Introduction
The prevalence of diabetes mellitus is on the
increase worldwide (1), leading to
escalating global health concerns. Diabetic cataracts (DC) is a
common complication of diabetes (2), which is an important cause of
blindness in patients with diabetes (3). Cataracts tend to be more prevalent
and deteriorate more rapidly in patients with diabetes compared
with the general population (4).
Human lens epithelial cells forms part of the main optical tissue
that is involved in nutrition and ions transportation, metabolism
and detoxification during lens development (5). In addition, these cells have been
reported to have a key role in cataract formation. In particular,
oxidative stress, apoptosis (6),
and epithelial-mesenchymal transition (EMT) (2) in human lens epithelial cells induced
by high glucose have been previously implicated in the pathogenesis
of cataract formation. Cataract surgery is currently the frontline
method of treatment for DC. However, cataract surgery in patients
with diabetes may lead to poor visual acuity caused by posterior
capsular opacification and postoperative cystoid macular edema
(7). Thus, optimization of
surgical and development of novel pharmacological methods may
increase the efficacy for patients with diabetes (8).
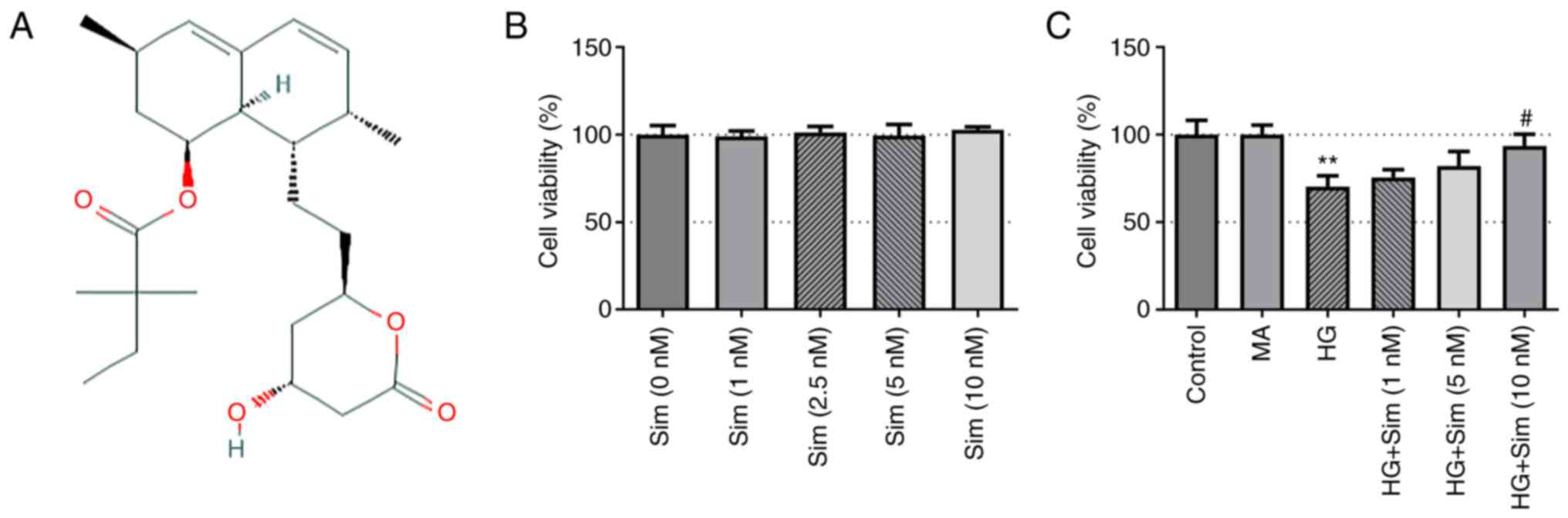
Simvastatin (Fig.
1A) is a recognized drug in the statin family that is commonly
used to reduce the risk of cardiovascular diseases associated with
hypercholesterolemia (9,10). High glucose (HG) contributes to
cardiomyocyte dysfunction and injury by promoting apoptosis and
decreasing autophagy (11).
Simvastatin has been shown to alleviate the injury induced by HG
levels in cardiomyocytes (12) and
the kidney (13). In addition,
findings of a previous showed that simvastatin conferred no adverse
effects on the lens (14).
Although simvastatin has been reported to reduce the apoptosis of
lens epithelial cells at HG levels (15), the precise mechanism underlying the
effects of simvastatin on DC remains poorly understood.
Simvastatin is capable of relieving neuropathic pain
by inhibiting RhoA activity (16).
RhoA is a small guanosine binding protein that is normally
localized to the plasma membrane (17). Results of a previous study showed
that RhoA/Rho-associated protein kinase (ROCK) signaling activation
exerts a key role in the occurrence and development of diabetes
complications (18). Additionally,
inhibition of RhoA/ROCK signaling, not only alleviated diabetic
retinopathy (19), but also
inhibited EMT in TGF-β-induced lens epithelial cells (20). However, the effects of simvastatin
on DC and the potential mechanism remain unclear. Therefore, the
aim of the present study was to investigate the biological effects
of simvastatin on oxidative stress and EMT in HG-induced lens
epithelial SRA01/04 cells and explore whether the RhoA/ROCK
signaling participates in simvastatin-regulated diabetic
cataracts.
Materials and methods
Cell culture and HG treatment
The human lens epithelial cell line SRA01/04 was
purchased from Guangzhou Jennio Biotech Co., Ltd. Cells were
maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere of 95% air and 5% CO2 at 37˚C. For
the establishment of an in vitro DC model, SRA01/04 cells
were grown in DMEM with HG (25 mM) for 24 h. Subsequently,
HG-induced cells were treated with 1, 5 and 10 nM simvastatin for
48 h at 37˚C. The cells were maintained in media containing 5 mM
glucose and served as the control group.
Cell counting kit-8 (CCK-8) assay
The CCK-8 assay was performed to measure cell
viability. SRA01/04 cells were seeded in 96-well plates at a
density of 5x103 cells/well and then incubated with
different doses of simvastatin for 48 h, followed by the addition
of 10 µl CCK-8 (Dojindo Molecular Technologies, Inc.) solution into
each well. The plates were then incubated for 2 h at 37˚C. The
optical density of each well was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.). All experiments
were performed three times.
