Introduction
Hepatitis C is a global public health burden and the
hepatitis C virus (HCV) infected ~71 million people worldwide by
2020(1). Acute HCV infection often
leads to chronic, long-term infection that can progress to liver
cirrhosis and hepatocellular carcinoma (HCC) after several decades
(1). Impaired immune responses to
viral antigens, as well as chronic immune cell infiltration and
activation in the liver may be associated with chronic HCV
infection and progression to liver disease (2). Novel direct-acting antiviral drugs
(DAAs) targeting nonstructural viral proteins of HCV are better
tolerated compared with interferon by patients and increase
sustained virological response rates (3). However, despite the ~100% cure rate,
DAA therapy does not prevent HCV reinfection (4). In addition, DAAs do not reduce the
risk of HCC in patients with HCV-related cirrhosis, and some
patients develop primary liver cancer or recurrent tumors shortly
after DAA treatment (5).
Therefore, it is clinically and economically important to study the
pathogenesis of chronic HCV infection and develop new
immunotherapeutic drugs.
Epidemiological studies have suggested that acute
HCV infection can be resolved without treatment in up to 20% of
cases, which implies that the outcome can be controlled by innate
and/or adaptive immune responses (6,7).
Inflammatory cells and cytokine cascades activate and recruit
monocytes or macrophages in chronic HCV infection. macrophages
differentiate from peripheral blood monocytes (PBMCs) and serve
important roles in regulating host inflammatory responses and
tissue pathology (8-12).
T helper (Th)1 cytokines, such as interferon-γ, Toll-like receptor
(TLR)4 ligand and lipopolysaccharide (LPS), polarize monocytes to
classically activated M1 macrophages, producing proinflammatory
cytokines, tumor necrosis factor (TNF)-α and IL-12, which in turn
promote clearance of pathogens and lead to tissue damage. However,
after exposure to Th2 cytokines, such as IL-4 and IL-13, monocytes
polarize towards alternately activated M2 macrophages and produce
anti-inflammatory mediators, including IL-10, which have an
anti-inflammatory and wound-healing role (13,14).
M2 macrophages are further differentiated into M2a, M2b and M2c
subtypes (15). A previous study
demonstrated that monocytes that are differentiated into M1 and M2
macrophages were both suppressed by HCV (16); however, it remains unclear as to
whether HCV affects the polarization of the three subtypes of M2
macrophages, and if this effect promotes the progression of chronic
disease. Thus, the present study hypothesized that HCV infection
could affect M2 macrophage polarization.
Materials and methods
Study subjects
The present study included 25 patients with chronic
hepatitis C (CHC) who visited the Digestive Diseases Hospital of
Shandong First Medical University in Jining between January 2019
and April 2022. This cohort met the diagnostic criteria of the
Guideline for Prevention and Treatment of Hepatitis C (2015)
(17). Inclusion criteria were: i)
Age, 18-70 years; ii) HCV infection for >6 months, or
epidemiological history 6 months ago prior to the enrollment; iii)
anti-HCV antibody and HCV-RNA positive; iv) evidence of
histopathological examination in line with the diagnostic criteria
for chronic hepatitis (17).
Exclusion criteria were: i) Other concomitant viral hepatitis, such
as chronic hepatitis B, D and other types of hepatitis; ii)
drug-induced liver disease; iii) alcoholic liver disease; iv)
autoimmune liver disease; v) HIV infection; vi) cancer; vii) severe
cardiovascular or cerebrovascular disease; viii) hematological or
thyroid disease; ix) diabetes; or x) receiving antiviral
treatment.
A total of 25 healthy controls (HCs) registered in
the Physical Examination Center of Digestive Diseases Hospital of
Shandong First Medical University between January 2019 and April
2022 were selected as the control group. Exclusion criteria were
the same as in the CHC group except for HCV infection. The present
study was approved by the Ethics Committee of Digestive Diseases
Hospital of Shandong First Medical University (approval code:
2018-LC-001), and all patients provided written informed consent
before participating in the study.
Sample collection
A total of 4-6 ml venous blood was collected from
patients and HCs in the morning on an empty stomach. The plasma was
separated from cells by centrifugation at 1,500 x g for 5 min and
stored at -80˚C. The plasma was used to determine alanine
aminotransferase (ALT), aspartate aminotransferase, alkaline
phosphatase, γ-glutamyl transferase, total bilirubin, albumin (ALB)
and HCV-RNA levels, and peripheral blood mononuclear cells (PBMCs)
were isolated and cultured. ALT, AST, ALP, GGT and ALB were
detected on the Toshiba TBA-120FR instrument by continuous
monitoring method. The HCV-RNA level was detected by real-time PCR
according to manufacturer's protocol.
Reagents
HCVc (amino acids 2-192) was purchased from Meridian
Bioscience, Inc. Human recombinant macrophage colony-stimulating
factor (M-CSF), LPS, IL-4, IL-13, IL-10 and Pam3CSK4 (TLR2/TLR1
agonist-synthetic triacylated lipoprotein), and a TLR2 antibody
(cat. no. MAB2616-SP) were obtained from R&D Systems, Inc.
Cell isolation and purification
PBMCs were isolated from the peripheral blood of HCs
and patients with HCV infection by Ficoll density gradient
separation (Axis-Shield Diagnostics, Ltd.). Monocytes were purified
by magnetic cell sorting with CD14 microbeads (BD Biosciences, cat.
no. 130-050-201) according to manufacturer's protocol. The purity
of the CD14+ monocytes was ≥95% as determined using flow
cytometry on a flow cytometer (FACScan; BD Biosciences) and the
acquired data were analyzed using FlowJo software (v7.6; FlowJo
LLC).
