Introduction
Atherosclerosis is a chronic vascular inflammatory
disease resulting from the buildup of cholesterol-rich fatty
deposits (plaques) in the artery walls and is a major contributor
to cardiovascular mortality (1).
Lipopolysaccharide (LPS), the principal surface membrane component
in the majority of Gram-negative bacteria, is known to cause
vascular inflammation (2). The
endothelial inflammatory response promotes leukocyte adhesion and
increases vascular permeability by increasing the expression levels
of several cell adhesion molecules, including vascular cell
adhesion molecule 1 (VCAM-1), intercellular adhesion molecule-1
(ICAM-1), and E-selectin, and via the release of pro-inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α), interleukin
(IL)-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1)
(3-5).
The activation of nuclear factor-κB (NF-κB) is a critical step in
the inflammatory response, as it regulates the expression of
various inflammatory mediators including cytokines and chemokines,
which promotes cell adhesion and increases endothelial permeability
(4,6,7).
Thus, medications or nutraceuticals that downregulate the
expression of inflammatory cytokines/chemokines and adhesion
molecules, and inhibit the NF-κB pathway, are promising candidates
for the treatment and prevention of atherosclerotic diseases.
Anti-inflammatory drugs are not equally effective in all patients
and are associated with adverse effects that limit their use.
Phytochemicals such as flavonoids, phenylpropanoids,
isothiocyanates, sulforaphanes, indole alkaloids, and sterol
glucosides can inhibit the production of pro-inflammatory cytokines
and subsequently their signaling pathways (8). Therefore, dietary agents with potent
anti-inflammatory activity and with few or no adverse effects are
promising for treating inflammatory diseases (9).
Spinach (Spinacia oleracea L.) is a
green leafy vegetable that a is rich source of vitamins, minerals,
phenolic compounds, and carotenoids. Its nutrient and phytochemical
contents are associated with a wide range of bioactivities,
including antioxidant, anti-inflammatory, hepatoprotective,
anti-cancer, anti-obesity, and hypolipidemic activity (10,11).
Vitamin K is naturally found in green leafy vegetables as
phylloquinone (vitamin K1), while menaquinones (vitamin
K2; MK-s, where s represents the number of
isoprenyl side-chain units) are produced by micro-organisms
including intestinal bacteria (12). Furthermore, higher concentrations
of vitamin K1 have been reported in spinach compared
with other foods and beverages (13). Vitamin K is a class of fat-soluble
vitamins and plays important roles in the coagulation cascade,
anti-inflammatory pathways, and in the regulation of serum calcium
levels and bone metabolism, all of which have an effect on
cardiovascular health (14).
Compared with vitamin K1, vitamin K2 has more
physiological benefits as it increases the regulation of
transcription factors that participate in steroid hormone
synthesis, and is associated with increased bone metabolism,
inhibition of vascular calcification, reduction of cholesterol, and
suppression of peripheral inflammation (15-17).
Lactic acid bacteria (LAB) as starter cultures,
probiotics, and producers of vitamins are vital components of
various food fermentation processes (18). Among LAB, Lactococcus lactis
are natural producers of vitamin K2 (19). Vitamin K2 production has
been observed in various bacteria that are involved in established
food fermentation processes, and the exact mechanism of vitamin
K2 production has been elucidated (20). However, earlier studies mainly
focused on the vitamin K2 levels in fermented dairy
products, with only a few studies focusing on vitamin K2
production by LAB itself (21-23).
Moreover, to the best of our knowledge, there are no available
reports on the fermentation of green vegetables such as spinach by
L. lactis.
In this study, it was hypothesized that
biotransformation of vitamin K1 to K2 in
spinach juice (S.juice) during L. lactis-mediated
fermentation can improve its health benefits. Therefore, the
objective the present work was to determine the anti-inflammatory
effects of fermented S.juice including rich vitamin K2
against the LPS-stimulated human umbilical vein endothelial cells
(HUVECs) and to elucidate the potential anti-vascular inflammatory
mechanism.
Materials and methods
Chemicals and reagents
Antibodies against VCAM-1 (cat. no. 13662), ICAM-1
(cat. no. 4915), and phospho-NF-κB p65 (cat. no. 3033), NF-κB p65
(cat. no. 8242), phospho-IκB-α (cat. no. 2859), IκB-α (cat. no.
9242), β-actin (cat. no. 4967) (all dilution, 1:1,000), and
goat-anti-rabbit IgG-horseradish peroxidase-conjugated (HRP)
secondary antibodies (dilution, 1:5,000; cat. no. 7074) were
purchased from Cell Signaling Technology, Inc. Enzyme-linked
immunosorbent assay (ELISA) kits for MCP-1 (Human MCP-1 ELISA Set;
cat. no. 555179) and IL-6 (Human IL-6 ELISA Set; cat. no. 555220)
were purchased from BD Bioscience. LPS was purchased from Sigma
Chemical Co. The NE-PER Nuclear and Cytoplasmic Extraction Reagents
kit (cat. no. 78833; Thermo Fisher Scientific, Inc.) were used.
Triton X-100 and 10% formalin solution were obtained from
Sigma-Aldrich; Merck KGaA. Additionally,
4',6-diamidino-2-phenylindole (DAPI) and Alexa Fluor dyes (488 and
555) were obtained from Thermo Fisher Scientific, Inc.
Isolation and identification of L.
lactis
A strain of L. lactis (KCCM12759P) was
isolated from sediment collected from the fish-farm tank at the
National Institute of Fisheries Science (Pohang, Republic of
Korea). The sediment sample was serially diluted, plated on De Man,
Rogosa and Sharpe (MRS; Sigma-Aldrich; Merck KGaA) Agar with
bromocresol purple, and incubated anaerobically at 30̊C for 24 h.
For 16s rDNA gene sequencing analysis, the isolated yellow colonies
were transferred to MRS broth, cultured, and DNA was subsequently
extracted using the Wizard Genomic DNA Purification kit (Promega
Corporation) following to the manufacturer's method. The 16s rDNA
gene was amplified using 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R
(5'-TACGGCTACCTTGTTACGACTT-3') primers. PCR was performed using the
AccPower PCR Premix kit (Bioneer Corporation) on a Takara PCR
Thermal Cycler Dice Gradient system (Takara Bio, Inc). The PCR
conditions were as follows: Initial denaturation at 95˚C for 5 min,
30 cycles of denaturation at 95˚C for 30 sec, annealing at 55˚C for
30 sec and extension at 72˚C for 90 sec and final extension at 72˚C
for 5 min. The PCR product was analyzed using 1% agarose gel
electrophoresis, purified with a NucleoSpin Gel and PCR clean-up
kit (cat. no. 740609.50; Macherey Nagel, Inc.), and sequenced using
an ABI 3730 sequencer (Applied Biosystems). Homology search was
performed using the Basic Local Alignment Search Tool (BLAST)
program from the NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The 16s
rDNA of a selected isolate showed 99% similarity with L.
lactis (acc. no. MG754653.1). The L. lactis used in this
study was deposited at the Korean Culture Center of Microorganisms
(KCCM12759P) and stored in vials of MRS broth with 50% (v/v)
glycerol at -70˚C until use.
