Introduction
Acute myocardial infarction (AMI) remains one of the
leading causes of mortality and disability worldwide (1,2).
Currently, the most effective way to rescue dying cardiomyocytes
induced by AMI is timely revascularization (3). However, re-establishing blood flow to
the ischemic area yields additional myocardial damage, which is
termed as myocardial ischemia/reperfusion (I/R) injury (MIRI)
(3). Although effective strategies
such as percutaneous coronary intervention, intravenous
thrombolysis and coronary artery bypass grafting timely limits
infarct expansion and decrease in-hospital mortality, the
subsequent occurrence of chronic heart failure and cardiac
remodeling has gradually increased (1-3).
Thus, MIRI affects the clinical efficacy of revascularization
strategies, and is an important factor that causes the
deterioration of myocardial function and electrophysiological
status (1-4).
Novel approaches for attenuating MIRI need to be explored in order
for the maximal benefits of myocardial revascularization under AMI
to be achieved.
Conventionally, the endoplasmic reticulum (ER) is
involved in the biosynthesis, enfoldment, processing, excretion and
transportation of proteins (3,5,6).
However, the disruption of ER homeostasis caused by MIRI, which is
referred to as ER stress (ERS), leads to inaccurate synthesis and
assembly of proteins (3,5,6).
Increasing evidence suggested that continuous activation of ERS and
the consequent elevation of apoptotic cascade events act as crucial
pathogenic mechanisms in MIRI (3,5,6).
Despite the confirmed benefits of ERS-induced apoptosis against
MIRI, the molecular mechanisms of MIRI and its related therapeutic
approaches remain to be elucidated (3,5,6).
Targeting ERS-related apoptosis may be a possible therapeutic
approach for MIRI.
Piperine (PIP) is a phenolic substance found in
black and long pepper that exerts multiple pharmacological effects,
such as liver protection and tumor inhibition (7,8). In
recent years, the protective effect of PIP in I/R injury has
received much attention (9,10). Vaibhav et al (10) found that piperine treatment
inhibited I/R-induced neuronal dysfunction and that its protective
function is achieved by reducing inflammation. Zou et al
(9) also verified the
neuroprotective effects of PIP in response to cerebral I/R injury,
the underlying mechanism of which is strictly related to the
regulation of complement and coagulation cascades. However, the
exact effects of PIP in MIRI are not clear and the possible
pathological mechanisms associated with ERS-related apoptosis
remains to be elucidated.
Accumulating data have proven that activation of the
PI3K/AKT signaling pathway acts as a crucial molecular mechanism by
which PIP exerts pleiotropic contributions under various
pathological conditions (11-13).
The activity of the PI3K/AKT pathway has multiple consequences in
response to MIRI; in particular, it has anti-apoptotic effects
against I/R injury related to ERS (3,5,6).
Further studies to investigate whether the anti-apoptotic effects
produced by PIP in a PI3K/AKT-related manner play a role in
cardioprotection during MIRI are required. The present study showed
that PIP administration repressesed myocardial apoptosis and ERS in
injury caused by 4 h of hypoxia followed by 6 h of reoxygenation
(H/R), and that this may be related to activation of the PI3K/AKT
pathway. Thus, the present study provided novel insights into the
benefits and potential mechanisms of PIP against MIRI, and may
offer a novel therapeutic target for MIRI treatment.
Materials and methods
Chemicals and reagents
PIP was purchased from Sigma-Aldrich; Merch KGaA.
The purity of PIP, which was >98%, was tested using
high-performance liquid chromatography as previously described
(12). DMEM, PBS, trypsin, FBS and
collagenase type II were purchased from Gibco; Thermo Fisher
Scientific, Inc. The CCK-8 assay and PI3K/AKT inhibitor LY294002
were purchased from Dojindo Molecular Technologies, Inc. and
Selleck Chemicals, respectively. Lactate dehydrogenase (LDH) and
creatine-kinase (CK) were detected using commercially available
ELISA kits (Nanjing Jiancheng Bioengineering Institute; cat.
A020-2-2 for LDH; cat. A032-1-1 for CK). The following primary
antibodies were purchased from Abcam: Phosphorylated (p)-PI3K
(1:600; cat. no. ab32509), PI3K (1:400; cat. no. ab191606), p-AKT
(1:1,000; cat. no. ab182651), endoplasmic reticulum chaperone BiP
(GRP78; 1:500; cat. no. ab21685), cleaved caspase-12 (1:600; cat.
no. ab62484) and GAPDH (1:1,000; cat. no. ab181602). Antibodies
against AKT (1:600; cat. no. 9272) and C/EBP-homologous protein
(CHOP; 1:600; cat. no. 2895) were purchased from Cell Signaling
Technology, Inc. Horseradish peroxidase-conjugated rabbit anti-rat
immunoglobulin G secondary antibody was purchased from BIOSS. The
BCA Protein Assay kit was purchased from Pierce; Thermo Fisher
Scientific, Inc.
Neonatal rat cardiomyocyte (NRCM)
culture
NRCMs were isolated from the ventricles of
1-3-day-old Sprague-Dawley rats as previously described (14). Animals were bred in a standard
environment with controlled temperature (20-25˚C), humidity
(40-60%) and light condition (12 h light/dark cycle). The
experiments and all animal care outlined in the present study were
performed in adherence with the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(NIH Publication, 8th Edition, 2011) (1,2), and
were approved by the Institutional Animal Care and Use Committee
from the Central Hospital of Wuhan, Tongji Medical College,
Huazhong University of Science and Technology. For cardiomyocyte
isolation, all rats were sacrificed by decapitation. Subsequently,
the hearts were quickly removed and large blood vessels were
carefully excised. The obtained ventricles were rinsed in ice-cold
PBS three times to remove residuary blood and placed in a dish with
ice-cold PBS. Heart tissues were digested with 0.08% collagenase
type II and 0.125% trypsin at room temperature for 5 min. NRCMs
were then centrifuged at 3,000 x g for 10 min at 37˚C and
resuspended in DMEM containing 10% FBS and 1%
penicillin/streptomycin (HyClone; GE Healthcare Life Sciences) in a
humidified incubator at 37˚C with 5% CO2 and 95%
O2.
