Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory
rheumatic disease that occurs in ~30% of patients with psoriasis.
Although the diagnosis of arthritis is usually established years
after skin involvement, the joint involvement sometimes can precede
it, with peripheral arthritis, as well as spine inflammatory
changes (1-4).
Enthesitis, inflammation of the origin and insertion of ligaments,
tendons, aponeuroses, annulus fibrosis and joint capsules, has been
suggested to be the underlying feature of PsA and it is reported to
be present in 30-50% of the cases (5,6). In
PsA, as stated by the Classification Criteria for Psoriatic
Arthritis (CASPAR) as well as by the Group for Research and
Assessment of Psoriasis and Psoriatic arthritis (GRAPPA)
recommendations, enthesitis identification is useful for diagnosis
and treatment (7,8).
Clinical examination of enthesis can be a challenge
in clinical practice, as its presentation can vary from
asymptomatic to inflammatory, mechanical or traumatic type of
involvement. Thus, it is highly necessary to use imaging
techniques, such as magnetic resonance imaging (MRI) or
ultrasonography (US), in order to properly detect the type and
nature of the changes. US has emerged as an indispensable tool for
evaluating all types of rheumatic conditions, offering the
advantage of being non-invasive, reproducible and easily to be used
by an experienced examiner (9-12).
In PsA patients, US seems to have a higher importance compared to
MRI, as it is more accurate in describing the structure, new bone
formation or vascularization and at a higher detail (13-15).
Several scores have been proposed in order to evaluate the extent
of entheseal abnormalities, among which is the MAdrid Sonographic
Enthesitis Index (MASEI), which has proven to be a reliable tool in
detecting signs of both subclinical and constituted disease
(13,16).
The present study aimed to analyze the type and
frequency of entheseal involvement in PsA patients, by US
examination, using MASEI and OMERACT definitions, as well as a
search for correlations between the presence of enthesitis and a
series of disease variables. The aim of our study was to provide a
possible pattern of involvement for enthesis in PsA patients, in
order to optimize the management of these patients.
Patients and methods
We performed a retrospective study on 41 patients
diagnosed with PsA based on CASPAR criteria (7), in a one-year interval between 2018 and
2019, admitted into the Rheumatology Department of the Emergency
County Hospital Craiova, Romania. We collected data that included
demographic, clinical, laboratory parameters and imagistic methods,
in accordance to the study protocol.
The study was performed in accordance with the
ethics and deontology principles of the Helsinki Human Right's
Declaration and was approved by the Emergency County Hospital
Craiova Ethics Committee (under the number of 28690/2019). Written
informed consent was obtained from each patient.
US
The examination was performed by an expert
sonographer (FAV), blinded to the history, clinical findings, and
biology of each patient, using an Esaote MyLab 25 machine, equipped
with a high frequency linear probe (10-18 MHz). Enthesitis was
evaluated and defined according to OMERACT (Outcome Measures in
Rheumatology) definitions (17).
The MASEI items were evaluated according to the description
(16) (Table I).
 | Table IMadrid Sonographic Enthesis Index
(MASEI). |
Table I
Madrid Sonographic Enthesis Index
(MASEI).
