Introduction
Under the circumstances of the increasing incidence
of dry eye disease, especially due to the extensive use of personal
computers and other gadget screens, amplified during the Covid-19
pandemic, and associated with global warming and pollution, the use
of artificial tears has become crucial for the comfort and
maintenance of ocular health (1).
The signs and symptoms of dry eye are of concern for
ophthalmologists because of their diversity and persistence,
sometimes despite adequate treatment (2-7).
The requirement of a good adherence of the patients to treatment is
a compulsory condition for success (8). The involvement of inflammatory
mechanism in dry eye has led to new challenges and the need for new
therapeutic agents (9-14).
Considering the recognition of inflammation associated with free
radical production, the present study focused on further
investigating some widely used artificial tears. Previous studies
dedicated to the evaluation of antioxidant activity of various
artificial tears revealed significant differences among them
(15-17).
Increasing exposure to pollution, global warming and
expanding work on video terminals requires permanent use of
artificial teardrops, so that the stability of their properties
becomes an important effectiveness condition. In addition,
artificial tears, as therapeutic agents in dry eye, may be more
effective if they also neutralize successfully some higher amounts
of free radicals. Thus, the aim of previous studies was to
investigate the artificial tear samples after opening of the vial,
a second time after one year of refrigeration and a third time
after having exposed the samples to UV irradiation, having in mind
the increase of natural environment UV irradiation caused by the
thinning of the ozone protective layer of the atmosphere and its
damaging potential for the eye (18,19).
The present study is a preliminary research aiming to evaluate the
impact of various environmental factors on these products, followed
by evaluation of the effectiveness of these products, in terms of
objective examination, patient preference and adherence criteria,
and how it is impacted by the conditions of use and storage. The
varying impact of the exposure to UV on the stability of the
artificial tears, may offer recommendations regarding a more
adequate medical behavior of dry eye subjects (3,4).
The term ‘dry eye’ includes a wide spectrum of
ocular surface alterations with different etiology and
pathophysiology, as multifactorial disease of the tears and ocular
surface that results in symptoms of discomfort, visual disturbance,
and tear film instability, with potential damage of the ocular
surface, which is evident in the ophthalmological examination and
quantified by special questionnaires (20-23).
The dry eye is accompanied by increased osmolarity of the tear film
and inflammation of the ocular surface (3,24).
The tested commercially available artificial tears
lack both the antioxidant content and UV-absorbing characteristics
of natural tears. Artificial tear formulations that help restore
natural antioxidant and UV-absorbing properties to the tear film of
the aging eye may help prevent or improve dry eye symptoms and
promote ocular health (25). The
literature data mention findings regarding the use of artificial
tears with an adequate antioxidative effect, preserved or
preservative-free, that may be beneficial in the treatment of dry
eyes caused by environmental factors (26-30).
The aim of the present study was to evaluate the
stability of the antioxidant activity of eight different artificial
tears, including three different active principles (hydroxypropyl
guar, hydroxypropyl methylcellulose, and carboxymethylcellulose),
in order to identify any existing correlation between the chemical
characteristics, the effectiveness of an artificial tear and the
stability in time under various circumstances, by storage at +4˚C
and to/by UV irradiation, hypothesizing that the effectiveness of
an artificial tear may be influenced by exposure to the environment
(31-33).
Thus, the artificial tear samples were irradiated with UVC
radiations at 254 nm wavelength.
Materials and methods
Types of artificial tears
For the experiment, artificial tear eye drops were
used for ophthalmic administration. The tear eye drops were
sterile, hypotone, soothing, moisturizing, lubricant solutions,
frecquently recommended for eye dry syndrome (moderate to severe).
A sample of each ophtalmic product was used. Tests were run on
eight different pharmaceutical ophtalmic products, various types of
artificial tears solutions, before and after exposure to UV
starting from the opening moment of the bottles in the first year
since their production, followed by preservation of the bottles at
+4˚C, and retesting after one year and two years from the opening
moment. The samples of artificial tears as eyedrops used in the
experiments, frequently commercialized on the Romanian market, were
required to have the following characteristics of active
principles:
i) Hydroxypropyl guar artificial tears
characterization
The artificial tears (samples 1, 2 and 3) based on
hydroxypropyl guar characteristics, were: Derived from guar gum, a
nonionic polymer made of naturally occurring guar or cluster bean,
Cyanopsis tetragonoloba (L.), which presents specific
properties such as developing a highly thickening effect,
containing 30% ethanol, good compatibility with electrolytes, good
stability in a large pH range of 5.0-7.0, and a yellow powder
water-soluble form. Hydroxypropyl guar is an excipient used in the
pharmaceutical industry as a thickening agent. It contains
nutritional polysaccharides and has numerous benefits; it increases
viscosity effectively, provides smooth skin feel and has
characteristic high level of lubricity, has favorable film-forming
properties, helps stabilize emulsions and has excellent salt and
alcohol tolerance in aqueous solutions and can be used for gel
products that can be pumped or sprayed (34). The effects of artificial tears based
on hydroxypropyl guar recommendations are that it temporarily
relieves the sensation of burning and irritation, due to dry eyes,
power of action, intensive care; re-wetting of contact lenses made
of silicone and soft hydrogel, is hydrophilic, in case of minor
irritations, discomfort or blurred vision (35).
