Introduction
Chronic heart failure (CHF), a major public health
concern worldwide, is a complex clinical syndrome, in which the
heart cannot pump enough blood to meet the body's requirements,
resulting in a series of clinical symptoms, including dyspnea,
weakness and lower limb swelling (1). The ASIAN-HF study reported that the
onset of heart failure (HF) is closely related to hypertension,
anemia, diabetes, coronary artery disease (CAD), atrial
fibrillation and obesity, contributing to a high morbidity
(2). In China. the overall
prevalence of HF has increased by 44% during the past 15 years in
patients aged >35 years (3).
Despite great advances in the treatment of CHF (4), further research is required to
alleviate its symptoms and decrease the morbidity, mortality and
re-hospitalization rates in CHF patients (5). Therefore, the development of novel
therapeutic approaches targeting CHF-associated pathways is of
great significance, to treat CHF more effectively.
CHF is a progressive disease, in which long-term
ischemia and hypoxia may lead to myocardial cell necrosis and
ventricular remodeling (6,7). Therefore, effective protection of
cardiomyocytes can deaccelerate the progression of CHF (8). Mitochondria may not only synthesize a
large amount of adenosine triphosphate (ATP) for myocardial cells,
but also participate in metabolism, signaling, redox balance and
ion homeostasis (9,10). Damaged mitochondria produce
reactive oxygen species (ROS), leading to oxidative stress and
cardiomyocyte death (11). The
homeostasis of myocardial mitochondria depends on mitochondrial
dynamics and autophagy. FUN14 domain-containing protein 1 (FUNDC1)
is an integral mitochondrial outer-membrane protein, which mediates
the formation of mitochondria-associated endoplasmic reticulum
membranes (12). In humans,
mitochondrial fusion is mediated by the dynamin-related GTPases
mitofusin 1 and mitofusin 2 (MFN1 and MFN2) and optic atrophy 1
(OPA1), which mediate outer mitochondrial membrane (OMM) and inter
mitochondrial membrane (IMM) fusion, respectively (13). However, under myocardial ischemic
and hypoxic conditions, FUNDC1 interacts with dynamin-related
protein 1 (DRP1), mitochondrial fission 1 protein (FIS1) and LC3 to
induce excessive mitochondrial fission and autophagy, leading to
cell death (14,15). In addition, de-phosphorylation of
phosphoglycerate mutase family member 5 (PGAM5) and phosphorylation
of Unc-51 Like Autophagy Activating Kinase 1 (ULK1) may activate
FUNDC1 to interact with LC3 for autophagosome recruitment (16,17).
Therefore, FUNDC1 plays an important role in myocardial
mitochondrial dynamics and autophagy.
Moxibustion (MOX), an important component of
traditional Chinese medicine (TCM), has been widely and effectively
used in the treatment of chronic diseases. Previous studies have
shown that MOX possesses therapeutic effects in coronary heart
disease (18,19). Our research group previously
explored its protective effects on the heart and demonstrated that
MOX can downregulate inflammatory factors while upregulating
anti-inflammatory factors (20).
MOX can regulate autophagy by inducing the activation of the
mechanistic target of rapamycin (mTOR) signaling pathway (21,22).
Hence, the present study aimed to investigate whether MOX could
mediate mitochondrial dynamics and autophagy to alleviate CHF in a
doxorubicin (DOX)-induced animal model.
Materials and methods
Laboratory animals
A total of 100 male Sprague-Dawley rats (aged, 8
weeks; body weight, 200-250 g) were obtained from the Anhui
University of Chinese Medicine [Hefei, China; animal license
number: SCXK (Shandong) 2019-003]. They were housed in accordance
with animal welfare regulations, under specific-pathogen-free
conditions at 25˚C, humidity of 50% and a 12-h light/dark cycle.
The rats had free access to food and water and were fasted 12 h
before the operation. All animal experiments were approved by the
Ethics Committee of the Anhui University of Chinese Medicine
(approval no. AHUCM-rats-2020026). The present study followed the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (23).
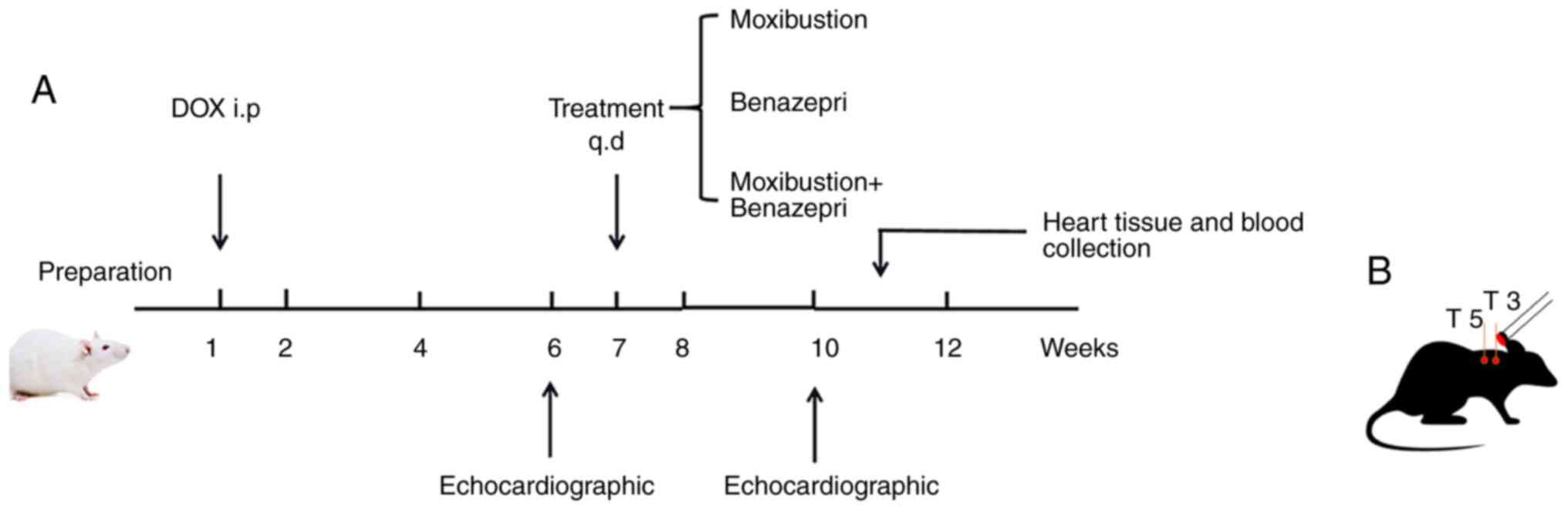
Animal group and CHF modeling
The 100 rats were assigned into Control group (C,
n=20), Doxorubicin group (DOX, n=20), Moxibustion group (MOX,
n=20), Benazepril (BEN, n=20) and Moxibustion + Benazepril group
(MOX + BEN, n=20), using a random number table.
The rats in the Control group were fed normally. CHF
models were established according to the method reported by
Leontyev et al (24) in all
groups except the control group. Doxorubicin injection solution (1
mg/ml) was prepared in 0.9% saline and 80 rats were injected with
doxorubicin at 2.5 mg/kg intraperitoneally once a week for 6
consecutive weeks, with a cumulative injection volume of 15 mg/kg.
Following the last injection, the successful model construction was
judged by echocardiography. After one week of adaptive feeding, the
intervention treatment was started after the 7th week.
Rats in the C group and DOX group were given the
equal dose of normal saline once a day for three consecutive weeks.
Rats in the MOX group were placed on a platform and underwent
moxibustion at Feishu (BL13, 7 mm below the third thoracic spinous
process on both sides) and Xinshu (BL15, 7 mm below the fifth
thoracic spinous process bilaterally) points using moxibustion
sticks (5x120 mm; Wolong Traditional Chinese Medicine Moxibustion
Factory) to make the temperature of the acupressure points up to
44±1˚C (25), once a day for three
consecutive weeks. Rats in the BEN group were administered with
0.86 mg/kg benazepril (Novartis International AG) by gavage once
per day for 3 consecutive weeks (26). Rats in the MOX + BEN group received
combined treatment with moxibustion and benazepril (0.86 mg/kg)
once a day for three consecutive weeks. The study flowchart is
shown in Fig. 1.
