Introduction
Malignant melanoma is an aggressive type of cancer
that has an increased rate of mortality and morbidity, being
responsible for >60% of all deaths from skin cancer. It arises
from transformed melanocytes and may occur on cutaneous or
non-cutaneous sites, such as the oral mucosa, paranasal sinuses,
urinary tract or eye (1). As the
incidence of melanoma has increased at a steady rate over the last
decades (it is rising by 3-8% per year in the Caucasian population)
(2), early detection of malignant
melanoma is vital for lowering both mortality and morbidity by
identifying patients prone to developing this type of neoplasia,
individuals with pre-cancerous lesions or with de novo
melanoma and adopting an appropriate management of the lesions,
with surgical excision frequently being curative for primary
cutaneous melanoma (3,4).
Pathological features of the primary melanoma, such
as tumor thickness (Breslow index), rate of mitosis and presence of
ulceration, are major prognostic factors. These characteristics may
be evaluated after localization and biopsy or surgical resection of
the tumor (5). On the other hand,
advanced metastatic melanoma requires a more apprehensive approach,
as in most of the cases, it cannot be managed only by surgery and
requires therapeutic alternatives. In order to attain a proper
management of this malignancy, potential metastatic lesions should
be detected in a timely manner due to high mortality rates. An
improved understanding of the molecular pathogenesis of malignant
melanoma proves to be valuable when assessing patients for the
requirement of newer therapeutic approaches, such as immunotherapy
(6).
Immunohistochemical staining for molecular markers
represents an important step not only in the diagnosis of malignant
melanoma, but also in staging, evaluating prognosis, establishing
treatment management and in predicting recurrence of the disease
(7). Actual molecular information
suggests that melanoma should be evaluated as a heterogeneous group
of lesions with different defects in molecular aspects that involve
distinct alterations of cellular processes including cell
signaling, cell differentiation, cell adhesion and apoptosis
(8). The histological features of
melanomas imitate those of lymphomas, sarcomas, neuroendocrine
tumors and Merkel cell carcinomas; for instance, both express
epithelial cytokeratin 20 and endothelial markers (9). In the present study, the correlations
between the specific biomarkers associated with malignant melanoma,
including S100 protein family, Ki67, HMB-45 and Melan A, as well as
the staging of the malignancy were highlighted, and the important
features of each prognostic factor were discussed.
Materials and methods
Patients and treatment
Immunohistochemical analysis was performed on 56
formalin-fixed paraffin-embedded cutaneous melanoma samples. All of
the cases covering a period of 2 years (January 2019-December 2020)
were retrieved from the archive of ‘Prof. Dr. Agrippa Ionescu’
Clinical Emergency Hospital (Bucharest, Romania). The cases
included the following histological subtypes of melanoma:
Lentiginous (n=10), nodular (n=18), superficial spreading melanomas
(SSM, n=17), acrallentiginous (n=10) and desmoplastic melanoma
(n=1). Out of all the lesions tested, 6 were metastatic malignant
melanoma and 11 cases suffered recurrence of the disease after
surgical removal of the neoplasia. All 56 patients underwent
further investigation by lymphoscintigraphy, with sentinel lymph
nodes being positive in 16 cases who were then subjected to
lymphadenectomy. A total of 10 patients presented with minor
postoperative complications (seroma, wound dehiscence) and special
dressings were used.
Patient analysis
The biomarkers tested in tumor samples were protein
S100, Ki67, HMB-45 and Melan A, the most important biomarkers used
for malignant melanoma (2).
Furthermore, each patient's characteristics, including sex, age,
smoking status, alcohol consumption, comorbidities and chronic
medication, as well as the particular features of the lesions,
including topography, lymph node metastasis and histological
aspects, were recorded. Regarding the histopathological features of
the melanomas, evaluation using the Clark and Breslow scales
(1) was performed, the mitotic
rate, the subtype of the lesion and the presence of ulceration were
determined. The anatomical sites of the lesions taken into
consideration were as follows: The face, trunk and extremities. The
entire information was inputted into a database on which
statistical analysis was performed.
Statistical analysis
Values are expressed as n (%) for count data and as
the mean ± standard deviation for continuous variables. Statistical
analysis was performed by using SPSS version 23.0 software (IBM
Corp.). Comparison of the averages for the continuous quantitative
variables between patients with and without relapse was performed
using the nonparametric Mann-Whitney U-test. Furthermore, the
frequencies were compared using both Fisher's exact test and the
χ2 test. In order to analyze the relationship between
recurrence and immunological or histopathological characteristics,
the odds ratios (OR) with a confidence interval (CI) of 95% were
determined.
