Introduction
Type 1 diabetes is an autoimmune disease
characterized by the gradual destruction of pancreatic B cells,
resulting in the loss of endogenous insulin production and
consequently hyperglycemia (1).
Therefore, the treatment of type 1 diabetes requires lifelong
insulin therapy to maintain normal blood glucose levels. The
limitations of insulin therapy are an increased risk of
hypoglycemia, excessive blood glucose fluctuation and weight gain,
which may prevent patients from titrating their daily insulin dose
sufficiently to reach the target level of glycosylated hemoglobin
A1c (HbA1c) (1,2). Insulin adjuvant drugs may solve these
unmet requirements, although most drugs, including pramlintide,
enteral insulin therapy and metformin, do not provide sufficient
benefits even if they are beneficial (3-9).
Sodium glucose cotransporter-2 (SGLT2) is a sodium-dependent
glucose transporter and is the major protein responsible for
glucose renal absorption. SGLT2 is mainly expressed in the proximal
renal tubules of the renal cortex and it is a therapeutic target
for blood glucose control (10).
SGLT2 inhibitors include dapagliflozin, empagliflozin, and
sotagliflozin among other drugs. Previously reviewed meta-analyses
have mostly focused on the efficacy and safety of SGLT2 inhibitors
as a whole for adjuvant treatment of type 1 diabetes (4). Dapagliflozin, as an SGLT2 inhibitor,
acts through an insulin-dependent mechanism to reduce renal glucose
reabsorption, thus promoting urinary glucose excretion (11). It has been widely used in patients
with type 2 diabetes to improve blood sugar control and minimize
hypoglycemia, and it is associated with weight loss and systolic
blood pressure drop (12-15).
Dapagliflozin has recently reached the third stage of development
in patients with type 1 diabetes with successful results and was
approved by the European market for specific purposes e.g. in type
1 diabetic adults with body mass index ≥27 kg/m2) in
2019 (16-21).
The randomized controlled trials (RCTs) to evaluate this drug were
so far not systematically evaluated (16-20).
In order to obtain conclusions from the evidence available on this
new therapeutic approach, a meta-analysis of RCTs was performed to
evaluate the efficacy and safety of dapagliflozin in patients with
type 1 diabetes.
Materials and methods
Data sources and article search
Studies published between January 2004 and February
5, 2020 in all languages were retrieved using the following
databases: Medline, Embase and Cochrane Library. The reference
lists in the experimental articles, review articles and reports
were manually scanned to identify any other relevant studies. The
search terms used were as follows: ‘dapagliflozin OR SGLT2 OR
sodium-glucose cotransporter 2 inhibitor OR SGLT2 inhibitor OR
sodium glucose transporter 2 inhibitors OR SGLT2 inhibitors’ and
‘diabetes mellitus, type 1 [Mesh] OR diabetes mellitus, type I OR
type 1 diabetes OR diabetes mellitus, ketosis-prone OR diabetes,
autoimmune OR diabetes mellitus, juvenile-onset OR juvenile-onset
diabetes OR diabetes mellitus, insulin-dependent OR IDDM OR
diabetes mellitus, insulin-dependent, 1 OR brittle diabetes
mellitus OR diabetes mellitus, sudden-onset’.
Study selection
The inclusion criteria were articles in English on
RCTs. Trial participants were aged 18 years and above and of any
gender or ethnic origin, and the trials compared dapagliflozin with
placebo or active comparators used as an additional drug to insulin
therapy in type 1 diabetes. Studies that were not on clinical
patients, non-RCTs, letters or case reports, as well as articles
not reporting outcomes of interest or primary data (editorials,
reviews) were excluded.
Outcome measures
The following outcomes were assessed compared with
the effects of the placebo: i) Efficacy outcomes: Changes in HbA1c,
body weight, mean daily glucose (MDG) and mean amplitude of glucose
excursion (MAGE). ii) Safety outcomes: Differences in adverse
events, serious adverse events, adverse events leading to
discontinuation, infections (urinary tract infections and
reproductive tract infections) and diabetic ketoacidosis (DKA). All
measures of dispersion were converted to standard deviations.
As an adverse event, any undesirable experience
associated with the use of a medical product in a patient (e.g.,
headache, diarrhea or upper respiratory tract infection) was
considered. The event was considered serious when it was
life-threatening, or if the outcome was patient death,
hospitalization (initial or prolonged), disability or permanent
damage (22).
Data extraction and risk of bias
assessment
A total of two reviewers (YH, ZJ) performed the data
extraction and risk of bias assessment and independently extracted
the data in duplicate according to the Cochrane Handbook of
Systematic Reviews of Interventions (23) using a pre-designed data collection
form. Any discrepancies were resolved through consensus. The
quality of the RCTs was assessed using the Cochrane bias risk tool
(23).
Data synthesis and analysis
The meta-analysis was performed in accordance with
the Cochrane Handbook of Systematic Reviews of Interventions
(23) using Revman version 5.3
(https://revman.cochrane.org/#/myReviews), and it was
reported according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses guidelines (24). Weighted mean differences (WMD) and
95% CIs were calculated for continuous outcomes using an inverse
variance random-effects model. With regard to dichotomous outcomes,
the risk ratio and 95% CI were calculated using the random-effects
Mantel-Haenszel approach with the significance threshold set at
P=0.05. An a priori random-effects model assuming a
substantial variability in the treatment effect size across studies
was used conservatively.
Heterogeneity analysis
Heterogeneity was evaluated using Cochrane Q and
I2 statistics. If heterogeneity was acceptable
(P>0.10, or P≤0.10, but I2=50%), the fixed effect
model was adopted. If heterogeneity did not meet these criteria,
the random effect model was adopted. A two-tailed P-value of ≤0.05
was considered to be statistically significant.
