Introduction
Ovarian cancer is one of the most common malignant
tumor types of the female reproductive system; it has a high
incidence rate and poses a serious threat to the lives of females
worldwide (1). Ovarian epithelial
cancer more commonly occurs in post-menopausal females, while
malignant germ cell tumors are more frequently encountered in
adolescent or young females (2).
The etiology of ovarian malignancies remains elusive and the major
reasons are genetic and endocrine factors (3). Ovarian epithelial cancer has no
obvious symptoms in the early stage, while 60-70% of patients with
ovarian malignant germ cell tumors are diagnosed and treated in the
early stage (4). To date, surgery
combined with chemotherapy is the major treatment for ovarian
tumors (5). In addition, targeted
therapy, endocrine therapy and radiation therapy have certain
curative effects (6). However,
after treatment, ovarian cancer recurs in numerous patients, and a
considerable proportion inevitably die after multiple
chemotherapies (7). Therefore, it
is important to explore novel therapeutic targets for ovarian
cancer treatment.
Long non-coding RNAs (lncRNAs) are a class of RNAs
of ~200 nt in length, which do not encode proteins (8). They have been indicated to be involved
in the regulation of development, differentiation and metabolism,
as well as numerous aspects of various diseases (9). To date, a large number of lncRNAs have
been confirmed to be involved in the development of ovarian cancer
(10,11). For instance, lncRNA human ovarian
cancer-specific transcript 2 was reported to regulate the
expression of microRNA (miR/miRNA) let-7b and affect the biological
behavior of epithelial ovarian cancer cells (10). Furthermore, carboplatin-docetaxel
caused upregulation of lncRNA PVT1, which was associated with its
anti-ovarian cancer effect (12).
Of note, lncRNA FOXD2-AS1 has been intensively researched in recent
years and its effect on the development of various cancer types,
including small cell lung cancer (13), colorectal cancer (14) and esophageal squamous cell carcinoma
(15), has been confirmed. However,
the mechanistic roles of lncRNA FOXD2-AS1 in ovarian cancer have
remained elusive. miR-4492 is also an important factor in cancer.
As reported in the study by Boo et al (16), miR-4492 was closely associated with
chemoresistance of breast cancer. Furthermore, it also participates
in the progression of the anti-viral immune response (17). Therefore, studying the association
of lncRNA FOXD2-AS1 and miR-4492 in ovarian cancer may have
clinical implications for the diagnosis and treatment of this
disease.
In the present study, the expression of lncRNA
FOXD2-AS1 and miR-4492 in ovarian cancer tissue and cells was
detected and the regulatory interaction was also assessed. More
importantly, the effect of lncRNA FOXD2-AS1 and miR-4492 in cell
proliferation and invasion of ovarian cancer was confirmed. The
present study may provide a novel biomarker for the prediction of
the disease.
Materials and methods
Collection of ovarian cancer
tissues
A total of 39 ovarian cancer patients (age range,
47±8.4 years) undergoing surgery at Laizhou People's Hospital
between January 2018 and December 2018 were included. Patients who
received radiotherapy or chemotherapy prior to surgery were
excluded from the current study. The ovarian cancer tissues and
paracancer tissues (at least 3 cm away from the tumor border and
with no microscopic evidence of tumor cells) were obtained and
immediately placed in liquid nitrogen for cryopreservation until
analysis. All patients provided written informed consent and the
study was approved by the ethics committee of Laizhou People's
Hospital.
Cell lines and culture
The ovarian cancer cell lines SKOV3, A2780 and
OVCAR3, and the human ovarian normal epithelial cell line IOSE80
were purchased from the Shanghai Cell Bank of the Chinese Academy
of Sciences. All cells were cultured in RPMI 1640 media with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin, and then
cultured at 37˚C in a humidified incubator with 5%
CO2.
Cell transfection
Ovarian cancer cells (SKOV3 and OVCAR3) in the
logarithmic growth phase were inoculated into 6-well cell culture
plates. When the confluence of cells reached 30-40%, the
transfection was performed according to the instructions of the
Lipofectamine 2000 kit (Invitrogen; Thermo Fisher Scientific,
Inc.), and small interfering (si)RNA targeting FOXD2-AS1
(si-FOXD2-AS1; 5'-GCGAAGAGUACGUUGCUAUTT-3'), miR-4492 mimics
(5'-GGGGCUGGGCGCGCGCC-3'), miR-4492 inhibitor
(5'-GGCGCGCGCCAGCCCC-3') and their corresponding controls
(5'-UCACAACCUCCUAGAAAGAGUAGA-3') were transfected separately. After
24 h of transfection, the medium was replaced with fresh medium.
The transfected siRNAs and miRNAs were synthesized by GenePharma
Co. Ltd.
Reverse transcription-quantitative
(RT-q)PCR assay
Total RNA was extracted from tissues or cells by
using the TRIzol® method (Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was obtained by RT using the
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.). qPCR
was performed with the SYBR Green I Supermix (Takara Biotechnology
Co., Ltd.) and quantification was performed using the
2-ΔΔCq method (18). In
brief, 3 µg total RNA were reverse-transcribed to cDNA with Moloney
murine leukemia virus reverse transcriptase (Invitrogen; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: Denaturation at 95˚C for 10 min; followed by 40 cycles of
denaturation at 95˚C for 15 sec and elongation at 60˚C for 1 min.
