Introduction
Listeria spp whose genus currently includes
28 species and six subspecies are Gram-positive, facultative
anaerobic bacteria (1).
Listeria species are separated into two phenotypically and
genotypically distinct groups: Listeria sensu lato and
Listeria sensu strict; this latter category includes
Listeria monocytogenes, a small Gram-positive intracellular
bacillus, which is widespread in the environment.
Farm animals can lead to the spread of L
monocytogenes in the agricultural environment and consequently
of his entry in the food chain if hygienic requirements are not
respected (1). Indeed, L.
monocytogenes is responsible for both foodborne diseases with
mild gastroenteritis (non-invasive listeriosis) and
life-threatening systemic infections (invasive listeriosis; e.g.,
sepsis and meningitis) (2).
L. monocytogenes has also been associated
with primary aortitis (only a few cases reported in the literature)
and serious vascular and graft infections in immunocompromised
hosts, patients with malignancy or previously treated aneurysms
(3-6).
Infections of L. monocytogenes may be resistant to several
antibiotics such as cephalosporins, first generation quinolones,
sulfamethoxazole, fosfomycin, oxacillin, and lincosamides but seem
to respond well to others such as penicillins, trimethoprim,
aminoglycosides, macrolides, and vancomycin (1).
In the present case report, to the best of our
knowledge, the first case of L. monocytogenes thrombotic
infection of an abdominal aorto-bi-iliac endograft in a patient who
had recently undergone a vascular intervention for repair of an
aortic abdominal aneurysm is described. As detailed below, serious
complications both affected and prolonged the clinical course of
the infection.
Case report
A 71-year-old man with arterial hypertension, type
II diabetes, dyslipidemia, previous splenectomy and abdominal
aortic aneurysm (anteroposterior diameter of 69 mm) was
hospitalized in a different hospital where he underwent an
aorto-bi-iliac endograft (Medtronic Endurant™) with suprarenal
attachment. The main body aortic endograft was implanted using a
percutaneous right femoral approach with no intraoperative issues
or abnormalities. However, the postoperative course was