| Data | Value |
|---|
| Inferior pole of the
calcaneus: Plantar aponeurosis enthesis | |
|
Plantar
aponeurosis structure | (0 or 1) |
|
Plantar
aponeurosis thickness >4.4 mm | (0 or 1) |
|
Inferior
pole of calcaneus erosion | (0 or 3) |
|
Inferior
pole of calcaneus enthesis calcification | (0, 1, 2 or 3) |
|
Plantar
aponeurosis enthesis power Doppler | (0 or 3) |
| Superior pole of the
calcaneus: Achilles tendon enthesis | |
|
Achilles
tendon structure | (0 or 1) |
|
Achilles
tendon thickness >5.29 mm | (0 or 1) |
|
Retrocalcaneal
bursitis | (0 or 1) |
|
Posterior
pole of calcaneus erosion | (0 or 3) |
|
Posterior
pole of calcaneus enthesis calcification | (0, 1, 2 or 3) |
|
Posterior
pole of calcaneus power Doppler | (0 or 3) |
| Tibial tuberosity:
Distal patellar ligament enthesis | |
|
Patellar
ligament structure | (0 or 1) |
|
Patellar
ligament thickness >4 mm | (0 or 1) |
|
Infrapatellar
bursitis | (0 or 1) |
|
Tibial
tuberosity erosion | (0 or 3) |
|
Tibial
tuberosity enthesis calcification | (0, 1, 2 or 3) |
|
Tibial
tuberosity enthesis power Doppler | (0 or 3) |
| Inferior pole of
the patella: Proximal patellar ligament enthesis | |
|
Patellar
ligament structure | (0 or 1) |
|
Patellar
ligament thickness >4 mm | (0 or 1) |
|
Inferior
pole of patella erosion | (0 or 3) |
|
Inferior
pole of patella enthesis calcification | (0, 1, 2 or 3) |
|
Inferior
pole of patella enthesis power Doppler | (0 or 3) |
| Superior pole of
the patella: Quadriceps tendon enthesis | |
|
Quadriceps
tendon structure | (0 or 1) |
|
Quadriceps
tendon thickness >6.1 mm | (0 or 1) |
|
Superior
pole of patella erosion | (0 or 3) |
|
Superior
pole of patella enthesis calcification | (0, 1, 2 or 3) |
|
Superior
pole of patella enthesis power Doppler | (0 or 3) |
| Oleocranon
tuberosity: Triceps tendon enthesis | |
|
Triceps
tendon structure | (0 or 1) |
|
Triceps
tendon thickness >6.1 mm | (0 or 1) |
|
Oleocranon
erosion | (0 or 3) |
|
Oleocranon
enthesis calcification | (0, 1, 2 or 3) |
|
Oleocranon
enthesis power Doppler | (0 or 3) |
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.5 (GraphPad Software, Inc.). Results are presented as mean
± SD and data were analyzed using t-test and one-way ANOVA for
comparing groups, and Pearson/Spearman's coefficient for evaluating
correlations. We considered a level of P<0.05 statistically
significant.
Results
We included 41 consecutive patients, 27 women and 14
men, with a mean age of 53.44±0.91 years and a mean disease
duration of 6.63±4.26 years, ranging from 0.5 to 12 years. We
registered a mean body mass index (BMI) of 27.44±6.35 [11 (26.82%)
patients were overweight and 13 (31.70%) obese]. The general
characteristics of the study group are presented in Table II.
 | Table IIGeneral characteristics of the study
group (N=41). |
Table II
General characteristics of the study
group (N=41).
| Patients (N) | Data values |
|---|
| Female, n (%) | 27 (65.86) |
| Male, n (%) | 14 (34.14) |
| Mean age
(years) | 54.34±0.91 |
| Disease duration
(years) | 6.63±4.26 |
| Type of psoriasis,
n (%) | |
|
Nail | 24 (58.53) |
|
Skin | 35 (85.36) |
|
Nail and
skin | 24 (58.53) |
|
Sine
psoriasis | 5 (12.19) |
| Type of psoriatic
arthritis, n (%) | |
|
Peripheral | 32 (78.04) |
|
Axial and
peripheral | 9 (21.95) |
| CRP (mg/dl) | 11.66±26.60 |
| ESR (mm/h) | 32.27±25.59 |
| DAPSA | 11.80±4.91 |
| PASI | 15.32±7.12 |
| BMI
(kg/m2) | 27.44±6.35 |
| Uric acid
(mg/dl) | 4.77±1.48 |
| Current medication,
n (%) | |
|
DMARD
non-biologic | 41(100) |
|
DMARD
biologic | 16 (39.02%) |
| MASEI score (mean ±
SD) | 13.2±5.8 |
In regard to inflammatory markers, we found a mean
value of 11.66±26.6 mg/dl for C reactive protein (CRP) and
33.27±25.59 for erythrocyte sedimentation rate (ESR).
Synthetic disease-modifying anti-rheumatic drugs
(DMARDs) were a therapeutic option for all the patients and
biologic DMARD for 39.02% (16 patients).
In regards to scoring the disease activity, we found
a mean disease activity in the Disease Activity Index for Psoriatic
Arthritis (DAPSA) score of 11.80±4.91, with limits between 2 and
25.8. For the Psoriasis Area Severity Index (PASI), we registered
values between 0 and 28, with a mean of 15.32±7.12.
