Introduction
Obesity poses a challenge to the health of pregnant
women. Stubert et al (1)
reported that the risk of pregnancy-related illness increases as
the severity of obesity increases. Maternal obesity rates have
already exceeded 30% in most European countries (2). Data from one study showed that obese
pregnant women are more likely to suffer from gestational diabetes,
pre-eclampsia, gestational hypertension, depression, caesarean
sections and surgical site infections than women with a healthy
weight (3). In addition to
affecting maternal health, maternal obesity may also have long-term
adverse effects on the health of the fetus and newborn. For
example, the fetuses of obese women are also subject to premature
births, stillbirths and fetal malformations (4). Maternal obesity has become a serious
public health problem that needs to be addressed, and therefore
exploring its pathogenesis is critical.
Growing evidence shows that the placenta plays an
important role in regulating fetal growth (1). However, maternal obesity is a risk
factor for placental dysfunction. According to a recent study,
obese pregnant women are prone to placental inflammation (5). This is due to the fact that excess
saturated fatty acid palmitic acid (PA) in the serum of obese
pregnant women creates a lipotoxic environment in the placenta,
which stimulates the production of pro-inflammatory cytokines,
including TNF-α, IL-6 and IL-8(6).
The release of excessive pro-inflammatory cytokines can cause
placental dysfunction and affect placental nutrient transport and
adipose tissue metabolism (7).
Recent studies have demonstrated that PA can cause inflammatory
release, impaired invasion and migration, and apoptosis in
trophoblasts (8,9).
C1q/TNF-related protein 9 (CTRP9) is the closest
analogue of adiponectin, a classic anti-inflammatory agent, and
plays a role in metabolic regulation, anti-atherosclerosis and
anti-inflammation (10,11). A recent study demonstrated that
CTRP9 expression level was decreased in the serum of obese patients
with eclampsia during pregnancy (12). CTRP9 has been shown to reduce high
fat diet-induced cardiac hypertrophy and cardiomyocyte lipotoxicity
(13). It has also been shown that
its expression is increased in the serum of patients with type 2
diabetes and is associated with insulin resistance (14). In addition, CTRP9 has been shown to
be able to activate the expression of AMP-activated protein kinase
(AMPK) signaling (15) and improve
the anti-contractile effect of diet-induced perivascular adipose
tissue in obese mice through the AMPK-endothelial nitric oxide
synthase pathway (eNOS) (16).
AMPK activation has been shown to suppress inflammatory responses
in various injury models, such as myocardial ischemia/reperfusion,
acute lung and liver injury (17).
Previous studies indicated that SREBP1c was the downstream gene of
AMPK, and AMPK could inhibit downstream SREBP1c expression level
(18-20).
SREBP1c is a key transcription factor for de
novo lipogenesis (21).
Studies have shown that SREBP1c expression level is increased in
both human trophoblasts and porcine placental trophoblasts with
lipid accumulation (22,23). Accordingly, the present study
focused on exploring whether CTRP9 could inhibit SREBP1c expression
through AMPK signaling, thereby reducing PA-induced inflammation,
apoptosis and impaired migration in gestational trophoblasts.
Materials and methods
Kyoto encyclopedia of genes and genome
(KEGG) analysis (24-26)
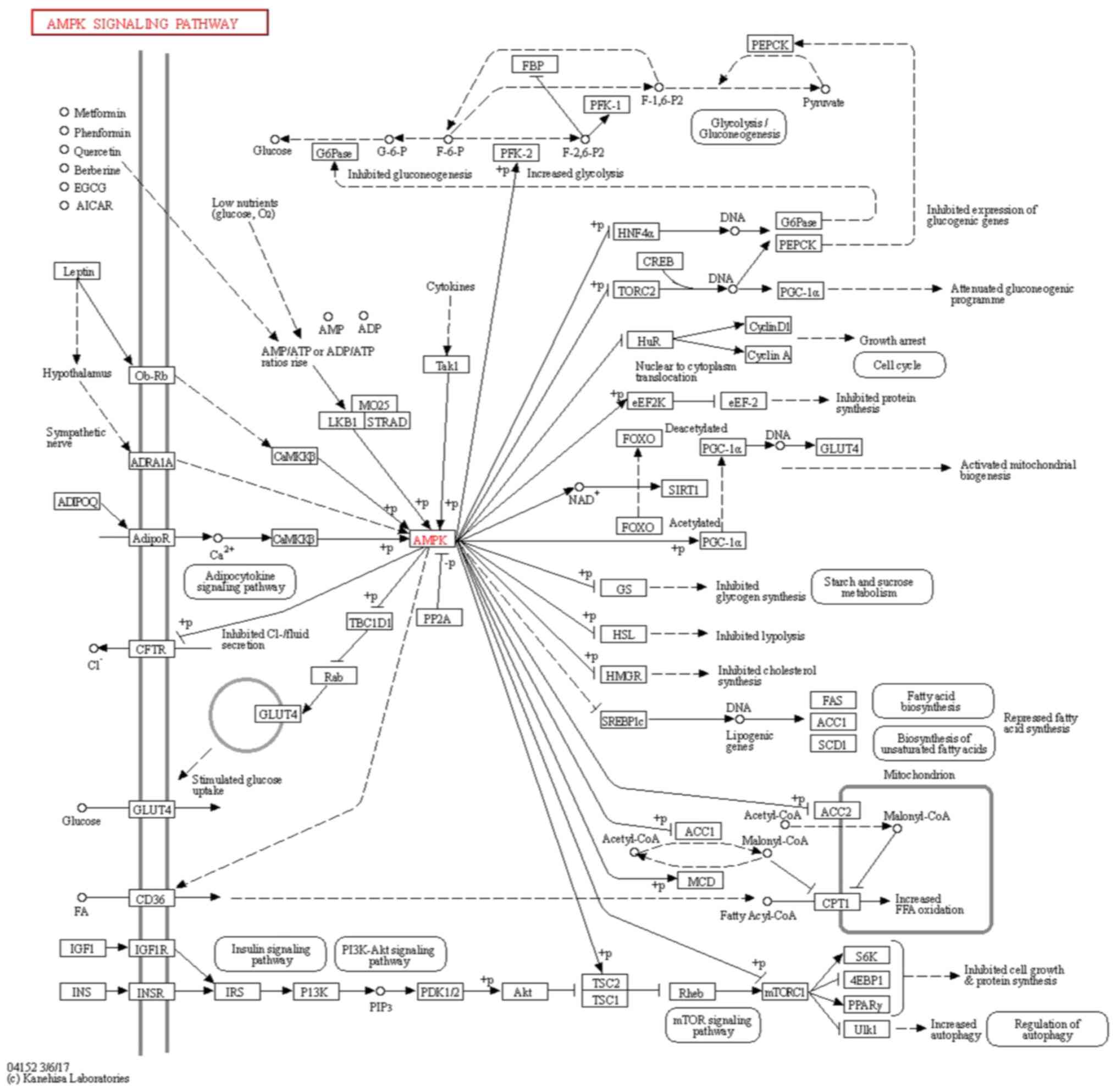
The KEGG pathway database (https://www.genome.jp/kegg/pathway.html) was used to
determine whether expression of SREBP1c, a downstream gene of AMPK,
was suppressed by AMPK in AMPK signaling pathway (map04152).
