Cirrhosis is a liver disease that is characterized
by widespread necrosis, inflammation and fibrosis in the liver
tissues (1). During cirrhosis,
nodular regeneration of residual hepatocytes also occurs, which is
caused by the chronic action of various factors including
inflammation, activation of hepatic stellate cells with ensuing
fibrogenesis, angiogenesis and parenchymal extinction lesions
caused by vascular occlusion (2).
In addition to liver damage, cirrhosis can also result in a series
of other systemic impairments. In 1953, Kowalski and Abelmann first
documented abnormal cardiac functions in patients with liver
cirrhosis (3). Since then, a series
of animal models and clinical studies have confirmed further that
myocardial contractility and stimulation in patients with cirrhosis
are weakened, where in severe cases heart failure can occur
(4-6).
This condition is known as cirrhotic cardiomyopathy (CCM) (4-6).
Previous studies have shown that echocardiography (ECG) can be used
to evaluate left ventricular diastolic functions in patients with
cirrhosis (7,8). In addition, other studies successfully
used ultrasound speckle tracking echocardiography to detect reduced
systolic functions in patients with cirrhosis during the early
stages of the disease (9).
Cirrhosis can hinder contractions and diastolic functions of the
heart and can cause heart failure in severe cases (9). Although myocardial remodeling has been
proposed to be a cause of this phenomena in cardiac functions
during cirrhosis (9), it remains
unclear how reconstruction of the myocardium occur in such cases
and how these pathological changes affect cardiac function.
The specific pathogenesis of CCM remain poorly
understood. Recent studies have suggested that abnormalities in the
β-adrenergic receptor (β-AR) signaling pathway are associated with
the development of CCM (10,11).
β-AR is the major class of receptor that regulates cardiac
functions by exerting positive chronotropic, inotropic and
dromotropic actions (10).
Downregulating the expression of β-AR at the onset of CCM has been
reported to reduce myocardial contractility (12). However, the effects of β-AR and
other inflammatory factors including tumour necrosis factor
(TNF)-α, interleukin (IL)-1β, IL-6 and IL-18(13), which are mediated by the β-AR
signaling pathway during myocardial injury, on cardiac physiology
during CCM remain poorly understood.
The heart is regulated by the autonomic nervous
system, where a dynamic balance is maintained by the homeostasis of
sympathetic and parasympathetic stimulation (14). However, no reports of changes in
parasympathetic myocardial stimulation during CCM have been
previously reported. Therefore, the present study established a
carbon tetrachloride (CCL4)-induced CCM rat model for assessing
changes in the expression profiles of β-AR and muscarinic
acetylcholine (M2) receptors along with the activation of their
corresponding signal transduction pathways. The aim is to explore
the underlying mechanism of CCM and to provide a theoretical and
practical basis for developing clinical prevention and treatment
strategies for CCM.
Materials and methods
Animals, modeling and grouping
In total, 40 male Sprague-Dawley (SD) rats aged 6-7
weeks weighing 180±20 g were provided by the Experimental Animal
Center of Xi'an Jiaotong University Medical College and randomly
divided into the following three groups: i) Control group (CON;
n=10); ii) 4-week CCM group (CCM4; n=15); and iii) 8-week CCM group
(CCM8; n=15). The rats in the present study were maintained on a
standard rat chow diet with free access to drinking water. Four or
five animals were housed per polycarbonate cage under controlled
conditions (22 ± 2˚C, 50-60% relative humidity and 12-h light/dark
cycles).
For treatment, CCL4 (99.5% analytical pure supplied
by Shanghai Siyan Biotechnology Co., Ltd.) and soybean oil were
formulated into 40 and 60% CCL4-oil solution for subcutaneous
injection into the neck of each rat. In the CCM4 group, rats
received an intraperitoneal injection of 40% CCL4-oil solution at
weeks 1 and 2, twice per week (Monday and Thursday), with the first
dose at 5 ml/kg and the remaining (including the second, the third
and the fourth) dose at 3 ml/kg, and fed with 10% ethanol in
drinking water (every other day). On weeks 3 and 4, each rat
received a subcutaneous injection of 60% CCL4-oil solution
twice/week (Monday and Thursday), with the dose as 3 ml/kg and fed
with 20% ethanol in drinking water (every other day). The rats were
then subcutaneously injected with the same amount of 0.9% normal
saline from weeks 5 to 8, twice per week (Monday and Thursday) and
feed with common feed and tap water.
For rats in the CCM8 group, they received an
intraperitoneal injection of 40% CCL4-oil solution on weeks 1 and 2
twice per week (Monday and Thursday), with the first dose as 5
ml/kg and the remaining dose at 3 ml/kg. During feeding, 10%
ethanol solution was added to drinking water every other day. On
weeks 3 to 8, rats received a subcutaneous injection of 60%
CCL4-oil solution, twice per week (Monday and Thursday), with the
dose set to 3 ml/kg and 20% ethanol solution was added to drinking
water every other day.
The rats in CON group were given normal drinking
water and granulated feed throughout the 8 weeks. The CON group was
intraperitoneally injected with the same amount of 0.9% normal
saline twice per week (Monday and Thursday) and then subcutaneously
injected with the same amount of 0.9% normal saline from weeks 3 to
8 (twice per week, on Monday and Thursday).
During this experimental period, the general
condition of the rats, including coat color, activity, appetite and
death, were observed every day, together with the final body weight
of each rat. When the rats developed cachexia or extreme weakness,
euthanasia would be immediately performed. The standard used to
assess the successful establishment of cirrhosis model in the rats
were as follows: i) Biochemical indices, including increased levels
of alanine aminotransferase (ALT) and aspartate aminotransferase
(AST), reduced levels of total protein (TP) compared with those in
the control group; ii) Imaging indices, which were assessed using
ultrasonography, whereby compared with those in the control group,
liver edges are round and blunt (sharp edge of the liver in the
control group rats) but the echo light spot of the parenchyma are
increased (uniform light spot in the control group); and iii)
Histopathological changes in the liver as assessed by H&E
staining, whereby hepatic lobules were destroyed, can be observed
with fatty deposits and fibrous hyperplasia wrapping around the
liver cell mass to form a typical pseudolobule (in the control
group, the structure of liver lobule should be normal, where
hepatocytes around the central portal vein are arranged radially)
(15). There were no deaths in the
CON group. A total of 3 rats died in the CCM4 group and 5 rats died
in the CCM8 group. Specifically, two rats reached the humane
endpoints by showing clear anorexia and depression, weight loss and
cachexia. In total, three rats had to be euthanized as they
developed severe weakness and were unable to stand. One rat
developed severe infection and ulceration at the injection site,
which was euthanized immediately. Two rats were found dead when fed
early morning. It was speculated that the death may have been
caused by cachexia and extreme weakness. All animal procedures
conformed to the guidelines for the Care and Use of Laboratory
Animals published by the National Institutes of Health (NIH
publication 85-23, revised in 1996) (16) and the approved regulations set by
the Laboratory Animal Care Committee at Xi'an Jiaotong University
(Xi'an, China).