Results
Patients
The patients included in the study had a mean age of
57.9±15.4 years, with no differences regarding the presence of
relapses. There was no difference in terms of age. Regarding
therapeutic approaches, in all 56 cases, surgical removal of the
lesions with oncological safety margins was performed.
The patients who suffered relapses did not exhibit
any differences in the prevalence of chronic diseases from those of
the patients without recurrences. Among patients with relapses,
90.9% were male patients and the risk of relapse was 8.75 times
higher in males (OR=8.75; 95% CI=1.03-74.18; Table I). A total of 3 (27.3%) of the
relapsing patients were smokers and 5 (45.5%) of them drank alcohol
occasionally. Furthermore, 45.5% (5 patients) of those who suffered
recurrences took long-term medication for other pathologies, most
commonly type II diabetes associated with chronic renal disease,
arterial hypertension or cardiopathies, which was higher than the
rate in those who did not relapse (n=21, 46.7%), but there was no
difference with this regard.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics | RELAPSING (N=11) N
(%) | NON-RELAPSING (N=45)
N (%) | P value |
|---|
| Age (years) average ±
SD | 58.2±16.7 | 57.8±15.2 | 0.826 |
| Sex | | | 0.022 |
|
Male | 10 (90.9) | 24 (53.3) | |
|
Female | 1 (9.1) | 21 (46.7) | |
| Comorbidities | | | |
|
Hypertension | 6 (54.5) | 18 (40.0) | 0.382 |
|
Diabetes
Mellitus | 1 (9.1) | 6 (13.3) | 0.703 |
|
Cardiopathies | 3 (27.3) | 9 (20.0) | 0.335 |
|
Venous
insufficiency | 3 (27.3) | 6 (13.3) | 0.259 |
|
Other cancer
types | 1 (9.1) | 7 (15.6) | 0.832 |
|
Surgical
history | 7 (63.6) | 19 (42.2) | 0.351 |
| Smoking | 3 (27.3) | 12 (26.7) | 0.973 |
| Alcohol use | 5 (45.5) | 17 (37.8) | 0.640 |
| Long-term use of
drugs | 5 (45.5) | 21 (46.7) | 0.942 |
Among the patients suffering recurrence of the
lesions, 90.9% (10 cases) were males. Furthermore, 63.6% of the
relapsing patients and 26.7% of the non-relapsing patients
presented with melanoma located in the cranial area. The average
mitosis rate in patients who suffered recurrences (9.0±3.7) was
significantly higher (P=0.023) compared with that in the patients
whose melanoma did not recur (5.7±4.3). Similarly, the Breslow
depth was significantly higher (P<0.001) in patients who
suffered recurrences than that in patients without (9.2±6.1 vs.
3.5±2.3; Table II).
 | Table IIImmunological and histopathological
characteristics and their association with relapse. |
Table II
Immunological and histopathological
characteristics and their association with relapse.
| Patient
characteristic | Relapsing
(n=11) | Non-relapsing
(n=45) | OR (95% CI) | P-value |
|---|
| Localization of
melanoma | | | | |
|
Head | 7 (63.6) | 12 (26.7) | 4.81
(1.2-19.4) | 0.027 |
|
Trunk | 0 (0.0) | 17 (37.8) | 0.08 (0.0-1.3) | 0.072 |
|
Limbs | 4 (36.4) | 16 (35.6) | 1.04 (0.3-4.1) | 0.960 |
| Lymph node
metastases | 6 (54.5) | 10 (22.2) | 4.20
(1.1-16.7) | 0.041 |
| Capsular
invasion | 10 (90.0) | 24 (53.4) | 8.75
(1.1-74.2) | 0.047 |
|
Immunophenotyping | | | | |
|
HMB-45 | 1 (9.1) | 15 (33.3) | 0.20 (0.1-1.7) | 0.111 |
|
S100 | 8 (72.8) | 16 (35.6) | 4.83
(1.2-20.8) | 0.034 |
|
Melan A | 3 (27.3) | 14 (31.1) | 0.83 (0.2-3.6) | 0.804 |
|
Ki67 | | | 5.41
(1.3-22.0) | 0.018 |
|
<30% | 4 (36.4) | 34 (75.6) | | |
|
>30% | 7 (63.6) | 11 (24.4) | | |
| Tumoral foci | 1.3±1.7 | 0.8±2.1 | 0.32
(-1.9-0.9) | 0.467 |
| Histological
subtype | | | | |
|
Lentiginous | 2 (18.2) | 8 (17.8) | 1.02 (0.2-5.7) | 0.975 |
|
Nodular | 1 (9.1) | 17 (37.8) | 0.16 (0.1-1.4) | 0.092 |
|
Superficial
spreading | 3 (27.3) | 14 (31.1) | 0.83 (0.2-3.6) | 0.804 |
|
Acrallentiginous | 5 (45.5) | 5 (11.1) | 6.67
(1.5-30.1) | 0.013 |
|
Others | 0 (0.0) | 1 (2.2) | 1.29
(0.1-33.8) | 0.878 |
| Mitoses | 9.0±3.7 | 5.7±4.3 | (-6.1-0.5) | 0.023 |
| Breslow depth | 9.2±6.1 | 3.5±2.3 | (-7.9-3.4) | <0.001 |
| Ulcerations | 2 (18.2) | 16 (35.6) | 0.40 (0.1-2.1) | 0.279 |
| Clark level | 2.8±1.5 | 3.1±1.3 | (-0.6-1.2) | 0.508 |
Lesions
Of the lesions tested, the most common subtypes of
malignant melanoma investigated at our clinic were nodular melanoma
and malignant melanoma with an adjacent component of SSM, followed
by the lentiginous, acrallentiginous and desmoplastic melanomas.