Preparation of fermented spinach juice
samples
Spinach (Spinacia oleracea L.) was obtained
in April 2020 from an herbal medicine cooperative situated in
Gyeongsang Province, Republic of Korea. The isolated L.
lactis strain (1x108 CFU/ml) was inoculated into 200
ml sterile S.juice and into MRS medium as control, and incubated
anaerobically at 26˚C with agitation for 48 h. S.juice was obtained
by pressing fresh spinach, lyophilized, and 5% (w/v) of S.juice was
calculated as the weight of the dried product, corresponding to the
volume of the juice for fermentation. After fermentation, the
culture solution including the pellet and the residues of spinach
were evaporated three times at 50˚C for 1 h using a rotary
evaporator (N-1000; Eyela); the cell pellet was subsequently washed
with 10 mM PBS (pH 7.4). Vitamin K2 (menaquinone), a
fat-soluble vitamin, is produced during fermentation by bacteria
such as lactic acid bacteria (20). Vitamin K2 is commonly
recovered from microbial cells by liquid-liquid extraction or
supercritical fluid extraction and stored at -70˚C until in
experiments for vitamin K analysis (22,23).
Quantitative analysis of vitamin K in
L. lactis
The vitamin K analysis was performed according to
Liu et al (21) and
Berenjian et al (24) with
minor modifications. Briefly, the cell pellet was added to 6 ml of
resuspension solution (10 mM PBS containing 1% lysozyme) and
incubated at 37˚C for 1 h. Subsequently, 24 ml of extraction buffer
(n-hexane-isopropanol=2:1, v/v; Sigma-Aldrich; Merck KGaA)
was added, vortexed twice at 25˚C for 30 sec, and centrifugation at
3,000 x g for 10 min. Next, the lower phase was collected, an equal
volume of n-hexane was added, then evaporated at 25˚C for 30
min. Subsequently, the dried pellet was dissolved by adding 2 ml
isopropanol; the sample was then filtered with a 0.45 µm syringe
filter (MilliporeSigma) for high-performance liquid chromatography
(HPLC) analysis using an Agilent C18 column (4.6x250 mm; Agilent
Technologies, Inc.), maintained at a temperature of 40˚C during the
analysis. The flow rate, injection volume, and detection wavelength
were 0.4 ml/min, 50 µl, and 254 nm, respectively. Reagent-grade
vitamin K1 and K2 (Sigma Chemical Co.),
dissolved in methanol (0.5% w/v), were used as reference standards.
The mobile phase was a mixture of methanol, isopropanol, and
n-hexane (25:37.5:37.5; v:v:v). The calibration curve was
obtained by plotting the peak area vs. concentration. The slope,
intercept, and correlation coefficients for the calibration curve
were then determined. The analysis was performed in triplicate
(25).
Cell culture and treatment
HUVECs were purchased from Lonza Group, Ltd. and
cultured in EBM-2 basal medium (cat. no. CC-3156; Lonza Group,
Ltd.) supplemented with EGM-2 SingleQuots supplement pack (cat. no.
CC-4176; Lonza Group, Ltd.). HUVECs were incubated at 37˚C with 5%
CO2, and the medium was replaced every 48 h.
Cytotoxicity assays
The cytotoxicity of S.juice and fermented S.juice
was measured using the Cell Counting Kit-8 assay (CCK-8; Dojindo
Molecular Technologies, Inc.). HUVECs were cultured in 48-well
plates (1x105 cells/well) and pretreated with S.juice or
fermented S.juice at 2 concentrations (200 and 400 µg/ml) for 1 h
at 37˚C, followed by stimulation with LPS (10 µg/ml) for an
incubation of 18 h at 37˚C. Subsequently, 400 µl of CCK-8 working
solution was added to each well and incubated at 37˚C for 1.5 h.
Cell viability was subsequently measured using CCK-8 solution and a
microplate reader set at a detection wavelength of 450 nm (Tecan
Group, Ltd.).
Enzyme-linked immunosorbent assay
(ELISA)
HUVECs were seeded in 24-well plates
(1x105 cells/well) and treated with LPS in the presence
or absence of S.juice and fermented S.juice at 200 or 400 µg/ml for
18 h. The cell-free supernatant fractions were collected, and the
levels of MCP-1 and IL-6 released into the culture supernatant were
measured using the Quantikine ELISA kits according to the
manufacturer's instructions.
Western blotting
The whole cell proteins were isolated using
radioimmunoprecipitation assay (RIPA) lysis (cat. no. MB-030-0050;
Rockland Immunochemicals Inc.) containing a 1x protease/phosphatase
inhibitor cocktail (cat. no. 5872; Cell Signaling Technology,
Inc.). The protein quantification was performed using the Bio-Rad
Protein Assay (Bio-Rad Laboratories, Inc.). Then, 30 µg of proteins
were separated by 12% SDS-PAGE and transferred to a PVDF membrane.
The membrane was blocked with EveryBlot Blocking Buffer (cat. no.
12010020; Bio-Rad Laboratories, Inc.) for 1 h at room temperature,
then incubated with 1:1,000 diluted primary antibodies: VCAM-1,
ICAM-1, phospho-NF-κB, NF-κB, phospho-IκB-α, IκB-α, and β-actin at
4˚C overnight. Following washing with TBST, the membrane was then
incubated with 1:5,000 diluted goat-anti-rabbit IgG-HRP-conjugated
secondary antibody for 1 h at room temperature. The bands were
detected using an enhanced chemiluminescence detection kit; Clarity
Max western ECL substrate (cat.no. 1705062; Bio-Rad Laboratories,
Inc.) and were analyzed using the ImageJ software (version 1.52;
National Institutes of Health).
Nuclear and cytosolic
fractionation
An NE-PER Nuclear and Cytoplasmic Extraction Reagent
kit (Thermo Fisher Scientific, Inc.) was used to extract nuclear
and cytoplasmic proteins. After treatment with S.juice and
fermented S.juice, the HUVEC were homogenized in Cytoplasmic
Extraction Reagent I buffer supplemented with protease inhibitor
cocktail (Thermo Fisher Scientific, Inc.). After homogenization,
the cells were isolated by centrifugation at 16,000 x g for 5 min
at 4˚C in the presence of Cytoplasmic Extraction Reagent Ⅱ (Thermo
Fisher Scientific, Inc.). The supernatant of cytoplasmic extract
and left pellet were homogenized in Nuclear Extraction Reagent
buffer supplemented with protease inhibitor cocktail (Thermo Fisher
Scientific, Inc.). The cells were subsequently isolated by
centrifugation at 16,000 x g for 10 min at 4˚C; the protein content
of the supernatant (representing the nuclear extract) was
quantified using Pierce™ Coomassie Plus (Bradford) Assay kit (cat.
no. 23236; Thermo Fisher Scientific, Inc.).