Western blot analysis
Total protein was extracted from SRA01/04 cells
after lysis on ice using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and quantified using a BCA kit (Beyotime Institute
of Biotechnology). A total of 30 µg protein samples per well were
then transferred to PDVF membranes after resolving using 10%
SDS-PAGE gels. Subsequently, the membranes were washed, blocked
with 5% skimmed milk for 1 h and then incubated with the following
primary antibodies at 4˚C overnight: Anti-E-cadherin antibody
(1:10,000; cat. no. ab40772; Abcam), anti-N-cadherin antibody
(1:5,000; cat. no. ab76011; Abcam), anti-Vimentin antibody
(1:1,000; cat. no. ab92547; Abcam), anti-α-smooth muscle act in
(α-SMA) antibody (1:1,000; cat. no. ab265588; Abcam), anti-RhoA
antibody (1:5,000; cat. no. ab187027; Abcam), anti-ROCK1 antibody
(1:1,000; cat. no. ab92547; Abcam) or anti-ROCK2 antibody (1:1,000;
cat. no. ab134181; Abcam). After washing with PBS, the membranes
were incubated with the HRP-conjugated goat anti-rabbit IgG
secondary antibody (1:2,000; cat. no. ab6721; Abcam) for 2 h at
room temperature. All antibodies utilized in the present study were
purchased from Abcam. GAPDH was used as the loading control. The
protein blots were visualized using enhanced chemiluminescence
(ECL) reagent and densitometry analysis of the bands was performed
using ImageJ (National Institutes of Health).
Measurements of reactive oxygen
species (ROS), superoxide dismutase (SOD) and glutathione
(GSH)/glutathione disulfide (GSSG)
Detection of ROS, SOD and GSH-GSSG was performed to
assess the extent of oxidative stress in SRA01/04 cells. ROS levels
were measured using the DCFH-DA measurement kit (Shanghai
Enzyme-linked Biotechnology Co., Ltd.) in the dark at 37˚C. SOD
activities were assessed using the SOD Assay Kit-WST (Dojindo
Molecular Technologies, Inc.), following the manufacturer's
protocols. GSH/GSSG activities were evaluated using the Glutathione