Cell culture and treatment
A human trophoblast HTR8/SVneo cell line was
obtained from the American Type Culture Collection (27). This cell line was originally
generated using freshly isolated extravillous cytotrophoblast from
first-trimester placenta and transfected with a plasmid containing
the simian virus 40 large T antigen, which included two
populations; one of epithelial and one of mesenchymal origin.
HTR8/SVneo cells were grown in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 5% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 at 37˚C.
Prior to treatment, PA (ChemicalBook) was dissolved
in 0.1 M NaOH at 70˚C to make 20 mM of stock solution and mixed
with 30% fatty-acid-free BSA (Shanghai Weiao Biotechnology Co.,
Ltd.) for 2 h to produce 5 mM PA-BSA conjugate solution. HTR8/SVneo
cells were treated with the PA-BSA conjugate solution at a
concentration of 200, 400, 600 and 800 µM for 24 h at 37˚C. In each
assay, 30% fatty-acid-free BSA was used as the vehicle. Compound C
is a potent and selective AMPK inhibitor. HTR8/SVneo cells were
pretreated with 10 µM Compound C (cat. no. S7840; Selleck
Chemicals) for 2 h prior to being exposed to PA for 24 h at
37˚C.
Plasmid construction of CTRP9 and
SREBP1c
The overexpression plasmid vectors (pcDNA 3.1)
targeting CTRP9 (Ov-CTRP9) and SREBP1c (Ov-SREBP1c), as well as
empty overexpression negative control vectors (Ov-NC), were
constructed by Shanghai GenePharma Co., Ltd. HTR8/SVneo cells were
seeded in 6-well culture plates (2x105 cells/well) and
cultured for 24 h at 37˚C. Next, HTR8/SVneo cells were transfected
with 200 nM Ov-CTRP9, 200 nM Ov-SREBP1c or 200 nM Ov-NC at 37˚C for
24 h using Lipofectamine® 2000 (Invitrogen; Thermo
Fischer Scientific, Inc.), according to the manufacturer's
instructions. The transfection efficiency was measured using
reverse transcription-quantitative PCR (RT-qPCR), and cells were
used for subsequent experiments 48 h after transfection.
Cell counting kit-8 (CCK-8)
HTR8/SVneo cells (2x104 cells/well) were
routinely seeded in 96-well plates for 24 h. Next, 10 µl CCK-8
solution (Glpbio) was added in each well and incubated for 2 h at
37˚C in an incubator. The optical density was measured at 450 nm
with a microplate reader (M3000; China Med Device).
RT-qPCR
The mRNA expression of CTRP9, TNF-α, IL-1β, IL-6 and
SREBP1c was examined using RT-qPCR. Total RNA extraction from
HTR8/SVneo cells was performed using TRIzol® reagent
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. cDNA was synthesized by reverse transcription of
total RNA using a PrimeScript™ RT Reagent Kit with gDNA
Eraser (Takara Biotechnology Co., Ltd.), according to the
manufacturer's instructions. qPCR was performed using a SYBR Green
RT-qPCR Master Mix kit (MedChemExpress) on an ABI 7500 quantitative
PCR instrument (PerkinElmer, Inc.). The thermocycling conditions
were as follows: 95˚C for 25 sec, followed by 40 cycles of 95˚C for
10 sec and 60˚C for 35 sec. The primer sequences used were as
follows: CTRP9 forward, 5'-GAGGATCCCCAGGAAAACAT-3' and reverse,
5'-AGCTTCTCCTTTGGGACCAG-3'; TNF-α forward,
5'-GGCGTGGAGCTGAGAGATAA-3' and reverse, 5'-TTGATGGCAGAGAGGAGGTT-3';
IL-1β forward, 5'-GCATCCAGCTACGAATCTCC-3' and reverse,
5'-TGAAGGGAAAGAAGGTGCTC-3'; IL-6 forward, 5'-TTCGGTCCAGTTGCCTTCT-3'
and reverse, 5'-GAGATGCCGTCGAGGATGTA-3'; SREBP1c forward,
5'-TGACTTCCCTGGCCTATTTG-3' and reverse, 5'-GCATGGACGGGTACATCTTC-3';
and GAPDH forward, 5'-CACCCATGGCAAATTCCATGGCA-3' and reverse,
5'-TCTAGACGGCAGGTCAGGTCCACC-3'. The relative mRNA expression of
genes was calculated using the 2-ΔΔCq method (28) following normalization to GAPDH.
Western blot analysis
Total protein was extracted from HTR8/SVneo cells
using RIPA buffer (MilliporeSigma) on ice. Protein concentration
was assessed using a BCA Protein Quantification Kit (Vazyme Biotech
Co., Ltd.), according to the manufacturer's instructions. Equal
amounts (20 µg) of protein samples were separated by SDS-PAGE on a
10% gel and transferred onto PVDF membranes (Roche Diagnostics).
Blocking with 5% non-fat milk for 2 h at room temperature was
performed, followed by incubation with primary antibodies against
CTRP9 (dilution 1:400; cat. no. LS-C373857; LifeSpan Biosciences,
Inc.), p-AMPK (dilution 1:1,000; cat. no. ab92701; Abcam), AMPK
(dilution 1:1,000; cat. no. ab32047; Abcam), SREBP1c (dilution,
1:1,000; cat. no. ab28481; Abcam) and GAPDH (dilution 1:2,500; cat.
no. ab9485; Abcam) at 4˚C overnight. Following three washes with
PBS, an HRP-conjugated antibody (1:2,000; cat. no. ab6721; Abcam)
was incubated together with the membranes at room temperature for a
further 1 h. Finally, the protein bands were visualized using
Bio-Rad ChemiDocTM XRS+ System (Bio-Rad Laboratories,
Inc.) and quantified using Image Lab 5.2.1 (Bio-Rad Laboratories,
Inc.). The experiment was performed in triplicate.
Cell apoptosis detection using TUNEL
assay
The apoptosis of HTR8/SVneo cells was assessed using
a One Step TUNEL Apoptosis Assay Kit (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
HTR8/SVneo cells were fixed with 4% paraformaldehyde for 30 min at
4˚C. Following washing with PBS, these cells were incubated with
PBS solution containing 0.3% Triton X-100 for 5 min at room
temperature. A TUNEL Assay Kit was prepared as required by the
configuration table. The samples were then incubated using 50 µl
TUNEL reagent in the dark for 60 min at 37˚C, followed by staining
of nuclear DNA with 10 µg/ml DAPI at 37˚C for 2-3 min. Following
three washes with PBS, cells with green fluorescence were observed
using fluorescence microscopy at a magnification of x200 in at
least 5 fields of view after blocking with 30 µl anti-fluorescence
quenching blocking solution (Beyotime Institute of Biotechnology)
at room temperature for 5 min. The total cells were defined as blue
dots and apoptotic cells were defined as green dots. The
quantification of the apoptotic cells was determined by Image J
1.51 software (National Institutes of Health).