Immunofluorescence
After treatment with S.juice and fermented S.juice,
HUVECs were fixed with 10% formalin for 10 min at room temperature
and immersed in 0.1% Triton X-100 (cat. no. T8787; Sigma-Aldrich;
Merck KGaA) in PBS for 15 min at room temperature. Subsequently,
the cells were blocked with 3% bovine serum albumin (BSA) (cat. no.
7906; Sigma-Aldrich; Merck KGaA) in PBS for 1 h at room temperature
and incubated overnight at 4˚C with primary antibody (NF-κB p65;
dilution, 1:200; cat. no. 8242; Cell Signaling Technology, Inc.).
For fluorescence detection, the cells were incubated for 1 h at 4˚C
in the dark with secondary goat anti-rabbit IgG Alexa Fluor 555 and
488 antibody (dilution 1:500; cat. no. 4413; Cell Signaling
Technology, Inc.) and goat anti-rabbit IgG Alexa Fluor 488 antibody
(dilution 1:200; cat. no. 4412; Cell Signaling Technology, Inc.).
Next, the cells were stained with DAPI solution for the imaging of
the cell nuclei through fluorescence microscopy (IX71; Olympus
Corporation) at the same gain and exposure time.
Statistical analysis
All the experiments were performed at least three
times and all graph bar data are presented as mean ± standard error
of the mean (SEM). The mean values of different groups were
compared using one-way analysis of variance (ANOVA) followed by
Tukey's tests (GraphPad Prism 8; GraphPad Software, Inc.). For all
data, statistical significance was defined as P<0.05.
Results
Effect of L. lactis fermentation on
vitamin K contents of S.juice
Table I shows the
respective vitamin K1 and vitamin K2 contents
of S.juice and fermented S.juice. Single-strain L.
lactis starters were used to ferment S.juice for 48 h at 26˚C.
The initial LAB cell count was ~1x108 CFU/ml. After 48 h
of fermentation with the L. lactis strains, the LAB cell
count in fermented S.juice was 3.58x1010 CFU/ml. The
vitamin K1 and K2 levels of fermented S.juice
as determined using HPLC were 1.77 and 1.89 µg/ml, respectively
(Table I). The vitamin
K2 content of fermented S.juice was approximately
47-fold higher than in S.juice. There were no significant
differences between the vitamin K1 contents of S.juice
and fermented S.juice.
 | Table IVitamin K1 and
K2 contents of S.juice and Lactococcus
lactis-fermented S.juice. |
Table I
Vitamin K1 and
K2 contents of S.juice and Lactococcus
lactis-fermented S.juice.
| | LAB counts
(CFU/ml) | |
|---|
| Sample | Initial | Final | Vitamin
K1 content (µg/ml) | Vitamin
K2 content (µg/ml) |
|---|
| S.juice | - | - | 1.75 | 0.04 |
| Fermented
S.juice |
1x108 |
3.58x1010 | 1.77 | 1.89 |
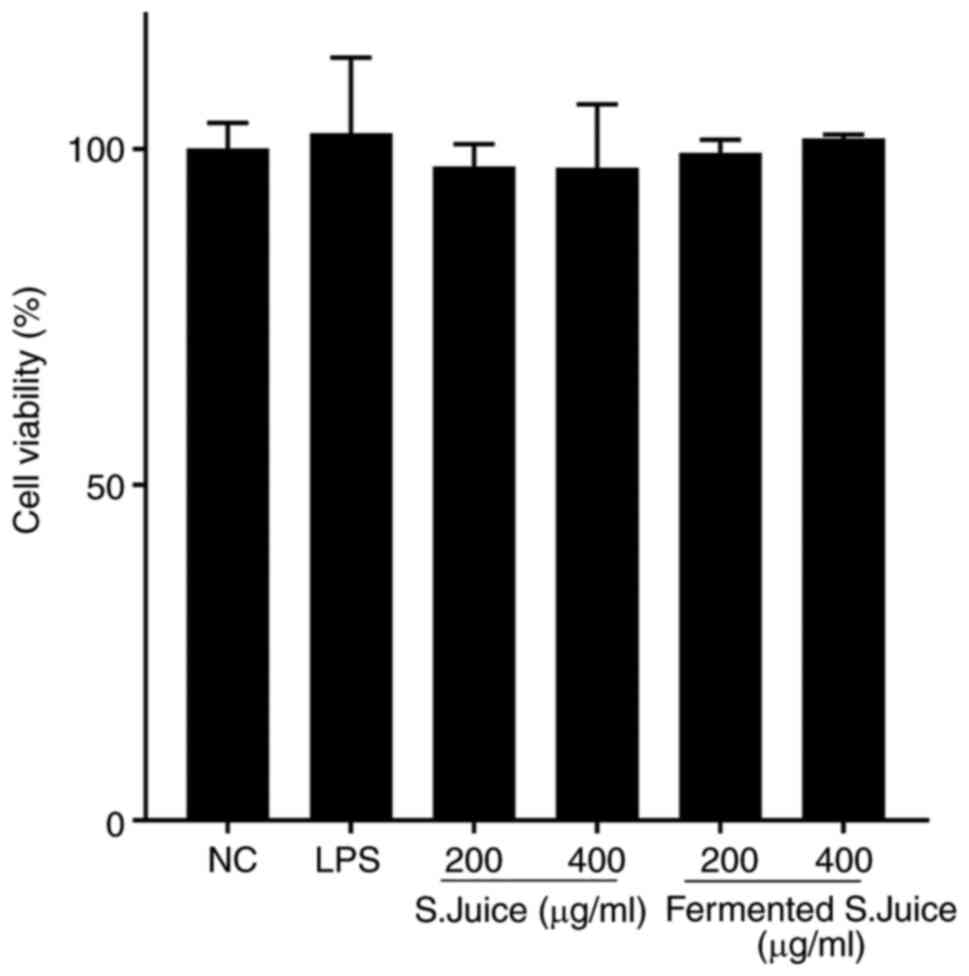
Cytotoxic effects of S.juice and
fermented S.juice in HUVECs
The potential cytotoxic effect of S.juice and
fermented S.juice against HUVECs was evaluated using CCK-8 assays
(Fig. 1). The cell viability was
not affected by S.juice and fermented S.juice at either of the test
concentrations (200 and 400 µg/ml), indicating no toxicity against
HUVECs at these concentrations.