During the experimental period, the general
condition of the rats, including diet, coat color, mental status,
activity and respiratory function of rats were observed every day
and body weight were measured every 2 days. When rats exhibited
extreme weakness, anorexia, weight loss (≥20%), multiple skin sores
that would not heal, respiratory disorders, cyanosis and continuous
poor sense of balance, sacrifice by overdose of anesthesia (150
mg/kg sodium pentobarbital intraperitoneal injection) was
immediately performed. There were no deaths in the C group. A total
of seven rats died in the DOX group, four rats died in the MOX
group, three rats died in the BEN group and two rats died in the
MOX + BEN group. Specifically, nine rats reached the humane
endpoints by showing clear anorexia and depression, weight loss
(≥20%) and extreme weakness. Three rats had to be sacrificed as
they developed dyspnea and were unable to stand. Two rats were
immediately sacrificed with severe infection and non-healing ulcers
on the abdomen. Two rats were found dead when fed early morning. It
was speculated that the death may have been caused by fatal
arrhythmias. Of the 100 rats, 84 rats completed the study and the
lethal rate of doxorubicin was 20% throughout the experiment
(24).
At the end of the experiment, surviving experimental
rats were intraperitoneally injected with 3% sodium pentobarbital
at a dose of 30 mg/kg and blood was collected from the abdominal
aorta. Then rats were euthanized (cervical dislocation) and the
heart tissue was removed after the rat's vital signs
disappeared.
Echocardiographic assessment
Intraperitoneal injection of 3% pentobarbital sodium
was performed for anesthesia at a dose of 30 mg/kg and left
ventricular cardiac function was assessed by echocardiography in
the two-dimensional B-mode and M-mode (Siemens Acuson Oxana 3;
Siemens AG), with left ventricular internal diameter in systole
(LVIDS), ejection fraction (EF) and fractional shortening (FS)
tested for at least three nonstop cardiac cycles. Transthoracic
echocardiography was performed immediately at 6 (to assess molding
success) and 10 (to assess treatment effect) weeks.
Pathological staining
Myocardial tissue samples were fixed with 4%
paraformaldehyde for 72 h at 4˚C and dehydrated using alcohol
gradient (70, 80, 90 and 100%) alcohol at room temperature. The
sections were infiltrated in paraffin for 30 min at 65˚C and
embedded in paraffin wax. The sections were cut into 5-µm slices
using a Leica Biosystems RM2245 microtome (Leica Microsystems
GmbH). After dewaxing with dimethyl benzene and rehydration with
descending alcohol series. Paraffin-embedded tissue sections were
stained with hematoxylin and eosin (H&E) and Masson's trichrome
to assess histopathological features and cardiac fibrosis,
respectively.
For the H&E staining, sections were stained with
hematoxylin at room temperature for 5 min, 1% HCl-alcohol
differentiation for 5-30 sec, staining with an eosin staining
solution for 5 min at room temperature. The sections were
dehydrated with 95% alcohol for 5 min at room temperature then were
cleared with xylene for 5 min and finally sealed with neutral
balsam. The tissue sections were subsequently visualized under a
light microscope at a magnification of x400 (Leica Microsystems
GmbH).
For Masson's trichrome staining, the sections were
stained with Wiegert iron hematoxylin solution (cat. no. G1340;
Beijing Solarbio Science and Technology Co., Ltd.) for 5 min at
room temperature, then were treated with Ponceau fuchsin acid
solution for 5-10 min. After washing in distilled water, the
sections were treated with 1% aqueous solution of phosphomolybdic
acid for 1-3 min. Without washing with water, the sections were
treated with aniline blue for 3-6 min. Finally, the sections were
treated with 1% glacial acetic acid for 1 min, dehydrated with
ethanol and were cleared with xylene for 5 min and sealed with
neutral balsam. Light microscopy was subsequently conducted at a
magnification of x400 (Nikon Corporation). The severity of
myocardial fibrosis was analyzed from tissue sections stained with
Masson's trichrome with the Image-Pro Plus 6.0 software (Media
Cybernetics Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples (1.5 ml) were collected from the
abdominal aorta, centrifuged at 1,500 x g at 4˚C for 15 min and
examined with an ELISA kit according to the manufacturer's
instructions (cat. no. E-EL-R0126c; Wuhan Elabscience Bio-Tech Co.,
Ltd.) to detect serum BNP levels.
Measurement of ATP content
Fresh myocardial tissue samples were obtained from
the left ventricle and 20 mg was added to 100 µl lysis buffer,
followed by thorough homogenization. After centrifugation at 11,290
x g at 4˚C for 5 min, the supernatant was collected. ATP was
detected with a specific kit (cat. no. S0026; Beyotime
Biotechnology) as directed by the manufacturer. Relative light
intensity (RLU) was assessed on a plate reader.
Transmission electron microscopy
(TEM)
Fresh myocardial tissue samples (1 mm3)
were fixed with 2% glutaraldehyde at 4˚C for at least 2 h, washed
with PBS 3 times (10 min each). This was followed by another
fixation with 1% osmium tetroxide at 20˚C for 2 h and 3 washes with
PBS. The tissue samples were dehydrated with graded ethanol (50,
70, 80 and 90%) for 15 min each and dehydrated with 100% acetate 3
times for 20 min each at room temperature. The tissue samples were
embedded using epoxy resin at room temperature overnight and sliced
into ultrathin sections (60-70 nm) with an ultramicrotome. The
ultrathin sections were stained with 2% uranium acetate saturated
alcohol solution and lead citrate 15 min each at room temperature.
Stained sections were visualized using HT7800 transmission electron
microscope (magnification x2,500 and x7,000) and autophagosomes
were counted with the Gatan Digital Micrograph software (v 3.5;
Gatan, Inc.).
Western blotting
Myocardial tissue samples were obtained from the
left ventricle and proteins were extracted with chilled
radio-immunoprecipitation assay (RIPA) buffer (Beyotime Institute
of Biotechnology). Following homogenization, the tissue was lysed
and centrifuged at 11,290 x g at 4˚C for 10 min and the resulting
supernatant was collected. Total protein levels were determined
with a BCA kit (Beijing Solarbio Science & Technology Co.,
Ltd.). To detect the expression levels of FUNDC1, phosphorylated
(p-) FUNDC1, PGAM5, ULK1, OPA1, DRP1, FIS1, LC3I, LC3II and p62 in
cardiac tissue specimens, 50 µg of proteins were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
followed by transfer onto polyvinylidene fluoride (PVDF) membranes.
The membranes were incubated with anti-FUNDC1 (1:2,000; cat. no.
49240; CST Biological Reagents Co., Ltd.), anti-p-FUNDC1 (1:2,000;
cat. no. AF0001; Affinity Biosciences), anti-PGAM5 (1:2,000; cat.
no. ab244218; Abcam), anti-ULK1 (1:5,000; cat. no. 8054; Abcam),
anti-OPA1 (1:2,000; cat. no. ab157457; Abcam), anti-DRP1 (1:2,000;
cat. no. ab184247; Abcam), anti-FIS1 (1:2,000; cat. no. ab156865;
Abcam), anti-LC3 II (1:2,000; cat. no. 43566; CST Biological
Reagents Co., Ltd.), anti-LC3 I (1:2,000; cat. no. 12741; CST
Biological Reagents Co., Ltd.), anti-P62 (1:2,000; cat. no.