Construction of a H/R injury model and
experimental groups
H/R injury was established as previously described
with minor modifications (15).
Cultured NRCMs were washed twice with PBS and preserved in
serum-free DMEM. Subsequently, the cells were incubated at 37˚C in
an anaerobic chamber for 4 h. The cells were then moved into a
normal incubator with 95% O2 and 5% CO2 at
37˚C for an additional 6 h for reoxygenation.
To determine the possible function of PIP in MIRI,
different dosage gradients of PIP (1, 5, 10 and 20 µM) were
administered to first measure the most effective dose against MIRI.
A total of 16 neonatal rats were used in each group (n=4 per
group). Another experiment was then designed to explore the precise
roles and molecular mechanisms of PIP in response to H/R. Primary
cardiomyocytes were randomly divided into four groups: The control
+ vehicle group, which consisted of NRCMs cultured in normoxic
conditions without PIP treatment; the control + PIP group, which
consisted of NRCMs cultured in normoxic conditions with PIP
treatment; the H/R + vehicle group, which consisted of NRCMs
cultured in H/R conditions without PIP treatment and the H/R + PIP
group, which consisted of NRCMs cultured in H/R conditions with PIP
treatment. During this experiment, 20 neonatal rats were used for
each group (n=5 per group). Additionally, to detect the underlying
mechanism of the association with the PI3K/AKT pathway, cells were
pre-treated with 10 µM LY294002 for 30 min prior to PIP
administration, and were then subjected to H/R intervention. During
this experiment, 24 neonatal rats were used for each group (n=6 per
group).
Cell viability assay
The CCK-8 assay was used to measure cell viability
in each group as previously described (16). The optical density values were
detected at a wavelength of 450 nm using a microplate reader
(Bio-Rad Laboratories, Hercules, CA). Cell viability was evaluated
as a percentage relative to the control group.
Measurement of markers of myocardial
injury
After the indicated procedures, the medium in each
group was collected using a sterile pipette. Commercially available
biochemical kits were used in line with the manufacturer's
instructions to detect LDH and CK.
Apoptosis detection
Apoptosis of NRCMs was measured by a flow cytometry
assay as previously described (16,17).
Cells were maintained in 100 µl of binding buffer, which included 5
µl Annexin V-APC and 5 µl 7-aminoactinomycin D. Stained cells were
observed using a flow cytometer (Beckman Coulter), followed by the
fluorescence-activated cell sorting on a flow cytometeric assay
(CytoFLEX; Beckman Coulter, Inc., Brea, CA, USA). NRCMs positive
for 7-AAD or Annexin V-APC were recognized as apoptotic cells.
Western blotting
Western blotting was used to detect protein
expression as previously described (3,16).
Myocardial samples were homogenized and lysed in RIPA buffer
(Beyotime Institute of Biotechnology) at a mass/volume ratio of 100
mg/ml Protein was then extracted and protein concentration was
detected using a BCA kit (Beyotime Institute of Biotechnology).
Then, 10% SDS-PAGE was performed to separate the proteins, and 50
µg of extracted proteins in each group were electrophoretically
transferred onto PVDF membranes (EMD Millipore). Next, the
membranes were blocked by 5% skimmed milk in TBST for 2 h at
37°C. Subsequently the membranes were incubated with
antibodies against CHOP, GRP78, caspase-12, PI3K, p-PI3K, AKT and
p-AKT overnight at 4°C. GAPDH was used as the internal
reference protein. The horseradish peroxidase-conjugated secondary
antibodies were added for another 2 h at 37°C. The
protein bands were visualized using an ECL kit (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to detect mRNA levels as
previously described (3,16). Total RNA was extracted from NRCMs
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturers protocol, after
the indicated treatments. qPCR was performed using a SYBR-Green
Master Mix kit (cat. no. 4472918; Thermo Fisher Scientific, Inc.)
on a 7500 ABI Prism system (Applied Biosystems). The following
thermocycling conditions were used for the qPCR: Initial denaturing
at 50˚C for 2 min, 40 cycles of denaturation at 95˚C for 30 sec and
final extension at 60˚C for 30 sec. The mRNA expression levels of
CHOP, GRP78 and caspase-12 were normalized to GAPDH levels. The
fold-change of the mRNA levels relative to the control cells was
calculated using the 2-ΔΔCq method (3-5).
The following primer pairs were used for the qPCR: CHOP forward,
5'-TAGCTTGGCTGACTGAGGAGC-3' and reverse,
5'-CTTCAGCAAGCTGTGCCACT-3'; GRP78 forward,
5'-GATAATCAGCCCACCGTAACAAT-3' and reverse,
5'-GCAAACTTCTCGGCGTCATT-3' and caspase-12 forward,
5'-CATTGCCAATTCCGACAAAC-3' and reverse,
5'-CCTTCCTTCTCCATCACTGGA-3'; GAPDH forward,
5'-ACAGCAACAGGGTGGTGGAC-3' and reverse,
5'-TTTGAGGGTGCAGCGAACTT-3'.