Results
Literature retrieval and
selection
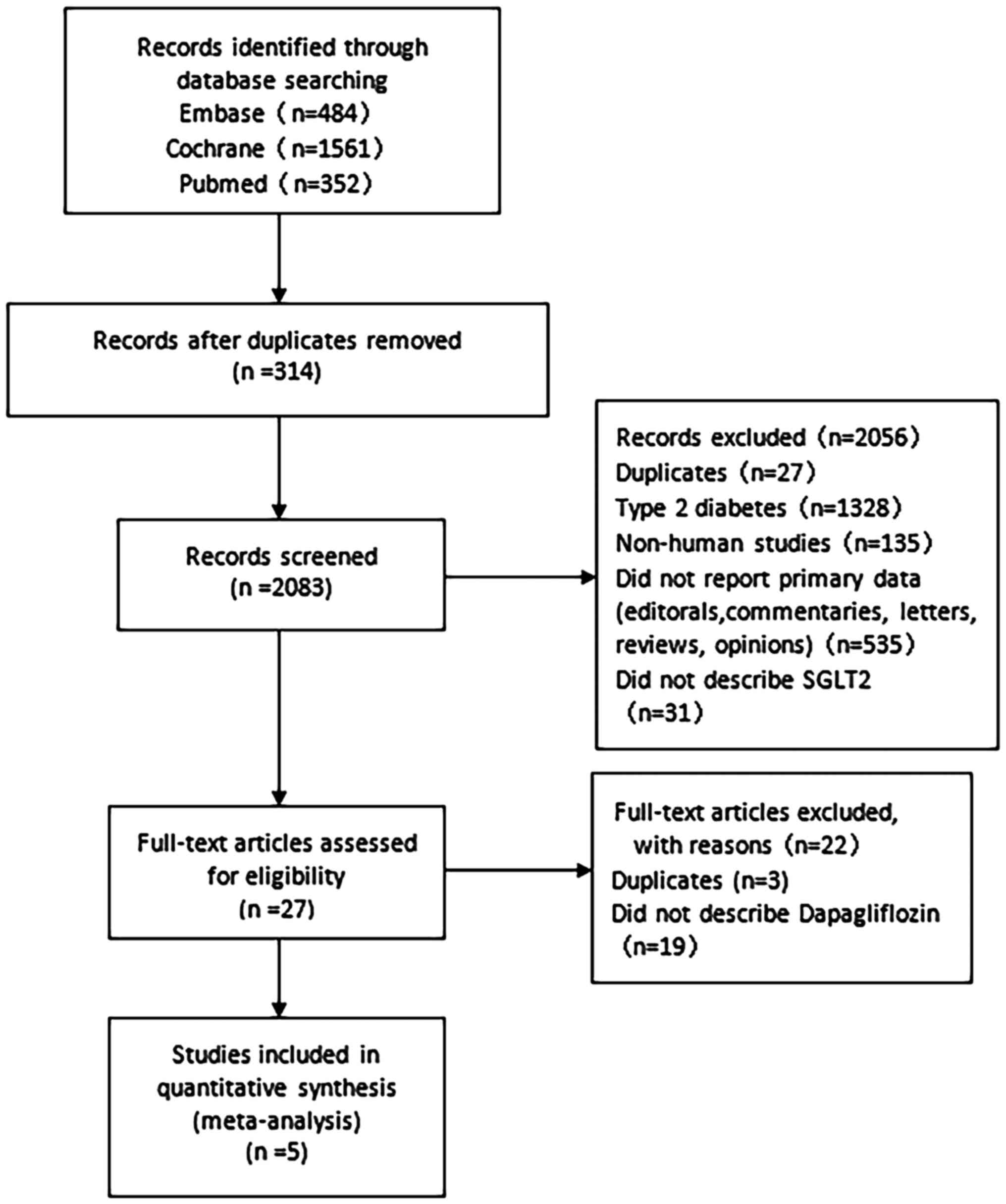
The literature search in the present study resulted
in the retrieval of the records of 2,397 eligible research
articles, and of these, 314 were excluded as they were duplicates.
After screening the titles and abstracts of the remaining 2,083
studies, a total of 2,056 were excluded according to the inclusion
and exclusion criteria, since 27 were duplicates, 1,328 were on
type 2 diabetes, 135 were on animal experiments, 535 had no
original data and 31 were irrelevant. Finally, a total of 27
full-text articles, including 24 original research articles, were
considered. However, 19 of these studies were not using
dapagliflozin and thus, they were not included in the present
meta-analysis. Therefore, the data of the five studies remaining,
which evaluated the effect of dapagliflozin combined with insulin
therapy in patients with type 1 diabetes mellitus (n=630), were
analyzed (16-20).
The studies by Dandona 2017 and Dandona 2018 (16,17)
were on the same trial but the follow-up duration was different (24
weeks and 52 weeks, respectively). In Dandona 2018, some patients
withdrew, resulting in an inconsistent number of patients compared
with Dandona 2017. In order to avoid data duplication, the data
from Dandona 2018 were excluded from this meta-analysis. The entire
process is summarized in Fig.
1.
Study characteristics
All of the studies included in the present
meta-analysis were double-blinded RCTs and were published as full
articles between 2016 and 2018. The follow-up duration in the
included studies ranged from 1 to 52 weeks. The baseline
characteristics were well-balanced in each individual study.
Placebo was used as a control in all trials. A summary of the
characteristics of the studies included is provided in Table I.
 | Table ICharacteristics of the studies
included. |
Table I
Characteristics of the studies
included.
| First author | Year | Country | Periodical | Institution | Follow- up
weeks | Intervention | Experimental group
(n) | Control group
(n) | Clinical Stage | Design | (Refs.) |
|---|
| Dandona | 2017 | USA | The Lancet Diabetes
and Endocrinology | Multi | 24 | Dapagliflozin | 518 | 260 | 3 | RCT | (16) |
| Dandona | 2018 | USA | Diabetes Care | Multi | 52 | Dapagliflozin | 518 | 260 | 3 | RCT | (17) |
| Henry | 2017 | USA | Diabetes, Obesity
and Metabolism | Single | 2 | Dapagliflozin | 57 | 13 | 2a | RCT | (18) |
| Kuhadiya | 2016 | USA | Journal of Clinical
Endocrinology and Metabolism | Single | 12 | Dapagliflozin | 20 | 10 | 4 | RCT | (19) |
| Mathieu | 2018 | USA | Diabetes Care | Multi | 24 | Dapagliflozin | 541 | 272 | 3 | RCT | (20) |
Efficacy outcomes HbA1c
Dapagliflozin therapy was associated with a
significant decrease in HbA1c levels compared with the placebo
(WMD: -0.42%, 95% CI: -0.45 to -0.40%, P<0.00001, I²=93%, 5
comparisons, 1,617 participants; Fig.
2). The effect of 5 and 10 mg dapagliflozin to reduce HbA1c did
not exhibit any significant difference.
