Introduction
Hemifacial spasm (HFS) is a movement disorder
characterized by involuntary spasms of the facial muscles, which
usually presents as recurrent paroxysmal and involuntary
convulsions of the muscles, including the orbicularis oculi muscle,
expression muscle, frontalis, platysma and orbicularis oris muscles
(1). When the patient is excited
or nervous, the condition may become more severe, and patient may
even have difficulty in opening their eyes, develop crooked mouth
corners or suffer from twitch-like noises in the ears (2-4).
The facial muscle spasm usually begins with the orbicularis oculi
muscle and eventually involves the ipsilateral facial muscles
innervated by the facial nerve; however, most patients only have
one side facial muscle spasm, and very few patients have bilateral
facial muscle spasm (5,6). The annual incidence rate of HFS is
~11 cases/1,000,000 individuals, with most cases beginning in
middle age (7). HFS with severe
symptoms will directly affect the quality of life (QOL) of
patients, so treatment is needed. The current treatments mainly
include botulinum toxin (BT) injection and microvascular
decompression (MVD). Lawrence et al (8) compared BT injection and MVD, and
pointed out that the two methods were effective in the treatment of
HFS, but that MVD was more effective in the treatment of vascular
HFS. At present, MVD is considered the mainstream surgical choice
for the treatment of HFS, and it is mainly performed under the
microscope. However, endoscopy has rapidly developed as a technique
and has the advantages of high brightness, a clear visual field and
flexible operation, so has been widely used in certain surgeries
instead of a microscope. Endoscopic MVD can identify offending
blood vessels, comprehensively assess whether decompression is
sufficient, reduce surgical trauma and complications, and improve
the QOL of a patient, which has been recognized and promoted by
experts in the field (9-11).
The present study report 5 cases of HFS treated by fully endoscopic
MVD, including the preoperative symptoms, intraoperative
conditions, postoperative efficacy and follow-up, in order to
further show that fully endoscopic MVD is a relatively safe,
feasible and effective method for the treatment of HFS.
Patients and methods
Patient summary
The cases of 5 patients with HFS treated by fully
endoscopic MVD in the Department of Neurosurgery, Chongqing General
Hospital (Chongqing, China) between May and December 2020, were
retrospectively reviewed and analyzed. The cases consisted of 1
female and 4 male patients, aged 46-64 years and the mean age was
54 years old, and the history of disease ranged from 2 months to 13