Cell culture
Purified monocytes were cultured in RPMI 1640 medium
(Corning, Inc.) supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and
streptomycin for 5 days at 37˚C in 5% CO2. The monocytes
were induced to polarize into M2 macrophages by adding 50 ng/ml
M-CSF during the culture. For the polarization of M2a, M2b and M2c
macrophages, M-CSF-induced macrophages were exposed to fresh
culture medium supplemented with IL-4 (25 ng/ml) + IL-13 (25
ng/ml), LPS (10 ng/ml) + immune complex (33 µl/ml), or IL-10 (25
ng/ml) for 24 h at 37˚C in 5% CO2, respectively
(18). The preparation method of
the immune complex was as follows: 1 µl 1.32 mg/ml bovine serum
albumin (BSA; Santa Cruz Biotechnology, Inc.) and 50 µl rabbit
polyclonal anti-BSA (Santa Cruz Biotechnology, Inc.) were mixed and
incubated for 1 h at 37˚C, whereupon they were stored at 4˚C until
use (typically overnight).
To study the effect of HCVc on macrophage
polarization, HCVc (10 µg/ml) or Pam3CSK4 (1 µg/ml) was added to
the M2 macrophage subtypes for 5 days. They were considered as HCVc
group and Pam3CSK4 group, compared with W/O group that the cells
were treated with medium alone. For mechanistic experiments,
monocytes were pretreated with TLR2 antibody (0.15 µg/ml) for 1 h
at 37˚C, and were then polarized to M2a, M2b and M2c macrophages
and treated with HCVc at 37˚C in 5% CO2 for 5 days and
then considered the HCVc/anti-TLR2 group.
Flow cytometry
Polarized M2 macrophages (0.1x106) were
collected and resuspended in staining buffer (PBS supplemented with
0.5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.))
and preincubated with FcR blocking reagent (Miltenyi Biotec, Inc.)
for 15 min at 4˚C. Cells were simultaneously stained for 20-40 min
at 4˚C with CD209-V450 (cat. no. 561275), CD86-FITC (cat. no.
555657) and CD163-PE (cat. no. 556018; all from BD Biosciences;
dilution 1:50), which are markers of M2a, M2b and M2c macrophages,
respectively. Cells were washed with staining buffer [PBS
supplemented with 0.5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.)] by centrifugation at 1,500 x g, at 4˚C for 5 min
and resuspended in PBS supplemented with 1% paraformaldehyde.
Finally, phenotypic analysis of M2 macrophages was performed on a
flow cytometer (FACScan; BD Biosciences) and the acquired data were
analyzed using FlowJo software (v7.6; FlowJo LLC).
ELISA
The cell culture supernatants were collected
following macrophage polarization in each experimental condition.
Concentrations of IL-1 receptor antagonist (IL-1RA) (cat. no.
CHC1183), TNF-α (cat. no. 88734677) and TGF-β (cat. no. 885039088)
in cell culture supernatants were measured by ELISA kits
(eBioscience; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and was reverse transcribed to cDNA using the reverse
transcription kit (TransGen Biotech Co., Ltd.) at 4˚C according to
manufacturer's protocols. qPCR was performed using the Faststart
Universal SYBR Green Master kit (Rox; Roche Diagnostics). The
primer sequence pairs were designed using NCBI online primer BLAST
software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/).
The following primers were used for qPCR: Fizz1, forward
5'-GTCAAAAGCCAAGGCAGACC-3' and reverse 5'-TGAACATCCCAC-GAACCACA-3';
TNF-α, forward 5'-TGGGGAGTGTGAGGGGTATC-3' and reverse
5'-TGCACCTTCTGTCTCGGTTT-3'; sphingosine kinase-1 (SPHK1), forward
5'-CTGGCAGCTTCCTTGAACCAT-3' and reverse
5'-TGTGCAGAGACAGCAGGTTCA-3'; and GAPDH, forward
5'-GACTTCAACAGCAACTCCCACTC-3' and reverse
5'-TAGCCGTATTCATTGTCATACCAG-3'. Reactions were performed using 3 µl
cDNA in a 20-µl reaction volume under the following thermocycling
conditions: Pre-denaturation at 94˚C for 10 sec, followed by
denaturation at 94˚C for 5 sec and final extension at 60˚C for 30
sec, for 40 cycles. Relative gene expression was determined by
normalizing the expression of each target gene to GAPDH. All the
experiments were performed in triplicate and the 2-ΔΔCq
method (19) was used to quantify
the relative fold change in the mRNA expression levels of Fizz1,
TNF-α and SPHK1.
Phagocytosis assay
Phagocytosis assay was performed as described
previously (20,21). Polarized M2 macrophages in the
presence or absence of HCVc were incubated with FITC-latex beads
(cat. no. L4655; MilliporeSigma; 0.05% of the stock concentration)
in refreshed culture medium for 1 h at 37˚C with 5% CO2.
Cells were then collected. Phagocytic activity of the cells was
detected using a flow cytometer (FACScan; BD Biosciences) and the
acquired data were analyzed using FlowJo software (v7.6; FlowJo
LLC).