ECG measurements
The instrument used for ECG measurement was Hitachi
HI VISION Preirus color ultrasound diagnostic instrument (Hitachi
High-Technologies Corporation) equipped with a EUP-L74M linear
array probe (probe frequency, 5.0-13.0 MHz; Hitachi
High-Technologies Corporation). All rats were weighed prior to ECG
analysis and were anesthetized with an intraperitoneal injection of
2% pentobarbital sodium (30 mg/kg). ECG was performed on
anesthetized rats fixed in a supine position. The parasternal long
and short axis sections of the left ventricle were taken for 2D
ultrasound measurements using an M-shaped curve. Parameters
measured included the following: i) Left ventricular posterior wall
dimension (LVPW); ii) interventricular septal dimension (IVS); iii)
left ventricular end-diastolic dimension (LVEDD); iv) left
ventricular end-systolic dimension (LVESD); v) left atrial diameter
(LAD); vi) left ventricular ejection fraction (LVEF); vii) left
ventricular short axis shortening rate (FS); and viii) cardiac
output (CO). Each index was measured over three consecutive cardiac
cycles and averaged.
Sampling of serum and tissue
specimens
After ECG measurements, the rats were euthanized by
CO2 inhalation (30% volume per minute displacement)
followed by cervical dislocation. Upon completion of the procedure,
death was confirmed by observation of cardiac and respiratory
arrest or fixed and dilated pupils. All the experimental operations
were in accordance with the requirements of the Experimental Animal
Ethics Committee, School of Medicine, Xi'an Jiaotong University.
All rats were cut open near the abdominal cavity and ~5 ml blood
was taken for sampling from the abdominal aorta. The blood samples
were then kept at room temperature for 30 min, followed by
centrifugation at 1,509 x g at 4˚C for 15 min before the serum was
collected and stored at -80˚C. In total, 500 µl serum from each
sample was taken out and thawed. The levels of myocardial enzymes,
specifically lactate dehydrogenase (LDH), creatine kinase isoenzyme
(CK-MB) and cardiac troponin T (cTnT), along with liver function
markers ALT, AST and TP, were determined using a Beckman Coulter
LH750 automatic biochemical analyzer (Beckman Coulter, Inc.) at the
Department of Clinical Laboratory, School of Medicine, The Second
Affiliated Hospital of Xi'an Jiaotong University. An incision was
then made in the chest cavity for collecting the liver and heart.
After the blood was rinsed using saline and the tissues were dried
using filter paper, their corresponding wet weights were measured.
In each rat, exactly the same region of the right liver lobe and
myocardial apex were collected, fixed in 4% paraformaldehyde at 4˚C
for 24 h and embedded in paraffin for H&E and Masson trichrome
staining. The rest of the myocardial and liver samples were placed
in separately cryotubes and rapidly frozen in liquid nitrogen,
which were stored at -80˚C for future protein detection
measurements. Before detecting the expression of interleukins in
myocardial tissue, the myocardial samples of rats were taken and
kept at ~2-8˚C with a certain amount of PBS (pH 7.4). The samples
were homogenized by homogenizer and centrifuged at 1,509 x g for
about 20 min at 4˚C. The supernatant was collected for detecting
the expression of interleukins in myocardial tissue. The levels of
IL-1, IL-2 and IL-6 in the serum and myocardial tissue were
measured with ELISA kits (Wuhan USCN Business Co., Ltd.) in
accordance with the manufacturer's protocol. The catalogue numbers
of IL-1, IL-2 and IL-3 were SEA563Ra, SEA073Ra and SEA079Ra,
respectively.
H&E staining and Masson trichrome
staining
For H&E staining, after the 5-µm thick paraffin
slice was dewaxed with xylene and then fully hydrated with ethanol,
it was stained with a hematoxylin solution for 3-8 min at room
temperature, followed by rinsing with water, 1% HCl-alcohol
differentiation for 5-30 sec, another water rinsing step,
incubation in 0.6% ammonia water solution for bluing for 1.5 h and
staining with an eosin dye solution for 1-3 min at room
temperature. The sections were then dehydrated and hyalinized in
xylene before finally being sealed in neutral gum. All the slides
were examined under a light microscope and images were captured
with a Nikon microscope (Y-THS; Nikon Corporation) (magnification,
x100).
For Masson trichrome staining, after the 5-µm thick
paraffin slice was dewaxed with xylene and then fully hydrated with
ethanol, it was placed in a dichromate-acetic acid solution for 40
min at room temperature, followed by a water rinsing, hematoxylin
staining for 10 min at room temperature, 2% HCl-alcohol
differentiation, 15-min staining in 1% ponceau acid fuchsin at room
temperature, 5-min treatment in 1% phosphomolybdic acid, 8-min
staining with 2% bright green solution at room temperature and 2 to
3-time color separation in 1% acetic acid. The samples were then
dehydrated/hyalinized before being finally sealed using in neutral
gum. All the slides were examined under a light microscope and
images were captured with a Nikon microscope (Y-THS; Nikon
Corporation) (magnification, x400).
Western blotting
The myocardial tissue was cryopreserved in liquid
nitrogen. Then, RIPA buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) and a protease inhibitor [Roche
Diagnostics (Shanghai) Co., Ltd.] were used for total protein
extraction. Protein concentrations were quantified by BCA assay
(cat. no. PA115-01, Tiangen Biotech Co, Ltd.) and diluted to a
final concentration of 5 µg/µl. The subsequent 10% SDS-PAGE was
performed as follows: i) 50 µg protein per lane; ii) 90 min 280 mA
constant-current electrophoresis; iii) 1 h blocking in 5% skim milk
at room temperature; iv) overnight incubation in primary antibody
at 4˚C; and v) 1 h incubation with secondary antibodies at 37˚C.
Primary antibodies used include anti-GAPDH (cat. no. sc-365062,
1:10,000; Santa Cruz Biotechnology, Inc.), anti-mAChR M2 (cat. no.
sc-33712, 1:1,000; Santa Cruz Biotechnology, Inc.) and anti-β1
adrenergic receptor (cat. no. sc-81577, 1:1,000; Santa Cruz
Biotechnology, Inc.). The secondary antibodies used were goat
anti-mouse IgG1-HRP (cat. no. SC-2060, 1:10,000; Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG2a-HRP (cat. no.