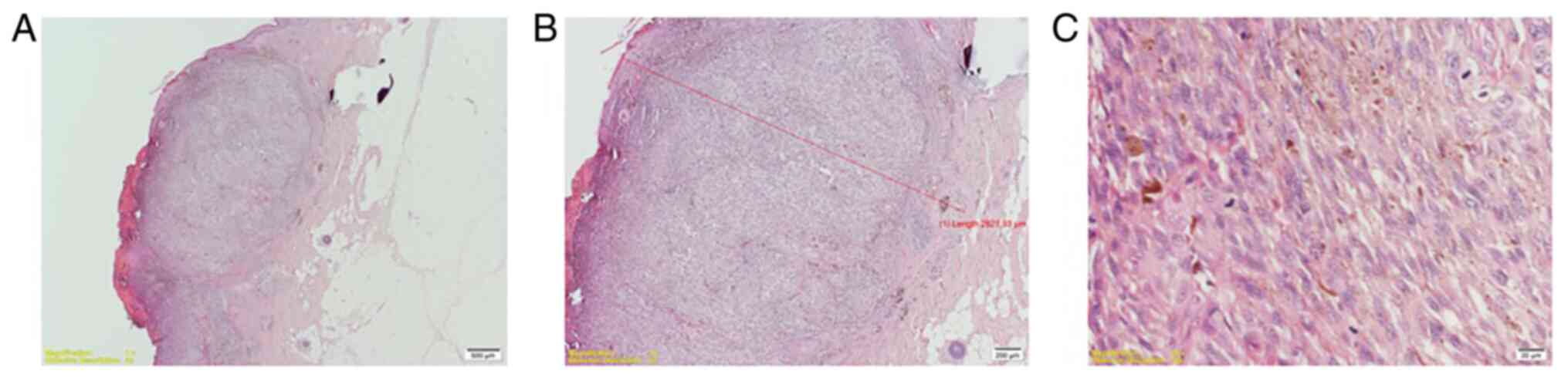
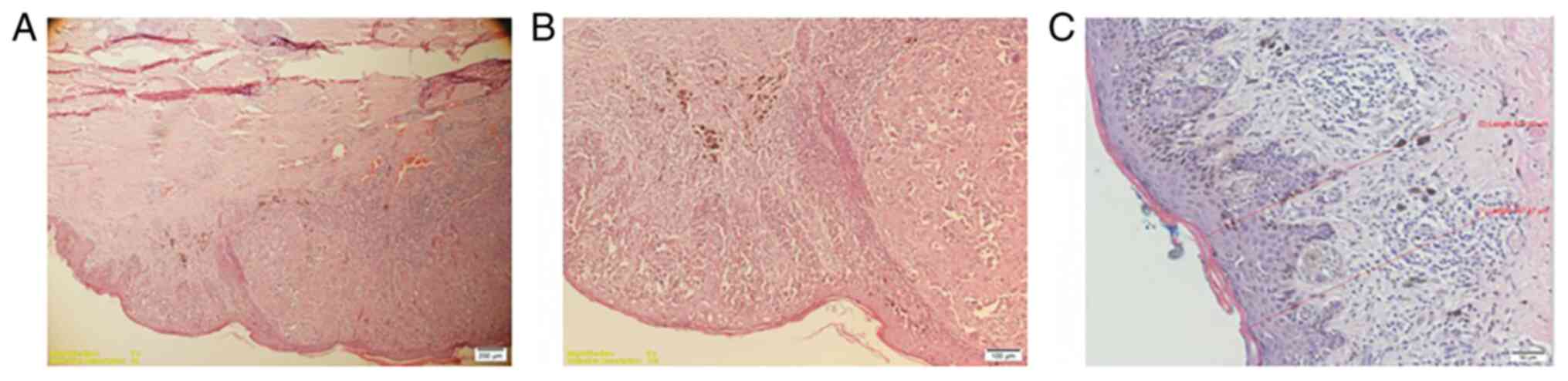
Nodular melanoma (Fig. 1) was found
in 18 patients. superficial spreading melanoma (Fig. 2) in 17 patients and 10 patients were
diagnosed with lentiginous melanoma (Fig. 3).