ab109012; Abcam) and anti-glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; 1:2,000; cat. no. K106389P; Beijing Solarbio Science &
Technology Co., Ltd.) primary antibodies for 1 h at room
temperature in a dark room. Then, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:3,000; cat. no. SE134; Beijing Solarbio Science & Technology
Co., Ltd.) at room temperature for 1-2 h and exposed to an ECL
Substrate kit (Beijing Solarbio Science & Technology Co.,
Ltd.). The protein levels were normalized to those of β-actin and
semi-quantification was performed with the ImageJ 1.8 software
(National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
ULK1, PGAM5 and FUNDC1 mRNA levels in heart tissue
samples were detected by RT-qPCR. According to the manufacturer's
protocol, total RNA from each sample was extracted with Trizol
reagent (cat. no. B511321; Shanghai Sangon Biotech Co., Ltd.). The
EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit
(cat. no. AE311-02; Beijing Transgen Biotech Co., Ltd.) was used
for reversing transcriptase at the conditions of 42˚C for 15 min
and cRNA was synthesized. A TransStart Green qPCR SuperMix kit
(cat. no. AQ131-01; Beijing Transgen Biotech Co., Ltd.) was used to
perform qPCR. The reaction volume was 20 µl (2X Top Green EX-Taq
Mix 10 µl, QF 0.5 µl, QR 0.5 µl, Template cDNA 2 µl, RNase free
dH2O 7 µl). The subsequent PCR amplification was carried
out for 2 min at 95˚C, followed by 40 cycles of 15 sec at 95˚C, 20
sec at 57˚C and 30 sec at 72˚C, with final extension at 95˚C for 15
sec. Each reaction was repeated in triplicate. The relative
expression levels were calculated by the 2-ΔΔCq method
and GAPDH was used as an internal control (27). The primers used for RT-qPCR are
shown in Table I.
 | Table IThe sequences of primers used for
reverse transcription-quantitative PCR. |
Table I
The sequences of primers used for
reverse transcription-quantitative PCR.
| Gene | Primers
(5'→3') |
|---|
| ULK1 |
GGCTCTATTGCAGCGTAACC |
| |
GCACAGGTGGGGATTTCTTGA |
| PGAM5 |
AGACTTGCTACGGGAAGGTG |
| |
GCATCAGCTCGGTGGATGTA |
| FUNDC1 |
TGTGATATCCAGCGGCTTCG |
| |
TGCTGCCACAGTCTTCCTCT |
| GAPDH |
GGAAAGCTGTGGCGTGAT |
| |
TCCACAACGGATACATTGGG |
Statistical analysis
The data were statistically analyzed with the SPSS
24.0 software (IBM Corp.) and expressed as mean ± standard
deviation (SD). Comparisons in multiple groups were performed by
one-way analysis of variance (ANOVA) and Dunnett's test was
utilized for group pair comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of MOX on cardiac function and
morphological alterations of the myocardium in the rat model of
CHF
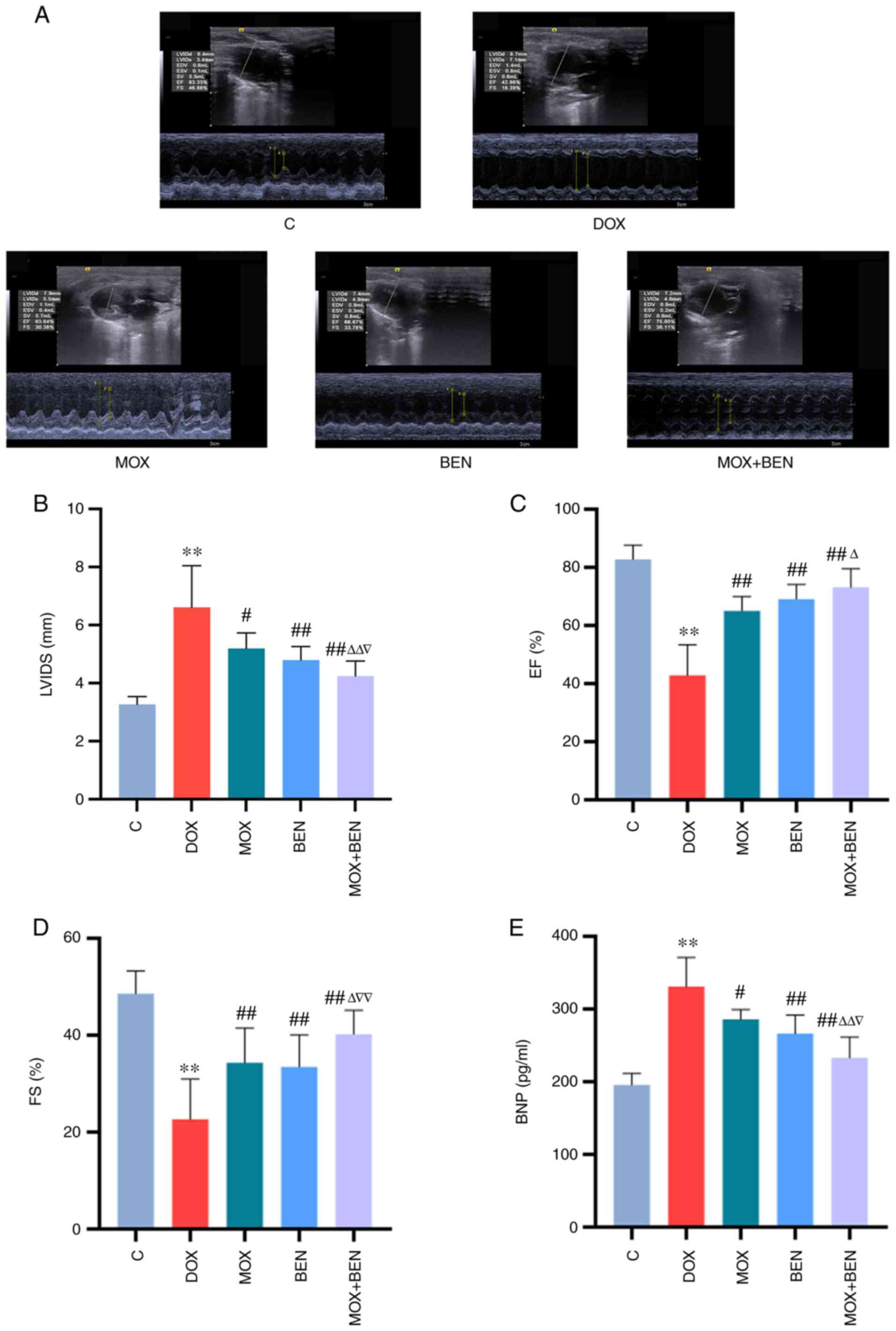
After 10 weeks, echocardiography revealed reduced EF
and FS in the DOX group compared with the C group (P<0.01,
Fig. 2). LVIDS values were
elevated in the DOX group (P<0.01, Fig. 2B), indicating alterations in
cardiac structure and function. Serum BNP levels were significantly
elevated in the DOX group compared with the C group (P<0.01,
Fig. 2E). After 3 weeks of
treatment, compared with the DOX group, EF and FS were
significantly increased in the MOX group, whereas serum BNP levels
were significantly decreased (EF and FS, P<0.01; BNP, P<0.05;
Fig. 2C-E). BEN and MOX had
similar effects and MOX + BEN exerted more pronounced effects
compared with each single therapy (Fig. 2).