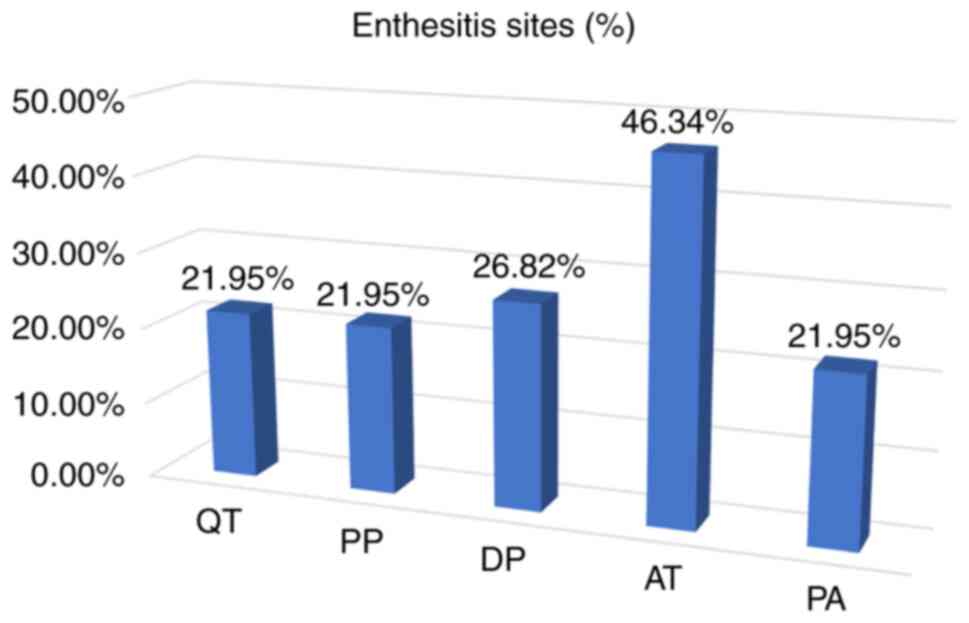
Enthesitis, according to OMERACT definitions, was
present in 26 of the included patients (63.41%). We identified
Achilles enthesis (AT) as the most common site [19 (46.34%)],
followed by distal patellar tendon (DP) [11 (26.82%)], quadriceps
tendon (QT) [11 (26.82%)], proximal patellar tendon (PP) [9
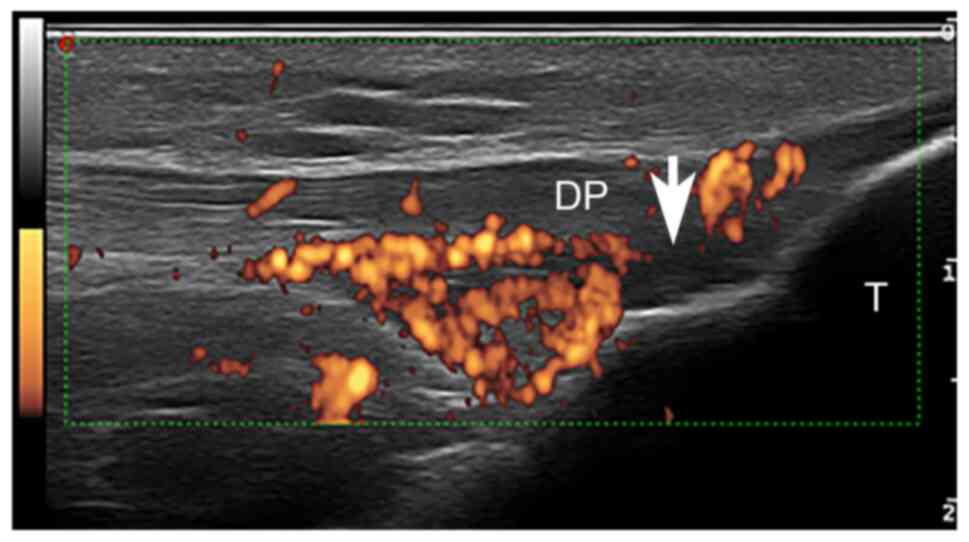
(21.94%)] and plantar aponeurosis (PA) [9 (21.94%)] (Table III, Fig. 1, Fig.