The most frequent location of acrallentiginous
melanoma (ALM) was on the lower limbs and it was associated with a
higher incidence of recurrence compared to any other subtype. The
histological subtype of the melanoma may be an important predictive
factor in the evolution of the lesion and patients with ALM had a
6.67-fold increased risk of developing recurrence (P=0.013)
compared to those with other histological patterns (Table II).
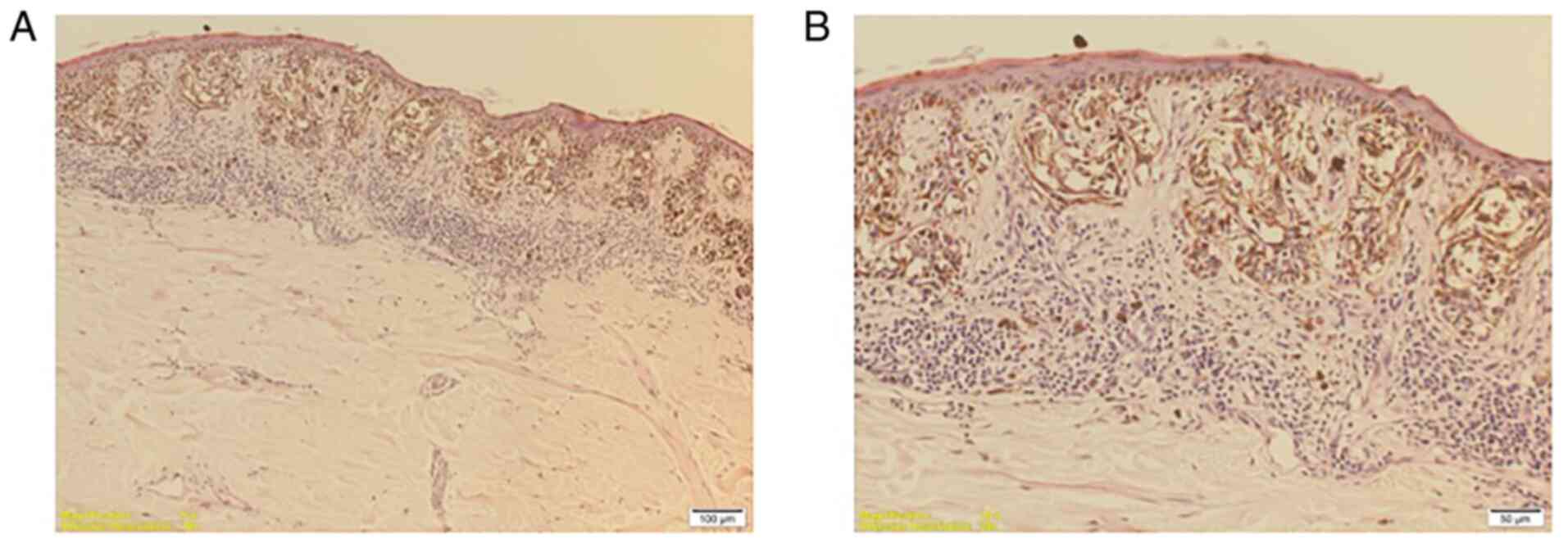
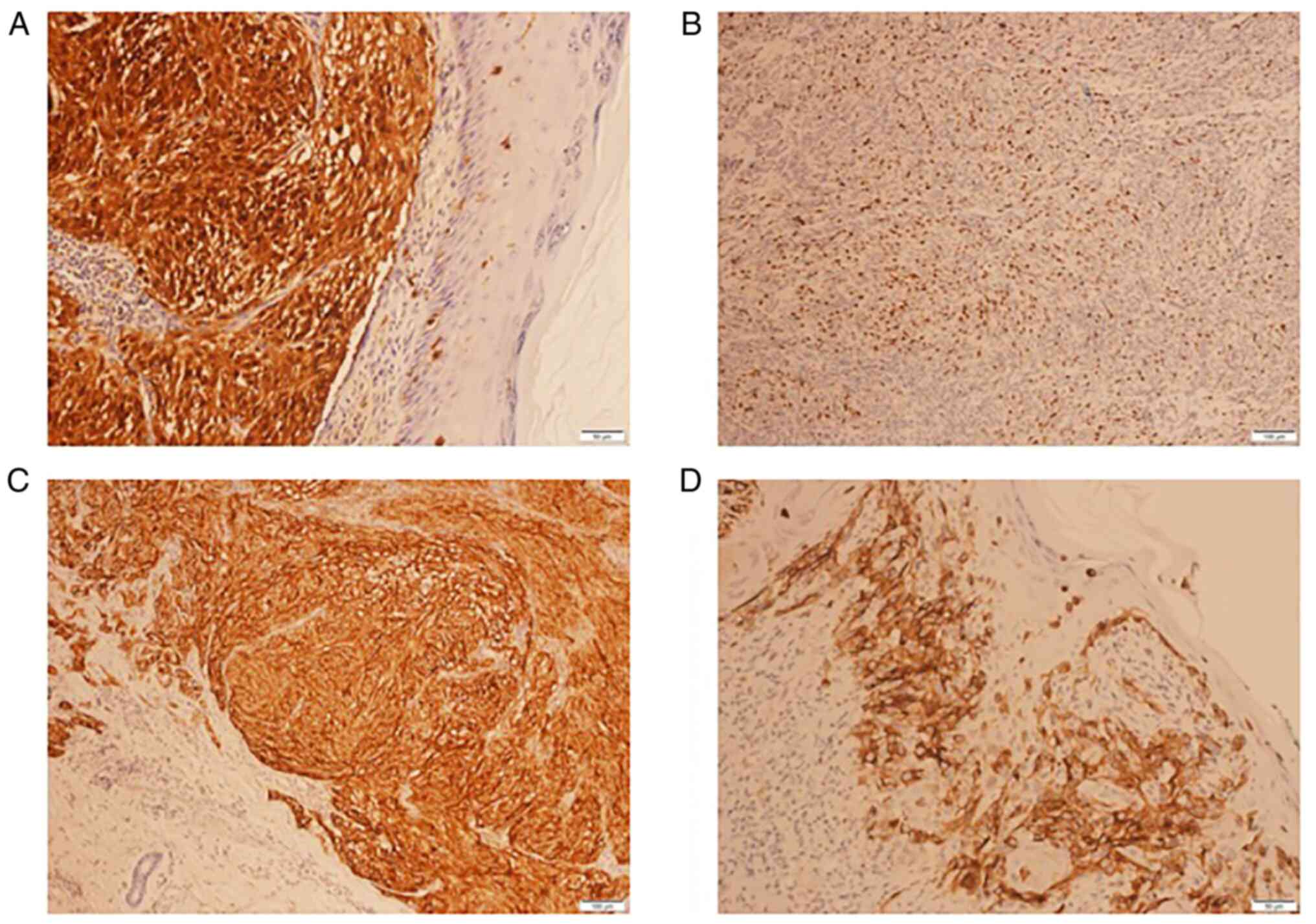
Regarding immunohistochemical analysis, the
specificity and sensitivity of S100 protein (Fig. 4A), Ki67 (Fig. 4B), HMB-45 (Fig. 4C) and Melan A (Fig. 4D) biomarkers were assessed. In the
present study, the S100 and Ki67 biomarkers were determined to be
predictive factors for recurrences. Patients with S100 expression
were 4.83 times (95% CI=1.2-20.8) more likely to suffer a relapse,
whereas patients with Ki67 expression of >30% had a 5.41-fold
higher risk (95% CI=1.3-22.0; Table
II). The correlation between S100 and the Breslow depth was
statistically significant (r-value: 0.43; P=0.027), the latter
being significantly higher in patients with S100 expression.
Discussion
Nodular melanoma accounts for 15-30% of melanoma
cases and is the second most common subtype after the superficial
spreading lesion (~70%) (10). It
consists mostly of lesions >2 mm in thickness, with an increased
rate of vertical growth and biologic aggressiveness, evaluated in
an advanced stage at initial presentation and higher incidence of
recurrence. Its cytologic features are epithelioid, resembling the
ones of the SSM, with a minimal or no demonstrable macular growth
phase (11).
SSM is the most frequent type of melanoma,
accounting for >50% and up to 70% of the cases of melanoma
diagnosed globally (12). It
commonly occurs on the extremities and on the trunk (13). During the last decades, there has
been an increase in the number of cases diagnosed during Stage I,
while there has also been an increase in the incidence of melanoma
(partly due to better diagnostic methods and a higher general
awareness), with a possibility for it to double over a period of
1-2 decades (12).