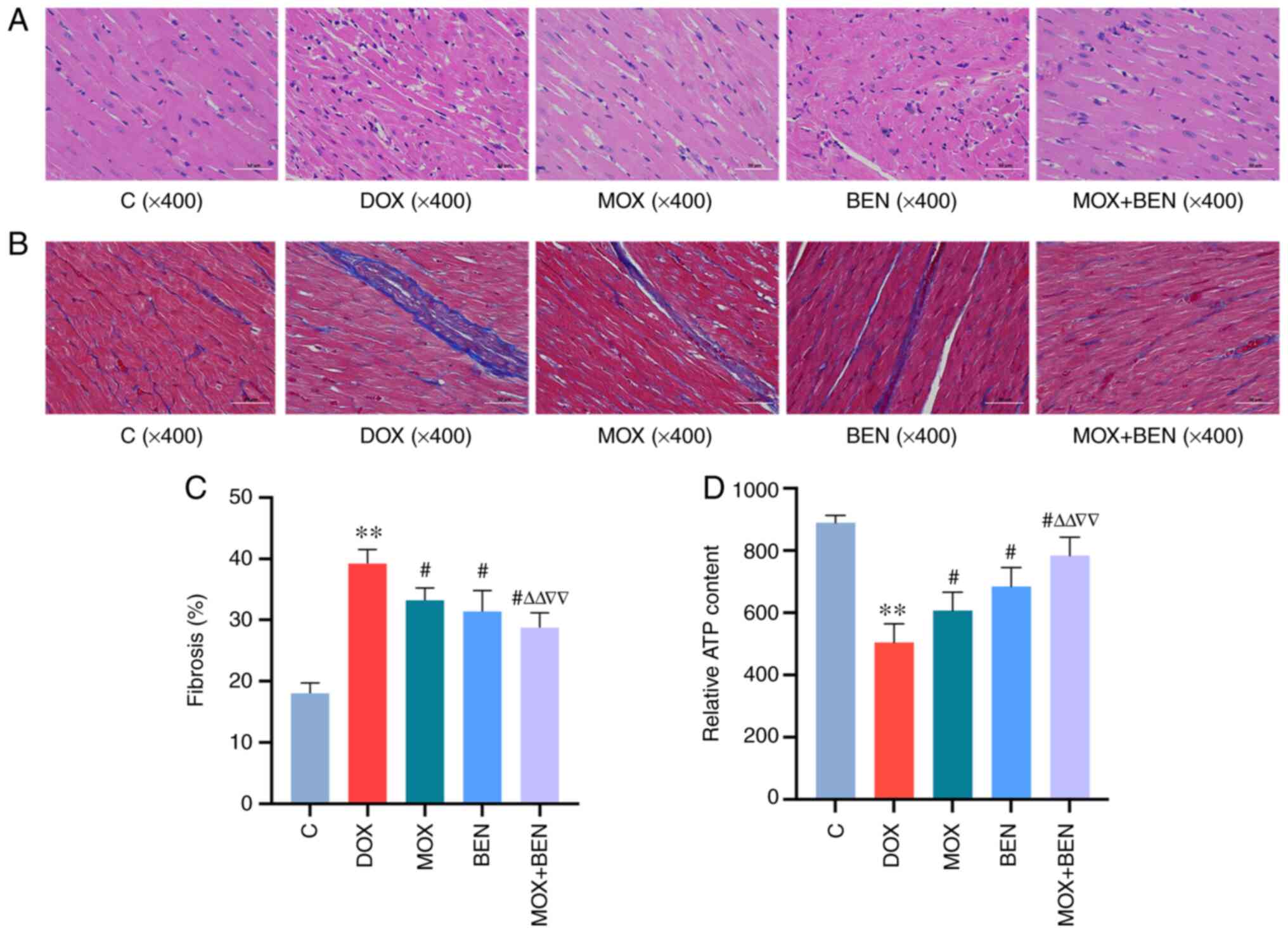
MOX ameliorates myocardial dysfunction
and fibrosis
H&E staining showed that cardiomyocytes in the
left ventricle were arranged in an orderly manner in the C group,
but loosely arranged in the DOX group. After 3 weeks of treatment,
these pathological changes were reversed in the MOX, BEN and MOX +
BEN groups compared with the C group (Fig. 3A). Masson's trichrome staining
showed a greater severity of cardiac fibrosis in the DOX group than
in the C group (P<0.01, Fig.
3B). After 3 weeks of treatment, compared with the DOX group,
collagen volume fractions were decreased in the MOX, BEN and MOX +
BEN groups (Fig. 3C). In addition,
MOX + BEN was more effective in improving vacuolar degeneration of
cardiomyocytes and myocardial fibrosis.
Moxibustion improves myocardial ATP
content
ATP levels were significantly reduced in the DOX
group compared with the C group (P<0.01, Fig. 3D). After 3 weeks of treatment,
compared with the DOX group, ATP levels in the MOX group were
significantly increased (P<0.01, Fig. 3D). BEN and MOX had similar effects
and MOX + BEN had more pronounced effects compared with each single
therapy (Fig. 3D).
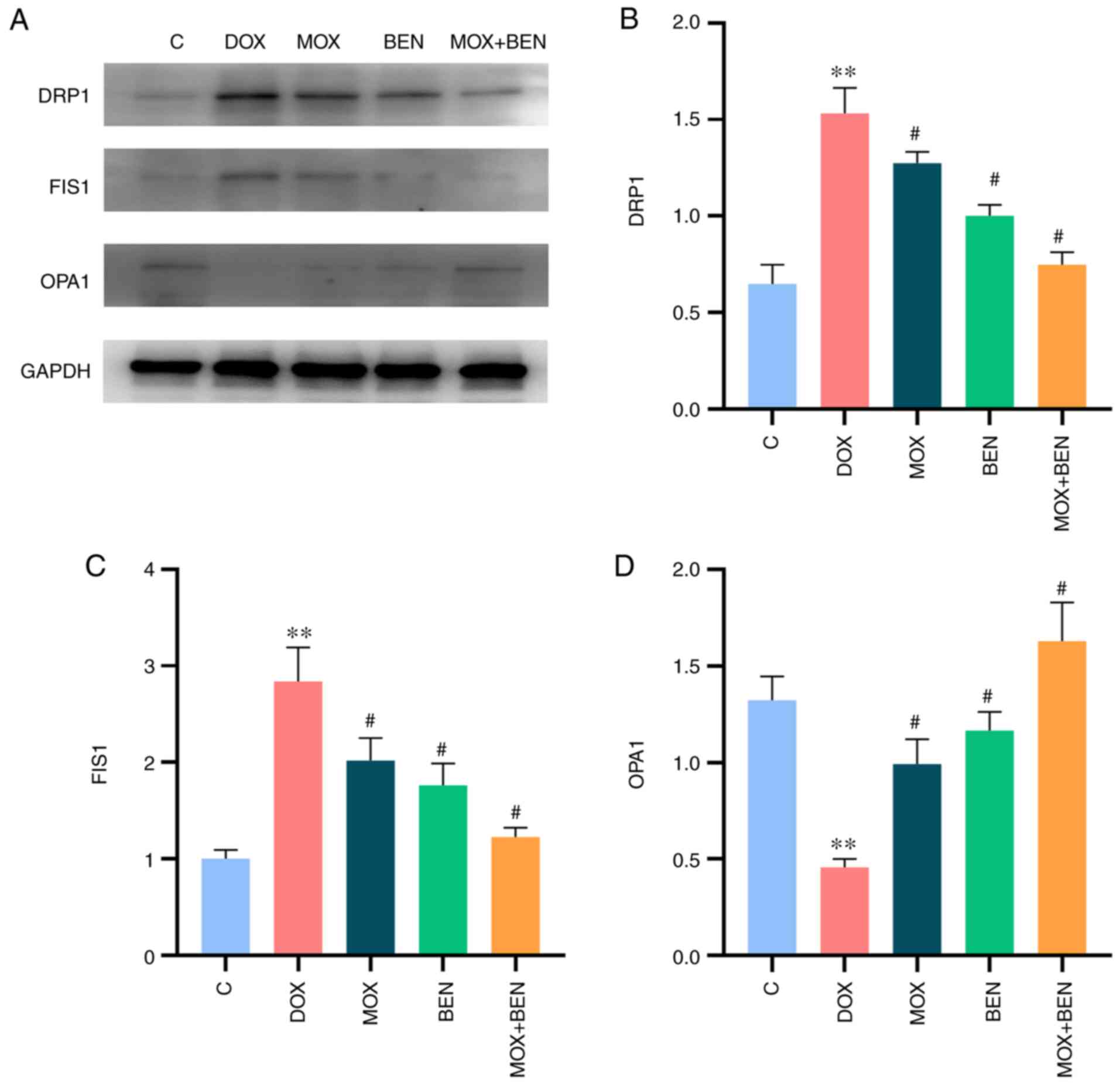
Effects of MOX on mitochondrial
dynamics in the rat model of CHF
Western blotting showed that OPA1 expression levels
were significantly decreased in the DOX group compared with the C
group (P<0.01, Fig. 4), while
DRP1 and FIS1 levels were significantly increased (P<0.01,
Fig. 4). These results indicated
reduced mitochondrial fusion and induced fission in the rat model
of CHF. Compared with the DOX group, the MOX, BEN and MOX + BEN
groups showed upregulated OPA1 (P<0.01, Fig. 4) and downregulated DRP1 and FIS1
(P<0.01, Fig. 4). In addition,
a greater regulation of mitochondrial dynamics was detected in the
MOX + BEN group compared with the other groups (Fig. 4).