(GSH) assay Kit (Abnova) in accordance with the manufacturer's
protocols.
Statistical analysis
All experimental data were calculated using SPSS
17.0 (SPSS, Inc.) and are presented as the mean ± standard
deviation (SD). Results were analyzed by one-way ANOVA followed by
Bonferroni post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Simvastatin increases the viability of
SRA01/04 cells treated with HG
To investigate the viability of SRA01/04 cells
before and after HG treatment, the CCK-8 assay was performed. No
changes were observed in the viability of SRA01/04 cells after
treatment with simvastatin at different concentrations (Fig. 1B). By contrast, HG treatment
significantly inhibited the viability of SRA01/04 cells, which was
reversed by simvastatin treatment (Fig. 1C). These results suggest that
simvastatin alone exerted no effect on SRA01/04 cells, but was able
to restore the viability of SRA01/04 cells treated with HG.
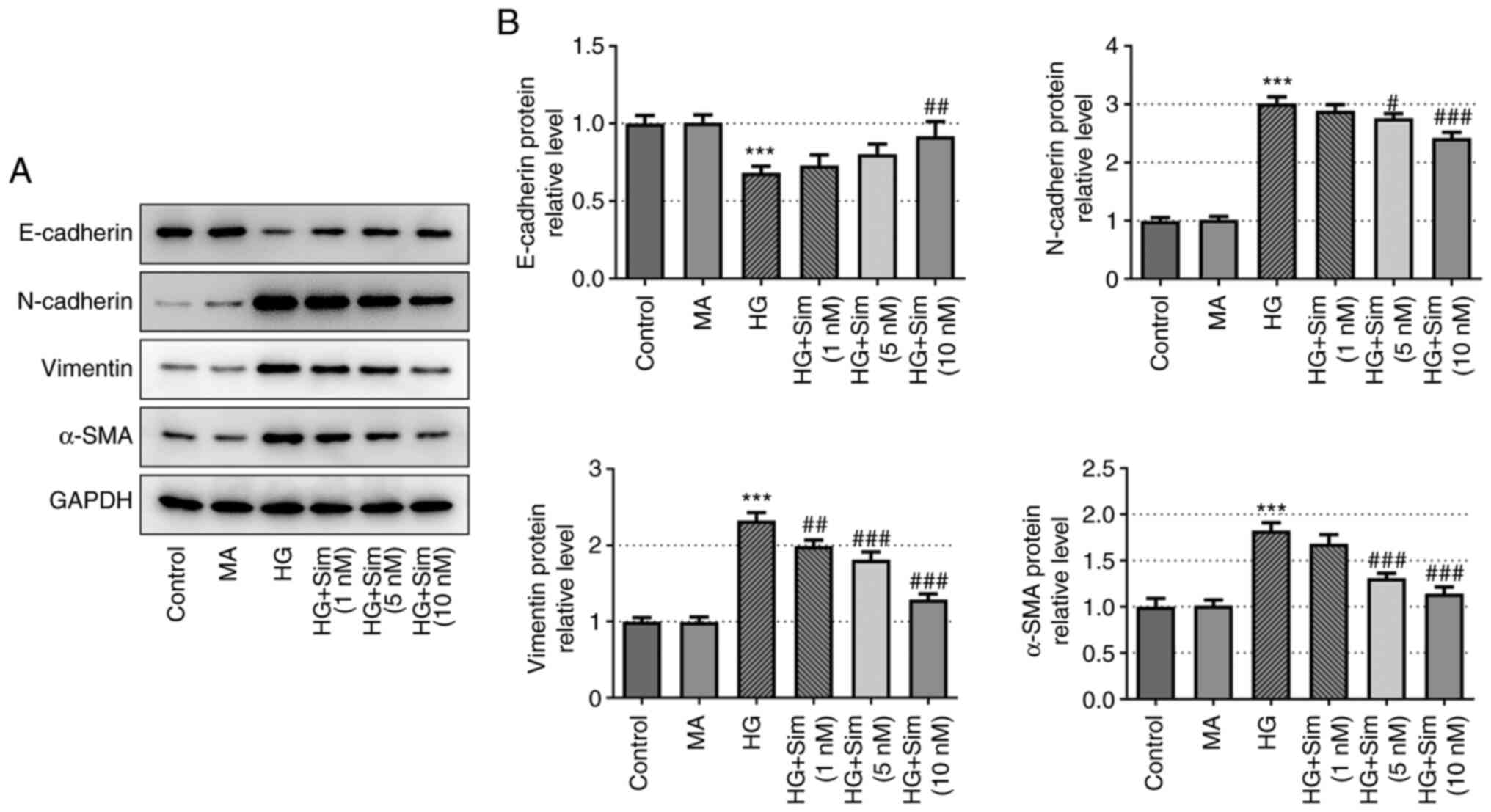
Simvastatin inhibits EMT in SRA01/04
cells treated with HG
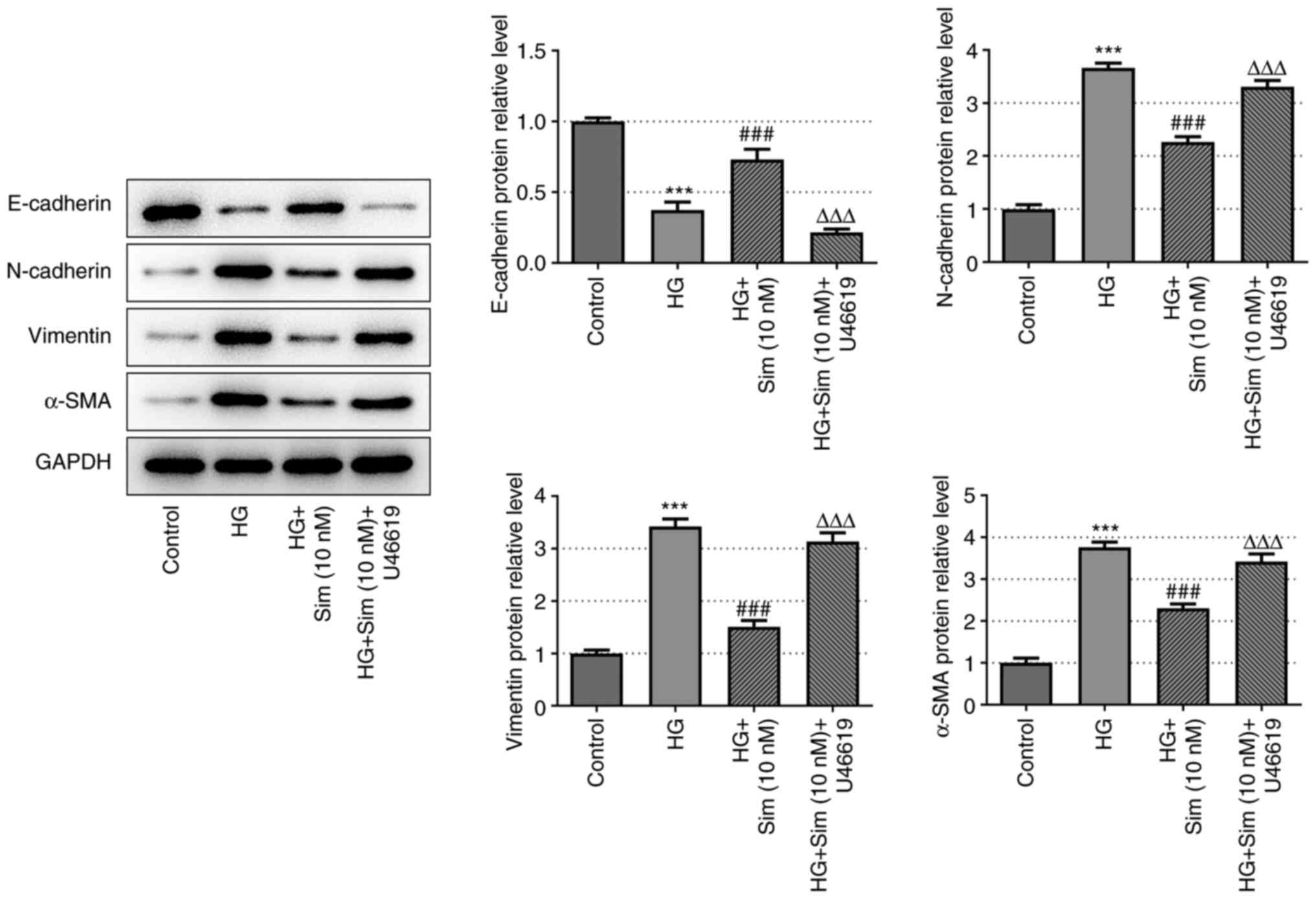
To further explore the effects of simvastatin on EMT
in SRA01/04 cells, the protein expression levels of E-cadherin,
N-cadherin, Vimentin and α-SMA were measured. Expression of the
epithelial cell marker E-cadherin was markedly decreased after HG
treatment, which was reversed following the addition of simvastatin
(Fig. 2). Additionally, the
expression levels of mesenchymal cell markers N-cadherin, Vimentin
and α-SMA exhibited the opposite trend compared with that of
E-cadherin, as they were increased by HG and the levels of the
three proteins were significantly decreased following treatment
with 10 nM of simvastatin compared with the HG-induced cells
(Fig. 2). This observation
provided an indicator on the EMT process and suggested that
simvastatin exerted an inhibitory effect on EMT in SRA01/04 cells
treated with HG.
