1. Introduction
The COVID-19 pandemic has called into question the
necessary drug-induced immune suppression in the hyperimmune
response of the body and the possibility of reactivation of the
hepatitis B virus (HBV) infection after these therapies. The
cytokine storm of SARS-CoV-2 severe infection requires treatment
with strong immunosuppressants (tocilizumab) or high doses of
cortisone (1). Although these
therapies are not needed for long periods of time, patients with
chronic or latent HBV infection run the risk of reactivation of HBV
during these therapies (2-5).
In severe forms of psoriasis, biological therapies
have brought marked therapeutic successes in recent decades. The
main estimated risk of these therapies was the development of
severe infections during treatment or the reactivation of latent
infections such as tuberculosis or HBV infection. The biological
therapies have significantly improved the quality of life of
psoriasis patients and are considered to be safer and more
effective than traditional systemic drugs (6). The risk of reactivation of the HBV
infection during these therapies depends on the status of this
infection and the type of immunosuppressant used (7).
The aim of the present study was to compare the
experience gained in monitoring immunosuppressive therapies in
psoriasis with preliminary clinical observations in COVID-19 and to
propose a strategy to monitor the risk of HBV reactivation and a
prophylactic therapy scheme for patients, requiring
immunosuppressive therapies, with severe forms of COVID-19 and HBV
infection.
2. Literature review methodology
An updated narrative review of studies published
between 2000 and 2021 in the current literature was performed,
which were focused on the number of patients in whom the
reactivation of chronic HBV infection was observed during various
long-term immunosuppressive therapies from psoriasis and short-term
immunosuppressive therapies from COVID-19. The testing protocols of
patients for chronic HBV infection before starting
immunosuppressive therapy and the patient profile at which
antiviral prophylaxis for HBV was initiated were also monitored.
Databases such as PubMed, Elsevier, Medline and ScienceDirect
Freedom Collection were used, in the search for related articles,
by introducing the terms: ‘reactivation of hepatitis B and
COVID-19’ or ‘Sars-CoV-2, reactivation of hepatitis B and
psoriasis’.
3. Evolution of hepatitis B
Chronic HBV infection was responsible worldwide for
approximately 257 million diseases (3.5% of the population) and
1.34 million deaths, in 2015, according to World Health
Organization (8).
HBV infection can evolve to acute hepatitis,
spontaneous viral clearance or chronic HBV infection. Clinical
trials note approximately 23% of cases of acute viral hepatitis
that achieve spontaneous viral clearance (6) with positive antibodies to hepatitis B
core antigen (anti-HBc), in low titers, and without the presence of
hepatitis B surface antigen (HBsAg). Acute forms of hepatitis with
HBV evolve in 94-98% of adults and 10% of infected children in the
first year of life, towards healing with negative HBV DNA and HBsAg
with the appearance of antibodies to hepatitis B surface antigen
(anti-HBs) and anti-HBc. A total of 2-6% of adults and 90% of
children infected in the first year of life, progress to chronic
persistence infection, with HBV DNA, HBsAg and total anti-HBc
positive and will be monitored at 6 months and treated with
specific antivirals in the case of HBV DNA with over 2,000 IU and
minimum F1 fibrosis or A1 inflammation on Fibromax or over 7 kPa on
Fibroscan (9,10).
HBV is a DNA virus that manages to graft its genome
onto the genome of the host hepatocyte; HBV covalently closed
circular DNA remains in the hepatocyte even in patients who manage
to have spontaneous viral clearance and in those who remain on
undetectable viremias in the blood, under antiviral therapies,
which explains the occurrence of hepatic adenocarcinoma in patients
with HBV infection, regardless of the degree of liver fibrosis and
the possible reactivation of latent infection in case of
immunosuppression. Reactivation of HBV may be an exacerbation of
chronic hepatitis B or reactivation of previous HBV infection
(11,12).
Patients with psoriasis treated with biologic
therapy due to an immunocompromised status have an increased risk
of reactivation of HBV infection and liver adenocarcinoma (13-16).
Other therapies used for comorbidities or other concomitant
infections, but also topically for psoriasis, can produce immediate
or long-term liver damage (17-19).
Patients with severe forms of COVID-19 requiring
immunosuppressive therapies are usually elderly, with multiple
comorbidities, a compromised immune status through underlying
pathology and severe viral disease, and thus a status that
predisposes them to reactivation of HBV infection (20-22).