Statistical analysis
All experiments were repeated three times. The data
were collated into an excel table. All continuous variables
presented as mean values ± standard deviation were normally
distributed and were analyzed and found to be significant using the
D'Agostino and Pearson omnibus normality test. Mean values were
compared using an unpaired Student's t-test (two groups) or one-way
ANOVA (more than two groups) followed by Bonferroni correction for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference. The statistical analysis was
performed using GraphPad Prism Version 5 (GraphPad Software,
Inc.).
Results
Baseline characteristics of study
subjects
To test the hypothesis that HCV affects the
polarization of monocytes to macrophages, 25 patients with CHC and
25 HCs were enrolled in the present study (Table I). The CHC group included 14 men
and 11 women, aged 48.88±1.20 years (range, 37-60 years), and the
HC group included 13 men and 12 women, aged 49.32±1.05 years
(range, 39-65 years). Plasma ALT and HCV-RNA levels were
significantly higher, and ALB levels were significantly lower in
the CHC group compared with those in the HC group (P<0.05;
Table I). There was no significant
difference in the sex ratio, age distribution and other biochemical
indexes between the two groups.
 | Table IComparison of baseline
characteristics between CHC and HC groups. |
Table I
Comparison of baseline
characteristics between CHC and HC groups.
| Clinical
indicators | HC group
(n=25) | CHC group
(n=25) | t-value | P-value |
|---|
| Age, years | 49.32±1.05 | 48.88±1.20 | 0.277 | 0.783 |
| Sex,
male/female, | 13/12 | 14/11 | | |
| ALT, U/l | 25.45±0.90 | 29.86±0.90 | 3.478 | 0.001a |
| AST, U/l | 26.91±1.13 | 29.00±1.10 | 1.329 | 0.190 |
| ALP, U/l | 68.72±1.89 | 67.29±2.17 | 0.496 | 0.622 |
| GGT, U/l | 42.43±2.62 | 48.86±1.97 | 1.962 | 0.056 |
| TBIL, U/l | 7.79±0.31 | 8.58±0.31 | 1.817 | 0.075 |
| ALB, g/l | 49.43±0.53 | 46.64±0.67 | 3.268 | 0.002a |
| HCV-RNA, IU/ml | <50 |
2.58x106±0.18x105 | 14.440 |
<0.0001a |
HCV infection inhibits monocyte
polarization to M2 macrophages
Purified monocytes from the HC and CHC groups were
polarized to M2a, M2b and M2c macrophages. The expression of cell
surface markers, CD209 (M2a), CD86 (M2b) and CD163 (M2c), of the
three types of M2 macrophages were detected using flow cytometry.
The secretion of the cytokines IL-1RA, TNF-α and TGF-β, which are
markers for M2a, M2b and M2c, respectively, were analyzed using
ELISA. The mRNA expression levels of Fizz1, TNF-α and SPHK1, which
are markers for M2a, M2b and M2c, respectively, were detected by
RT-qPCR. The expression levels of CD209, CD86 and CD163 on M2a
macrophages (P<0.05; Fig.
1A-F), CD86 on M2b macrophages (P<0.05; Fig. 1C and D) and CD163 on M2c macrophages
(P<0.05; Fig. 1E and F) were significantly lower in patients
with CHC than those in the HC group. The release levels of IL-1RA
(P<0.05; Fig. 1G) and the mRNA
expression levels of Fizz1 and SPHK1 (P<0.05; Fig. 1J and L) in M2a macrophages; the release levels
of TNF-α (P<0.05; Fig. 1H) and
the mRNA expression levels of TNF-α (P<0.05; Fig. 1K) in M2b macrophages; and the
release levels of TGF-β (P<0.05; Fig. 1I) and the mRNA expression levels of
SPHK1 (P<0.05; Fig. 1L) in M2c
macrophages, were all significantly decreased in patients with CHC
compared with those in the HC group. These results suggested that
peripheral monocytes derived from patients with CHC were less
polarizable towards the three types of M2 macrophages compared with
the HC group. Therefore, the ability of human PBMCs from patients
with CHC to polarize to M2 macrophages could be impaired.