SC-2970, 1:10,000; Santa Cruz Biotechnology, Inc.). The gel strips
were then photographed by luminescent imaging (Tanon 6200
Luminescent Imaging Workstation; Tanon Science and Technology Co.,
Ltd.). Quantification was performed using Image J v.1.8.0 (National
Institutes of Health). GAPDH was used as the loading control.
Statistical analysis
The data were expressed as the mean ± SD. SPSS 18.0
(SPSS, Inc.) statistical analysis software was used to perform
one-way ANOVA followed by the LSD post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in liver structure and
function in the CCM rat models
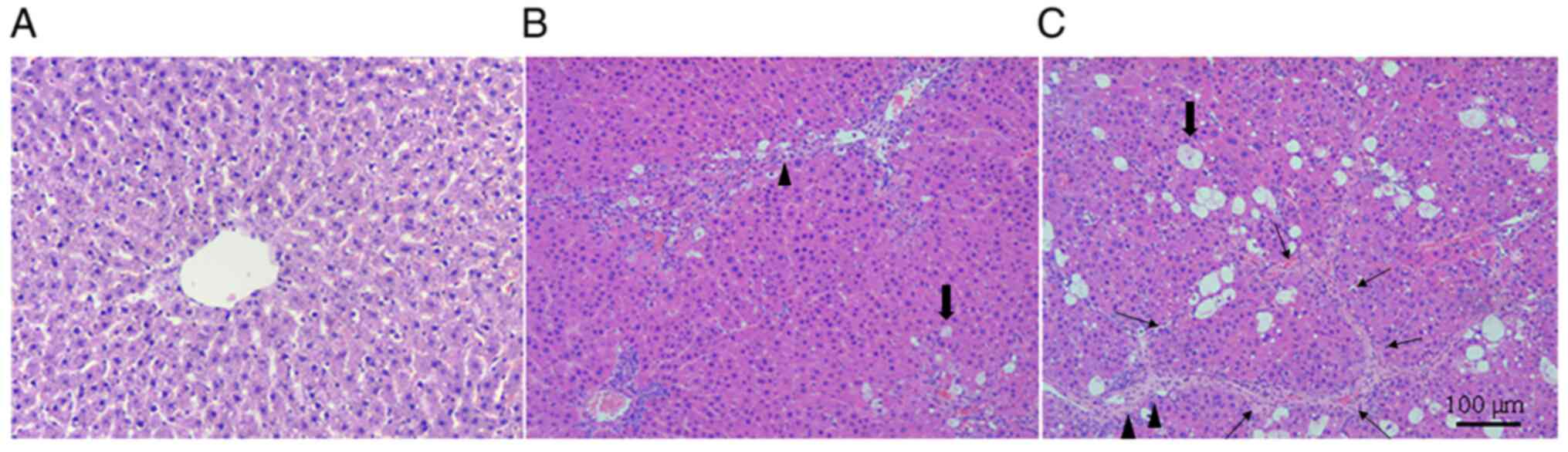
H&E staining under light microscopy showed that
after CCM induction, the hepatocytes present in liver tissues had
varying degrees of hepatic lobules destroyed and steatosis, where
the fibers proliferated significantly and formed a typical
pseudolobule around the hepatocyte mass (Fig. 1). In addition, the extent of this
appeared to be more severe in liver tissues from rats in the CCM8
group (Fig. 1). This suggested that
the CCM model was successfully established.
Serum levels of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) are indicators of hepatocyte
injury (17). ALT and AST combined
with liver histopathology were used in the present study to confirm
whether the model group was successfully modeled and the degree of
hepatocyte injury. Compared with those in the CON group, the
concentrations of ALT and AST were significantly increased, whilst
those of TP were significantly decreased, in both the CCM4 and the
CCM8 groups. ALT and AST levels were also significantly increased
in the CCM8 group compared with those in the CCM4 group (Table I).
 | Table IComparison of liver function
parameters among the three groups. |
Table I
Comparison of liver function
parameters among the three groups.
| Parameter | CON | CCM4 | CCM8 |
|---|
| Aspartate
aminotransferase | 72.3±7.5 |
102.8±10.8b |
198.7±12.5b |
| Alanine
aminotransferase | 65.1±7.0 |
89.4±9.6a |
132.0±11.9b |
| Total protein | 54.7±6.2 |
46.2±4.3a |
37.2±4.0b |
Changes in the myocardial structure
and function after CCM induction
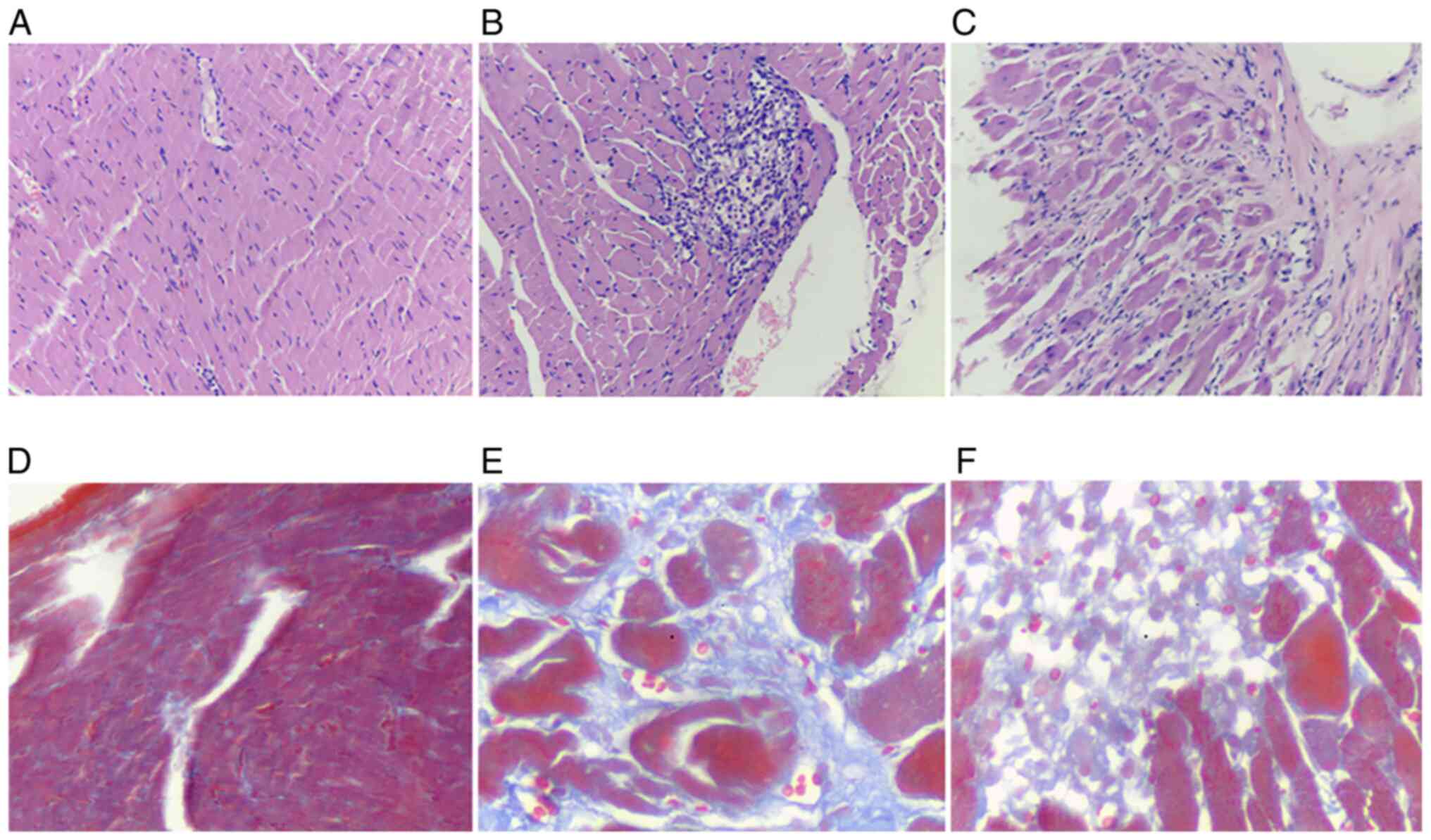
Morphological changes in the myocardial tissue of
rats after CCM induction was assessed using H&E staining.
