Introduction
Lung cancer is an aggressive cancer with a 5-year
survival rate of 8% worldwide (1).
Overall, 20-30% of lung cancer cases are lung squamous cell cancer
(LSCC) and >60% of patients with LSCC are diagnosed with locally
metastatic or advanced disease globally. Therefore, conventional
chemotherapy, radiotherapy, immunotherapy and supportive treatment
are the common options for patients with LSCC (2,3). To
date, molecularly targeted therapies have not demonstrated an
overall survival advantage for early-stage (stages I, II and IIIA)
patients, for these patients, surgical resection is recommended
(4). Although platinum-based
adjuvant chemotherapy is recommended for stage II-IIIA disease, the
recurrence rate is 30-70% (5).
Most patients with stage III LSCC are candidates for non-surgical
therapy, and concurrent chemoradiotherapy followed by immunotherapy
is the current standard of therapy (4). Nevertheless, the outcomes of these
treatments are still unsatisfactory (6).
The present report describes a rare case of a male
patient diagnosed with LSCC with pericardial, cervical and
extensive mediastinal lymph node metastases, who still survives
today, in March 2022, >10 years after chemotherapy and
radiotherapy. Therefore, this clinical case presents a promising
therapeutic regimen for patients with a similar condition.
Case report
A 50-year-old man was admitted to the Pneumology
Department in The Third Affiliated Hospital of Qiqihar Medical
University, Qiqihar, P.R. China on May 16, 2011 after exhibiting
cough with bloody sputum for 1 week. Other than the cough, he had
no other symptoms such as chest pain, shortness of breath, fever,
difficulty in breathing, fatigue, poor appetite or weight loss. The
patient had a free medical history. He had a smoking history of 20
packs years (1 pack of cigarettes/day for 20 years), and had been a
social drinker (1 bottle of beer/week) for ~20 years.
For physical examination, the patient's temperature
was 36.5˚C, heart rate 71 bpm, respiratory rate 15 breaths/min,
blood pressure 130/80 mmHg and oxygen saturation in room air 100%.
A painless hard nodule (4x3 cm) was found in the right