Statistical analysis
SPSS 17.0 (SPSS, Inc.) was used for statistical
analysis. Data were expressed as the mean ± SD. The Student's
t-test was used for comparisons within groups. The statistical
comparisons among multiple groups were performed using analysis of
variance (ANOVA) followed by a post hoc Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
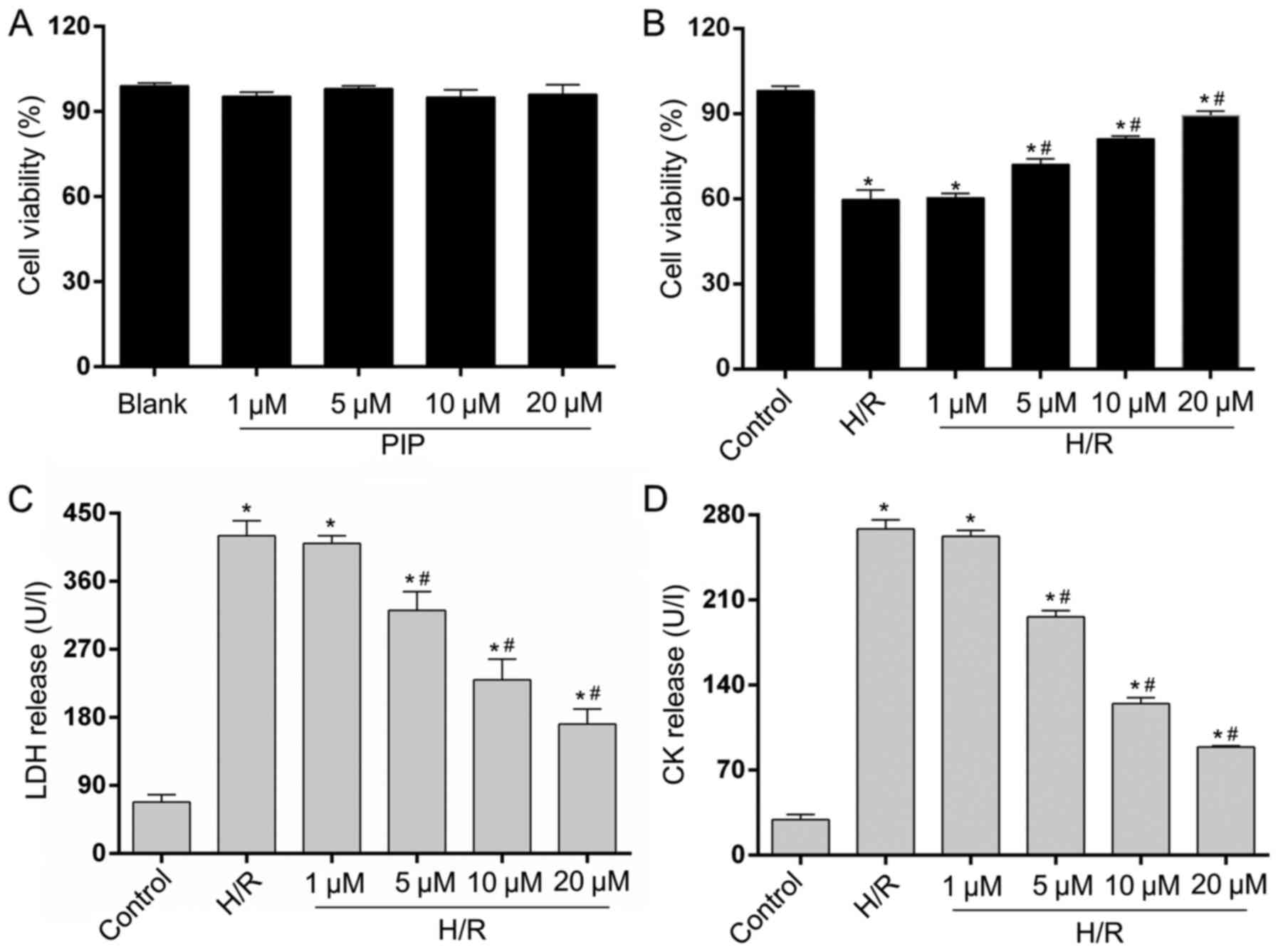
PIP attenuates H/R-induced NRCM
injury
A CCK-8 assay was performed to assess cell viability
(17). The four different PIP
concentrations (1, 5, 10 and 20 µM) had no effect on cell viability
and exhibited very low cytotoxicity in control NRCMs (Fig. 1A). To evaluate the possible effects
of PIP on H/R injury, NRCMs underwent 4 h of hypoxia and 6 h of
reoxygenation. As shown in Fig. 1B,
cell viability was reduced following H/R insult compared with
controls. In addition, the various doses of PIP (5, 10 and 20 µM)
increased cell viability compared with H/R-induced cells. Cell
viability significantly increased following treatment with 5 and 10
µM PIP, and the highest improvement in cell viability compared with
the H/R group was obtained following treatment with 20 µM PIP. LDH
and CK release were detected to assess NRCM death. The release of
LDH (Fig. 1C) and CK (Fig. 1D) caused by H/R injury was limited
starting at a concentration of 5 µM. Minimal levels of LDH and CK
were measured in cells treated with 20 µM PIP compared with other
concentrations. No statistical difference in LDH and CK release or
cell viability was found between the 1 µM PIP-treated and H/R
groups. A total of 20 µM PIP was used for subsequent experiments.
The results suggested that PIP could increase cell survival and
repress cell damage in NRCMs under H/R injury.
