Introduction
Cardiac fibrosis is a common pathological process in
cardiovascular disease (1,2). It is primarily characterized by
fibroblast accumulation and excess deposition of extracellular
matrix proteins (3,4). Activation of cardiac fibroblasts
(CFs) is a key step in cardiac fibrosis progression; CFs
proliferate and differentiate into myofibroblasts (5,6).
Myofibroblasts, the primary effector cells of cardiac fibrosis,
contain large amounts of α-smooth muscle actin (α-SMA) and secrete
abundant collagen and cytokines (7,8).
Isoproterenol (ISO) has been indicated to increase the levels of
proinflammatory cytokines (9),
which further causes cardiac fibrosis by increasing matrix
metalloproteinase expression in CFs (9). Thus, confirming the potential
mechanisms by which CF activation is regulated may provide novel
insights for the prevention and treatment of heart failure.
MicroRNAs (miRNAs/miRs) are endogenous small
non-coding RNAs, 22 nucleotides in length, that are capable of
post-transcriptionally modulating gene expression by binding to the
3'-untranslated region (UTR) of their target mRNAs (10). Increasing evidence suggests that
miRNAs are key regulators of cell differentiation, cancer and
cardiac disease (11,12). In addition, several miRNAs have
been reported to participate in cardiac fibrosis development. For
example, miR-223 modulates cardiac fibrosis following myocardial
infarction by regulating RASA1 expression (13). Furthermore, miR-155 knockdown
inhibits high glucose-triggered cardiac fibrosis via transforming
growth factor (TGF)-β signaling (14). miR-146a has been reported to
exhibit an anti-inflammatory and antifibrosis effect (15,16).
For example, miR-146a-5p inhibits fibrosis-associated molecules
(such as α-SMA and collagen I) in irradiated and TGF-β1-induced
hepatic stellate cells via protein tyrosine phosphatase receptor
type A/SRC signaling (17).
Moreover, Pan et al (18)
indicated that miR-146a serves a role in myocardial protection by
regulating early growth response (EGR)1. However, the role of
miR-146a-5p in cardiac fibrosis progression remains unknown.
Fibroblast growth factor 2 (FGF2) is an
intra-ovarian peptide that belongs to the FGF family. It has been
reported that FGF2 mediates various biological processes, such as
viability, differentiation and angiogenesis (19). A previous study demonstrated that
FGF2 exerts its function in radiation-induced fibrosis by enhancing
interstitial cell viability and stimulating fibroblast
trans-differentiation into myofibroblasts (20,21).
However, the function of FGF2 in cardiac fibrosis remains
unclear.
The present study aimed to investigate the
biological role of miR-146a-5p in cardiac fibrosis and the results
demonstrated that miR-146a-5p suppresses ISO-treated cardiac
fibrosis in rats by decreasing FGF2 expression, providing promising
therapeutic modality for cardiac fibrosis.
Materials and methods
Animal studies
A total of 32 male Sprague-Dawley (SD) rats (age, 8
weeks; weight, 200-220 g) were obtained from the Laboratory Animal
Center of Nanjing the First Hospital, Nanjing Medical University.
The rats were maintained under conditions of 50% relative humidity,
a 12-h light/dark cycle and 22˚C, and received food and water ad
libitum. All animal experiments were approved by the Animal
Care and Use Ethics Committee of Nanjing First Hospital (Nanjing,
China; approval no. NFH-081241-2). Rats were randomly divided into
two groups (n=16 per group) and intraperitoneally injected with
either isoproterenol (ISO; 5 mg/kg/day) for consecutive 10 days or
an equal volume of saline. On the 11th day, the rats were
sacrificed by cervical dislocation following anesthesia with sodium
pentobarbital (50 mg/kg) and hearts were collected. Tissue sections
(5 µm thick) were fixed in 4% formaldehyde solution at 37˚C for 24
h and stained with haematoxylin and eosin (H&E) or Masson
staining for 10 min at room temperature to observe histological
changes in the cardiac tissue. Some samples were immediately frozen
and stored at -80˚C for RNA and protein analysis.
CF isolation and ISO treatment
CFs were isolated from SD rats as previously
described (22). Ventricles were
immediately placed in D-Hank's solution, cut into 1-mm3
pieces and subsequently digested using a mixture of 0.2%
collagenase and 0.25% trypsin at 37˚C for 20 min. Cells were
resuspended in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin at 37˚C for 2 h to allow fibroblast
attachment to the culture plates. The third passage of CFs were
used in the present study. Cells (1x104) were seeded
into 12-well plates and treated with 10 µM ISO or saline in
serum-free medium for 24 h.