characterized by the appearance of left calf claudication 2 months
after having performed the index endovascular aneurysm repair
(EVAR) procedure. For this reason, catheter-directed thrombolysis
was performed in another hospital without restoring the left limb
perfusion.
Subsequently, after a further 8 months, due to the
presence of abdominal pain and persistent left calf claudication,
the patient was admitted to our hospital ‘Campus Bio-Medico’
University of Rome (Rome, Italy) on February 2019, where color
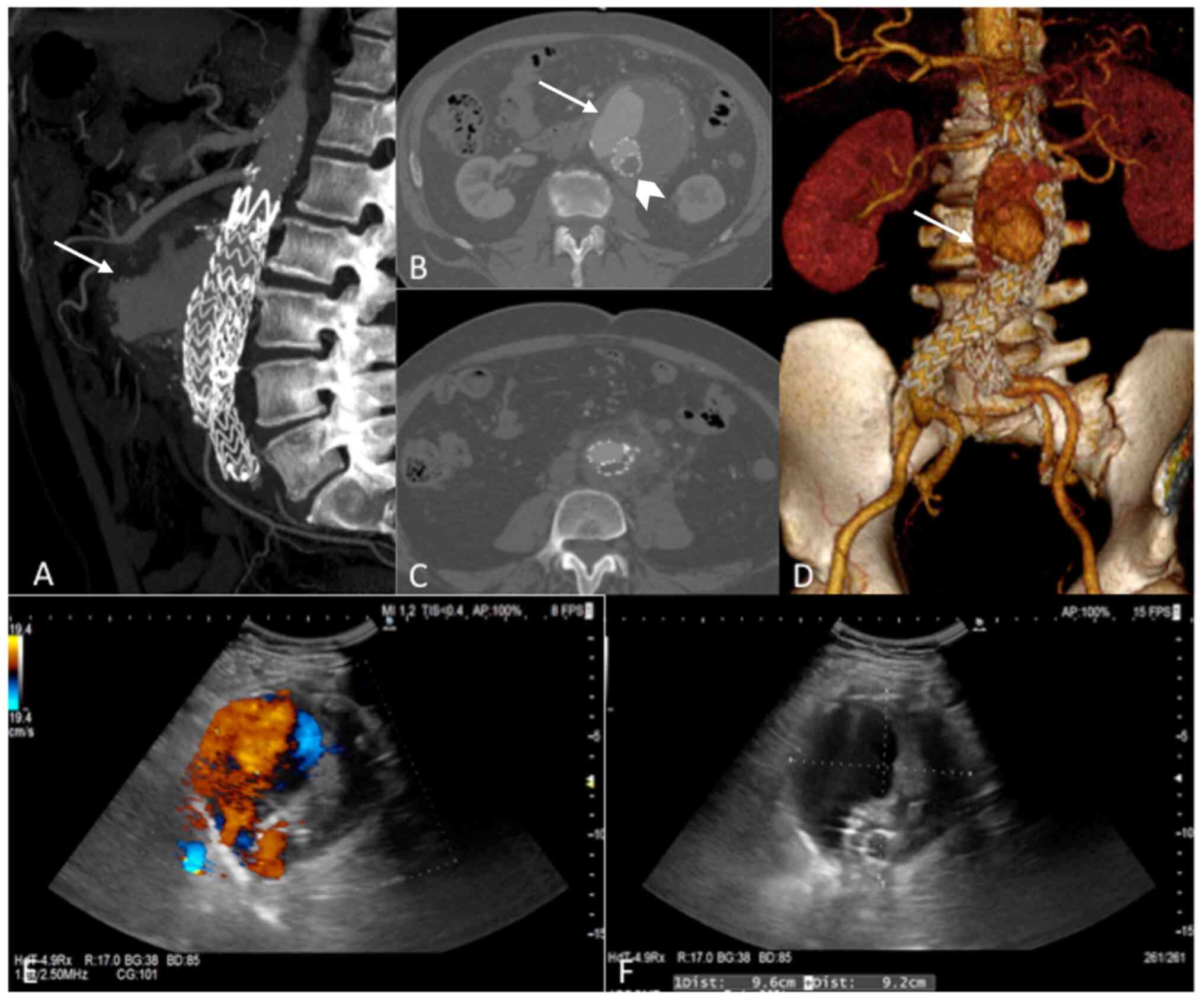
Doppler sonography and computed tomography angiography (CTA)
revealed an abdominal aortic type Ia endoleak, with graft
thrombotic occlusion and a maximum diameter of the aneurysm of 91
mm (Fig. 1A-F). Neither gas
bubbles nor any obvious indications of graft infection were
present. Similarly, laboratory investigations failed to reveal any
abnormalities, with the exception of the white blood cell count
(13,800 cells/µl), a C reactive protein concentration of 2.1 mg/dl
(normal value <0.5 mg/dl), and a creatinine reading of 1.3
mg/dl, albeit without clinical signs of systemic infection.
Considering the stent graft limb occlusion (with a
previously failed revascularization attempt) and the type Ia
endoleak without graft migration (i.e., the proximal stent graft
was in a good position relative to the lowest renal artery), a
proximal aortic positioning was excluded. A fenestrated endograft
and chimney procedures were also excluded, given the good clinical
condition (preoperative work-up revealed a 60% ejection fraction
and normal pulmonary function tests) and the relatively young age
of the patient (7); therefore, an
open surgical approach was preferred, and a partial stent-graft
explant was planned.
Using a left lombotomic extraperitoneal access, the
aorta was exposed from the left iliac bifurcation up to the
suprarenal portion without dissection of the native right iliac
axis; after systemic heparinization (70 IU/kg), the aortic
cross-clamp was placed below the lowest renal artery (including the
first covered stent of the endograft), and the left external and
hypogastric arteries were clamped in the proximal region.