ii) Hydroxypropyl methylcellulose or
hypromellose artificial tears characterization
The artificial tears (samples 4, 5 and 6) based on
hydroxypropyl methylcellulose or hypromellose characteristics,
were: A non-ionic cellulose ether made from natural cotton fiber
under a series of chemical processing, which presents specific
properties, including being an odorless, tasteless and non-toxic
white powder, soluble in cold water to form a transparent viscous
solution with the properties of thickening, binding, dispersing,
emulsifying, film coating, suspending, absorbing, gelling, water
retention and colloid protection (36). It is most commonly used in
hydrophilic matrix fabrication and it allows for the controlled
release of drug substances, thereby increasing the duration of
therapeutic effects. The physical characteristics of this drug
resemble natural tears, providing lubrication to the ocular surface
and maintaining corneal hydration in dry eye syndromes (37). Known as an anti-caking agent, a
polymer with moisturizing and vascoelastic properties that protect
and lubricate the ocular surface, reducing the feeling of
discomfort, it is the product with an epithelializing, a
disinfecting and a moisturizing protective effect. At the surface
of the cornea and conjunctiva, hydroxypropyl methylcellulose
creates a temporary protective film, which moistens the outer
surface of the eye and offers an alternative in case of
insufficient tear secretion. Additionally, it induces rapid
relaxation in the case of minor eye irritations of non-infectious
origin and removes dry eye sensation. The presence of dexpanthenol,
the alcoholic form of vitamin B5, an active substance
with a similar effect as pantothenic acid, helps the development
and regeneration of mucous membranes and skin, and contributes to
the rapid regeneration of cells on the surface of the cornea
(epithelialization). The effects of artificial tears based on
hydroxypropyl methylcellulose recommendations are that it offers
calming and lubricating qualities and is used to relieve eye
discomfort and irritation manifestations associated with dry eye
syndrome and environmental factors such as wind, salt water, smoke
or computer work (38).
iii) Sodium carboxymethylcellulose or
cellulose gum artificial tears characterization
The artificial tears (samples 7 and 8) based on
sodium carboxymethylcellulose or cellulose gum, a sodium salt of
polycarboxymethyl cellulose characteristics (39), were: It is obtained from cellulose,
the main polysaccharide and constituent of wood and all plant
structures. It is obtained for commercial purposes from wood and is
chemically modified. Sodium carboxymethylcellulose is used as a
thickening agent, but also as a filler, dietary fiber, anti-caking
agent and emulsifier. It is similar to cellulose, but is very
soluble in water (40,41). The effects of artificial tears based
on sodium carboxymethylcellulose recommendations are that it
lubricates the eye surface and moistens the cells on the eye
surface by restoring the natural osmotic balance and it has a
triple action: lubricating, humidifying, and osmoprotective
(42-44).
Sample preparation
After opening, the ophthalmic pharmaceutical
artificial tears solutions were stored at ambient temperature.
Subsequently, 50 µl of each ophthalmic product sample were pipetted
in the transparent well plate support and directly UVC-irradiated
for 2 min, with 254 nm wavelength radiation. After irradiation, 5
µl working volume was taken from these samples and analyzed
according to the Antioxidant Capacity of Lipid soluble substances
(ACL) procedure (Analytik Jena AG).
UV irradiation
It is known from the literature that all bacteria
and viruses tested, including various coronaviruses, respond to UVC
disinfection. An Ultra-Lum Hand Held Portable Ultra Violet Lamp
(Claremont) at 254 nm wavelength (UVC), specific for disinfection,
was used to provide exposure of the samples to UV. Exposure to UV
is commonly applied for disinfection as it generally affects the
DNA structures of microorganisms, causing a photochemical effect on
thymines, producing dimerization, which means that two adjacent
information carriers are improperly linked. This molecular change
means that DNA cannot be used for the essential process of
transcription (metabolism) and replication (cell division) and as a
result, the microorganism becomes harmless and dies. This study
emphasized the impact of UVC radiation exposure on eight marketed
artificial tears solutions, by irradiation of the samples. Despite
the fact that in the natural environment there is no exposure to
UVC radiation, because it is entirely absorbed by the ozone layer
from the atmosphere, under the circumstances of Covid-19 pandemics
we have witnessed new, increased disinfection needs followed by an
enormous development of a very wide range of UVC disinfecting
devices for both public, household and individual use. This reality
led to higher risks of environmental accidental UVC irradiation of
humans and objects, including medicine vials or disposals.
Unintentional irradiation is favored by inadequate beam
orientation, reflective surfaces or extended irradiation
duration.
Total antioxidant capacity
determination
According to the ACL-specific procedure, the total
antioxidative capacity (TEAC) is provided by the superoxide
anion-free radicals being produced by irradiation of a
photosensitizer substance, these being partially eliminated from
the sample by reaction with the antioxidants present in the sample.
By optical excitation of a photosensitizing substance added in
standardized volumes to the sample to be measured, superoxide anion
radicals are being produced. Residual radicals cause the detector
substance luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) to
luminesce, which is then determined in a separate cell by means of
a photomultiplier tube. In the measuring cell, the remaining
radicals cause the detector substance, Luminol, to luminesce and
thereby the antioxidant capacity of the sample is determined
(45-47).
The measuring signal produced by the luminescence is traced over
120 sec and the measuring curves show varied behavior. The
calibration curve is constructed by measuring a series of 0.5, 1.0,
2.0 and 3.0 nmol Trolox (Hoffman-LaRoche's trade name for
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standard
solutions, an antioxidant used in biological applications to reduce
oxidative stress or cell damage. Trolox equivalent antioxidant
activity (TEAC) is a measure of a complex mixture of antioxidant
strength, in units called Trolox equivalents (TE), nmol/sample, and
used as a benchmark for the total antioxidant capacity of
artificial tear mixture compounds considered (48,49).
Apparatus used for TEAC was a photochemiluminometer PHOTOCHEM
(Analytik Jena AG).
Statistical analysis
The Student's t-test emphasized two-sample assuming
unequal variances with P(T<=t) 0.05.