Transfection
Small interfering (si)RNAs targeting FGF2 (siFGF2;
50 nM; 5'-CAGGUGACAGACUUUAUCAAA-3'), negative control (siNC; 50 nM;
5'-UUCUCCGAACGUGUC ACGUTT-3'), miR-146a-5p mimics (50 nM;
5'-GCCCAAA GGUGAAUUUUUUGGG-3'), NC mimics (50 nM; 5'-UCA
CAACCUCCUAGAAAGAGUAGA-3'), miR-146a-5p inhibitor (50 nM;
5'-CCCAAAAAAUUCACCUUUGGGC-3'), NC inhibitor (50 nM;
5'-UUUGUACUACACAAAAGUACUG-3'), pcDNA3.1/FGF2 and pcDNA3.1 were
synthesized by Shanghai GenePharma, Co., Ltd. Transfection was
performed using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37˚C for 20 min and subsequent
experiments were performed 48 h post-transfection.
Dual-luciferase reporter assay
The StarBase database (starbase.sysu.edu.cn) revealed that FGF2 is a target
gene of miR-146a-5p. The sequences of FGF2 3'-UTR containing
putative miR-146a-5p binding sites were inserted into a pmirGLO
plasmid (Promega Corporation) to form wild-type (WT) and mutant
(MUT) FGF2. Subsequently, miR-146a-5p mimics and vectors were
co-transfected into 293 cells using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After a 48-h
transfection, luciferase activity was detected using a
Dual-Luciferase reporter assay system (Promega Corporation).
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Blood sample collection and
measurement of cardiac markers
After sacrificing the animals, 2 ml blood samples
were collected from the carotid artery of rats. Creatine kinase
(CK) activity was measured using an auto-analyzer (AU640; Olympus
Diagnostics) according to the manufacturer's instructions. The CK
isozyme (CK-MB) and cardiac troponin I (cTnI) levels in serum were
performed via routine biochemical testing and detected using a 7600
auto-chemistry analyzer (Hitachi Ltd.) according to the
manufacturer's instructions.
Cell Counting Kit-8 (CCK-8)
Cell viability was detected via CCK-8 assay (Dojindo
Molecular Technologies, Inc.). Briefly, CFs (1x104
cells/well) were seeded into 96-well plates and transfected with
miR-146a-5p mimic, miR-146a-5p inhibitor and NCs. After 36 h, cells
were treated with ISO for 24 h and 10 µl CCK-8 reagent was
subsequently added into each well. Following incubation for 4 h at
37˚C, absorbance was measured at a wavelength of 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
To detect mRNA and miRNA expression levels, total
RNA was extracted from cardiac tissue and CFs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT was performed using a PrimeScript™ RT reagent kit (Takara
Bio, Inc.) according to the manufacturer's protocol and qPCR was
subsequently performed using a SYBR Green Detection kit (both from
Takara Bio, Inc.). The thermocycling conditions were as follows:
Pre-denaturation at 95˚C for 1 min, followed by 40 cycles of 95˚C
for 15 sec, 60˚C for 30 sec and 72˚C for 30 sec. mRNA expression
levels were normalized to GAPDH, while miR-146a-5p expression
levels were normalized to U6. Relative expression levels were
calculated using the 2-ΔΔCq method (23). The primer sequences were as
follows: miR-146a-5p forward, 5'-CGAGTCCAGTTTTC CCAGGA-3' and
reverse, 5'-GTCGTATCCAGTGCAGGG-3'; FGF2 forward,
5'-GCAAAAACGGGGGCTTCTTC-3' and reverse, 5'-AACGGTTAGCACACACTCCT-3';
U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCT
TCACGAATTTGCGT-3'; and GAPDH forward, 5'-CAAGCT CATTTCCTGGTATGAC-3'
and reverse, 5'-CAGTGAGGGTC TCTCTCTTCCT-3'.
Western blot assay
Total protein was extracted from CFs using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and quantified
using the BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Proteins (20 µg/lane) were separated by 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore). After blocking with
5% non-fat milk for 2 h at room temperature, the membrane was
incubated with primary antibodies against FGF2 (1:1,000; cat. no.
PB0619; Boster Biological Technology, Ltd.) and GADPH (1:1,000;
cat. no. ab9485; Abcam) overnight at 4˚C. Subsequently, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody (1:1,000; cat. no. ab205718; Abcam) for 2 h at
room temperature. Finally, protein bands were visualized using an
enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology) and band densities were determined using ImageJ
software (version 1.48; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 software (IBM Corp.). All experiments were performed
in triplicate and data are presented as the mean ± SD. Unpaired
Student's t-test was used to compare differences between two
groups, while one-way ANOVA followed by Tukey's post hoc test were
used to compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-146a-5p expression is
downregulated in ISO-induced CFs and rat heart
H&E and Masson staining confirmed the cardiac
fibrosis model was successfully constructed following treatment
with ISO (Fig. 1A). Biomarkers,
such as CK, CK-MB and cTnI, are widely applied in diagnosis and
monitoring of myocardial lesion (24). Thus, the serum levels of CK, CK-MB
and cTnI were detected; the results demonstrated that CK, CK-MB and
cTnI levels were upregulated in the serum of ISO-treated rats
(Fig. 1B-D). To determine whether
miR-146a-5p is implicated in cardiac fibrosis, RT-qPCR analysis was
performed to detect changes in miR-146a-5p expression in
ISO-treated rat heart. The results demonstrated that miR-146a-5p
expression was downregulated in ISO-treated rat heart tissue
(Fig. 1E). However, the expression
levels of collagen I and α-SMA increased following ISO injection in
rat heart tissue (Fig. 1F). In
vitro, RT-qPCR analysis demonstrated that miR-146a-5p
expression was downregulated in ISO-induced CFs, while collagen I
and α-SMA expression levels significantly increased following
treatment of CFs with ISO (Fig. 1G
and H).