Effects of MOX on autophagy in the rat
model of CHF
Mitochondria and autophagosomes were observed by
TEM. In the control group, mitochondrial structure was normal and
very few autophagosomes were observed. In the DOX group,
mitochondria were sparsely swollen and vacuolated, accompanied by
lipid deposition and the number of autophagosomes was significantly
increased. The MOX and BEN groups also had mitochondrial damage,
but the number of autophagosomes was decreased compared with DOX
group. The structure and arrangement of mitochondria in the MOX +
BEN group were relatively normal and the number of autophagosomes
was significantly reduced (Fig.
5). Western blotting revealed elevated LC3II/LC3I ratio in the
DOX group compared with the C group (P<0.01, Fig. 5C and E), while p62 was markedly downregulated
(P<0.01, Fig. 5C and F). Meanwhile LC3II/LC3I ratio were
reduced in the MOX, BEN and MOX + BEN groups compared with the DOX
group, whereas the p62 was upregulated (Fig. 5C and E). Taken together, MOX played a
protective role in DOX-induced CHF in rats by inhibiting excessive
mitochondrial autophagy.
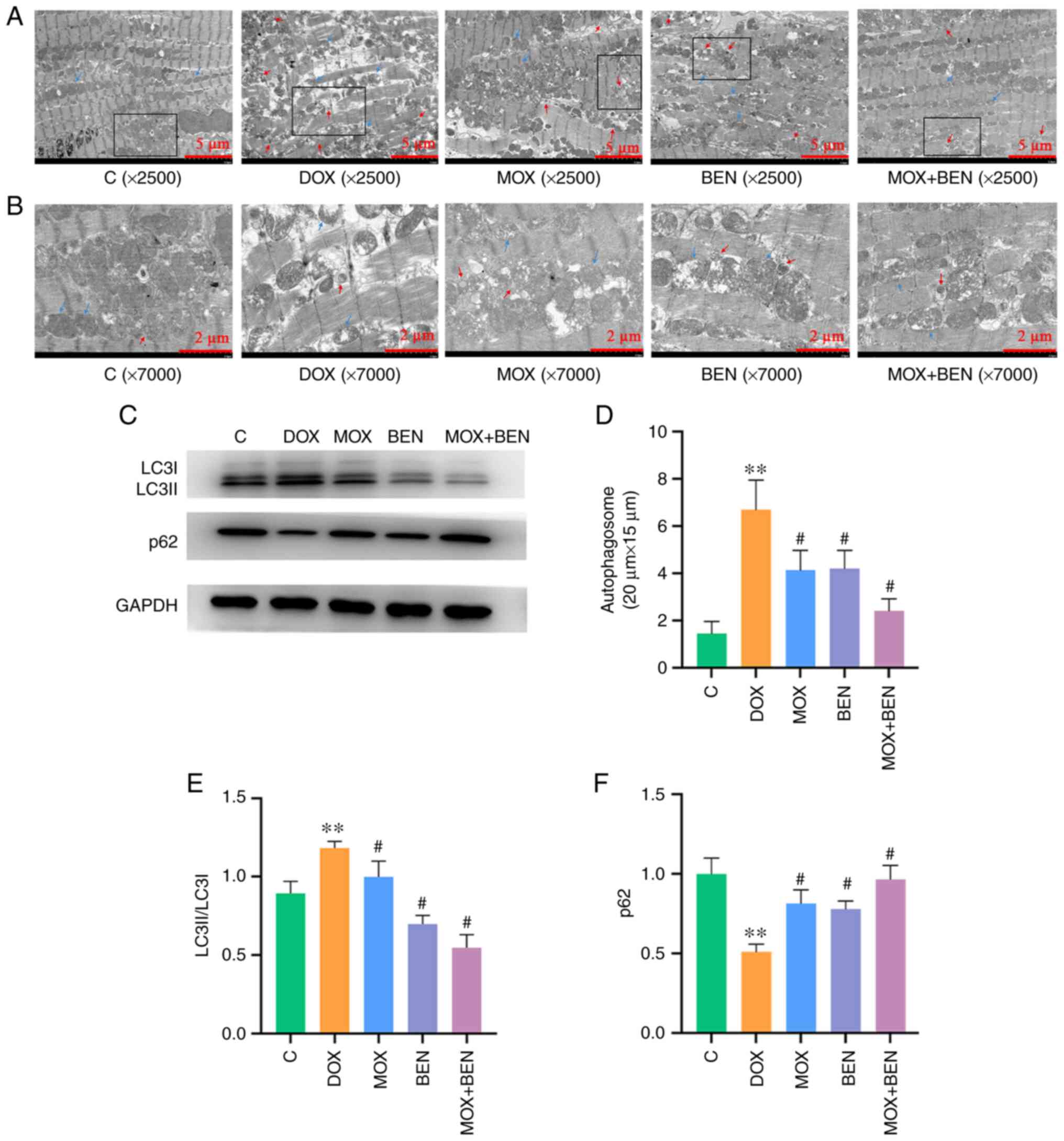 | Figure 5Effects of MOX on mitophagy in the
rat model of CHF. (A) Transmission electron microscopy images. Blue
arrow, mitochondria; red arrow, autophagosome. Magnification,
x2,500; scale bar=5 µm. (B) Enlarged image of autophagosome
structure in black frame area. Blue arrow, mitochondria; red arrow,
autophagosome. Magnification, x7000; scale bar=2 µm. (C) Expression
levels of LC3I, LC3II, p62 and GAPDH. (D) The number of
autophagosomes in each group in the 20x15 µm area. (E) LC3II/LC3I
relative to GAPDH. (F) p62 relative to GAPDH.
**P<0.01 vs. C group and #P<0.01 vs.
DOX group. Data are mean ± SD from five independent experiments
(n=13-20). MOX moxibustion; CHF, chronic heart failure; DOX,
doxorubicin; BEN, benazepril C, control. |
MOX inhibits the FUNDC1 pathway in the
rat model of CHF
The present study further investigated the role of
the FUNDC1 pathway, which is closely correlated with mitochondrial
dynamics and mitophagy (14,15).
ULK1 and PGAM5, as upstream effectors of FUNDC1, could activate the
FUNDC1 pathway in cardiomyocytes under hypoxic conditions or
mitochondrial damage (16,17). Western blotting demonstrated that
ULK1, PGAM5, FUNDC1 and p-FUNDC1 protein levels were significantly
increased in the DOX group compared with the C group (P<0.01,
Fig. 6) and significantly reduced
in the MOX group compared with the DOX group (P<0.01, Fig. 6). Similarly, these proteins were
markedly downregulated in the BEN and MOX + BEN groups. Finally,
RT-qPCR revealed that ULK, PGAM5 and FUNDC1 mRNA levels were
elevated in the DOX group compared with the C group (P<0.01,
Fig. 6) and decreased in the MOX
group compared with the DOX group (P<0.01, Fig. 6).