GAPDH and U6 were used as references. The primers were as follows:
FOXD2-AS1 forward, 5'-CACTGAGGGACAGCCAAGA-3' and reverse,
5'-GGCGGCGTGTAATTGGTA-3'; GAPDH forward,
5'-GGAGCGAGATCCCTCCAAAAT-3'; miR-4492 forward,
5'-AACGAGACGACGACAGAC-3' and reverse, 5'-GGGGCUGGGCGCGCGCC-3'; U6
forward 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Cell-Counting-Kit-8 (CCK-8) assay for
detecting cell proliferation
si-FOXD2-AS1, miR-4492 and their corresponding
controls were transfected into SKOV3 and OVCAR3 cells using
Lipofectamine™ 2000, and cells in the logarithmic growth phase
expressing si-FOXD2-AS1 were individually seeded into 96-well
plates (2.0x103 cells/well). At 24, 48 and 72 h, 20 µl
CCK8 solution was added to each well, followed by incubation for 4
h at 37˚C. The optical density of each well at 450 nm was measured
with a microplate reader (Berthold Technologies GmbH & Co KG).
The optical density values of the four wells were measured, the
average value was taken, and the cell growth curve was plotted on
the abscissa of the cell culture time. Three parallel wells were
set for each sample and the experiment was repeated three times
independently.
Migration and invasion assays
Single-cell suspensions of transfected SKOV3 and
OVCAR3 cells were prepared and cells were counted. Subsequently,
5x105 cells in 100 µl RPMI 1640 medium were added to the
upper chambers of a Transwell insert. Furthermore, 900 µl RPMI-1640
medium containing 20% fetal bovine serum was added to the lower
chamber. For each experimental condition, 3 replicate wells were
used. Following culture for 16 h in a cell incubator, the cells in
the upper chamber were wiped off with a cotton swab, and the
remaining cells were fixed with 95% ethanol for 30 min and stained
with 0.5% crystal violet for 20 min at room temperature. The
chamber was rinsed twice with PBS and dried at room temperature.
Images of the migrated cells were captured under the microscope
cells were counted. For the invasion experiment, the above protocol
was followed with the modification of the 8-µm Millicell chamber
being coated with Matrigel®.
Luciferase reporter assay
The potential binding site between FOXD2-AS1 and
miR-4492 was predicted using the miRDB tool (http://mirdb.org/miRDB/index.html). Sequences