2 and Fig. 3). Given the fact
that all patients received DMARD therapy, synthetic with/without
biologic, the US evaluation of our study group did not show a high
percentage of active Power Doppler (PD) enthesitis in the evaluated
sites, except AT (24.32%) (Table
III, Fig. 2).
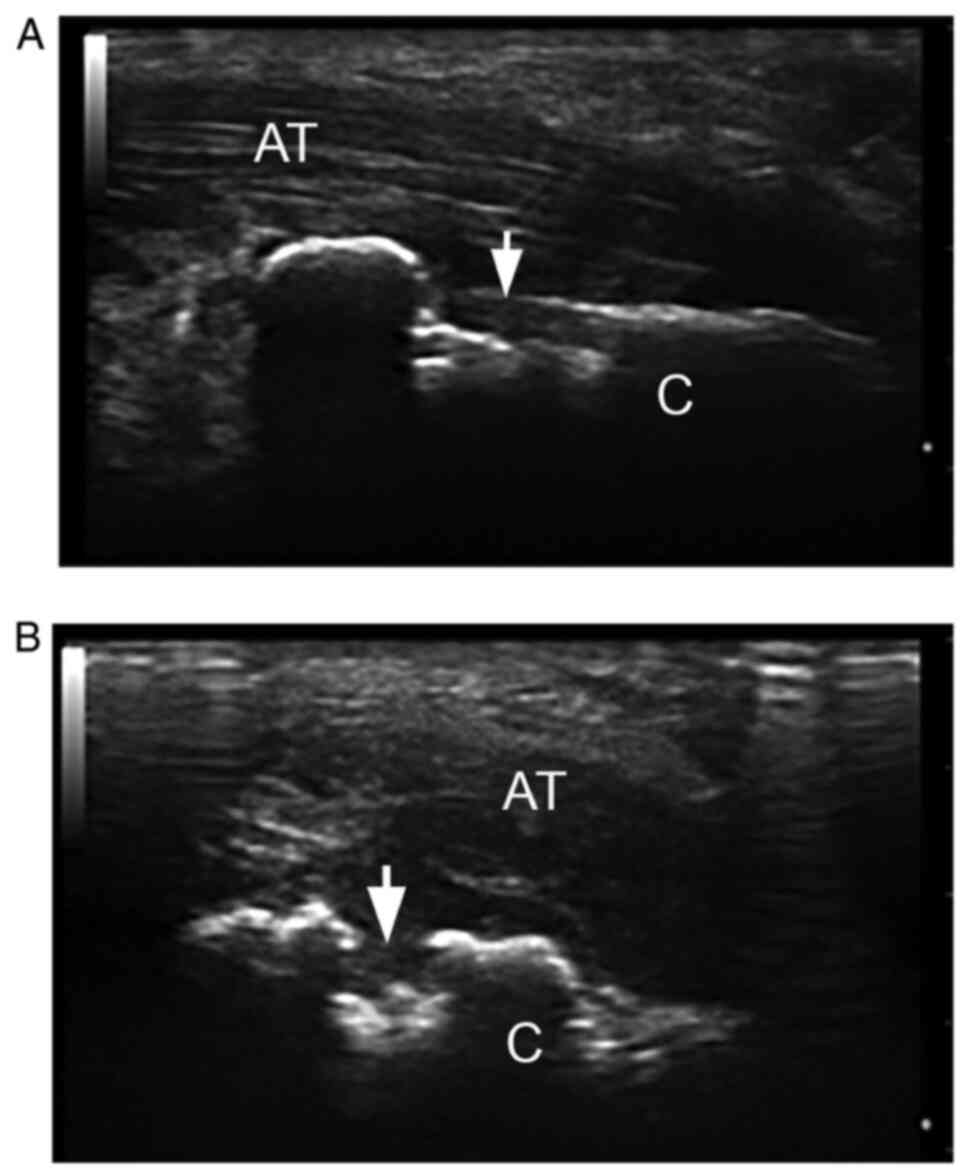 | Figure 2Grey scale US of Achilles tendon. (A)
Longitudinal and (B) transverse scan: Hypoechoic Achilles tendon
(AT), with loss of the fibrillar pattern, mostly near to the
cortical calcaneus (C in the images) bone (<2 mm), findings
suggestive for enthesitis. Moreover, we observed erosion (arrow),
like step-down changes, identified in two perpendicular views. US,
ultrasonography; AT, Achilles tendon; C, calcaneus (Esaote, MyLab25
18 MHz). |
 | Table IIIUltrasonography (US) changes
according to MASEI. |
Table III
Ultrasonography (US) changes
according to MASEI.