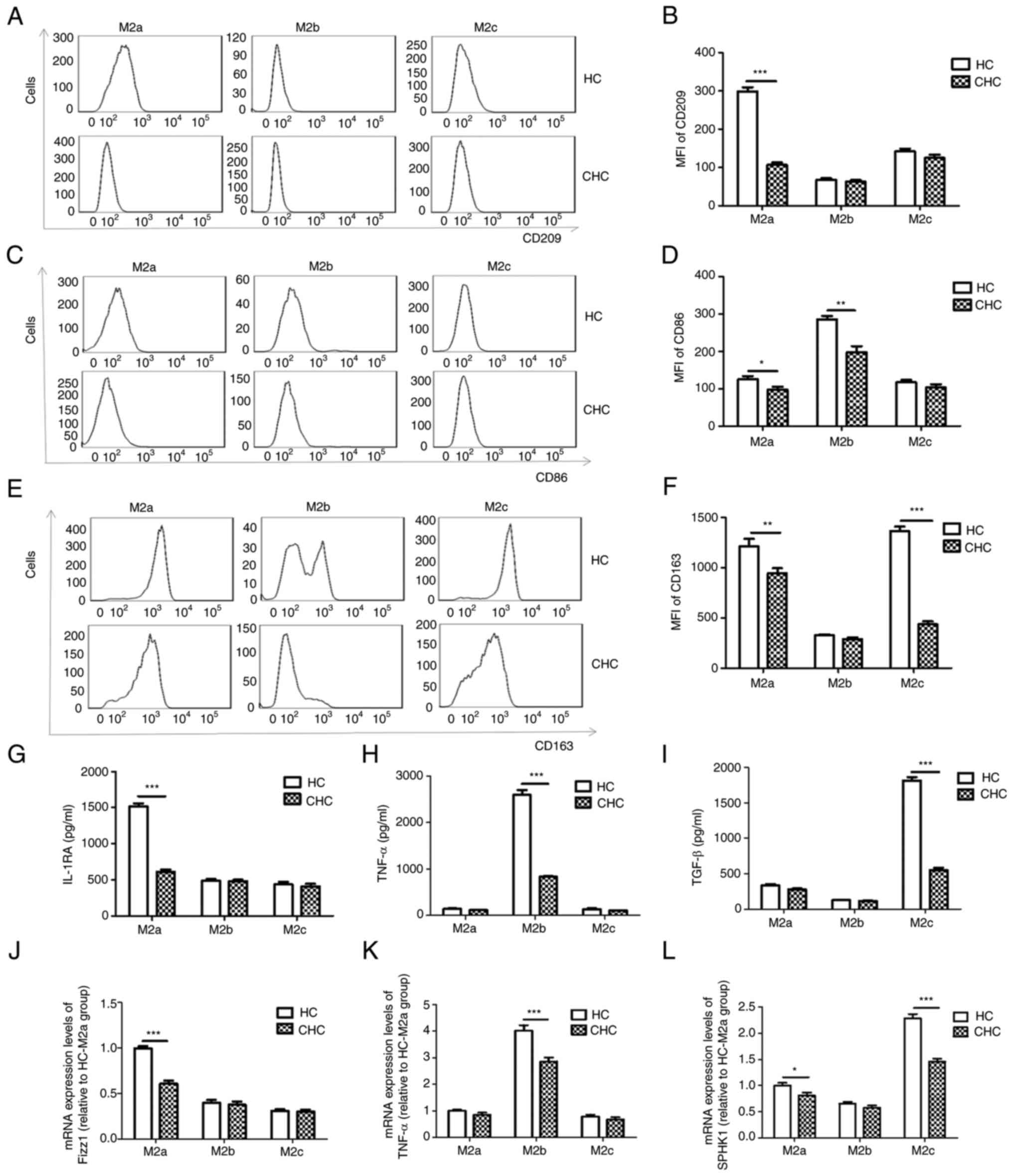 | Figure 1Differentiation of peripheral blood
monocytes into M2a, M2b and M2c macrophages is impaired in patients
with CHC. (A and B) M2a macrophages, which were differentiated from
peripheral blood monocytes derived from patients with CHC,
expressed lower levels of CD209 compared with those in the HC
group. (C and D) M2b macrophages, which were differentiated from
peripheral blood monocytes derived from patients with CHC,
expressed lower levels of CD86 compared with those in the HC group.
(E and F) M2c macrophages, which were differentiated from
peripheral blood monocytes derived from patients with CHC,
expressed lower levels of CD63 compared with those in the HC group.
Secreted levels of (G) IL-1RA, (H) TNF-α and (I) TGF-β were
decreased in M2a, M2b and M2c macrophages differentiated from
peripheral blood monocytes of patients with CHC compared with those
in the HC group, respectively. mRNA expression levels of (J) Fizz1,
(K) TNF-α and (L) SPHK1 were decreased in the M2a, M2b and M2c
macrophages differentiated from peripheral blood monocytes of
patients with CHC compared with those in the HC group,
respectively. To simplify the results, mRNA expression levels were
calculated relative to HC-M2a group'. n=25. *P<0.05,
**P<0.01, ***P<0.001. CHC, chronic
hepatitis C; HC, healthy control; MFI, mean fluorescence intensity;
IL-1RA, IL-1 receptor antagonist; SPHK1, sphingosine kinase 1. |
HCVc suppresses M2 macrophage
polarization
According to our previous study, HCVc can inhibit
the polarization of monocytes towards M1 and M2 macrophages
(16). The present study
investigated whether the impaired polarization of the three types
of M2 macrophages in patients with CHC was also affected by HCVc.
Therefore, purified monocytes derived from the healthy people were
polarized to M2a, M2b and M2c macrophages in the presence or
absence of HCVc to study the effect of HCVc on M2 macrophages
polarization in vitro. The optimal HCVc concentration was
chosen according to the results of previous studies (16,22);
10 µg/ml was used as the standard concentration in all experiments.
The results of the present study demonstrated that HCVc
downregulated the expression levels of CD209, CD86 and CD163
(P<0.05; Fig. 2A-F) on M2a
macrophages, as well as the release of IL-1RA (P<0.05; Fig. 2G) and the mRNA expression levels of
Fizz1 (P<0.05; Fig. 2J),
compared with W/O group. HCVc also downregulated the expression
levels of CD86 (P<0.05; Fig. 2C
and D) on M2b macrophages, as well
as the release of IL-1RA and TNF-α (P<0.05; Fig. 2G and H) and the mRNA expression levels of TNF-α
(P<0.05; Fig. 2K), compared
with the medium controls. In addition, HCVc downregulated the
expression levels of CD209 and CD163 (P<0.05; Fig. 2A, B, E and
F) on M2c macrophages, as well as
the release of TGF-β (P<0.05; Fig.