Transwell assay
HTR8/SVneo cells (1x105) were incubated
in 200 µl serum-free RPMI-1640 medium for 24 h before plating them
in the upper chamber of 24-well Transwell plates. Cells in the
lower chamber were incubated for 24 h with 600 µl RPMI-1640
containing 20% FBS. After discarding the culture medium, the plates
were washed twice with calcium-free PBS and then fixed in methanol
for 30 min at room temperature. Subsequently, after cells were
stained with 0.1% crystal violet for 20 min at room temperature,
non-migrating cells in the upper layer were gently wiped off with a
cotton swab and washed 3 more times with PBS. A total of five areas
of cells were randomly selected and observed under a light
microscope at a magnification of x100 (Shenzhen Boshida Instrument
Co., Ltd.; https://www.cn-microscope.com/) for counting using
EVOS M7000 Imaging System (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 (SPSS, Inc.). All data are presented as the mean ± SD.
One-way ANOVA followed by Tukey's post hoc test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed in triplicate.
Results
CTRP9 expression is reduced in
PA-treated HTR8/SVneo cells
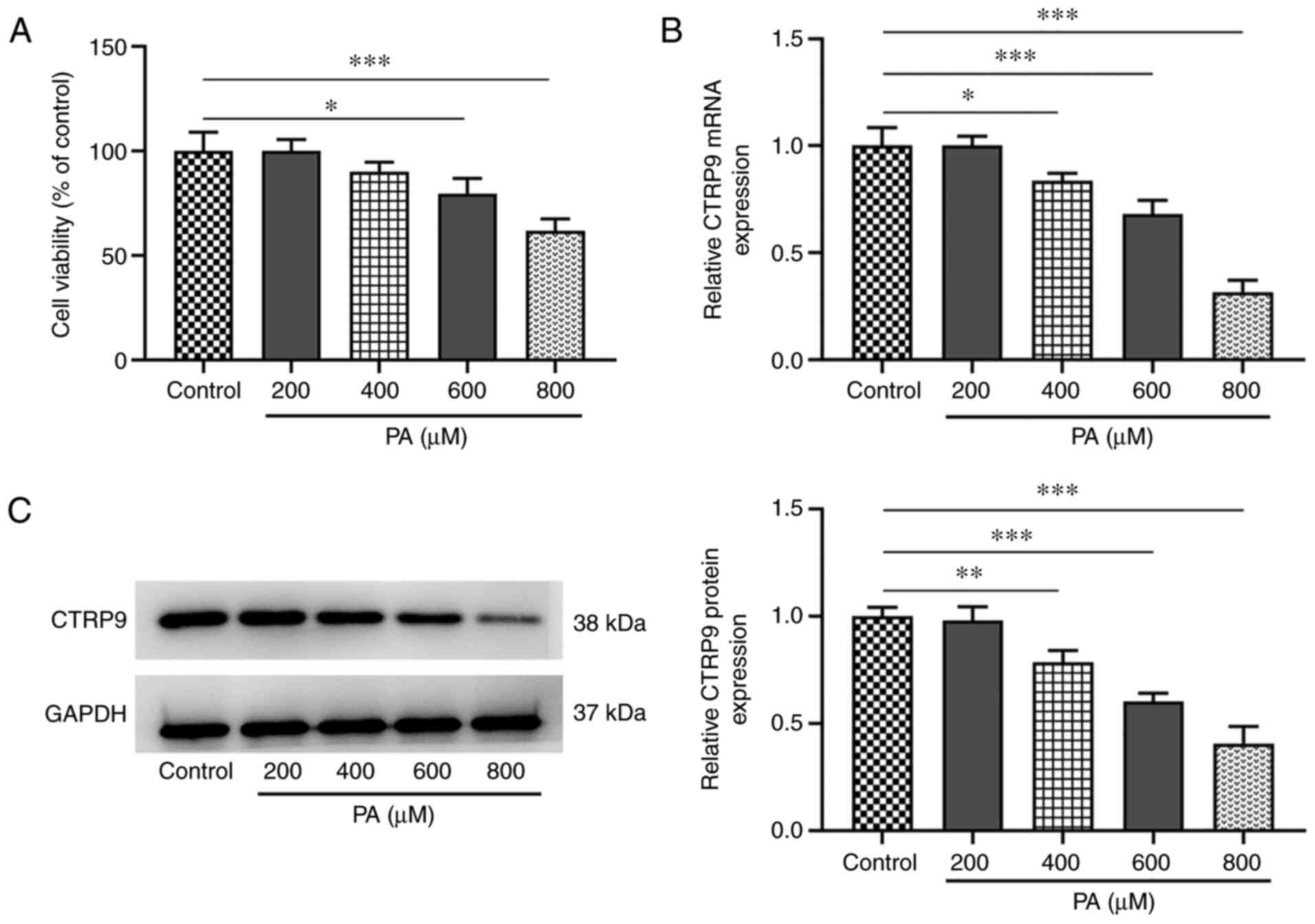
Cell viability and CTRP9 expression in HTR8/SVneo
cells with or without PA treatment at concentrations of 200, 400,
600 and 800 µM was measured using CCK-8, western blotting and
RT-qPCR analyses. HTR8/SVneo cell viability was gradually reduced
in response to PA induction, compared with that of the control
group. Higher PA concentrations resulted in lower cell viability,
with the lowest viability at 800 µM PA (Fig. 1A). The mRNA and protein expression
levels of CTRP9 were significantly downregulated in PA-treated
HTR8/SVneo cells (vs. control) in a dose-dependent manner, with the
lowest expression level observed at 800 µM PA (Fig. 1B and C). Therefore, the group treated with 800
µM PA was selected for the next experiments.