Results
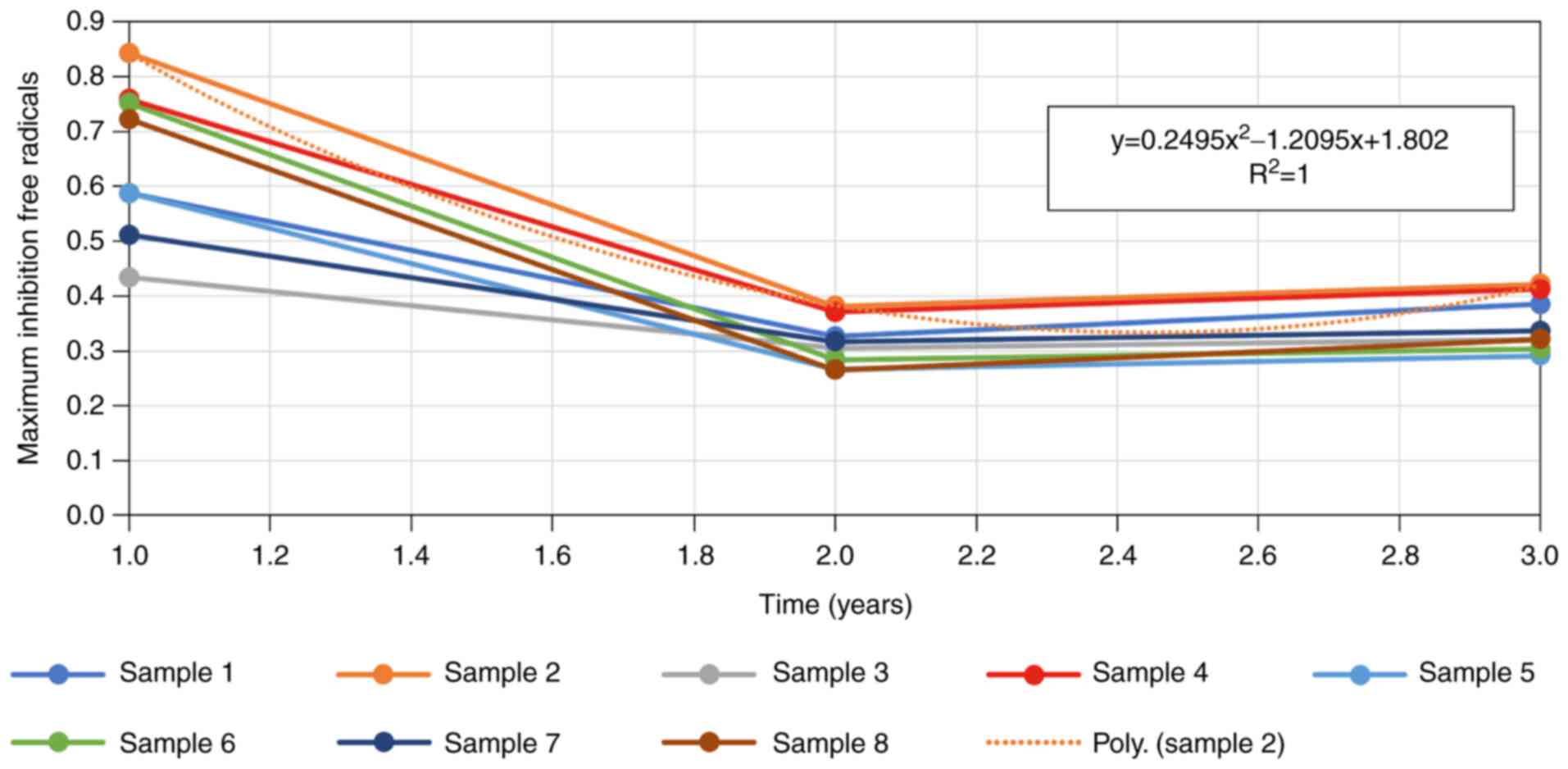
Maximum inhibition-free radicals
The results obtained for the evolution in time,
along two years (the study was performed on 2 years interval of
time, if we consider the first measurement which was done at the
end of the first year of the product fabrication time, while the
product was still covered by the guarantee of the producer, still
sealed, and respecting all the parameter that the manufacturer was
written on its label) since the first exposure to UV, of total
antioxidative capacity (TEAC) of artificial tear samples, before
and after UVC irradiation (Figs. 1
and 2, respectively) for 2 min, at
254 nm wavelengths were assessed. For the observation period, the
ophthalmic product samples were preserved tightly closed, at
ambient temperature and by storage in the refrigerator (+4˚C).
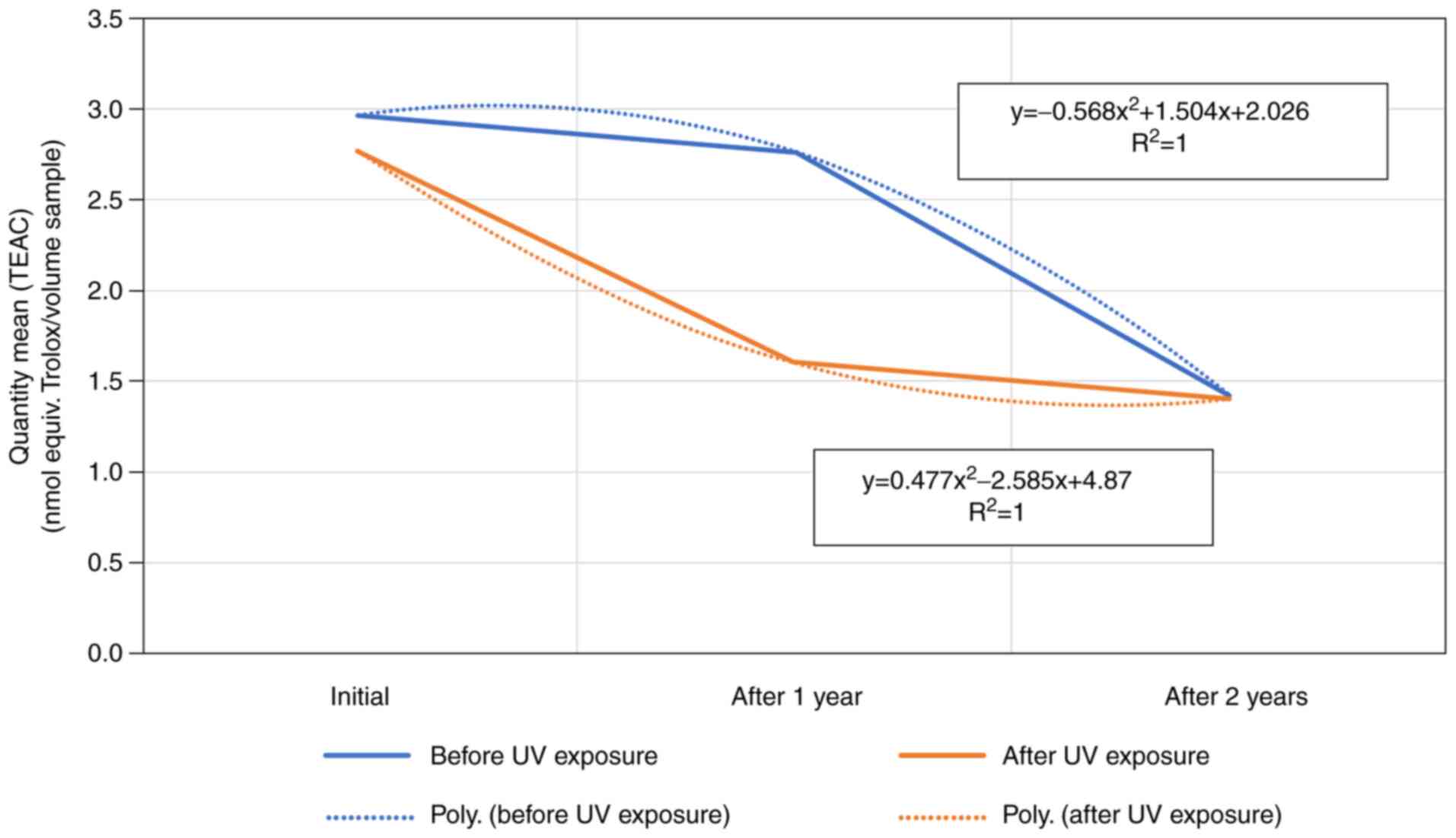
All artificial tear samples showed a reduction in
maximum inhibition-free radicals in the first year following
opening of the vial. The drop was fitting to a polynomial equation
of the 2nd order. Samples 2, 4 and 6 show a more marked decrease
compared to the remaining samples studied.
The data analysis emphasized there were no
statistically significant differences between the properties of the