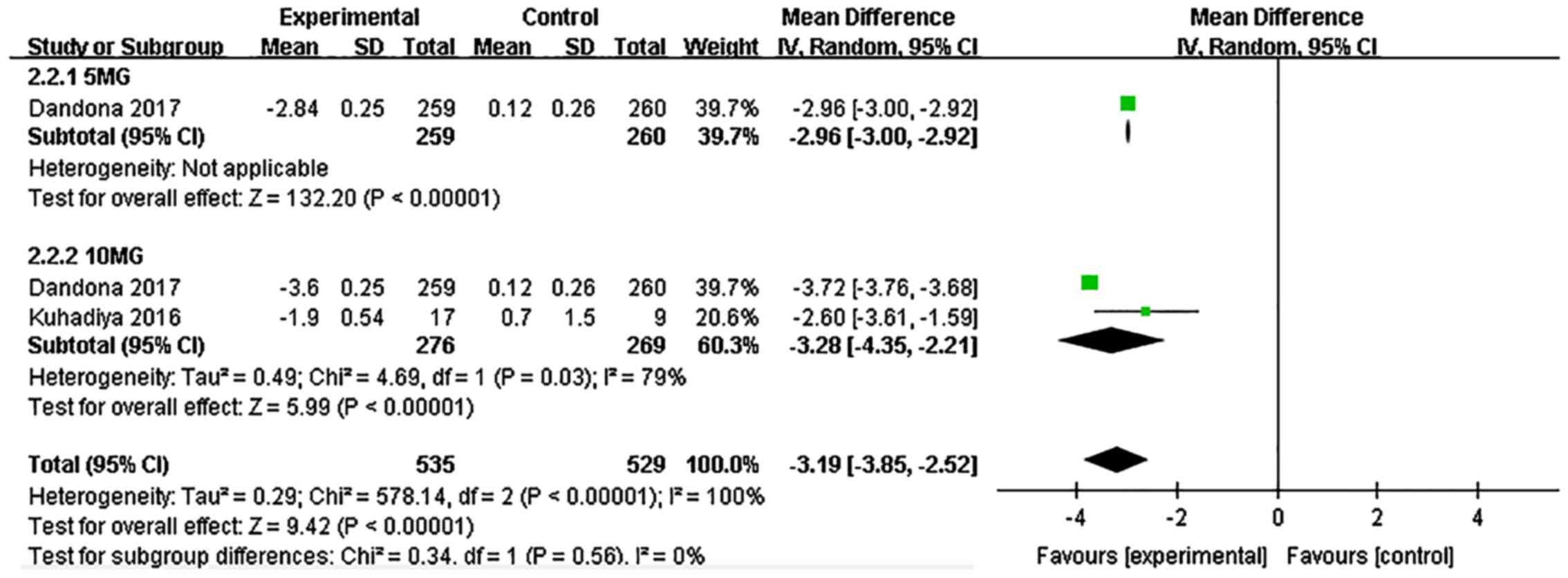
Body weight
Dapagliflozin significantly reduced the body weight
compared with the placebo (WMD: -3.19, 95% CI: -3.85 to -2.52,
P<0.00001, I²=100%, 3 comparisons, 804 participants; Fig. 3). Dapagliflozin at 5 and 10 mg
exerted a similar effect on weight loss.
MDG
Dapagliflozin significantly reduced the MDG compared
with the placebo (WMD: -0.88, 95% CI: -0.99 to -0.76, P<0.00001,
I²=99%, 7 comparisons, 1,533 participants; Fig. 4). Dapagliflozin at 5 and 10 mg did
not exert any differential effect to reduce MDG.
MAGE
Dapagliflozin significantly reduced the average
blood glucose fluctuation compared with the placebo (WMD: -0.80,
95% CI: -1.07 to -0.54, P<0.00001, I²=100%, 6 comparisons, 1,507
participants; Fig. 5).
Dapagliflozin at 5 and 10 mg exerted a similar effect to reduce
MAGE.
Safety outcomes Adverse events
The incidence rate of adverse events associated with
dapagliflozin treatment was significantly higher than that in the
placebo group (risk ratio: 1.11, 95% CI: 1.04-1.17, P=0.001, I²=0%,
6 comparisons, 1,688 participants; Fig.
6). No significant difference in the incidence of adverse
events caused by dapagliflozin between the doses of 5 and 10 mg was
observed (P>0.1).
Serious adverse events
The incidence of serious adverse events associated
with dapagliflozin treatment was significantly higher than that in
the placebo group (risk ratio: 1.61, 95% CI: 1.10-2.34, P=0.01,
I²=0%, 6 comparisons, 1,143 participants; Fig. 7). No significant difference in the
incidence of serious adverse events caused by dapagliflozin between
the doses of 5 and 10 mg was observed (P>0.1).
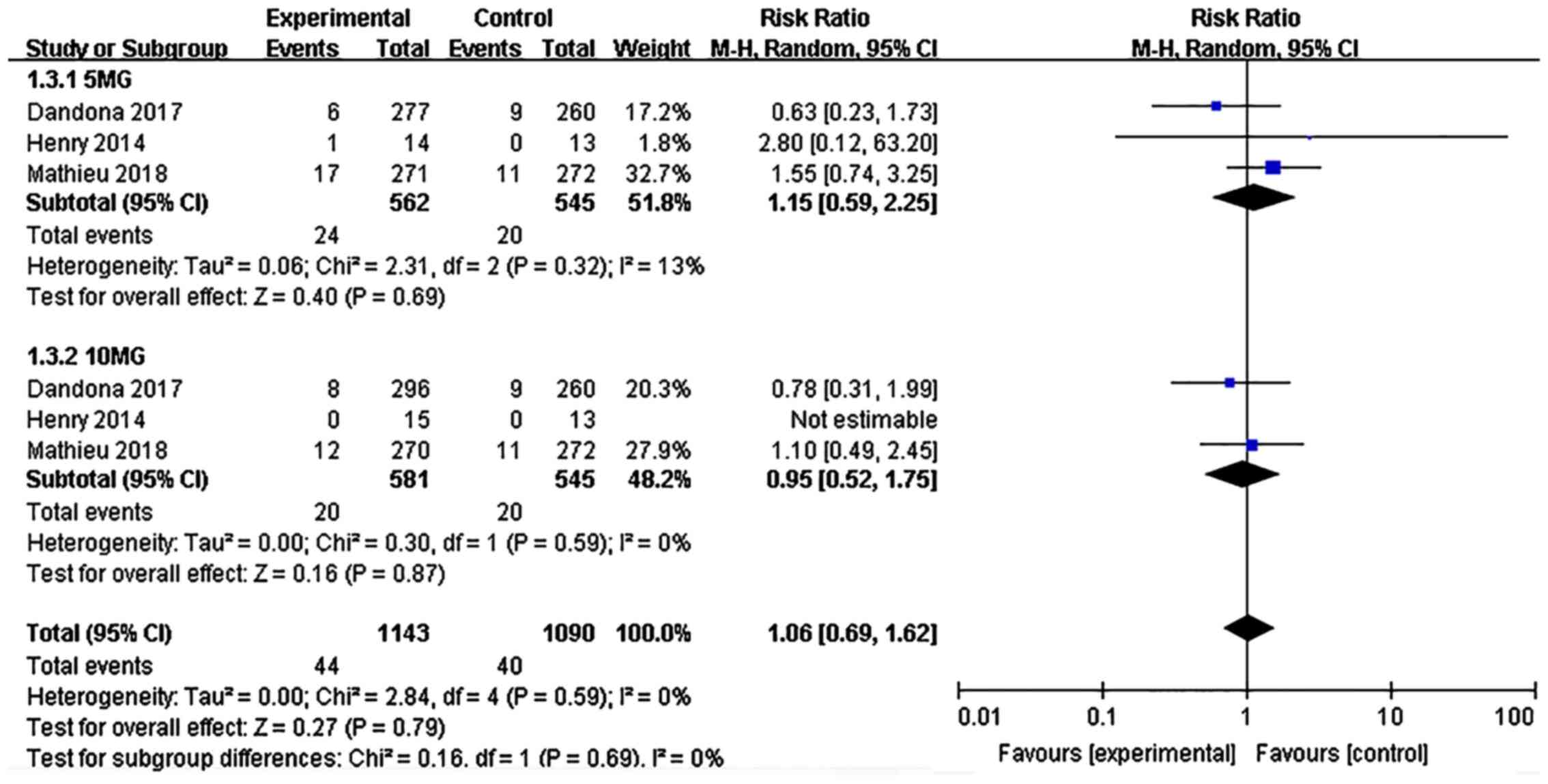
Adverse events leading to
discontinuation
The proportion of patients who stopped the treatment
due to adverse events was evaluated. The incidence of
discontinuation due to adverse events in the dapagliflozin
treatment group was not significantly different from that in the
placebo group (risk ratio: 1.06, 95% CI: 0.69-1.62, P=0.79, I²=0%,
6 comparisons, 1,688 participants; Fig.