| | Abnormal tendon
structure n (%) | Thickened tendon n
(%) | Erosion n (%) | Enthesis
calcification/ enthesophyte n (%) | Enthesis PD n
(%) | Bursitis n (%) |
|---|
| TT | 2 (4.87) | 1 2.43 | 0 | 4 (9.75) | 0 | 0 |
| QT | 8 (19.51) | 3 (7.31) | 1 (2.43) | 7 (17.07) | 3 (7.31) | 0 |
| PP | 5 (12.19) | 4 (9.75) | 1 (2.43) | 3 (7.31) | 1 (2.43) | 0 |
| DP | 6 (14.63) | 5 (12.19) | 0 | 2 (4.87) | 2 (4.87) | 0 |
| AT | 12 (29.26) | 7 (17.07) | 6 (14.63) | 7 (17.07) | 10 (24.39) | 2 (4.87) |
| PA | 6 (14.63) | 3 (7.31) | 2.43 | 9.75 | 0 | 0 |
We carried out further statistical analysis on the
possible correlations between the presence of enthesitis and
certain variables. We found a moderately positive correlation
between the presence of enthesitis and inflammatory markers
(r=0.42, P=0.005 for CRP and r=0.36, P=0.020 for ESR). Another
significant correlation was established between US enthesitis,
patient age (r=0.37, P=0.05) and PASI score (r=0.43, P=0.004). Of
the 18 patients with a moderate/severe PASI score, 13 had entheseal
involvement at US evaluation.
When we analyzed our data for the effect of body
mass index (BMI) on entheseal damage, although the value of Pearson
correlation coefficient was 0.35, we noted that US examination
found a high percentage of enthesitis in overweight and obese
patients (16 of 22, compared to 10 of the 19 patients with a normal
BMI).
Discussion
PsA is a disease that requires early diagnosis and
therapeutic approach in order to prevent future tendon and
articular damage and its consequent functional impairment.
Entheseal involvement is a hallmark of the disease, more commonly
found in PsA patients compared to other inflammatory or
non-inflammatory conditions, directly related to both peripheral
and axial structural damage (1).
Clinical examination can be challenging in identifying enthesitis,
both for asymptomatic patients as for those presenting signs and
symptoms similar to other conditions and often requires additional
imaging techniques (18).
US is a well-established validated method for
detecting enthesitis (19), both
subclinical and clinical manifestations, in patients with PsA and
psoriasis, providing an accurate information on both structural and
inflammatory changes. Moreover, it has been reported to be a
real-time, reproducible, and cost-effective technique. Several
previous studies, which have focused on enthesis evaluation in PsA
patients, have proven that this condition is particularly known to
be an entheseal disease, with significant impact on disease
activity and quality of life. In order to obtain a proper indicator
of disease activity and treatment response, patient evaluation
should mandatorily include US entheseal assessment (7,15-18).
The involvement of US entheseal in PsA patients, as
aimed by our research, revealed it to be present in a high
percentage of patients. The data found in our study (63.41%) is
similar to data reported by Gutierrez et al, in a study
conducted on 45 patients (20).
Other publications have also demonstrated similar data (1,21-24).
The prevalence of tendon structure abnormalities and
enthesophytes as reported by significant scientific reports is
similar to our results, underlying the importance of chronic
inflammation on entheseal sites (1,21).