Subsequently, the aneurysm was opened through a longitudinal
arteriotomy extended cranially below the first covered stent, and
distally at the level of the iliac branches of the stent-graft. At
this point, the main body of the stent-graft was carefully
transected below the first covered stent, and the right iliac limb
was resected at its proximal portion; back-bleeding from the right
iliac branch was rapidly achieved using a catheter used for
intraluminal control (Bard Medical Division). The left stent-graft
limb was resected at the proximal portion and, together with the
thrombus, sent for culture, whereas the distal part was left in
place as it was chronically occluded. Once the stent-graft had been
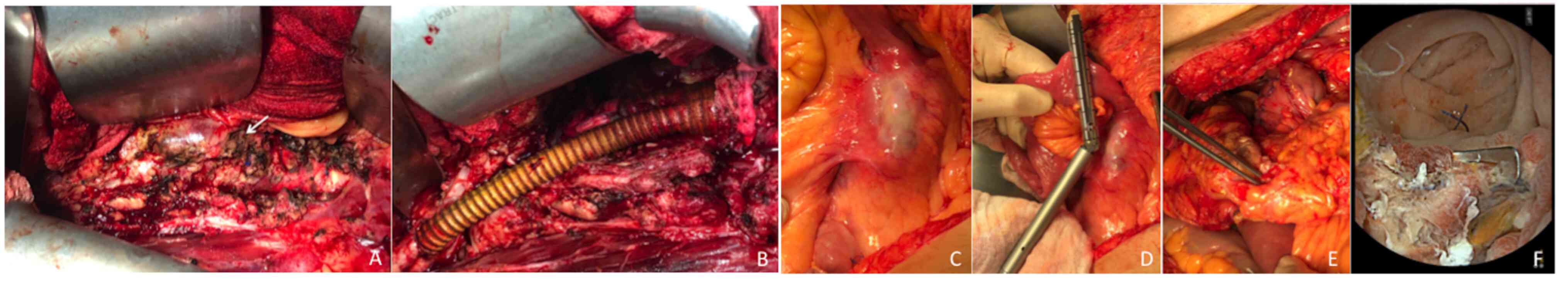
partially explanted, the proximal anastomosis was performed using a
3/0-polypropylene suture in an end-to-end fashion with a Silver
Dacron bifurcated graft (18x9 mm), which included all the first
covered stent of the endograft; to obtain a tight reinforced
anastomosis, a Teflon stripe (10-cm x 6-mm regular
polytetrafluoroethylene) was used (Fig. 2A and B). However, the postoperative clinical
condition of the patient quickly worsened with the onset of a 39˚C
fever, malaise, nausea, vomiting, abdominal swelling and tenderness
and development of an acute abdomen. A course of adequate
parenteral hydration with 1,000 ml glucose and saline solution
(1,000 ml a day) was promptly started. Laboratory investigations
showed a C reactive protein concentration of 27.3 mg/dl (normal
value, <0.5 mg/dl), a procalcitonin level of 1.43 ng/dl (normal
value, <0.5 ng/dl), an adrenomedullin level of 4.17 nmol/l
(normal value, <0.5 nmol/l), a white blood cell count of 23,690
cells/µl, a neutrophil count of 19,460 cells/µl (82.2%), a red
blood cell count of 3,410,000 cells/µl, a hemoglobin level of 9.2
g/dl, a platelet count of 277,000/µl, and a creatinine level of
1.43 mg/dl.
On the 4th day after surgery, CTA was performed,
which led to the detection of the presence of aorto-enteric fistula
(AEF), abdominal effusion and left pneumothorax. Emergency surgery,
consisting of duodenal resection with duodenojejunal anastomosis
packaging and omentum transposition to cover the aneurismatic sac,
was performed a few hours after obtaining the CTA findings
(Fig. 2C-E). Before knowing the
culture results, a multiple empiric intravenous antibiotic
treatment (4.5 g piperacillin/tazobactam administered every 8 h, 50
mg tigecycline once a day and 250 mg ciprofloxacin administered
every 12 h) was started and continued for several days, albeit
without the patient receiving any clear benefit. Laboratory
investigations showed a C reactive protein level of 10.18 mg/dl, a
procalcitonin level of 0.21 ng/dl, a white blood cell count of
25,390 cells/µl, a neutrophil count of 20,600 cells/µl (81.1%), a
red blood cell count of 2,790,000 cells/µl, a hemoglobin level of
8.4 g/dl, a platelet count of 451,000/µl, a creatinine level of
0.67 mg/dl, as well as measurements of 128 mmol/l sodium, 3.6
mmol/l potassium and 1.9 g/dl albumin.
When tested, the culture of material from the
abdominal and left thorax drainages, placed near the abdominal
aorta and in the posterior basal region of the left lung, was found
to be positive for Candida lusitanae. The culture
examination of the removed graft and the thrombotic material found
inside the graft revealed the growth of L. monocytogenes.