supraclavicular area, indicating abnormal enlarged lymph node, and
coarse rales could be heard in the right pulmonary base indicating
inflammation.
For laboratory examination, the serum tumor markers
carcinoembryonic antigen and α-fetoprotein were 7.78 and 1.09
ng/ml, respectively. They were in the reference range (<20 and
<5 ng/ml, respectively). Mycobacterium tuberculosis was
not found in sputum culture.
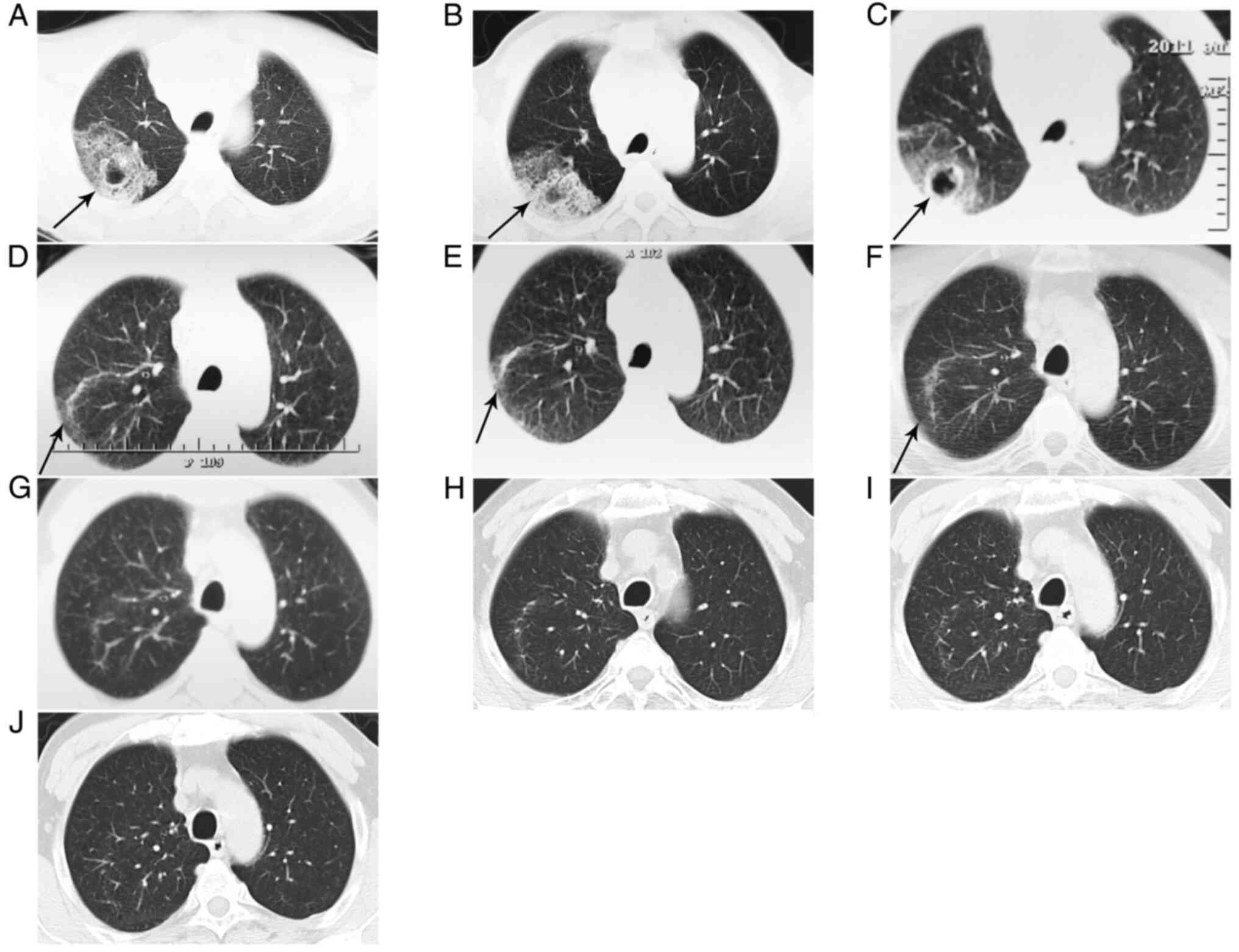
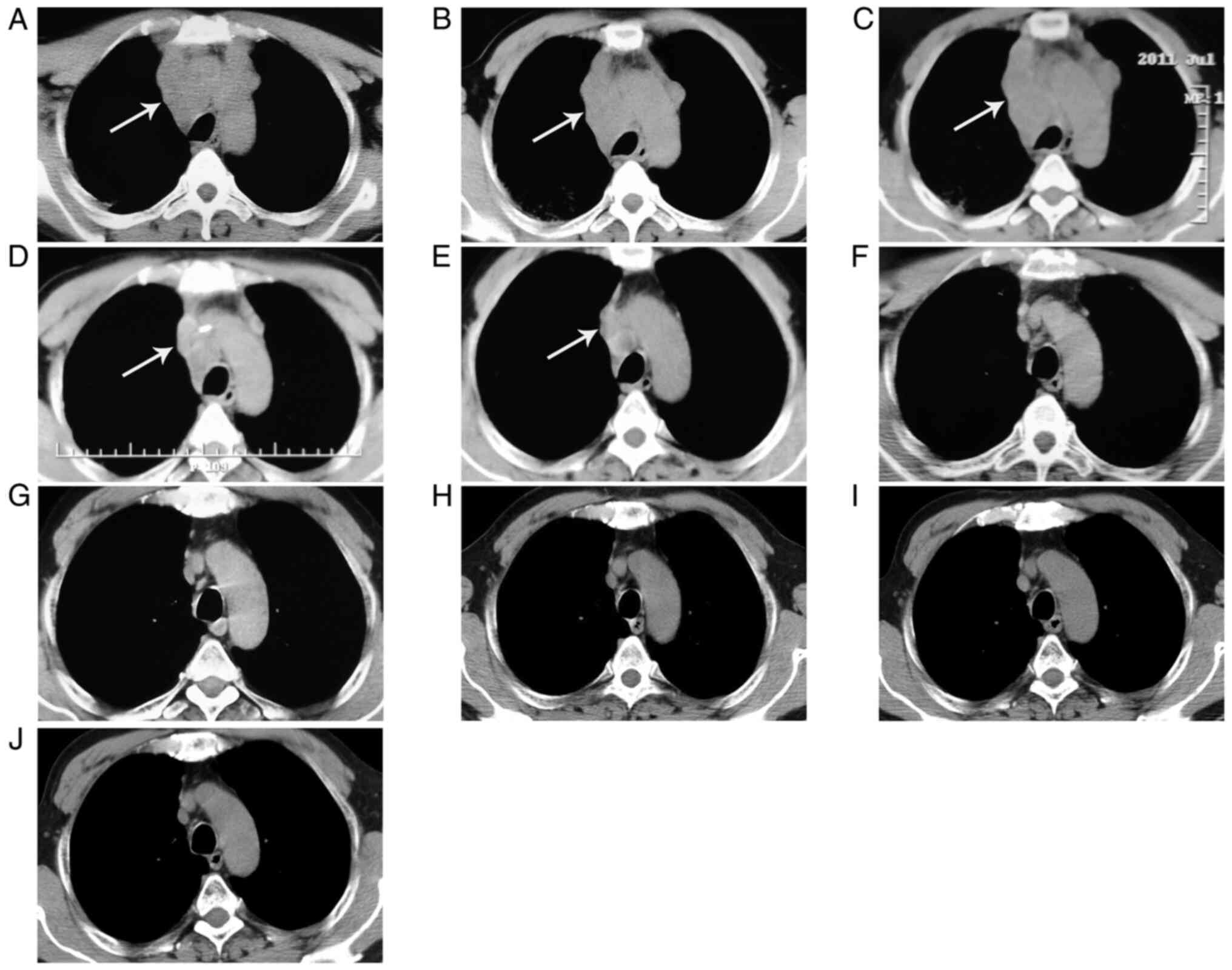
For imaging examination, Computed tomography (CT)
(Fig. 1A), revealed a flocculent
mass (7x4 cm) with some empty bubbles (the largest being 2x1.5 cm)
in the upper lobe of the right lung, indicating malignancy or
inflammation. In addition, extensively enlarged lymph nodes were
detected in the upper mediastinal regions (Fig. 2A), indicating metastases or
inflammation, and pericardial thickening was also found.
Ultrasonography revealed an abnormally blended and enlarged lymph
node (4x3 cm) in the right cervical region, suggesting the
possibility of metastases. Furthermore, head, abdominal and pelvic
CT identified no abnormalities.