2I) and the mRNA expression levels of SPHK1 (P<0.05;
Fig. 2L), compared with the medium
controls. The present results demonstrated that HCVc inhibited all
the three types of M2 macrophage polarization, which is in
accordance with chronic HCV infection.
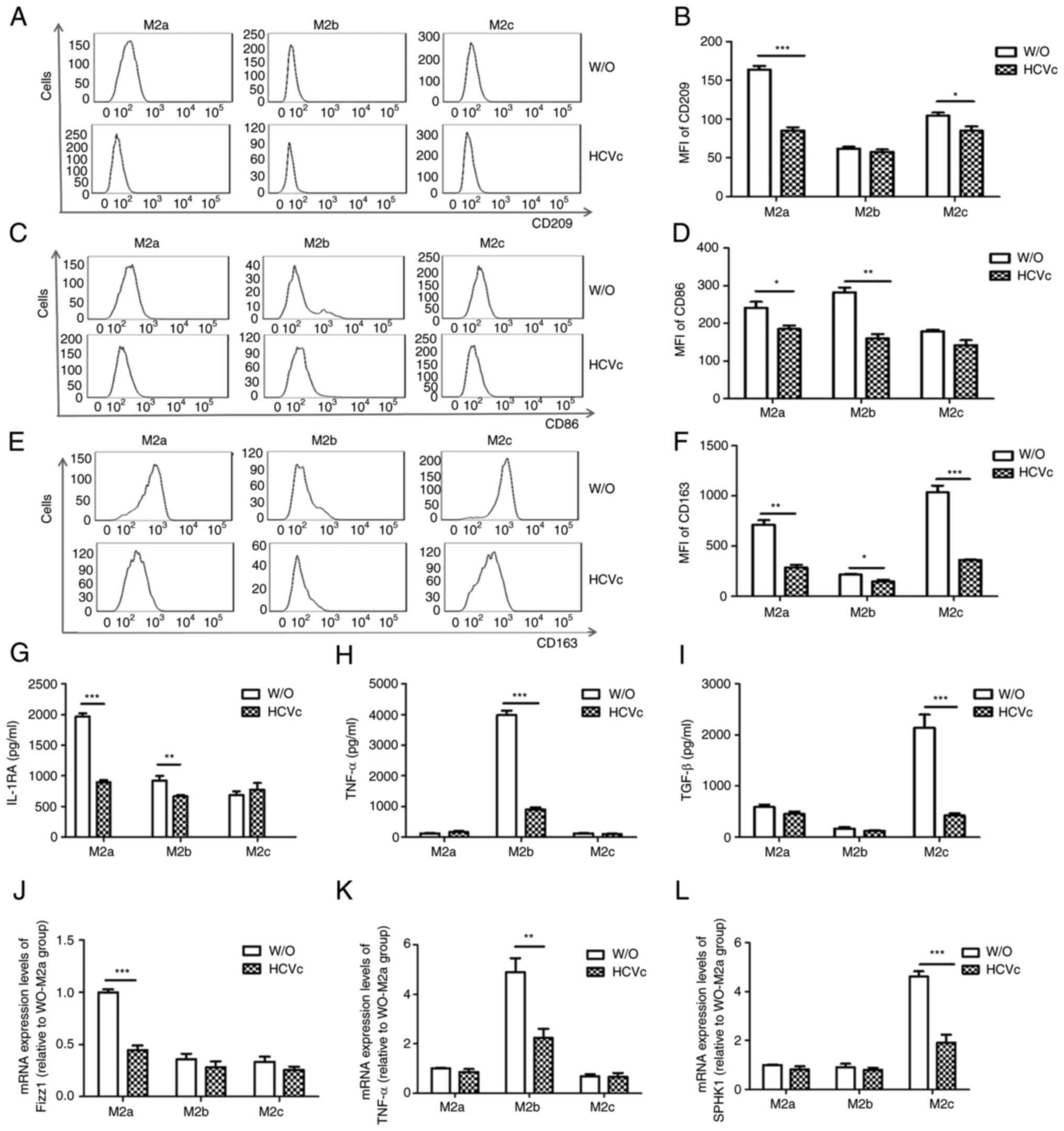 | Figure 2Differentiation of peripheral blood
monocytes derived from the HC group to the three subtypes of M2
macrophages is inhibited by exposure to HCVc. (A and B) Expression
of CD209 on M2a macrophages was inhibited by HCVc. (C and D)
Expression of CD86 on M2b macrophages was inhibited by HCVc. (E and
F) Expression of CD163 on M2c macrophages was inhibited by HCVc.
Secretion levels of (G) IL-1RA, (H) TNF-α and (I) TGF-β were
decreased in the M2a, M2b and M2c macrophages that were treated
with HCVc, respectively. mRNA expression levels of (J) Fizz1, (K)
TNF-α and (L) SPHK1 were decreased in the M2a, M2b and M2c
macrophages treated with HCVc, respectively. Representative
histograms of three independent experiments are shown.
*P<0.05, **P<0.01,
***P<0.001. MFI, mean fluorescence intensity; HCVc,
hepatitis C virus core protein; W/O, control group (W/O, without
stimulant; other groups received HCVc); IL-1RA, IL-1 receptor
antagonist; SPHK1, sphingosine kinase 1. |
HCVc suppresses M2 macrophage
polarization through TLR2 signaling
Based on our previous research, HCVc may inhibit
TLR2-mediated polarization of M1 and M2 macrophages (16). In the present study, the
involvement of the TLR2 signaling pathway was further investigated
in the three types of M2 macrophage polarization. Purified
monocytes were pretreated with a TLR2 antibody, and in turn
polarized into M2a, M2b and M2c macrophages and treated with HCVc.
