Introduction
Psoriasis is a common autoimmune disease that is
characterized by thick erythematous skin plaques, affecting
0.91-8.5% of the global population (1,2). The
high prevalence and long disease course of psoriasis cause
substantial economic and psychological burdens to individuals and
their families (3,4). In addition, psoriasis is a type of
chronic inflammatory condition and is classified as a T-helper
(Th)1 disease, as the cytokines involved in the Th1 pathway
[including tumor necrosis factor α (TNF-α), interleukin (IL)-6,
IL-17 and IL-22 expression] are increased in the lesional skin and
peripheral blood of patients (5).
Although several therapeutic approaches (including topical therapy,
phototherapy, systemic non-biologic treatments and biologic
treatment) are able to decrease the disease activity in the
majority of circumstances, there is still no cure for psoriasis
(6,7). Therefore, it is paramount to
investigate the underlying mechanisms of the pathology of psoriasis
to further explore potential treatment options.
MicroRNAs (miRNAs/miRs) are a group of small
non-coding RNAs comprised of 22 nucleotides (8). Accumulating studies have revealed that
miRNAs are widely distributed in animals, plants and certain
viruses, and they have been determined to be key regulators of
numerous important biological processes, including cell
proliferation, differentiation and inflammation (9-11).
As one of the commonly investigated miRNAs, miR-146b has been
implicated in the malignant proliferation of various cancer cell
types (including ovarian and papillary thyroid cancer cells) and
several immune activities, including innate immune sensing and
pro-inflammatory cytokine release (12-16).
Several studies have revealed that miR-146b expression is increased
in several autoimmune diseases [including rheumatoid arthritis
(RA), multiple sclerosis and autoimmune thyroid diseases (ATD)]
(17-19).
Considering that miR-146b promotes cell proliferation and has a
role in the pathology of several autoimmune diseases, and that
psoriasis is a typical autoimmune disease associated with malignant
proliferation of keratinocytes, the present study hypothesized that
miR-146b may participate in the development and progression of
psoriasis. Of note, the role of miR-146b in the pathology of
psoriasis has so far remained elusive. Therefore, the present study
aimed to explore the expression of miR-146b in psoriatic tissue and
to determine its association with psoriasis activity and
inflammation. In addition, the present study explored the effect of
miR-146b overexpression on the regulation of keratinocyte
proliferation and apoptosis.
Materials and methods
Patients
A total of 110 patients with psoriasis were enrolled
at the Affiliated Hospital of Hebei University of Engineering
(Handan, China) between January 2017 and June 2018. The inclusion
criteria were as follows: i) Diagnosed with psoriasis vulgaris; ii)
age >18 years; iii) consent to donate psoriasis-affected skin
tissue samples and normal skin tissue samples. The exclusion
criteria were as follows: i) Complicated with inflammatory skin
diseases other than psoriasis; ii) complicated with other systemic
immune or inflammatory diseases, including systemic lupus
erythematosus or inflammatory bowel disease; iii) complicated with
malignancies. The present study was approved by the Ethics Review
Board of the Affiliated Hospital of Hebei University of Engineering
(Handan, China). All patients provided written informed
consent.
Disease assessment
After enrollment, the characteristics of the
patients, including their age, gender, body mass index (BMI),
disease duration and treatment options, were documented. The
psoriasis-affected body surface area (BSA) and psoriasis area
severity index (PASI) scores were subsequently determined (20,21).
Sample collection and reverse
transcription-quantitative (RT-q)PCR
Psoriasis-affected skin and normal skin tissue
samples were obtained via punch biopsy. The expression of miR-146b,
TNF-α, IL-6, IL-17 and IL-22 in each sample were measured via
RT-qPCR. Total RNA was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), after which the concentration,
purity and integrity of samples was determined. Complementary DNA
was then produced by RT using the ReverTra Ace® qPCR RT
kit (Toyobo Life Science). PCR was performed using SYBR®
Green Realtime PCR Master mix (Toyobo Life Science). The primers
utilized are listed in Table I. The
quantitative results of the PCR analysis were calculated using
2-ΔΔCq method (22). U6
was used as a reference gene for miR-146b, while GAPDH was used as
a reference for the remaining mRNAs. The RT was performed using the
following conditions: 65˚C for 5 min, 37˚C for 20 min and then 98˚C
for 5 min. The qPCR amplification was performed using the following
program: 95˚C for 60 sec, followed by 40 cycles of 95˚C for 15 sec
and 61˚C for 30 sec.
 | Table IPrimer list. |
Table I
Primer list.
| Gene | Forward primer | Reverse primer |
|---|
| miR-146b |
5'-ACACTCCAGCTGGGTGAGAACTGAATTCCA-3' |
5'-TGTCGTGGAGTCGGCAATTC-3' |
| U6 |
5'-CGCTTCGGCAGCACATATACTA-3' |
5'-ATGGAACGCTTCACGAATTTGC-3' |
| TNF-α |
5'-TGTTCCTCAGCCTCTTCTCCTT-3' |
5'-CTCTCAGCTCCACGCCATTG-3' |
| IL-6 |
5'-CTTCGGTCCAGTTGCCTTCTC-3' |
5'-AGGTGAGTGGCTGTCTGTGT-3' |
| IL-17 |
5'-ATTACTACAACCGATCCACCTCAC-3' |
5'-CCACGGACACCAGTATCTTCTC-3' |
| IL-22 |
5'-TATCACCAACCGCACCTTCAT-3' |
5'-CTCATACTGACTCCGTGGAACA-3' |
| GAPDH |
5'-GAGTCCACTGGCGTCTTCAC-3' |
5'-ATCTTGAGGCTGTTGTCATACTTCT-3' |
Cell culture and transfection
The spontaneously immortalized human keratinocyte
cell line HaCaT was purchased from Kunming Cell Bank and cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% inactivated fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
U/ml) and streptomycin (100 mg/ml; both from Sigma-Aldrich; Merck
KGaA). miR-146b overexpression and control overexpression plasmids
(with non-coding DNA fragment) were constructed by Shanghai QeeJen
Bio-Tech Co., Ltd. and then transfected into HaCaT cells, which
were correspondingly designated as the miR-146b+ group and the
Control+ group. The relative expression of miR-146b was detected at
24 h post-transfection via RT-qPCR as described above. All cell
experiments were performed in triplicate.