years. The HFS symptoms occurred on the left side in 2 patients, on
the right side in 2 patients and on both sides in 1 patient. Before
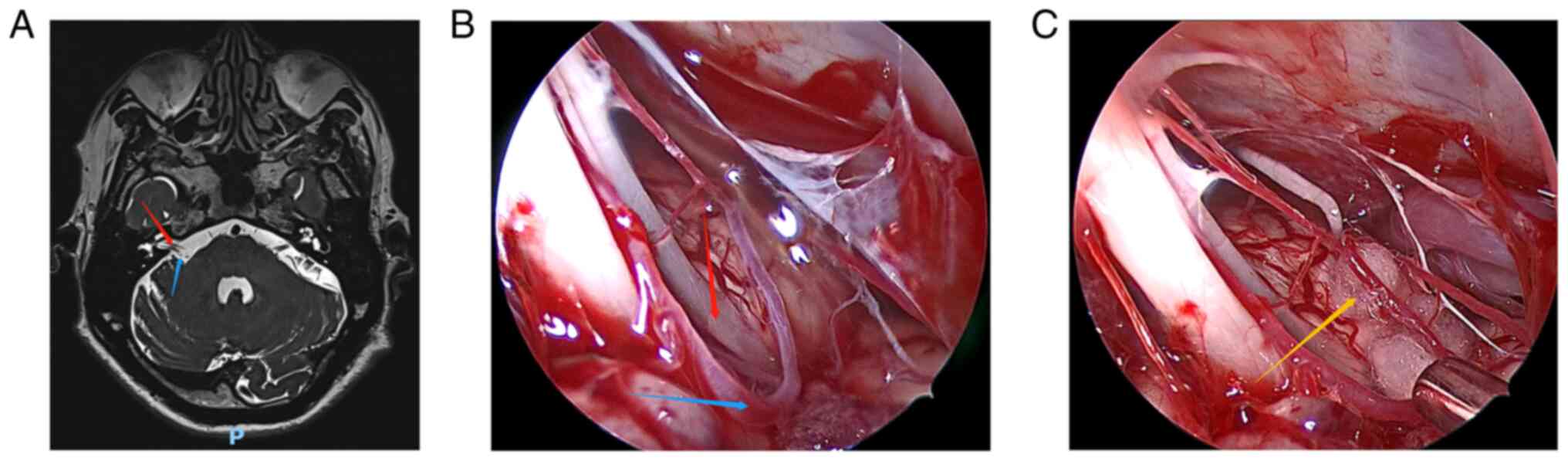
surgery, all patients underwent cranial magnetic resonance imaging
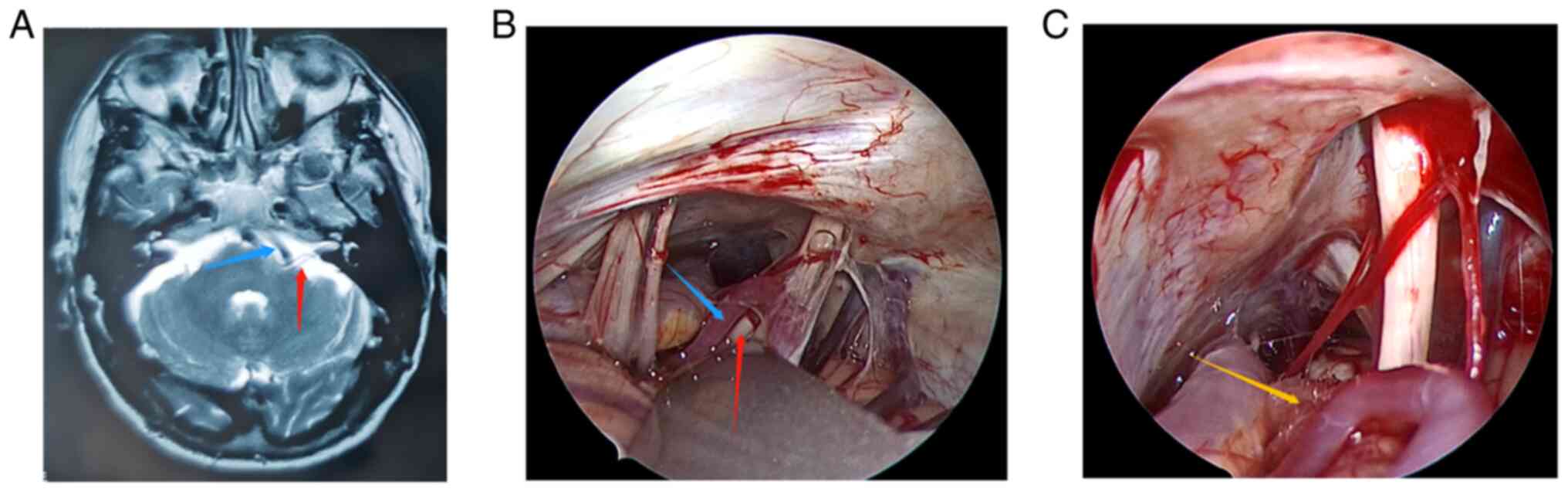
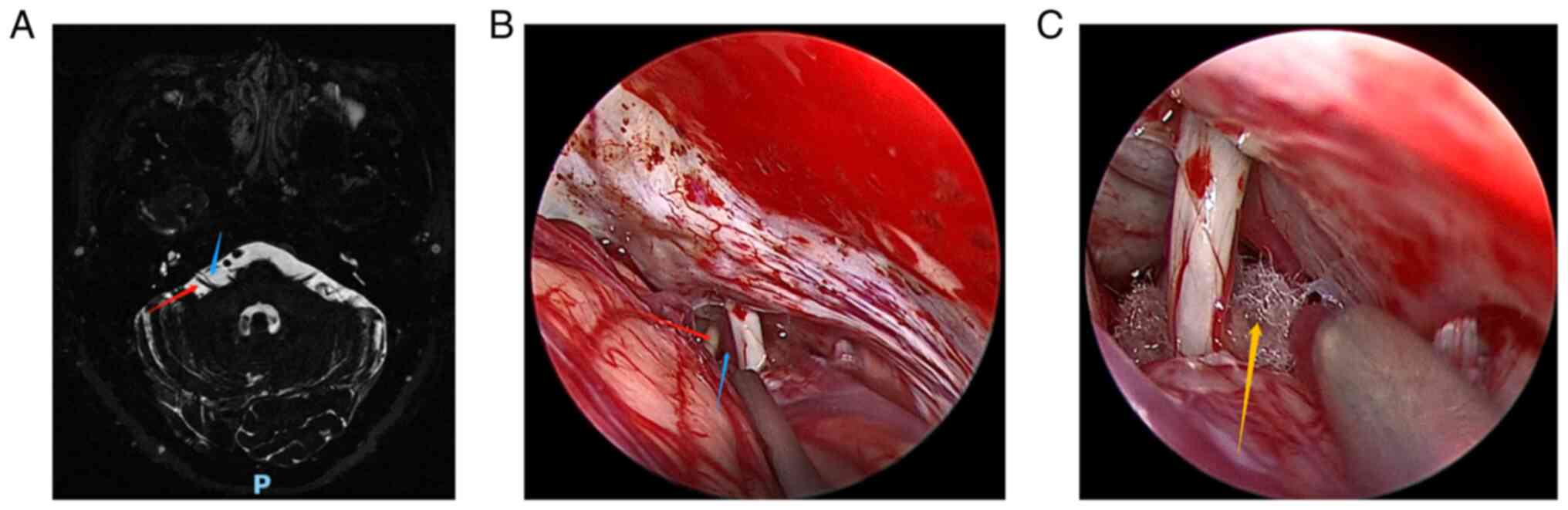
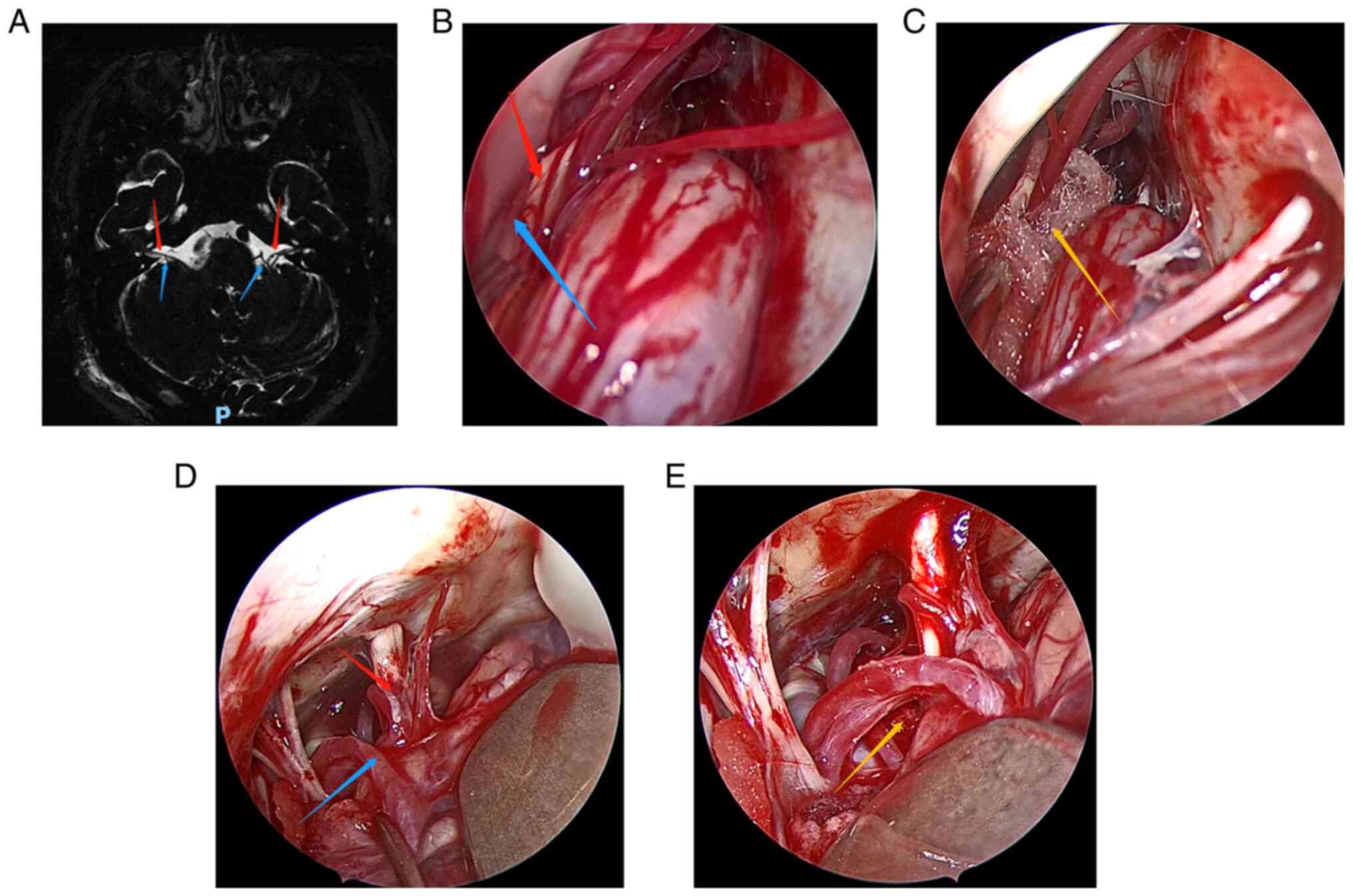
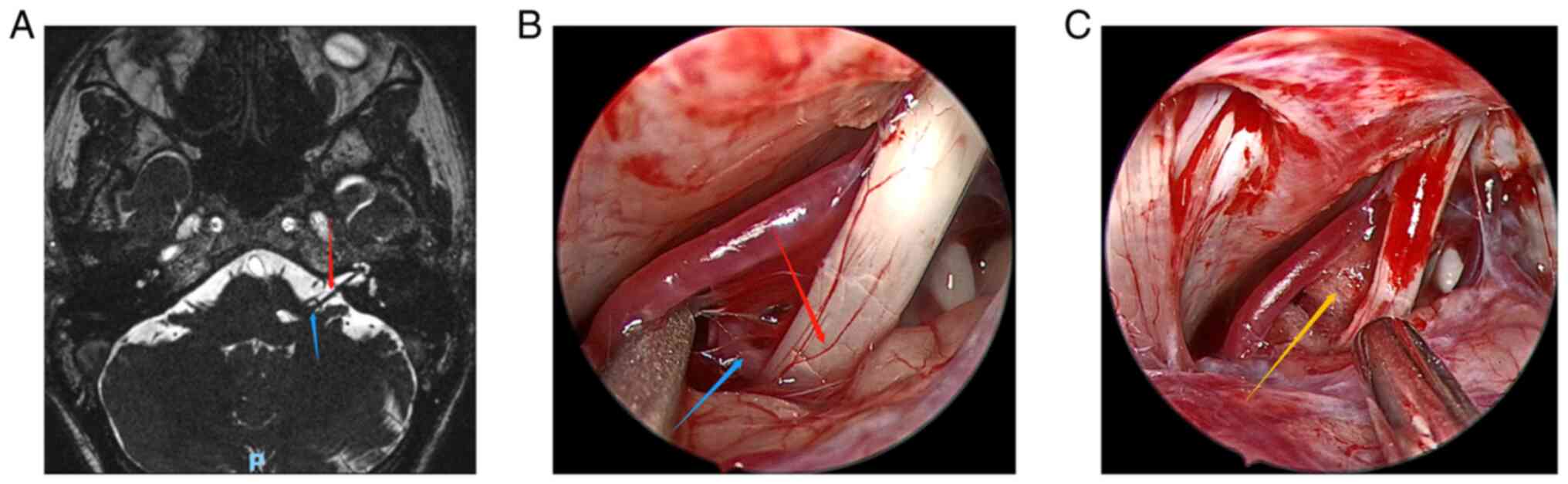
(MRI) (Fig. 1A, Fig. 2A, Fig.
3A, Fig. 4A and Fig. 5A) to exclude tumors or other
intracranial diseases, and electrophysiological examination to
evaluate their condition. The correct muscle location for surgery
was determined according to the anatomy of the patient, clinical
research and cranial MRI.
Patients Patient 1
A 57-year-old male had suffered from left-sided
facial twitches for 5 years. His left facial twitch was paroxysmal
and could be resolved spontaneously. The patient had been treated
with acupuncture and moxibustion, but with little effect. He was
admitted to hospital on May 5, 2020. A physical examination showed
that the left-sided facial twitches were involuntary, and that
hearing and extremity muscle tone were normal.
Patient 2
A 46-year-old female had experienced right-sided
facial twitches for 13 years. The initial clinical manifestation of
the patient was twitching of the right eyelid, and later the
clinical symptoms gradually became the whole right facial
twitching. The patient had been treated with BT injection four
times, and after each treatment, the symptoms were relieved for
several months before relapsing again. She was admitted to hospital
on May 25, 2020.
Patient 3
A 54-year-old male had experienced involuntary
bilateral lateral twitching for 2 months. His bilateral facial
twitches were paroxysmal, and these clinical manifestations
aggravated when he was tense and disappeared when he slept. The
patient was admitted to the hospital on May 7, 2020 and November
23, 2020. He received no treatment prior to his first admission to
the hospital.
Patient 4
A 50-year-old male had suffered from left-sided
facial twitches for 6 years. The patient had received acupuncture
and moxibustion therapy with poor results. The patient had also
received BT injections several times, and after each treatment, the
symptoms were only relieved for a few months before the condition
relapsed again. He was admitted to hospital on July 1, 2020.
Patient 5
A 64-year-old male had experienced right-sided
facial twitches for 2 years. His left facial twitch was paroxysmal
and could be resolved spontaneously. The patient was admitted to
hospital on July 1, 2020. He had not received any treatment prior
to his admission to the hospital.