4. Therapeutic perspective for the
prevention of HBV reactivation in patients using
immunosuppressants
The perspective towards a patient with psoriasis who
is about to start biological therapy, in order to prevent the
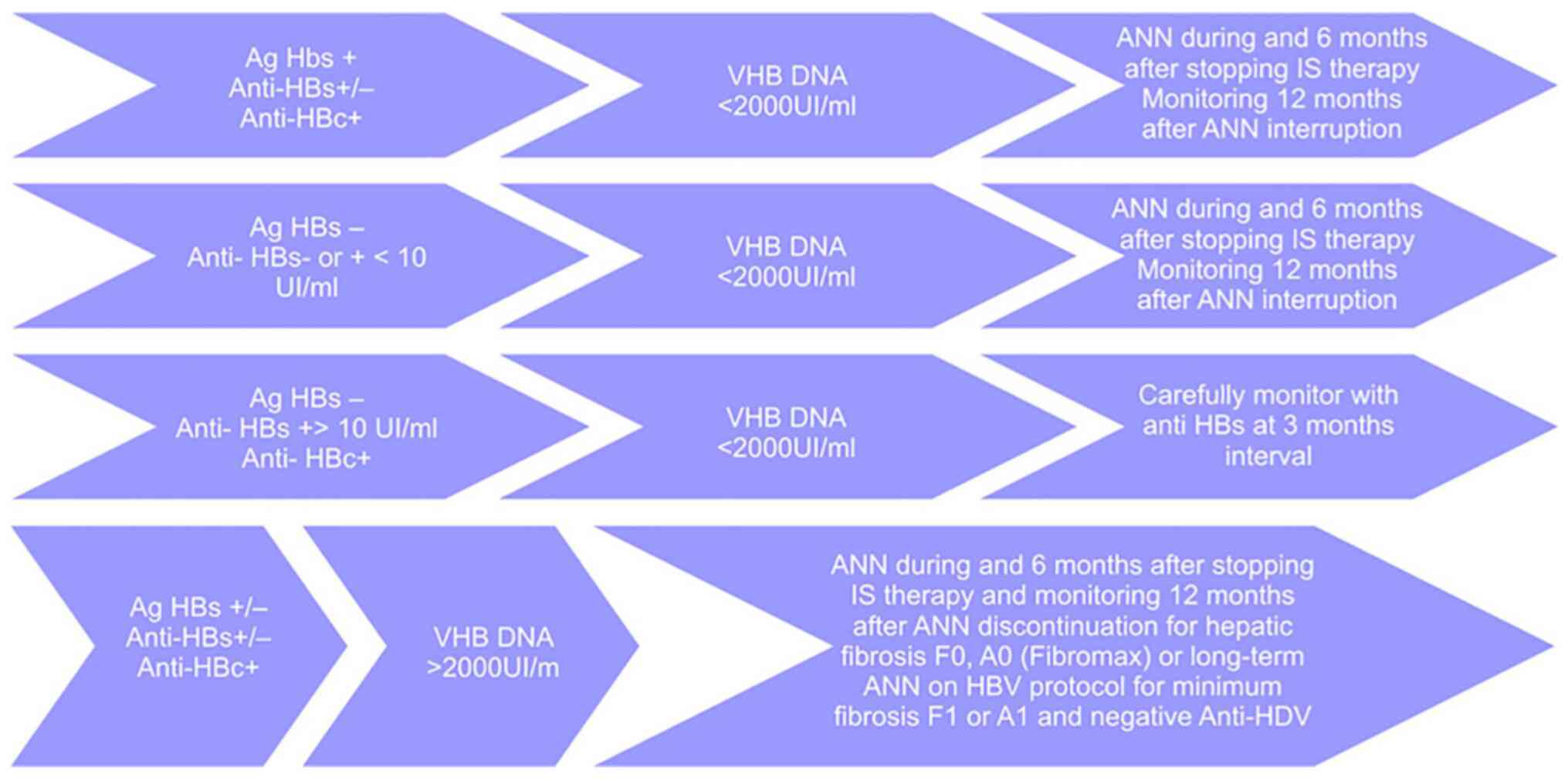
reactivation of a latent HBV infection is the following (23-25):
i) For HBV seronegative patients, the hepatitis B vaccine is
indicated before starting biological therapy; ii) in patients with
acute HBV infection and detectable HBV DNA in the serum,
immunoglobulin M antibodies to hepatitis B core (anti-HBc
IgM)-positive, elevated hepatic transaminases it is indicated to
discontinue biological therapy for psoriasis or delay its onset by
6-12 months; iii) patients with chronic HBV infection, according to
the Romanian protocol (December 2019), depending on the status of
the infection will be monitored according to the protocols in
Fig. 1; and iv) therapeutic options
with nucleoside/nucleotide analogues (ANN) are: Entecavir 0.5
mg/daily, in adults and children weighing ≥32.6 kg and older than 3
years; or tenofovir 245 mg/daily, in adults and adolescents aged 12
to 18 years weighing ≥35 kg, and doses are adjusted for creatinine
clearance.
All HBV-positive patients must be monitored at 6
months for liver adenocarcinoma with abdominal ultrasound and
alpha-fetoprotein dosing.
5. Risk of reactivation of HBV in patients
undergoing biological therapies for psoriasis
The risk of reactivation of the HBV during
immunosuppressive therapies for psoriasis, is high risk (≥10%),
moderate risk (1-10%) and low risk (<1%), depending on the
presence or the absence of HBsAg and anti-HBc and the type of
immunosuppressive therapy.
Depending on the serological status, this risk is:
i) High, HBsAg-positive and HBV DNA >2,000 IU/ml; ii) medium,
HBsAg-negative, anti-HBc IgG-positive and anti-HBs-negative; or
low, HBsAg-negative, anti-HBc IgG-positive and anti-HBs-positive
(7).
Depending on the type of biological therapy used in
psoriasis, for both TNF-α inhibitors and other cytokine inhibitors
this risk is medium (1-10%) (7).