containing the wild-type (WT) or site-mutated (Mut) region of
FOXD2-AS1 were synthesized by Sangon Biotech Co., Ltd., and
inserted into the pGL3 vector (Promega Corporation). SKOV3 and
OVCAR3 cells were seeded in 24-well plates (2x104 cells
per well) and transfected with the WT or Mut reporter gene vector
and miR-4492 mimics or negative controls for 24 h, followed by
assessment of the luciferase activity using the dual luciferase
assay system (Promega Corp.) according to the manufacturer's
protocol.
Statistical analysis
Data analysis was performed using SPSS 22.0 software
(IBM Corp.). All values are expressed as the mean ± standard
deviation. A Student's t-test was used to analyze the differences
between two groups. One-way analysis of variance followed by a
Tukey's post-hoc test was used for multiple comparisons. A
Pearson's correlation coefficient analysis was used to determine
the correlation between expression levels. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of lncRNA FOXD2-AS1 in
ovarian cancer tissues and cell lines
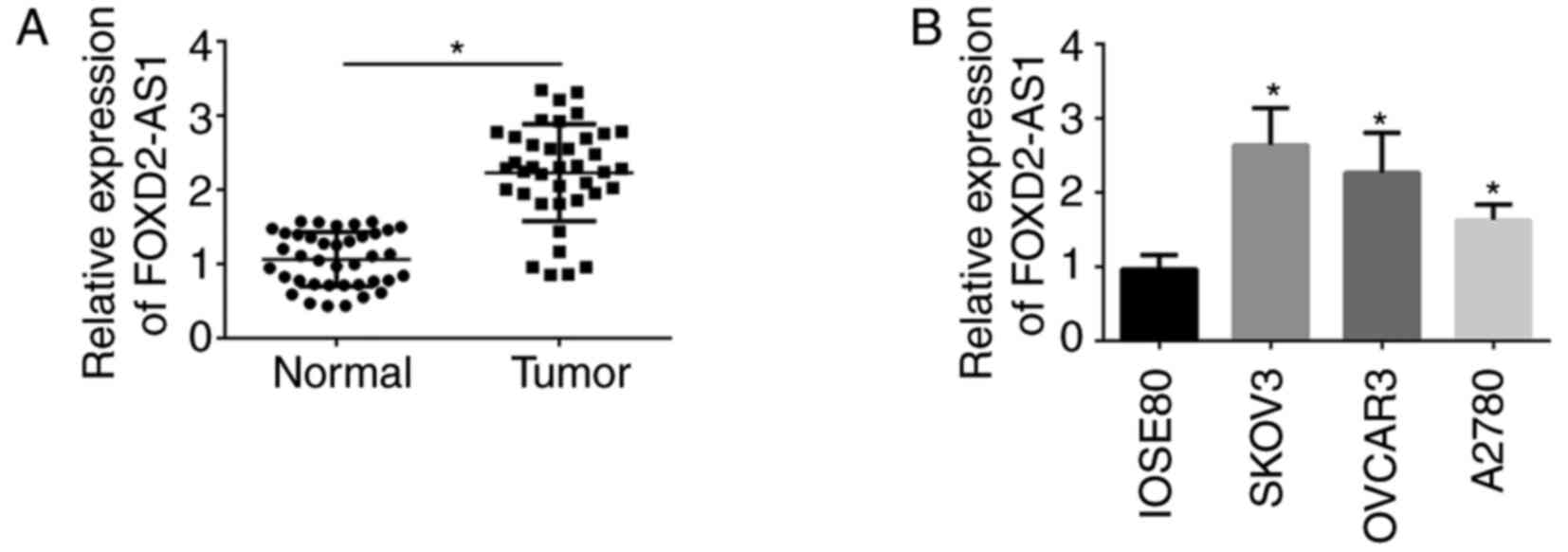
The expression of lncRNA FOXD2-AS1 was detected in a
total of 39 paired specimens from ovarian cancer patients. As
presented in Fig. 1A, the
expression of lncRNA FOXD2-AS1 was significantly higher in ovarian
cancer tissues compared with that in paracancer tissues. Similar
results were also obtained for the cell lines. Compared with that
in the IOSE80 cell line, the expression of lncRNA FOXD2-AS1 was
significantly higher in the SKOV3, A2780 and OVCAR3 cell lines
(Fig. 1B). In conclusion, lncRNA
FOXD2-AS1 was upregulated in ovarian cancer tissues and cell
lines.
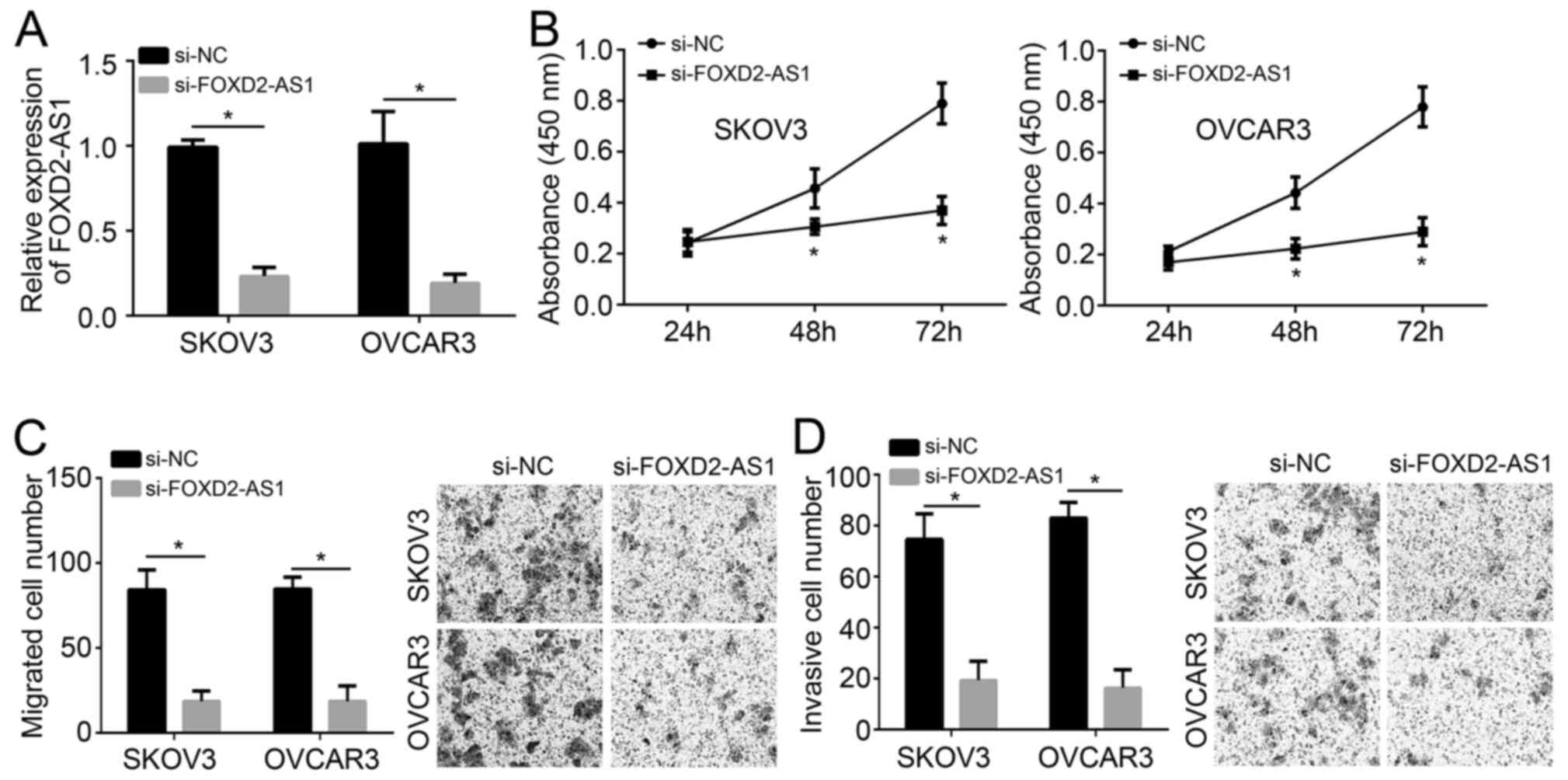
si-FOXD2-AS1 inhibits the
proliferation, migration and invasion of ovarian cancer cells
After transfection with si-FOXD2-AS1, the expression
of FOXD2-AS1 was significantly downregulated in SKOV3 and OVCAR3
cell lines (Fig. 2A), which
indicated that the knockdown was successful. As indicated by the
CCK-8 assay, si-FOXD2-AS1 significantly inhibited the proliferation
of ovarian cancer cells after 48 h (P<0.05; Fig. 2B). In addition, the migration and
invasion of si-FOXD2-AS1-transfected SKOV3 and OVCAR3 cells was
significantly suppressed (Fig. 2C
and D).