CTRP9 overexpression attenuates
PA-induced apoptosis and impaired cell viability in HTR8/SVneo
cells
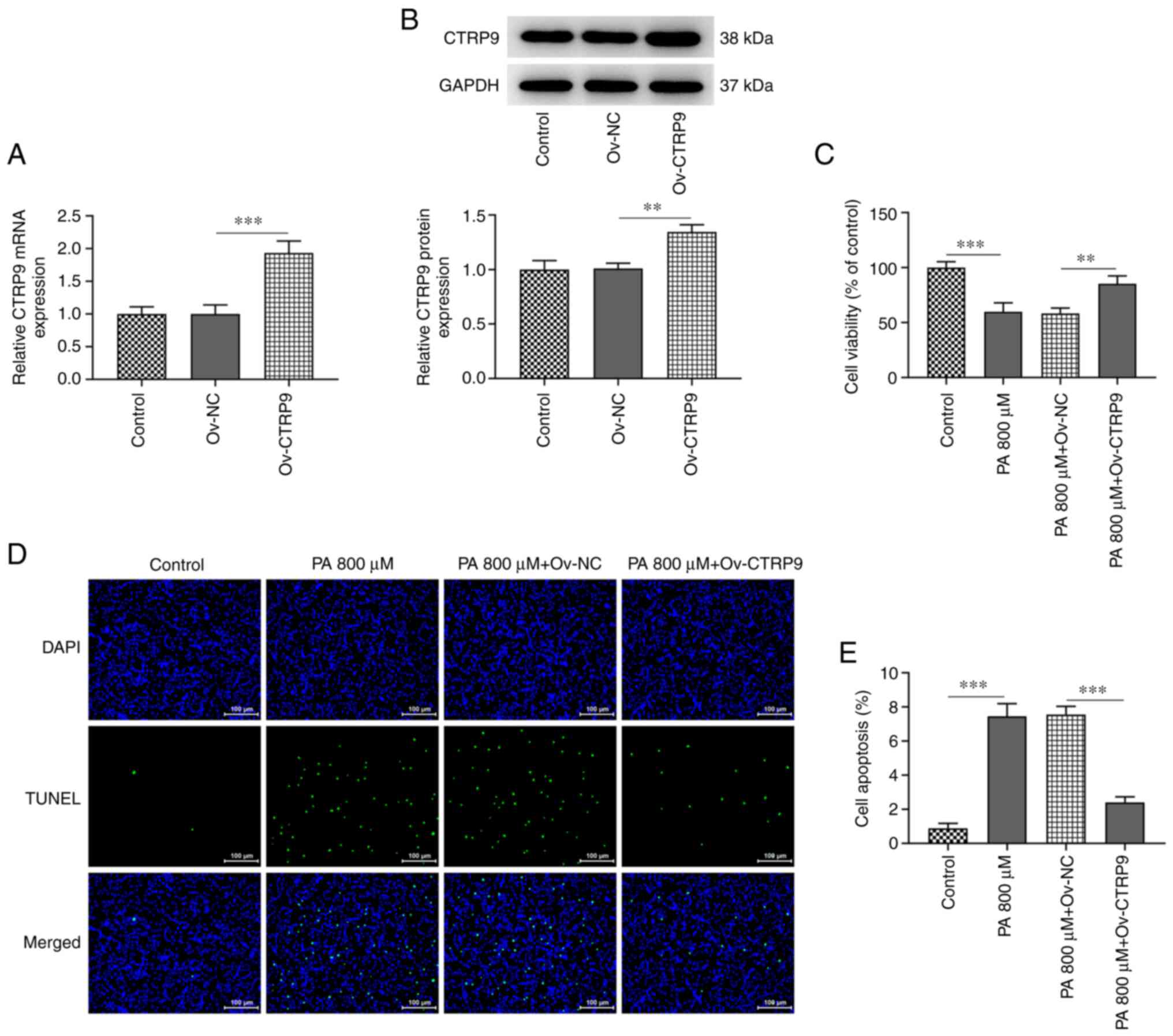
Following the transfection of HTR8/SVneo cells with
Ov-CTRP9, the mRNA and protein expression levels of CTRP9 were
significantly elevated compared with those in the Ov-NC group
(Fig. 2A and B). As indicated in Fig. 2C, the viability of HTR8/SVneo cells
increased in the PA+Ov-CTRP9 group, compared with that in the Ov-NC
group. In addition, Fig. 2D and
E revealed more TUNEL-positive
HTR8/SVneo cells in the PA group (PA group vs. control) but notably
less TUNEL-positive cells in the PA+Ov-CTRP9 group (PA+Ov-CTRP9 vs.
PA+Ov-NC). Accordingly, the increased CTRP9 expression level in
HTR8/SVneo cells could largely reduce the PA-induced impairment in
cell viability and apoptosis.
CTRP9 overexpression attenuates
inflammation and impaired migration in PA-treated HTR8/SVneo
cells
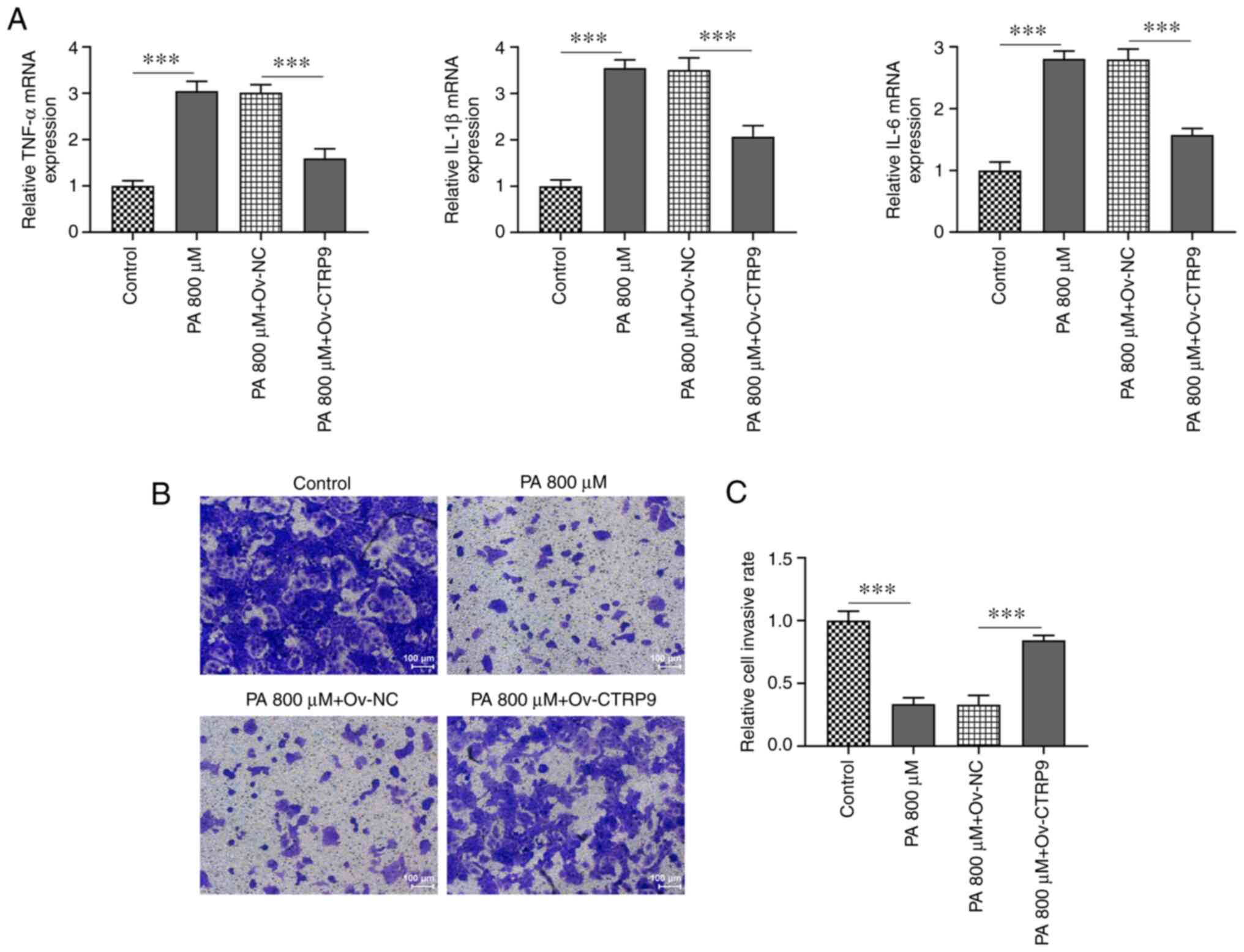
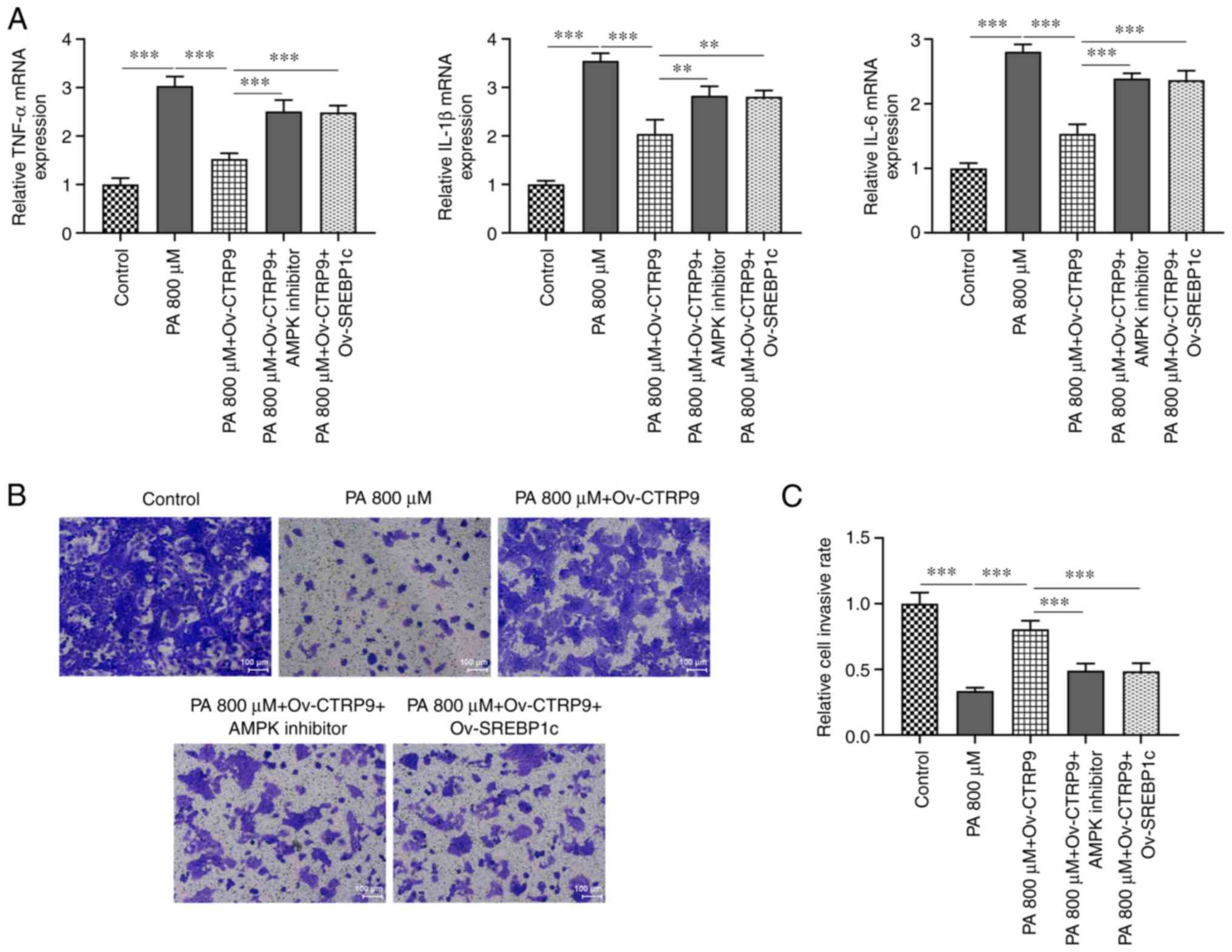
RT-qPCR revealed that the mRNA levels of
pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were increased in
PA-treated HTR9/SVneo cells compared with the Control group and
were reduced by CTRP9 overexpression compared with the PA 800 µM +
Ov-NC group (Fig. 3A). The
Transwell assay revealed decreased migratory ability in PA-treated
HTR8/SVneo cells compared with that in the control group. However,