A retrospective, observational study was performed
on 81 patients (average age, 63 years; 83% males) admitted to the
Dermatology Clinic of ‘Sf. Cuv. Parascheva’ Hospital of Infectious
Diseases Galati and Braila Emergency County Hospital between June
1, 2016-1, 2020 with the diagnosis of moderate and severe forms of
disseminated psoriasis, undergoing biological therapy in which HBV
infection was monitored and treated prophylactically.
Criteria for inclusion in the study were: Adults
with psoriasis, undergoing biological therapy; patients who signed
informed consent for participation in the study, staff in full
knowledge.
Exclusion criteria from the study were: Unconscious
patients or inability to sign informed consent; patients who
refused to participate in the study; pregnant or breastfeeding
women; patients under 18 years, comedication contraindicated in
psoriasis (for example beta blockers) (26).
Patients were monitored during the hospitalization
period and every 3 months after discharge, they were evaluated in
the hospital.
The treatment of the study group was: Of the 81
patients monitored for psoriasis, in biological therapies, 12
patients were treated with etanercept, 42 with adalimumab, 3 with
infliximab, 5 with ixekizumab, 9 with secukinumab, 10 with
ustekinumab. Of these, 6 patients (Table I) were detected with positive
markers for HBV. The average age of these patients was 63 years, of
which 83.33% were men, diagnosed with psoriasis between 1969 and
2016. They started immunosuppressive therapy between 2012 and 2019
with an average Psoriasis Area Severity Index (PASI) (27) of 32.9 and an average Dermatology
Life Quality Index (DLQI) (28) of
22. All 6 patients were HBsAg-negative, anti-HBc positive,
undetectable HBV DNA and anti-HDV negative. Anti-HBs in 2 patients
was below 10 IU/l and they received prophylactic treatment with
entecavir 0.5 mg per day and the other 4 patients with anti-HBs
titers over 10 IU/l were monitored at 3 months.
 | Table ICharacteristics of the patients with
psoriasis and chronic infection with HBV. |
Table I
Characteristics of the patients with
psoriasis and chronic infection with HBV.
|
Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|
| Sex | Male | Female | Male | Male | Male | Male |
| Age | 58 | 71 | 69 | 60 | 63 | 57 |
| The time of
diagnosis of psoriasis | 1992 | 1969 | 2000 | 1993 | 2016 | 1985 |
| The date at the
start of the IS therapy | 03.2018 | 06.2019 | 06.2016 | 2014 | 12.2018 | 2012 |
| PASI score at the
start of IS therapy (35) | 36.8 | 23.7 | 32 | 23.7 | 39.6 | 41.6 |
| DLQI score at the
start of IS therapy (36) | 30 | 17 | 16 | 21 | 23 | 25 |
| Special areas | Scalp, nails,
palms, plants, genital, folds | Palms, plants | Scalp, nails | Scalp, plants | Palms, plants | Scalp, nails,
palms, plants |
| Anti-HBc | Positive | Positive | Positive | Positive | Positive | Positive |
| HBs Ag | Negative | Negative | Negative | Negative | Negative | Negative |
| Anti-HBs | Below 2 UI/l | 1,568-2,000
UI/l | 58-64 UI/l | 3 UI/l | 124-362 UI/l | 923-1,328 UI/l |
| HBV DNA
undetectable | Yes | Yes | Yes | Yes | Yes | Yes |
| Anti-HDV | Negative | Negative | Negative | Negative | Negative | Negative |
| AST (U/l) | 17.5 | 21.1 | 20.3 | 23 | 22 | 40.4 |
| ALT (U/l) | 25.5 | 26.5 | 18.5 | 34 | 23 | 48.5 |
| IS therapy | Adalimumab | Adalimumab | Etanercept | Adalimumab | Secukinumab | Etanercept |
| HBV therapy | Entecavir 0.5
mg/day | Monitoring at 3
months | Monitoring at 3
months | Entecavir 0.5
mg/day | Monitoring at 3
months | Monitoring at 3
months |
| Favorable skin
evolution | Yes | Yes | Yes | Yes | Yes | Yes |
All patients had a favorable evolution of
dermatological lesions without reactivation of HBV infection.
The literature has noted cases between 1.14-34.3% of
HBV reactivations in patients with psoriasis in biological
therapies and a selection of 2 meta-analyses are presented
(6,10): i) A meta-analysis from 2019, on
2,060 patients with psoriasis (3,562 episodes of treated disease)
who received biological therapies between 2009 and 2018, of which
359 patients had HBV infection (561 treatment episodes), 88
therapeutic episodes were observed with HBV reactivations.
Reactivations were more common in patients with chronic HBV
infection than in those with occult HBV infection (34.3 vs. 3.2%,
P=0.001). Patients who were HBsA-positive and hepatitis B envelope
antigen (HBeAg)-positive were statistically the most prone to
reactivation of HBV (6); ii) a
meta-analysis from 2017, on 312 patients followed for a mean of
30.9 months, with psoriasis, treated with biological agents,
observed 2 cases of HBV reactivation out of the 175 patients who
were anti-HBc-positive and 8 reactivations in the 40 patients with
chronic HBV infection (10).