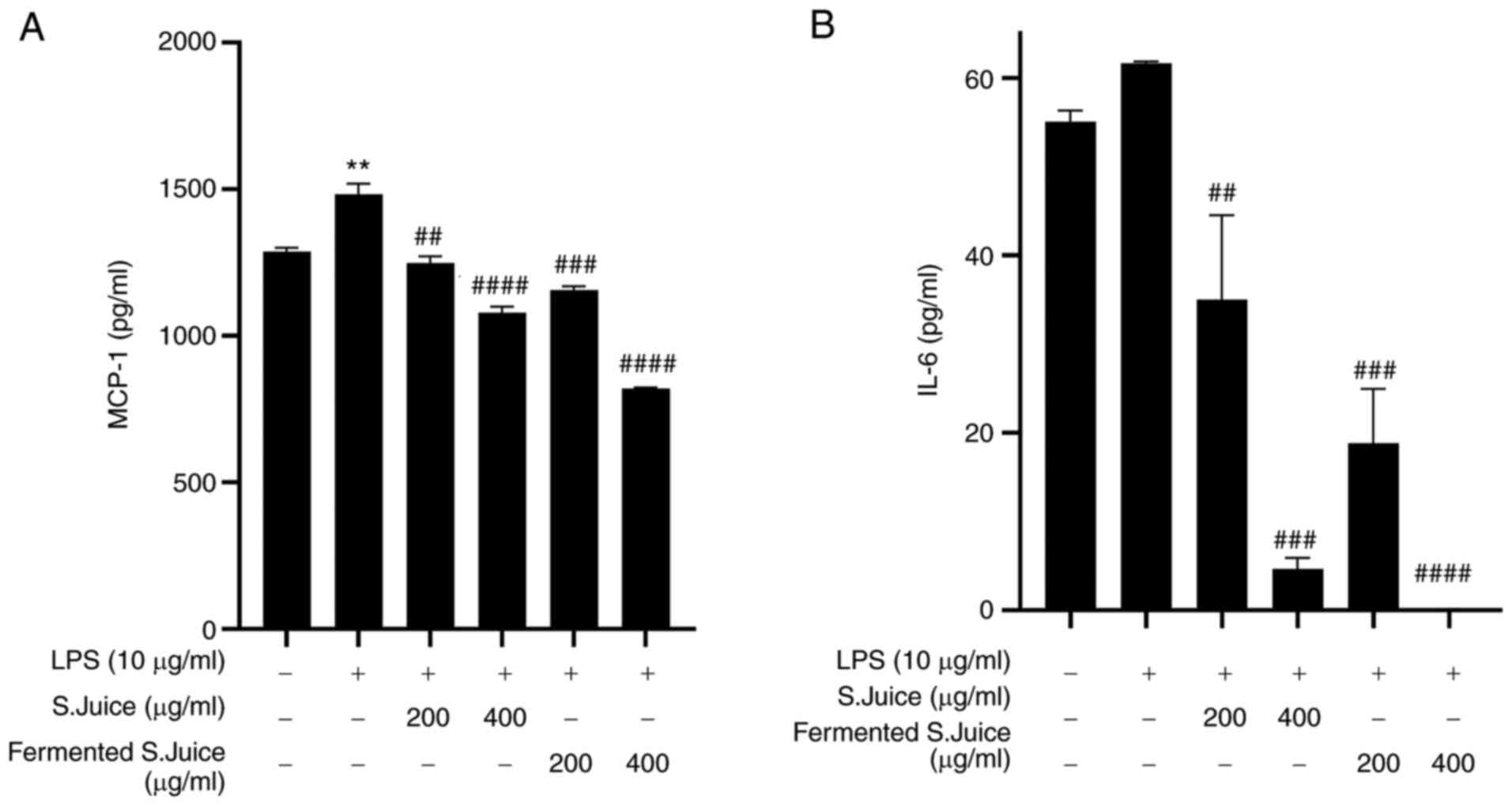
S.juice and fermented S.juice suppress
the levels of pro-inflammatory cytokines and chemokines in
LPS-stimulated HUVECs
Inflammatory cytokines and chemokines, such as MCP-1
and IL-6, play critical roles in inflammation (26). Therefore, to investigate the
potential anti-inflammatory effects of LPS-stimulated HUVECs, the
levels of MCP-1 and IL-6 were determined. Stimulation with LPS
significantly increased the production of inflammatory cytokines
such as MCP-1 and IL-6 in HUVECs (Fig.
2). However, the treatment with fermented S.juice of 400 µg/ml
markedly inhibited the levels of MCP-1 and IL-6 compared with the
treatment with S.juice, in a dose-dependent manner.
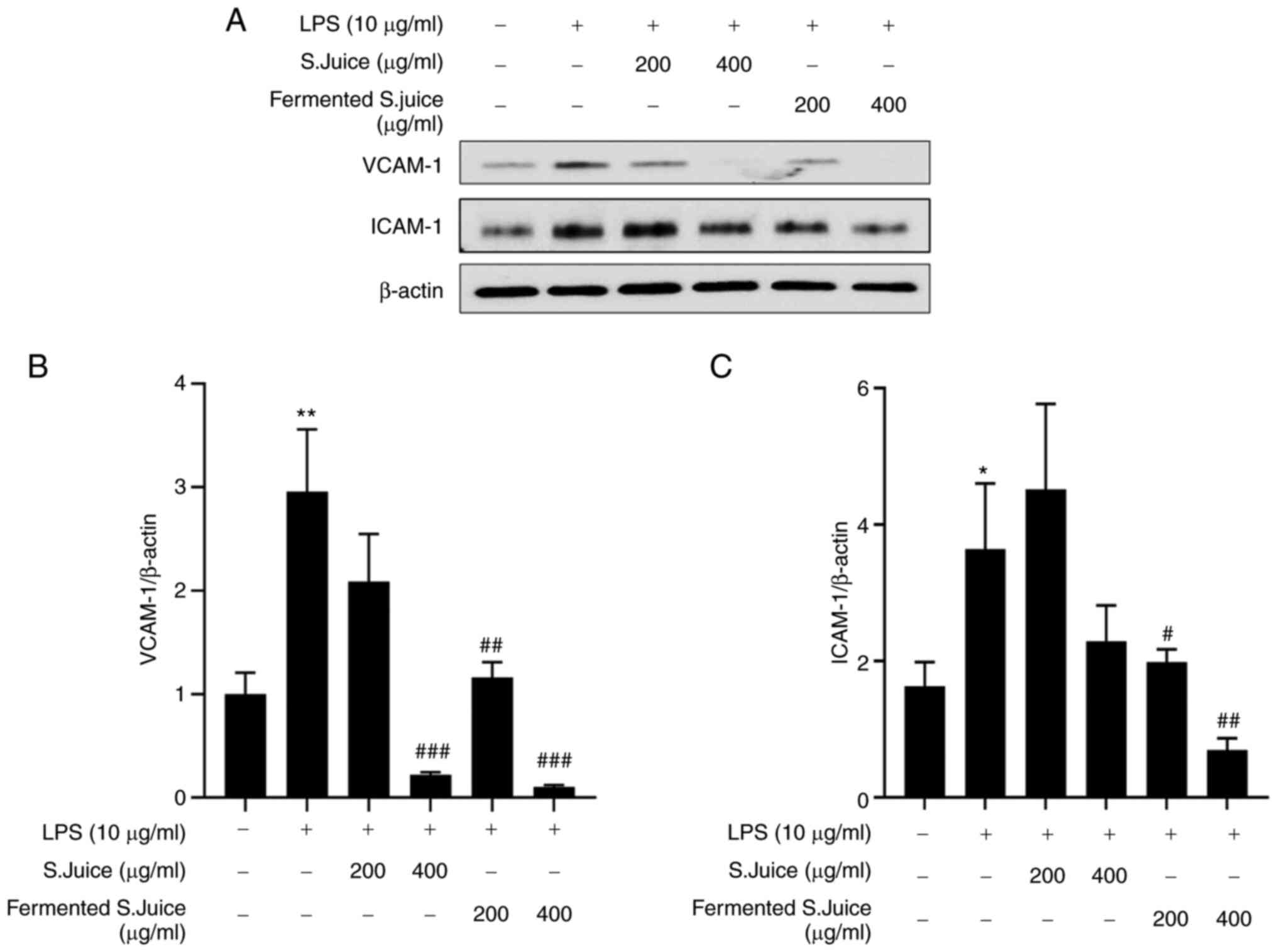
S.juice and fermented S.juice
downregulate the expression of adhesion molecules in LPS-stimulated
HUVECs
Adhesion molecules such as VCAM-1 and ICAM-1 play
major roles in the initial adhesion and subsequent
trans-endothelial migration of leukocytes into inflamed vessels
(26). In this study, the effects
of S.juice and fermented S.juice on the expression VCAM-1 and
ICAM-1 following LPS treatment were determined by western blotting.