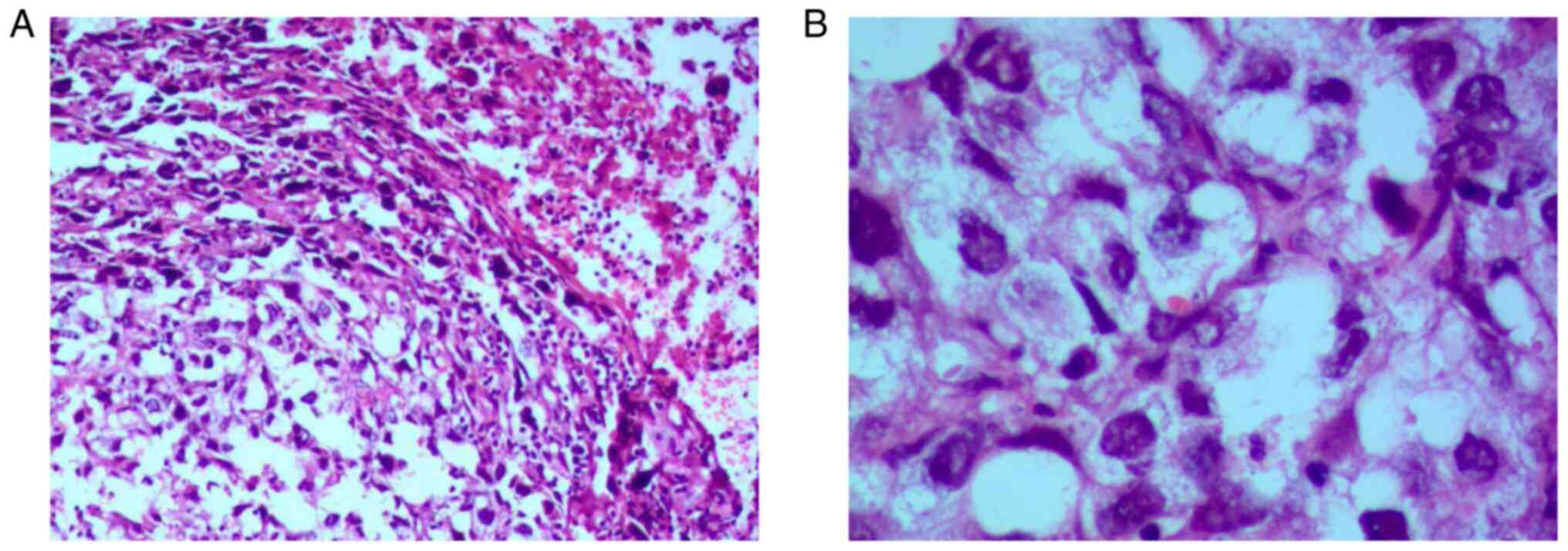
For biopsy, tissue specimens were fixed in 10%
formalin at 20˚C for 12 h, sectioned at a thickness of 5 µm,
stained with hematoxylin for 10 min and eosin for 20 sec at 20˚C,
and observed by light microscopy. Bronchoscopy confirmed bronchitis
in the upper lobe of the right lung. Subsequent biopsy of the right
cervical lymph node confirmed a poorly differentiated invasive
squamous cell carcinoma, which may have metastasized from the lung
(Fig. 3). Unfortunately, the
patient refused further immunohistochemical examination because of
economic concerns.
The pulmonary lesion was not improved after 2 weeks
of treatment of piperacillin sodium and tazobactam sodium and
aztreonam (approved by chest CT; Figs.
1B and 2B). After taking into
consideration the patient's condition, the cervical lymphatic
metastases were confirmed, and the patient was transferred to the
Department of Medical Oncology in the same hospital. Combined
therapy of docetaxel and carboplatin was started on June 16, 2011.
Nevertheless, after two courses of chemotherapy, the pulmonary
lesion and extensively enlarged lymph nodes were not improved
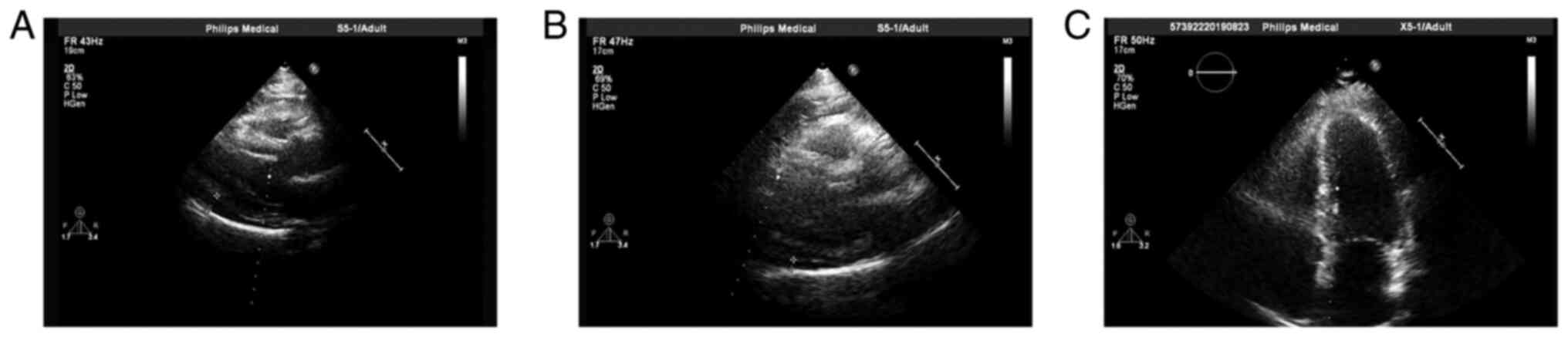
(Figs. 1C and 2C). In addition, pericardial effusion was
observed (Fig. 4A). Between July
27 and December 9, 2011, the patient was subjected to four courses
of gemcitabine and cisplatin as a second-line regimen. Furthermore,
radiotherapy of 60 Gy (2 Gy/day x 30 days) was administered on
December 1, 2011. The side effects of these treatments were mild
leukopenia, mild anemia, mild thrombocytopenia, mild hepatic
function impairment, mild radiation esophagitis, poor sleep
quality, decreased appetite and intermittent constipation.