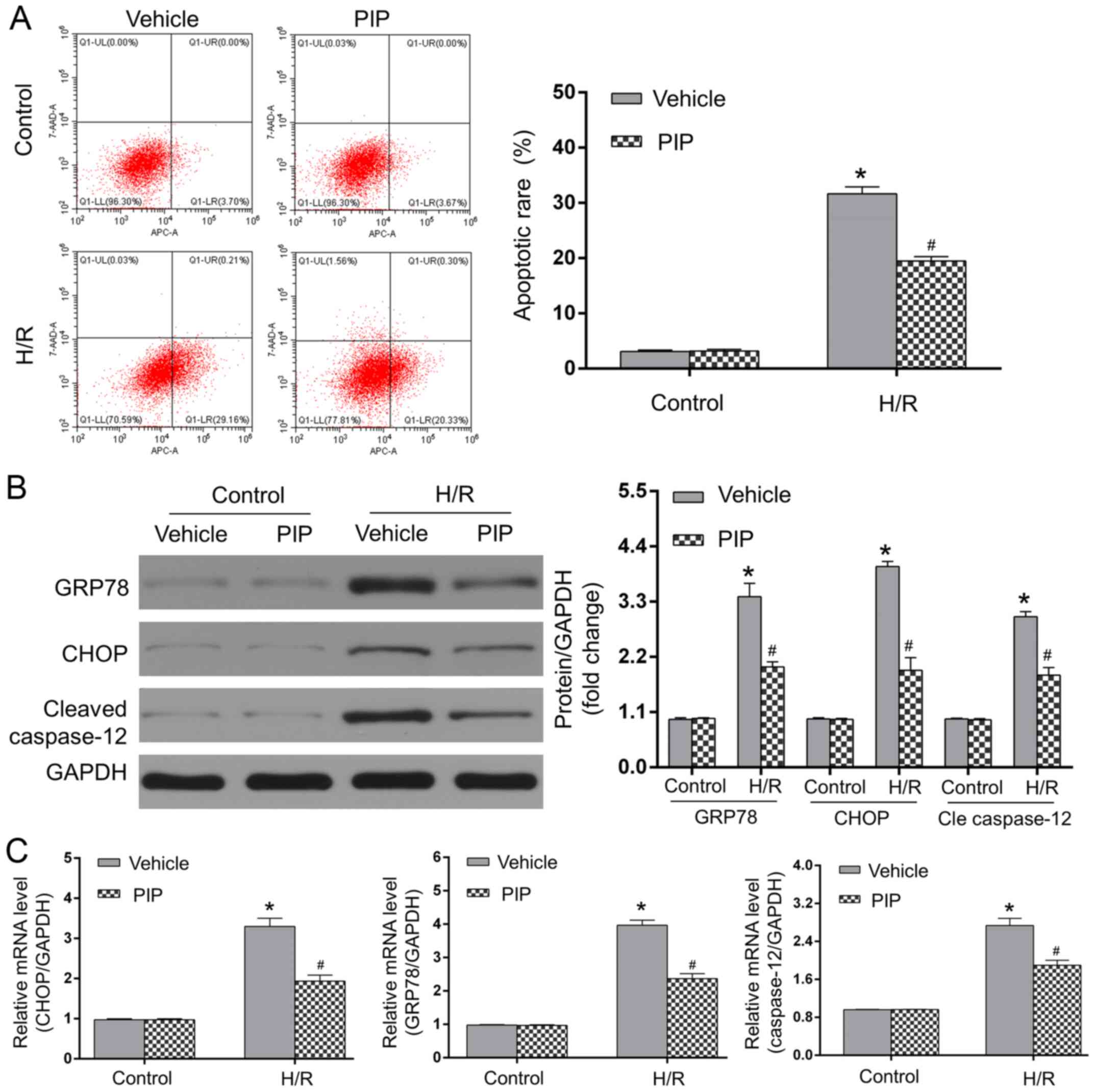
PIP treatment alleviates ERS-induced
apoptosis during H/R injury
Myocardial H/R injury is related to the activation
of ERS-induced apoptosis and PIP was indicated to be involved in
ERS progression (3,18,19).
As an ER chaperone, GRP78 is crucial for myocardial apoptosis
during H/R, particularly since ERS can drive the apoptotic cascade,
including the activation of caspase-12 in a CHOP-dependent manner
(3). To determine whether 20 µM PIP
affects H/R injury in an ERS-related manner, the protein and mRNA
expressions of GRP78, CHOP and caspase-12 in apoptotic NRCMs were
assessed. As shown in Fig. 2A,
minimal apoptotic rates were observed in the control + vehicle and
control + PIP groups, whereas compared to the controls, H/R injury
significantly increased cardiomyocyte apoptosis. PIP treatment
significantly decreased the number of apoptotic cells under H/R
conditions compared with vehicle-treated cells following H/R
(P<0.05). The expression of ERS-related proteins, CHOP, GRP78
and caspase-12 in each group was detected using western blotting
and RT-qPCR. As shown in Fig. 2B
and C, H/R injury elevated protein
and mRNA expressions of GRP78, CHOP and caspase-12 compared with
the control groups (P<0.05). However, PIP administration
significantly decreased the levels of these proteins and mRNAs in
response to H/R injury compared with those in the H/R + vehicle
group (P<0.05). Therefore, the above data indicated that PIP
mitigated H/R-led ERS and subsequent apoptosis in NRCMs.