The previous antibiotic regime was discontinued and, according to
antibiogram sensitivity, specific antimycotic and antibiotic
treatments (intravenous fluconazole at 200 mg once a day and
intravenous meropenem at 1 g every 8 h) were started. In order to
broaden the antibacterial spectrum, vancomycin (500 mg) and
clindamycin (600 mg), both intravenously administered every 8 h,
were also added for a period of 21 days according to the C reactive
protein dosage and clinical status.
On the 10th day following surgical intervention, the
patient had an episode of hematemesis due to serious bleeding
originating from the duodenojejunal anastomosis; this caused severe
anemia (red blood cell count, 2,280,000 cells/µl; hemoglobin, 6.9
g/dl) requiring a red blood cell transfusion, endoscopic hemostasis
and positioning of metallic clips (Fig. 2F), and subsequent placement of both
naso-gastric and naso-jejunal tubes. The patient was maintained in
a fasting state with infusion of total parenteral nutrition and
albumin to allow the healing of the intestinal mucosa, to ensure
sufficient caloric intake and to restore electrolyte and protein
balance.
After 1 week, the abdominal and pleural drainages
were removed and, 1 week later, the patient could resume feeding on
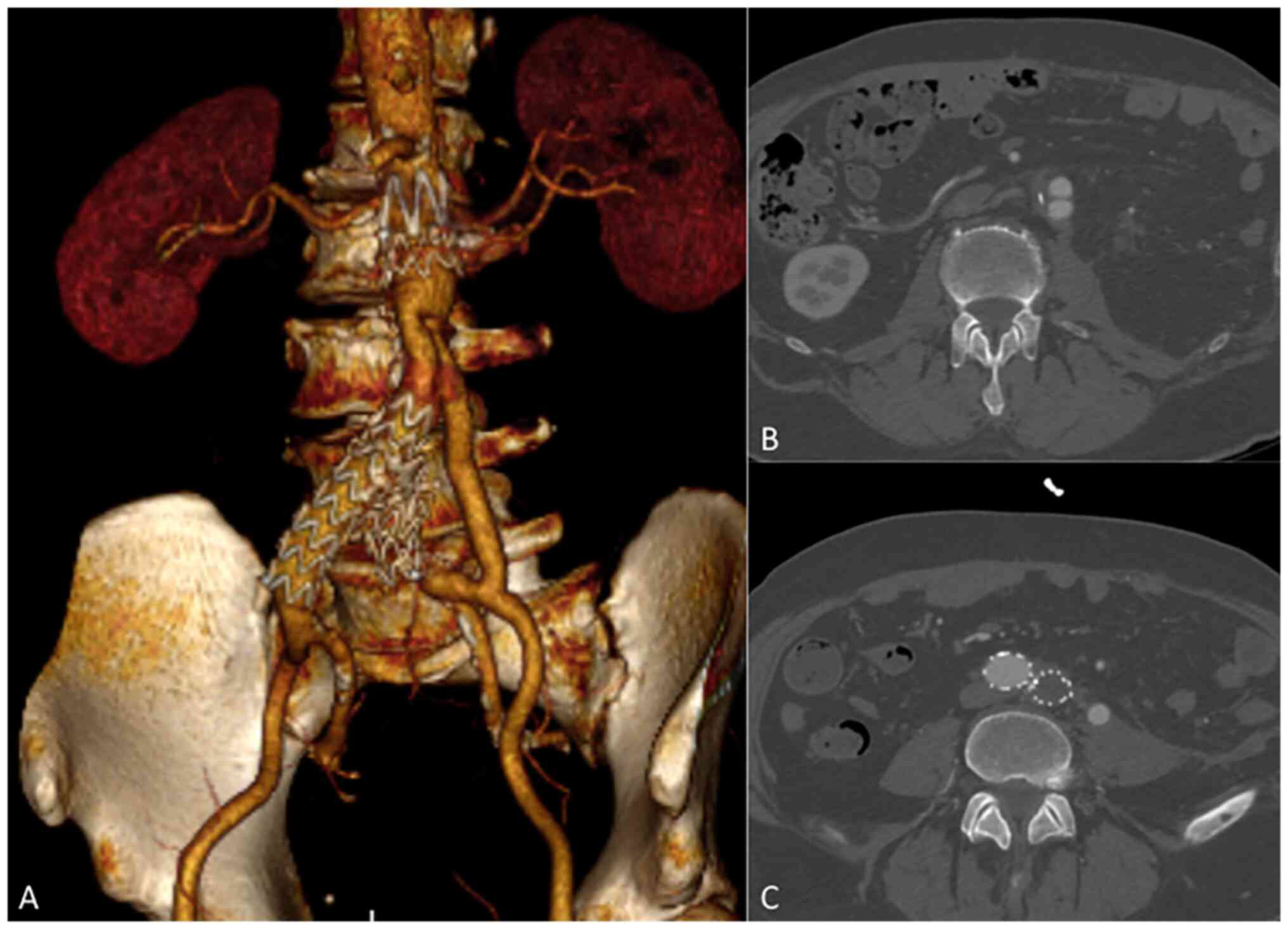
a soft diet, without any signs of bleeding. A control CTA revealed
both a significant reduction in pleural and abdominal effusions and
patency of the bifurcated Silver Dacron graft (Fig. 3A-C); therefore, the intravenous
antibiotic therapy was discontinued, and treatment with oral
clarithromycin (500 mg every 12 h) was started. After a further
week, the patient was transferred to a Rehabilitation Centre, where
he followed a plan of re-education with a continuance of oral
antibiotic therapy for 3 months in accordance with his clinical
conditions and the laboratory tests. After 3 months, the patient
was discharged from the Rehabilitation Centre and after 9 months of
follow-up, the patient was in good clinical condition without signs
or symptoms of reinfection.
Discussion
Aortic endograft is an efficacious option of
treatment for aortic aneurysm burdened with several ischemic and
vascular complications (8).
However, only partial and limited information is available on
graft-associated infective complications, as that they occur
infrequently (with an estimated incidence of 6.2/1,000
person-years), are difficult to manage and have high mortality
rates (9-12).
Reports in the literaure indicate that
Staphylococci, Escherichia coli and Pseudomonas
aeruginosa are the most common organisms isolated in endograft