Cardiomyocytes from rats in the CON group were arranged tightly in
a regular manner, with their full structure intact and clear cell
boundaries clearly visible. In the CCM model of rats, the
myocardial structure was more disordered with nuclei accumulation
(Fig. 2B and C). Furthermore, the myocardial fibers were
either swollen, dissolved or broken (Fig. 2B and C). Masson trichrome staining is frequently
used for detecting tissue collagens, which can distinguish among
muscle fibers, collagen fibers and nuclei (18). Masson trichrome staining results
revealed that the cardiomyocytes of the rats in the CON group were
orderly arranged with intact muscle bundles. The myocardial tissue
had a small quantity of collagen fibers, which stain blue and were
distributed evenly among the red cardiomyocytes. After CCM
induction, myocardial tissue damage appeared to have occurred in
rats in both CCM4 and CCM8 groups (Fig.
2). Features observed include basic tissue destruction,
large-areas fibrotic tissues displacing the healthy myocardial
tissue, excessive myocardial interstitial collagen deposition, a
large quantity of fibrous, meshed connective tissues and damaged
myocardial muscle bundles, which were either separated, enveloped
or fused (Fig. 2).
Compared with those in the CON group, the body
weights (BW) and wet heart weights (HW) of the rats in the CCM
groups were significantly lower, though the magnitude of decrease
was greater in those in the CCM8 group. This may be due to
reductions in appetite and water intake in rats in the CCM groups.
However, the left ventricular weight (LVW), HW/BW and LVW/HW in
rats in the CCM group were significantly higher compared with those
in the CON group (Table II). This
suggest that the heart, especially the left ventricle, had
undergone myocardial tissue remodeling.
 | Table IIComparison of BW and HW among the
three groups. |
Table II
Comparison of BW and HW among the
three groups.
| Parameter | CON | CCM4 | CCM8 |
|---|
| BW (g) | 345±11.4 |
328.3±13.0a |
303.0±9.7b |
| HW (mg) | 987.2±61.9 |
967.4±49.6a |
923.7±46.3a |
| LVW (mg) | 684.8±33.7 |
701.5±30.1a |
725.6±15.0a |
| HW/BW | 2.83±0.11 |
2.95±0.16a |
3.04±0.21a |
| LVW/HW | 0.73±0.03 |
0.82±0.05b |
0.83±0.04b |
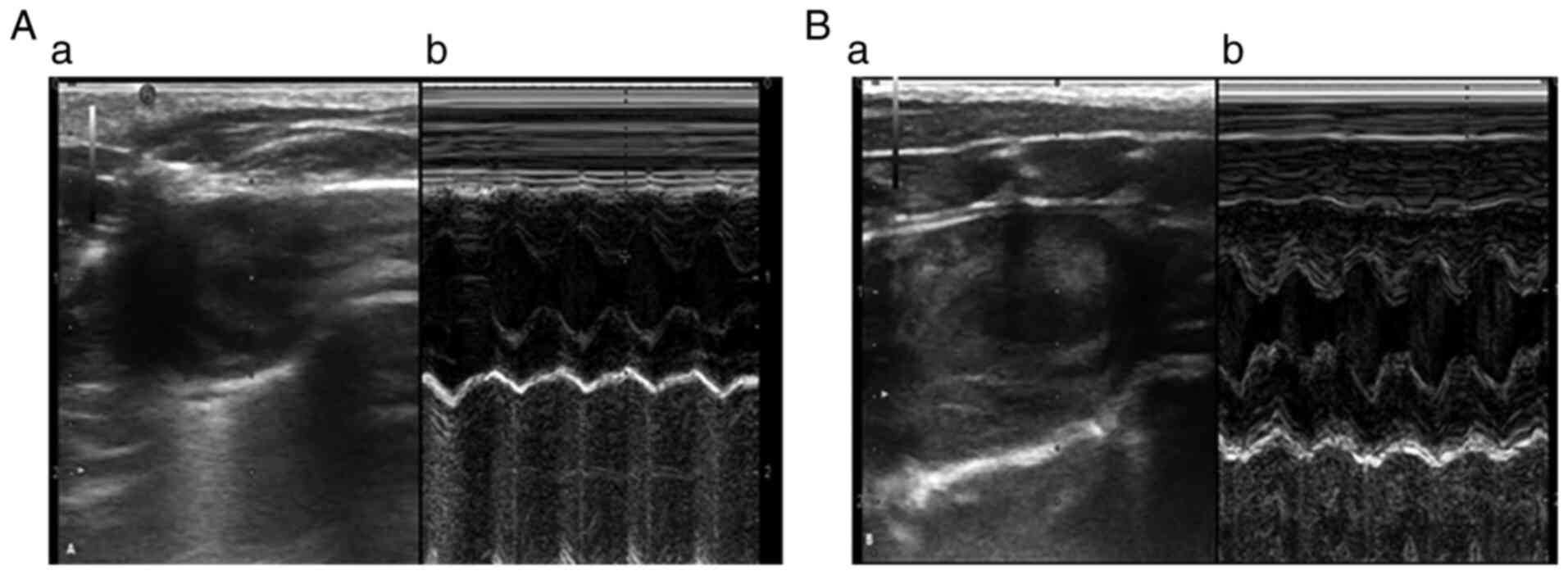
Compared with those in rats in the CON groups,
LVESD, LVEDD, IVS and LAD values in the CCM8 group were
significantly increased (Table
III). Rats in the CCM8 group also exhibited statistically
increased CO, decreased VE/VA ratio and prolonged deceleration time
(DT; Table III). There were no
significant changes in the posterior wall thickness of the left
ventricle and the LVEF values (Table
III, Fig. 3).