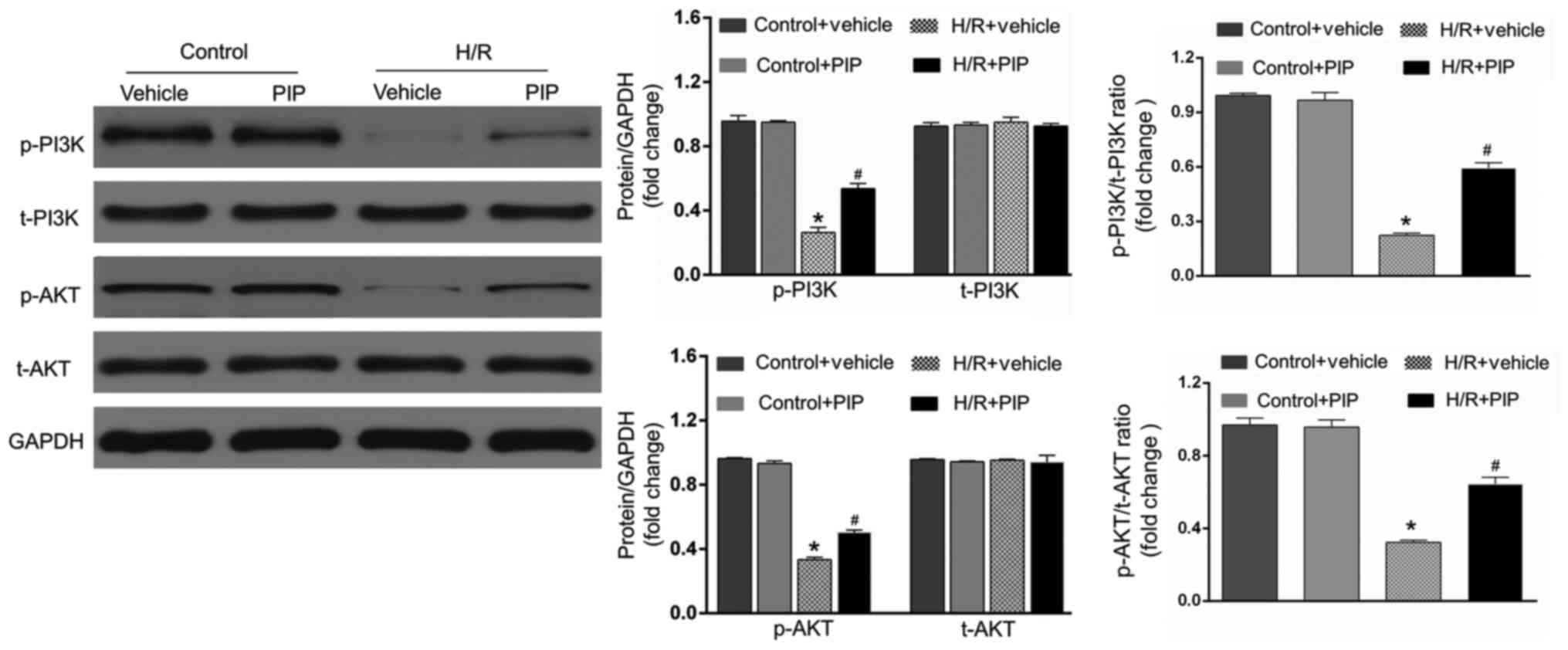
PIP activates the PI3K/AKT signaling
pathway
To further investigate the underlying mechanisms
behind PIP in alleviating I/R injury, the expression of genes
involved in the PI3K/AKT signaling pathway was determined. As shown
in Fig. 3, H/R significantly
downregulated the expression of p-PI3K and p-AKT compared with the
control group (P<0.05). However, pretreatment with PIP at the
onset of H/R increased the expression of p-PI3K and p-AKT compared
with the H/R + vehicle group. No significant difference in the
expression of PI3K and AKT was observed between the different
groups. Therefore, it can be speculated that H/R injury is
ameliorated by PIP, possibly through activating the PI3K/AKT
signaling pathway.
PI3K/AKT repression reversed the
inhibitory effects of PIP on H/R injury
To further validate whether the PI3K/AKT pathway
served as a causative mediator of PIP-induced repression of H/R,
NRCMs were pre-administrated with the specific PI3K/AKT inhibitor
LY294002 and subjected to H/R injury. The results showed that
LY294002 rescued H/R-induced cell death and ERS-related apoptosis,
as demonstrated by the restored LDH and CK activity (Fig. 4B), apoptotic rate (Fig. 4C), restored GRP78, CHOP and
caspase-12 mRNA levels (Fig. 4D)
and suppression of cell viability (Fig.
4A). PIP lost the ability to reverse these alternations in the
presence of LY294002 under H/R insult (Fig. 4A-D). Furthermore, in the presence of
LY294002, there was no significant difference in these alterations
with or without PIP administration. Thus, these findings indicated
that PIP pretreatment alleviates ERS-induced apoptosis in
H/R-exposed NRCMs mainly by activating the PI3K/AKT pathway.
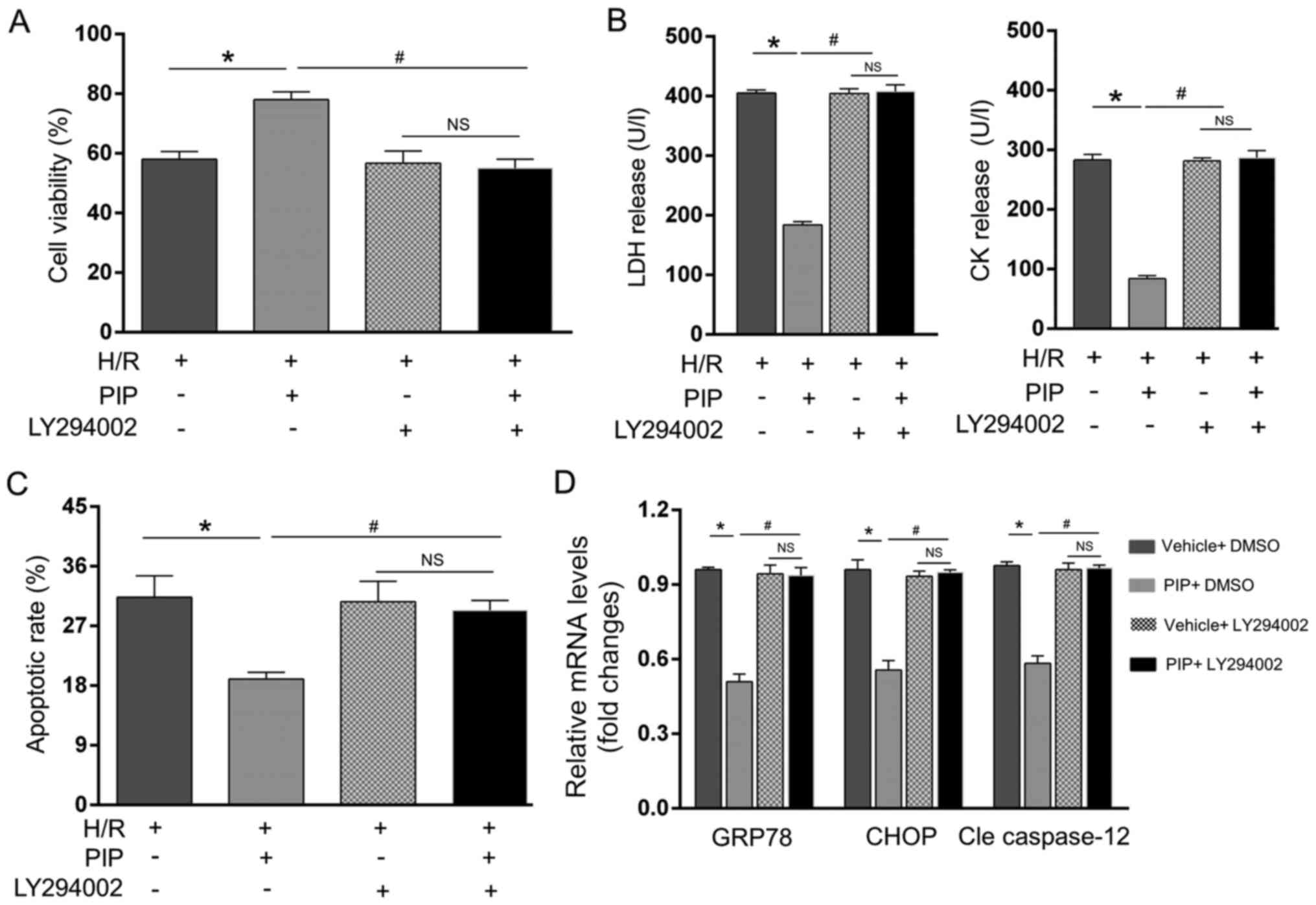 | Figure 4PI3K/AKT inhibition reverses the
inhibitory effects of PIP on H/R. CCK-8 and ELISA assays were used
to detect (A) cell viability and (B) LDH and CK release,
respectively. Apoptosis was measured by (C) flow cytometry and (D)
GRP78, CHOP and caspase-12 mRNA levels were detected by reverse
transcription-quantitative PCR. n=4. *P<0.05 vs. the
H/R group and #P<0.05 vs. the PIP + H/R group. NS,
not significant; PIP, piperine; H/R, hypoxia/reoxygenation; LDH,
lactate dehydrogenase; CK, creatine-kinase; GRP78, endoplasmic
reticulum chaperone BiP; CHOP, C/EBP-homologous protein; Cle
caspase-12, cleaved caspase-12. |
Discussion
Previous studies found that PIP could alleviate
pressure overload-led cardiac remodeling and neuronal I/R injury
(9,12), but its role in MIRI and the possible
mechanisms in association with the PI3K/AKT pathway remain to be
elucidated. The present study observed that PIP treatment reduced
myocardial H/R injury, which was characterized by the upregulation