Depending on the type of immunosuppressant used,
clinical trials note small differences in the percentages of
reactivation of the HBV infection. Among the TNF-α inhibitors
(etanercept, infliximab, adalimumab and certolizumab), clinical
trials note more HBV reactivations with infliximab and adalimumab
than with etanercept (29-31).
In our study, there were 3 patients treated with adalimumab and 2
patients treated with etanercept of which 2 patients with
adalimumab received prophylaxis with entecavir and the others were
monitored once every 3 months. No patient experienced HBV
reactivation.
A meta-analysis from 2017, on 187 cases of psoriasis
treated with TNF-α inhibitors and chronic or occult HBV infections,
monitored between 7.8 and 72 months, of which only 2 cases received
prophylaxis with lamivudine or entecavir noted 3 HBV reactivations
(9). In another study on 468
patients who were anti-HBc-positive and treated with infliximab,
the HBV reactivation rate was 1.7% (29). In this group, HBsAg was present in
12.3% of patients.
A 2018 meta-analysis of 200 patients who received
TNF-α inhibitors for psoriasis, who were followed for 24 weeks to 6
years, noted 3 patients with reactivated HBV (2 patients with DNA
HBV-positive and one with HBsAg-positive and DNA HBV-negative), all
without antiviral prophylactic treatment (7).
IL-12/IL-23 inhibitor is ustekinumab. IL-12 has an
important role in triggering a cellular immune response against
intracellular pathogens (32-34),
so that IL-12 inhibitory therapies could contribute to HBV
reactivation. A 2018 meta-analysis of 28 cases of psoriasis treated
with ustekinumab that was undertaken between 4 months and 3 years,
noted 3 cases that experienced HBV reactivation, inactive or occult
carrier that did not receive antiviral prophylaxis (35).
IL-17 inhibitors include secukinumab, ixekizumab and
brodalumab. A study of 46 patients treated with secukinumab,
without prophylactic antiviral therapy, noted 7 (15.2%) patients
with HBV reactivation (36). In our
study there was one patient that was treated with secukinumab,
which was monitored once every 3 months, that did not exhibit HBV
reactivation.
Clinical trials of SPIRIT-P1 and SPIRIT-P2,
performed on 1,118 patients with psoriasis that were treated with
ixekizumab, noted discontinuation of therapy due to an HBV
reactivation (37).
IL-23 inhibitors include guselkumab, tildrakizumab
and risankizumab. Data were not found in the literature on HBV
reactivation during the therapies with brodalumab and IL-23
inhibitors, but the guidelines recommend the same surveillance
measures as for other biologic therapies (38).
6. Risk of reactivation of HBV in patients
undergoing immunosuppressive therapies for severe forms of
COVID-19
Severe cases of COVID-19 reveal an inadequate
inflammatory response, with multiorgan damage whose common cause is
a process of prothrombotic endotheliitis. The role of
anti-inflammatory and immunomodulatory therapy is to stabilize this
damaged endothelium (39,40). The recovery study shows that, in
selected cases, corticosteroids save lives. The benefits were
present only among patients who required a form of respiratory
support, non-invasive or invasive, usually instituted after 7 days
(1). Anti-cytokines represent a
rescue therapy, off-label. The most commonly used agent is
tocilizumab [humanized monoclonal antibody type IgG1, anti-human
receptor for interleukin-6 (IL-6)] (41-43).
Selection criteria for tocilizumab therapy are: IL-6 >40 pg/ml,
D-dimers >1,500 ng/ml, ferritin >1,000 ng/ml, C-reactive
protein >50 mg/l. Drug toxicity of tocilizumab treatment may
increase liver enzymes and very rarely severe liver injury
(44). In patients with rheumatoid
arthritis, tocilizumab increases the risk of HBV reactivation
(45,46).
A study of 12,997 patients with previous HBV
exposure, who received systemic corticosteroid therapy, revealed
that they had low risk of liver failure. Liver aggression is
dependent on the dose of cortisone and its duration. Thus, alanine
aminotransferase (ALT) elevation was observed starting from
equivalent prednisone doses of 20-40 mg with a duration of
administration of at least 7 days. The increase in ALT was
progressive at administrations of 7-28 days and over 28 days. The
study concluded that patients who were HBsAg-negative and
anti-HBc-positive who received high doses of corticosteroids were
at risk of a hepatitis flare and should be monitored frequently for
early detection of hepatitis flares (47).
Direct aggression of SARS-COV-2 on the liver, which
may add to the aggression caused by HBV, should also be considered.
Liver damage in COVID-19 has been attributed to SARS-CoV-2 direct
aggression, hypoxia caused by pneumonia, acute inflammatory damage
and drug toxicity of COVID-19 therapy. SARS-CoV-2 direct aggression
on the liver includes the direct cytopathic effect on hepatocytes
and cholangiocytes, the effects of coagulopathy and endothelial
aggression in small intrahepatic vessels (48-50).
Liver injury has been observed in 14-53% of the
patients with COVID-19 (51-53).
Abnormal hepatic biochemical tests in patients with COVID-19 were
associated with increased disease severity and risk of mortality
(54,55).
A meta-analysis of studies on pre-existing liver
lesions, in COVID-19 estimated the pooled prevalence of HBV as 0.9%
(56).