The most common site of inflammation found in our
patients was represented by Achilles enthesis. Michelsen et
al assessed 141 patients and revealed percentages of over 50
for structural damage and 16.3 for inflammatory activity, when
examining AT, similar to our results (25). The observation made by the
aforementioned study, that AT insertion is the site of major
entheseal abnormalities, was also confirmed by Perrotta et
al (26). A recent multi-center
study, that enrolled a total number of 1,130 PsA patients, reported
that 22.2% presented with active enthesitis (27).
The observation that the PASI score was correlated
with enthesitis is in full agreement with the report of Moshrif
et al (21), as well as with
other studies (28,29).
Analyzing the associations between different
variables and the presence of enthesitis, we observed a moderately
positive correlation with body mass index (BMI). Although the
calculated Pearson correlation coefficient was 0.35, overweight and
obese patients presented a higher prevalence of entheseal
abnormalities. BMI, a variable with significant role in entheseal
findings, is generally higher in PsA patients, compared to healthy
subjects. The mean BMI calculated for our group was 27.44±6.35. US
scores, such as MASEI, and more specifically AT abnormalities and
entesophytes presence, were demonstrated to be positively
correlated to an increased BMI (1,30-33).
In our study group, we also obtained a moderately positive
inter-relationship between the two variables. Nevertheless, BMI is
also a factor of biomechanical stress, which can input certain
abnormalities of this structure.
The lack of Doppler activity in our patients may
have been related as already mentioned to the fact that most
patients were already receiving disease-modifying anti-rheumatic
drug (DMARD) treatment, but, at the same time, we realized the fact
that the machine's sensitivity on vascularization might have
influenced the results. Other limitations of the study include the
small number of patients and the fact that we had only one US
examiner and no other imaging technique was used to confirm the
findings.
In conclusion, enthesitis, the defining feature of
PsA and an important part of the disease pathogenesis, predicts
patient outcome, future structural changes and noticeably impacts
the quality of life of these patients. US examination has proven to
be a reliable imaging method, with significant and continuous
improvement, which is clearly a requisite part of the current
understanding and diagnosis of enthesitis and patient follow-up
algorithm.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BAC, ALB, RMD and FAV conceived and designed the
current study. BAC, ALB, CDP, SCF and SCD provided administrative
support. BAC, ALB, CDP, SCF, RMD, SBC, MVB, SCD, HVP, RAI, ATS, AMV
and FAV searched the literature for pertinent data and findings.
BAC, CDP, SCF, RMD, SBC, SCD, AMV and FAV collected and collated
the data. BAC, CDP, SCF, RMD, SBC, PLC, SCD, MVB, HVP, RAI, ATS and
FAV analyzed and interpreted the data. All authors wrote the
manuscript and all authors read and approved the final manuscript
for publication.
Ethics approval and consent to
participate
The study complied with the Declaration of Helsinki
and was approved by the Emergency County Hospital Craiova Ethics
Committee (under the number of 28690/2019). Written informed
consent was obtained from each patient.
Patient consent for publication
The patients consented to the publication of their
data and images in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaeley GS, Eder L, Aydin SZ, Gutierrez M
and Bakewell C: Enthesitis: A hallmark of psoriatic arthritis.
Semin Arthritis Rheum. 48:35–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mease PJ, Gladman DD, Papp KA, Khraishi
MM, Thaci D, Behrens F, Northington R, Fuiman J, Bananis E, Boggs R
and Alvarez D: Prevalence of rheumatologist-diagnosed psoriatic
arthritis in patients with psoriasis in European/North American
dermatology clinics. J Am Acad Dermatol. 69:729–735.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Catanoso M, Pipitone N and Salvarani C:
Epidemiology of psoriatic arthritis. Reumatismo. 64:66–70.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chisălău BA, Crînguș LI, Vreju FA,
Pârvănescu CD, Firulescu SC, Dinescu ȘC, Ciobanu DA, Tica AA, Sandu
RE, Siloși I, et al: New insights into IL-17/IL-23 signaling in
ankylosing spondylitis (Review). Exp Ther Med. 20:3493–3497.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gladman DD: Clinical features and
diagnostic considerations in psoriatic arthritis. Rheum Dis Clin
North Am. 41:569–579. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Polachek A, Li S, Chandran V and Gladman
D: Clinical enthesitis in a prospective longitudinal psoriatic
arthritis cohort: Incidence, prevalence, characteristics and
outcome. Arthritis Care Res (Hoboken). 69:1685–1691.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taylor W, Gladman D, Helliwell P,
Marchesoni A, Mease P and Mielants H: CASPAR Study Group.