of cell activity and the decreased release of LDH and CK. In
addition, PIP administration significantly alleviated myocardial
apoptosis and inhibited the protein expression of ERS-related
molecules, such as GRP78, CHOP and caspase-12. Mechanistic studies
observed that PIP markedly promoted the PI3K/AKT signaling pathway,
while LY294002 reversed its protective function against H/R-induced
damage and ERS-related apoptosis. The above results therefore
indicated that PIP ameliorated H/R-induced and ERS-related
apoptosis mainly via the PI3K/AKT-dependent pathway.
Evidence has suggested that PIP has pleiotropic
impacts on a variety of pathological conditions, such as glutamate
release from rat hippocampal nerve terminals, lupus nephritis,
6-hydroxydopamine-induced Parkinson's disease and prostate cancer
development, possibly due to its multiple bioactivities (20-23).
Recent findings revealed that PIP also seems to be beneficial in
the treatment of cardiovascular disease. In a rat model of
isoproterenol (ISO)-induced myocardial infarction, Dhivya et
al (24) demonstrated that PIP
pretreatment acted as a potent therapeutic agent with antioxidant
and anti-dyslipidemic actions against ISO-caused myocardial
ischemiaIn addition, Ma et al (12) indicated that pretreatment with PIP
can attenuate pathological cardiac fibrosis following pressure
overload and ISO injection. Latest research indicated that PIP
exerts a pivotal effect on the course of the pathological process
of I/R (9). For example, in a rat
model of cerebral I/R injury, Zou et al (9) showed that PIP pretreatment protected
neurocytes from I/R damage by the regulation of complement and
coagulation cascades. Vaibhav et al (10) found that PIP administration rescued
ischemic penumbral zone neurons via its anti-inflammatory
properties, thereby suppressing ischemic cell death. However, to
the best of our knowledge, the involvements and potential
mechanisms of PIP in MIRI remain to be elucidated. A recent study
has demonstrated that PIP is an important mediator in the
initiation and development of ERS and subsequent apoptotic
activation (18). Yaffe et
al (25) demonstrated that PIP
treatment inhibited the apoptosis of human colon cancer cells
triggered by ERS. PIP was verified to regulate ERS progression and
thus is a possible therapeutic agent that limited ERS-related renal
apoptosis and cell death (19). In
terms of the possible involvement of PIP in I/R injury and
ERS-related apoptosis, it was hypothesized that PIP could exert
protective roles on cardiomyocytes and play inhibitory roles in
ERS-related apoptosis in response to H/R insult. Consequently, the
present research provided evidence that PIP has an advantageous
role in H/R-injured NRCMs and can also alleviate myocardial I/R
disorder.