LncRNA FOXD2-AS1 targets miR-4492
Previous evidence has demonstrated that lncRNAs act
as sponges for miRNAs via interacting with the response elements of
their target miRNAs (19,20). Thus, FOXD2-AS1 may be a sponge for
specific miRNAs. A mutual binding site of lncRNA FOXD2-AS1 and
miR-4492 was predicted by using the miRDB database. The predicted
complementary sequences are presented in Fig. 3A. To confirm the binding
interaction, a luciferase reporter assay was performed. The
relative luciferase activity of SKOV3 and OVCAR3 cells transfected
with reporter plasmid containing the mutated or wild-type sequence
from lncRNA FOXD2-AS1 and co-transfected with miR-4492 mimics or
miR-NC was determined. After transfection with miR-4492 mimics, the
expression of miR-4492 was significantly increased, indicating that
the overexpression was successful (Fig.
3B). When the SKOV3 and OVCAR3 cells were co-transfected with
the wild-type reporter plasmid and miR-4492, the luciferase
activity was significantly inhibited compared with that in the
group co-transfected with miR-NC (P<0.05), while the luciferase
activity of the mutant control vector was comparable in WT and Mut
groups (P>0.05; Fig. 3C and
D). These results demonstrated that
lncRNA FOXD2-AS1 reduced the amount of available miR-4492 by
binding to the response element of miR-4492, suggesting that lncRNA
FOXD2-AS1 directly regulates miR-4492 levels and activity. In
addition, the level of miR-4492 was significantly upregulated by
si-FOXD2-AS1 (Fig. 3E).
Furthermore, miR-4492 was downregulated in tumor vs. paracancerous
tissues (Fig. 3F). Of note, the
expression of FOXD2-AS1 and miR-4492 was negatively correlated in
tumor tissues (r=-0.699; P<0.05; Fig. 3G).
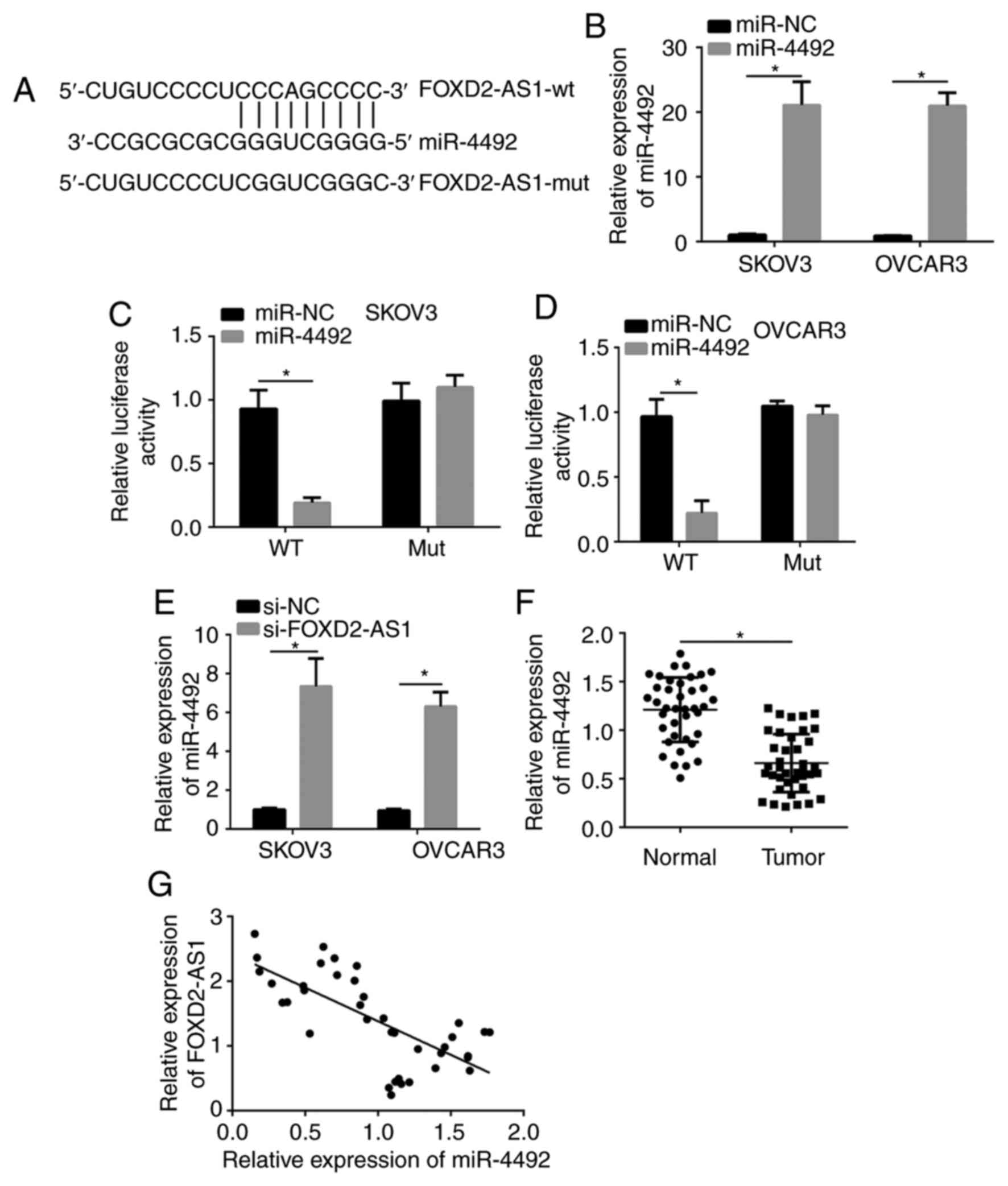 | Figure 3lncRNA FOXD2-AS1 targets miR-4492. (A)
The mutual binding site of lncRNA FOXD2-AS1 and miR-4492 was
predicted with the miRDB database. (B) RT-qPCR was used to confirm
successful transfection of miR-4492 mimics. (C and D) Luciferase