Measurement of cell proliferation and
apoptosis
After transfection, cell proliferation was detected
at 0, 24, 48 and 72 h using a Cell Counting Kit-8 (Dojindo
Molecular Technologies, Inc.). The rate of cell apoptosis was also
detected at 24 h following transfection with an FITC Annexin V
Apoptosis Detection kit II (BD Biosciences). The flow cytometer (BD
Biosciences) was used for the Annexin V assay. All procedures were
performed according to the manufacturers' protocols.
Measurement of TNF-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis
At 48 h post-transfection, 200 ng/ml TRAIL
(Sigma-Aldrich; Merck KGaA) was added to HaCaT cells and the cells
were incubated for a further 24 h. The rate of cell apoptosis was
then detected using an FITC Annexin V Apoptosis Detection kit II
(BD Biosciences) to determine TRAIL-induced apoptosis.
Statistical analysis
SPSS Software 23.0 (IBM Corp.) was used for
statistical analysis and GraphPad Prism Software 7.01 (GraphPad,
Inc.) was used for visualization of quantitative results.
Comparisons between two groups were performed using Wilcoxon's
signed rank-sum tests. Comparisons between two individual groups
were determined by Wilcoxon's rank-sum tests and Student's t-tests.
Differences in miR-146b expression among groups were determined
using the Kruskal-Wallis H-test. Correlations between two
parameters were determined by Spearman's correlation test. In
addition, receiver operating characteristic (ROC) curves were drawn
and the area under the curve (AUC) was calculated to determine the
ability of the parameter to distinguish psoriasis-affected skin
tissue from normal skin tissue. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients
The characteristics of the patients are presented in
Table II. The mean age of the 110
patients with psoriasis included in the present study was 47.4±10.8
years, and the cohort comprised 65 (59.1%) males and 45 (40.9%)
females. The mean psoriasis-affected BSA was 19.0±5.4% and the PASI
score was 9.6±3.8 (Table II). The
number of patients that received topical therapy, phototherapy,
systemic non-biologic treatment and systemic biologic treatment was
99 (90.0%), 92 (83.6%), 77 (70.0%) and 8 (7.3%), respectively.
 | Table IICharacteristics of the patients
(n=110). |
Table II
Characteristics of the patients
(n=110).
| Parameter | Value |
|---|
| Age (years) | 47.4±10.8 |
| Sex | |
|
Male | 65 (59.1) |
|
Female | 45 (40.9) |
| BMI
(kg/m2) | 23.2±3.9 |
| Disease duration
(years) | 10.0
(6.0-14.3) |
| Psoriasis-affected
BSA (%) | 19.0±5.4 |
| PASI score | 9.6±3.8 |
| Clinical course, n
(%) | |
| Progressive
stage | 59 (53.6) |
| Quiescent
stage | 24 (21.8) |
| Regressive
stage | 27 (24.5) |
| Treatment | |
|
Topical
therapy | 99 (90.0) |
|
Phototherapy | 92 (83.6) |
|
Systemic
non-biologic treatment | 77 (70.0) |
|
Systemic
biologic treatment | 8 (7.3) |
Comparison of miR-146b expression
between psoriasis-affected tissue and unaffected tissue
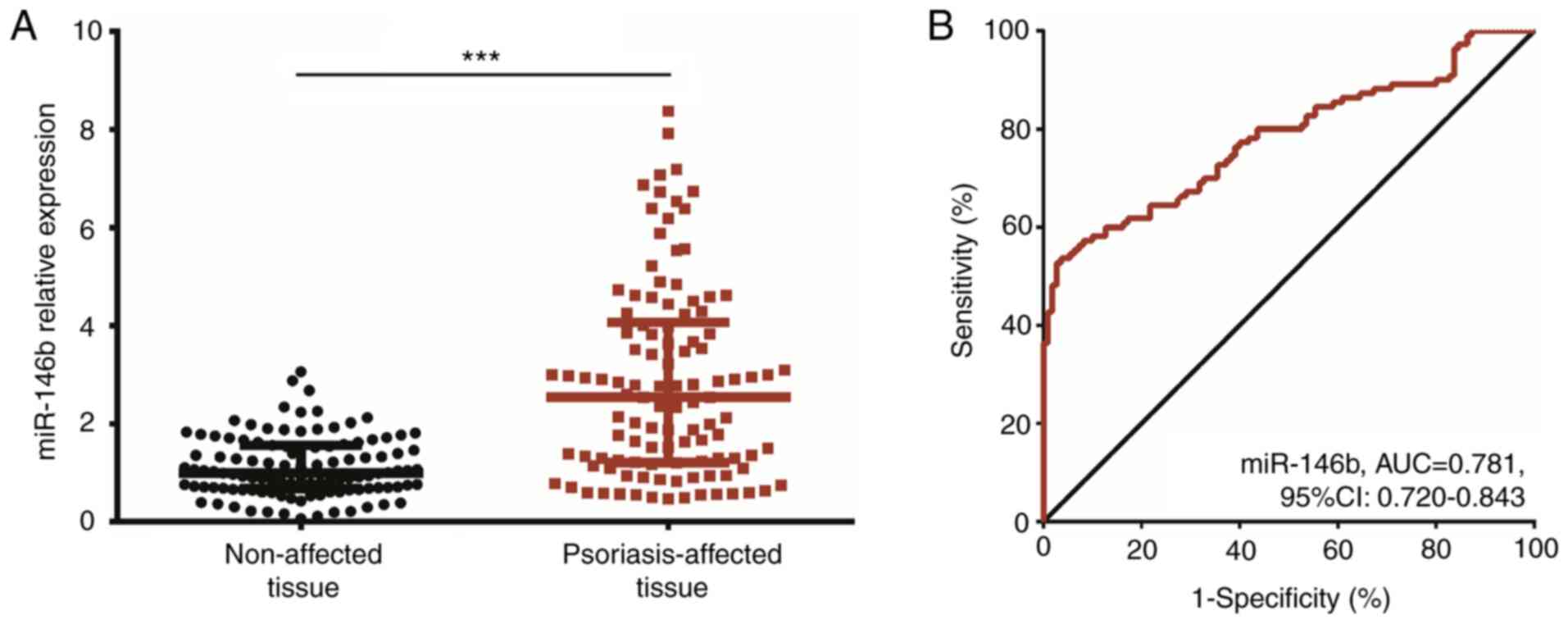
The expression of miR-146b was increased in
psoriasis-affected tissue when compared with that in unaffected
tissue (P<0.001; Fig. 1A).