A meta-analysis of 28 studies that included 235
patients with COVID-19 and chronic HBV infection, with a mean age
of 49.8 years, revealed a death rate of 6% and a transfer rate in
intensive care of 14.1% for patients with HBV infection,
statistically significantly higher than patients without this
comorbidity (57).
A study of 3 patients who were HBsAg-positive and 69
patients who were HBsAg-negative and anti-HBc-positive, with severe
forms of COVID-19 and treated with an immune modulator, revealed
prophylaxis for HBV reactivation, as follows: HBsAg-positive
patients received entecavir 0.5 mg/day for at least 6 months; those
with only anti-HBc markers received entecavir 0.5 mg/day for 1
month (doses adjusted according to renal function); or did not
receive antivirals and were closely monitored. They detected only
two patients with positive HBV-DNA, who did not have entecavir
prophylaxis. These patients were anti-HBs-negative,
HBV-DNA-positive, below the limit of quantification and with normal
ALT. The study noted low risk of HBV reactivation in patients with
severe forms of COVID-19, treated with immunomodulators and
resolved HBV infection. Monitoring of these patients at discharge
is necessary, but if pandemic conditions do not allow it, a short
course of entecavir may be helpful in preventing HBV reactivation
in patients without anti-HBs (2,58).
Aldhaleei et al published the first case of
HBV reactivation in a patient with severe COVID-19, specifically, a
36-year-old man who was HBsAg-positive, anti-HBc IgM-positive,
HBeAg-negative, and antibodies to hepatitis B envelope antigen
(anti-HBe)-positive, HBV-DNA 2,490 IU/ml, ALT 4,758 IU/l and mental
disturbances, at admission. HBV reactivation was interpreted in the
context of immunosuppression caused by severe COVID-19 because the
patient did not receive immunosuppressive therapy (3).
A study of 20 patients with COVID-19 and chronic HBV
infection noted 3 cases of HBV reactivation and did not observe
significant differences from the group without HBV infection in
terms of increased liver transaminases and bilirubin (59).
A retrospective study including 72 patients
diagnosed with COVID-19 and HBV carriers revealed that SARS-CoV-2
does not directly activate the HBV, and the risk of liver cell
damage of HBV carriers with COVID-19 does not increase (60).
An observational, retrospective study was performed
on 958 patients with COVID-19, hospitalized between March 1, 2020
to 30, 2021, at the Second Clinic of the Clinical Hospital of
Infectious Diseases ‘Sf. Cuv. Parascheva’ Galati. Of these, 17
patients had a history of chronic infection with HBV. The
statistical comparison (MedCalc v. 15.8) of the demographic,
clinical and paraclinical characteristics of these two groups are
presented in Table II. The group
with chronic HBV infection did not statistically significantly
differ from the total group with COVID-19, in terms of length of
hospitalization or unfavorable evolution towards death or transfer
to the intensive care. Of the 17 patients with chronic HBV
infection, only 9 received systemic corticosteroid therapy between
5 and 13 days, no patient received tocilizumab, 10 patients had
consistently elevated values of the transaminases, during
hospitalization, and two patients required intensive care for
severe forms of COVID-19. Patients were monitored for HBV
reactivation, did not receive ANN therapy, and no cases of
reactivation of chronic HBV infection were observed.
 | Table IIDemographic and clinical
characteristics of the patients with COVID-19. |
Table II
Demographic and clinical
characteristics of the patients with COVID-19.
|
Characteristics | Total COVID-19
patients | Patients with
chronic infection with HBV |
|---|
| Age (years) | | |
|
Minimum-maximum | 0.083-97 | 32-64 |
|
Average | 50.62 | 52.58
(P=0.6885) |
|
95% CI | 49.55-51.90 | 48.21-56.96 |
| Female (%) | 54.27 | 47.05
(P=0.7288) |
| BMI | | |
|
Minimum-maximum | 14.42-58.59 | 21.16-37.87 |
|
Average | 28.31 | 28.32
(P=1.0001) |
|
95% CI | 27.90-28.74 | 25.10-31.54 |
| Charlson score (%
patients) | | |
|
0 | 39.85 | 5.88
(P=0.0095) |
|
(1-2) | 33.15 | 64.70
(P=0.0135) |
|
(3-4) | 17.25 | 23.52
(P=0.7237) |
|
(5-11) | 9.72 | 5.88
(P=0.9067) |
|
Hypertension
(%) | 28.91 | 0 (P=0.0188) |
|
Diabetes
(%) | 11.58 | 0 (P=0.2691) |
|
Obesity
(%) | 32.87 | 0 (P=0.009) |
|
Cancer
(%) | 1.56 | 0 (P=0.6335) |
|
Chronic
respiratory diseases (%) | 3.75 | 0 (P=0.8696) |
| Number of days of
hospitalization | | |
|
Minimum-maximum | 1-80 | 4-18 |
|
Average | 11.00 | 10.29
(P=0.685) |
|
95% CI | 10.54-11.46 | 8.19-12.38 |
| Curb 65 score
(%) | | |
|
0 | 11.70 | 17.64
(P=0.7094) |
|
1 | 60.61 | 76.47
(P=0.2815) |
|
2 | 24.39 | 5.88
(P=0.1381) |
|
3 | 3.28 | 0 (P=0.9476) |
| Unfavorable
evolution (death or transfer to intensive care) (%) | 4.38 | 11.76
(P=0.3876) |
7. Conclusions
In our clinical experience and clinical studies,
immunosuppressive therapies for patients with psoriasis, proved
safe from the point of view of HBV infection reactivation if
provided at the initial screening of all patients for HBV
infection, regardless of serological profile: HBsAg, anti-HBc IgG,
anti-HBs and their monitoring and treatment according to Fig. 1.