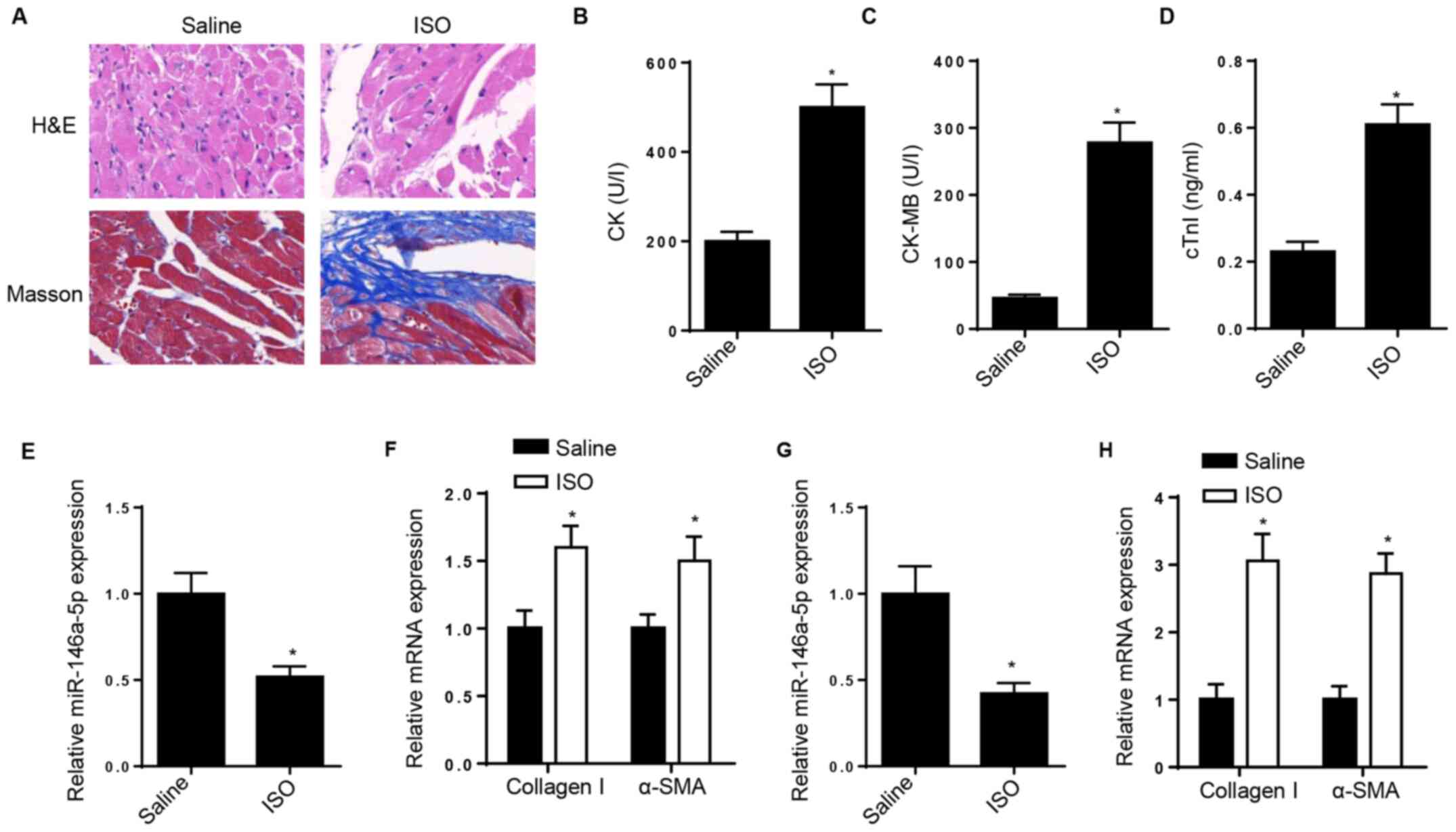 | Figure 1miR-146a-5p expression is
downregulated in ISO-induced CFs and rat heart. (A) H&E and
Masson staining determined myocardial collagen deposition in
myocardial tissue following ISO injection. (B) CK, (C) CK-MB and
(D) cTnI concentrations were measured in the serum of ISO-treated
and normal control rats. RT-qPCR analysis determined expression
levels of (E) miR-146a-5p and (F) collagen I and α-SMA in
ISO-treated rat heart tissue. RT-qPCR analysis determined levels of
(G) miR-146a-5p and (H) collagen I and α-SMA in ISO-treated CFs.