infections whereas very few cases are due to L.
monocytogenes (13).
This case study, to the best of our knowledge,
presents the first-ever described case of L. monocytogenes
aorto-bi-iliac endograft and thrombotic infection. Prior to
surgery, clinical signs of systemic infection were not reported.
Preoperative blood investigations were found to be normal, with the
exception of the white blood cell count and a small increase in the
level of C reactive protein; however, over the course of the
previous 10 years, the patient had shown persistent mild
leukocytosis that was related to a previous splenectomy. A critical
revision of the preoperative CTA revealed only a slight
hyperdensity of periaortic adipose tissues, and consequently this
was judged to be a case of purely dormant stent-graft infection
incidentally detected on culture. It was possible to speculate that
AEF already existed at the time of conversion, even though it had
not been recognized, a likely consequence of L.
monocytogenes infection acquired through ingesting food.
Since an infection was not suspected, given the good
clinical condition and relatively young age of the patient, a
partial stent-graft explant was performed, according to our
standard approach in case of EVAR failure requiring conversion
(14).
Certain cases of AEF following aortic repair have
been reported in the literature (15-18),
and although it is known that its onset may not occur until 15
years after (17), data regarding
its incidence are lacking. It has been generally recognized that,
when AEF following aortic repair does occur, it prolongs the stay
in hospital extensively. The appearance of symptoms depends on the
site of origin of the AEF; in our case study, AEF was severe, and
therefore a duodenal resection and omental transposition were
necessary.
Although only a few cases of endograft infections
caused by L. monocytogenes, for example, two cases of
infective endocarditis via L. monocytogenes (one of which
was complicated by a distal popliteal embolization of a mycotic
aneurysm) and one case of thrombotic infection of the vascular
graft of the right leg, have been reported (2,4-5,19-24),
to the best of our knowledge no case of aortic endograft associated
with thrombotic infection has ever been reported, making sure that
our case is novel. Certain risk factors, including
immunosuppression, immunization against human papilloma virus in
vaccinated subjects, infective endocarditis, HIV infection,
intravenous drug use, giant cell aortic aneurysm and sepsis, as
well as the presence of aneurysm, may predispose a patient to L.
monocytogenes endograft infection (11,25,26).