sample-product before and after exposure to UV-254 nm, either from
the opening of the vial to the final point of the experiment, 3
years later. Although the measured values showed improvement in the
maximum inhibition-free radicals parameter in the first year of the
study, in most cases this was followed by a decrease of this
activity in the second year.
Total antioxidative capacity
values
An important total antioxidative capacity (TEAC)
value modification in time was observed at the working solution
volume of 5 µl. A possible reason for this is that all the samples
taken into consideration have lost the antioxidative capacity
(quantified by TEAC values), even if they were subjected or not to
UV exposure. The manner in which the characteristic was lost varied
from sample to sample and there were differences between samples
exposed to UV radiation and samples without exposure. Changes of
this parameter for each sample in part are shown in Fig. 3, Fig.
4 and Fig. 5.
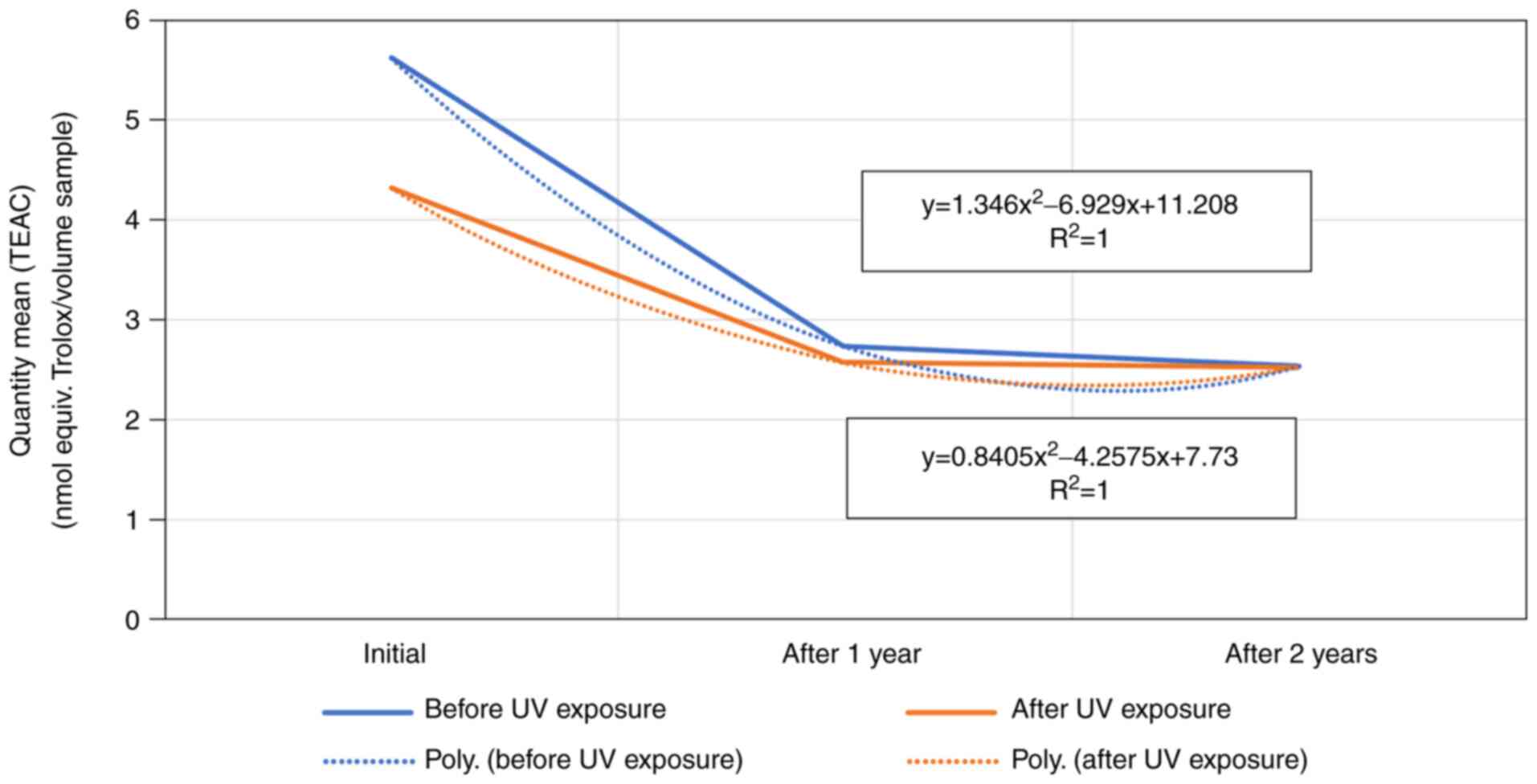
A comparison between the mean quantity (TEAC) of the
product based on hydroxypropyl guar, both before and after exposure
of the samples as well as during the study period, a TEAC decrease
in function (sample 2) correlated to a polynomial function, whereas
the other two product samples showed a linear decrease (Fig. 3).