Timely and effective reperfusion of ischemic heart
tissue was confirmed to be the most efficacious remedy for AMI
(1-3).
However, the accompanying I/R injury largely offsets this
beneficial effect (1-3).
Effective methods for mitigating the adverse influences of
reperfusion still need to be further determined. Myocardial
apoptosis is considered to be one of the most critical mechanisms
of MIRI pathogenesis, and the ERS-elicited apoptotic cascade has
drawn considerable attention (3,5).
Previous findings showed that MIRI could result in severe ERS as
GRP78 levels are drastically enhanced following MIRI (3). GRP78 activation then causes a
depolymerization reaction between ER-transmembrane transducers and
GRP78, resulting in the activation of caspase-12 and apoptosis
(3). CHOP is a specific
transcription factor involved in ERS-induced apoptosis (3), and prolonged ERS promotes the
expression of CHOP (3). The present
study demonstrated that MIRI could cause significant myocardial
damage and ERS. However, PIP administration reduced GRP78, CHOP and
caspase-12 expression, ameliorating the parameters relative to the
severity of the H/R injury. Therefore, PIP has the ability to
protect cardiomyocytes mainly by mitigating ERS-related
apoptosis.
Based on the above findings, the potential molecular
mechanisms by which PIP exerts its protective effects against H/R
injury and ERS-related apoptosis were further explored. PI3K and
its downstream target serine/threonine kinase AKT belong to a
conserved family of signal transduction enzymes (3,5,6).
Activation of the PI3K/AKT pathway is considered an endogenous
regulatory mechanism that facilitates cell survival in response to
harmful external stimuli (3,5,6).
Numerous studies provided evidence that the PI3K/AKT signaling
pathway plays a critical role in the pathological process of MIRI
(3,5,6), and
compromised ERS-related apoptosis was verified to be an important
mechanism for the protective role of the PI3K/AKT activation
(3,5,6). Zhang
et al (3) showed that the
PI3K/AKT axis participated in anti-I/R effects via ERS repression,
resulting in reduced GRP78, CHOP and caspase-12 expression and
apoptotic inhibition. Deng et al (26) demonstrated that activation of the
PI3K/AKT signaling pathway promoted myocardial survival by
attenuating ERS-induced apoptosis. Moreover, there is a close
relationship between PIP and the activation of the PI3K/AKT pathway
(13). Chen et al (13) revealed that PIP promoted Leydig cell
development but inhibited spermatogenesis in rats by activating
AKT. Consistent with a previous study (13), the present study validated that
pretreatment with PIP not only inhibited apoptosis induced by H/R
injury, but also upregulated the expression of p-PI3K and p-AKT. To
further verify the causal relationship between the activation of
the PI3K/AKT signaling pathway and PIP administration, LY294002 was
added to cells at the onset of H/R injury. The cardioprotective
efficacy and inhibitory effects of PIP on ERS-related apoptosis
were significantly reversed in the presence of LY294002. Therefore,
the above data indicated that PIP administration can decrease
ERS-associated apoptosis and H/R-induced cell damage in a
PI3K/AKT-dependent manner. Of note, the LY294002 supplement at the
onset of control group was missing in this experiment. This is a
limitation of the manuscript that it would be beneficial to include
this group to confirm the H/R-inhibitory roles of PIP. Furthermore,
the specific roles of PIP in alterations in the PI3K/AKT pathway
remain controversial. Ma et al (12) found that PIP attenuated cardiac
fibrosis via the inhibition of AKT after pressure overload,
inconsistent with previous reports elucidating that PIP repressed
migration and proliferation potentially via a reduction in the AKT
pathway in non-cardiomyocytes (11). These discrepancies might be
partially explained by the fact that the actions of PIP on the
activation of the PI3K/AKT pathway vary in a cell-type and
stimulus-dependent manner.
In conclusion, results of the present study have
demonstrated that pretreatment with PIP alleviated MIRI by
attenuating ERS-associated apoptosis in a PI3K/AKT-dependent
manner. Numerous other signaling pathways including the TLR4/NF-κB
pathway (16) and the LncRNA/miRNA
pathway (2) are involved in the
pathophysiological process of MIRI. Further studies are necessary
to explore whether any other signaling pathways involved in the
protective effect of PIP in MIRI. Taken together, the present
results indicated that PIP might be a prospective therapeutic
option for MIRI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPL and YHC conceived and designed the experiments.
ZC and YPL performed majority of the experiments, collected the
data and finished the statistical analysis. All authors contributed
to manuscript preparation and revision. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zeng G, Lian C, Yang P, Zheng M, Ren H and
Wang H: E3-ubiquitin ligase TRIM6 aggravates myocardial
ischemia/reperfusion injury via promoting STAT1-dependent
cardiomyocyte apoptosis. Aging (Albany NY). 11:3536–3550.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ruan Z, Wang S, Yu W and Deng F: LncRNA
MALAT1 aggravates inflammation response through regulating PTGS2 by
targeting miR-26b in myocardial ischemia-reperfusion injury. Int J
Cardiol. 288(122)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang BF, Jiang H, Chen J, Guo X, Li Y, Hu
Q and Yang S: Nobiletin ameliorates myocardial ischemia and
reperfusion injury by attenuating endoplasmic reticulum
stress-associated apoptosis through regulation of the PI3K/AKT
signal pathway. Int Immunopharmacol. 73:98–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chang JC, Lien CF, Lee WS, Chang HR, Hsu
YC, Luo YP, Jeng JR, Hsieh JC and Yang KT: Intermittent
intermittent hypoxia prevents myocardial mitochondrial
Ca2+ overload and cell death during
ischemia/reperfusion: The role of reactive oxygen species. Cells.