Pam3CSK4, a canonical TLR2 ligand, was used as a control for the
HCVc binding to TLR2. Blockade of the HCVc/TLR2 interaction using
the TLR2 antibody upregulated the expression levels of CD209 and
CD163 (P<0.05; Fig. 3A and
C) on M2a macrophages, as well as
the release of IL-1RA (P<0.05; Fig.
3D) and the mRNA expression levels of Fizz1 (P<0.05;
Fig. 3G), compared with HCVc
group. Blockade of HCVc/TLR2 engagement using the TLR2 antibody
also upregulated the expression levels of CD86 (P<0.05; Fig. 3B) on M2b macrophages, as well as
the release of IL-1RA and TNF-α (P<0.05; Fig. 3D and E) and the mRNA expression levels of TNF-α
(P<0.05; Fig. 3H), compared
with HCVc alone. In addition, blockade of HCVc/TLR2 engagement
using the TLR2 antibody upregulated the expression levels of CD163
(P<0.05; Fig. 3C) on M2c
macrophages, as well as the release of TGF-β (P<0.05; Fig. 3F) and the mRNA expression levels of
SPHK1 (P<0.05; Fig. 3I),
compared with HCVc alone. By contrast, Pam3CSK4 significantly
suppressed M2 macrophage polarization compared with W/O group. In
summary, HCVc inhibited M2a, M2b and M2c macrophage polarization
via TLR2, and there was no difference in the effect of HCVc on the
polarization of the three subtypes.
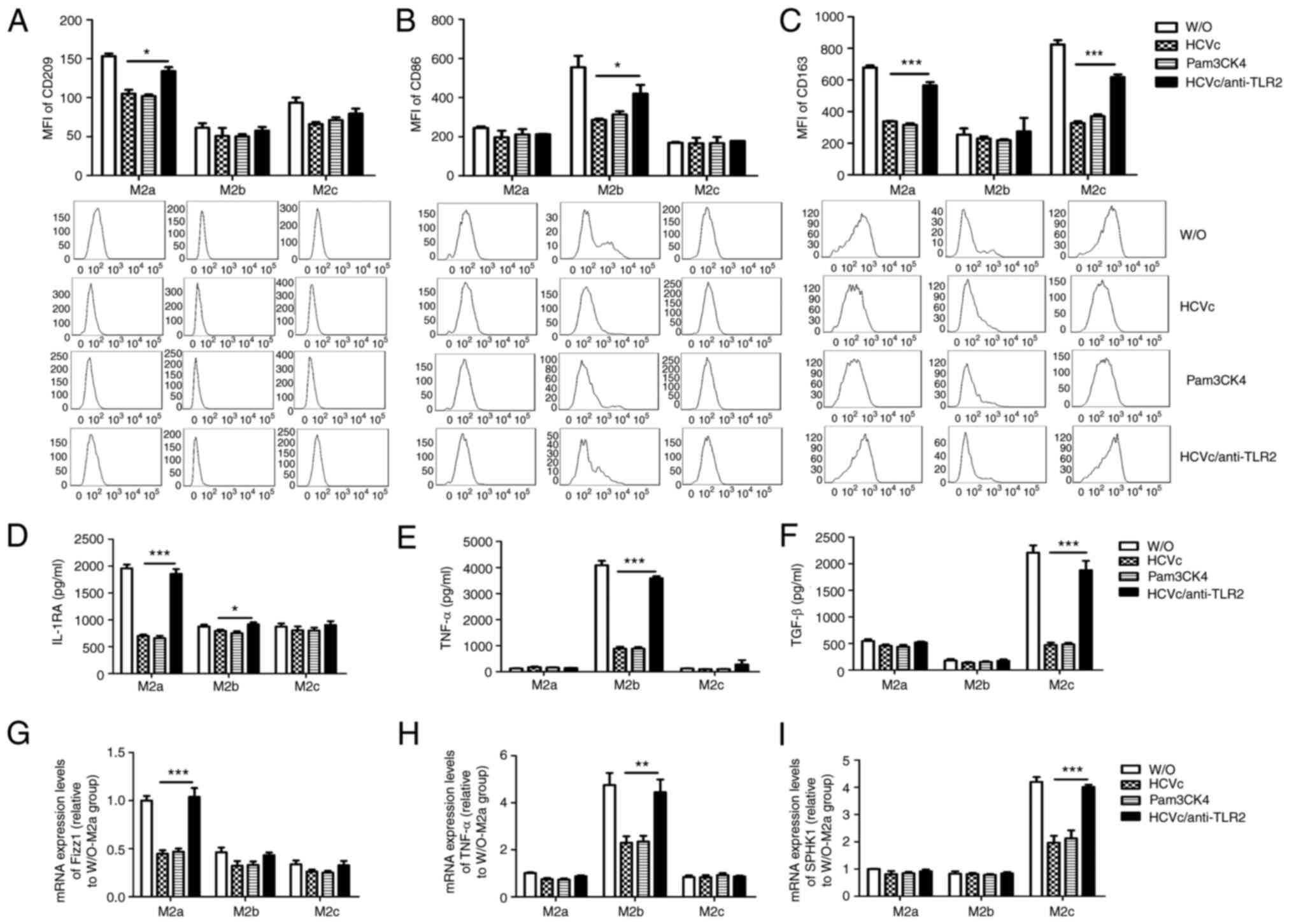 | Figure 3Macrophage polarization is inhibited
by HCVc via TLR2 signaling. Expression levels of (A) CD209 in M2a
macrophages, (B) CD86 in M2b macrophages and (C) CD163 in M2c
macrophages were decreased by exposure to HCVc or Pam3CSK4.