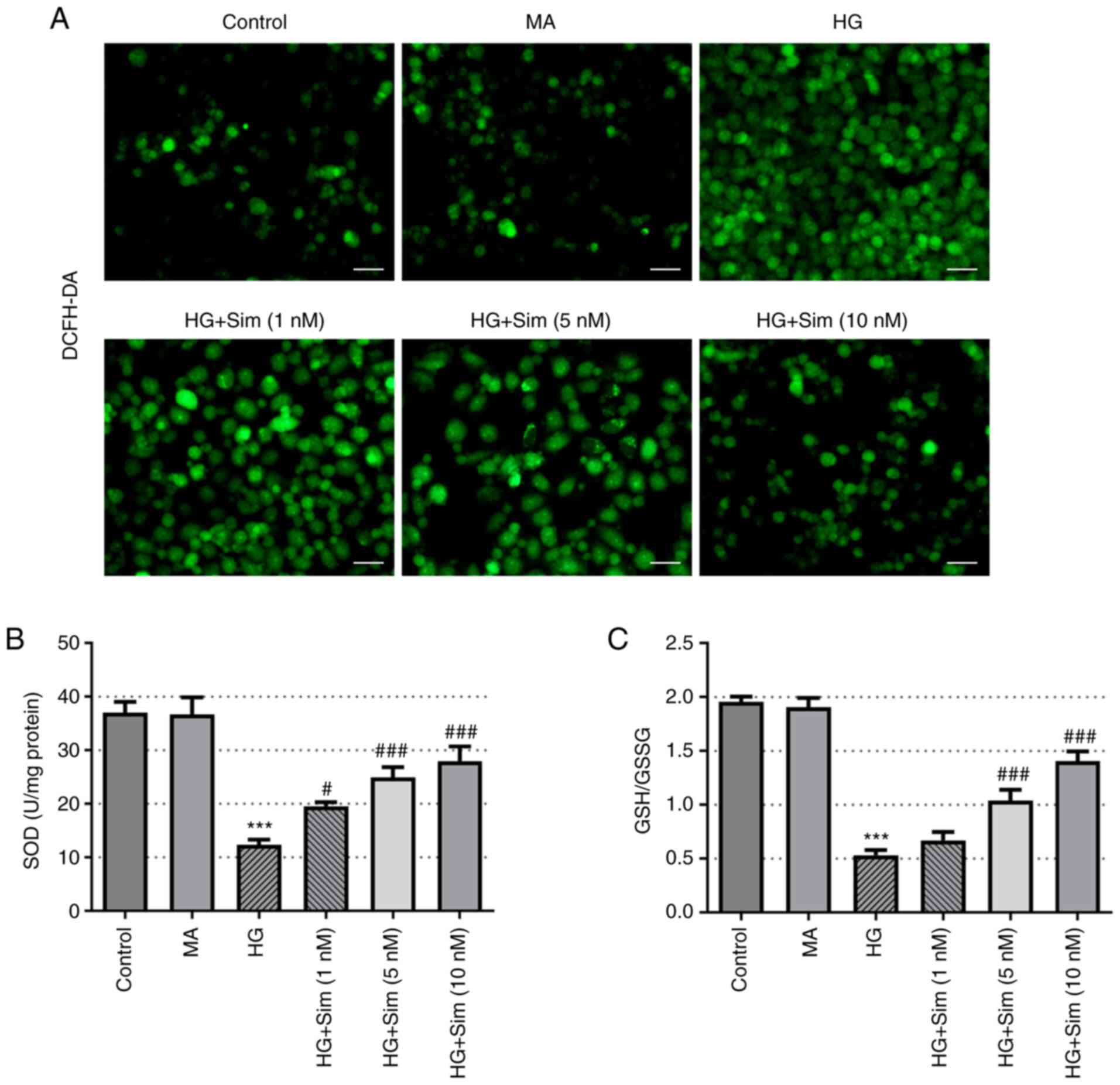
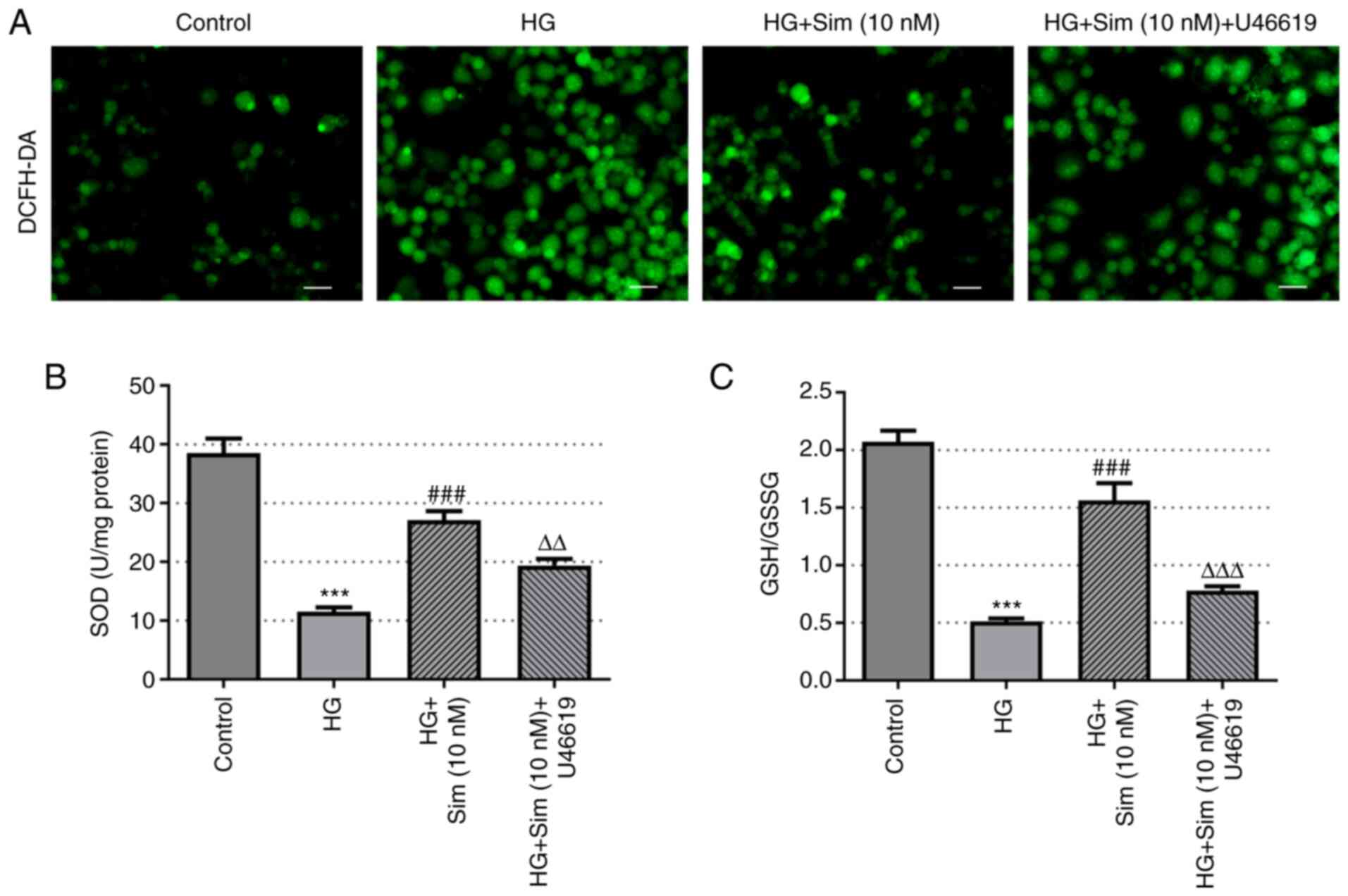
Simvastatin alleviates oxidative
stress in SRA01/04 cells treated with HG
To investigate the role of simvastatin on oxidative
stress in SRA01/04 cells, the levels of ROS, SOD and GSH-GSSG were
measured. ROS levels were found to be markedly increased after
culturing with HG compared with those in the control group, whilst
the addition of simvastatin markedly reduced the levels of ROS
(Fig. 3A). In addition, HG
treatment markedly reduced the activity of SOD, which recovered
following the addition of simvastatin (Fig. 3B). Similarly, the levels of
GSH/GSSG were reduced by HG but were reversed by simvastatin
(Fig. 3C). These findings suggest