CTRP9 overexpression increased the migration of PA-treated
HTR8/SVneo cells compared with that in the Ov-NC group (Fig. 3B and C). Therefore, CTRP9 upregulation could
reduce the release of inflammatory cytokines and protect against
the impairment of PA-promoted migratory ability of HTR8/SVneo
cells.
CTRP9 overexpression attenuates
PA-induced cell viability impairment and apoptosis in HTR8/SVneo
cells through AMPK/SREBP1c signaling
According to the KEGG pathway database, SREBP1c is a
downstream gene of AMPK (Fig. 4).
As shown in Fig. 5A, the protein
expression level of p-AMPK was markedly decreased in the PA group
compared with that in the control group, but was increased
following CTRP9 overexpression in PA-induced HTR8/SVneo cells
compared with the Ov-NC group. Treatment with the AMPK inhibitor
caused a decrease in the protein level of p-AMPK compared with that
in the PA+Ov-CTRP9 group. In addition, there were no obvious
changes in AMPK protein level in the different groups. Furthermore,
the protein expression level of SREBP1c increased in the PA group
compared with the control group, and decreased in the PA+Ov-CTRP9
group compared with that in the PA+Ov-NC group. The protein
expression level of SREBP1c was elevated following the addition of
AMPK inhibitor, which indicated that CTRP9 overexpression played a
regulatory role in AMPK/SREBP1c signaling in PA-induced HTR8/SVneo
cells (Fig. 5A).