The final diagnosis of the presented case was LSCC
with pericardial, cervical lymph node and extensive mediastinal
lymph node metastases. The effectiveness of treatment was evaluated
by imaging examination (Figs.
1D-J, 2D-J and 4B and C), which was supported by a gradually
diminished pulmonary lesion, lymph node enlargement and pericardial
effusion.
Changes of the lesion over time by chest CT scan of
the lung: Before any treatment (2011-5-16, 8x5 cm); after
anti-inflammatory treatment (2011-6-3, 8x5 cm); during therapy of
docetaxel + carboplatin (2011-7-9, 8x4.5 cm); during therapy of
gemcitabine + cisplatin (2011-9-19, 6x3.5 cm); during therapy of
gemcitabine + cisplatin (2011-11-30, 4x1.5 cm); after therapy of
gemcitabine + cisplatin + radiation (2012-10-8, 3.5x1 cm); review
(2014-4-11, disappeared); review (2016-8-18, 2018-7-3 and
2020-2-10, no relapse).
Changes of the enlarged lymph nodes over time by
chest CT scan of the mediastinum: Before any treatment (2011-5-16,
extensively enlarged lymph nodes); after anti-inflammatory
treatment (2011-6-3, extensively enlarged lymph nodes); during
therapy of docetaxel + carboplatin (2011-7-9, extensively enlarged
lymph nodes); during therapy of gemcitabine + cisplatin (2011-9-19,
some enlarged lymph nodes); during therapy of gemcitabine +
cisplatin (2011-11-30, few enlarged lymph nodes); after therapy of
gemcitabine + cisplatin + radiation (2012-10-8, no enlarged lymph
nodes); review (2014-4-11, 2016-8-18, 2018-7-3 and 2020-2-10, no
enlarged lymph nodes).
Changes of the pericardial effusion over time by
echocardiography: Moderate pericardial effusion after therapy of
docetaxel and carboplatin (2011-7-20); little pericardial effusion
during therapy of gemcitabine + cisplatin + radiation (2011-12-7);
no pericardial effusion after therapy of gemcitabine + cisplatin +
radiation (2012-2-1).
Changes of the blood routine over time: Before any
treatment (2011-5-17, all items were in the reference range); after
anti-inflammatory treatment (2011-6-4, all items were in the
reference range); during therapy of docetaxel + carboplatin
(2011-7-7, all items were in the reference range); during therapy
of gemcitabine + cisplatin [2011-8-8; WBC 3.9x109/l
(reference range, 4.0-10.0x109/l); PLT
109x109/l (reference range, 120-380x109/l)];
during therapy of gemcitabine + cisplatin [2011-8-16; WBC
3.6x109/l; RBC 3.59x1012/l (reference range,
3.80-5.30x1012/l); HGB 106 g/l (reference range, 110-170
g/l)]; during therapy of gemcitabine + cisplatin (2011-10-13; RBC
3.26x1012/l); after therapy of gemcitabine + cisplatin +
radiation (2012-1-8; RBC 3.16x1012/l); review
(2012-11-19, 2013-9-28, 2014-4-14, 2015-7-7, 2017-5-28, 2019-9-10
and 2020-5-5, all items were in the reference range).