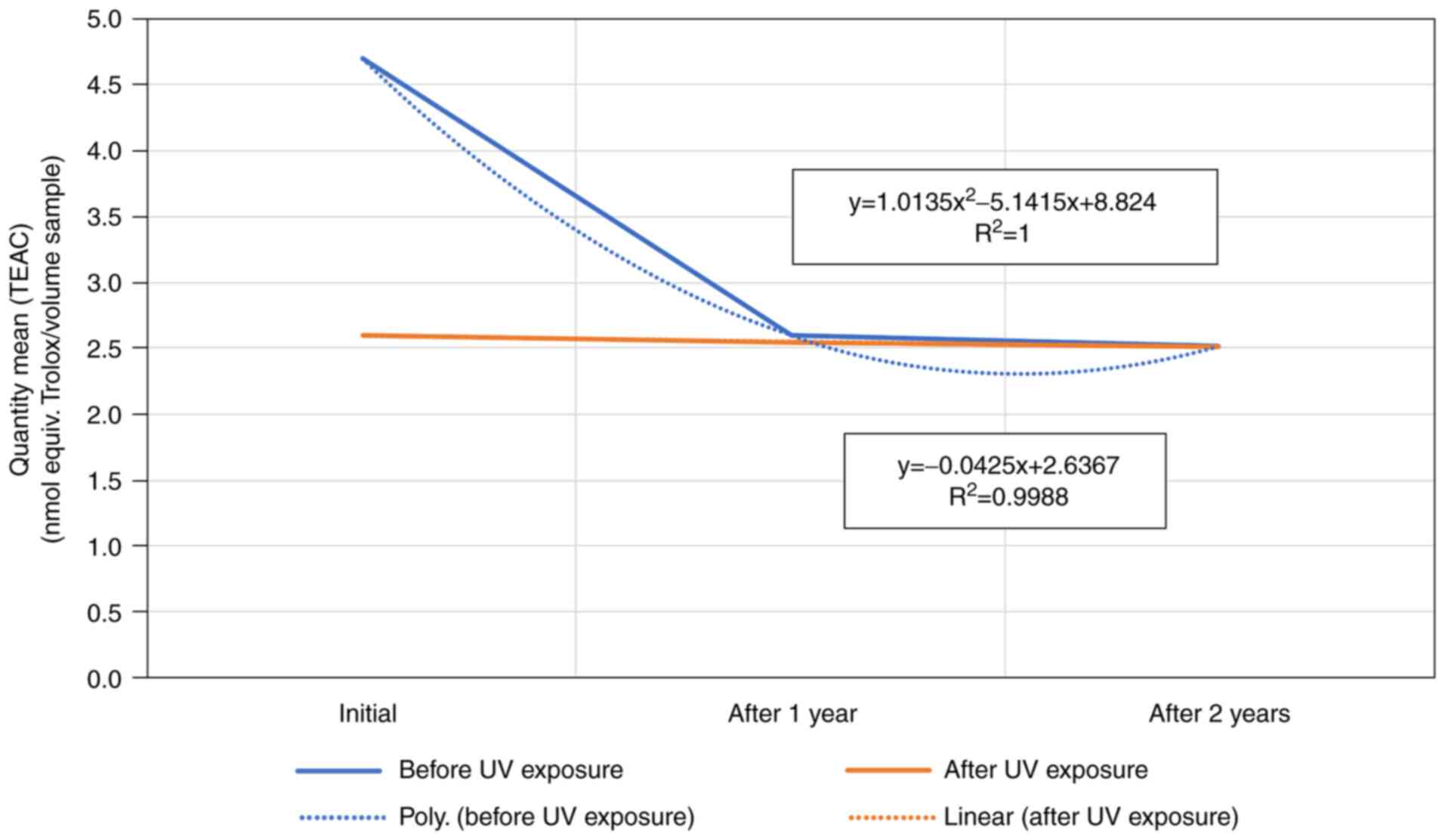
Sample 4 mean quantity (TEAC) expressed as a nmol
equivalent Trolox/volume sample, showed a linear behavior for the
sample exposed to UV-254 nm over the 3-year study. By contrast,
unexposed samples showed a sudden drop immediately after opening of
the vial in the first year, and then remained almost constant,
meaning the maximum decay after the first year was reached
(Fig. 4).
The sample 5 product, based on hydroxypropylmethyl
cellulose, had the most unusual behavior compared with the other
two products. Without exposure to radiation, after opening the
vial, the product favorably maintained the mean quantity (TEAC)
initially, with the decrease manifesting in the second year.
Following exposure to UV-254 nm sample 5 had a sudden polynomial
drop in the first year of the (TEAC), albeit in the end, both
exposed and unexposed samples to UV reached the same mean quantity
(TEAC) at the end of the 3-year study (Fig. 5).