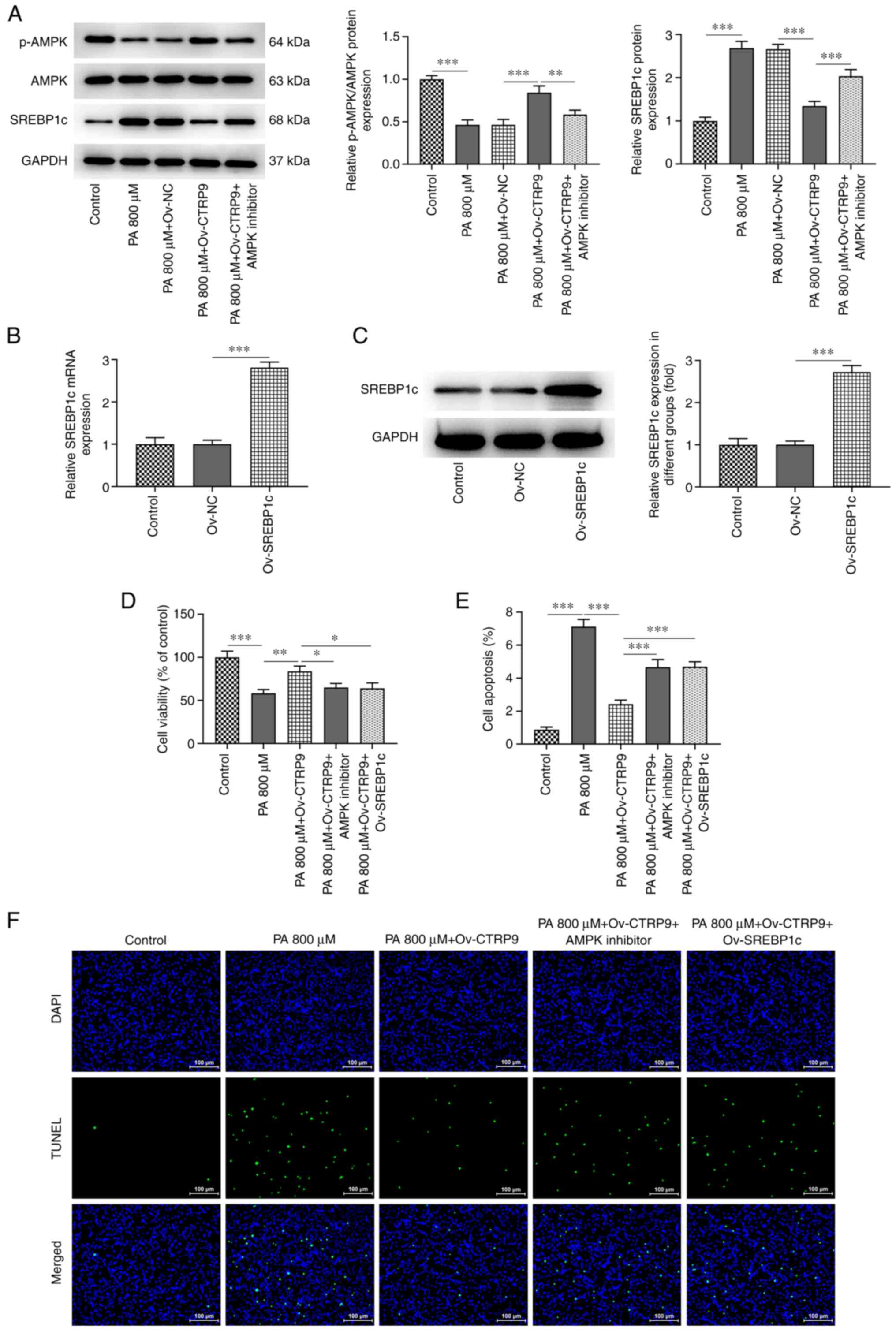 | Figure 5CTRP9 overexpression attenuates
PA-induced cell viability impairment and apoptosis in HTR8/SVneo
cells through AMPK/SREBP1c signaling. (A) Protein expression levels
of p-AMPK, AMPK and SREBP1c in PA-induced HTR8/SVneo cells
transfected with Ov-NC or Ov-CTRP9 were measured by western
blotting after addition of AMPK inhibitor. SREBP1c overexpression
in HTR8/SVneo cells transfected with none, Ov-NC, Ov-CTRP9 was
detected by (B) reverse transcription-quantitative PCR and (C)
western blotting. (D) Cell viability of PA-induced HTR8/SVneo cells
was assessed by cell counting kit-8 in the groups of Ov-CTRP9+AMPK
inhibitor and Ov-CTRP9+Ov-SREBP1c. (E) The quantification of (F)
apoptosis of PA-induced HTR8/SVneo cells was evaluated by TUNEL
assay in the Ov-CTRP9+AMPK inhibitor and Ov-CTRP9+Ov-SREBP1c
groups. *P<0.05, **P<0.01 and
***P<0.001. SREBP1c, sterol-regulatory element
binding protein 1c; AMPK, AMP-activated protein kinase; CTRP9,
C1q/TNF-related protein 9; PA, palmitic acid; p-, phospho-; Ov-,
overexpression vector; NC, negative control. |
In the present study, HTR8/SVneo cells transfected
with Ov-SREBP1c expressed a higher level of SREBP1c than those of
the Ov-NC groups (Fig. 5B and
C). In the aforementioned results,
CTRP9 overexpression improved the viability of PA-induced
HTR8/SVneo cells. However, the viability of these cells was
decreased both in the Ov-CTRP9+AMPK inhibitor and
Ov-CTRP9+Ov-SREBP1c groups (Fig.
5D). Similarly, Ov-CTRP9 reduced PA-induced apoptosis in the
HTR8/SVneo cells. Nevertheless, apoptotic cells were increased in
the Ov-CTRP9+AMPK inhibitor and Ov-CTRP9+Ov-SREBP1c groups
(Fig. 5E and F). These results suggested that the
increased CTRP9 expression level could prevent PA-induced impaired
cell viability and reduce the apoptosis of HTR8/SVneo cells through
AMPK/SREBP1c signaling.
CTRP9 overexpression attenuates
inflammation-induced impairment in PA-induced HTR8/SVneo cell
migration through AMPK/SREBP1c signaling
According to the aforementioned experimental
findings, it was demonstrated that CTRP9 overexpression could
reduce inflammatory cytokine release in PA-induced HTR8/SVneo
cells. The mRNA levels of TNF-α, IL-6 and IL-1β were higher in both
the Ov-CTRP9+AMPK inhibitor and Ov-CTRP9+Ov-SREBP1c groups compared
with those in the PA+Ov-CTRP9 group (Fig. 6A). The migratory ability of
PA-induced HTR8/SVneo cells was promoted by CTRP9 overexpression,
as aforementioned. However, according to the data presented in
Fig. 6B and C, a notable decline in the migratory
ability of PA-induced HTR8/SVneo cells was observed in the
Ov-CTRP9+AMPK inhibitor and Ov-CTRP9+Ov-SREBP1c groups. These
results suggested that CTRP9 overexpression could reduce PA-induced
inflammation and migration impairment in HTR8/SVneo cells through
AMPK/SREBP1c signaling.
Discussion
Maternal obesity has a serious impact on the health
of both the mother and the fetus. Improving the metabolic
environment of obese pregnant women and reducing the future
economic, social and personal burden of maternal obesity is
therefore of great significance. Studies have shown that placental
cells from obese pregnant women contain high levels of PA, inducing
an inflammatory response in trophoblasts and attenuating cell
migration through a Pediocin PA-1-mediated mechanism, leading to
placental dysfunction and apoptosis (8). CTRP9 has been reported to be
downregulated in obese patients, and can affect the placental
system and suppress pro-inflammatory genes (12,29).
To explore the role of CTRP9 on placental inflammation in obese
women, in the present study, trophoblasts were treated with high PA
levels to mimic the lipotoxic environment of the placenta. Based on
the experimental results, it was observed that CTRP9 expression was
reduced in PA-induced cells. By contrast, CTRP9 overexpression
attenuated the impairment in cellular activity and apoptosis, and
reduced inflammation and the impaired migratory ability of cells.
In addition, CTRP9 was shown to activate AMPK to inhibit SREBP1c,
and subsequent experiments also demonstrated that CTRP9
overexpression attenuated PA-induced impaired cell activity and
apoptosis through AMPK/SREBP1c signaling by reducing the
inflammatory and migration impairment.
CTRP9 belongs to the adipokine family and is
considered an adipocytokine with cardioprotective properties. Sun
(30) reported that CTRP9 could
attenuate oxidized-low density lipoprotein (ox-LDL)-induced human
umbilical vein endothelial cell (HUVEC) proliferation, apoptosis,
migration and angiogenesis. In the present study, CTRP9 was poorly
expressed in HTR8/SVneo cells treated with a high PA concentration.