Secretion levels of (D) IL-1RA in M2a macrophages, (E) TNF-α in M2b
macrophages and (F) TGF-β in M2c macrophages were decreased by
exposure to HCVc or Pam3CSK4. mRNA expression levels of (G) Fizz1
in M2a macrophages, (H) TNF-α in M2b macrophages and (I) SPHK1 in
M2c macrophages were decreased by exposure to HCVc or Pam3CSK4.
(A-I) Blockade of the TLR2 signaling pathway using a TLR2 antibody
partially restored macrophage polarization. Representative
histograms of three independent experiments are shown.
*P<0.05, **P<0.01,
***P<0.001. MFI, mean fluorescence intensity; HCVc,
hepatitis C virus core protein; W/O, control group (W/O, without
stimulant; other groups received HCVc, Pam3CSK4 and TLR2 antibody);
IL-1RA, IL-1 receptor antagonist; SPHK1, sphingosine kinase 1;
TLR2, Toll-like receptor 2. |
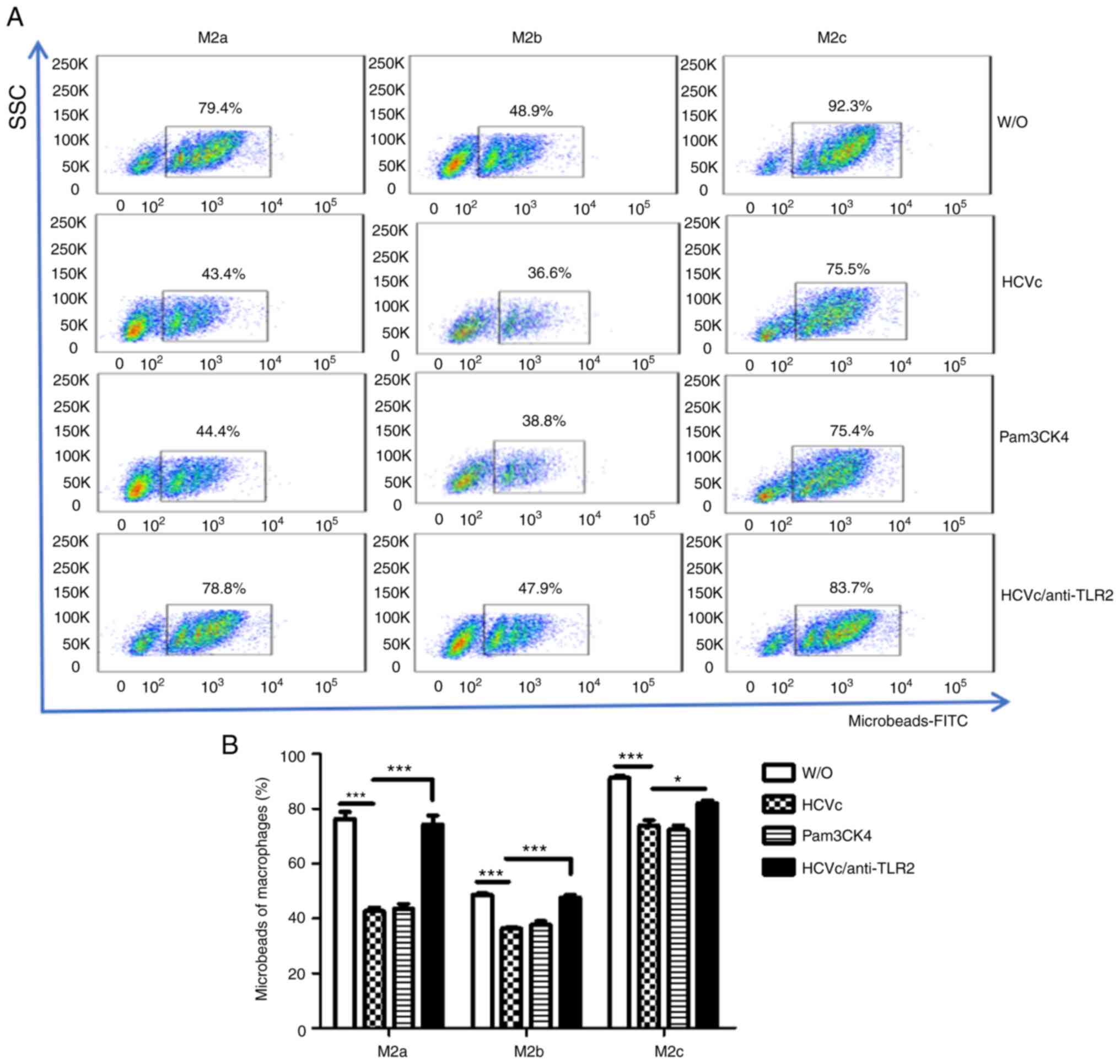
HCVc suppresses the phagocytic
activity of M2 macrophages via TLR2 signaling
Macrophages have a vital role in immune surveillance
through phagocytic activity; therefore, the effect of HCVc on the
phagocytic activity of the three M2 subtypes was investigated.