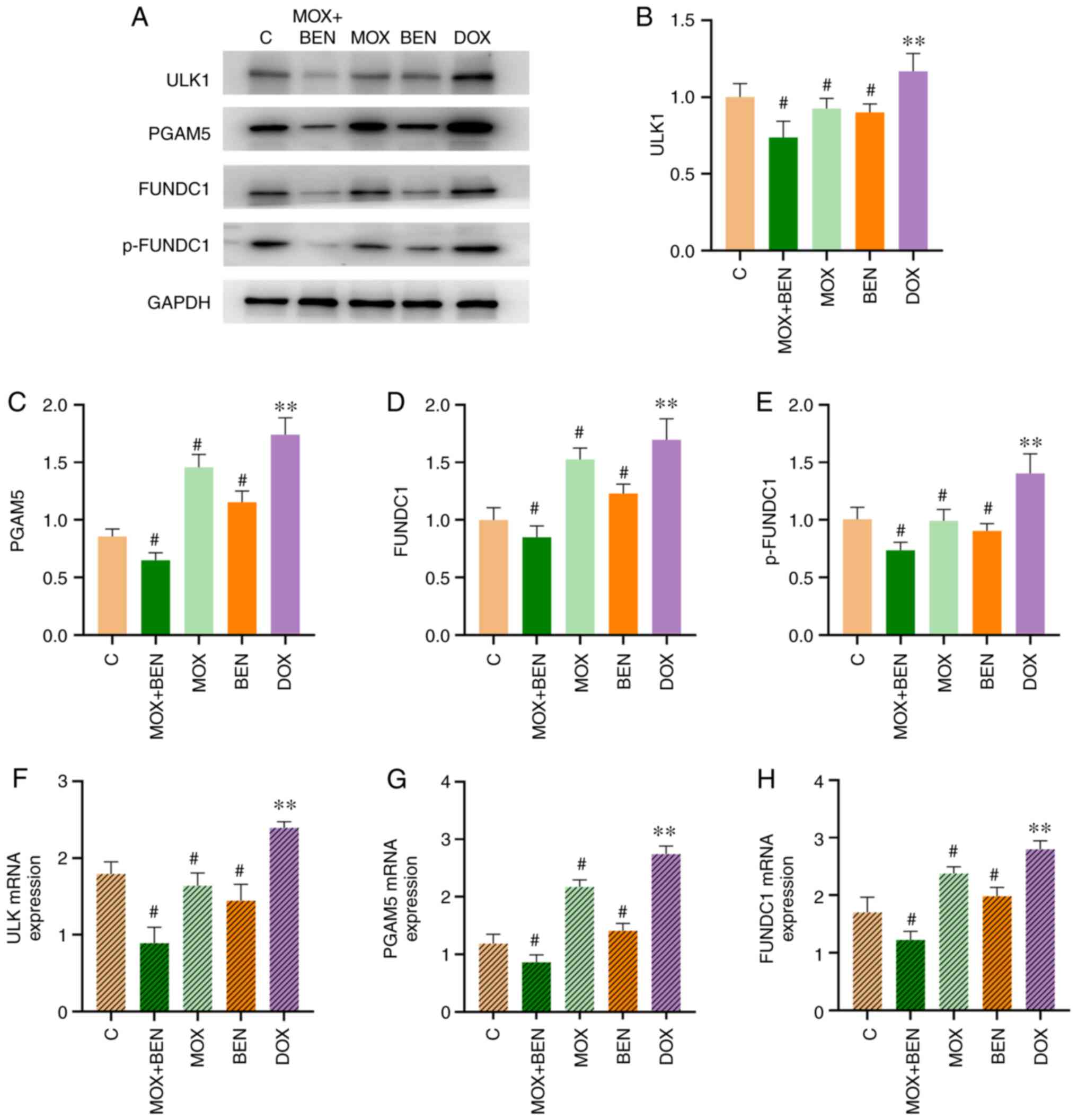 | Figure 6The effects of MOX on FUNDC1 pathway.
(A) The expression levels of ULK1, PGAM5, FUNDC1, p-FUNDC1 and
GAPDH. (B) ULK1 relative to GAPDH, (C) PGAM5 relative to GAPDH, (D)
FUNDC1 relative to GAPDH. (E) p-FUNDC1 relative to GAPDH. (F) ULK
mRNA levels. (G) PGAM5 mRNA levels. (H) FUNDC1 mRNA levels.
**P<0.05 vs. C group and #P<0.05 vs.
DOX group. Protein levels were measured by western blotting. ULK1,
PGAM5 and FUNDC1 mRNA levels were measured by RT-qPCR. Data are
mean ± standard deviation from five independent experiments
(n=13-20). MOX moxibustion; FUNDC1, FUN14 domain-containing protein
1; ULK1, Unc-51 Like Autophagy Activating Kinase 1; PGAM5,
phosphoglycerate mutase family member 5; p-, phosphorylated; DOX,
doxorubicin; BEN, benazepril C, control. |
Discussion
In the present study, in vivo experiments
were conducted to assess the protective effects of MOX in CHF and
to explore the underlying molecular mechanism. The main findings
could be summarized as follows: i) MOX improved the cardiac
function of rats with DOX-induced CHF by regulating mitochondrial
dynamics and inhibiting mitophagy; ii) The effects on mitochondrial
dynamics and mitophagy could be related to the regulation of the
FUNDC1 pathway; and, iii) MOX + BEN therapy showed a higher
efficacy compared with the single administration of MOX or BEN.
MOX is a form of heat therapy that utilizes the
burning of the herb Artemisia Argyi (also known as Chinese
mugwort) on or around acupuncture points. This herb specifically
relieves respiratory difficulty, alleviates aches and pain in
joints and muscles and promotes blood circulation (28-31).
As an important component of TCM, it has been widely used in the
treatment of a variety of diseases, including primary insomnia,
breast cancer-related lymphedema, primary dysmenorrhea, knee
osteoarthritis and inflammatory bowel disease, with significant
therapeutic effects (32-37).
In recent years, MOX has also been used to treat cardiovascular
diseases. A pilot controlled clinical trial showed that MOX could
lower blood pressure in patients with prehypertension or stage I
hypertension (38). Furthermore,
acupuncture and MOX have been widely applied to treat
hyperlipidemia in clinical practice (39). In addition, the combination of
acupuncture and MOX could improve cardiac function of patients with
HF in clinical practice (40). In
the present study, MOX increased the levels of indicators of
cardiac function (e.g., BNP, LVIDS, EF and FS) and attenuated
myocardial fibrosis.
Maintenance of mitochondrial function and integrity
is imperative for the myocardium and other tissues with high energy
demand (41). However, prolonged
and/or high-level cardiac stress causes mitochondrial damage and
ATP level reduction, which are associated with HF progression and
ventricular remodeling (42). The
present study found that DOX decreased ATP levels in the
myocardium, which were increased by moxibustion. In addition, it
was observed that after moxibustion treatment, mitochondrial
structure and morphology were somewhat different. These findings
indicated that moxibustion improves the damaged mitochondria.
Mitochondrial dynamics and mitophagy cooperatively
act to determine cardiac mitochondrial function and integrity, as
well as to promote cardiomyocyte cell survival (43). OPA1, a mitochondrial fusion
protein, is required for inner mitochondrial membrane fusion and
interacts with FUNDC1 under normal conditions, contributing to
mitochondrial maintenance (44). A
previous study showed that OPA1 knockdown results in mitochondrial
dysfunction, mitochondrial morphological changes and HF in mice
(45). The present study found
that mitochondrial fusion was reduced in rats with CHF and
increased following moxibustion treatment, an effect which may be
associated with the induction of healthy mitochondrial fusion or
the recycling of damaged mitochondria by mitophagy-related
clearance. Mitochondrial fission is a precursor of mitophagy
(43). DRP1 and FIS1 are involved
in mitochondrial fission, while LC3 and p62 are the hallmark
molecules of autophagy (46,47).
FUNDC1 is an essential regulator of cardiac mitochondrial fission
and mitophagy, recruiting DRP1 to facilitate mitochondrial fission
in response to hypoxia and directly binding with LC3 to mediate
mitophagy (48). FUNDC1 regulates
FIS1 at the transcriptional level by activating CREB in a
Ca2+-dependent manner (49). The present study indicated that
DRP1, FIS1 levels and LC3II/LC3I ratio were elevated, while p62 was
downregulated in the DOX group; electron microscopy also showed
significantly increased number of autophagosomes, due to excessive
mitochondrial fission and mitophagy under stress. Following
moxibustion treatment, DRP1, FIS1 levels and LC3II/LC3I ratio as
well as the amounts of autophagosomes were reduced, while p62 was
increased. These findings revealed that the protective effect of
MOX on the myocardium may be related to the inhibition of excessive
mitochondrial fission and mitophagy.
DOX is a non-selective class I anthracycline
antibiotic, which is extensively utilized in the treatment of
diverse types of cancer (50).
However, its application is clinically restricted because of
cumulative dose-dependent cardiotoxic effects, leading to cardiac
dysfunction, cardiomyopathy, dilated cardiomyopathy and eventually
CHF and mortality (51,52). A number of studies have
demonstrated that DOX could produce excessive ROS in the myocardium
and activate several mitochondria-related apoptotic signals,
leading to myocardial cell death (53-55).
In addition, studies have reported that DOX-induced HF is
associated with mitochondrial fission and autophagy (56,57).
In a rat model of DOX-induced cardiomyopathy, the present study
aimed to examine whether MOX inhibited mitochondrial fission and
autophagy to alleviate DOX-induced HF and myocardial cell death.
BEN was chosen as the positive control. A previous study found that
angiotensin II (Ang II) serves a direct role in CHF development and
exerts pro-autophagic effects in cardiomyocytes (58). A study demonstrated that captopril
has a protective effect on prion-mediated neuronal cell death via
autophagy inhibition (59).