that simvastatin effectively alleviated oxidative stress in
SRA01/04 cells induced by HG.
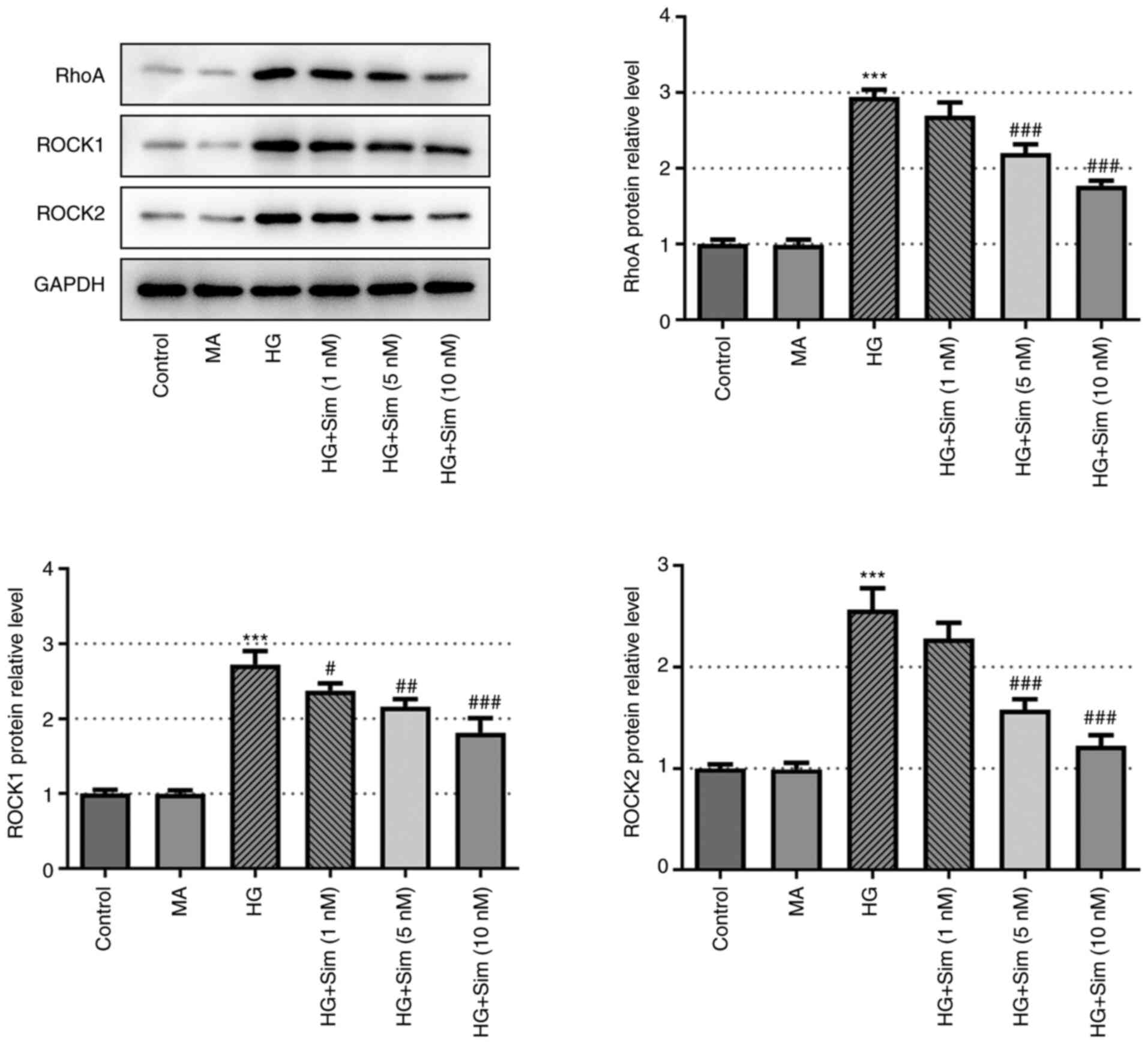
Simvastatin inhibits RhoA/ROCK
signaling
To assess the specific impact of simvastatin on
RhoA/ROCK signaling, western blot analysis was performed to measure
the expression levels of Rho family proteins RhoA, ROCK1 and ROCK2
in SRA01/04 cells treated with HG and then simvastatin. As
indicated in Fig. 4, the protein
expression levels of RhoA, ROCK1 and ROCK2 were found to be
markedly increased by HG treatment but reversed as simvastatin was
added. These observations suggest that simvastatin exerted an
inhibitory effect on RhoA/ROCK signal transduction in the presence
of HG.