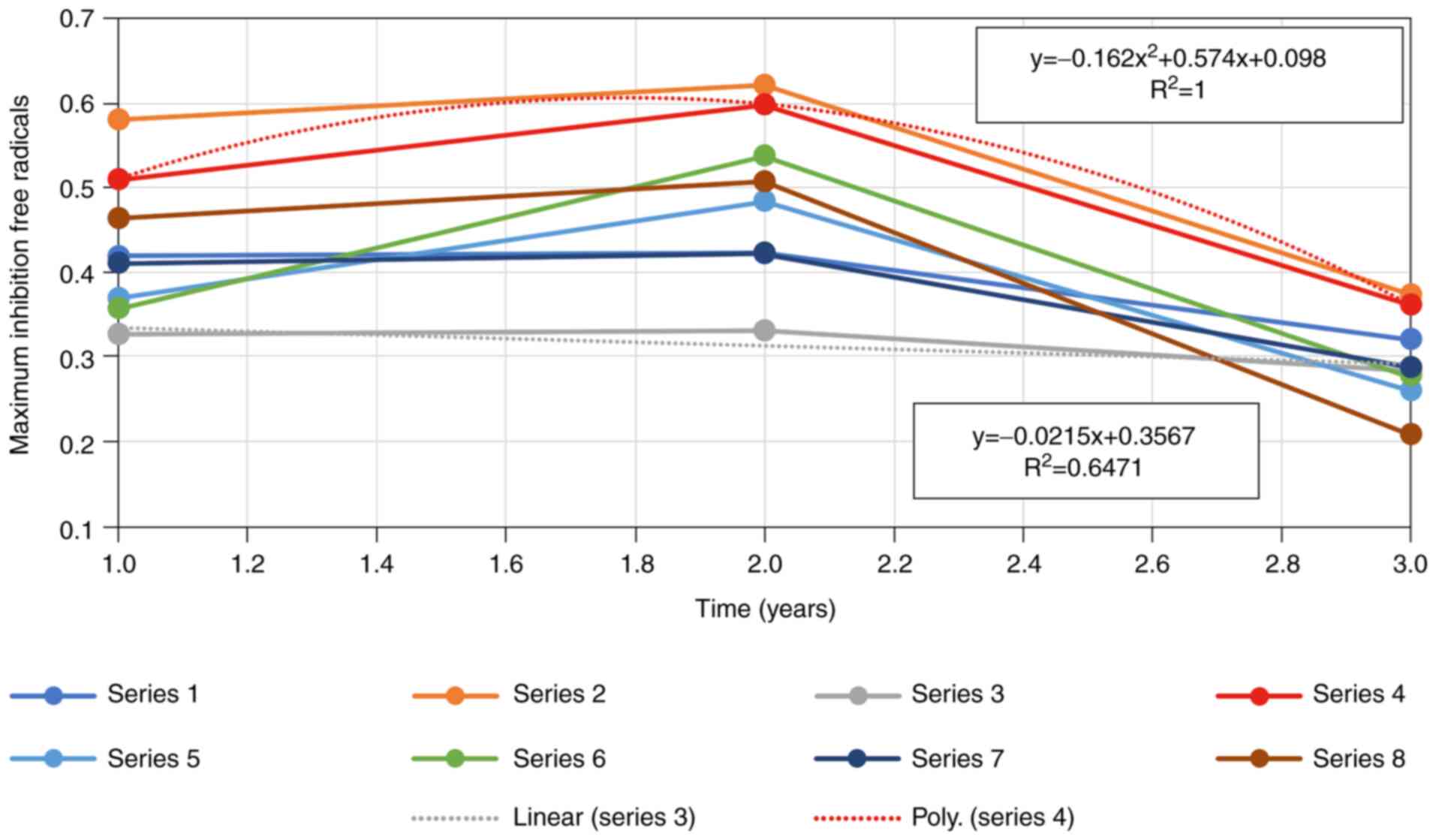
Discussion
The statistical t-test emphasize that two-sample
assuming unequal variances, performed for all samples of the
artificial tears before and after exposure to UVC-254 nm wavelength
radiation along the 3-years study, did not show significant
differences between the samples over the given period. However, a
clear decrease of the maximum inhibition-free radicals value was
evident, indicated by a linear drop starting from the beginning
point, which is the opening of the vial, and ending after 3 years
of conservation at +4˚C. There was one exception to this rule, that
of sample 6. The sample drop in maximum inhibition-free radicals
was of a polynomial type. This means a higher and more rapid rate
of deterioration of the activity with and without exposure to UVC
at a 254 nm wavelength (50-52).
The quantity mean of total antioxidant capacity
(TEAC) (nmol equiv. Trolox/volume sample) had a rather linear drop,
except for samples 2 and 5, where the decrease was of a polynomial
type. Sample 5 tended to maintain an almost constant TEAC for the
unexposed sample for almost one year, prior to its decrease.
Furthermore, after exposure to UVC-254 nm wavelength the TEAC
sample had a rapid decrease for the investigated samples (13,14,53).
Thus, ophthalmic product storage at +4˚C for a long
period with UV irradiation of 254 nm wavelength, decreased TEAC for
all the tested artificial tears.
The comparative determinations of the total
antioxidant capacity activity emphasized the pharmaceutical product
samples 2 and 4, which present at the bottle opening, before
UVC-irradiation, the most increased values of TEAC.
Significant results after the first UVC irradiation
with 254 nm wavelength radiations were registered, TEAC values were
modified, decreasing for all considered dry eye products. Under
these circumstances the highest scores of total antioxidative
capacity suggested sample 2 artificial tears (based on
hydroxypropyl guar), compounds with a favorable stability at first
UVC irradiation.
The highest final TEAC values, for hydroxypropyl
guar and hydroxypropylmethyl cellulose were registered, which
emphasized a favorable stability over the 3-year study period.
The lowest final TEAC value, for lipidic artificial
tears, followed by carboxymethylcellulose tears, was registered,
which appeared to have an increased stability.