Furthermore, miR-146b was able to distinguish psoriasis-affected
tissue from unaffected tissue with an AUC value of 0.781 (95% CI:
0.720-0.843; miR-146b cutoff value: 2.293; Fig. 1B) and the specificity and
sensitivity was 96.4 and 53.6% respectively.
Correlation of miR-146b expression in
psoriatic tissue with disease duration, psoriasis-affected BSA and
PASI scores in patients with psoriasis
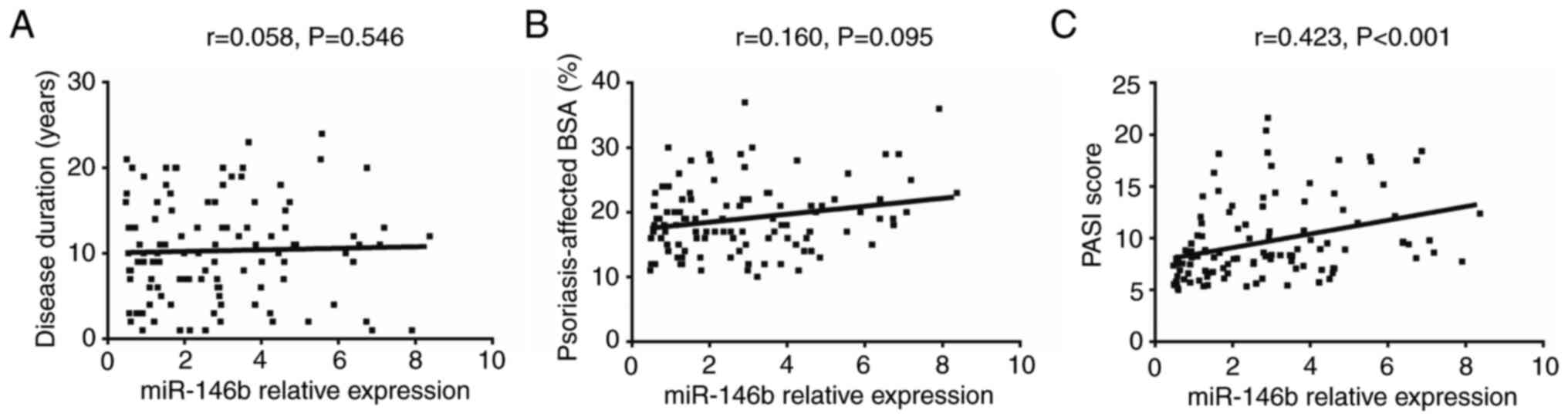
The results revealed that there was no correlation
between miR-146b expression in psoriatic tissue and disease
duration (r=0.058, P=0.546; Fig.
2A) or psoriasis-affected BSA (r=0.160, P=0.095; Fig. 2B). However, miR-146b expression was
positively correlated with the PASI score (r=0.423, P<0.001;
Fig. 2C), indicating that the
expression of miR-146b was associated with increased disease
activity in patients with psoriasis. In addition, the number of
patients with psoriasis at the progressive, quiescent and
regressive stage was 59, 24 and 27, respectively. The expression of
miR-146b was highest in patients at the progressive stage, followed
by that in patients at the quiescent stage and regressive stage
(P<0.001; Fig. S1).