The expression of both VCAM-1 and ICAM-1 in LPS treatment was
significantly increased compared with the control (Fig. 3A and 3B). However, the increase in the VCAM-1
and ICAM-1 levels following LPS treatment was attenuated both by
S.juice and fermented S.juice in a dose-dependent manner, with the
fermented S.juice exerting a greater effect.
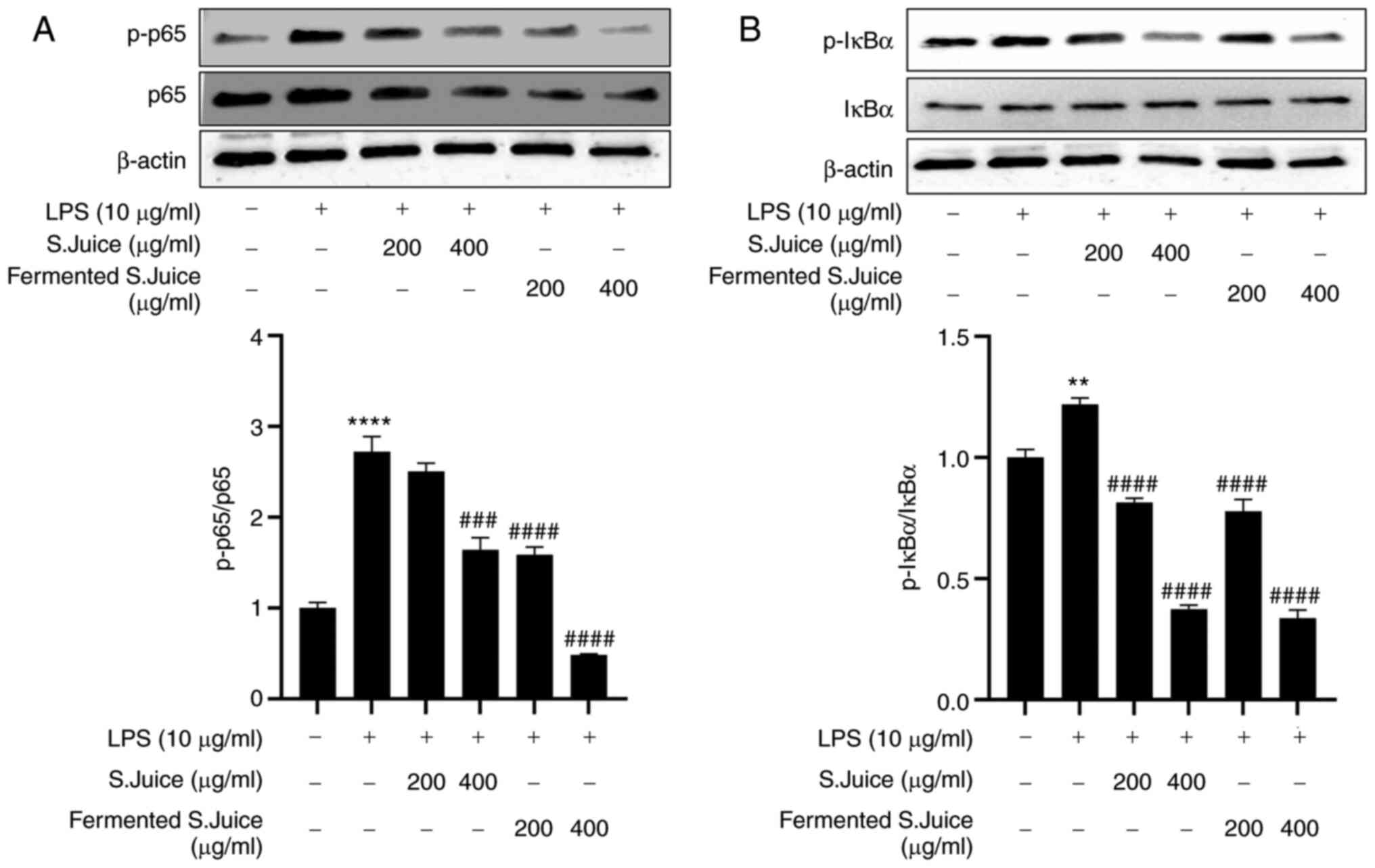
S.juice and fermented S.juice suppress
the LPS-induced activation of NF-κB
NF-κB plays an important role in regulating the
production of inflammatory mediators (27). Cytokines promote inflammation by
promoting NF-κB phosphorylation and IκB-α degradation (28). In this study, the effects of the
S.juice and fermented S.juice on NF-κB activation following LPS
treatment were investigated. The levels of NF-κB activation and
IκB-α degradation were greater following LPS treatment, compared
with the control (Fig. 4).
However, the pre-treatment with S.juice and fermented S.juice
significantly inhibited the activation of NF-κB (Fig. 4A) as well as the degradation of
IκB-α (Fig. 4B) induced by LPS in
a concentration-dependent manner.