According to an interesting survey published
previously, the clinical course of vascular infection caused by
L. monocytogenes was found to be milder than that caused by
other bacteria, such as Staphylococcus aureus,
Enterobacteriaceae and anaerobic flora (27). On the other hand, embolization
following endocarditis by L. monocytogenes and the mortality
rates associated with aneurysmal or vascular endograft infection
had rates of frequency similar to those found with other bacteria
(27). Although the mortality rate
due to endocarditis caused by L. monocytogenes was similar
to that of S. aureus, it was 10 times higher than that
reported for Streptococcus (27).
Although, in the majority of cases, the source of
L. monocytogenes infection is unknown, it is known that it
may depend on eating habits, such as the consumption of raw meat
and the intake of unpasteurized milk and other milk products, not
forgetting the possible role of transmission from asymptomatic
personnel handling such products. Moreover, the possibility of
L. monocytogenes contamination occurring during the first
surgical procedure or the secondary reintervention for limb
occlusion cannot be eliminated. Even though a correlation between
hostile anatomy and AEF has not been clearly established in the
literature, it is well known that EVAR is associated with a high
reintervention rate during follow-up (14-25%) (28,29).
Although a great majority of such secondary procedures are
endovascularly managed and are associated with low mortality rates
(30), reinterventions are,
however, accompanied by a high risk of EVAR infection (10). The high percentage of
reinterventions is likely associated with the largescale use of
EVAR, and also applies to patients with suboptimal or an hostile
anatomy (31).
In the present case study, the patient showed limb
occlusion and a Type Ia endoleak at short-term follow-up,
suggesting the presence of suboptimal or hostile anatomy at the
time of the EVAR procedure, although reliable data and details on
aortic neck and iliac anatomy were missing (the EVAR procedure was
not performed at our Centre, and performing CTA prior to EVAR was
no longer an available option). Consequently, the initial decision
to use EVAR as the first treatment was not ideal, particularly as
the patient was relatively young and in good clinical condition at
the time of EVAR. It may be speculated that the patient would have
successfully tolerated an open repair as a first-line treatment,
which, in hindsight, would probably have been the most appropriate
action to have taken in the light of the subsequent
complications.
The underlying mechanism of the pathogenesis of
thrombotic L. monocytogenes infection remains unclear,
although it is likely that, in the present case study, a persistent
aortitis favored thrombotic infection. In this regard, an
interesting in vivo animal study has provided some evidence
for the pivotal role of a lethal listeriosis infection in inducing
coagulopathy and thrombus formation through the upregulation of
factor XI of the coagulation cascade (32).
It remains impossible at the present time to either
confirm or deny that this is the mechanism through which L.
monocytogenes induced graft thrombotic occlusion in our
patient; instead, it is desirable that the role of L.
monocytogenes infection in favoring a thrombophilic state be
explored in greater detail in subsequent studies.
At present, there are no available therapeutic
recommendations or guidelines for L. monocytogenes endograft
infection (27); however, we do
know that surgical treatment may be unsuccessful, and with or
without lifelong antibiotic treatment, mycotic aneurysms are
burdened by moderate rates of mortality (12%) and morbidity,
although to a lesser extent than for endocarditis (27). In the present study, our patient
benefited from a rapidly performed surgical aortic resection and
new silver graft positioning, as well as a specific long-term
antibiotic treatment strategy based on an antibiogram against both
L. monocytogenes and C. lusitanae. This is likely to
account for why our patient was again asymptomatic at the 9 months'
follow-up stage.
In the event of a complication requiring open
conversion, even if not suspected from the patient's medical
history, an underlying infection should always be excluded with an
in-depth preoperative work-up, including
18F-fluoro-D-deoxyglucose positron emission tomography
with low dose CT (18F-FDG PET/TC) or white blood cell
scintigraphy (33); in addition,
an esophagogastroduodenoscopy may be useful both for appreciation
of an occult AEF and to determine the subsequent course of
treatment.