Correlation of miR-146b expression in
psoriatic tissue with treatment approaches in psoriasis
patients
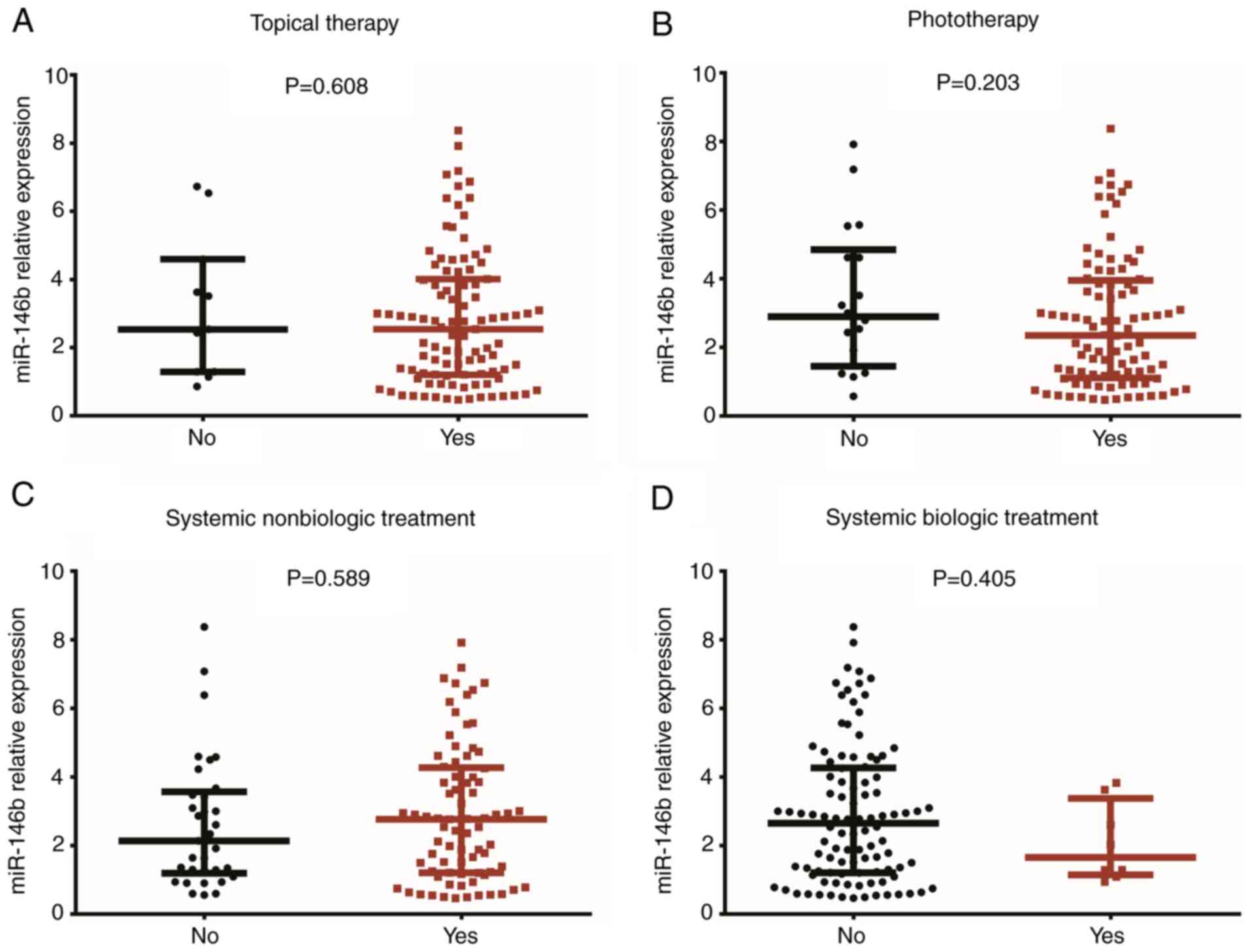
No correlation was identified between the expression
of miR-146b in psoriatic tissue and topical therapy (P=0.608;
Fig. 3A), phototherapy (P=0.203;
Fig. 3B), systemic non-biologic
treatment (P=0.589; Fig. 3C) or
systemic biologic treatment (P=0.405; Fig. 3D).
Correlation of miR-146b expression
with inflammatory cytokine levels in psoriatic tissue
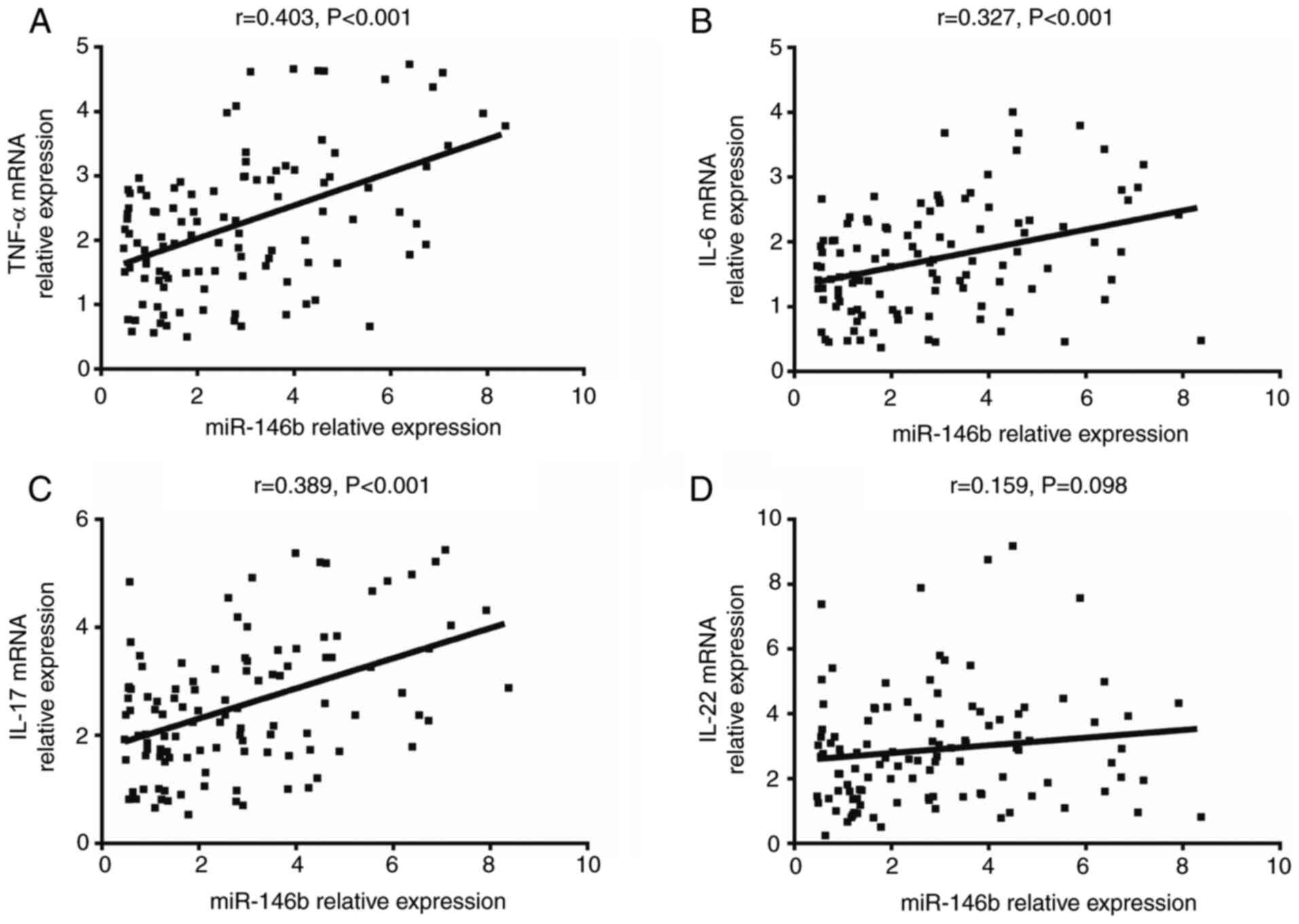
The expression of miR-146b in psoriatic tissue was
determined to be positively correlated with the mRNA expression of
TNF-α (r=0.403, P<0.001; Fig.