However, the elevated cell viability and decreased number of
apoptotic cells following CTRP9 overexpression also indicated that
CTRP9 overexpression could attenuate PA-induced HTR8/SVneo cell
activity impairment and apoptosis.
By contrast, CTRP9 has been reported to inhibit
pro-inflammatory factors and reduce inflammation (31). For instance, CTRP9 inhibits the
expression of pro-inflammatory cytokines in macrophages (29). CTRP9 significantly reduces
ox-LDL-induced TNF-α and monocyte chemoattractant protein-1
expression through the inhibition of the NF-κB signaling pathway in
macrophages (32). In addition,
IL-6 and TNF-α have been shown to be increased in the placenta of
obese women (33,34), as well as to increase the activity
of the amino acid transporter A system (35). IL-1β downregulates
insulin-stimulated A system transport in primary trophoblasts
(36), thereby affecting placental
nutrient transport. In the present study, the pro-inflammatory
factors TNF-α, IL-6 and IL-1β were upregulated in PA-treated
HTR8/SVneo cells, but decreased following CTRP9 overexpression,
suggesting that the increased CTRP9 expression level could
alleviate inflammation in PA-induced HTR8/SVneo cells. In addition,
TNF-α has also previously been shown to promote trophoblast
apoptosis in the placenta (37).
CTRP9 has previously been demonstrated to inhibit pro-inflammatory
factors (31). Consequently, CTRP9
may also reduce apoptosis by inhibiting TNF-α release.
There is abundant evidence that CTRP9 activates AMPK
signaling. For example, CTRP9 has been shown to increase AMPK
phosphorylation in ischemic hearts (38). In addition, CTRP9 promotes AMPK,
Akt and eNOS phosphorylation in HUVECs (39). In the present study, the elevated
p-AMPK level in PA-induced HTR8/SVneo cells following CTRP9
overexpression suggested that CTRP9 overexpression did activate the
expression of AMPK signaling. SREBP1c has been shown to be involved
in pro-inflammatory processes. In a previous study, the activation
of SREBP1c in de novo lipid synthesis was associated with
the increased activation of Akt-mechanistic target of rapamycin
(mTOR) signaling (40). The
phosphorylation of Akt inhibits mTOR complex 1, leading to the
downregulation of SREBP1c and the upregulation of peroxisome
proliferator-activated receptor (PPARs), thereby preventing obesity
and hepatic steatosis, and reducing the release of inflammatory
factors (41). In the present
study, SREBP1c expression was markedly decreased in PA-induced
HTR8/SVneo cells following CTRP9 overexpression. However, the AMPK
inhibitor rescued the decreased SREBP1c expression, which also
confirmed that AMPK suppressed SREBP1c expression. In addition,
SREBP1c overexpression, similar to AMPK inhibitors, reduced cell
viability and promoted apoptosis in PA-induced HTR8/SVneo cells
following CTRP9 overexpression, which suggested that CTRP9
overexpression attenuated PA-induced HTR8/SVneo cell viability
impairment and apoptosis through AMPK/SREBP1c signaling. Similarly,
increased TNF-α, IL-6 and IL-1β levels, and decreased migratory
ability in PA-induced HTR8/SVneo cells transfected with Ov-CTRP9,
also verified that CTRP9 overexpression mitigated inflammatory
cytokine release and migration impairment in PA-treated HTR8/SVneo
cells through AMPK/SREBP1c signaling.
In conclusion, in the present study, CTRP9
overexpression ameliorated inflammation, apoptosis and migration
impairment in PA-induced HTR8/SVneo cells through AMPK/SREBP1c
signaling. Therefore, CTRP9 is a key molecule in the study of the
pathogenesis of obesity in pregnant women. However, the present
study had certain limitations. The TUNEL assay was conducted to
analyze apoptosis; however, the western blot analysis was missing
for cleaved caspases 3/7 and/or PARP. Also, TNF-α, IL-1β and IL-6
RT-qPCR data have been presented, but ELISA data is missing.
Clinical samples will be used in future to confirm the association
between CTRP9, AMPK, SERBP1c and clinical features. Additional cell
lines and animal experiments will also be conducted to strengthen
the conclusion of this study based on these cell experiments. Since
other cellular signals within the trophoblast, such as those of
PPARs, STAT3, NF-κB and p38 MAPK, are also involved in regulating
the expression of placental nutrient transport proteins, future
studies will focus on the association between CTRP9 and the
aforementioned cellular signals to further identify the mechanisms
regulating the intrinsic cellular functions of obesity in pregnant
women. In addition, the association between AMPK and autophagy and
pyroptosis will be investigated in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and JZ designed the study. LL performed the
experiments with the help of ZG. LL made considerable contributions
to the drafting of the manuscript. JZ revised the manuscript for
important intellectual content. All authors have read and approved
the final manuscript. LL and ZG confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stubert J, Reister F, Hartmann S and Janni
W: The risks associated with obesity in pregnancy. Dtsch Arztebl
Int. 115:276–283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flegal KM, Carroll MD, Ogden CL and Curtin
LR: Prevalence and trends in obesity among US adults, 1999-2008.
JAMA. 303:235–241. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marchi J, Berg M, Dencker A, Olander EK
and Begley C: Risks associated with obesity in pregnancy, for the
mother and baby: A systematic review of reviews. Obes Rev.
16:621–638. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vasudevan C, Renfrew M and McGuire W:
Fetal and perinatal consequences of maternal obesity. Arch Dis
Child Fetal Neonatal Ed. 96:F378–F382. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Basu S, Haghiac M, Surace P, Challier JC,
Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano
PM, et al: Pregravid obesity associates with increased maternal
endotoxemia and metabolic inflammation. Obesity (Silver Spring).
19:476–482. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang X, Haghiac M, Glazebrook P, Minium J,
Catalano PM and Hauguel-de Mouzon S: Saturated fatty acids enhance
TLR4 immune pathways in human trophoblasts. Hum Reprod.
30:2152–2159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pantham P, Aye IL and Powell TL:
Inflammation in maternal obesity and gestational diabetes mellitus.
Placenta. 36:709–715. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rampersaud AM, Dunk CE, Lye SJ and Renaud
SJ: Palmitic acid induces inflammation in placental trophoblasts
and impairs their migration toward smooth muscle cells through
plasminogen activator inhibitor-1. Mol Hum Reprod. 26:850–865.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu G, Luo H, Zhang N, Wang Y, Li Y, Huang
H, Liu Y, Hu Y, Liu H, Zhang J, et al: Loss of p53 sensitizes cells
to palmitic acid-induced apoptosis by reactive oxygen species
accumulation. Int J Mol Sci. 20(6268)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Okamoto Y, Kihara S, Ouchi N, Nishida M,
Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, et
al: Adiponectin reduces atherosclerosis in apolipoprotein
E-deficient mice. Circulation. 106:2767–2770. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Whitehead JP, Richards AA, Hickman IJ,
Macdonald GA and Prins JB: Adiponectin - a key adipokine in the
metabolic syndrome. Diabetes Obes Metab. 8:264–280. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aksin S and Andan C: Protein-9 (CTRP9)
levels associated with C1q tumor necrosis factor in obese
preeclamptic, non-obese preeclamptic, obese and normal pregnant
women. J Matern Fetal Neonatal Med. 34:2540–2547. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zuo A, Zhao X, Li T, Li J, Lei S, Chen J,
Xu D, Song C, Liu T, Li C and Guo Y: CTRP9 knockout exaggerates
lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac
hypertrophy through inhibiting the LKB1/AMPK pathway. J Cell Mol
Med. 24:2635–2647. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jia Y, Luo X, Ji Y, Xie J, Jiang H, Fu M
and Li X: Circulating CTRP9 levels are increased in patients with
newly diagnosed type 2 diabetes and correlated with insulin
resistance. Diabetes Res Clin Pract. 131:116–123. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Niemann B, Li L, Siegler D, Siegler BH,
Knapp F, Hanna J, Aslam M, Kracht M, Schulz R and Rohrbach S: CTRP9
mediates protective effects in cardiomyocytes via AMPK- and
adiponectin receptor-mediated induction of anti-oxidant response.