In conclusion, it may be said that L.
monocytogenes infection, though not widespread, should be
considered in patients with prosthetic devices, especially those
with graft thrombotic occlusion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors confirmed the authenticity of all the
raw data. EMZ was responsible for the conception and design of the
report, and was the major contributor in writing the article. NM,
VC, TG and DMZ analyzed and interpreted the patient data, and
revised the article. MC was responsible for the duodenectomy and
latero-lateral duodeno-jejunal anastomosis and interpretation of
data. FSp, FSt and NM were responsible for the prosthetic endograft
replacement and acquisition of data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lourenco A, Linke K, Wagner M and Stessl
B: The saprophytic lifestyle of Listeria monocytogenes and
entry into the food-processing environment. Front Microbiol.
13(789801)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schlech WF: Foodborne listeriosis. Clin
Infect Dis. 31:770–775. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Arzola LH, Anaya-Ayala JE, Lopez-Pena G,
Luna L, Ruben-Castillo C, Contreras-Jimenez E, Laparra-Escareno H
and Hinojosa CA: Favorable conservative management of a rare
primary aortitis secondary to Listeria monocytogenes: Case
report and review of the literature. Vasc Endovascular Surg.
55:744–748. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gauto AR, Cone LA, Woodard DR, Mahler RJ,
Lynch RD and Stoltzman DH: Arterial infections due to Listeria
monocytogenes: Report of four cases and review of world
literature. Clin Infect Dis. 14:23–28. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Clouse WD, DeWitt CC, Hagino RT, DeCaprio
J and Kashyap VS: Rapidly enlarging iliac aneurysm secondary to
Listeria monocytogenes infection: A case report. Vasc
Endovascular Surg. 37:145–149. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Molacek J, Treska V, Baxa J, Certik B and
Houdek K: Acute conditions caused by infectious aortitis. Aorta
(Stamford). 2:93–99. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Glorion M, Coscas R, McWilliams RG,
Javerliat I, Goëau-Brissonniere O and Coggia M: A comprehensive
review of in situ fenestration of aortic endografts. Eur J Vasc
Endovasc Surg. 52:787–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sousa LHD, Baptista-Silva JC, Vasconcelos
V, Flumignan RL and Nakano LC: Internal iliac artery
revascularisation versus internal iliac artery occlusion for
endovascular treatment of aorto-iliac aneurysms. Cochrane Database
Syst Rev. 7(CD013168)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sharif MA, Lee B, Lau LL, Ellis PK,
Collins AJ, Blair PH and Soong CV: Prosthetic stent graft infection
after endovascular abdominal aortic aneurysm repair. J Vasc Surg.
46:442–448. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Benedetto F, Lentini S, Passari G, Stilo
F, De Caridi G, Cascio A and Spinelli F: Endovascular repair of
aortic rupture due to Brucella aortitis. Vasa. 40:150–156.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Capoccia L, Speziale F, Menna D, Esposito
A, Sirignano P, Rizzo AR, Mansour W, Montelione N, Sbarigia E and
Setacci C: Collaborators. Preliminary results from a national
enquiry of infection in abdominal aortic endovascular repair
(R.I.-EVAR). Ann Vasc Surg. 30:198–204. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Montelione N, Menna D, Sirignano P,
Capoccia L, Mansour W and Speziale F: Open conversion after aortic
endograft infection caused by colistin-resistant,
carbapenemase-producing klebsiella pneumoniae. Tex Heart Inst J.
43:453–457. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ducasse E, Calisti A, Speziale F, Rizzo L,
Misuraca M and Fiorani P: Aortoiliac stent graft infection: Current
problems and management. Ann Vasc Surg. 18:521–526. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stilo F, Montelione N, Catanese V,
Vigliotti RC and Spinelli F: Minimally invasive open conversion for
late EVAR failure. Ann Vasc Surg. 63:92–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chenu C, Marcheix B, Barcelo C and
Rousseau H: Aorto-enteric fistula after endovascular abdominal
aortic aneurysm repair: case report and review. Eur J Vasc Endovasc
Surg. 37:401–406. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kakkos SK, Bicknell CD, Tsolakis IA and
Bergqvist D: Hellenic Co-operative Group on Aortic Surgery.