reporter assays were performed to confirm the predicted binding
site. Relative luciferase activity of lncRNA FOXD2-AS1 in (C) SKOV3
and (D) OVCAR3 cells after miR-4492 transfection. (E) The
expression of miR-4492 was significantly upregulated in cells
transfected with si-FOXD2-AS1. (F) Relative expression of miR-4492
in pairs of tumor tissues and adjacent normal controls determined
by RT-qPCR. (G) The expression of FOXD2-AS1 and miR-4492 was
negatively correlated. *P<0.05 as indicated. lncRNA,
long non-coding RNA; FOXD2-AS1, forkhead box D2 antisense 1; miR,
microRNA; wt, wild-type; mut, mutant; NC, negative control;
si-FOXD2-AS1, siRNA targeting FOXD2-AS1; si-NC, negative control
siRNA; siRNA, small interfering RNA; RT-qPCR, reverse
transcription-quantitative PCR. |
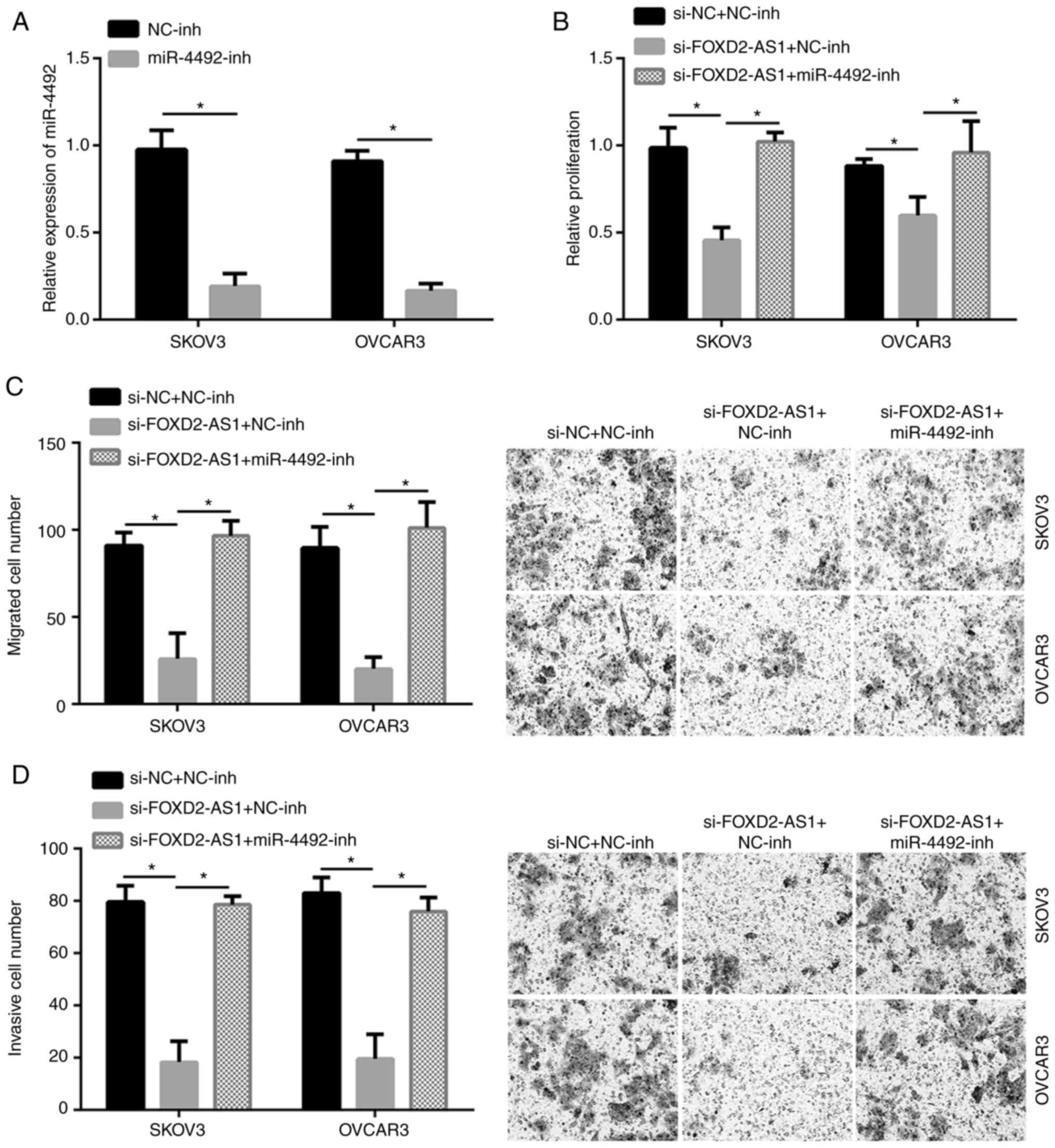
miR-4492 inhibitor rescues the effect
of siFOXD2-AS1
After transfection with miR-4492-inhibitor, the
level of miR-4492 was confirmed to be significantly downregulated
(Fig. 4A). si-FOXD2-AS1
significantly inhibited the proliferation, migration and invasion
of SKOV3 and OVCAR3 cell lines (P<0.05). However, in a rescue
experiment, co-transfection with miR-4492-inhibitor abrogated this
effect (Fig. 4B-D). Therefore,
miR-4492 inhibitor rescued the effect of siFOXD2-AS1 to inhibit the
proliferation, migration and invasion of SKOV3 and OVCAR3 cell
lines.
Discussion
Ovarian cancer has obvious genetic characteristics
and there is a requirement for early diagnostic biomarkers and
therapeutic targets (21). Thereby,
more accurate biomarkers are urgently required to be screened
(22,23). In the present study, the
implications of lncRNA FOXD2-AS1 and miR-4492 in the proliferation,
migration and invasion of ovarian cancer cells were investigated.
The results indicated that lncRNA FOXD2-AS1 was highly expressed in
ovarian cancer tissues and cells as compared with that in
paracancerous tissues and a normal cell line, respectively.
siFOXD2-AS1 inhibited the proliferation, invasion and migration of