S.juice and fermented S.juice suppress
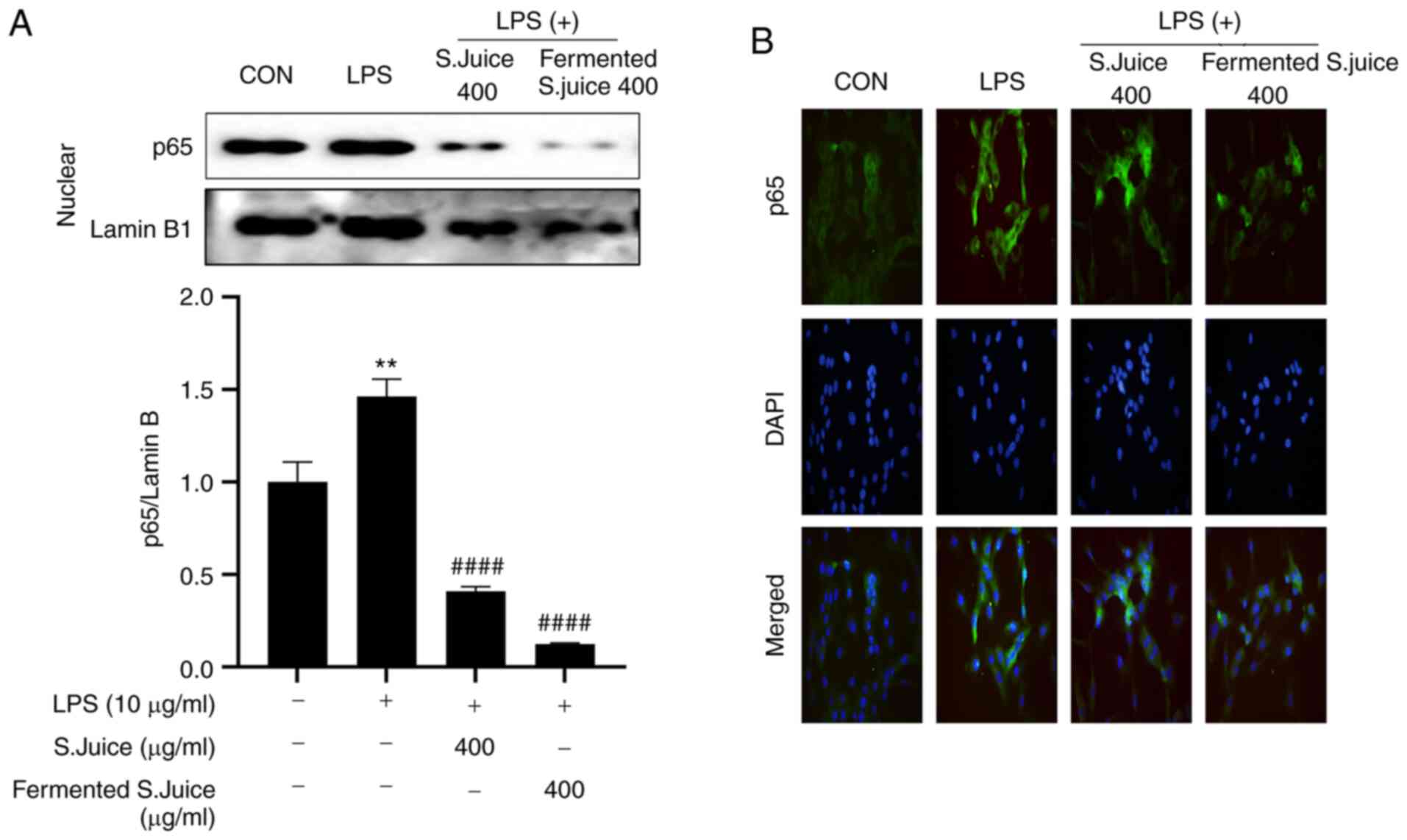
the NF-κB p65 signaling pathway in HUVECs
Once activated, the NF-κB p65 subunit is
translocated from the cytoplasm to the nucleus and regulates the
expression of target genes (28).
The effect of S.juice and fermented S.juice on LPS-induced nuclear
translocation of NF-κB p65 was investigated. Immunofluorescence
assays demonstrated that the LPS-induced nuclear translocation of
NF-κB p65 was inhibited by both S.juice and fermented S.juice
(Fig. 5). Overall, this indicated
that S.juice and fermented S.juice inhibited the production of
inflammatory factors by suppressing the NF-κB pathway. Furthermore,
fermented S.juice attenuated the LPS-induced expression of NF-κB
p65 to a greater extent than S.juice.
Discussion
The present study is, to the best of our knowledge,
the first characterization of the mechanism involved in the
anti-inflammatory effects of fermented S.juice in HUVECs. Although
spinach has been reported to have strong protective effects against
vascular inflammation, the anti-inflammatory activity of fermented
spinach products remains unclear (10). Fermentation of spinach by L.
lactis may synthesize into bioactive forms such as vitamin
K2, MK4, MK7, MK9 and to more forms (20). First, it has been demonstrated that
vitamin K2, a major bioactive component of the fermented
S.juice, inhibits the endothelial inflammatory response induced by
LPS (16,27). The anti-inflammatory effects
include the suppression of the secretion of pro-inflammatory
cytokines and chemokines and the downregulation of molecules that
facilitate adhesion to endothelial cells (10). Our mechanistic analysis revealed
that the anti-inflammatory effect of fermented S.juice was possibly
mediated via the suppression of NF-κB activation. Thus, to the best
of our knowledge, this is the first study to show that fermented
S.juice attenuates HUVECs-associated inflammatory responses
possibly via the inhibition of the NF-κB signaling pathway.
Spinach is a rich source of vitamins, minerals,
phenolic compounds, and carotenoids that are responsible for its
various bioactivities (10). The
main vitamin in spinach is vitamin K1, which is used for
the microbial biosynthesis of vitamin K2, including that
in intestinal bacteria (12,13).
An in silico study of LAB genome revealed that the genes
encoding enzymes of the vitamin K2 biosynthesis pathway
are different and vary depending on the strain (22,29).
LAB play vital roles in food fermentation processes including
vitamin K2 production (30); among the LAB, L. lactis is
associated with particularly high levels of vitamin K2
production (19,31). Our results indicate that the
vitamin K2 levels in S.juice increase during
fermentation by L. lactis. The fat-soluble vitamin K class,
including vitamin K1 and K2, regulates
vascular calcification, bone metabolism, and inflammation, all of
which may affect cardiovascular health (32). A previous study reported that the
intake of vitamin K2, but not vitamin K1,
reduced the risk of coronary heart disease mortality, all-cause
mortality, as well as severe aortic calcifications (33). The typical recommended dietary
intake of vitamin K in North America varies from 50 to >600
µg/day for vitamin K1, and from 5 to 600 µg/day for
vitamin K2 (34).