*P<0.05 vs. saline. miR, microRNA; ISO,
isoproterenol; CF, cardiac fibroblast; H&E, hematoxylin and
eosin; CK, creatine kinase; CK-MB, creatine kinase isozyme; cTnI,
cardiac troponin I; RT-q, reverse transcription-quantitative;
α-SMA, α-smooth muscle actin. |
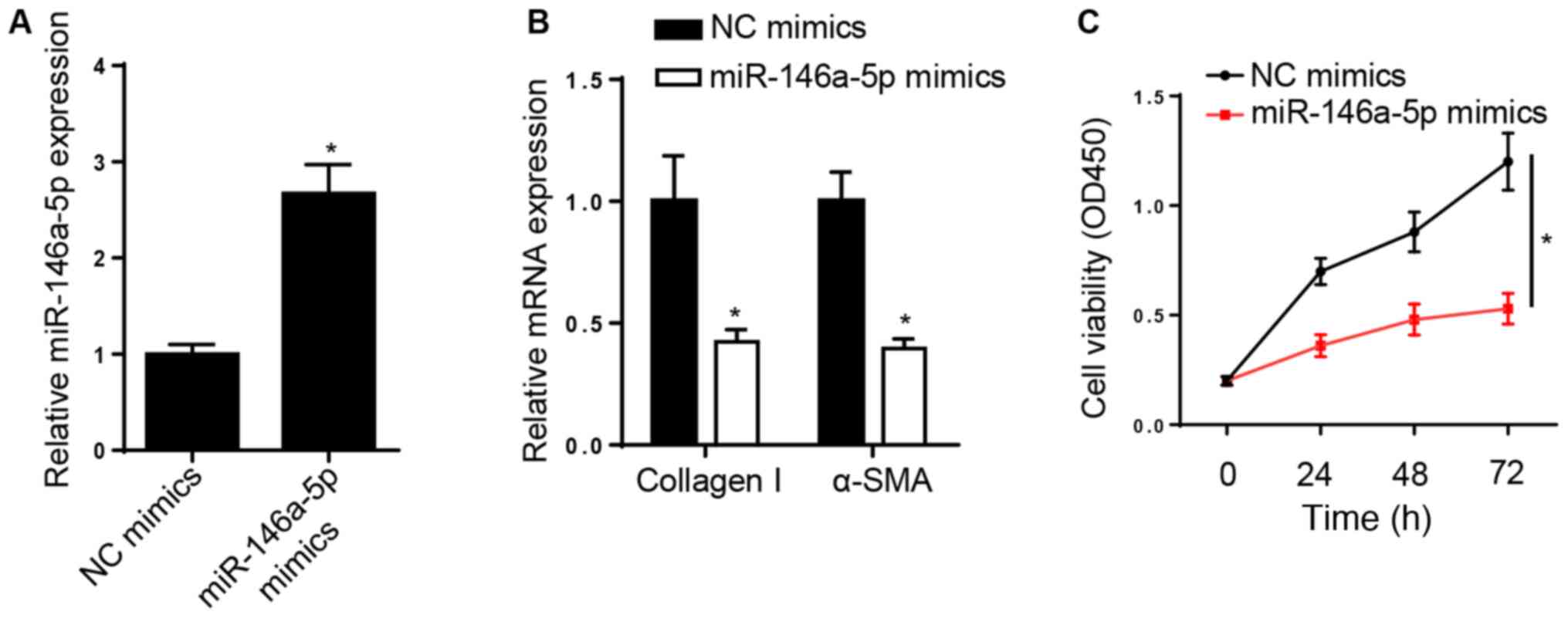
miR-146a-5p suppresses the viability
and differentiation of ISO-induced CFs
The effect of miR-146a-5p on cardiac fibrosis was
assessed. miR-146a-5p expression increased in rat CFs transfected
with miR-146a-5p mimics (Fig. 2A).
RT-qPCR analysis demonstrated that overexpression of miR-146a-5p
downregulated the expression levels of collagen I and α-SMA
(Fig. 2B). In addition,
overexpression of miR-146a-5p decreased ISO-induced cell viability
(Fig. 2C). Taken together, these
results suggest that miR-146a-5p suppressed the viability and
differentiation of CFs following treatment with ISO.
miR-146a-5p directly targets FGF2 and
FGF2 knockdown decreases collagen I and α-SMA expression levels in
CFs
The StarBase database revealed that FGF2 may be a
potential target of miR-146a-5p (Fig.
3A). The dual-luciferase reporter assay demonstrated that
overexpression of miR-146a-5p repressed the luciferase activity of
the 3'-UTR of WT-FGF2, but no changes were observed in the MUT-FGF2
group in 293 cells (Fig. 3B). In
addition, RT-qPCR and western blot analysis demonstrated that FGF2
expression decreased following overexpression of miR-146a-5p but
increased following miR-146a-5p knockdown in ISO-treated CFs
(Fig. 3C and D). Moreover, RT-qPCR and western blot
analysis demonstrated that FGF2 expression decreased following FGF2
knockdown in rat CFs (Fig. 3E and
F). The expression levels of
fibrosis-associated collagen I and α-SMA were also inhibited
following FGF2 knockdown (Fig.