8: pii(E564)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shu Z, Yang Y, Yang L, Jiang H, Yu X and
Wang Y: Cardioprotective effects of dihydroquercetin against
ischemia reperfusion injury by inhibiting oxidative stress and
endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt
pathway. Food Funct. 10:203–215. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shen D, Chen R, Zhang L, Rao Z, Ruan Y, Li
L, Chu M and Zhang Y: Sulodexide attenuates endoplasmic reticulum
stress induced by myocardial ischaemia/reperfusion by activating
the PI3K/Akt pathway. J Cell Mol Med. 23:5063–5075. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Manayi A, Nabavi SM, Setzer WN and Jafari
S: Piperine as a potential anti-cancer agent: A review on
preclinical studies. Curr Med Chem. 25:4918–4928. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abdel-Daim MM, Sayed AA, Abdeen A, Aleya
L, Ali D, Alkahtane AA, Alarifi S and Alkahtani S: Piperine
enhances the antioxidant and anti-inflammatory activities of
thymoquinone against Microcystin-LR-induced hepatotoxicity and
neurotoxicity in mice. Oxid Med Cell Longev.
2019(1309175)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zou Y, Gong P, Zhao W, Zhang J, Wu X, Xin
C, Xiong Z, Li Z, Wu X, Wan Q, et al: Quantitative iTRAQ-based
proteomic analysis of piperine protected cerebral
ischemia/reperfusion injury in rat brain. Neurochem Int. 124:51–61.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vaibhav K, Shrivastava P, Javed H, Khan A,
Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, et
al: Piperine suppresses cerebral ischemia-reperfusion-induced
inflammation through the repression of COX-2, NOS-2, and NF-κB in
middle cerebral artery occlusion rat model. Mol Cell Biochem.
367:73–84. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zeng Y and Yang Y: Piperine depresses the
migration progression via downregulating the Akt/mTOR/MMP-9
signaling pathway in DU145 cells. Mol Med Rep. 17:6363–6370.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma ZG, Yuan YP, Zhang X, Xu SC, Wang SS
and Tang QZ: Piperine attenuates pathological cardiac fibrosis via
PPAR-γ/AKT pathways. Ebiomedicine. 18:179–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen X, Ge F, Liu J, Bao S, Chen Y, Li D,
Li Y, Huang T, Chen X, Zhu Q, et al: Diverged effects of piperine
on testicular development: Stimulating leydig cell development but
inhibiting spermatogenesis in rats. Front Pharmacol.
9(244)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu S, Cheng M, Guo X, Wang S, Liu B, Jiang
H, Huang C and Wu G: Down-regulation of miR-200c attenuates
AngII-induced cardiac hypertrophy via targeting the MLCK-mediated
pathway. J Cell Mol Med. 23:2505–2516. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng F, Yuan W, Cao M, Chen R, Wu X and
Yan J: Cyclophilin a protects cardiomyocytes against
hypoxia/reoxygenation-induced apoptosis via the AKT/Nox2 pathway.
Oxid Med Cell Longev. 2019(2717986)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo X, Jiang H, Chen J, Zhang BF, Hu Q,
Yang S, Yang J and Zhang J: RP105 ameliorates hypoxia̸reoxygenation
injury in cardiac microvascular endothelial cells by suppressing
TLR4̸MAPKs̸NF-κB signaling. Int J Mol Med. 42:505–513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Y, Liu L, Yuan J, Sun Q, Wang N and
Wang Y: RP105 protects PC12 cells from oxygenglucose
deprivation/reoxygenation injury via activation of the PI3K/AKT
signaling pathway. Int J Mol Med. 41:3081–3089. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo J, Cui Y, Liu Q, Yang Y, Li Y, Weng L,
Tang B, Jin P, Li XJ, Yang S and Li S: Piperine ameliorates SCA17
neuropathology by reducing ER stress. Mol Neurodegener.
13(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hammad AS, Ravindran S, Khalil A and
Munusamy S: Structure-activity relationship of piperine and its
synthetic amide analogs for therapeutic potential to prevent
experimentally induced ER stress in vitro. Cell Stress Chaperones.
22:417–428. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Peng X, Yang T, Liu G, Liu H, Peng Y and
He L: Piperine ameliorated lupus nephritis by targeting
AMPK-mediated activation of NLRP3 inflammasome. Int
Immunopharmacol. 65:448–457. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hsieh TY, Chang Y and Wang SJ:
Piperine-mediated suppression of voltage-dependent Ca2+
influx and glutamate release in rat hippocampal nerve terminals
involves 5HT1A receptors and G protein βγ activation. Food Funct.
10:2720–2728. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh S and Kumar P: Piperine in
combination with quercetin halt 6-OHDA induced neurodegeneration in
experimental rats: Biochemical and neurochemical evidences.
Neurosci Res. 133:38–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
George K, Thomas NS and Malathi R:
Piperine blocks voltage gated K+ current and inhibits
proliferation in androgen sensitive and insensitive human prostate
cancer cell lines. Arch Biochem Biophys. 667:36–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dhivya V, Priya LB, Chirayil HT,
Sathiskumar S, Huang CY and Padma VV: Piperine modulates
isoproterenol induced myocardial ischemia through antioxidant and
anti-dyslipidemic effect in male Wistar rats. Biomed Pharmacother.
87:705–713. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yaffe PB, Power Coombs MR, Doucette CD,
Walsh M and Hoskin DW: Piperine, an alkaloid from black pepper,
inhibits growth of human colon cancer cells via G1 arrest and
apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog.
54:1070–1085. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Deng T, Wang Y, Wang C and Yan H: FABP4
silencing ameliorates hypoxia reoxygenation injury through the
attenuation of endoplasmic reticulum stress-mediated apoptosis by
activating PI3K/Akt pathway. Life Sci. 224:149–156. 2019.PubMed/NCBI View Article : Google Scholar
|