4A), IL-6 (r=0.327, P<0.001; Fig. 4B) and IL-17 (r=0.389, P<0.001;
Fig. 4C). However, no significant
correlation was obtained between miR-146b and IL-22 expression
(r=0.159, P=0.098; Fig. 4D). These
results indicated that the expression of miR-146b was associated
with elevated inflammation in psoriatic tissue.
Effect of miR-146b overexpression on
cell proliferation and apoptosis of HaCaT cells
To investigate the potential role of miR-146b in the
development of psoriasis, in vitro experiments were
performed by transfecting miR-146b overexpression and control
plasmids into HaCaT cells. The results revealed that the expression
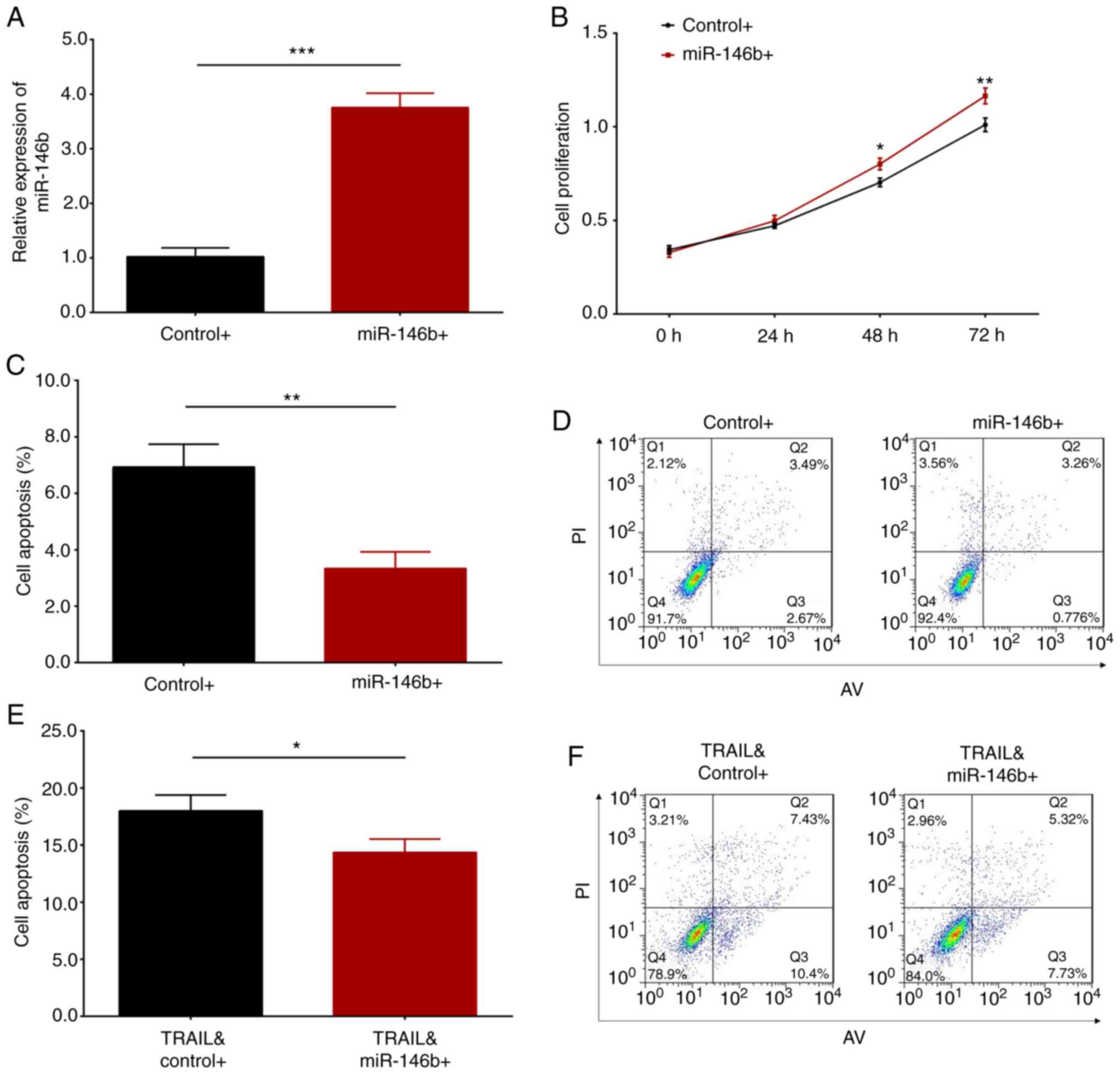
of miR-146b was significantly elevated following transfection of
miR-146b overexpression plasmid (P<0.001; Fig. 5A). This result indicated that
plasmid-mediated overexpression was successful. Further experiments
demonstrated that miR-146b overexpression enhanced cell
proliferation at 48 h (P<0.05) and 72 h (P<0.01; Fig. 5B), and suppressed cell apoptosis
(P<0.01; Fig. 5C and D) compared with control HaCaT cells. To
investigate the impact of miR-146b overexpression on keratinocytes
under inflammatory conditions, HaCaT cells were treated with TRAIL
following transfection. The results revealed that miR-146b
overexpression attenuated TRAIL-induced cell apoptosis (P<0.05;
Fig. 5E and F). Overall, it was indicated that miR-146b
overexpression promoted cell proliferation and inhibited HaCaT cell
apoptosis.