ovarian cancer cells. The expression of lncRNA FOXD2-AS1 and
miR-4492 had a negative correlation. Furthermore, miR-4492
inhibitor rescued the suppressive effect of siFOXD2-AS1 on the
malignant behavior of ovarian cancer cells.
Previous studies have confirmed that lncRNA
FOXD2-AS1 has an important role in various cancer types (13-15).
For instance, lncRNA FOXD2-AS1 was confirmed to be highly expressed
in non-small cell lung cancer and to participate in the progression
of this disease via the Wnt/β-catenin signaling pathway (14). Furthermore, it was reported to
participate in the Notch signaling pathway and
epithelial-to-mesenchymal transition (EMT), and to affect the
development of colorectal cancer (15). Of note, the Wnt/β-catenin pathway
and EMT signaling have been indicated to be important in the
development of ovarian cancer (24,25).
In addition, An et al (26)
confirmed that lncRNA FOXD2-AS1 regulated the expression of miR-143
to thereby improve the gemcitabine resistance of bladder cancer. Of
note, miR-143 has been confirmed to inhibit the progression of
ovarian cancer by targeting cellular communication network factor
2(27). Furthermore, lncRNA
FOXD2-AS1 was also indicated to regulate the expression of
miR-3663-5p and S100A1, and aggravate the genesis of nasopharyngeal
carcinoma (28). In ovarian cancer,
S100A1 was reported to be highly expressed and to have an important
role in cell migration and proliferation (29). For these reasons, lncRNA FOXD2-AS1
was likely to be a key lncRNA in ovarian cancer; however, this has
so far remained to be demonstrated. The present study aimed to
explore the biological function of lncRNA FOXD2-AS1 in ovarian
cancer and the results indicated that the proliferation, as well as
the migratory and invasive ability of ovarian cancer cells were
decreased after silencing of FOXD2-AS1. This indicates that
FOXD2-AS1 may participate in the progression of ovarian cancer,
mainly by promoting cell proliferation, migration and invasion.