SSM usually consists of intraepidermal spotted
(pagetoid) lesions, with voluminous epithelioid melanocytes spread
throughout the epidermis, either alone or in packs or nests which
vary in size and shape and which may frequently converge (13). The level of atypia of the
melanocytes is variable. The lesions are usually flat and barely
protuberant, having erratic shapes and edges (12). The SSM is positive for a variety of
markers used in the diagnosis of melanoma, which include S100,
HBM45 and Melan-A/MART1, which, however, cannot differentiate SSM
from benign melanocytomas (14).
ALM is a rare subtype with a higher incidence in
people of color (15). It usually
implies a worse prognosis than that of all other known malignant
skin lesions. Early clinical diagnosis of ALM is essential, but in
numerous cases, it is delayed due to atypical location of the
lesions, mainly arising on the palms, soles and nail beds. ALM
occurring in individuals with dark-colored skin has been
demonstrated to have a predilection for lower limb locations,
particularly on plantar regions (16). Lentiginous melanoma usually arises
on sun-exposed surfaces, such as the face and upper part of the
trunk and is a slowly growing entity that may remain in situ
for a prolonged duration and patients may at times suffer local
recurrence after the oncological removal of the tumor. Desmoplastic
melanoma is commonly associated with the lentiginous subtype and it
consists of bulky tumoral masses that are usually amelanotic. Cells
of this type of neoplasia have a storiform pattern and
spindle-shaped morphology with high mitotic rates (17).
In the present study, the histopathological findings
revealed that 10 lesions were lentiginous melanomas, with the most
common topography on the face, and 2 patients had recurrence of the
disease. Furthermore, one case was documented as desmoplastic
melanoma with facial localization, lymph node involvement, strong
positivity for S100 protein and no affinity for Melan A and HMB-45
biomarkers.
The most common chronic disease was hypertension
(18), detected in 6 cases (54.5%)
of the patients who relapsed and in 18 individuals (40%) of those
who did not. Regarding the recurrence of the lesions, 90.9%
appeared in male patients, thus making the melanoma 8.75 times more
likely to recur in males than in females (OR=8.75; 95%
CI=1.03-74.18).
The risk of recurrence was 4.81 times higher in
patients with cranial localizations of the melanoma compared to
other sites. Those patients with lymph node metastases and those
who presented with capsular invasion had a significantly higher
risk of recurrence (P=0.041 and P=0.047, respectively).
The ulceration and mitosis rate represented a
prognostic factor for melanoma and it provided significant
information regarding the aggressiveness of the tumor. It is
frequently a characteristic of thick tumors and it is associated
with a higher proliferative status of nodular melanomas rather than
superficial spreading ones (10).
This feature was evaluated by the frequency of mitoses detected for
each category. Greater rates of mitosis have been observed to be
linked to a fast tumoral size increase, indifferent to the lesion's
dimensional characteristics (11).
The Breslow thickness is a crucial variable and is the most
important prognostic factor in cutaneous melanoma (3).
The level of mitoses was significantly positively
correlated with the Breslow depth (r-value: 0.45; P=0.017), meaning
that for an increase in the level of mitoses by one unit, the
Breslow depth increases by 0.45 mm.
S100 protein is a biomarker used in the evaluation
of tumors with a low degree of differentiation, with an almost 100%
sensitivity for melanoma (19). It
is involved in the process of calcium binding and it is also a
regulating component of the microtubules. The protein is involved
in the cellular division, in the metabolism of calcium, in protein
phosphorylation and secretion, in cellular growth and in the
regulation of cellular proliferation (20). S100 has been indicated to be
expressed in a variety of poorly-differentiated types of cancer and
also in diseases such as neurodegenerative disorders, inflammatory
diseases and cardiomyopathies. Recently, it was proven that S100
has a close association with various cancer types, including
melanoma (21). This may, in part,
be due to the localization of the S100 genes on chromosome 1q21,
which is highly susceptible to mutations (22). Among the various subtypes of S100,
S100B, S100P, S100A4 (Metastatin), S100A6 and S100A13 are
frequently present in melanoma, with S100P being positive in all
melanoma subtypes. The association is lesser in oral malignant
melanoma than in cutaneous melanoma, the former exhibiting both a
lower grade of staining for S100 and a higher biological
aggressiveness than the latter (21).
Ki67 is a cell cycle control protein whose specific
antibody is used to ascertain the existence of a nuclear antigen
only present in tissues with a high cellular proliferation rate,
while it is otherwise absent in normal tissue. Ki67 is also
involved in the transcription of RNA (23). It is absent during the G0 resting
phase but present during the active cellular division phases G1, S,
G2 and mitosis (24).