Evaluation of the clinical criteria regarding
effectiveness and adherence of the patients, such as TBUT (tear
breakup time, used to evaluate the stability of lacrimal film), DEQ
(Dry eye questionnaire) and OSDI (Ocular surface disease index),
used to evaluate the dryness symptoms, are in progress to determine
whether there are relevant differences between effectiveness of
irradiated and non-irradiated artificial tears.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the first author on reasonable
request.
Authors' contributions
SJ, TNP, MH, MV and BSNP were involved in literature
research and wrote the manuscript. MV supported the statistical
analysis and reviewed the results. SJ, TNP, RC and BSNP conceived,
planned and followed the execution of the experiments. All authors
contributed to manuscript revision, read and approved the final
version. SJ, TNP, MV and BSNP confirmed the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mehra D and Galor A: Digital screen use
and dry eye: A review. Asia Pac J Ophthalmol (Phila). 9:491–497.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Savini G, Prabhawasat P, Kojima T,
Grueterich M, Espana E and Goto E: The challenge of dry eye
diagnosis. Clin Ophthalmol. 2:31–55. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Salmon J: Kanski's Clinical Ophthalmology:
A Systematic Approach. 9th edition. Elsevier, pp190-230, 2019.
|
|
4
|
Nelson JD: Dry eye. Br J Ophthalmol.
81(426)1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Javadi MA and Feizi S: Dry eye syndrome. J
Ophthalmic Vis Res. 6:192–198. 2011.PubMed/NCBI
|
|
6
|
McMonnies CW: Why the symptoms and
objective signs of dry eye disease may not correlate. J Optomol.
14:3–10. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
de Araújo DML and Galera PD: Ocular
lubricants: What is the best choice? Ciência Rural. 46:2055–2063.
2016.
|
|
8
|
Rouen PA and White ML: Dry eye disease:
Prevalence, assessment, and management. Home Health Now. 36:74–83.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Phadatare SP, Momin M, Nighojkar P,
Askarkar S and Singh KK: A comprehensive review on dry eye disease:
Diagnosis, medical management, recent developments, and future
challenges. Adv Pharm J. 2015:1–12. 2015.
|
|
10
|
Aragona P, Giannaccare G, Mencucci R,
Rubino P, Cantera E and Rolando M: Modern approach to the treatment
of dry eye, a complex multifactorial disease: A.P.I.C.A.S.S.O.
board review. Br J Ophthalmol. 105:446–453. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shiraishi A and Sakane Y: Assessment of
dry eye symptoms: Current trends and issues of dry eye
questionnaires in Japan. Invest Ophthalmol Vis Sci. 59:DES23–DES28.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lin H and Yiu SC: Dry eye disease: A
review of diagnostic approaches and treatments. Saudi J Ophthalmol.
28:173–181. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pucker AD, Ng SM and Nichols JJ: Over the
counter (OTC) artificial tear drops for dry eye syndrome. Cochrane
Database Syst Rev. 2(CD009729)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
She Y, Li J, Xiao B, Lu H, Liu H, Simmons
PA, Vehige JG and Chen W: Evaluation of a novel artificial tear in
the prevention and treatment of dry eye in an animal model. J Ocul
Pharmacol Ther. 31:525–530. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jurja S, Negreanu-Pirjol T and
Negreanu-Pirjol BS: Comparative antioxidant activity of some ocular
tissue lubricants from Romanian market. In: Proceedings of the 17th
International Multidisciplinary Scientific GeoConferences-SGEM,
Albena, pp593-600, 2017.
|
|
16
|
Jurja S, Negreanu-Pirjol T,
Negreanu-Pirjol B-S and Roncea FN: UV Radiations influence on the
total antioxidant capacity of artificial tears (ocular lubricant).
In: Proceedings of the 16th International Multidisciplinary
Scientific GeoConferences-SGEM, Sofia, pp395-402, 2016.
|
|
17
|
Jurja S, Negreanu-Pirjol T, Lepadatu AC,
Sirbu R and Negreanu-Pirjol BS: Different artificial tears,
different eyes. How to choose? In: Proceedings of the 15th
International Multidisciplinary Scientific GeoConferences-SGEM,
Albena, pp405-410, 2015.
|
|
18
|
Jurja S, Coman M and Hincu MC: The
ultraviolet influence upon soft eye tissues. Rom J Morphol Embryol.
58:45–52. 2017.PubMed/NCBI
|
|
19
|
Jurja S, Hincu M, Dobrescu MA, Golu AE,
Balasoiu AT and Coman M: Ocular cells and light: Harmony or
conflict? Rom J Morphol Embryol. 55:257–261. 2014.PubMed/NCBI
|
|
20
|
Buckley RJ: Assessment and management of
dry eye disease. Eye. 32:200–203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hantera MM: Trends in dry eye disease
management worldwide. Clin Ophthalmol. 15:165–173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tomlinson A, Madden LC and Simmons PA:
Effectiveness of dry eye therapy under conditions of environmental
stress. Curr Eye Res. 38:229–236. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Simpson TL, Situ P, Jones LW and Fonn D:
Dry eye symptoms assessed by four questionnaires. Optom Vis Sci.
85:692–699. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Willshire C, Buckley RJ and Bron AJ:
Estimating basal tear osmolarity in normal and dry eye subjects.
Cont Lens Anterior Eye. 41:34–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Choy CK, Cho P and Benzie IF: Antioxidant
content and ultraviolet absorption characteristics of human tears.
Optom Vis Sci. 88:507–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rieger G: Anti-oxidative capacity of
various artificial tear preparations. Graefes Arch Clin Exp
Ophthalmol. 239:222–226. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abelson M, Ousler G and Stein L: Oxidative
stress reduction for dry eye. Antioxidants' effects on harmful
reactive oxygen species may be helpful in the fight against dry
eye. Rev Ophthalmol. 33:96–115. 2016.
|
|
28
|
Daxer A, Blumthaler M, Schreder J and Ettl
A: Effectiveness of eye drops protective against ultraviolet
radiation. Ophthalmic Res. 30:286–290. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ribeiro MVMR, Barbosa FT, Ribeiro LEF,
Sousa-Rodrigues CF and Ribeiro EAN: Effectiveness of using
preservative-free artificial tears versus preserved lubricants for
the treatment of dry eyes: A systematic review. Arq Bras Oftalmol.