It was previously shown that the FUNDC1 signaling
pathway, a critical regulator of cardiac dynamics and mitophagy, is
involved in HF (15). Therefore,
regulation of mitochondrial fusion, fission and autophagy by MOX
could be associated with the FUNDC1 pathway. The present study
further investigated the effects of MOX on the expression levels of
FUNDC1 pathway-related proteins to explore its potential protective
mechanism. FUNDC1 is activated by phosphorylated/dephosphorylated
ULK1 and PGAM5 to promote mitophagy, thereby controlling
mitochondrial dynamics and autophagy (17) (Fig.
7). The present study showed that MOX decreased the expression
levels of ULK1, PGAM5, FUNDC1 and p-FUNDC1, indicating that MOX
regulates mitochondrial dynamics and mitophagy and the underlying
mechanism may be related to the FUNDC1 signaling pathway. However,
the FUNDC1 pathway has only been discussed initially and further
FUNDC1 activators studies are needed to confirm these findings,
such as the upstream protein ULK1 activator (LYN-1604
hydrochloride) or FUNDC1 gene knockout can be used for further
research.
 | Figure 7MOX protects the myocardium by
inhibiting excessive mitochondrial fission and mitophagy through
the FUNDC1 pathway. (A) DOX leads to chronic heart failure by
promoting excessive mitochondrial fission and mitophagy;
moxibustion could inhibit excessive mitochondrial fission and
mitophagy for myocardial protection. (B) Moxibustion downregulates
the mitochondrial outer membrane protein FUNDC1 and reduces the
recruitment of the mitochondrial mitotic proteins DRP1, FIS1 and
LC3, inhibiting excessive mitochondrial fission and mitophagy.
After scavenging via mitophagy, mitochondrial fragments further
occur through the binding of the mitochondrial fusion protein OPA1
to FUNDC1, resulting in mitochondrial fusion. (C) Effectors of the
FUNDC1 signaling pathway. MOX moxibustion; FUNDC1, FUN14
domain-containing protein 1; DOX, doxorubicin; DRP1,
dynamin-related protein 1; FIS1, fission 1 protein; OPA1, optic
atrophy 1; ULK1, Unc-51 Like Autophagy Activating Kinase 1; PGAM5,
phosphoglycerate mutase family member 5; DRP1, dynamin-related
protein 1. |
In summary, these findings confirmed that MOX
improves the heart function of CHF rats by regulating mitochondrial
dynamics and inhibiting autophagy. The underlying mechanism may be
related to the inhibition of the FUNDC1 signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81574084),
Key Research and Development Program of Anhui Province (grant no.
202004j07020045) and the Open Fund Project of Key Laboratory of
Xin'an Medicine of the Ministry of Education (grant no.
2020xayx07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW conceived and supervised the study; JW, RX and XD
designed the experiments; RX, QL and WW performed the experiments;
QL, QM and BG analyzed the data; RX and JW wrote the manuscript; RX
and JW performed manuscript revision. JW and RX confirm the
authenticity of all the raw data. All authors reviewed the results
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Anhui University of Chinese Medicine [Approval No.
SCXK (Shandong) 2019-003]. It was conducted on the basis of the
Regulations for the Administration of Laboratory Animals in
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ewen S, Nikolovska A, Zivanovic I,
Kindermann I and Böhm M: Chronic heart failure-new insights. Dtsch
Med Wochenschr. 141:1560–1564. 2016.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
2
|
Tromp J, Teng TH, Tay WT, Hung CL,
Narasimhan C, Shimizu W, Park SW, Liew HB, Ngarmukos T, Reyes EB,
et al: Heart failure with preserved ejection fraction in Asia. Eur
J Heart Fail. 21:23–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hao G, Wang X, Chen Z, Zhang L, Zhang Y,
Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, et al: Prevalence of heart
failure and left ventricular dysfunction in China: The China
hypertension survey, 2012-2015. Eur J Heart Fail. 21:1329–1337.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van der Meer P, Gaggin HK and Dec GW:
ACC/AHA versus ESC guidelines on heart failure: JACC guideline
comparison. J Am Coll Cardiol. 73:2756–2768. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boorsma EM, Ter Maaten JM, Damman K, Dinh
W, Gustafsson F, Goldsmith S, Burkhoff D, Zannad F, Udelson JE and
Voors AA: Congestion in heart failure: A contemporary look at
physiology, diagnosis and treatment. Nat Rev Cardiol. 17:641–655.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dick SA and Epelman S: Chronic heart
failure and inflammation: What do we really know? Circ Res.
119:159–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanai E and Frantz S: Pathophysiology of
heart failure. Compr Physiol. 6:187–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tian R, Colucci WS, Arany Z, Bachschmid
MM, Ballinger SW, Boudina S, Bruce JE, Busija DW, Dikalov S, Dorn
GW II, et al: Unlocking the secrets of mitochondria in the
cardiovascular system: Path to a cure in heart failure-a report
from the 2018 national heart, lung, and blood institute workshop.
Circulation. 140:1205–1216. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tahrir FG, Langford D, Amini S, Mohseni
Ahooyi T and Khalili K: Mitochondrial quality control in cardiac
cells: Mechanisms and role in cardiac cell injury and disease. J
Cell Physiol. 234:8122–8133. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chaanine AH, LeJemtel TH and Delafontaine
P: Mitochondrial pathobiology and metabolic remodeling in
progression to overt systolic heart failure. J Clin Med.
9(3582)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y,
Xiao H, Yu H, Zheng Y, Liang Y, et al: Targeting
mitochondria-inflammation circuit by β-hydroxybutyrate mitigates
HFpEF. Circ Res. 128:232–245. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Muñoz JP and Zorzano A: FUNDC1: A novel
protein in cardiac health. Circulation. 136:2267–2270.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meyer JN, Leuthner TC and Luz AL:
Mitochondrial fusion, fission, and mitochondrial toxicity.
Toxicology. 391:42–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li
Y, Han Z, Chen L, Gao R, Liu L and Chen Q: Mitophagy receptor
FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy.
12:689–702. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu W, Li W, Chen H, Jiang L, Zhu R and
Feng D: FUNDC1 is a novel mitochondrial-associated-membrane (MAM)
protein required for hypoxia-induced mitochondrial fission and
mitophagy. Autophagy. 12:1675–1676. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma K, Zhang Z, Chang R, Cheng H, Mu C,
Zhao T, Chen L, Zhang C, Luo Q, Lin J, et al: Dynamic PGAM5
multimers dephosphorylate BCL-xL or FUNDC1 to regulate
mitochondrial and cellular fate. Cell Death Differ. 27:1036–1051.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu W, Tian W, Hu Z, Chen G, Huang L, Li W,
Zhang X, Xue P, Zhou C, Liu L, et al: ULK1 translocates to
mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO
Rep. 15:566–575. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang L, Deng Z, Zhang H, Li Y, Wang T and
Xie W: Acupoint for angina pectoris: A protocol for systematic
review and meta-analysis. Medicine (Baltimore).
100(e24080)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun H, Li X, Lou J, Zhang Y, Jiang Y and
Fang J: Acupuncture and related therapies for treating stable
angina pectoris: A protocol of an overview of systematic reviews
and meta-analysis. Medicine (Baltimore). 99(e23701)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang J, Zeng YL, Wu FQ, Sun RR, Chen J,
Jia XZ and Xi YH: Effect of moxibustion stimulation of ‘Feishu’ (BL
13) and ‘Xinshu’ (BL 15) on expression of myocardial MyD 88 protein
and caspase 3 mRNA in chronic heart failure rats. Zhen Ci Yan Jiu.
41:429–434. 2016.PubMed/NCBI(In Chinese).
|
|
21
|
Liu NN, Jia XZ, Wang J, Zhu GQ, Li D, Li
QL and Ma Q: Moxibustion improves cardiac function by up-regulating
autophagy-related proteins of cardiomyocytes in rats with chronic
heart failure. Zhen Ci Yan Jiu. 44:25–30. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Li Q, Wang W, Ma Q, Xia R, Gao B, Zhu G
and Wang J: Moxibustion improves chronic heart failure by
inhibiting autophagy and inflammation via upregulation of mTOR
expression. Evid Based Complement Alternat Med.