Since the protein's discovery in 1983, Ki67 proved
to be a reliable index in both the diagnosis and the prognosis of
various types of cancer (23).
Specifically, in melanoma, Ki67 is useful for the prevention of
false-negative diagnoses of melanoma during the differential
diagnosis from benign nevi (24).
The level of Ki67 is closely related to the rate of cell
proliferation, thus allowing accurate assessment of the presence of
the growth fraction of a certain cellular population (25). Furthermore, the expression of Ki67
also corresponds to the evolution of the disease: A higher level of
Ki67 is associated with thicker tumors and, consequently, with less
favorable prognosis for the patients (25).
HMB-45, which stands for ‘human melanoma black’,
that was discovered in 1986 and recognizes a melanosomal
glycoprotein (Pmel17) involved in the synthesis of the melanosomal
fibrils and in the process of evolution from stage I
pre-melanosomes to stage II. HMB-45 is one of the markers widely
used for the positive diagnosis of malignant melanoma and in the
assessment of sentinel lymph nodes for ruling out the presence of
micrometastases (26). The
sensitivity is 95% when using common antigen-retrieval techniques
and it increases when using aggressive antigen retrieving
techniques (this way, spindle cell melanomas may also be
recognized) (27). HMB-45 staining
is usually negative in desmoplastic melanoma. HMB-45 has 100%
specificity in diagnosing malignant melanoma (26). In malignant melanoma, the level of
staining to HMB-45 is proportional to the degree of cellular atypia
(27).
Melan A, or MART-1, is a protein occurring in
melanocytes, which may be used as a histopathological marker for
detecting tumors derived from melanocytic precursors (4). It has been demonstrated that Melan A
has the ability to differentiate between melanoma-in-situ in
its early stages and senile keratosis (8). It is a sensitive and specific marker
for the diagnosis of melanoma, but it may also be found in other
tumors of melanocytic origin, such as clear cell sarcoma, benign
nevi, melanotic neurofibroma or perivascular epithelioid cell
tumors (28-30).
Malignant melanoma is considered one of the most
virulent diseases, so the importance of a multidisciplinary team
including a plastic surgeon, anatomopathologist and oncologist in
the treatment patients with malignant melanoma should be
highlighted.
Acknowledgements
The authors thank Dr Obrocea Florin, Dr Tianu Elena
and Dr Costache Simona from the Department of Anatomopathology of
the ‘Professor Dr Agrippa Ionescu’ Clinical Emergency Hospital
(Bucharest, Romania) for their help with the interpretation of the
figures.
Funding
Funding: This research received no external funding.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DEGM, AA, AEBS, MM, DGB, LAB, AMP, DI, BMC, LFT, CRJ
and LR designed the study, analysed and interpreted datasets and
wrote the manuscript. DEGM, AA, AEBS and MM collected the data and
analysed the datasets. DEGM, AA, AEBS, MM, DGB, LAB, AMP, DI, BMC,
LFT, CRJ and LR performed a literature search and selected the
studies to be included. CRJ and LR critically revised the
manuscript. All authors read and approved the final manuscript. CRJ
and LR checked and approved the authenticity of the raw data of the
study.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the Ethics Committee
of Professor Dr ‘Agrippa Ionescu’ Clinical Emergency Hospital
(protocol no. 1736615/05.08.2014). Informed consent was obtained
from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gloster HM and Brodland DG: The
epidemiology of skin cancer. Dermatol Surg. 22:217–226.
1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Becker JC, Kirkwood JM, Agarwala SS,
Dummer R, Schrama D and Hauschild A: Molecularly targeted therapy
for melanoma: Current reality and future options. Cancer.
107:2317–2327. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferrone CR, Ben Porat L, Panageas KS,
Berwick M, Halpern AC, Patel A and Coit DG: Clinicopathological
features of and risk factors for multiple primary melanomas. JAMA.
294:1647–1654. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bevona C, Goggins W, Quinn T, Fullerton J
and Tsao H: Cutaneous melanomas associated with nevi. Arch
Dermatol. 139:1620–1624. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Balch CM, Murad TM, Soong SJ, Ingalls AL,
Halpern NB and Maddox WA: A multifactorial analysis of melanoma:
Prognostic histopathological features comparing Clark's and
Breslow's staging methods. Ann Surg. 188:732–742. 1978.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoon DS, Bostick P, Kuo C, Okamoto T, Wang
HJ, Elashoff R and Morton DL: Molecular markers in blood as
surrogate prognostic indicators of melanoma recurrence. Cancer Res.