MVD treatment
All operations were performed via the
suboccipito-retrosigmoid approach under full endoscopy. The
patients were placed in an upside-down position on the surgical
site, with the head drooping 15˚ and rotating 10˚ to the opposite
side. The neck was slightly forward, the mandible was ~2 transverse
fingers from the sternum, and the mastoid on the surgical side was
roughly parallel to the operating table and in the highest
position. The head was fixed and slightly turned to the surgical
site, in order to help the cerebellum leave the petrous bone due to
its own gravity, without the use of a brain plate. A straight
incision parallel to the inner edge of the hairline or a transverse
incision through the root of the mastoid was performed, at a length
of 4-6 cm. The upper and the outer edges of the bone window were
located under the transverse sinus and exposed the edge of the
sigmoid sinus. Usually, the diameter of the bone window is only 2-3
cm. In order to prevent damage to the venous sinus, drill a hole
farthest from the venous sinus, then grind the skull, and gradually
expand the bone window to the transverse sinus and sigmoid sinus.
In order to make the bone window as close to the sigmoid sinus as
possible, the mastoid air chamber can be opened if necessary, but
it must be blocked with bone wax in time. After suspending and
cutting the dura mater, the arachnoid membrane was cut to slowly
release cerebrospinal fluid, so that the cerebellar hemisphere
collapsed naturally and formed enough space for surgery. Next,
under the neuroendoscope, the arachnoid surrounding the trigeminal
nerve, the acoustic-facial bundle and the lower cranial nerves was
completely cleared and dissected, and the neurovascular conflict
area was identified. The offending blood vessels in contact with
the facial nerve roots were separated and shifted, and decompressed
using a Teflon pad. Meanwhile, a small piece of Teflon pad cotton
was placed parallel to the root outlet zone (REZ) along the nerve
to prevent blood vessels from compressing the REZ. Arteries with
severe atherosclerosis should be avoided during the operation, so
as not to distort arteries and cause blood flow obstruction. Next,
under the neuroendoscope, checks were performed to confirm that no
offending blood vessels were missed, that decompression was
sufficient, and that the size and location of the Teflon pads were
appropriate. Finally, the dura mater was sutured and the skull
defect was repaired with titanium plates and screws, and sutured
closed layer by layer.
Results
Overall results
Fig. 1, Fig. 2, Fig.
3, Fig. 4 and Fig. 5 show the relevant images of cases
1-5, respectively, including the preoperative MRI and
intraoperative images. From the intraoperative images, the
offending blood vessels are clearly visible in all 5 cases,
including 3 cases in which the anterior inferior cerebellar artery
was affected (Figs. 1, 3 and 5),
2 cases in which the posterior inferior cerebellar artery was
affected (Figs. 2 and 4) and 1 case where the small arteries
were affected (Fig. 3). Notably,
the offending blood vessels were found on both sides of the left
and right face of the same patient, with the left side affected by
the small arteries and the right side by the inferior cerebellar
artery. (Fig. 3). Generally, the
criteria for judging the curative effect of HFS after surgery are
divided into four levels using a postoperative efficacy
classification based on previous literature and clinical symptoms
(12): i) Excellent: The symptoms
of HFS completely disappear. ii) Good: The symptoms of HFS
basically disappear and can only be induced occasionally when the
patient is emotionally intense or during certain facial movements.
In addition, when the symptoms have basically disappeared and the
patient feels subjectively satisfied. iii) Fair: The symptoms of
HFS are relieved but still frequent, and the patient feels
subjectively dissatisfied. iv) Poor: The symptoms of HFS did not
change and even worsened. According to the aforementioned
classification, the surgical efficacy of the 5 cases was evaluated,
from which 2 cases were graded as excellent and 3 cases were graded
as good, with an effective rate of 100%. This showed that fully
endoscopic MVD was a safe and effective method for the treatment of
HFS. In addition, 4 cases had no postoperative complications, while
only 1 patient experience postoperative aseptic meningitis, but
fully recovered after follow-up treatment.
Individual patient results Patient
1
The symptoms of left-sided HFS were significantly
improved after fully endoscopic MVD, but twitching around the eyes
occasionally occurred. The patient also suffered from aseptic
meningitis after the surgery, but recovered well after lumbar
puncture and drainage. After 12 months of follow-up, the clinical
symptoms disappeared without recurrence and complications. The
offending blood vessel was identified as the anterior inferior
cerebellar artery during the operation, and the postoperative
curative effect was evaluated as good.
Patient 2
The symptoms of HFS basically disappeared after
fully endoscopic MVD, and there was no recurrence during 12 months
of follow-up. The offending blood vessel was identified as the
posterior inferior cerebellar artery during the operation, and the
postoperative curative effect was evaluated as good.
Patient 3
After the first fully endoscopic MVD, the
right-sided facial symptoms were completely relieved, but the left
facial symptoms were only slightly improved. At 5 months
post-surgery, the patient underwent a second fully endoscopic MVD
and the left-sided facial symptoms were also completely relieved.
After 12 months of follow-up, there was no recurrence on either
side of the face. In the two operations, the responsible blood
vessels were identified as the anterior inferior cerebellar artery
on the right side and the small artery on the left side. The
postoperative curative effect was evaluated as excellent.