8). No difference in the incidence of discontinuation due to
adverse events was observed between the 5 and 10 mg dapagliflozin
groups (P>0.1).
Infections
The incidence of urinary tract infections and
reproductive tract infections was analyzed. The incidence of
infection associated with dapagliflozin treatment was not
significantly different from that in the placebo group (risk ratio:
1.38, 95% CI: 0.86-2.22, P=0.19, I²=53%, 7 comparisons, 1,714
participants; Fig. 9). No
significant difference was observed in the infection rate between
dapagliflozin at 5 and 10 mg (P>0.1).
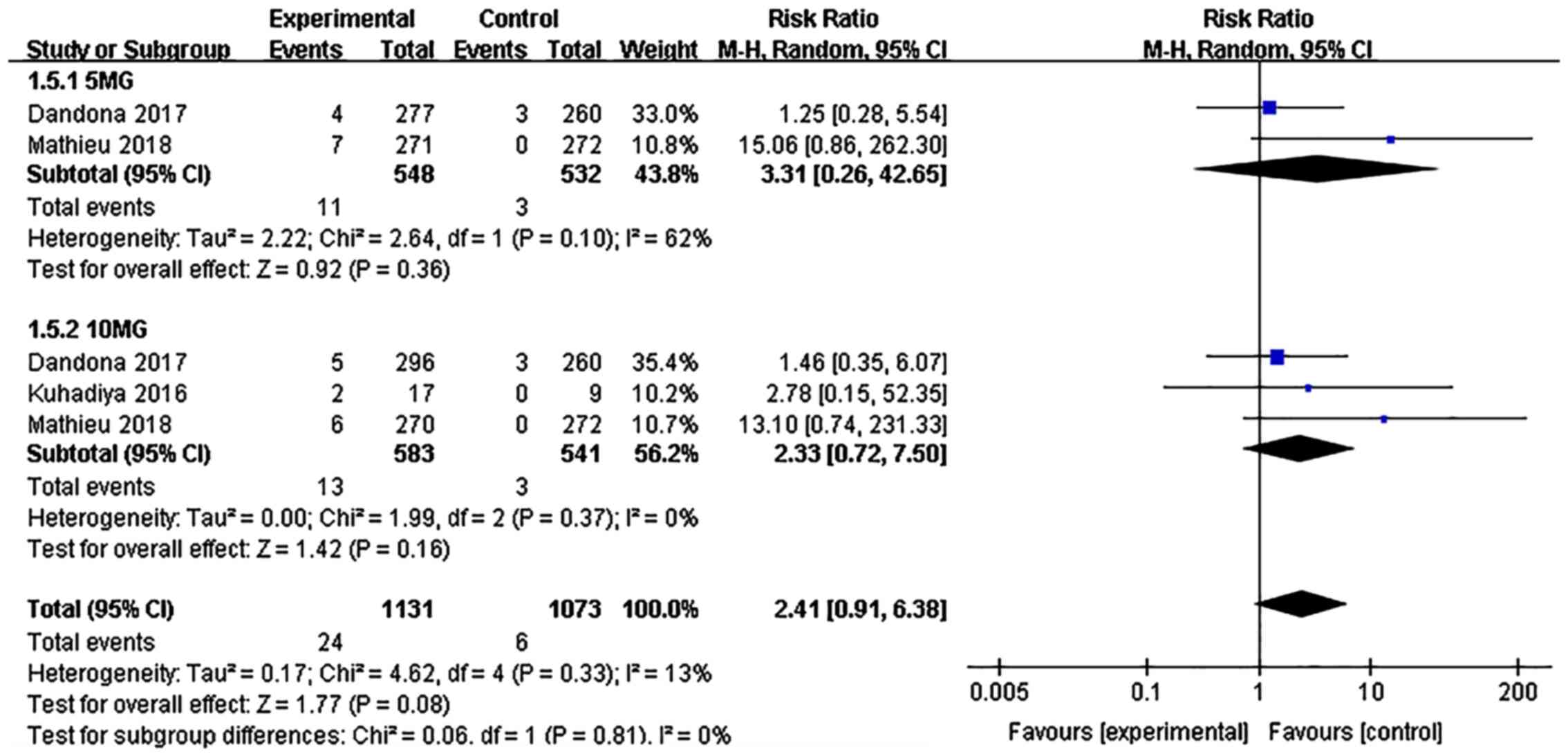
DKA
The incidence of DKA associated with dapagliflozin
treatment was not significantly different from that in the placebo
group (risk ratio: 2.41, 95% CI: 0.91-6.38, P=0.08, I²=13%, 5
comparisons, 1,672 participants; Fig.
10). No significant difference in the incidence of DKA was
observed between the dapagliflozin 5 and 10 mg groups
(P>0.1).