 | Table IIIHigh-frequency ultrasound data among
the two groups. |
Table III
High-frequency ultrasound data among
the two groups.
| Groups | n | LVESD (mm) | LVEDD (mm) | IVS (mm) | LVPW (mm) | LAD (mm) | LVEF (%) | CO (l) | VE/VA | DT (msec) |
|---|
| CON | 10 | 3.8±0.55 | 6.8±0.45 | 1.31±0.09 | 1.63±0.08 | 4.08±0.33 | 77.20±2.41 | 0.16±0.02 | 1.26±0.29 | 18±2.06 |
| CCM8 | 10 |
4.4±0.64a |
7.5±0.64a |
1.85±0.25a | 1.84±0.07 |
6.67±0.58a | 75.02±1.88 |
0.38±0.05a |
1.01±0.16a |
21±1.23a |
Changes in levels of myocardial
enzymes and inflammatory factors in the serum and myocardial
tissues
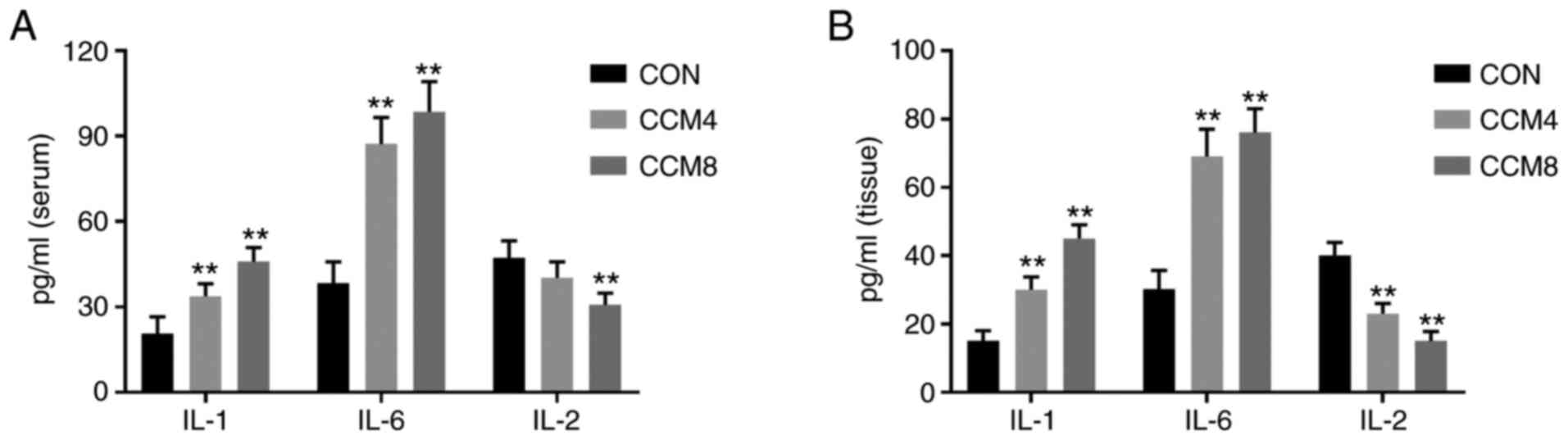
Compared with those in the CON group, the levels of
LDH, CK-MB and cTnT in CCM4 and CCM8 groups were significantly
increased (Table IV). In addition,
compared with those in the CON group, the levels of
pro-inflammatory factors IL-1 and IL-6 in the serum and myocardial
tissues were significantly increased in the CCM4 and CCM8 groups
(Fig. 4). By contrast, the levels
of IL-2 were decreased, but only those in the CCM8 group exhibited
statistically significant differences (Fig. 4).
 | Table IVChanges in the expression of
biomarkers for myocardial injury in rats with cirrhosis. |
Table IV
Changes in the expression of
biomarkers for myocardial injury in rats with cirrhosis.
| Biomarker | CON | CCM4 | CCM8 |
|---|
| Lactate
dehydrogenase | 219.6±35.6 |
407.1±40a |
567.4±47.3a |
| Creatine
kinase-isoenzyme | 308.23±32.9 |
451±66.2a |
658±50.5a |
| Cardiac tropinin
T | 258.7±38.2 |
398.5±56.3a |
603.3±48.4a |
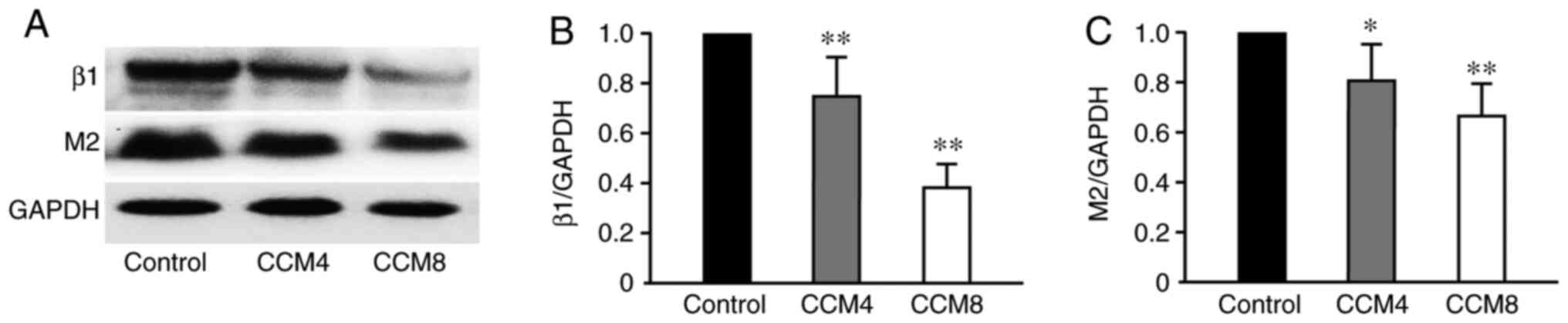
Downregulation of myocardial β1 and M2
receptor expression in CCM rats
Semi-quantitative analysis of β1 and M2 receptor
protein expression in the myocardial tissues was performed using
western blotting. Compared with that in the CON group, expression
of β1 and M2 receptor proteins in the CCM4 and CCM8 groups were
significantly downregulated, which was more prominent in the CCM8
group (Fig. 5).