Patient 4
The patient underwent fully endoscopic MVD and the
symptoms on the left side of the face were significantly improved.
There was no recurrence during a follow-up period of 12 months. The
offending blood vessel was identified as the posterior inferior
cerebellar artery during the operation, and the postoperative
curative effect was evaluated as good.
Patient 5
The patient underwent fully endoscopic MVD and the
symptoms were completely relieved. After 12 months of follow-up,
there was no recurrence. The offending blood vessel was identified
as the anterior inferior cerebellar artery during the operation,
and the postoperative curative effect was evaluated as
excellent.
Discussion
HFS is a common clinical cranial nerve disease that
manifests as paroxysmal involuntary facial muscle twitching. When
the symptoms are relatively severe, it can cause serious
psychological obstacles to patients, and even affect their QOL,
work and social communication. Therefore, the treatment desire of
patients is strong. In 1947, Campbell and Keedy (13) reported 2 cases of HFS caused by
abnormal blood vessels in the posterior fossa compressing the
facial nerve roots. Both patients underwent surgery through the
suboccipital approach, and cirsoid aneurysms of the basilar artery
were both observed and separated from the facial nerve root during
surgery. The involuntary facial convulsion symptoms were relieved
to a certain extent after the surgery. In 1962, Gardner (3) first proposed that the HFS was caused
by vascular compression of the facial nerve roots. In 1966,
Jannetta and Rand (14)
hypothesized that the REZ of the facial nerve was compressed by the
offending blood vessels, resulting in demyelination of the facial
nerve and an impulse short circuit between the afferent and
efferent nerve fibers, which was the root cause of HFS. The
vascular compression etiology theory was thus further improved.
Based on this, MVD was pioneered to treat HFS (14). At present, MVD is mainly performed
using a microscope (microscopic MVD), a microscope combined with an
endoscope (endoscope-assisted MVD) or using an endoscope only
(fully endoscopic MVD). Among these methods, microscopic MVD is the
most traditional and is commonly used. However, the straight
viewing field of the microscope limits the observation of deep
structures and the angle, making it difficult for doctors to
accurately determine the compression points of all offending blood
vessels. A study has highlighted that ~23% of double vessel
compression is difficult to detect under the microscope (15). With the rapid development of
endoscopic technology, endoscopy now has advantages such as the
wide field of vision, bright light, no obstruction of the field of
vision and flexible operation, and so has become a standard
operative tool for minimally invasive neurosurgery of the sellar
and parasellar regions, and the ventricular system (16-18).
As a representative of minimally invasive neurosurgery,
endoscopy-assisted MVD has been applied in clinical treatment. For
MVD, compared with the microscope, the endoscope has the greatest
advantage of accurately identifying the offending blood vessels.
Magnan et al (19) reported
the first 43 cases of patients who received endoscopy-assisted MVD
and suggested the endoscopy could accurately identify the offending
blood vessels causing the facial nerve compression. It was then
reported that for the same 60 patients, the confirmation rate of
vascular nerve compression location by endoscopy was 65% higher
than that by the microscope, which may be due to the complexity of
local neurovascular anatomy, local vessels abnormalities or even
branch entanglement, and the offending blood vessels being located
in the deep part of the abnormal vascular plexus (20). Teo et al (21) also reported that in
endoscopy-assisted MVD, endoscopy accurately identified 8% of
offending blood vessels missed by microscope. In the same period,
fully endoscopic MVD was also trialed to treat HFS. The first
report on 3 cases of HFS treated with fully endoscopic MVD was
published by Eby et al (22) in 2001, and the HFS symptoms of the
patients were completely relieved after the operation. Cheng et
al (23) reported that 10
cases with HFS were cured by fully endoscopic MVD without serious
complications. Flanders et al (24) reported 27 cases of fully endoscopic
MVD treatment for HFS, with the cure rate and postoperative
complication rate recorded as 60.7 and 26.0%, respectively. Feng
et al (11) reported 45
cases of HFS that were treated with fully endoscopic MVD, for which
the cure rate was 93.3%, the total effective rate was 97.8% and the
postoperative complication rate was only 4.4%. At total of two case
series with complete data, including 56 cases of HFS treated with
fully endoscopic MVD, were reviewed (11,25),
for which the postoperative effective rate reached ~92.8% and the
postoperative complication rate was ~7.2% (including 2 cases of
temporary facial paralysis, 1 case of mild but significant hearing
loss and 1 case of intracranial infection). In the present study,
all 5 HFS patients underwent fully endoscopic MVD, and the
offending blood vessels were accurately identified during the
operation. The postoperative effective rate was 100%, and no
serious complications occurred. Only 1 patient developed aseptic
meningitis, but fully recovered after treatment. There was no
hearing loss, facial paralysis or cerebrospinal fluid leakage
recorded.