3G).
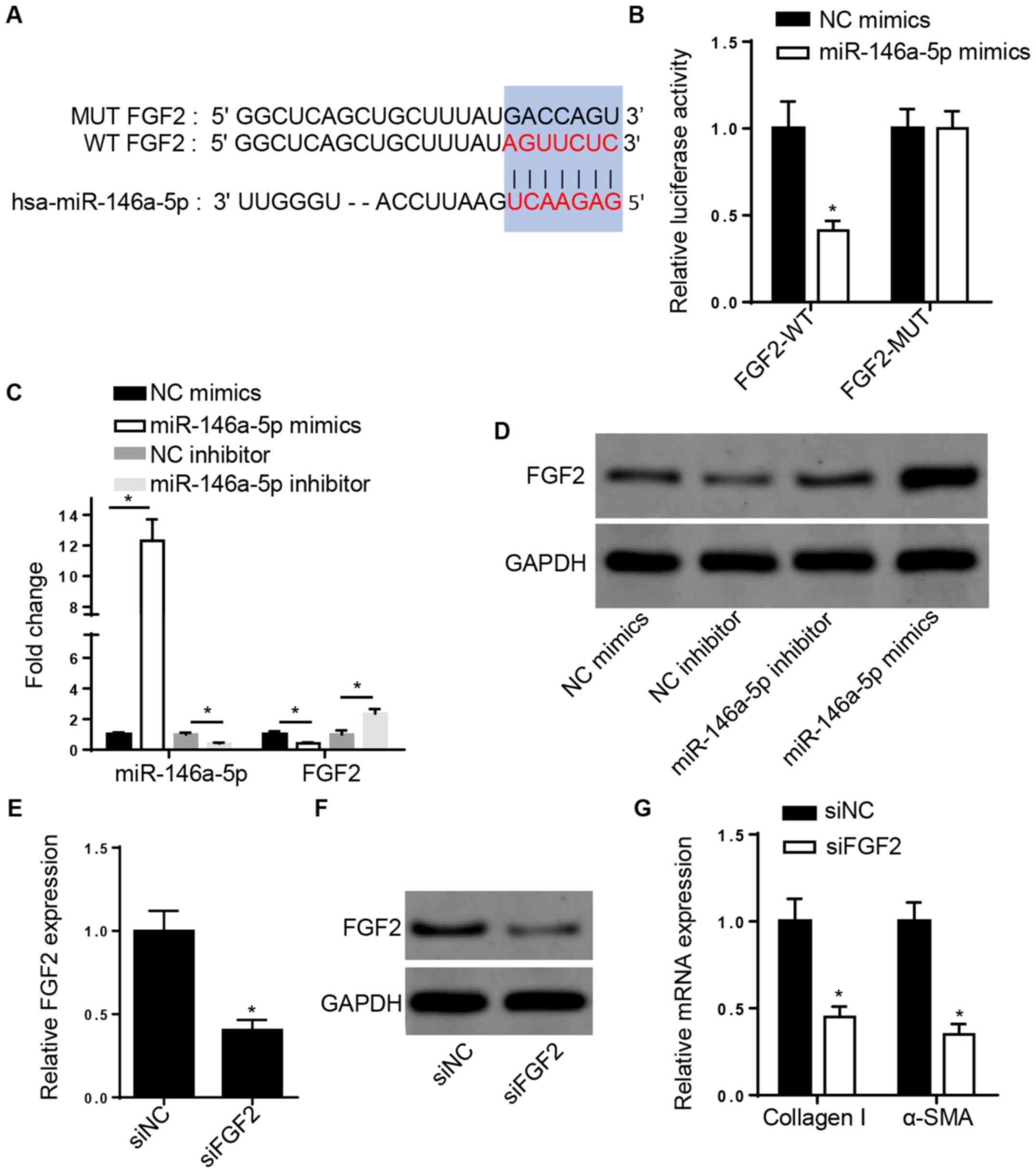 | Figure 3miR-146a-5p directly targets FGF2 and
FGF2 knockdown decreases collagen I and α-SMA expression levels in
CFs. (A) StarBase database showed the potential binding sites
between FGF2 and miR-146a-5p. (B) Luciferase reporter assay was
used to determine the binding ability between FGF2 and miR-146a-5p
in 293 cells. *P<0.05 vs. NC mimics. (C) RT-qPCR and
(D) western blotting were used to assess the mRNA and protein
levels of FGF2 in ISO-treated CFs transfected with NC or
miR-146a-5p mimics or inhibitor or inhibitor. (E) RT-qPCR and (F)
western blotting showed mRNA and protein levels of FGF2 in CFs
transfected with siNC or siFGF2. (G) RT-qPCR analysis showed
collagen I and α-SMA mRNA levels in ISO-treated CFs transfected
with siNC or siFGF2. *P<0.05 vs. siNC. miR, microRNA;
FGF2, fibroblast growth factor 2; α-SMA, α-smooth muscle actin; NC,