2021(6635876)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US), Washington (DC).
|
|
24
|
Leontyev S, Schlegel F, Spath C, Schmiedel
R, Nichtitz M, Boldt A, Rübsamen R, Salameh A, Kostelka M, Mohr FW
and Dhein S: Transplantation of engineered heart tissue as a
biological cardiac assist device for treatment of dilated
cardiomyopathy. Eur J Heart Fail. 15:23–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qi Q, Liu YN, Jin XM, Zhang LS, Wang C,
Bao CH, Liu HR, Wu HG and Wang XM: Moxibustion treatment modulates
the gut microbiota and immune function in a dextran sulphate
sodium-induced colitis rat model. World J Gastroenterol.
24:3130–3144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Y, Zhao J, Han Q, Wang Z, Wang Z, Dong
X, Li J, Liu L and Shen X: The effect and mechanism of Chinese
herbal formula sini tang in heart failure after myocardial
infarction in rats. Evid Based Complement Alternat Med.
2018(5629342)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shin NR, Ryu HW, Ko JW, Park SH, Yuk HJ,
Kim HJ, Kim JC, Jeong SH and Shin IS: Artemisia argyi
attenuates airway inflammation in ovalbumin-induced asthmatic
animals. J Ethnopharmacol. 209:108–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shin NR, Park SH, Ko JW, Ryu HW, Jeong SH,
Kim JC, Shin DH, Lee HS and Shin IS: Artemisia argyi
attenuates airway inflammation in lipopolysaccharide induced acute
lung injury model. Lab Anim Res. 33:209–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee H, Jang D, Jeon J, Cho C, Choi S, Han
SJ, Oh E, Nam J, Park CH, Shin YS, et al: Seomae mugwort and
jaceosidin attenuate osteoarthritic cartilage damage by blocking
IκB degradation in mice. J Cell Mol Med. 24:8126–8137.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ge YB, Wang ZG, Xiong Y, Huang XJ, Mei ZN
and Hong ZG: Anti-inflammatory and blood stasis activities of
essential oil extracted from Artemisia argyi leaf in
animals. J Nat Med. 70:531–538. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang C, Yang M, Fan Y and Pei X:
Moxibustion as a therapy for breast cancer-related lymphedema in
female adults: A preliminary randomized controlled trial. Integr
Cancer Ther. 18(1534735419866919)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun YJ, Yuan JM and Yang ZM: Effectiveness
and safety of moxibustion for primary insomnia: A systematic review
and meta-analysis. BMC Complement Altern Med.
16(217)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ma F, Yan X, Yu Y, Du D, Li S, Chen C,
Zhang X, Dong Z and Ma Y and Ma Y: Effects of herb-partitioned
moxibustion for primary dysmenorrhea: A protocol for systematic
review and meta-analysis. Medicine (Baltimore).
99(e21253)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu F, Huang M, Jin Y, Kong Q, Lei Z and
Wei X: Moxibustion treatment for primary osteoporosis: A systematic
review of randomized controlled trials. PLoS One.
12(e0178688)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen L, Huang Z, Cheng K, Wu F, Deng H,
Lin L, Zhao L and Shen X: The efficacy of jade moxibustion in knee
osteoarthritis. Medicine (Baltimore). 99(e19845)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stein DJ: Massage acupuncture,
moxibustion, and other forms of complementary and alternative
medicine in inflammatory bowel disease. Gastroenterol Clin North
Am. 46:875–880. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shin KM, Park JE, Yook TH, Kim JU, Kwon O
and Choi SM: Moxibustion for prehypertension and stage I
hypertension: A pilot randomized controlled trial. Integr Med Res.
8:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yao Q, Zhang X, Huang Y, Wang H, Hui X and
Zhao B: Moxibustion for treating patients with hyperlipidemia: A
systematic review and meta-analysis protocol. Medicine (Baltimore).
98(e18209)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liang B, Yan C, Zhang L, Yang Z, Wang L,
Xian S and Lu L: The effect of acupuncture and moxibustion on heart
function in heart failure patients: A systematic review and
meta-analysis. Evid Based Complement Alternat Med.
2019(6074967)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Koch RE, Josefson CC and Hill GE:
Mitochondrial function, ornamentation, and immunocompetence. Biol
Rev Camb Philos Soc. 92:1459–1474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Morales PE, Arias-Durán C, Ávalos-Guajardo
Y, Aedo G, Verdejo HE, Parra V and Lavandero S: Emerging role of
mitophagy in cardiovascular physiology and pathology. Mol Aspects
Med. 71(100822)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vásquez-Trincado C, García-Carvajal I,
Pennanen C, Parra V, Hill JA, Rothermel BA and Lavandero S:
Mitochondrial dynamics, mitophagy and cardiovascular disease. J
Physiol. 594:509–525. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wai T, Garcia-Prieto J, Baker MJ,
Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas C, Ibañez B and
Langer T: Imbalanced OPA1 processing and mitochondrial
fragmentation cause heart failure in mice. Science.
350(aad0116)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen L, Gong Q, Stice JP and Knowlton AA:
Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res.
84:91–99. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Horbay R and Bilyy R: Mitochondrial
dynamics during cell cycling. Apoptosis. 21:1327–1335.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jiang P and Mizushima N: LC3- and
p62-based biochemical methods for the analysis of autophagy
progression in mammalian cells. Methods. 75:13–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chai P, Cheng Y, Hou C, Yin L, Zhang D, Hu
Y, Chen Q, Zheng P, Teng J and Chen J: USP19 promotes
hypoxia-induced mitochondrial division via FUNDC1 at
ER-mitochondria contact sites. J Cell Biol.
220(e202010006)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X,
Huang K, Xie Z and Zou MH: Binding of FUN14 domain containing 1
with inositol 1,4,5-trisphosphate receptor in
mitochondria-associated endoplasmic reticulum membranes maintains
mitochondrial dynamics and function in hearts in vivo. Circulation.
136:2248–2266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gong J, Yan J, Forscher C and Hendifar A:
Aldoxorubicin: A tumor-targeted doxorubicin conjugate for relapsed
or refractory soft tissue sarcomas. Drug Des Devel Ther.
12:777–786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Green PS and Leeuwenburgh C: Mitochondrial
dysfunction is an early indicator of doxorubicin-induced apoptosis.
Biochim Biophys Acta. 1588:94–101. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Koleini N and Kardami E: Autophagy and
mitophagy in the context of doxorubicin-induced cardiotoxicity.
Oncotarget. 8:46663–46680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Songbo M, Lang H, Xinyong C, Bin X, Ping Z
and Liang S: Oxidative stress injury in doxorubicin-induced
cardiotoxicity. Toxicol Lett. 307:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotoxicity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pan JA, Zhang H, Lin H, Gao L, Zhang HL,
Zhang JF, Wang CQ and Gu J: Irisin ameliorates doxorubicin-induced
cardiac perivascular fibrosis through inhibiting
endothelial-to-mesenchymal transition by regulating ROS
accumulation and autophagy disorder in endothelial cells. Redox
Biol. 46(102120)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Catanzaro MP, Weiner A, Kaminaris A, Li C,
Cai F, Zhao F, Kobayashi S, Kobayashi T, Huang Y, Sesaki H and
Liang Q: Doxorubicin-induced cardiomyocyte death is mediated by
unchecked mitochondrial fission and mitophagy. FASEB J.
33:11096–11108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mancilla TR, Davis LR and Aune GJ:
Doxorubicin-induced p53 interferes with mitophagy in cardiac
fibroblasts. PLoS One. 15(e0238856)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Porrello ER, D'Amore A, Curl CL, Allen AM,
Harrap SB, Thomas WG and Delbridge LM: Angiotensin II type 2
receptor antagonizes angiotensin II type 1 receptor-mediated
cardiomyocyte autophagy. Hypertension. 53:1032–1040.
2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Moon JH, Jeong JK, Hong JM, Seol JW and
Park SY: Inhibition of Autophagy by captopril attenuates prion
peptide-mediated neuronal apoptosis via AMPK activation. Mol
Neurobiol. 56:4192–4202. 2019.PubMed/NCBI View Article : Google Scholar
|