Regarding the risk of reactivation of HBV in the
severe acute episode of COVID-19, there are few clinical studies to
monitor this situation. It has been revealed in clinical trials
that liver damage worsens the prognosis of COVID-19 and that severe
forms of COVID-19 can spontaneously reactivate HBV. The need for
immunosuppressive therapies in severe forms of COVID-19 and
evidence from clinical trials on other pathologies, that these
therapies may reactivate HBV imposes special attention in
monitoring patients with HBV infection and severe forms of COVID-19
and, if such monitoring is not possible, initiation of ANN
prophylaxis, according to the protocol applicable to biological
therapies in psoriasis.
More studies are indeed needed to fully evaluate the
positive and adverse effects of ANN prophylaxis in patients with
either long-term immunosuppressive therapies such as psoriasis or
short-term as COVID-19 and the patient profile that requires this
prophylaxis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was academically supported by the
‘Dunarea de Jos’ University of Galati, Romania, via the
Multidisciplinary Integrated Center of Dermatological Interface
Research Center.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
LB and LA conceptualized the study, and prepared and
wrote the original draft of the manuscript. The methodology,
writing-review and editing of the manuscript, as well as software
use were performed by ACL, AIS, ALT, and EN. Formal analysis was
performed by AI, FN and CD. The investigation and data curation was
performed by LB, SF and MD. The validation of data was performed by
LB, AI, CD and DCV. The review was supervised by LB, ALT, AN and
AIS. LB, ALT, MD confirm the authenticity of all the raw data. All
authors agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki, and approved by the Ethics
Committee of ‘Sf. Cuv. Parascheva’ Clinical Hospital of Infectious
Diseases of Galati, Romania (Decisions no. 104, 105, date of
approval: 30.12.2020). All patients signed informed consent to
participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
RECOVERY Collaborative Group. Horby P, Lim
WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,
Brightling C, Ustianowski A, et al: Dexamethasone in hospitalized
patients with Covid-19. N Engl J Med. 384:693–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rodríguez-Tajes S, Miralpeix A, Costa J,
López-Suñé E, Laguno M, Pocurull A, Lens S, Mariño Z and Forns X:
Low risk of hepatitis B reactivation in patients with severe
COVID-19 who receive immunosuppressive therapy. J Viral Hepat.
28:89–94. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aldhaleei WA, Alnuaimi A and Bhagavathula
AS: COVID-19 induced hepatitis B virus reactivation: A novel case
from the United Arab Emirates. Cureus. 12(e8645)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baroiu L, Dumitru C, Iancu A, Leșe AC,
Drăgănescu M, Baroiu N and Anghel L: COVID-19 impact on the liver.
World J Clin Cases. 9:3814–3825. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Trifan A, Stanciu C, Iliescu L, Sporea I,
Baroiu L, Diculescu M, Luca MC, Miftode E, Cijeveschi C, Mihai C,
et al: Effectiveness of 8- and 12-week treatment with
ombitasvir/paritaprevir/ritonavir and dasabuvir in treatment-naïve
HCV patients in a real-life setting in Romania: The AMETHYST study.
J Gastrointestin Liver Dis. 30:88–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chiu HY, Chiu YM, Chang Liao NF, Chi CC,
Tsai TF, Hsieh CY, Hsieh TY, Lai KL, Chiu TM, Wu NL, et al:
Predictors of hepatitis B and C virus reactivation in patients with
psoriasis treated with biologic agents: A 9-year multicenter cohort
study. J Am Acad Dermatol. 85:337–344. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aygen B, Demir AM, Gumus M, Karabay O,
Kaymakoğlu S, Köksal AŞ, Köksal İ, Örmeci N and Tabak F:
Immunosuppressive therapy and the risk of hepatitis B reactivation:
Consensus report. Turk J Gastroenterol. 29:259–269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
WHO: Global Hepatitis Report 2017.
https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
Accessed February 8, 2021.
|
|
9
|
Cannizzaro MV, Franceschini C, Esposito M,
Bianchi L and Giunta A: Hepatitis B reactivation in psoriasis
patients treated with anti-TNF agents: Prevention and management.