Classification criteria for psoriatic arthritis: Development of new
criteria from a large international study. Arthritis Rheum.
54:2665–2673. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Coates LC, Kavanaugh A, Mease PJ, Soriano
ER, Acosta-Felquer L, Armstrong M, Bautista-Molano AW, Boehncke W,
Campbell WH, Cauli W, et al: Group for research and assessment of
psoriasis and psoriatic arthritis 2015 treatment recommendations
for psoriatic arthritis. Arthritis Rheumatol. 68:1060–1071.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barbulescu AL, Ciurea PL, Mitran C,
Chisalau BA, Parvanescu CD, Firulescu SC, Balasoiu M, Boldeanu MV,
Popoviciu H and Vreju FA: High frequency ultrasonography of the
hand versus anti-RA33 evaluation in early rheumatoid arthritis-a
pilot study. Med Ultrason. 19:166–171. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Vreju FA, Ciurea ME, Popa D, Popa F,
Parvanescu CD, Chisalau BA, Barbulescu AL, Parvanescu V, Rosu A and
Ciurea PL: Ultrasonography in the diagnosis and management of
noninflammatory conditions of the hand and wrist. Med Ultrason.
18:90–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ciurea ME, Ciurea RN, Bărbulescu AL,
Chisălău AB, Pârvănescu CD, Firulescu SC, Covei Bănicioiu S, Ciurea
PL and Vreju AF: Intramuscular hemangioma of the arm:
Ultrasonography and pathology features. Rom J Morph Embryol.
57:521–524. 2016.PubMed/NCBI
|
|
12
|
Filippou G, Scirè CA, Adinolfi A, Damjanov
NS, Carrara G, Bruyn GAW, Cazenave T, D'Agostino MA, Delle Sedie A,
Di Sabatino V, et al: Identification of calcium pyrophosphate
deposition disease (CPPD) by ultrasound: Reliability of the OMERACT
definitions in an extended set of joints-an international
multiobserver study by the OMERACT Calcium Pyrophosphate Deposition
Disease Ultrasound Subtask Force. Ann Rheum Dis. 77:1194–1199.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Miguel E, Cobo T, Munoz-Fernandez S,
Naredo E, Uson J, Acebes JC and Martin-Mola E: Validity of enthesis
ultrasound assessment in spondyloarthropathy. Ann Rheum Dis.
68:169–174. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Micu MC and Fodor D: Concepts in
monitoring enthesitis in patients with spondylarthritis-the role of
musculoskeletal ultrasound. Med Ultrason. 18:82–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Poggenborg RP, Eshed I, Østergaard M,
Sørensen IJ, Møller JM, Madsen OR and Pdersen SJ: Enthesitis in
patients with psoriatic arthritis, axial spondyloarthritis and
healthy subjects assessed by ‘headto-toe’ whole-body MRI and
clinical examination. Ann Rheum Dis. 74:823–829. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wervers K, Vis M, Rasappu N, Van Der Ven
M, Tchetverikov I, Kok MR, Gerards AH, Hazes J and Luime JJ:
Modification of a sonographic enthesitis score to differentiate
between psoriatic arthritis and young healthy volunteers. Scand J
Rheumatol. 47:291–294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Balint PV, Terslev L, Aegerter P, Bruyn
AWG, Chary-Valckenaere I, Gandjbakhch F, Iagnocco AM, Jousse-Joulin
SJ, Möller I, Naredo E, et al: On behalf of the Omeract ultrasound
task force members, reliability of a consensus-based ultrasound
definition and scoring for enthesitis in spondyloarthritis and
psoriatic arthritis: An OMERACT US initiative. Ann Rheum Dis.