Kawashima et al (35)
reported anti-atherosclerotic effects in hypercholesterolemic
rabbits treated with vitamin K2 (MK-7; 1 to 10 mg/kg
body weight/day) including reduced intimal thickening and
ester-cholesterol deposition in the aorta, as well as a slower
progression of atherosclerotic plaques. Vitamin K2
(MK-4) decreased the levels of inflammatory markers such as IL-6,
MCP-1, and TNF-α in the liver (36); this might be attributed to the
anti-inflammatory effects of vitamin K2. Vitamin
K2 (MK-4; 0.1-10 µM) reduced the levels of IL-6, IL-1β,
and TNFα in LPS-induced MG6 mouse microglia-derived cells (37). Excess vitamin K attenuated the
general plasma inflammatory index (38). The overall expression of
pro-inflammatory cytokines and chemokines decreased in this study;
therefore, vitamin K2 might directly suppress the
vascular NF-κB pathway; this is in line with a previous report
(37). In the present study, the
treatment levels of fermented S.juice were 200 and 400 µg/ml; 400
µg/ml fermented S.juice contained approximately 211 µg of vitamin
K2 (MK-4).
Vascular inflammatory responses contribute to the
pathogenesis of atherosclerosis (39), and endothelial cells play an
important role in vascular inflammation (40). Pro-inflammatory stimuli such as LPS
activate the endothelial inflammatory response, resulting in the
secretion of pro-inflammatory cytokines that contribute to the
pathogenesis of cardiovascular disease (41). Endothelial inflammatory responses
are characterized by the overproduction of inflammatory mediators,
including pro-inflammatory chemokines (e.g., MCP-1), cytokines
(e.g., IL-6), and adhesion molecules (e.g., VCAM-1 and ICAM-1). In
the present study, LPS significantly upregulated the expression of
MCP-1, IL-6, VCAM-1, and ICAM-1; however, this pro-inflammatory
effect of LPS was significantly inhibited by pretreatment of HUVECs
with S.juice and in particular fermented S.juice.
The inhibitory effect of fermented S.juice on the
expression of inflammatory mediators was similar to that of vitamin
K2, an inhibitor of the NF-κB signaling pathway
(37). These findings indicated
that the LPS-induced secretion of MCP-1, IL-6, VCAM-1, and ICAM-1
in HUVECs was mediated via the NF-κB signaling pathway. In
addition, the inhibitory effect of fermented S.juice on
inflammatory mediators was likely mediated via inhibition of the
NF-κB pathway.
NF-κB is involved in the LPS-induced inflammation
response (42). LPS induces the
phosphorylation and degradation of IκBα, as well as the
phosphorylation, release, and nuclear translocation of NF-κB to
activate target gene expression (43,44).
To the best of our knowledge, the present study characterized for
the first time the effects of S.juice and fermented S.juice on the
LPS-induced activation of NF-κB.
These results are not direct evidence that the NF-κB
pathway is suppressed by vitamin K2 to exert a
protective effect against vascular inflammation; however, previous
data that demonstrated the role of vitamin K2 in
suppressing LPS-induced microglial inflammation (36), combined with our results, suggest
that vitamin K2-enriched fermented S.juice may inhibit
LPS-induced inflammation by suppressing the NF-κB signaling
pathway.
In conclusion, this is, to the best of our
knowledge, the first report of the inhibitory effect of fermented
S.juice against LPS-induced inflammation via suppression of NF-κB
signaling and downregulation of ICAM-1, VCAM-1, IL-6, and MCP-1 in
HUVECs. Our present findings indicate that fermented S.juice might
be a potential novel anti-inflammatory agent against vascular
inflammation or cardiovascular disease induced by inflammation.
Acknowledgements
The authors thank Dr Yumi Kim (Korea Food Research
Institute, Iseo-myeon, South Korea) for helping with the
immunofluorescence assay.
Funding
Funding: This study was supported by grants from the Korea Food
Research Institute (grant no. E0210102) and the Korea Institute of
Planning and Evaluation for Technology in Food, Agriculture, and
Forestry (grants no. GA119027 and GA121047).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SMH and SHL conceived and designed the study. ARH
performed the experiments. MJS and BMK performed the data analysis.
SHL wrote the original draft. SHL, SMH and MJS confirm the
authenticity of all the raw data. All authors discussed the results
and reviewed the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Awan Z and Genest J: Inflammation
modulation and cardiovascular disease prevention. Eur J Prev
Cardiol. 22:719–733. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Doherty TM, Shah PK and Arditi M:
Lipopolysaccharide, toll-like receptors, and the immune
contribution to atherosclerosis. Arterioscler Thromb Vasc Biol.
25(e38)2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liang J, Yuan S, Wang X, Lei Y, Zhang X,
Huang M and Ouyang H: Attenuation of pristimerin on TNF-α-induced
endothelial inflammation. Int Immunopharmacol.
82(106326)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang W, Huang M, Ouyang H, Peng J and
Liang J: Oridonin inhibits vascular inflammation by blocking NF-κB
and MAPK activation. Eur J Pharmacol. 826:133–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang L and Wang F, Zhang Q, Kiang Q, Wang
S, Xian M and Wang F: Anti-inflammatory and anti-apoptotic effects
of Stybenpropol A on human umbilical vein endothelial cells. Int J
Mol Sci. 20(5383)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao J, Zhao WX, Zhou LJ, Zeng BX, Yao SL,
Liu D and Chen ZQ: Protective effects of propofol on
lipopolysaccharide-activated endothelial cell barrier dysfunction.
Inflamm Res. 55:385–392. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rao C, Liu B, Huang D, Chen R, Huang K, Ki
F and Dong N: Nucleophosmin contributes to vascular inflammation
and endothelial dysfunction in atherosclerosis progression. J Thora
Cardiovasc Surg. 161:e377–e393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Manach C, Scalbert A, Morand C, Rémésy C
and Jiménez L: Polyphenols: Food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Patil KR, Mahajan UB, Unger BS, Goyal SN,
Belemkar S, Surana SJ, Ojha S and Patil CR: Animal models of
inflammation for screening of anti-inflammatory drugs: Implications
for the discovery and development of phytopharmaceuticals. Int J
Mol Sci. 20(4367)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ishii M, Nakahara T, Araho D, Murakami J
and Nishimura M: Glycolipids from spinach suppress LPS-induced
vascular inflammation through eNOS and NK-κB signaling. Biomed
Pharmacother. 91:111–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roberts JL and Moreau R: Functional
properties of spinach (Spinacia oleracea L.) phytochemicals
and bioactives. Food Funct. 7:3337–3353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shearer MJ and Newman P: Metabolism and
cell biology of vitamin K. Thromb Haemost. 100:530–547.
2008.PubMed/NCBI
|
|
13
|
Booth SL, Davidson KW and Sadowski JA:
Evaluation of an HPLC method for the determination of phylloquinine
(vitamin K1) in various food matrixes. J Agric Food Chem.