Simvastatin suppresses EMT in SRA01/04
cells induced by HG by inhibiting RhoA/ROCK signal
transduction
Simvastatin has been previously revealed to inhibit
RhoA/ROCK signal transduction (21,22).
Therefore, to investigate whether simvastatin suppressed EMT in
SRA01/04 cells by altering RhoA/ROCK signal transduction, the
present study subsequently designated the following four groups:
Control; HG; HG + 10 nM simvastatin; HG + 10 nM simvastatin +
U46619. U46619 functions as an activator of RhoA/ROCK. The protein
levels of E-cadherin, N-cadherin, Vimentin and α-SMA were measured
using western blot analysis. As shown in Fig. 5, reduced expression of E-cadherin
and increased expression of N-cadherin, Vimentin and α-SMA were
observed in the HG + 10 nM simvastatin + U46619 group. These data
suggest that EMT in SRA01/04 cells induced by HG was inhibited by
simvastatin through the RhoA/ROCK pathway.
Simvastatin alleviates oxidative
stress in SRA01/04 cells induced by HG through inhibition of
RhoA/ROCK signaling
As shown in Fig. 6,
the levels of ROS, SOD and GSH-GSSG in SRA01/04 cells after the
addition of the RhoA activator U46619 were investigated using
DCFH-DA, SOD and GSH/GSSG kits. In the HG + 10 nM simvastatin +
U46619 group, the levels of ROS were increased, whereas the levels
of SOD and GSH-GSSG were reduced compared with those in the HG +
simvastatin group. These results highlight the role of simvastatin
in attenuating oxidative stress in SRA01/04 cells induced by HG
through inhibiting the RhoA/ROCK pathway.
Discussion
As the incidence of DC increases, so does the
importance of developing an effective therapeutic strategy for
treating this condition. Although agents are available for treating
DC, including aldose reductase inhibitors (23) and aspirin (24), their therapeutic effects remain
unsatisfactory. Simvastatin is a type of statin that is widely
applied for controlling hypercholesterolemia (25). Emerging evidence has indicated that
simvastatin is involved in the development of diabetic
complications. Simvastatin has been documented to reverse the
increase in apoptosis, while also increasing autophagy after
treatment with HG (12). The aim
of the present study was to investigate the mechanism underlying
the effects of simvastatin on DC. Specifically, the focus was on
the potential effects of simvastatin on EMT and oxidative stress in
the human lens epithelial cell line SRA01/04 induced by HG.
Lens epithelial cell damage in patients with
diabetes is mainly caused by oxidative stress, EMT and cell
apoptosis induced by HG. Previous findings showed that simvastatin
does not result in any deleterious effects on the lens (26). In the present study, 25 mM HG was
used to stimulate SRA01/04 cells in order to simulate a HG
condition and detect the effects of HG on cell viability, EMT and
oxidative stress. The results showed that 25 mM HG significantly
inhibited cell viability, promoted the EMT process and oxidative
stress, accompanied with higher ROS, lower levels of SOD and
GSH/GSSG, which indicated that a DC model was established
successfully. In addition, the viability of SRA01/04 cells did not
exhibit any significant differences after 48 h of treatment with
different doses of simvastatin. Additionally, the viability of
SRA01/04 cells was markedly decreased by HG, mimicking the
physiological condition that occurs in the lens of patients with
diabetes (27). In the present
study, to assess cell viability, SRA01/04 cells were treated with
25 mM HG to establish an in vitro model of DC before
simvastatin treatment. The results showed that after treatment with
simvastatin, cell viability was markedly restored, suggesting that
simvastatin can increase lens epithelial cell viability. If
SRA01/04 cells were cultured for 7 days without continuous passage
culture, the cells would be too old to maintain normal cell
morphology and function. Thus, treatment of simvastatin for one
week is inappropriate. Therefore, the action time of simvastatin
was selected according to another study (15).
RhoA/ROCK signaling serves key roles in a variety of
cellular processes (28).
Simvastatin was previously shown to inhibit the TGF-β1-induced
RhoA/ROCK signaling pathway by blocking Rho geranylation (29). Another study reported significant
and specific decreases in RhoA/ROCK activity following treatment
with simvastatin (30). In
addition, simvastatin reduced RhoA activity by suppressing the
levels of isoprenoid intermediates (31). In the present study, HG increased
RhoA, ROCK1 and ROCK2 protein expression, all of which was reversed
by simvastatin. This suggests that simvastatin exerted inhibitory
effects on RhoA/ROCK signaling.
The RhoA/ROCK pathway can also regulate HG-induced
EMT (32). EMT has been previously
reported to serve a key role in cataract formation. It was found
that simvastatin can block EMT in human alveolar epithelial cells
(33) and in human prostate cancer
cells (34) induced by TGF-β1. In
addition, Fan et al (35)
reported that atorvastatin partially suppressed the EMT process in
A549 cells induced by TGF-β1. The present study showed that under
both HG and simvastatin presence, the protein expression levels of
E-cadherin were elevated whereas the expression levels of
N-cadherin, Vimentin and α-SMA were decreased. Downregulation of
E-cadherin and upregulation of N-cadherin, vimentin and α-SMA are
closely associated with EMT injury (36), which was contrary to the results
and further suggested the inhibitory effects of simvastatin on EMT
in SRA01/04 cells induced by HG. However, after U46619, the
activator of RhoA/ROCK, was added, and the opposite trend in the
expression of these EMT protein markers was observed, suggesting
that RhoA/ROCK is important for mediating EMT in this cell type.