Changes of the blood biochemistry over time: Before
any treatment (2011-5-17, all items were in the reference range);
after anti-inflammatory treatment (2011-6-4, all items were in the
reference range); during therapy of docetaxel + carboplatin
(2011-7-7, all items were in the reference range); during therapy
of gemcitabine + cisplatin [2011-10-8; γ-glutamyl transpeptidase
(GGT) 59 U/l (reference range: 0-50 U/l)]; during therapy of
gemcitabine + cisplatin (2011-10-31; GGT 58 U/l); after therapy of
gemcitabine + cisplatin + radiation (2012-1-8, all items were in
the reference range); review (2012-11-19, 2013-9-28, 2014-4-14,
2015-7-7, 2017-5-28, 2019-9-10 and 2020-5-5, all items were in the
reference range).
The tumor biomarkers, carcinoembryonic antigen,
α-fetoprotein, neuron-specific enolase, glycoantigen-199 and
squamous epithelial cell carcinoma antigen were in the reference
range (on 2020-5-5).
The changes of blood routine and biochemistry
demonstrated that the patient had gradually recovered from
leukopenia, anemia and thrombocytopenia. The patient still survives
healthily today, in March 2022, more than 10 years after
diagnosis.
Discussion
This patient had a flocculent mass with some empty
bubbles in the upper lobe of the right lung; extensively enlarged
lymph nodes in the upper mediastinal regions with pericardial
thickening; and an abnormally blended and enlarged lymph node in
the right cervical region; all of which confirmed a poorly
differentiated invasive squamous cell carcinoma with histological
features similar to lung cancer. These findings suggested a primary
lung cancer with multiple metastases.
The patient had no primary heart disease or
pericardial effusion. After two courses of chemotherapy (docetaxel
+ carboplatin), the pulmonary lesion and enlarged lymph nodes were
not improved, and pericardial effusion was observed. All the drugs
(including docetaxel + carboplatin) had been administrated to him
have no side effect of causing pericardial effusion. Furthermore,
pericardial effusion is the most common clinical manifestation of
pericardial metastatic disease. Therefore, it was concluded that
pericardial effusion was due to pericardial metastasis from lung
cancer.
At present, cisplatin is the preferred regimen used
concurrently with radiotherapy for locally advanced non-small-cell
lung cancer (NSCLC) (7). For
patients with LSCC and distant metastasis, the National
Comprehensive Cancer Network guidelines recommend cisplatin and
gemcitabine as the first-line regimen (8). In the present case, the patient had
been treated with docetaxel and carboplatin as first-line
chemotherapy for two courses. However, lesions were not improved
and pericardial effusion emerged. Therefore, a new regimen of
gemcitabine and cisplatin combined with radiotherapy was
administered. After the second-line treatment, a complete response
of the pulmonary lesion and cervical and mediastinal lymph node
enlargement and pericardial effusion were confirmed using chest CT,
cervical ultrasound imaging and echocardiography. Some researchers
have reported promising clinical outcomes of gemcitabine, cisplatin
and radiotherapy. Ma et al (9) reported that patients with squamous
cell lung cancer treated with gemcitabine and cisplatin as an
adjuvant therapy achieve improved outcomes. Yang et al
(10) reported that gemcitabine
with cisplatin was an effective induction therapy before surgery
for patients with NSCLC. Zwitter et al (11) reported that gemcitabine, cisplatin
and radiotherapy achieved improved disease control compared with
traditional regimens for advanced NSCLC. These studies echoed the
present report. Furthermore, Das et al (12) reported that a trial of docetaxel +
carboplatin + radiotherapy results in promising outcomes in stage
III patients with NSCLC. Nevertheless, docetaxel + carboplatin as a
first-line treatment option was not effective in the present
case.