42:295–300. 1994.
|
|
14
|
McCann JC and Ames BN: Vitamin K, an
example of triage theory: Is micronutrient inadequacy linked to
diseases of aging? Am J Clin Nutr. 90:889–907. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ichikawa T, Horie-Inoue K, Ikeda K,
Blumberg B and Inoue S: Steroid and xenobiotic receptor SXR
mediates vitamin K2-activated transcription of extracellular
matrix-related genes and collagen accumulation in osteoblastic
cells. J Biol Chem. 281:16927–16934. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ohsaki Y, Shirakawa H, Miura A, Giriwono
PE, Sato S, Ohashi A, Iribe M, Goto T and Komai M: Vitamin K
suppresses the lipopolysaccharide-induced expression of
inflammatory cytokines in cultured macrophage-like cells via the
inhibition of the activation of nuclear factor κB through the
repression of IKKα/β phosphorylation. J Nutr Biochem. 21:1120–1126.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yokoyama T, Miyazawa K, Naito M, Toyotake
J, Tauchi T, Itoh M, You A, Hayashi Y, Georgescu MM, Kondo Y, et
al: Vitamin K2 induces autophagy and apoptosis simultaneously in
leukemia cells. Autophagy. 4:629–640. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bintsis T: Lactic acid bacteria as starter
cultures: An update in their metabolism and genetics. AIMS
Microbiol. 4:665–684. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bøe CA and Holo H: Engineering
Lactococcus lactis for increased vitamin K2 production.
Front Bioeng Biotechnol. 8(191)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Z, Liu L, Liu C, Sun Y and Zhang D:
New aspects of micrbial vitamin K2 production by expanding the
product spectrum. Microb Cell Fact. 20:84–96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Y, van Bennekom EO, Zhang Y, Abee T
and Smid EJ: Long-chain vitamin K2 production in Lactococcus
lactis is influenced by temperature, carbon source, aeration
and mode of energy metabolism. Micro Cell Fact.
18(129)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chollet M, Guggisberg D, Portmann R, Risse
MC and Walther B: Determination of menaquinone production by
Lactococcus spp. and propionibacteria in cheese. Int Dairy J.
75:1–9. 2017.
|
|
23
|
Morishita T, Tamura N, Makino T and Kudo
S: Production of menaquinones by lactic acid bacteria. J Dairy Sci.
82:1897–1903. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Berenjian A, Mahanama R, Talbot A, Biffin
R, Regtop H, Valtchev P, Kavanagh J and Dehghani F: Efficient media
for high menaquinone-7 production: Response surface methodology
approach. N Biotechnol. 28:665–672. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Irvan Atsuta Y, Saeki T, Daimon H and
Fujie K: Supercritical carbon dioxide extraction of ubiquinones and
menaquinones from activated sludge. J Chromatogr A. 1113:14–19.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bhaskar S, Sudhakaran PR and Helen A:
Quercetin attenuates atherosclerotic inflammation and adhesion
molecule expression by modulating TLR-NF-κB signaling pathway. Cell
Immunol. 310:131–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Tang Y, Ma B, Wang Z, Jiang J, Hou
S, Wang S, Zhang J, Deng M, Duan Z, et al: The peptide lycosin-I
attenuates TNF-α-induced inflammation in human umbilical vein
endothelial cells via IκB/NF-κB signaling pathway. Inflamm Res.
67:455–466. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Han BH, Song CH, Yoon JJ, Kim HY, Seo CS,
Kang DG, Lee YJ and Lee HS: Anti-vascular inflammatory effect of
ethanol extract from Securinega suffruticosa in human umbilical
vein endothelial cells. Nutrients. 12(3448)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Watthanasakphuban N, Virginia LJ, Haltrich
D and Peterbauer C: Analysis and reconstitution of the menaquinone
biosynthesis pathway in lactiplantibacillus plantarum and
lentilactibacillus buchneri. Microorganisms. 9(1476)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Y, Charamis N, Boeren S, Blok J, Lewis
AG, Smid EJ and Abee T: Physiological roles of short-chain and
long-chain menaquinones (vitamin K2) in Lactococcus cremoris. Front
Microbiol. 13(823623)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Garrigues C and Pederson MB:
Lactococcus lactis strain with high vitamin K2 production.
US Patent 8765118B2. Filed August 11,. 2011, issued March 15,
2012.
|
|
32
|
Fusaro M, Gallieni M, Rizzo MA, Stucchi A,
Delanaye P, Cavalier E, Moysés RMA, Jorgetti V, Iervasi G, Giannini
S, et al: Vitamin K plasma levels determination in human health.
Clin Chem Lab Med. 55:789–799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Geleijnse JM, Vermeer C, Grobbee DE,
Schurgers LJ, Knapen MH, van der Meer IM, Hofman A and Witteman JC:
Dietary intake of menaquinone is associated with a reduced risk of
coronary heart disease: The Rotterdam study. J Nutr. 134:3100–3105.
2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Marles RJ, Roe AL and Oketch-Rabah HA: US
pharmacopeial convention safety evaluation of menaquinone-7, a form
of vitamin K. Nutr Rev. 75:553–578. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawashima H, Nakajima Y, Matubara Y,
Nakanowatari J, Fukuta T, Mizuno S, Takahashi S, Tajima T and
Nakamura T: Effects of vitamin K2 (menatetrenone) on
atherosclerosis and blood coagulation in hypercholesterolemic
rabbits. Jpn J Pharmacol. 75:135–143. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Weisell J, Ruotsalainen AK, Näpänkangas J,
Jauhiainen M and Rysä J: Menaquinone 4 increases plasma lipid
levels in hypercholesterolemic mice. Sci Rep.
11(3014)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saputra WD, Aoyama N, Komai M and
Shirakawa H: Menaquinone-4 suppresses lipopolysaccharide-induced
inflammation in MG6 mouse microglia-derived cells by inhibiting the
NF-κB signaling pathway. Int J Mol Sci. 20(2317)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shea MK, Booth SL, Massaro JM, Jacques PF,
D'Agostino RB Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ,
Kathiresan S, Keaney JF Jr, et al: Vitamin K and vitamin D status:
Associations with inflammatory markers in the Framingham offspring
study. Am J Epidemiol. 167:313–320. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chistiakov DA, Sobenin IA and Orekhov AN:
Regulatory T cells in atherosclerosis and strategies to induce the
endogenous atheroprotective immune response. Immunol Lett.
151:10–22. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Makó V, Czúcz J, Weiszhár Z, Herczenik E,
Matkó J, Prohászka Z and Cervenak L: Proinflammatory activation
pattern of human umbilical vein endothelial cells induced by IL-1β,
TNF-α, and LPS. Cytometry A. 77:962–970. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xiao L, Liu Y and Wang N: New paradigms in
inflammatory signaling in vascular endothelial cells. Am J Physiol
Heart Circ Physiol. 306:H317–H325. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fu Y, Wei Z, Zhou E, Zhang N and Yang Z:
Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-induced
inflammatory response in mouse mastitis model. J Lipid Res.
55:1111–1119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Baker RG, Hayden MS and Ghosh S: NF- κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Khakpour S, Wilhelmsen K and Hellman J:
Vascular endothelial cell Toll-like receptor pathway in sepsis.
Innate Immun. 21:827–846. 2015.PubMed/NCBI View Article : Google Scholar
|