Editor's choice-management of secondary aorto-enteric and other
abdominal arterio-enteric fistulas: A review and pooled data
analysis. Eur J Vasc Endovasc Surg. 52:770–786. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lirici MM, Tierno SM, Giudice R,
Coscarella C, Graziani MG and Pogany G: Secondary aortoenteric
fistula successfully treated with staged endovascular repair and
duodenal resection without graft removal. Minim Invasive Ther
Allied Technol. 29:114–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kadhim MMK, Rasmussen JBG and Eiberg JP:
Aorto-enteric Fistula 15 years after uncomplicated endovascular
aortic repair with unforeseen onset of endocarditis. EJVES Short
Rep. 8:16–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Murphy K, Al-Jundi W and Nawaz S: Mycotic
aneurysms of the abdominal aorta due to Listeria
monocytogenes. Int J Surg Case Rep. 4:626–628. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rahmati E, Jan Geiseler P and She RC:
Lower extremity mycotic aneurysm in a patient with Listeria
monocytogenes-associated prosthetic valve endocarditis. JMM
Case Rep. 4(e005095)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Van Noyen R, Reybrouck R, Peeters P,
Verheyen L and Vandepitte J: Listeria monocytogenes
infection of a prosthetic vascular graft. Infection. 21:125–126.
1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Segura J, Anguita M, Vivancos R, Franco M,
Romo E, Suárez de Lezo J and Vallés F: Listeria
monocytogenes endocarditis in a patient with mitral prosthesis,
left auricular thrombus and adenocarcinoma of the colon. Rev Esp
Cardiol. 45:483–485. 1992.PubMed/NCBI(In Spanish).
|
|
23
|
Ahadzada Z, Ghaly P, Farmer E and Ahmad M:
Listeria monocytogenes endograft infection after fenestrated
endovascular aneurysm repair-a case report. J Vasc Surg Cases Innov
Tech. 8:1–4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miranda JA, Khouqeer A, Livesay JJ and
Montero-Baker M: Very late aortic endograft infection with
Listeria monocytogenes in an elderly man. Tex Heart Inst J.
49(e207298)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fares E, McCloskey CB, Gutierrez A,
Princiotta M, Salinas LJ and Drevets DA: Vaccine strain Listeria
monocytogenes bacteremia occurring 31 months after
immunization. Infection. 47:489–492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Silvestri V and Isernia G: Suspected giant
cell aortitis: From multiple aortic structural damage to fatal
listeria sepsis, a case report. Ann Vasc Surg. 42:307.e1–307.e6.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shoai-Tehrani M, Pilmis B, Maury MM, Maury
MM, Robineau O, Disson O, Jouvion G, Coulpier G, Thouvenot P,
Bracq-Dieye H, Valès G, et al: Listeria
monocytogenes-associated endovascular infections: A study of 71
consecutive cases. J Infect. 79:322–331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang RW, Goodney P, Tucker LY, Okuhn S,
Hua H, Rhoades A, Sivamurthy N and Hill B: Ten-year results of
endovascular abdominal aortic aneurysm repair from a large
multicenter registry. J Vasc Surg. 58:324–332. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mehta M, Sternbach Y, Taggert JB,
Kreienberg PB, Roddy SP, Paty PS, Ozsvath KJ and Darling RC III:
Long-term out-comes of secondary procedures after endovascular
aneurysm repair. J Vasc Surg. 52:1442–1449. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Paravastu SCV, Jayarajasingam R, Cottam R,
Palfreyman SJ, Michaels JA and Thomas SM: Endovascular repair of
abdominal aortic aneurysm. Cochrane Database Syst Rev.
1(CD004178)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schanzer A, Greenberg RK, Hevelone N,
Robinson WP, Eslami MH, Goldberg RJ and Messina L: Predictors of
abdominal aortic aneurysm sac enlargement after endovascular
repair. Circulation. 123:2848–2855. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo D, Szaba FM, Kummer LW, Johnson LL,
Tucker EI, Gruber A, Gailani D and Smiley ST: Factor XI-deficient
mice display reduced inflammation, coagulopathy, and bacterial
growth during listeriosis. Infect Immun. 80:91–99. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Reinders Folmer EI. Von Meijenfeldt GCI,
Van der Laan MJ, Glaudemans AWJM, Slart RHJA, Saleem BR and
Zeebregts CJ: Diagnostic imaging in vascular graft infection: A
systematic review and meta-analysis. Eur J Vasc Endovasc Surg.
56:719–729. 2018.PubMed/NCBI View Article : Google Scholar
|