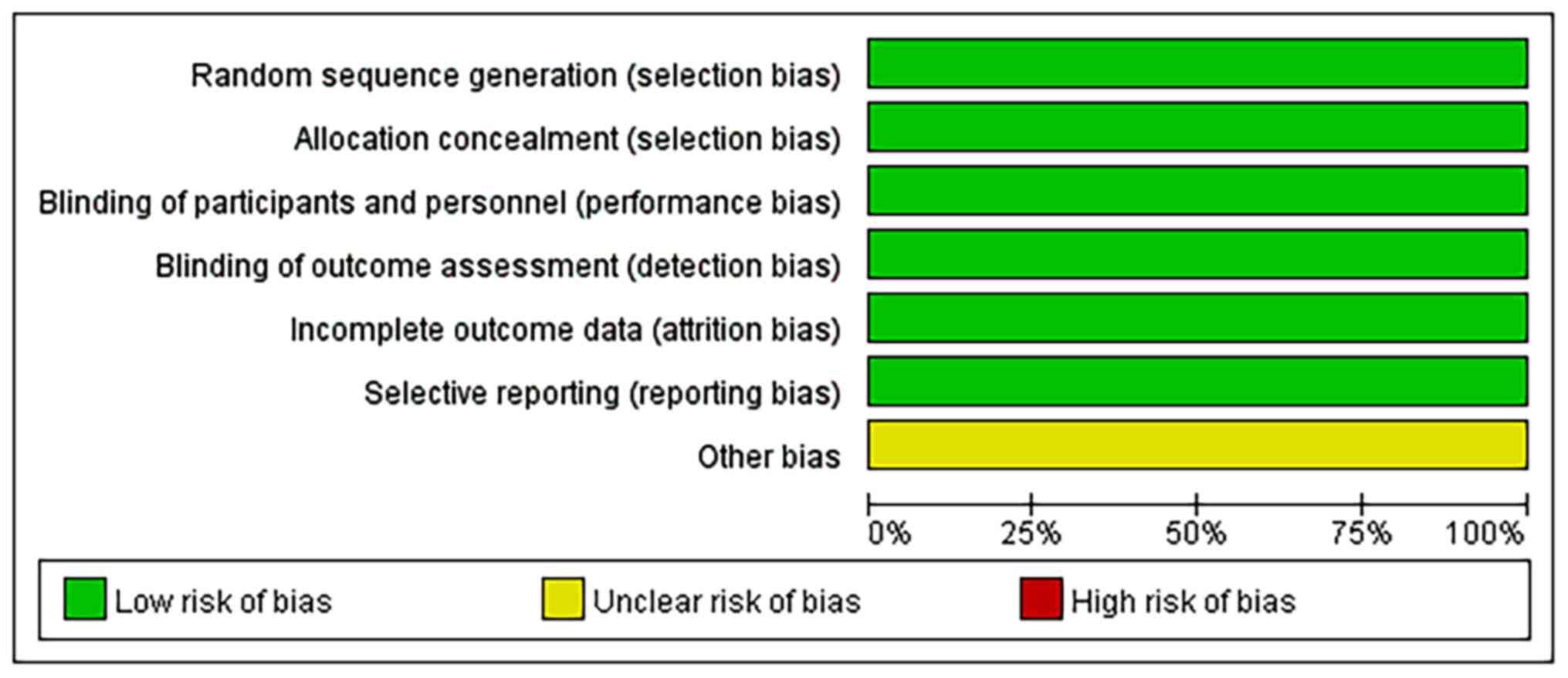
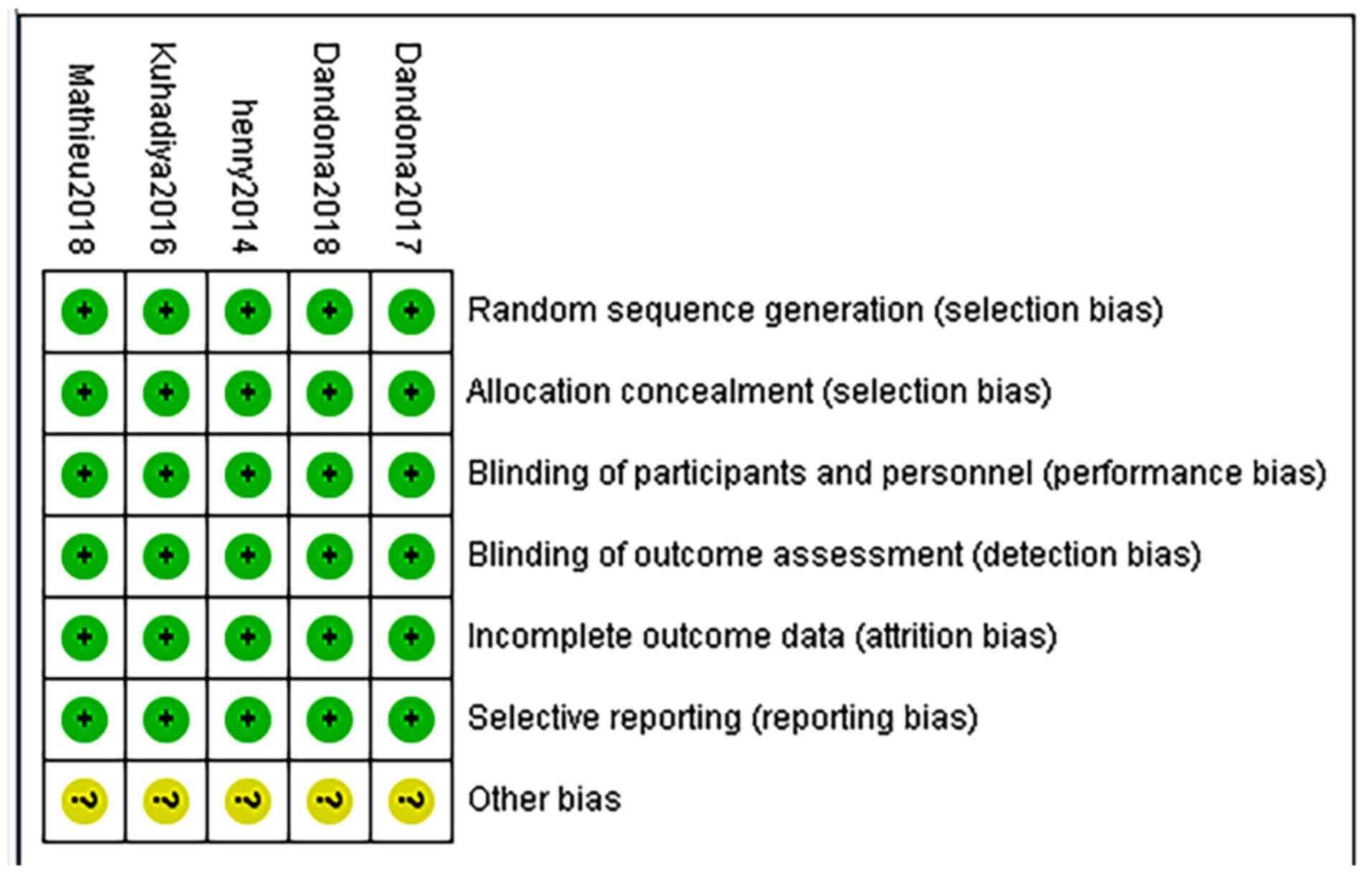
Quality of the included studies
Risk of a bias assessment using the Cochrane
Collaboration's Tool was performed to evaluate the quality of all
of the included studies. The overall risk of bias of the five
studies was low, other bias are not clear. The results in the
quality assessment domain of the included trials are presented in
Figs. 11 and 12.