Discussion
In the present study, it was determined that the
expression of miR-146b was increased in psoriasis-affected tissue
when compared with that in unaffected tissue. The results also
revealed that the expression of miR-146b was associated with
elevated disease activity in patients with psoriasis and with
aggravated inflammation in psoriatic tissue. Furthermore,
overexpression of miR-146b enhanced keratinocyte proliferation,
inhibited keratinocyte apoptosis and reduced TRAIL-induced
keratinocyte apoptosis.
Numerous studies have demonstrated that miRNAs
participate in various cell activities and signaling pathways.
Investigating the role of these miRNAs in complex diseases
(including psoriasis) may facilitate the discovery of novel
biomarkers for their early diagnosis, also contributing to the
elucidation of disease etiology. As a well-known miRNA, miR-146b is
closely associated with inflammatory activities (12,23,24).
For instance, a previous study reported that miR-146b modulated the
Toll-like receptor 4 signaling pathway by directly targeting
myeloid differentiation primary response 88 (MYD88), TRAF6 and IL-1
receptor-associated kinase 1 (IRAK-1), thereby regulating pathogen
detection and the initiation of the inflammatory response (25). A further study demonstrated that the
upregulation of miR-146b attenuated bacterial
lipopolysaccharide-induced inflammatory responses and reduced the
expression of IRAK-1 and TRAF6 in human umbilical vein endothelial
cells (26). In addition, miR-146b
has been determined to be upregulated during human monocyte
differentiation into immature dendritic cells (imDCs) and mature
DCs (mDCs). Furthermore, silencing of miR-146b in imDCs and mDCs
markedly prevented cell apoptosis, while miR-146b overexpression
stimulated cell apoptosis (27).
These studies indicated that miR-146b exerts multiple functions in
the regulation of inflammation- and immune-associated signaling
pathways.
Limited information exists regarding the clinical
implications of miR-146b in autoimmune diseases. Only a small
number of studies have reported that miR-146b is highly expressed
in certain autoimmune conditions (including RA, giant cell
arteritis and ATD), indicating that miR-146b may contribute to the
pathology of autoimmune diseases (17,18,28).
Considering the potential role of miR-146b in the pathology of
certain autoimmune diseases and the inflammation/autoimmune
characteristics of psoriasis (a common and typical autoimmune
disease), the present study hypothesized that miR-146b may also
serve a role in the development and progression of psoriasis. To
the best of our knowledge, only one study has determined that the
expression of miR-146b was higher in psoriasis-affected skin
compared with that in unaffected skin from patients with psoriasis
and that of healthy controls. However, due to the small sample size
of the aforementioned study (n=30), the statistical power of this
result was relatively low (29).
Thus, further studies including more patients are essential. In the
present study, the clinical implications of miR-146b in patients
with psoriasis were investigated using 110 patients. The analyses
revealed that miR-146b expression was increased in
psoriasis-affected tissue when compared with that in unaffected
tissue. Furthermore, miR-146b expression was highest in patients at
the progressive stage, followed by patients at the quiescent stage
and regressive stage. An explanation for these results may be that
miR-146b regulates its target genes or proteins (including MYD88,
TRAF6 and IRAK-1), which subsequently promote keratinocyte
proliferation and inhibit keratinocyte apoptosis, thereby leading
to the formation of psoriatic lesions and acceleration of disease
progression (25). Thus, miR-146b
expression was increased in psoriasis-affected tissue, and was also
highly expressed in patients with psoriasis at the progressive
stage.
According to previous studies, psoriasis is a
recurrent, chronic autoimmune disease of the skin that involves the
infiltration of white blood cells, abnormal proliferation of
keratinocytes and activation of T cells in psoriasis-affected skin
tissue, which secrete various cytokines (including TNF-α, IL-6,
IL-17 and IL-22) that commonly contribute to the pathological
processes of psoriasis (2,30-33).
Therefore, to explore the correlation between miRNA-146 expression
and inflammation in patients with psoriasis, the expression of the
most common inflammatory cytokines, including TNF-α, IL-6, IL-17
and IL-22, were detected in the present study. The results revealed
that miR-146b expression was correlated with higher PASI scores in
patients with psoriasis and with elevated TNF-α, IL-6 and IL-17
mRNA expression in psoriatic tissue. These results indicated that
miR-146b expression was correlated with increased disease activity
and inflammation. The possible explanations for this result may be
as follows: i) miR-146b mediates TRAF6 and IRAK-1 expression,
promoting the release of pro-inflammatory cytokines (including
TNF-α, IL-6 and IL-17), thereby increasing disease activity and
inflammation observed in patients with psoriasis (12,34,35).
ii) miR-146b may have activated several inflammation-associated
signaling pathways (including NF-κB), causing a higher disease
activity and aggravated inflammation in patients with psoriasis.