Psoriasis (Auckl). 7:35–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Snast I, Atzmony L, Braun M, Hodak E and
Pavlovsky L: Risk for hepatitis B and C virus reactivation in
patients with psoriasis on biologic therapies: A retrospective
cohort study and systematic review of the literature. J Am Acad
Dermatol. 77:88–97.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gheorghe G, Pantea Stoian A, Gaman MA,
Socea B, Neagu TP, Stanescu AMA, Bratu OG, Mischianu DLD, Suceveanu
AI and Diaconu CC: The benefits and risks of antioxidant treatment
in liver diseases. Rev Chim. 70:651–655. 2019.
|
|
12
|
Lopes H, Baptista-Leite R, Franco D,
Eclemea I, Bratu EC, Furtunescu FL, Pop CS and Pana BC: Modeling
the puzzle of hepatitis C epidemiology in Romania: A pathway to
control. J Gastrointestin Liver Dis. 29:377–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Niculet E, Radaschin SD, Nastase F,
Draganescu M, Baroiu L, Miulescu M, Arbune M and Tatu AL: Influence
of phytochemicals in induced psoriasis (Review). Exp Ther Med.
20:3421–3424. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tatu AL, Baroiu L, Fotea S, Anghel L,
Drima Polea E, Nadasdy T, Chioncel V and Nwabudike LC: A working
hypothesis on vesicular lesions related to COVID-19 infection,
koebner phenomena type V, and a short review of related data. Clin
Cosmet Investig Dermatol. 14:419–423. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nwabudike LC and Tatu AL: Using
complementary and alternative medicine for the treatment of
psoriasis. A step in the right direction. JAMA Dermatol.
155(636)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nwabudike LC and Tatu AL: Response to:
‘Use of complementary and alternative medicine by patients with
psoriasis’. J Am Acad Dermatol. 81(e105)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baroiu L, Beznea A, Plesea Condratovici C,
Onisor C, Grigore CA, Topor G and Rugină S: Comparative
Effectiveness of vancomycin and metronidazole for the initial
episode of nonsevere clostridium difficile infection, Rev. Chim.
70:3741–3745. 2019.
|
|
20
|
Pantea Stoian A, Pricop-Jeckstadt M, Pana
A, Ileanu BV, Schitea R, Geanta M, Catrinoiu D, Suceveanu AI,
Serafinceanu C, Pituru S, et al: Death by SARS-CoV 2: A Romanian
COVID-19 multi-centre comorbidity study. Sci Rep.
10(21613)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ancuceanu R, Dinu M, Furtunescu F and Boda
D: An inventory of medicinal products causing skin rash: Clinical
and regulatory lessons. Exp Ther Med. 18:5061–5071. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stoian AP, Catrinoiu D, Rizzo M and
Ceriello A: Hydroxychloroquine, COVID-19 and diabetes. Why it is a
different story. Diabetes Metab Res Rev. 37(e3379)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Terrault NA, Bzowej NH, Chang KM, Hwang
JP, Jonas MM and Murad MH: American Association for the Study of
Liver Diseases. AASLD guidelines for treatment of chronic hepatitis
B. Hepatology. 63:261–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan
HL, Chen CJ, Chen DS, Chen HL, Chen J, Chien RN, et al:
Asian-Pacific clinical practice guidelines on the management of
hepatitis B: A 2015 update. Hepatol Int. 10:1–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
European Association For The Study Of The
Liver. EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tatu AL, Elisei AM, Chioncel V, Miulescu M
and Nwabudike LC: Immunologic adverse reactions of β-blockers and
the skin. Exp Ther Med. 18:955–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Torsten Fredriksson: PASI score.
https://www.mdcalc.com/psoriasis-area-severity-index-pasi.
Accessed June 8, 2020.
|
|
28
|
Derm Calculator: DLQI score. http://www.dermcalculator.com/dlqi/. Accessed
June 8, 2020.
|
|
29
|
Calabrese LH, Zein NN and Vassilopoulos D:
Hepatitis B virus (HBV) reactivation with immunosuppressive therapy
in rheumatic diseases: Assessment and preventive strategies. Ann
Rheum Dis. 65:983–989. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ilie MA, Caruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging
of skin inflammation: Clinical applications and research
directions. Exp Ther Med. 17:1004–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lee YH, Bae SC and Song GG: Hepatitis B
virus (HBV) reactivation in rheumatic patients with hepatitis core
antigen (HBV occult carriers) undergoing anti-tumor necrosis factor
therapy. Clin Exp Rheumatol. 31:118–121. 2013.PubMed/NCBI
|
|
33
|
Schurich A, Pallet LJ, Lubowiecki M, Singh
HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W and
Maini MK: The third signal cytokine IL-12 rescues the anti-viral
function of exhausted HBV-specific CD8 T cells. PLoS Pathog.
9(e1003208)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zeuzem S and Carreño V: Interleukin-12 in
the treatment of chronic hepatitis B and C. Antiviral Res.
52:181–188. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bonifati C, Lora V, Graceffa D and Nosotti
L: Management of psoriasis patients with hepatitis B or hepatitis C
virus infection. World J Gastroenterol. 22:6444–6455.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chiu HY, Hui RC, Huang YH, Huang RY, Chen
KL, Tsai YC, Lai PJ, Wang TS and Tsai TF: Safety profile of
secukinumab in treatment of patients with psoriasis and concurrent
hepatitis B or C: A multicentric prospective cohort study. Acta
Derm Venereol. 98:829–834. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Combe B, Rahman P, Kameda H, Cañete JD,
Gallo G, Agada N, Xu W and Genovese MC: Safety results of
ixekizumab with 1822.2 patient-years of exposure: An integrated
analysis of 3 clinical trials in adult patients with psoriatic
arthritis. Arthritis Res Ther. 22(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Menter A, Strober BE, Kaplan DH,
Kivelevitch D, Prater EF, Stoff B, Armstrong AW, Connor C, Cordoro
KM, Davis DMR, et al: Joint AAD-NPF guidelines of care for the
management and treatment of psoriasis with biologics. J Am Acad
Dermatol. 80:1029–1072. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Teuwen LA, Geldhof V, Pasut A and
Carmeliet P: COVID-19: The vasculature unleashed. Nat Rev Immunol.