Heterogeneity analysis
Significant heterogeneity (I2≥50%) was
identified among all efficacy outcomes but the heterogeneity among
all safety outcomes was low (I2<50%). Since the
number of the included studies was small, it was not possible to
perform a large number of subgroup analyses, and thus, only the
doses were grouped.
Discussion
The present meta-analysis reported three major
results. First of all, dapagliflozin-assisted insulin therapy for
type 1 diabetes exerted significant beneficial effects on blood
sugar control, blood sugar stability and weight loss. No
significant difference in therapeutic efficacy between 5 and 10 mg
dapagliflozin was identified. Furthermore, the use of dapagliflozin
significantly increased the incidence of adverse events and serious
adverse events compared with placebo. Finally, during the treatment
of type 1 diabetes with dapagliflozin, the overall risk of
infection, DKA and discontinuation caused by adverse events did not
increase compared to placebo.
The results of the present study indicated that
dapagliflozin-assisted insulin therapy significantly reduced HbA1c,
body weight, daily average blood glucose and average blood glucose
fluctuation in patients with type 1 diabetes. Further exploration
regarding the association between dose and effect revealed no
significant difference in effective outcomes between the two
subgroups by dose (5 and 10 mg). Due to the complexity of the
treatment, hypoglycemia and the possibility of weight gain,
achieving and maintaining the target level of HbA1c through insulin
optimization strategies remain a major challenge. Although progress
has been made in insulin preparation, drug delivery systems and
blood glucose monitoring, only one third of the patients are able
to reach the blood glucose target, while numerous patients become
overweight or obese (25,26). Due to the glucose-dependent and
insulin-independent mechanism of dapagliflozin (27), its function is to increase urine
glucose excretion without giving rise to a risk of hypoglycemia,
thus reducing the degree of fluctuation of the level of glucose in
the blood. Therefore, dapagliflozin may not only reduce the daily
average blood sugar level and HbA1c, but also reduce blood sugar
volatility, thus helping patients with type 1 diabetes to achieve
the goal of gaining control of their blood sugar levels. A previous
study indicated that weight loss under dapagliflozin was associated
with heat loss caused by diabetes (28). Moderate weight loss also has
beneficial effects on cardiovascular risk factors (29,30),
suggesting that the beneficial effects of dapagliflozin on weight
may help reduce cardiovascular risk factors. In addition, based on
the positive results from the DEPICT studies (16,17),
dapagliflozin 5 mg received market approval in Europe in March 2019
as an additional drug to insulin therapy in patients with type 1
diabetes with a body mass index (BMI) ≥27 kg/m2 (the BMI
restriction reflects safety concerns regarding the DKA risk in
those patients with a lower BMI) (31). The present study confirmed that the
effect of dapagliflozin at the dose of 5 mg was sufficiently
effective. Although the effect of 10 mg was more pronounced, the
difference between the two doses was not evident.
A previous study suggested that the use of the
lowest dose (dapagliflozin 5 mg) of the SGLT inhibitor was able to
reduce the risk of DKA and other adverse events (32). By contrast, the present study
indicated that dapagliflozin significantly increased the risk of
adverse events and serious adverse events compared with placebo
(however, no significant difference between the doses of 5 and 10
mg was observed; P>0.1). This is inconsistent with the
association between dose and adverse events indicated by a previous
study (32). The heterogeneity of
the outcome of adverse reactions was low and this may indicate that
the results are credible. Therefore, the correlation between drug
dosage and safety requires further exploration and research in the
future. With regard to adverse events leading to discontinuation
and infections, no significant difference in outcomes was
identified and the heterogeneity of the two outcomes was also low.
The results actually demonstrated no significant risk of adverse
events leading to discontinuation and infections associated with
the use of dapagliflozin. Different from previous meta-analyses on
SGLT2 (33-35)
and clinical studies (16-17), the present
meta-analysis on dapagliflozin did not indicate an increase in the
risk of DKA and no significant difference was observed between the
two different doses. However, a degree of caution should be
exercised when interpreting these results as most of the studies
included in the present analysis had a duration of 24 weeks. It may
only be suggested that dapagliflozin-assisted insulin therapy does
not increase the risk of DKA in the short term (24 weeks) in
patients with type 1 diabetes, while the risk during long-term
treatment requires further research. In view of the conclusions
made by previous studies (16-20)
on the risks of DKA during treatment, it may be assumed that the
risk of DKA remains an important issue that cannot be ignored
during the treatment with dapagliflozin, and doctors should inform
patients of the potential risk of DKA and provide information on
how to mitigate the risk. The education of patients and providers
should include insulin titration guidance, as mismanagement of this
is a major factor responsible for DKA development (36). A reasonable insulin titration
strategy, based on SALM-1 testing, is to reduce the insulin dose by
no more than 20% after starting to use SGLT-2 inhibitor
dapagliflozin, and then titrate back to the initial insulin dose
(37). Patients should receive
education on the possibility of normal blood glucose DKA and
understand the risk factors for DKA, including acute diseases and
infections; heavy drinking, strenuous exercise, reduced
carbohydrate intake and insufficient insulin dose (e.g. dose
omission or pump failure) (37). If
the patient's ketone level is abnormal or DKA symptoms occur, the
drug should be stopped immediately and medical care should be
sought (36).
Previous meta-analyses discussed the efficacy and
safety of SGLT2 inhibitors as a whole in adjuvant treatment of type
1 diabetes (36,38). Yang et al (38) analyzed the role of SGLT2 inhibitors
as a whole in three randomized controlled trials, namely, three
different drugs (dapagliflozin, empagliflozin, sotagliflozin),
among which the study on dapagliflozin was included in the present
article (18). El Masri et
al (34) analyzed the role of
SGLT2 inhibitors as a whole in four randomized controlled trials,
namely, four different drugs (dapagliflozin, empagliflozin,
sotagliflozin and canagliflozin), of which the study on
dapagliflozin was included in the present article (19). SGLT2 inhibitors include
dapagliflozin, empagliflozin, sotagliflozin, canagliflozin and
other drugs, and their efficacy and safety may be different. The
present meta-analysis took dapagliflozin as the research object and
included four randomized controlled trials to study the same drug.
In addition, subgroup analysis was conducted to analyze the
significance of differences in efficacy and safety between 5 mg and
10 mg of dapagliflozin treatment.