negative control; CF, cardiac fibroblast; RT-q, reverse
transcription-quantitative; ISO, isoproterenol; si, small
interfering; WT, wild-type; MUT, mutant. |
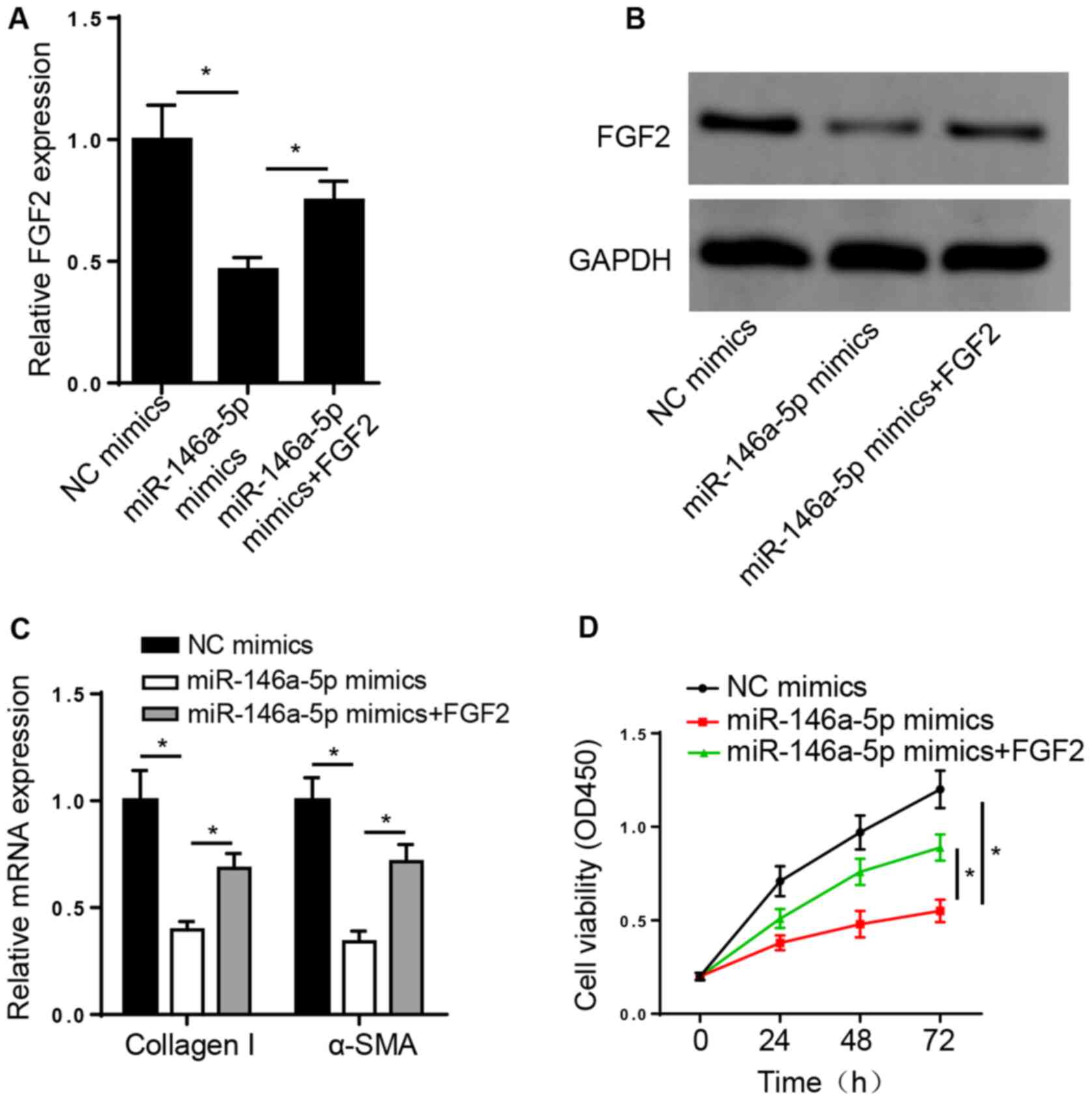
miR-146a-5p suppresses ISO-treated
cardiac fibrosis by targeting FGF2
To determine whether miR-146a-5p exerts its
biological role in fibrogenesis via FGF2, ISO-induced CFs were
transfected with NC or miR-146a-5p mimics and miR-146a-5p mimics +
pcDNA3.1/FGF2. RT-qPCR and western blot analysis demonstrated that
pcDNA3.1/FGF2 transfection rescued the suppressive effect of
miR-146a-5p overexpression on FGF2 mRNA and protein expression
levels in CFs (Fig. 4A and
B). Furthermore, overexpression of
miR-146a-5p suppressed the expression levels of collagen I and
α-SMA and inhibited cell viability; these effects were reversed
following overexpression of FGF2 (Fig.