Cells. 9(1229)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao SJ, Shen YF, Li Q, He YJ, Zhang YK,
Hu LP, Jiang YQ, Xu NW, Wang YJ, Li J, et al: SLIT2/ROBO1 axis
contributes to the Warburg effect in osteosarcoma through
activation of SRC/ERK/c-MYC/PFKFB2 pathway. Cell Death Dis.
9(390)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Myerburg MM, King JD Jr, Oyster NM, Fitch
AC, Magill A, Baty CJ, Watkins SC, Kolls JK, Pilewski JM and
Hallows KR: AMPK agonists ameliorate sodium and fluid transport and
inflammation in cystic fibrosis airway epithelial cells. Am J
Respir Cell Mol Biol. 42:676–684. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ren L, Sun D, Zhou X, Yang Y, Huang X and
Li Y, Wang C and Li Y: Chronic treatment with the modified Longdan
Xiegan Tang attenuates olanzapine-induced fatty liver in rats by
regulating hepatic de novo lipogenesis and fatty acid
beta-oxidation-associated gene expression mediated by SREBP-1c,
PPAR-alpha and AMPK-alpha. J Ethnopharmacol. 232:176–187.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kohjima M, Higuchi N, Kato M, Kotoh K,
Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, et al:
SREBP-1c, regulated by the insulin and AMPK signaling pathways,
plays a role in nonalcoholic fatty liver disease. Int J Mol Med.
21:507–511. 2008.PubMed/NCBI
|
|
20
|
Jayachandran M, Zhang T, Wu Z, Liu Y and
Xu B: Isoquercetin regulates SREBP-1C via AMPK pathway in skeletal
muscle to exert antihyperlipidemic and anti-inflammatory effects in
STZ induced diabetic rats. Mol Biol Rep. 47:593–602.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee G, Jang H, Kim YY, Choe SS, Kong J,
Hwang I, Park J, Im SS and Kim JB: SREBP1c-PAX4 axis mediates
pancreatic β-Cell compensatory responses upon metabolic stress.
Diabetes. 68:81–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Larkin JC, Sears SB and Sadovsky Y: The
influence of ligand-activated LXR on primary human trophoblasts.
Placenta. 35:919–924. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tian L, Wen A, Dong S and Yan P: Molecular
characterization of microtubule affinity-regulating kinase4 from
sus scrofa and promotion of lipogenesis in primary porcine
placental trophoblasts. Int J Mol Sci. 20(1206)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Kanehisa M, Furumichi M, Sato Y,
Ishiguro-Watanabe M and Tanabe M: KEGG: Integrating viruses and
cellular organisms. Nucleic Acids Res. 49D:D545–D551.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li J, Zhang P, Li T, Liu Y, Zhu Q, Chen T,
Liu T, Huang C, Zhang J, Zhang Y and Guo Y: CTRP9 enhances carotid
plaque stability by reducing pro-inflammatory cytokines in
macrophages. Biochem Biophys Res Commun. 458:890–895.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun H, Zhu X, Zhou Y, Cai W and Qiu L:
C1q/TNF-related protein-9 ameliorates Ox-LDL-induced endothelial
dysfunction via PGC-1α/AMPK-mediated antioxidant enzyme induction.
Int J Mol Sci. 18(1097)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moradi N, Fadaei R, Emamgholipour S,
Kazemian E, Panahi G, Vahedi S, Saed L and Fallah S: Association of
circulating CTRP9 with soluble adhesion molecules and inflammatory
markers in patients with type 2 diabetes mellitus and coronary
artery disease. PLoS One. 13(e0192159)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang P, Huang C, Li J, Li T, Guo H, Liu
T, Li N, Zhu Q and Guo Y: Globular CTRP9 inhibits oxLDL-induced
inflammatory response in RAW 264.7 macrophages via AMPK activation.
Mol Cell Biochem. 417:67–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Challier JC, Basu S, Bintein T, Minium J,
Hotmire K, Catalano PM and Hauguel-de Mouzon S: Obesity in
pregnancy stimulates macrophage accumulation and inflammation in
the placenta. Placenta. 29:274–281. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El-Haggar SM and Mostafa TM: Adipokines
and biochemical changes in Egyptian obese subjects: Possible
variation with sex and degree of obesity. Endocrine. 48:878–885.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jones HN, Jansson T and Powell TL: IL-6
stimulates system A amino acid transporter activity in trophoblast
cells through STAT3 and increased expression of SNAT2. Am J Physiol
Cell Physiol. 297:C1228–C1235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aye IL, Jansson T and Powell TL:
Interleukin-1β inhibits insulin signaling and prevents
insulin-stimulated system A amino acid transport in primary human
trophoblasts. Mol Cell Endocrinol. 381:46–55. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Knöfler M, Mösl B, Bauer S, Griesinger G
and Husslein P: TNF-alpha/TNFRI in primary and immortalized first
trimester cytotrophoblasts. Placenta. 21:525–535. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kambara T, Ohashi K, Shibata R, Ogura Y,
Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, et
al: CTRP9 protein protects against myocardial injury following
ischemia-reperfusion through AMP-activated protein kinase
(AMPK)-dependent mechanism. J Biol Chem. 287:18965–18973.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yamaguchi S, Shibata R, Ohashi K, Enomoto
T, Ogawa H, Otaka N, Hiramatsu-Ito M, Masutomi T, Kawanishi H,
Murohara T and Ouchi N: C1q/TNF-related protein 9 promotes
revascularization in response to ischemia via an eNOS-dependent
manner. Front Pharmacol. 11(1313)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yecies JL, Zhang HH, Menon S, Liu S,
Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS,
Lee CH and Manning BD: Akt stimulates hepatic SREBP1c and
lipogenesis through parallel mTORC1-dependent and independent
pathways. Cell Metab. 14:21–32. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kwon D, Kim SH, Son SW, Seo J, Jeong TB,
Kim KM, Jung JC, Jung MS, Lee YH and Jung YS: Germinated soybean
embryo extract ameliorates fatty liver injury in high-fat diet-fed
obese mice. Pharmaceuticals (Basel). 13(380)2020.PubMed/NCBI View Article : Google Scholar
|