Discussion
CCM is one of the complications that can occur
during cirrhosis (19). Although
patients may not exhibit symptoms under normal circumstances, other
factors, including stress, infection, transjugular intrahepatic
portosystemic shunts (TIPS) or liver transplantation, can result in
serious complications (20). Due to
CCM being difficult to diagnose early, an increasing number of
studies have placed focus on investigating the pathogenesis of left
ventricular systolic and diastolic dysfunctions during CCM. The
ultimate aim was to devise a method for a more accurate diagnosis
and prevention of such complications (21,22). A
number of studies (23,24) have reported that compensatory
adaptions occur in cardiac function during the early stages of
cirrhosis, reported a submaximal increase in cardiac output, heart
rate and increase in plasma volume, but the arterial pressure drops
and peripheral resistance is reduced. Previous clinical studies
have also found that patients with cirrhosis may have systolic and
diastolic dysfunctions (4,25). Myocardial remodeling may be the
cause of cardiac dysfunction in CCM (20), but the cellular mechanism involved
in this remodeling process and how these pathological changes
affect cardiac functions remain poorly understood. Since it is
difficult to obtain myocardial tissues from patients with
cirrhosis, the present study used rats models of CCM to simulate
myocardial damage. In the present study, a CCM model was
established by CCL4 treatment, which resulted in ventricular
remodeling and fibrosis, in turn leading to left ventricular
systolic and diastolic dysfunction. In addition, CCM induced
inflammatory reaction in the body, which was characterized by
increased levels of proinflammatory factors IL-1 and IL-6 and
decreased levels of the anti-inflammatory factor IL-2. Therefore,
it can be speculated that the release of inflammatory mediators
serve an important role in myocardial remodeling during CCM
(26). Liver cirrhosis can affect a
variety of organs and systems in the body, including the
cardiovascular and autonomic nervous systems (27,28).
Myocardial remodeling and abnormal inflammatory reactions in CCM
may be caused by defects in its autonomic nervous regulatory
system.
Patients with cirrhosis, especially those who had
undergone liver transplantation previously received TIPS before,
frequently have compromised circulatory systems and lead to fatal
arrhythmia, heart failure, or even death (20). Therefore, studies into CCM warrants
further study (29). Due to a
combination of neurohumoral factors such as, troponin I, B-type
natriuretic peptide (BNP) and pro-BNP, ventricular load is
aggravated, which together with increased sodium retention and
insufficient myocardial blood supply, result in myocardial
hypertrophy and myocardial interstitial fibrosis (30). Ventricular remodeling is a
pathological characteristic that occurs during CCM (31). Previous studies have shown that the
proportion of heart enlargement in patients with cirrhosis is
significantly increased compared with the healthy control group,
where the structural cardiac changes are mainly focused in the left
atrium and ventricle (8,9). Extensive thickening of the left
ventricular wall and histomorphological changes, including cardiac
hypertrophy and interstitial/intracellular edema, can also be seen
in patients with cirrhosis (32,33).
In the present study, the liver weight and heart weight of rats
with cirrhosis were significantly lower compared with those in the
control rats, but the cardiac coefficient (heart/body ratio) and
left ventricular weight/heart weight ratio were significantly
increased, suggesting the occurrence of myocardial remodeling in
rats with cirrhosis to some extent. In addition, cirrhosis not only
causes myocardial cell hypertrophy but can also cause myocardial
interstitial hyperplasia (33). In
the present study, rats with cirrhosis, myocardial collagen fibers
were found to be disordered, which can lead to hyperplasia and
stiffness (34). Therefore,
myocardial compliance indicated by the enlargement of left atrium
and the decrease of VE/VA ratio in the present study decreased,
which caused diastolic dysfunction. In addition, an increase in the
levels of collagen fibers may interfere with the arrangement,
communication and connection of myocardial cells, adversely
affecting the production and transmission of force to induce
systolic dysfunction (34).
Myocardial fibrosis is an important manifestation of ventricular
remodeling and also an intrinsic cause of cardiac insufficiency
that can lead to heart failure (35).
The present study also examined changes in
conventional ultrasound parameters in rats 8 weeks after CCM
induction. Among them, the LVESD, LVEDD, IVS, and LAD values were
significantly increased whilst an increase in the levels of CO was
observed. However, the ratio of VE/VA was decreased and the DT was
prolonged. These results were consistent with results from the
ultrasound diagnosis in patients with clinical cirrhosis in our
previous study (7), suggesting that
this modeling method can mimic myocardial damage in patients with
cirrhosis and lead to cardiac function. In addition, this also
suggests that high-frequency ultrasound can clearly detect the
various indices of rat heart function. The hemodynamic indices such
as CO, LVEF and VE/VA of rats with cirrhosis appeared to be lower
than those of control rats, suggesting that this is an effective
model for studying the pathogenesis of CCM.
Inflammatory responses are the body's major
responses to tissue damage and serves an important role in cardiac
damage (36). The degree of the
inflammatory response is an important factor in determining the
prognosis of patients with myocardial injuries (36). Proinflammatory factors IL-6 and
IL-1β are not normally expressed in the myocardium, where their
production and upregulation results in intrinsic stress and
myocardial damage (37). Various
reports (37-39)
revealed that during cirrhosis, IL-6 and IL-1β can be stimulated by
endotoxemia and other factors such as lipopolysaccharide and T cell
immunoglobulin and mucin domain 3, which can induce fibrosis in
damaged myocardial fibers. The present study also revealed that the
levels of IL-6 and IL-1β in the serum and myocardial tissues of
rats with cirrhosis were significantly higher compared with those
in the CON group, which increased the severity of cirrhosis. By
contrast, the anti-inflammatory factor IL-2 showed a significant
decrease, suggesting that an aberrant inflammatory reaction is
involved in the damage caused by cirrhosis to the myocardium and
accelerated ventricular remodeling.
Cirrhosis causes hyperdynamic circulation and is
regulated by neurohumoral factors (40). The hyperdynamic circulation begins
in the portal venous bed as a consequence of portal hypertension
due to the increased resistance to flow from altered hepatic
vascular morphology of chronic liver disease (41). Previous studies have reported that
myocardial β-adrenergic receptor (β-AR) downregulation may be an
important pathogenic mechanism in cirrhosis-induced myocardial
injury (42,43). The adrenaline receptor subtype
predominantly expressed in cardiac tissues is β1, where its density
reduction and/or desensitization is closely associated with the
development of cardiovascular diseases, including heart failure and
hypertension (44). The present
study also found that the expression of the β1-AR protein in the
myocardium gradually decreased with prolongation of the CCM course.