82:436–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seen S and Tong L: Dry eye disease and
oxidative stress. Acta Ophthalmol. 96:e412–e420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jurja S, Pirjol TN, Costea DO and Pirjol
BSN: Correlation between effectiveness and antioxidant activity of
some anti cataract eye drops. Rev Chim (Bucharest). 67:1004–1007.
2016.
|
|
32
|
Jurja S, Negreanu-Pirjol T, Roncea F,
Negreanu-Pirjol BS, Sîrbu R, Lepadatu AC and Miresan H: Correlation
between antioxidant activity of vegetal supplement and age related
maculopathy. In: Proceedings of the 14th International
Multidisciplinary Scientific GeoConferences, Surveying Geology
& mining Ecology Management-SGEM, Albena, pp321-328, 2014.
|
|
33
|
Jurja S, Negreanu-Pirjol T, Roncea F,
Negreanu-Pirjol BS, Paraschiv G and Miresan H: Aging effect on
ocular tissues of antioxidant vegetal supplements from Romanian
market. In: Proceedings of the 14th International Multidisciplinary
Scientific GeoConferences ‘Surveying Geology & mining Ecology
Management-SGEM, Albena, pp225-230, 2014.
|
|
34
|
Manjunath M, Anjali Gowda DV, Kumar P,
Srivastava A, Osmani RA, Shinde C and S Hatna: Guar Gum and its
pharmaceutical and biomedical applications. Adv Sci Eng Med.
8:1–14. 2016.
|
|
35
|
Bouyer E, Mekhloufi G, Rosilio V,
Grossiord JL and Agnely F: Proteins, polysaccharides, and their
complexes used as stabilizers for emulsions: Alternatives to
synthetic surfactants in the pharmaceutical field? Int J Pharm.
436:359–378. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu H, Du S, Lu Y, Li Y and Wang D: The
application of biomedical polymer material hydroxy propyl methyl
cellulose (HPMC) in pharmaceutical preparations. J Chem Pharm Res.
6:155–160. 2014.
|
|
37
|
Al-Tabakha MM: HPMC capsules: Current
status and future prospects. J Pharm Pharm Sci. 13:428–442.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Li CL, Martini LG, Ford JL and Roberts M:
The use of hypromellose in oral drug delivery. J Pharm Pharmacol.
57:533–546. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Garrett Q, Simmons PA, Xu S, Vehige J,
Zhao Z, Ehrmann K and Willcox M: Carboxymethylcellulose binds to
human corneal epithelial cells and is a modulator of corneal
epithelial wound healing. Invest Ophthalmol Vis Sci. 48:1559–1567.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Karabinos JV and Hindert M:
Carboxymethylcellulose. Adv Carbohydr Chem. 9:285–302.
1954.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bruix A, Adan A and Casaroli-Marano RP:
Efficacy of sodium carboxymethylcellulose in the treatment of dry
eye syndrome. Arch Soc Esp Oftalmol. 81:85–92. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yao K, Bao Y, Ye J, Lu Y, Bi H, Tang X,
Zhao Y, Zhang J and Yang J: Efficacy of 1% carboxymethylcellulose
sodium for treating dry eye after phacoemulsification: Results from
a multicenter, open-label, randomized, controlled study. BMC
Ophthalmol. 15(28)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cohen S, Martin A and Sall K: Evaluation
of clinical outcomes in patients with dry eye disease using
lubricant eye drops containing polyethylene glycol or
carboxymethylcellulose. Clin Ophthalmol. 8:157–164. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Labetoulle M, Chiambaretta F, Shirlaw A,
Leaback R and Baudouin C: Osmoprotectants, Carboxymethylcellulose
and hyaluronic acid multi-ingredient eye drop: A randomised
controlled trial in moderate to severe dry eye. Eye (Lond).
31:1409–1416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Popov I and Lewin G: Antioxidative
homeostasis: Characterization by means of chemiluminescent
technique. Methods Enzymol. 300:437–456. 1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Popov IN and Lewin G:
Photochemiluminescent detection of antiradical activity; IV:
Testing of lipid-soluble antioxidants. J Biochem Biophys Methods.
31:1–8. 1996.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Popov IN and Lewin G: Photosensitized
Chemiluminescence. Labo. 8:498–527. 1997.
|
|
48
|
Dinardo JC, Lewis JA, Neudecker BA and
Maibach HI: Antioxidants compared in a new protocol to measure
protective capacity against oxidative stress-part II1. J
Am Acad Dermatol. 50 (Suppl)(P30)2004.
|
|
49
|
Pegg RB, Amarowicz R, Naczk M and Shahidi
F: Photochem for determination of antioxidant capacity of plant
extract,. 2007, ACS Symposium Series 956:140-158, Publisher:
American Chemical Society, In book: Antioxidant Measurement and
Applications, pp.140-158, DOI:10.1021/bk-2007-0956.ch011.
|
|
50
|
Bartollino S, Palazzo M, Semeraro F,
Parolini B, Caruso C, Merolla F, Guerra G and Costagliola C:
Effects of an antioxidant protective topical formulation on retinal
tissue of UV-exposed rabbits. Int Ophthalmol. 40:925–933.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Palazzo M, Vizzarri F, Ondruška L, Rinaldi
M, Pacente L, Guerra G, Merolla F, Caruso C and Costagliola C:
Corneal UV protective effects of a topical antioxidant formulation:
A pilot study on in vivo rabbits. Int J Mol Sci.
21(5426)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ivanov IV, Mappes T, Schaupp P, Lappe C
and Wahl S: Ultraviolet radiation oxidative stress affects eye
health. J Biophotonics. 11(e201700377)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cejka C and Cejkova J: Oxidative stress to
the cornea, changes in corneal optical properties, and advances in
treatment of corneal oxidative injuries. Oxid Med Cell Longev.
2015(591530)2015.PubMed/NCBI View Article : Google Scholar
|