Therefore, the present study indicated that miR-146b may serve as a
novel biomarker for the predication of elevated psoriasis activity
and aggravated inflammation in the clinical setting. However, the
present study had certain limitations. First, psoriatic tissue was
not easy to obtain, which means that it may not be feasible to
predict disease activity levels based on measurements of miR-146b
in the samples collected. Therefore, investigations into the
predictive value of circulating miR-146b expression are required
for determining psoriasis activity. In addition, the present study
had a single-centered design, meaning that most of the patients
were from North China, which may result in selection bias.
Therefore, multi-center studies are required for future
determinations.
Considering the malignant proliferation of
keratinocytes in psoriatic tissue, and to further explore the
underlying mechanism of miR-146b in the development of psoriasis,
the miR-146b overexpression and control plasmids were transfected
into HaCaT cells. The impact of miR-146b overexpression on HaCaT
cell proliferation and apoptosis was subsequently evaluated. The
present results revealed that miR-146b overexpression stimulated
cell proliferation and suppressed cell apoptosis, indicating that
miR-146b increased psoriasis activity and inflammation, potentially
by enhancing keratinocyte proliferation and reducing keratinocyte
apoptosis. To better understand the effect of miR-146b
overexpression on keratinocytes under inflammatory conditions,
HaCaT cell apoptosis following treatment with TRAIL (a protein that
induces cell apoptosis) was also evaluated in the present study.
The results demonstrated that miR-146b overexpression inhibited
TRAIL-induced cell apoptosis. The reasons for this result may be as
follows: i) miR-146b regulates multiple signaling pathways
(including NF-κB), thereby directly accelerating keratinocyte
proliferation and inhibiting apoptosis (23,36).
ii) miR-146b mediates various proteins (including TRAF6 and IRAK-1)
to promote the secretion of pro-inflammatory cytokines (including
TNF-α, IL-6 and IL-17), whose secretions may indirectly stimulate
keratinocyte proliferation (37).
Taken together, the present study provided novel insight into the
pathogenesis of psoriasis and may facilitate the discovery of novel
biomarkers for the prediction of psoriasis activity, as well as the
discovery of novel treatment options.
In summary, miR-146b expression was elevated in
psoriasis-affected tissue when compared with that in unaffected
tissue, and its high expression was associated with increased
disease activity in patients with psoriasis as well as aggravated
inflammation in psoriatic tissue. Furthermore, miR-146b
overexpression promoted keratinocyte proliferation and inhibited
keratinocyte apoptosis in vitro.
Supplementary Material
Association of miR-146b with the
clinical course. Comparisons of miR-146b expression among groups
were determined using the Kruskal-Wallis H-test.
***P<0.001 (significant differences were found
between all groups). miR, microRNA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the experiments. LZ and SZ
performed the experiments. JW analyzed the data. SZ and JW wrote
the main manuscript text. All authors revised the manuscript. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Review Board
of the Affiliated Hospital of Hebei University of Engineering
(Handan, China) and all patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM: Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team. Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
von Csiky-Sessoms S and Lebwohl M: What's
new in psoriasis. Dermatol Clin. 37:129–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Christophers E and van de Kerkhof PCM:
Severity, heterogeneity and systemic inflammation in psoriasis. J
Eur Acad Dermatol Venereol. 33:643–647. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Golińska J, Sar-Pomian M and Rudnicka L:
Dermoscopic features of psoriasis of the skin, scalp and nails-a
systematic review. J Eur Acad Dermatol Venereol. 33:648–660.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hwang YJ, Jung HJ, Kim MJ, Roh NK, Jung
JW, Lee YW, Choe YB and Ahn KJ: Serum levels of LL-37 and
inflammatory cytokines in plaque and guttate psoriasis. Mediators
Inflamm. 2014(268257)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Menter A, Strober BE, Kaplan DH,
Kivelevitch D, Prater EF, Stoff B, Armstrong AW, Connor C, Cordoro
KM, Davis DMR, et al: Joint AAD-NPF guidelines of care for the
management and treatment of psoriasis with biologics. J Am Acad
Dermatol. 80:1029–1072. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Elmets CA, Leonardi CL, Davis DMR, Gelfand
JM, Lichten J, Mehta NN, Armstrong AW, Connor C, Cordoro KM,
Elewski BE, et al: Joint AAD-NPF guidelines of care for the
management and treatment of psoriasis with awareness and attention
to comorbidities. J Am Acad Dermatol. 80:1073–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo WT and Wang Y: Dgcr8 knockout
approaches to understand microRNA functions in vitro and in vivo.
Cell Mol Life Sci. 76:1697–1711. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Asadzadeh Z, Mansoori B, Mohammadi A,
Aghajani M, Haji-Asgarzadeh K, Safarzadeh E, Mokhtarzadeh A, Duijf
PHG and Baradaran B: microRNAs in cancer stem cells: Biology,
pathways, and therapeutic opportunities. J Cell Physiol.
234:10002–10017. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Basso MF, Ferreira PCG, Kobayashi AK,
Harmon FG, Nepomuceno AL, Molinari HBC and Grossi-de-Sa MF:
MicroRNAs and new biotechnological tools for its modulation and
improving stress tolerance in plants. Plant Biotechnol J.