4C and D). Taken together,
these results suggest that FGF2 was involved in cardiac fibrosis
progression in ISO-induced CFs.
Discussion
The results of the present study demonstrated that
miR-146a-5p served a key role in ISO-treated cardiac fibrosis:
miR-146a-5p suppressed cardiac fibrosis and downregulated the
expression levels of collagen I and α-SMA by modulating FGF2.
Increasing evidence suggests that miRNAs are
implicated in cardiac fibrosis development. For example, Yuan et
al (25) demonstrated that
miR-21 accelerates cardiac fibrosis following myocardial infarction
by regulating Smad7. Wei et al (26) indicated that downregulation of
miR-155 suppresses cardiac fibrosis during angiotensin II-induced
cardiac remodeling. Zhang et al (27) reported that miR-323a-3p facilitates
pressure overload-induced cardiac fibrosis via TIMP3. A previous
study reported that miR-146a-5p expression is downregulated in the
liver of CCl4-treated rats and that miR-146a-5p attenuates liver
fibrosis by suppressing the profibrogenic effects of TGF-β1 and
lipopolysaccharide (28).
Combination of miR-21 and miR-146a attenuates cardiac dysfunction
and apoptosis during acute myocardial infarction in mice (29). The present study investigated the
role of miR-146a-5p in cardiac fibrosis. H&E and Masson
staining confirmed that the model of cardiac fibrosis was
successfully induced by ISO treatment of CFs. Furthermore,
miR-146a-5p expression was downregulated in ISO-induced rat heart
tissue and CFs, whereas expression levels of collagen I and α-SMA
increased following treatment with ISO. Overexpression of
miR-146a-5p suppressed collagen I and α-SMA expression levels and
attenuated viability of ISO-treated CFs. Consistently, transfection
with miR-146a-5p mimics decreased the viability of ISO-induced
CFs.
FGF2 is a pro-angiogenic factor involved in wound
repair (30) and mediates several
biological functions, such as viability, differentiation and
angiogenesis (19). A recent study
demonstrated that miR-203 attenuates keloid fibroblast viability
and invasion by regulating early growth response 1 and
FGF2(31). FGF2 has been reported
to mediate ISO-treated cardiac hypertrophy by activating ERK
signaling (32). In the present
study, StarBase database and dual-luciferase reporter assay
demonstrated that miR-146a-5p targeted FGF2. In addition, RT-qPCR
analysis demonstrated that overexpression of miR-146a-5p suppressed
FGF2 expression. Notably, miR-146a-5p overexpression decreased the
expression levels of collagen I and α-SMA in CFs; these effects
were reversed following overexpression of FGF2 in ISO-treated CFs.
Taken together, these results suggest that miR-146a-5p suppressed
ISO-treated cardiac fibrosis by decreasing FGF2 expression.
In conclusion, the results of the present study
suggest that miR-146a-5p served a vital role in the development of
ISO-treated cardiac fibrosis by regulating FGF2. Thus, the
miR-146a-5p/FGF2 axis may be a potential novel target for the
treatment of cardiac fibrosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YH designed the present study. HZ, HW and YH
performed the experiments, analyzed the data and prepared the
figures. HZ and YH drafted the initial manuscript. HZ and YH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Ethics Committee of Nanjing First Hospital (Nanjing,
China; approval no. NFH-081241-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Travers JG, Kamal FA, Robbins J, Yutzey KE
and Blaxall BC: Cardiac fibrosis: The fibroblast awakens. Circ Res.
118:1021–1040. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Broughton KM, Wang BJ, Firouzi F,
Khalafalla F, Dimmeler S, Fernandez-Aviles F and Sussman MA:
Mechanisms of cardiac repair and regeneration. Circ Res.
122:1151–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Segura AM, Frazier OH and Buja LM:
Fibrosis and heart failure. Heart Fail Rev. 19:173–185.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fan D, Takawale A, Lee J and Kassiri Z:
Cardiac fibroblasts, fibrosis and extracellular matrix remodeling
in heart disease. Fibrogenesis Tissue Repair. 5(15)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alex L and Frangogiannis NG: The cellular
origin of activated fibroblasts in the infarcted and remodeling
myocardium. Circ Res. 122:540–542. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun
L, Davis K, Weisel RD and Li RK: Role of miR-145 in cardiac
myofibroblast differentiation. J Mol Cell Cardiol. 66:94–105.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ma ZG, Yuan YP, Wu HM, Zhang X and Tang
QZ: Cardiac fibrosis: New insights into the pathogenesis. Int J
Biol Sci. 14:1645–1657. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Penas FN, Carta D, Dmytrenko G, Mirkin GA,
Modenutti CP, Cevey ÁC, Rada MJ, Ferlin MG, Sales ME and Goren NB:
Treatment with a new peroxisome proliferator-activated receptor
gamma agonist, pyridinecarboxylic acid derivative, increases
angiogenesis and reduces inflammatory mediators in the heart of
Trypanosoma cruzi-infected mice. Front Immunol.