Of note, lncRNA FOXD2-AS1 was confirmed to have a
mutual binding site with miR-4492 in the present study, and
miR-4492 inhibitor was able to rescue the suppressive effect of
siFOXD2-AS1 in ovarian cancer cells. In 2016, Boo et al
(16) published a comprehensive
bioinformatics analysis and confirmed that miR-4492 was
differentially expressed in breast cancer cells. Furthermore, the
expression of miR-4492 was also associated with the immune response
(17,30). It has become apparent that the
mechanisms of the immune response in ovarian cancer have a
significant prognostic significance in the clinic (31). Overall, it is indicated that lncRNA
FOXD2-AS1 is able to negatively regulate the levels of miR-4492 and
further affect the course of ovarian cancer.
While the implication of lncRNA FOXD2-AS1 and
miR-4492 in cell proliferation, migration and invasion of ovarian
cancer cells was comprehensively assessed in the present study,
certain limitations are worth mentioning. First, the regulation at
the molecular level is usually complex, and further lncRNAs, genes
and miRNAs require to be investigated. In addition, most of the
data were obtained from in vitro experiments, and more in
vivo experiments, including animal models, require to be
performed. Furthermore, the number of clinical samples was small
and the results require to be confirmed in more patients. The above
issues will be the focus of future research performed by our group.
There are also several limitations regarding the molecular
biological analysis. For instance, the expression of EMT markers
was not measured by western blot analysis, which requires
investigation in the future. Furthermore, the downstream targets of
miR-4492 were not analyzed, which will also be determined in future
work performed by our group.
In conclusion, the present study indicated that
lncRNA FOXD2-AS1 promotes the proliferation, migration and invasion
of ovarian cancer cells at least partially by regulating the
expression of miR-4492. It may potentially serve as a novel
diagnostic biomarker and therapeutic target for ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG and PZ contributed to the conception and design
of the present study. In addition, PZ analyzed and interpreted the
results and wrote the manuscript. FL and XZ performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Laizhou People's Hospital. Written informed consent
was obtained from all patients enrolled.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Golan A, Joosting AC and Orchard ME: Mumps
virus and ovarian cancer. S Afr Med J. 56:18–20. 1979.PubMed/NCBI
|
|
2
|
Skates SJ, Menon U, Macdonald N, Rosenthal
AN, Oram DH, Knapp RC and Jacobs IJ: Calculation of the risk of
ovarian cancer from serial CA-125 values for preclinical detection
in postmenopausal Women. J Clin Oncol. 21 (10 Suppl):206s–210s.
2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Makar AP and Tropé CG: Endometrial and
ovarian malignancies: Epidemiology, etiology and prognostic
factors. Acta Obstet Gynecol Scand. 71:331–336. 1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Du K, Gong HY and Gong ZM: Influence of
serum VEGF levels on therapeutic outcome and diagnosis/prognostic
value in patients with cervical cancer. Asian Pac J Cancer Prev.
15:8793–8796. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Suzuki M, Ohwara M, Sekiguchi I and Sato
I: Radical cytoreductive surgery combined with
platinums-carboplatin and cisplatin chemotherapy for advanced
ovarian cancer. Int J Gynecol Cancer. 9:54–60. 2010.
|
|
6
|
Slotman BJ and Rao BR: Ovarian cancer
(review) Etiology, diagnosis, prognosis, surgery, radiotherapy,
chemotherapy and endocrine therapy. Anticancer Res. 8:417–434.
1988.PubMed/NCBI
|
|
7
|
Tu H, Huang H, Huang QD, Li Z, Feng YL and
Liu JH: Treatment and prognostic analysis of ovarian cancer
patients with isolated region of lymph node recurrence. Zhonghua Fu
Chan Ke Za Zhi. 47:928–933. 2012.PubMed/NCBI(In Chinese).
|
|
8
|
Vance KW and Ponting CP: Transcriptional
regulatory functions of nuclear long noncoding RNAs. Trends Genet.
30:348–355. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Y, Zhang J, Huo C, Ding N, Li J, Xiao
J, Lin X, Cai B, Zhang Y and Xu J: Dynamic organization of lncRNA
and Circular RNA regulators collectively controlled cardiac
differentiation in humans. Ebiomedicine. 24:137–146.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao
T, Liu Y, Ou J, Wang D, Yao L, et al: LncRNA-HOST2 regulates cell
biological behaviors in epithelial ovarian cancer through a
mechanism involving microRNA let-7b. Hum Mol Genet. 24:841–852.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chai Y, Liu J, Zhang Z and Liu L:
HuR-regulated lncRNA NEAT1 stability in tumorigenesis and
progression of ovarian cancer. Cancer Med. 5:1588–1598.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Liu E, Liu Z and Zhou Y:
Carboplatin-docetaxel-induced activity against ovarian cancer is
dependent on up-regulated lncRNA PVT1. Int J Clin Exp Pathol.