The present patient survives healthily >10 years
after diagnosis, which is much longer compared with the median
overall survival of 21.8 months reported by Driesen et al
(13) after induction with
gemcitabine and cisplatin followed by concurrent chemoradiotherapy
for patients with unresectable locally advanced NSCLC. The present
study considered that the reasonable treatment plan, a free medical
history and a positive psychological state contributed greatly to
the patient's survival. Although it is only a rare case, it may
offer a promising therapeutic regimen to patients with a similar
condition.
In conclusion, administration of gemcitabine +
cisplatin + radiotherapy (performed after chemotherapy) was
considered to be an effective regimen against pericardial, cervical
lymph node and extensive mediastinal lymph nodes metastases from
squamous cell lung carcinoma. This case may offer a promising
therapeutic regimen to patients with a similar condition.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL is the patient's physician, reviewed the
literature, and contributed to acquisition, analysis and
interpretation of data and manuscript drafting. JY contributed to
manuscript drafting and acquisition of data. XJS and SNL analyzed
and interpreted the imaging findings. SL reviewed the literature,
and contributed to conception and design of the study, analysis and
interpretation of data, and drafted, reviewed and edited the
manuscript. SL and YL confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient signed informed written consent about
treatment interventions, his data collection and submission for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan PS, Bilger M, de Lima Lopes G,
Acharyya S and Haaland B: Meta-analysis of first-line therapies
with maintenance regimens for advanced non-small-cell lung cancer
(NSCLC) in molecularly and clinically selected populations. Cancer
Med. 6:1847–1860. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alexander M, Kim SY and Cheng HY: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE collaborative group. J Clin Oncol.
26:3552–3559. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu TT, He Z, Dang J and Li G: Comparative
efficacy and safety for different chemotherapy regimens used
concurrently with thoracic radiation for locally advanced non-small
cell lung cancer: A systematic review and network meta-analysis.
Radiat Oncol. 14(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ettinger DS, Aisner DL, Wood DE, Akerley
W, Bauman J, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ,
Dobelbower M, et al: NCCN guidelines insights: Non-small cell lung
cancer, version 5.2018. J Natl Compr Canc Netw. 16:807–821.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma D, Wang J, Hao X, Wang Y, Hu X, Xing P
and Li J: Gemcitabine combined with cisplatin as adjuvant
chemotherapy for non-small cell lung cancer: A retrospective
analysis. Thorac Cancer. 8:482–488. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang CH, Tsai CM, Wang LS, Lee YC, Chang
CJ, Lui LT, Yen SH, Hsu C, Cheng AL, Liu MY, et al: Gemcitabine and
cisplatin in a multimodality treatment for locally advanced
non-small cell lung cancer. Br J Cancer. 86:190–195.
2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zwitter M, Kovac V, Smrdel U and Strojan
P: Gemcitabine, cisplatin, and hyperfractionated accelerated
radiotherapy for locally advanced non-small cell lung cancer. J
Thorac Oncol. 1:662–666. 2006.PubMed/NCBI
|
|
12
|
Das M, Donington JS, Murphy J, Kozak M,
Eclov N, Whyte RI, Hoang CD, Zhou L, Le QT, Loo BW and Wakelee H:
Results from a single institution phase II trial of concurrent
docetaxel/carboplatin/radiotherapy followed by surgical resection
and consolidation docetaxel/carboplatin in stage III non-small-cell
lung cancer. Clin Lung Cancer. 12:280–285. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Driesen P, Lambrechts M, Kraaij K,
Soldatenkova V, Chouaki N and Colinet B: A phase II single-arm
study of induction chemotherapy with cisplatin and gemcitabine
followed by concurrent cisplatin and gemcitabine with thoracic
radiation for unresectable locally advanced non-small cell lung
cancer. Ther Adv Med Oncol. 5:159–168. 2013.PubMed/NCBI View Article : Google Scholar
|