Together, these results suggest that simvastatin can inhibit EMT in
SRA01/04 cells, at least partially by suppressing RhoA/ROCK
signaling.
Oxidative stress is caused by the imbalance between
ROS production and activity in the anti-oxidant defense system in
the body (37). Oxidative stress
and subsequent oxidative damage to lens proteins is a frequently
reported causative factor in cataract formation (38). A previous study has found that
simvastatin can prevent cardiac hypertrophy in diabetic rats by
attenuating oxidative stress and inflammation caused by the
calpain-1-mediated activation of NF-κB (39). Statins have been found to alleviate
inflammatory and oxidative stress damage (40). In the present study, the levels of
representative oxidative stress markers ROS, SOD and GSH-GSSG were
measured after treatment with HG and simvastatin. Significantly
decreased levels of ROS and elevated levels of SOD and GSH-GSSG
were observed after simvastatin treatment, suggesting that
simvastatin inhibited oxidative stress in SRA01/04 cells. By
contrast, the addition of U46619 reversed the effects of
simvastatin on SRA01/04 cells, suggesting that RhoA/ROCK signaling
also exerts an inhibitory role in oxidative stress injury. These
observations suggest that simvastatin alleviates oxidative stress
in SRA01/04 cells induced by HG through inhibition of RhoA/ROCK. In
addition, in vivo experiments are far more complex than cell
experiments; thus it is not equivalent to treatment in humans. The
dose of simvastatin used in this study was selected according to
that of a previous study (15).
However, the appropriate doses of simvastatin used in clinical
trials need to be further investigated.
To conclude, simvastatin exerted a
concentration-dependent therapeutic effect on the human lens
epithelial cell line SRA01/04 induced by HG. In addition, reversal
experiments using the RhoA/ROCK activator revealed that simvastatin
could reduce EMT and oxidative stress by inhibiting RhoA/ROCK
signaling. Therefore, simvastatin has the potential for application
as a therapeutic agent for treating DC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JF and XH designed the study, performed the
experiment, drafted and revised the manuscript. JF analyzed the
data and searched the literature. All authors read and approved the
final manuscript. JF and XH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cole JB and Florez JC: Genetics of
diabetes mellitus and diabetes complications. Nat Rev Nephrol.
16:377–390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ye W, Ma J, Wang F, Wu T, He M, Li J, Pei
R, Zhang L, Wang Y and Zhou J: LncRNA MALAT1 regulates miR-144-3p
to facilitate epithelial-mesenchymal transition of lens epithelial
cells via the ROS/NRF2/Notch1/snail pathway. Oxid Med Cell Longev.
2020(8184314)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sreedharan R and Abdelmalak B: Diabetes
mellitus: Preoperative concerns and evaluation. Anesthesiol Clin.
36:581–597. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Panozzo G, Staurenghi G, Dalla Mura G,
Giannarelli D, Alessio G, Alongi S, Appolloni R, Baldascino A,
Boscia F, Caporossi A, et al: Prevalence of diabetes and diabetic
macular edema in patients undergoing senile cataract surgery in
Italy: The DIabetes and CATaract study. Eur J Ophthalmol.
30:315–320. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Andley UP: The lens epithelium: Focus on
the expression and function of the alpha-crystallin chaperones. Int
J Biochem Cell Biol. 40:317–323. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gong W, Zhu G, Li J and Yang X: LncRNA
MALAT1 promotes the apoptosis and oxidative stress of human lens
epithelial cells via p38MAPK pathway in diabetic cataract. Diabetes
Res Clin Pract. 144:314–321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peterson SR, Silva PA, Murtha TJ and Sun
JK: Cataract surgery in patients with diabetes: Management
strategies. Semin Ophthalmol. 33:75–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kelkar A, Kelkar J, Mehta H and Amoaku W:
Cataract surgery in diabetes mellitus: A systematic review. Indian
J Ophthalmol. 66:1401–1410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fattah TA, Saeed A and Shehzadi SA:
Synthetic approaches towards antihypercholesterolemic drug
simvastatin. Curr Org Synth. 16:652–670. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu A, Wu Q, Guo J, Ares I, Rodríguez JL,
Martínez-Larrañaga MR, Yuan Z, Anadón A, Wang X and Martínez MA:
Statins: Adverse reactions, oxidative stress and metabolic
interactions. Pharmacol Ther. 195:54–84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Rasheed NM, Al-Rasheed NM, Hasan IH,
Al-Amin MA, Al-Ajmi HN, Mohamad RA and Mahmoud AM: Simvastatin
ameliorates diabetic cardiomyopathy by attenuating oxidative stress
and inflammation in rats. Oxid Med Cell Longev.
2017(1092015)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
E L and Jiang H: Simvastatin protects high
glucose-induced H9c2 cells from injury by inducing autophagy. Pharm
Biol. 58:1077–1084. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Rasheed NM, Al-Rasheed NM, Bassiouni
YA, Hasan IH, Al-Amin MA, Al-Ajmi HN and Mahmoud AM: Simvastatin
ameliorates diabetic nephropathy by attenuating oxidative stress
and apoptosis in a rat model of streptozotocin-induced type 1
diabetes. Biomed Pharmacother. 105:290–298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harris ML, Bron AJ, Brown NA, Keech AC,
Wallendszus KR, Armitage JM, MacMahon S, Snibson G and Collins R:
Absence of effect of simvastatin on the progression of lens
opacities in a randomised placebo controlled study. Oxford
cholesterol study group. Br J Ophthalmol. 79:996–1002.
1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang Z, Yao K and Jin C: Apoptosis of
lens epithelial cells induced by high concentration of glucose is
associated with a decrease in caveolin-1 levels. Mol Vis.
15:2008–2017. 2009.PubMed/NCBI
|
|
16
|
Qiu Y, Chen WY, Wang ZY, Liu F, Wei M, Ma
C and Huang YG: Simvastatin attenuates Neuropathic pain by
inhibiting the RhoA/LIMK/cofilin pathway. Neurochem Res.