20:389–391. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tatu AL, Nadasdy T and Bujoreanu FC:
Inflammation and vascular injury as the basis of COVID-19 skin
changes: Preliminary analysis of 23 patients from the literature
[Letter]. Clin Cosmet Investig Dermatol. 14:185–186.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Levi M: Tocilizumab for severe COVID-19: A
promising intervention affecting inflammation and coagulation. Eur
J Intern Med. 76:21–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sciascia S, Aprà F, Baffa A, Baldovino S,
Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, et
al: Pilot prospective open, single-arm multicentre study on
off-label use of tocilizumab in patients with severe COVID-19. Clin
Exp Rheumatol. 38:529–532. 2020.PubMed/NCBI
|
|
43
|
Niculet E, Chioncel V, Elisei AM, Miulescu
M, Buzia OD, Nwabudike LC, Craescu M, Draganescu M, Bujoreanu F,
Marinescu E, et al: Multifactorial expression of IL-6 with update
on COVID-19 and the therapeutic strategies of its blockade
(Review). Exp Ther Med. 21(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tocilizumab. LiverTox: Clinical and
research information on drug-induced liver injury [Internet].
Bethesda (MD): National Institute of Diabetes and Digestive and
Kidney Diseases, 2012. https://www.ncbi.nlm.nih.gov/books/NBK548243.
Accessed May 11, 2021.
|
|
45
|
Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH
and Dai L: Short-course tocilizumab increases risk of hepatitis B
virus reactivation in patients with rheumatoid arthritis: A
prospective clinical observation. Int J Rheum Dis. 20:859–869.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sonneveld MJ, Murad SD, van der Eijk AA
and de Man RA: Fulminant liver failure due to hepatitis B
reactivation during treatment with tocilizumab. ACG Case Rep J.
6(e00243)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC,
Luk HW, Lui GC and Chan HL: Risk of hepatitis B surface antigen
seroreversion after corticosteroid treatment in patients with
previous hepatitis B virus exposure. J Hepatol. 72:57–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Voicu DF, Anghel L, Baroiu L and Stan D:
About minimal hepatic encephalopathy. BRAIN. 11:70–77. 2020.
|
|
49
|
Luca L, Baroiu L, Ciubara AB, Anghel R,
Bulgaru-Iliescu AI, Anghel L and Ciubara A: Covid-19 and the
Spanish flu. From suffering to re-silience. BRAIN. 11:01–07.
2020.
|
|
50
|
Marin IM, Petropolou M, Baroiu L, Chirosca
AC, Anghel L and Luca L: Schizophrenia and the family burden during
the pandemic. BRAIN. 11:89–97. 2020.
|
|
51
|
Xu L, Liu J, Lu M, Yang D and Zheng X:
Liver injury during highly pathogenic human coronavirus infections.
Liver Int. 40:998–1004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma
CL, Li SB, Wang HY, Zhang S, Gao HN, et al: Clinical findings in a
group of patients infected with the 2019 novel coronavirus
(SARS-Cov-2) outside of Wuhan, China: Retrospective case series.
BMJ. 368(m606)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fan Z, Chen L, Li J, Cheng X, Yang J, Tian
C, Zhang Y, Huang S, Liu Z and Cheng J: Clinical features of
COVID-19-related liver functional abnormality. Clin Gastroenterol
Hepatol. 18:1561–1566. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y,
Li Z, Zhou G, Gou J, Qu J, et al: COVID-19: Abnormal liver function
tests. J Hepatol. 73:566–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kunutsor SK and Laukkanen JA: Hepatic
manifestations and complications of COVID-19: A systematic review
and meta-analysis. J Infect. 81:e72–e74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mirzaie H, Vahidi M, Shokoohi M,
Darvishian M, Sharifi H, Sharafi H and Karamouzian M: COVID-19
among patients with hepatitis B or hepatitis C: A systematic
review. Hepat Mon. 20(e111617)2020.
|
|
58
|
Halichidis S, Dumea E and Cambrea CS:
Seroclearance of hepatitis B surface antigen after entecavir
treatment. J Gastrointestin Liver Dis. 22(236)2013.PubMed/NCBI
|
|
59
|
Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou
G, He Q, Wang FS, Liu L and Chen J: Longitudinal changes of liver
function and hepatitis B reactivation in COVID-19 patients with
pre-existing chronic hepatitis B virus infection. Hepatol Res.
50:1211–1221. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lu J, Hu M, Zhou X, Zhu H, Wang F, Huang
J, Guo Z, Li Q, Yin Q and Yang Z: Clinical Characteristics and
outcomes in HBV carriers with COVID-19 in Wuhan, China: A
retrospective cohort study. Res Sq. 2–17. 2020.
|