8(1738)2017.
|
|
9
|
Periasamy S, Chen SY and Liu MY: The study
of ISO induced heart failure rat model, Exp Mol Pathol.
2010;88:299-304. Exp Mol Pathol 90: 84, 2011.
|
|
10
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu X, Xu Y, Deng Y and Li H: MicroRNA-223
regulates cardiac fibrosis after myocardial infarction by targeting
RASA1. Cell Physiol Biochem. 46:1439–1454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang D, Cui Y, Li B, Luo X, Li B and Tang
Y: miR-155 regulates high glucose-induced cardiac fibrosis via the
TGF-β signaling pathway. Mol Biosyst. 13:215–224. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee HM, Kim TS and Jo EK: MiR-146 and
miR-125 in the regulation of innate immunity and inflammation. BMB
Rep. 49:311–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Morishita Y, Imai T, Yoshizawa H, Watanabe
M, Ishibashi K, Muto S and Nagata D: Delivery of microRNA-146a with
polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int
J Nanomedicine. 10:3475–3488. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yuan BY, Chen YH, Wu ZF, Zhuang Y, Chen
GW, Zhang L, Zhang HG, Cheng JC, Lin Q and Zeng ZC:
MicroRNA-146a-5p Attenuates fibrosis-related molecules in
irradiated and TGF-beta1-treated human hepatic stellate cells by
regulating PTPRA-SRC signaling. Radiat Res. 192:621–629.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pan J, Alimujiang M, Chen Q, Shi H and Luo
X: Exosomes derived from miR-146a-modified adipose-derived stem
cells attenuate acute myocardial infarction-induced myocardial
damage via downregulation of early growth response factor 1. J Cell
Biochem. 120:4433–4443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shi H, Xu J, Zhao R, Wu H, Gu L and Chen
Y: FGF2 regulates proliferation, migration, and invasion of ECA109
cells through PI3K/Akt signalling pathway in vitro. Cell Biol Int.
40:524–533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu J, Ye J, Zhu J, Xiao Z, He C, Shi H,
Wang Y, Lin C, Zhang H, Zhao Y, et al: Heparin-based coacervate of
FGF2 improves dermal regeneration by asserting a synergistic role
with cell proliferation and endogenous facilitated VEGF for
cutaneous wound healing. Biomacromolecules. 17:2168–2177.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schreier T, Degen E and Baschong W:
Fibroblast migration and proliferation during in vitro wound
healing. A quantitative comparison between various growth factors
and a low molecular weight blood dialysate used in the clinic to
normalize impaired wound healing. Res Exp Med (Berl). 195–205.
1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu J, Wang QY, Zhou Y, Lu XC, Liu YH, Wu
Y, Guo Q, Ma YT and Tang YQ: Astragaloside Ⅳ against cardiac
fibrosis by inhibiting TRPM7 channel. Phytomedicine. 30:10–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
No authors listed. Myocardial infarction
redefined - a consensus document of The Joint European Society of
Cardiology/American College of Cardiology Committee for the
redefinition of myocardial infarction. Eur Heart J. 21:1502–1513.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y,
Gu M, Zhou Y, Zhu J, Ge T, et al: Mir-21 promotes cardiac fibrosis
after myocardial infarction via targeting Smad7. Cell Physiol
Biochem. 42:2207–2219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei Y, Yan X, Yan L, Hu F, Ma W, Wang Y,
Lu S, Zeng Q and Wang Z: Inhibition of microRNA 155 ameliorates
cardiac fibrosis in the process of angiotensin II induced cardiac
remodeling. Mol Med Rep. 16:7287–7296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang J, Lang Y, Guo L, Pei Y, Hao S,
Liang Z, Su G, Shu L, Liu H, Huang C, et al: MicroRNA-323a-3p
promotes pressure overload-induced cardiac fibrosis by targeting
TIMP3. Cell Physiol Biochem. 50:2176–2187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zou Y, Cai Y, Lu D, Zhou Y, Yao Q and
Zhang S: MicroRNA-146a-5p attenuates liver fibrosis by suppressing
profibrogenic effects of TGFβ1 and lipopolysaccharide. Cell Signal.
39:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang W, Tian SS, Hang PZ, Sun C, Guo J
and Du ZM: Combination of microRNA-21 and microRNA-146a attenuates
cardiac dysfunction and apoptosis during acute myocardial
infarction in mice. Mol Ther Nucleic Acids. 5(e296)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shi K, Qiu X, Zheng W, Yan D and Peng W:
miR-203 regulates keloid fibroblast proliferation, invasion, and
extracellular matrix expression by targeting EGR1 and FGF2. Biomed
Pharmacother. 108:1282–1288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
House SL, House BE, Glascock B, Kimball T,
Nusayr E, Schultz JE and Doetschman T: fibroblast growth factor 2
mediates isoproterenol-induced cardiac hypertrophy through
activation of the extracellular regulated kinase. Mol Cell
Pharmacol. 2:143–154. 2010.PubMed/NCBI View Article : Google Scholar
|