Of note, the present study had certain strengths and
limitations. The present meta-analysis included four experiments
with dapagliflozin as the research object and is, to the best of
our knowledge, the first systematic evaluation and meta-analysis of
dapagliflozin-assisted insulin therapy in type 1 diabetes. The risk
of bias assessment for inclusion in the trial and the grading of
the quality of the evidence were included in the present study. All
of the five studies were of high quality. The results of the
present analysis strongly support the conclusion drawn from a
previous relevant review that SGLT-2 inhibitors as adjunctive
therapy to insulin provide additional glycemic and non-glycemic
benefits for patients with type 1 diabetes (37). However, certain limitations were
also present. These include the relatively small number of trials
included in the present meta-analysis and the short duration of the
treatment (no more than 52 weeks), which did not allow for a robust
assessment of long-term results (e.g. pertaining to the risk of
DKA). A subgroup and regression analysis was conducted on the
follow-up time, which found that the follow-up time had no obvious
significance for heterogeneity. Subgroup analysis of the follow-up
time in so few test groups may not be meaningful and would not make
a positive contribution to the review findings or help to compare
and synthesize information about the characteristics of
interventions in the study. Confidence in the results comes from
the high quality of the included trials. Due to the small number of
studies, marked heterogeneity was present for the outcome
indicators. The source of heterogeneity in this systematic review
was not determined, since more clinical research data is required,
and a subgroup analysis by age, drug injection method and region
will be considered in further studies. In addition, the high
heterogeneity between the results of each group and/or the small
number of trials and the contribution of the participants to each
subgroup gave rise to uncertainty about the importance of these
subgroup differences.
In March 2019, the European Commission approved the
adjuvant treatment of type 1 diabetes with dapagliflozin.
Similarly, Japan's Ministry of Health, Labor and Welfare approved
dapagliflozin as an adjuvant drug for patients with type 1 diabetes
using insulin (21). Previous
studies pointed out that the use of SGLT2 inhibitors increases the
risk of DKA in patients using insulin (39,40).
Additional research is required on risk factors of DKA and risk
mitigation to determine the characteristics of high-risk patients
and prevent such events. However, the present results suggest that
dapagliflozin does not increase the risk of DKA in the short term
(24 weeks). Although 5 mg dapagliflozin gained marked approval in
Europe in March 2019, our study showed that dapagliflozin
significantly increased the risk of adverse events and serious
adverse events compared with placebo (no significant difference was
observed between doses of 5 and 10 mg; P>0.1).
In conclusion, the present meta-analysis indicated
that dapagliflozin, as an adjuvant drug for insulin therapy in
patients with type 1 diabetes, provided a significant benefit. The
present results provided a reference for the clinical application
and further research on dapagliflozin in the future. Additional
prospective RCTs with larger sample sizes and a longer duration are
required to further assess the efficacy and safety of dapagliflozin
in the treatment of type 1 diabetes.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 81560345 and
81860379), Preeminence Youth Fund of Jiangxi Province (grant no.
20162BCB23058), China Postdoctoral Science Foundation Grant (grant
no. 2017M610401) and the Science and Technology Planning Project at
the Department of Science and Technology of Jiangxi Province, China
(grant nos. 20151BBG70165 and 20171BAB205075).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZJ, YH and YW conceived and designed the study; ZJ
and YH performed the database search, study selection and data
extraction; ZJ, YH and YW performed the quality assessment of the
screened studies; ZJ and YH wrote the manuscript; All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Chiang JL, Kirkman MS, Laffel LM and
Peters AL: Type 1 Diabetes Sourcebook Authors. Type 1 diabetes
through the life span: A position statement of the American
diabetes association. Diabetes Care. 37:2034–2054. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aschner P, Horton E, Leiter LA, Munro N
and Skyler JS: Global Partnership For Effective Diabetes
Management. Practical steps to improving the management of type 1
diabetes: Recommendations from the Global partnership for effective
diabetes management. Int J Clin Pract. 64:305–315. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miller KM, Foster NC, Beck RW, Bergenstal
RM, DuBose SN, DiMeglio LA, Maahs DM and Tamborlane WV: T1D
Exchange Clinic Network. Current state of type 1 diabetes treatment
in the U.S.: Updated data from the T1D exchange clinic registry.
Diabetes Care. 38:971–978. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bode BW and Garg SK: The emerging role of
adjunctive noninsulin antihyperglycemic therapy in the management
of type 1 diabetes. Endocr Pract. 22:220–230. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lyons SK, Hermann JM, Miller KM, Hofer SE,
Foster NC, Rami-Merhar BM, Aleppo G, Seufert J, DiMeglio LA, Danne
T, et al: Use of adjuvant pharmacotherapy in type 1 diabetes:
International comparison of 49,996 individuals in the prospective
diabetes follow-up and T1D exchange registries. Diabetes Care.
40:e139–e140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Petrie JR, Chaturvedi N, Ford I, Brouwers
MC, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein
BE, et al: Cardiovascular and metabolic effects of metformin in
patients with type 1 diabetes (REMOVAL): A double-blind,
randomised, placebo-controlled trial. Lancet Diabetes Endocrinol.
5:597–609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garg S, Moser E, Bode B, Klaff L, Hiatt W,
Beatson C and Snell-Bergeon J: Effect of sitagliptin on
post-prandial glucagon and GLP-1 levels in patients with type 1
diabetes: Investigator initiated, double-blind, randomized, placebo
controlled trial. Endocr Pract. 19:19–28. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ellis SL, Moser EG, Snell-Bergeon JK,
Rodionova AS, Hazenfield RM and Garg SK: Effect of sitagliptin on
glucose control in adult patients with type 1 diabetes: A pilot,
double-blind, randomized, crossover trial. Diabet Med.
28:1176–1181. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mathieu C, Zinman B, Hemmingsson JU, Woo
V, Colman P, Christiansen E, Linder M and Bode B: ADJUNCT ONE
Investigators. Efficacy and safety of liraglutide added to insulin
treatment in type 1 diabetes: The ADJUNCT ONE treat to-target
randomized trial. Diabetes Care. 39:1702–1710. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen J, Williams S, Ho S, Loraine H, Hagan
D, Whaley JM and Feder JN: Quantitative PCR tissue expression
profiling of the human SGLT2 gene and related family members.
Diabetes Ther. 1:57–92. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chao EC and Henry RR: SGLT2 inhibition-a
novel strategy for diabetes treatment. Nat Rev Drug Discov.
9:551–559. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Sun YN, Zhou Y, Chen X, Che WS and Leung
SW: The efficacy of dapagliflozin combined with hypoglycaemic drugs
in treating type 2 diabetes mellitus: Meta-analysis of randomised
controlled trials. BMJ Open. 4(e004619)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang M, Zhang L, Wu B, Song H, An Z and
Li S: Dapagliflozin treatment for type 2 diabetes: A systematic
review and meta-analysis of randomized controlled trials. Diabetes
Metab Res Rev. 30:204–221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fioretto P, Giaccari A and Sesti G:
Efficacy and safety of dapagliflozin, a sodium glucose
cotransporter 2 (SGLT2) inhibitor in diabetes mellitus. Cardiovasc
Diabetol. 14(142)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Weber MA, Mansfield TA, Cain VA, Iqbal N,
Parikh S and Ptaszynska A: Blood pressure and glycaemic effects of
dapagliflozin versus placebo in patients with type 2 diabetes on
combination antihypertensive therapy: A randomised, double blind,
placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol.