The heart is also dually regulated by the sympathetic and
parasympathetic nervous systems, which antagonize each other to
maintain the dynamic balance required for basal cardiac function
under various stress-inducing states (45). In the present study, in addition to
the reduction in the levels of β1-AR expression in CCM rat tissues,
the expression of M2 receptor (M2), a major receptor for myocardial
parasympathetic neurotransmitters (46), was also reduced. However, this
downward trend was not as pronounced as that of β1. These findings
suggest that there is a decrease in autonomic regulation during
cirrhosis-induced cardiac dysfunction, but the balance between
sympathetic and parasympathetic in cardiac regulation requires
further study.
To conclude, rat CCM model established by CCL4
treatment in the present study can simulate the pathogenesis of
cirrhosis to cause myocardial damage. There was a clear sign of
myocardial remodeling present in rats with CCM. High-frequency
ultrasound electrocardiography can be used to effectively observe
changes in cardiac functions in rats. The levels of
pro-inflammatory factors IL-6 and IL-1 were increased, whilst the
expression of β1-AR protein and M2 receptor were decreased in rats
with cirrhosis, suggesting that abnormal inflammatory reaction,
autonomic regulation and other mechanisms may be involved in
cirrhosis-related damage to the myocardium and accelerate
ventricular remodeling.
Acknowledgements
Not applicable.
Funding
Funding: The present study was sponsored by the National Natural
Science Foundation of China (grant no. 82001843).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and LS performed the experiments, analysis and
interpretation of the data and revision for intellectual content.
HW and JJ were involved in the experiments, and acquisition,
analysis and interpretation of the data. QZ was responsible for the
conception, design, and acquisition, analysis and interpretation of
the data. SY and LS confirmed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol has been reviewed and approved by the Institutional
Animal Care and Use Committee of Xi'an Jiaotong University (Xi'an,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Romanelli RG and Stasi C: Recent
advancements in diagnosis and therapy of liver cirrhosis. Curr Drug
Targets. 17:1804–1817. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsochatzis EA, Bosch J and Burroughs AK:
Liver cirrhosis. Lancet. 383:1749–1761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kowalski HJ and Abelmann WH: The cardiac
output at rest in Laennec's cirrhosis. J Clin Invest. 32:1025–1033.
1953.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ruiz-del-Arbol L and Serradilla R:
Cirrhotic cardiomyopathy. World J Gastroenterol. 21:11502–11521.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khare J, Srivastava P, Wadhwa J and Deb P:
Cardiac cirrhosis-an uncommon manifestation of common disease. OGH
Reports. 6:28–30. 2017.
|
|
6
|
Jarkovska D, Bludovska M, Mistrova E,
Krizkova V, Kotyzova D, Kubikova T, Slavikova J, Erek SN,
Djordjevic A and Chottova Dvorakova M: Expression of classical
mediators in hearts of rats with hepatic dysfunction. Can J Physiol
Pharmacol. 95:1351–1359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X, Yu S, Li L, Han D, Dai S and Gao Y:
Cirrhosis-related changes in left ventricular function and
correlation with the model for end-stage liver disease score. Int J
Clin Exp Med. 7:5751–5757. 2014.PubMed/NCBI
|
|
8
|
Cesari M, Fasolato S, Rosi S and Angeli P:
Cardiac dysfunction in patients with cirrhosis: Is the systolic
component its main feature? Eur J Gastroenterol Hepatol.
27:660–666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sampaio F, Pimenta J, Bettencourt N,
Fontes-Carvalho R, Silva AP, Valente J, Bettencourt P, Fraga J and
Gama V: Systolic and diastolic dysfunction in cirrhosis: A
tissue-Doppler and speckle tracking echocardiography study. Liver
Int. 33:1158–1165. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gregolin CS, do Nascimento M, Borges de
Souza SL, Ferreira Mota GA, Bomfim GF, de Azevedo Melo Luvizotto R,
Sugizaki MM, Zanati Bazan SG, Salomé de Campos DH, Dias MC, et al:
Myocardial dysfunction in cirrhotic cardiomyopathy is associated
with alterations of phospholamban phosphorylation and IL-6 levels.
Arch Med Res. 52:284–293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Silvestre OM, Farias AQ, Ramos DS, Furtado
MS, Rodrigues AC, Ximenes RO, de Campos Mazo DF, Yoshimura Zitelli
PM, Diniz MA, Andrade JL, et al: β-blocker therapy for cirrhotic
cardiomyopathy: A randomized-controlled trial. Eur J Gastroenterol
Hepatol. 30:930–937. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Accornero F, van Berlo JH, Correll RN,
Elrod JW, Sargent MA, York A, Rabinowitz JE, Leask A and Molkentin
JD: Genetic analysis of connective tissue growth factor as an
effector of transforming growth factor β signaling and cardiac
remodeling. Mol Cell Biol. 35:2154–2164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao H, Li H, Wang JJ, Zhang JS, Shen J,
An XB, Zhang CC, Wu JM, Song Y, Wang XY, et al: IL-18 cleavage
triggers cardiac inflammation and fibrosis upon β-adrenergic
insult. Eur Heart J. 39:60–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ziemssen T and Siepmann T: The
investigation of the cardiovascular and sudomotor autonomis nervous
system-a review. Front Neurol. 10(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang CH, Ting WJ, Day CH, Ju DT, Yeh YL,
Chung LC, Tsai FJ, Tsai CH, Tsai Y and Huang CY: SHSST cyclodextrin
complex prevents the fibrosis effect on CCl4-induced
cirrhotic cardiomyopathy in rats through TGF-β pathway inhibition
effects. Int J Mol Sci. 15:8037–8048. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: The 1996 guide for the care and use of laboratory
animals. ILAR J. 38:41–48. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kasarala G and Tillmann H: Standard liver
tests. Clin Liver Dis (Hoboken). 8:13–18. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Lo RC and Kim H: Histopathological
evaluation of liver fibrosis and cirrhosis regression. Clin Mol
Hepatal. 23:302–307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moller S and Henriksen JH: Cirrhotic
cardiomyopathy. J Hepatol. 53:179–190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Naqvi IH, Mahmood K, Naeem M, Vashwani AS
and Ziaullah S: The heart matters when the liver shatters!