8:3803–3810. 2015.PubMed/NCBI
|
|
13
|
Rong L, Zhao R and Lu J: Highly expressed
long non-coding RNA FOXD2-AS1 promotes non-small cell lung cancer
progression via Wnt/β-catenin signaling. Biochem Biophys Res
Commun. 484:586–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang X, Duan B and Zhou X: Long non-coding
RNA FOXD2-AS1 functions as a tumor promoter in colorectal cancer by
regulating EMT and Notch signaling pathway. Eur Rev Med Pharmacol
Sci. 21:3586–3591. 2017.PubMed/NCBI
|
|
15
|
Bao J, Zhou C, Zhang J, Mo J, Ye Q, He J
and Diao J: Upregulation of the long noncoding RNA FOXD2-AS1
predicts poor prognosis in esophageal squamous cell carcinoma.
Cancer Biomark. 21:527–533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boo L, Ho WY, Ali NM, Yeap SK, Ky H, Chan
KG, Yin WF, Satharasinghe DA, Liew WC, Tan SW, et al: MiRNA
transcriptome profiling of spheroid-enriched cells with cancer stem
cell properties in human breast MCF-7 cell line. Int J Biol Sci.
12:427–445. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Egaña-Gorroño L, Guardo AC, Bargalló ME,
Planet E, Vilaplana E, Escribà T, Pérez I, Gatell JM, García F,
Arnedo M, et al: MicroRNA profile in CD8+ T-lymphocytes from
HIV-infected individuals: Relationship with antiviral immune
response and disease progression. PLoS One.
11(e0155245)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang A, Jin C, Li H, Qin Q and Li L:
LncRNA ADAMTS9-AS2 regulates ovarian cancer progression by
targeting miR-182-5p/FOXF2 signaling pathway. Int J Biol Macromol.
120:1705–1713. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan H, Li H, Li P, Li X, Lin J, Zhu L,
Silva MA, Wang X, Wang P and Zhang Z: Long noncoding RNA MLK7-AS1
promotes ovarian cancer cells progression by modulating
miR-375/YAP1 axis. J Exp Clin Cancer Res. 37(237)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Soegaard M, Kjaer SK, Cox M, Wozniak E,
Høgdall E, Høgdall C, Blaakaer J, Jacobs IJ, Gayther SA and Ramus
SJ: BRCA1 and BRCA2 mutation prevalence and clinical
characteristics of a population-based series of ovarian cancer
cases from Denmark. Clin Cancer Res. 14:3761–3767. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kozak KR, Amneus MW, Pusey SM, Su F, Luong
MN, Luong SA, Reddy ST and Farias-Eisner R: Identification of
biomarkers for ovarian cancer using strong anion-exchange
ProteinChips: Potential use in diagnosis and prognosis. Proc Natl
Acad Sci USA. 100:12343–12348. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang B, Liu M, Zhuang R, Jiang J, Gao J,
Wang H, Chen H, Zhang Z, Kuang Y and Li P: Long non-coding RNA
CCAT2 promotes epithelial-mesenchymal transition involving
Wnt/β-catenin pathway in epithelial ovarian carcinoma cells. Oncol
Lett. 15:3369–3375. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Usongo M, Li X and Farookhi R: Activation
of the canonical WNT signaling pathway promotes ovarian surface
epithelial proliferation without inducing β-catenin/Tcf-mediated
reporter expression. Dev Dyn. 242:291–300. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
An Q, Zhou L and Xu N: Long noncoding RNA
FOXD2-AS1 accelerates the gemcitabine-resistance of bladder cancer
by sponging miR-143. Biomed Pharmacother. 103:415–420.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, He J, Xu H, Xu L and Li N: MiR-143
targets CTGF and exerts tumor-suppressing functions in epithelial
ovarian cancer. Am J Transl Res. 8:2716–2726. 2016.PubMed/NCBI
|
|
28
|
Chen G, Sun W, Hua X, Zeng W and Yang L:
Long non-coding RNA FOXD2-AS1 aggravates nasopharyngeal carcinoma
carcinogenesis by modulating miR-363-5p/S100A1 pathway. Gene.
645:76–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tian T, Li X, Hua Z, Ma J, Liu Z, Chen H
and Cui Z: S100A1 promotes cell proliferation and migration and is
associated with lymph node metastasis in ovarian cancer. Discov
Med. 23:235–245. 2017.PubMed/NCBI
|
|
30
|
Xun M, Ma CF, Du QL, Ji YH and Xu JR:
Differential expression of miRNAs in enterovirus 71-infected cells.
Virol J. 12(56)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gavalas NG, Karadimou A, Dimopoulos MA and
Bamias A: Immune response in ovarian cancer: How is the immune
system involved in prognosis and therapy: Potential for treatment
utilization. Clin Dev Immunol. 2010(791603)2011.PubMed/NCBI View Article : Google Scholar
|