77:1730–1735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zabotti A, Bandinelli F, Batticciotto A,
Scirè CA, Iagnocco A and Sakellariou G: Musculoskeletal
ultrasonography for psoriatic arthritis and psoriasis patients: A
systematic literature review. Rheumatology. 56:1518–1532.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gandjbakhch F, Terslev L, Joshua F,
Wakefield RJ, Naredo E and D'Agostino MA: OMERACT Ultrasound Task
Force. Ultrasound in the evaluation of enthesitis: Status and
perspectives. Arthritis Res Ther. 13(R188)2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Gutierrez M, Filippucci E, De Angelis R,
Salaffi F, Filosa G, Ruta S, Bertolazzi C and Grassi W: Subclinical
entheseal involvement in patients with psoriasis: An ultrasound
study. Semin Arthritis Rheum. 40:407–412. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moshrif A, Mosallam A, Mohamed EEM, Gouda
W and Doma M: Subclinical enthesopathy in patients with psoriasis
and its association with other disease parameters: A power Doppler
ultrasonographic study. Eur J Rheumatol. 4:24–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Naredo E, Möller I, De Miguel E,
Batlle-Gualda E, Acebes C, Brito E, Mayordomo L, Moragues C, Uson
J, De Agustín JJ, et al: High prevalence of ultrasonographic
synovitis and enthesopathy in patients with psoriasis without
psoriatic arthritis: A prospective case-control study. Rheumatology
(Oxford). 50:1838–1848. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eder L, Barzilai M, Peled N, Gladman DD
and Zisman D: The use of ultrasound for the assessment of
enthesitis in patients with spondyloarthritis. Clin Radiol.
68:219–223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kristensen S, Christensen JH, Schmidt EB,
Olesen JL, Johansen MB, Arvesen KB and Schlemmer A: Assessment of
enthesitis in patients with psoriatic arthritis using clinical
examination and ultrasound. Muscles Ligaments Tendons J. 6:241–247.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Michelsen B, Diamantopoulos AP, Soldal DM,
Hammer HB, Kavanaugh A and Haugeberg G: Achilles enthesitis defined
by ultrasound is not associated with clinical enthesitis in
patients with psoriatic arthritis. RMD Open.
3(e000486)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Perrotta FM, Astorri D, Zappia M,
Reginelli A, Brunese L and Lubrano E: An ultrasonographic study of
enthesis in early psoriatic arthritis patients naive to traditional
and biologic DMARDs treatment. Rheumatol Int. 36:1579–1583.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sunar I, Ataman S, Nas K, Kilic E, Sargin
B, Kasman SA, Alkan H, Sahin N, Cengiz G, Cuzdan N, et al:
Enthesitis and its relationship with disease activity, functional
status, and quality of life in psoriatic arthritis: A multi-center
study. Rheumatol Int. 40:283–294. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sakkas LI, Alexiou I, Simopoulou T and
Vlychou M: Enthesitis in psoriatic arthritis. Semin Arthritis
Rheum. 43:325–334. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Husic R, Anja Ficjan A, Christina Duftner
C and Dejaco C: Use of ultrasound for diagnosis and follow-up of
psoriatic arthritis. EMJ Rheumatol. 1:65–72. 2014.
|
|
30
|
Gisondi P, Tinazzi I, El-Dalati G, Gallo
M, Biasi D, Barbara LM and Girolomoni G: Lower limb enthesopathy in
patients with psoriasis without clinical signs of arthropathy: A
hospital-based case-control study. Ann Rheum Dis. 67:26–30.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Eder L, Jayakar J, Thavaneswaran A, Haddad
A, Chandran V, Salonen D, Rosen CF and Gladman DD: Is the Madrid
Sonographic Enthesitis Index useful for differentiating psoriatic
arthritis from psoriasis alone and healthy controls? J Rheumatol.
14:466–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aydin SZ, Can M, Alibaz-Oner F, Keser G,
Kurum E, Inal V, Yazisiz V, Birlik M, Emmungil H, Atagunduz P, et
al: A relationship between spinal new bone formation in ankylosing
spondylitis and the sonographically determined Achilles tendon
enthesophytes. Rheumatol Int. 36:397–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Aydin SZ, Filippucci E, Atagunduz P, Yavuz
S, Grassi W and Direskeneli H: Sonographic measurement of Achilles
tendon thickness in seronegative spondyloarthropathies. Eur J
Rheumatol. 1:7–10. 2014.PubMed/NCBI View Article : Google Scholar
|