Cirrhotic cardiomyopathy: Frequency, comparison, and correlation
with severity of disease. Prz Gastroenterology. 11:247–256.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Berzigotti A: Advances and challenges in
cirrhosis and portal hypertension. BMC Med. 15(200)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chayanupatkul M and Liangpunsakul S:
Cirrhotic cardiomyopathy: Review of pathophysiology and treatment.
Hepatol Int. 8:308–315. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Hu Y, Zeng Z, Li Y, Su H, Li Y,
Wang R, Zhang M, Yang Y and Deng J: Influence of androgen on
myocardial apoptosis and expression of myocardial IR and IRS1 in
chronic heart failure rat models. Mol Med Rep. 17:1057–1064.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gassanov N, Caglayan E, Semmo N,
Massenkeil G and Er F: Cirrhotic cardiomyopathy: A cardiologist's
perspective. World J Gastroenterol. 20:15492–15498. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiao J, Wang F, Wong NK, He J, Zhang R,
Sun R, Xu Y, Liu Y, Li W, Koike K, et al: Global liver disease
burdens and research trends: Analysis from a Chinese perspective. J
Hepatol. 71:212–221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu L, Ong S, Talor MV, Barin JG,
Baldeviano GC, Kass DA, Bedja D, Zhang H, Sheikh A, Margolick JB,
et al: Cardiac fibroblasts mediate IL-17A-driven inflammatory
dilated cardiomyopathy. J Exp Med. 211:1449–1464. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fede G, Privitera G, Tomaselli T, Spadaro
L and Purrello F: Cardiovascular dysfunction in patients with liver
cirrhosis. Ann Gastroenterol. 28:31–40. 2015.PubMed/NCBI
|
|
28
|
Karagiannakis DS, Papatheodoridis G and
Vlachogiannakos J: Recent advances in cirrhotic cardiomyopathy. Dig
Dis Sci. 60:1141–1151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu H, Jayakumar S, Traboulsi M and Lee
SS: Cirrhotic cardiomyopathy: Implications for liver
transplantation. Liver Transpl. 23:826–835. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Saner FH, Neumann T, Canbay A, Trechmann
JW, Hartmann M, Goerlinger K, Bertram S, Beckebaum S, Cicinnati V
and Paul A: High brain-natriuretic peptide level predicts cirrhotic
cardiomyopathy in liver transplant patients. Transpl Int.
24:425–432. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang YY and Lin HC: The heart:
Pathophysiology and clinical implications of cirrhotic
cardiomyopathy. J Chin Med Assoc. 75:619–623. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zaky A and Lang JD: Cirrhosis-associated
cardiomyopathy. J Anesth Clin Res. 3:1–7. 2012.
|
|
33
|
Ortiz-Olvera NX, Castellanos-Pallares G,
Gómez-Jiménez LM, Cabrera-Muñoz ML, Méndez-Navarro J, Morán-Villota
S and Dehesa-Violante M: Anatomical cardiac alterations in liver
cirrhosis: An autopsy study. Ann Hepatol. 10:321–326.
2011.PubMed/NCBI
|
|
34
|
Naveed M, Han L, Hasnat M, Baig MMFA, Wang
W, Mikrani R, Zhiwei L, Sembatya KR, Xie D and Zhou X: Suppression
of TGP on myocardial remodeling by regulating the NF-κB pathway.
Biomed Pharmacother. 108:1460–1468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Briasoulis A, Mallikethi-Reddy S, Palla M,
Alesh I and Afonso L: Myocardial fibrosis on cardiac magnetic
resonance and cardiac outcomes in hypertrophic cardiomyopathy: A
meta-analysis. Heart. 101:1406–1411. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jou C, Shah R, Figueroa A and Patel JK:
The role of inflammatory cytokines in cardiac arrest. J Intensive
Care Med. 35:219–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bageghni SA, Hemmings KE, Zava N, Denton
CP, Porter KE, Ainscough JFX, Drinkhill MJ and Turner NA: Cardiac
fibroblast-specific p38α MAP kinase promotes cardiac hypertrophy
via a putative paracrine interleukin-6 signaling mechanism. FASEB
J. 32:4941–4954. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Soresi M, Giannitrapani L, D'Antona F,
Florena AM, La Spada E, Terranova A, Cervello M, D'Alessandro N and
Montalto G: Interleukin-6 and its soluble receptor in patients with
liver cirrhosis and hepatocellular carcinoma. World J
Gastroenterol. 12:2563–2568. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu X, Li C, Zhu J, Li W and Zhu Q:
Dysregulation of FTX/miR-545 signaling pathway downregulates Tim-3
and is responsible for the abnormal activation of macrophage in
cirrhosis. J Cell Biochem: Oct 10, 2018 (Epub ahead of print).
|
|
40
|
Pagourelias ED, Sotiriou P, Papadopoulos
CE, Cholongitas E, Giouleme O and Vassilikos V: Left ventricular
myocardial mechanics in cirrhosis: A speckle tracking
echocardiographic study. Echocardiography. 33:223–232.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Blendis L and Wong F: The hyperdynamic
circulation in cirrhosis: An overview. Pharmacol Ther. 89:221–231.
2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Moller S, Hove JD, Dixen U and Bendtsen F:
New insights into cirrhotic cardiomyopathy. Int J Cardiol.
167:1101–1108. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Muñoz-Ortega MH, Llamas-Ramírez RW,
Romero-Delgadillo NI, Elías-Flores TG, Tavares-Rodríguez EJ,
Campos-Esparza MR, Cervantes-García D, Muñoz-Fernández L,
Gerardo-Rodríguez M and Ventura-Juárez J: Doxazasin treatment
attenuates carbon tetrachloride-induced liver fibrosis in hamsters
through a decrease in transforming growth factor β secretion. Gut
Liver. 10:101–108. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Communal C, Singh M, Menon B, Xie Z,
Colucci WS and Singh K: Beta1integrins expression in adult rat
ventricular myocytes and its role in the regulation of
beta-adrenergic receptorstimulated apoptosis. J Cell Biochem.
89:381–388. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Parvaneh S, Howe CL, Toosizadeh N,
Honarvar B, Slepian MJ, Fain M, Mohler J and Najafi B: Regulation
of cardiac autonomic nervous system control across frailty
statuses: A systematic review. Gerontology. 62:3–15.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ma G, Wang Y, Hou D, Liu J, Zhang J, Xu L,
Wang H, Zhao W, Zhang Y and Zhang L: Association of autoantibodies
against the M2-muscarinic receptor with long-term outcomes in
peripartum cardiomyopathy patients: A 5-year prospective study. J
Cardiol. 74:251–257. 2019.PubMed/NCBI View Article : Google Scholar
|