4:211–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dandona P, Mathieu C, Phillip M, Hansen L,
Griffen SC, Tschöpe D, Thorén F, Xu J and Langkilde AM: DEPICT-1
Investigators. Efficacy and safety of dapagliflozin in patients
with inadequately controlled type 1 diabetes (DEPICT-1): 24 week
results from a multicentre, double-blind, phase 3, randomised
controlled trial. Lancet Diabetes Endocrinol. 5:864–876.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dandona P, Mathieu C, Phillip M, Hansen L,
Tschöpe D, Thorén F, Xu J and Langkilde AM: DEPICT-1 Investigators.
Efficacy and safety of dapagliflozin in patients with inadequately
controlled type 1 diabetes: The DEPICT-1 52-Week Study. Diabetes
Care. 41:2552–2559. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Henry RR, Dandona P, Pettus J, Mudaliar S,
Xu J and Hansen L: Dapagliflozin in patients with type 1 diabetes:
A post hoc analysis of the effect of insulin dose adjustments on
24-hour continuously monitored mean glucose and fasting
β-hydroxybutyrate levels in a phase IIa pilot study. Diabetes Obes
Metab. 19:814–821. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuhadiya ND, Ghanim H, Mehta A, Garg M,
Khan S, Hejna J, Torre B, Makdissi A, Chaudhuri A, Batra M and
Dandona P: Dapagliflozin as additional treatment to liraglutide and
insulin in patients with type 1 diabetes. J Clin Endocrinol Metab.
101:3506–3515. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mathieu C, Dandona P, Gillard P, Senior P,
Hasslacher C, Araki E, Lind M, Bain SC, Jabbour S, Arya N, et al:
Efficacy and safety of dapagliflozin in patients with inadequately
controlled type 1 diabetes (the DEPICT-2 Study): 24-week results
from a randomized controlled trial. Diabetes Care. 41:1938–1946.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aguillo'n AR, Mascarello A, Segretti ND,
de Azevedo HF, Guimaraes CR, Miranda LS and de Souza RO: Synthetic
strategies toward SGLT2 inhibitors. Org Process Res Dev.
22:467–488. 2018.
|
|
22
|
NIA Adverse Event and Serious Adverse
Event Guidelines. National Institutes on Aging, National Institutes
of Health, 2011.
|
|
23
|
Higgins J and Green S (eds): Cochrane
Handbook for Systematic Reviews of Interventions, version 5.1.0,
March 2011. The Cochrane Collaboration. John Wiley & Sons,
Ltd., New Jersey, 2014.
|
|
24
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McKnight J, Wild S, Lamb M, Cooper M,
Jones T, Davis E, Hofer S, Fritsch M, Schober E, Svensson J, et al:
Glycaemic control of type 1 diabetes in clinical practice early in
the 21st century: An international comparison. Diabet Med.
32:1036–1050. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weinstock RS, Schutz-Fuhrmann I, Connor
CG, Hermann JM, Maahs DM, Schütt M, Agarwal S, Hofer SE, Beck RW
and Holl RW: T1D Exchange Clinic Network; DPV Initiative. T1D
exchange clinic network; DPV initiative. Type 1 diabetes in older
adults: Comparing treatments and chronic complications in the
United States T1D exchange and the German/Austrian DPV registries.
Diabetes Res Clin Pract. 122:28–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chao EC: SGLT-2 inhibitors: A new
mechanism for glycemic control. Clin Diabetes. 32:4–11.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bolinder J, Ljunggren O, Kullberg J,
Johansson L, Wilding J, Langkilde AM, Sugg J and Parikh S: Effects
of dapagliflozin on body weight, total fat mass, and regional
adipose tissue distribution in patients with type 2 diabetes
mellitus with inadequate glycemic control on metformin. J Clin
Endocrinol Metab. 97:1020–1031. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wing RR, Lang W, Wadden TA, Safford M,
Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A and
Wagenknecht L: Look AHEAD Research Group. Benefits of modest weight
loss in improving cardiovascular risk factors in overweight and
obese individuals with type 2 diabetes. Diabetes Care.
34:1481–1486. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Van Gaal LF, Wauters MA and De Leeuw IH:
The beneficial effects of modest weight loss on cardiovascular risk
factors. Int J Obes Relat Metab Disord. 21 (Suppl 1):S5–S9.
1997.PubMed/NCBI
|
|
31
|
Evans M, Hicks D, Patel D, Patel V, McEwan
P and Dashora U: Optimising the benefits of SGLT2 inhibitors for
type 1 diabetes. Diabetes Ther. 11:37–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McCrimmon RJ and Henry RR: SGLT inhibitor
adjunct therapy in type 1 diabetes. Diabetologia. 61:2126–2133.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li K and Xu G: Safety and efficacy of
sodium glucose co-transporter 2 inhibitors combined with insulin in
adults with type 1 diabetes: A meta-analysis of randomized
controlled trials. J Diabetes. 11:645–655. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El Masri D, Ghosh S and Jaber LA: Safety
and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors
in type 1 diabetes: A systematic review and meta-analysis. Diabetes
Res Clin Pract. 137:83–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamada T, Shojima N, Noma H, Yamauchi T
and Kadowaki T: Sodium-glucose co-transporter-2 inhibitors as
add-on therapy to insulin for type 1 diabetes mellitus: Systematic
review and meta-analysis of randomized controlled trials. Diabetes
Obes Metab. 20:1755–1761. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baker C, Wason S, Banks P, Sawhney S,
Chang A, Danne T, Gesty-Palmer D, Kushner JA, McGuire DK, Mikell F,
et al: Dose-dependent glycometabolic effects of sotagliflozin on
type 1 diabetes over 12 weeks: The inTandem4 trial. Diabetes Obes
Metab. 21:2440–2449. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boeder S and Edelman SV: Sodium-glucose
co-transporter inhibitors as adjunctive treatment to insulin in
type 1 diabetes: A review of randomized controlled trials. Diabetes
Obes Metab. 21 (Suppl 2):S62–S77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang Y, Pan H, Wang B, Chen S and Zhu H:
Efficacy and safety of SGLT2 inhibitors in patients with type 1
diabetes: A meta-analysis of randomized controlled trials. Chin Med
Sci J. 32:22–27. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Peters AL, Henry RR, Thakkar P, Tong C and
Alba M: Diabetic ketoacidosis with canagliflozin, a sodium-glucose
cotransporter 2 inhibitor, in patients with type 1 diabetes.
Diabetes Care. 39:532–538. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Peters AL, Buschur EO, Buse JB, Cohan P,
Diner JC and Hirsch IB: Euglycemic diabetic ketoacidosis: A
potential complication of treatment with sodium-glucose
cotransporter 2 inhibition. Diabetes Care. 38:1687–1693.
2015.PubMed/NCBI View Article : Google Scholar
|