41:2457–2469. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Z, Wu X, Li W, Peng H, Shen X, Ma L,
Liu H and Li H: RhoA/rock signaling mediates peroxynitrite-induced
functional impairment of Rat coronary vessels. BMC Cardiovasc
Disord. 16(193)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yan Q, Wang X, Zha M, Yu M, Sheng M and Yu
J: The RhoA/ROCK signaling pathway affects the development of
diabetic nephropathy resulting from the epithelial to mesenchymal
transition. Int J Clin Exp Pathol. 11:4296–4304. 2018.PubMed/NCBI
|
|
19
|
Zhu L, Wang W, Xie TH, Zou J, Nie X, Wang
X, Zhang MY, Wang ZY, Gu S, Zhuang M, et al: TGR5 receptor
activation attenuates diabetic retinopathy through suppression of
RhoA/ROCK signaling. FASEB J. 34:4189–4203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Korol A, Taiyab A and West-Mays JA:
RhoA/ROCK signaling regulates TGFβ-induced epithelial-mesenchymal
transition of lens epithelial cells through MRTF-A. Mol Med.
22:713–723. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Serra N, Rosales R, Masana L and Vallvé
JC: Simvastatin increases fibulin-2 expression in human coronary
artery smooth muscle cells via RhoA/Rho-kinase signaling pathway
inhibition. PLoS One. 10(e0133875)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ohsawa M, Ishikura K, Mutoh J and Hisa H:
Involvement of inhibition of RhoA/Rho kinase signaling in
simvastatin-induced amelioration of neuropathic pain. Neuroscience.
333:204–213. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Quattrini L and La Motta C: Aldose
reductase inhibitors: 2013-Present. Expert Opin Ther Pat.
29:199–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shastri GV, Thomas M, Victoria AJ,
Selvakumar R, Kanagasabapathy AS, Thomas K and Lakshmi :
Effect of aspirin and sodium salicylate on cataract development in
diabetic rats. Indian J Exp Biol. 36:651–657. 1998.PubMed/NCBI
|
|
25
|
Cho YE, Moon PG, Lee JE, Singh TS, Kang W,
Lee HC, Lee MH, Kim SH and Baek MC: Integrative analysis of
proteomic and transcriptomic data for identification of pathways
related to simvastatin-induced hepatotoxicity. Proteomics.
13:1257–1275. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lundh BL and Nilsson SE: Lens changes in
matched normals and hyperlipidemic patients treated with
simvastatin for 2 years. Acta Ophthalmol (Copenh). 68:658–660.
1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Song XL, Li MJ, Liu Q, Hu ZX, Xu ZY, Li
JH, Zheng WL, Huang XM, Xiao F, Cui YH and Pan HW:
Cyanidin-3-O-glucoside protects lens epithelial cells against high
glucose-induced apoptosis and prevents cataract formation via
suppressing NF-κB activation and Cox-2 expression. J Agric Food
Chem. 68:8286–8294. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deng Z, Jia Y, Liu H, He M, Yang Y, Xiao W
and Li Y: RhoA/ROCK pathway: Implication in osteoarthritis and
therapeutic targets. Am J Transl Res. 11:5324–5331. 2019.PubMed/NCBI
|
|
29
|
Wei YH, Liao SL, Wang SH, Wang CC and Yang
CH: Simvastatin and ROCK inhibitor Y-27632 inhibit myofibroblast
differentiation of graves' ophthalmopathy-derived orbital
fibroblasts via RhoA-mediated ERK and p38 signaling pathways. Front
Endocrinol (Lausanne). 11(607968)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rattan S: 3-Hydroxymethyl coenzyme A
reductase inhibition attenuates spontaneous smooth muscle tone via
RhoA/ROCK pathway regulated by RhoA prenylation. Am J Physiol
Gastrointest Liver Physiol. 298:G962–G969. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wong MJ, Kantores C, Ivanovska J, Jain A
and Jankov RP: Simvastatin prevents and reverses chronic pulmonary
hypertension in newborn rats via pleiotropic inhibition of RhoA
signaling. Am J Physiol Lung Cell Mol Physiol. 311:L985–L999.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang W, Yi B, Zhang K, Li A, Yang S,
Huang J, Liu J and Zhang H: 1,25-(OH)2D3 and
its analogue BXL-628 inhibit high glucose-induced activation of
RhoA/ROCK pathway in HK-2 cells. Exp Ther Med. 13:1969–1976.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang T, Chen M and Sun T: Simvastatin
attenuates TGF-β1-induced epithelial-mesenchymal transition in
human alveolar epithelial cells. Cell Physiol Biochem. 31:863–874.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xie F, Liu J, Li C and Zhao Y: Simvastatin
blocks TGF-β1-induced epithelial-mesenchymal transition in human
prostate cancer cells. Oncol Lett. 11:3377–3383. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fan Z, Jiang H, Wang Z and Qu J:
Atorvastatin partially inhibits the epithelial-mesenchymal
transition in A549 cells induced by TGF-β1 by attenuating the
upregulation of SphK1. Oncol Rep. 36:1016–1022. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Peluso I, Morabito G, Urban L, Ioannone F
and Serafini M: Oxidative stress in atherosclerosis development:
The central role of LDL and oxidative burst. Endocr Metab Immune
Disord Drug Targets. 12:351–360. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Braakhuis AJ, Donaldson CI, Lim JC and
Donaldson PJ: Nutritional strategies to prevent lens cataract:
Current status and future strategies. Nutrients.
11(1186)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Han Q, Liu Q, Zhang H, Lu M, Wang H, Tang
F and Zhang Y: Simvastatin improves cardiac hypertrophy in diabetic
rats by attenuation of oxidative stress and inflammation induced by
calpain-1-mediated activation of nuclear factor-κB (NF-κB). Med Sci
Monit. 25:1232–1241. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao J, Zhang X, Dong L, Wen Y and Cui L:
The many roles of statins in ischemic stroke. Curr Neuropharmacol.
12:564–574. 2014.PubMed/NCBI View Article : Google Scholar
|