The manner in which complications can be reduced and
the efficiency of surgery can be improved is a concern for all
neurosurgeons. The complications of MVD treatment for HFS mainly
include cranial nerve dysfunction, cerebellar brainstem injury,
intracranial hypotension syndrome, cerebrospinal fluid leakage,
vertebral artery injury, aseptic meningitis and wound infection
(26-30).
Specifically, when MVD is used to treat HFS, whether it is
microscopic MVD, endoscopic-assisted MVD or fully endoscopic MVD,
it is necessary to first identify the offending blood vessels, and
then place a Teflon pad between the seventh and eighth pairs of
cranial nerves (facial and auditory nerves) and the offending blood
vessels to relieve vascular compression. Among the three types of
MVD, fully endoscopic MVD has many advantages in the treatment of
HFS. Firstly, the excellent visual field of endoscopy helps
surgeons to accurately identify the blood vessels and nerves in the
surgical area, so as to avoid damaging the surrounding brain
tissue, blood vessels and nerves, and to reduce postoperative
complications. In addition, during the operation, endoscopy is
helpful to evaluate the position of the Teflon pad, the
decompression effect and other injuries in a timely manner, so that
surgeons can adjust the operation strategy according to the
intraoperative situation. Finally, fully endoscopic MVD can shorten
the length of the surgical incision, eliminate excessive craniotomy
exposure, avoid excessive separation of arachnoids, and reduce the
traction of brain tissues and cranial nerves. In addition, the
average operation time of fully endoscopic MVD is equivalent to
that of microscopic MVD, which helps to avoid the wasted time of
endoscopic-assisted MVD in terms of changing from the microscope to
the endoscope to the microscope again. In addition to the
aforementioned advantages, fully endoscopic MVD also has some
disadvantages. For example, the endoscope occupies a certain
operational space during surgery and can only provide
two-dimensional images. Intraoperative bleeding may also pollute
the endoscope lens, affecting the image quality and the continuity
of the operation. Furthermore, it should be mentioned that the
present study has certain limitations, as the follow-up time was
relatively short and only 5 patients were studied. At the same
time, it was designed as a non-randomized retrospective study and
does not completely rule out potential selection biases.
In conclusion, with the rapid development of
endoscopic technology, fully endoscopic MVD for the treatment of
HFS is widely recognized, and its advantages are gradually obvious
compared with other technologies. The present study reviewed 5
cases of HFS that were successfully treated using a fully
endoscopic MVD technique. Fully endoscopic MVD should be considered
as safe and effective to treat HFS. For surgeons, fully endoscopic
MVD technology has certain challenges, but sufficient training,
rich experience and constantly updated equipment can help them to
successfully master this technology.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HTJ, PW, DWZ, LWZ, BL and NW participated in the
conception, design and data acquisition of the article. HTJ
participated in drafting and revising the manuscript. PW critically
revised the article. NW ensured that questions related to the
integrity of any part of the work were appropriately investigated
and resolved. HTJ, PW, DWZ, LWZ, BL and NW confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Chongqing General Hospital (Chongqing, China).
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of anonymized data and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blue R, Li C, Spadola M, Saylany A,
McShane B and Lee JYK: Complication rates during endoscopic
microvascular decompression surgery are low with or without
petrosal vein sacrifice. World Neurosurg. 138:e420–e425.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jannetta PJ: Neurovascular compression in
cranial nerve and systemic disease. Ann Surg. 192:518–525.
1980.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gardned WJ: Concerning the mechanism of
trigeminal neuralgia and hemifacial spasm. J Neurosurg. 19:947–958.
1962.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McLaughlin MR, Jannetta PJ, Clyde BL,
Subach BR, Comey CH and Resnick DK: Microvascular decompression of
cranial nerves: Lessons learned after 4400 operations. J Neurosurg.
90:1–8. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Teton ZE, Blatt D, Holste K, Raslan AM and
Burchiel KJ: Utilization of 3D imaging reconstructions and
assessment of symptom-free survival after microvascular
decompression of the facial nerve in hemifacial spasm. J Neurosurg:
1-8, 2019 (Epub ahead of print).
|
|
6
|
Felício AC, Godeiro-Junior Cde O, Borges
V, Silva SM and Ferraz HB: Bilateral hemifacial spasm: A series of
10 patients with literature review. Parkinsonism Relat Disord.
14:154–156. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haller S, Etienne L, Kövari E, Varoquaux
AD, Urbach H and Becker M: Imaging of neurovascular compression
syndromes: Trigeminal neuralgia, hemifacial spasm, vestibular
paroxysmia, and glossopharyngeal neuralgia. AJNR Am J Neuroradiol.