60:2253–2257. 2000.PubMed/NCBI
|
|
8
|
Takata M and Saida T: Genetic alterations
in melanocytic tumors. J Dermatol Sci. 43:1–10. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ilie MA, Caruntu C, Lupu M, Lixandru D,
Georgescu SR, Bastian A, Constantin C, Neagu M, Zurac SA and Boda
D: Current and future applications of confocal laser scanning
microscopy imaging in skin oncology. Oncol Lett. 17:4102–4111.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ali Z, Yousaf N and Larkin J: Melanoma
epidemiology, biology and prognosis. EJC Suppl. 11:81–91.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Demierre MF, Chung C, Miller DR and Geller
AC: Early detection of thick melanomas in the United States: Beware
of the nodular subtype. Arch Dermatol. 141:745–750. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Urist MM and Karnell LH: The national
cancer data base. Report on melanoma. Cancer. 74:782–788.
1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weyers W, Euler M, Diaz-Cascajo C, Schill
WB and Bonczkowitz M: Classification of cutaneous malignant
melanoma: A reassessment of histopathologic criteria for the
distinction of different types. Cancer. 86:288–299. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jaeger J, Koczan D, Thiesen HJ, Ibrahim
SM, Gross G, Spang R and Kunz M: Gene expression signatures for
tumor progression, tumor subtype, and tumor thickness in
laser-microdissected melanoma tissues. Clin Cancer Res. 13:806–815.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arrington JH, Reed RJ, Ichinose H and
Krementz ET: Plantar lentiginous melanoma: A distinctive variant of
human cutaneous malignant melanoma. Am J Surg. Pathol. 1:131–143.
1977.PubMed/NCBI
|
|
16
|
Feibleman GE, Stoll H and Maize JC:
Melanomas of the palm, sole, and nailbed: A clinicopathologic
study. Cancer. 46:2492–2504. 1980.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kossard S, Commens C, Symons M and Doyle
J: Lentinginous dysplastic naevi in the elderly: A potential
precursor for malignant melanoma. Australas J Dermatol. 32:27–37.
1991.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mandita A, Timofte D, Balcangiu-Stroescu
AE, Balan DG, Raducu L, Tanasescu MD, Diaconescu AC, Dragos D,
Cosconel CI, Stoicescu SM, et al: Treatment of high blood pressure
in patients with chronic renal disease. Rev Chim. 70:993–995.
2019.
|
|
19
|
Banerjee SS and Harris M: Morphological
and immunophenotypic variations in malignant melanoma.
Histopathology. 36:387–402. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marenholz I, Heizmann CW and Fritz G: S100
proteins in mouse and man: From evolution to function and pathology
(including an update of the nomenclature). Biochem Biophys Res
Commun. 322:1111–1122. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sedaghat F and Notopoulos A: S100 protein
family and its application in clinical practice. Hippokratia.
12:198–204. 2008.PubMed/NCBI
|
|
22
|
Ravasi T, Hsu K, Goyette J, Schroder K,
Yang Z, Rahimi F, Miranda LP, Alewood PF, Hume DA and Geczy C:
Probing the S100 protein family through genomic and functional
analysis. Genomics. 84:10–22. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
El Halal Schuch L, Azevedo MM, Furian R,
Rigon P, Reiter KC, Crivelatti I, Riccardi F and Bica CG:
Evaluation of Kindlin-1 and Ki-67 immunohistochemical expression in
primary cutaneous malignant melanoma: A clinical series. Appl
Cancer Res. 39(10)2019.
|
|
24
|
Bengtsson E and Ranefall P: Image analysis
in digital pathology: Combining automated assessment of Ki67
staining quality with calculation of Ki67 cell proliferation index.
Cytometry A. 95:714–716. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thompson JJ, Herlyn MF, Elder DE, Clark
WH, Steplewski Z and Koprowski H: Use of monoclonal antibodies in
detection of melanoma-associated antigens in intact human tumors.
Am J Pathol. 107:357–361. 1982.PubMed/NCBI
|
|
27
|
Sun J, Morton TH Jr and Gown AM: Antibody
HMB-45 identifies the cells of blue nevi. An immunohistochemical
study on paraffin sections. Am J Surg Pathol. 14:748–751.
1990.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kaufmann O, Koch S, Burghardt J, Audring H
and Dietel M: Tyrosinase, melan-A, and KBA62 as markers for the
immunohistochemical identification of metastatic amelanotic
melanomas on paraffin sections. Mod Pathol. 11:740–746.
1998.PubMed/NCBI
|
|
29
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013.
|
|
30
|
Ancuceanu R, Dinu M, Neaga I, Laszlo FG
and Boda D: Development of QSAR machine learning-based models to
forecast the effect of substances on malignant melanoma cells.
Oncol Lett. 17:4188–4196. 2019.PubMed/NCBI View Article : Google Scholar
|