17:1482–1500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hou T, Liao J, Zhang C, Sun C, Li X and
Wang G: Elevated expression of miR-146, miR-139 and miR-340
involved in regulating Th1/Th2 balance with acute exposure of fine
particulate matter in mice. Int Immunopharmacol. 54:68–77.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tu Z, Xiong J, Xiao R, Shao L, Yang X,
Zhou L, Yuan W, Wang M, Yin Q, Wu Y, et al: Loss of miR-146b-5p
promotes T cell acute lymphoblastic leukemia migration and invasion
via the IL-17A pathway. J Cell Biochem. 120:5936–5948.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kirchmeyer M, Servais FA, Hamdorf M,
Nazarov PV, Ginolhac A, Halder R, Vallar L, Glanemann M, Rubie C,
Lammert F, et al: Cytokine-mediated modulation of the hepatic
miRNome: miR-146b-5p is an IL-6-inducible miRNA with multiple
targets. J Leukoc Biol. 104:987–1002. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yan M, Yang X, Shen R, Wu C, Wang H, Ye Q,
Yang P, Zhang L, Chen M, Wan B, et al: miR-146b promotes cell
proliferation and increases chemosensitivity, but attenuates cell
migration and invasion via FBXL10 in ovarian cancer. Cell Death
Dis. 9(1123)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu C, Zhang L, Luo D, Yan F, Liu J, Shao
S, Zhao L, Jin T, Zhao J and Gao L: MicroRNA-146b-3p promotes cell
metastasis by directly targeting NF2 in human papillary thyroid
cancer. Thyroid. 28:1627–1641. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakasa T, Miyaki S, Okubo A, Hashimoto M,
Nishida K, Ochi M and Asahara H: Expression of microRNA-146 in
rheumatoid arthritis synovial tissue. Arthritis Rheum.
58:1284–1292. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martínez-Hernández R, Sampedro-Núñez M,
Serrano-Somavilla A, Ramos-Leví AM, de la Fuente H, Triviño JC,
Sanz-García A, Sánchez-Madrid F and Marazuela M: A MicroRNA
signature for evaluation of risk and severity of autoimmune thyroid
diseases. J Clin Endocrinol Metab. 103:1139–1150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Venkatesha SH, Dudics S, Song Y, Mahurkar
A and Moudgil KD: The miRNA expression profile of experimental
autoimmune encephalomyelitis reveals novel potential disease
biomarkers. Int J Mol Sci. 19(3990)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wallace AB: The exposure treatment of
burns. Lancet. 1:501–504. 1951.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fredriksson T and Pettersson U: Severe
psoriasis-oral therapy with a new retinoid. Dermatologica.
157:238–244. 1978.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cho S, Lee HM, Yu IS, Choi YS, Huang HY,
Hashemifar SS, Lin LL, Chen MC, Afanasiev ND, Khan AA, et al:
Differential cell-intrinsic regulations of germinal center B and T
cells by miR-146a and miR-146b. Nat Commun. 9(2757)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Z, Dai X, Qi J, Ao Y, Yang C and Li
Y: Astragalus mongholicus (Fisch.) bge improves peripheral treg
cell immunity imbalance in the children with viral myocarditis by
reducing the levels of miR-146b and miR-155. Front Pediatr.
6(139)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Curtale G, Mirolo M, Renzi TA, Rossato M,
Bazzoni F and Locati M: Negative regulation of Toll-like receptor 4
signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA.
110:11499–11504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Echavarria R, Mayaki D, Neel JC, Harel S,
Sanchez V and Hussain SN: Angiopoietin-1 inhibits toll-like
receptor 4 signalling in cultured endothelial cells: Role of
miR-146b-5p. Cardiovasc Res. 106:465–477. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Croci S, Zerbini A, Boiardi L, Muratore F,
Bisagni A, Nicoli D, Farnetti E, Pazzola G, Cimino L, Moramarco A,
et al: MicroRNA markers of inflammation and remodelling in temporal
arteries from patients with giant cell arteritis. Ann Rheum Dis.
75:1527–1533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hermann H, Runnel T, Aab A, Baurecht H,
Rodriguez E, Magilnick N, Urgard E, Šahmatova L, Prans E,
Maslovskaja J, et al: miR-146b probably assists miRNA-146a in the
suppression of keratinocyte proliferation and inflammatory
responses in psoriasis. J Invest Dermatol. 137:1945–1954.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Grine L, Dejager L, Libert C and
Vandenbroucke RE: An inflammatory triangle in psoriasis: TNF, type
I IFNs and IL-17. Cytokine Growth Factor Rev. 26:25–33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang WM and Jin HZ: Interleukin-6 in
psoriasis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 40:284–288.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Mease PJ: Inhibition of interleukin-17,
interleukin-23 and the TH17 cell pathway in the treatment of
psoriatic arthritis and psoriasis. Curr Opin Rheumatol. 27:127–133.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hao JQ: Targeting interleukin-22 in
psoriasis. Inflammation. 37:94–99. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Viladomiu M, Hontecillas R, Pedragosa M,
Carbo A, Hoops S, Michalak P, Michalak K, Guerrant RL, Roche JK,
Warren CA and Bassaganya-Riera J: Modeling the role of peroxisome
proliferator-activated receptor γ and microRNA-146 in mucosal
immune responses to Clostridium difficile. PLoS One.
7(e47525)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cai F, Wu F, Cao J and Chen X:
MicroRNA-146b-3p regulates the development and progression of
cerebral infarction with diabetes through RAF1/P38MAPK/COX-2
signaling pathway. Am J Transl Res. 10:618–628. 2018.PubMed/NCBI
|
|
36
|
Weng Z, Patel AB, Vasiadi M, Therianou A
and Theoharides TC: Luteolin inhibits human keratinocyte activation
and decreases NF-κB induction that is increased in psoriatic skin.
PLoS One. 9(e90739)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009.PubMed/NCBI View Article : Google Scholar
|