37:1384–1392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lawrence JD, Frederickson AM, Chang YF,
Weiss PM, Gerszten PC and Sekula RF: An investigation into quality
of life improvement in patients undergoing microvascular
decompression for hemifacial spasm. J Neurosurg. 128:193–201.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mizobuchi Y, Nagahiro S, Kondo A, Arita K,
Date I, Fujii Y, Fujimaki T, Hanaya R, Hasegawa M, Hatayama T, et
al: Prospective, multicenter clinical study of microvascular
decompression for hemifacial spasm. Neurosurgery. 88:846–854.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Artz GJ, Hux FJ, Larouere MJ, Bojrab DI,
Babu S and Pieper DR: Endoscopic vascular decompression. Otol
Neurotol. 29:995–1000. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng BH, Zhong WX, Li ST and Wang XH:
Fully endoscopic microvascular decompression of the hemifacial
spasm: Our experience. Acta Neurochir (Wien). 162:1081–1087.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng BH, Zheng XS, Wang XH, Ying TT, Yang
M, Tang YD and Li ST: Management of vessels passing through the
facial nerve in the treatment of hemifacial spasm. Acta Neurochir
(Wien). 157:1935–1940. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Campdell E and Keedy C: Hemifacial spasm;
a note on the etiology in two cases. J Neurosurg. 4:342–347.
1947.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jannetta PJ and Rand RW: Transtentorial
retrogasserian rhizotomy in trigeminal neuralgia by
microneurosurgical technique. Bull Los Angeles Neurol Soc.
31:93–99. 1996.PubMed/NCBI
|
|
15
|
Dubey A, Yadav N, Ratre S, Parihar VS and
Yadav YR: Full endoscopic vascular decompression in trigeminal
neuralgia: Experience of 230 patients. World Neurosurg.
113:e612–e617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jho HD and Carrau RL: Endoscopy assisted
transsphenoidal surgery for pituitary adenoma. Technical note. Acta
Neurochir (Wien). 138:1416–1425. 1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adappa ND, Learned KO, Palmer JN, Newman
JG and Lee JY: Radiographic enhancement of the nasoseptal flap does
not predict postoperative cerebrospinal fluid leaks in endoscopic
skull base reconstruction. Laryngoscope. 122:1226–1234.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagata Y, Watanabe T, Nagatani T, Takeuchi
K, Chu J and Wakabayashi T: The multiscope technique for
microvascular decompression. World Neurosurg. 103:310–314.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Magnan J, Chays A, Lepetre C, Pencroffi E
and Locatelli P: Surgical perspectives of endoscopy of the
cerebellopontine angle. Am J Otol. 15:366–370. 1994.PubMed/NCBI
|
|
20
|
Magnan J, Caces F, Locatelli P and Chays
A: Hemifacial spasm: Endoscopic vascular decompression. Otolaryngol
Head Neck Surg. 117:308–314. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Teo C, Nakaji P and Mobbs RJ:
Endoscope-assisted microvascular decompression for trigeminal
neuralgia: Technical case report. Neurosurgery. 59 (4 Suppl
2):ONSE489–90; discussion ONSE490. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eby JB, Cha ST and Shahinian HK: Fully
endoscopic vascular decompression of the facial nerve for
hemifacial spasm. Skull Base. 11:189–197. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cheng WY, Chao SC and Shen CC: Endoscopic
microvascular decompression of the hemifacial spasm. Surg Neurol.
70 (Suppl 1):S40–S46. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Flanders TM, Blue R, Roberts S, McShane
BJ, Wilent B, Tambi V, Petrov D and Lee JYK: Fully endoscopic
microvascular decompression for hemifacial spasm. J Neurosurg.
131:813–819. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cai Q, Li Z, Guo Q, Wang W, Ji B, Chen Z,
Dong H and Mao S: Microvascular decompression using a fully
transcranial neuroendoscopic approach. Br J Neurosurg: 1-4, 2021
(Epub ahead of print).
|
|
26
|
Huh R, Han IB, Moon JY, Chang JW and Chung
SS: Microvascular decompression for hemifacial spasm: Analyses of
operative complications in 1582 consecutive patients. Surg Neurol.
69:153. –157; discussion 157. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wilkins RH: Hemifacial spasm: A review.
Surg Neuro. 36:251–277. 1991.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barker FG II, Jannetta PJ, Bissonette DJ,
Shields PT, Larkins MV and Jho HD: Microvascular decompression for
hemifacial spasm. J Neurosurg. 82:201–210. 1995.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jannetta PJ: Typical or atypical
hemifacial spasm. J Neurosurg. 89:346–347. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang A and Jankovic J: Hemifacial spasm:
Clinical findings and treatment. Muscle Nerve. 21:1740–1747.
1998.PubMed/NCBI View Article : Google Scholar
|