Polarized M2 macrophages treated with HCVc or Pam3CSK4 were
cultured in the presence of FITC-latex beads. The phagocytic
activity was then analyzed by flow cytometry. HCVc and Pam3CSK4
significantly suppressed the phagocytic activity of all three
subtypes (P<0.05; Fig. 4). To
determine if the suppression of phagocytosis caused by HCVc was
regulated through its interaction with TLR2, purified monocytes
were pretreated with a TLR2 antibody and polarized to M2a, M2b and
M2c macrophages in the presence of HCVc; Pam3CSK4 was used as a
control. Blocking the binding of HCVc to TLR2 using a TLR2 antibody
partially restored the phagocytic activity of all three subtypes of
M2 macrophages (P<0.05; Fig.
4).
Discussion
Macrophages serve a vital role in the primary immune
response to pathogenic agents, inflammation, repair, resolution of
inflammation and tissue homeostasis (23). macrophages exhibit a range of
different phenotypes, in connection with macrophage polarization.
They take part in host defense, immunoregulation and tissue
recovery, and are defined by surface markers and the release of
soluble cytokines (24). Activated
macrophages can be divided into M1 and M2 subtypes under different
stimulation conditions. In addition, M2 macrophages can be further
subdivided into M2a, M2b and M2c in the appropriate context
(25). IL-4 and IL-10 cytokines
can activate M2a macrophages, expressing high levels of surface
markers, such as IL-1RA, CD209, Fc ε receptor and Dectin-1(26). IL-1, LPS, immune complexes such as
viral antigens and TLR/IL-1R induce activation of M2b macrophages,
which are involved in immunoregulation (27). Notably, M2b macrophages produce
cytokines, such as IL-1, IL-6 and TNF-α. M2c macrophages are
induced by anti-inflammatory cytokines IL-10 and TGF-β (28), and produce IL-10, TGF-β and
α1-antitrypsin. M2c macrophages also take part in extracellular
matrix remodeling, tissue repair and phagocytosis of apoptotic
cells (29).
During HCV infection, monocytes and macrophages
mediate an abnormal inflammatory response that affects the natural
history of infection (30).
Plasticity and functional polarization are hallmarks of
macrophages, leading to phenotypic diversity in macrophage
populations. Dysregulation of macrophage polarization is associated
with the pathogenesis of various diseases (31,32).
According to our previous research, the polarization of monocytes
in patients with CHC to both M1 and M2 macrophages was impaired
compared with that of healthy monocytes (16). In the present study, the
differences in M2a, M2b and M2c subtypes between patients with CHC
and HCs were investigated. It was demonstrated that HCV inhibited
the polarization of all three subtypes. Despite the relatively
small sample size of 25 patients with CHC and 25 HCs, the results
of all indicators were consistently significant between the two
groups; therefore, the effect of chance could be excluded. In the
context of HCV infection, impaired macrophage polarization may
contribute to chronic infection, where the subsequent
immunosuppression promotes liver disease.
HCVc is found in the cytoplasm and nuclei of
infected cells, including hepatocytes and other cells in the liver,
from which it can be secreted as well (33). A previous study showed that HCVc
could inhibit the expression of TNF-α and transferrin receptor
protein 1 expression and phagocytosis of HCVc particles in M1
macrophages, which should be enhanced in the conversion of M1 to
M2(34). The differentiation of
peripheral blood monocytes into M1/M2 macrophages in the presence
of HCVc was studied in our previous study, which showed that HCVc
could inhibit monocyte polarization to M1 and M2 macrophages
(16). In the present study, the
effect of HCVc on the differentiation of M2a, M2b and M2c subtypes
was further investigated. As expected, HCVc inhibited polarization
of all three subtypes.
It has been reported that HCVc can activate TLR2,
which is expressed in human monocytes, macrophages, Kupffer cells
and regulatory T cells, to induce the production of inflammatory
cytokines by activating the MyD88-dependent TLR signaling pathway
(35-37).
HCV induces the production of myeloid derived suppressor cells
(MDSCs) like suppressive monocytes via the TLR2/PI3K/AKT/STAT3
signaling pathway, which in turn induces
CD4+Foxp3+ regulatory T cells and inhibits
autologous CD4+ T cell activation (38). In our previous study, HCVc
inhibited the polarization of M1 and M2 macrophages by binding to
TLR2(16). Therefore, it was
hypothesized that HCVc engagement with TLR2 on monocytes could
regulate the subtypes of M2 macrophages polarization. In
vitro, peripheral blood monocytes derived from the HC group
were differentiated into M2a, M2b and M2c macrophages in the
presence of HCVc, and the results showed that HCVc could suppress
the polarization of monocytes to all three subtypes of M2
macrophages. Moreover, the TLR2 ligand Pam3CSK4 inhibited the
polarization of M1/M2 macrophages, whereas blocking the binding of
HCVc to TLR2 partially alleviated the HCVc-induced inhibition of
macrophage polarization. These results suggested that HCV may
modulate the polarization of various subtypes of M2 macrophages
through the interaction of HCVc with TLR2 on monocytes.
Macrophages have a large capacity for phagocytosis,
which is the first step for the presentation of antigens of foreign
pathogens. macrophages engulf HCV intracellularly by phagocytosis
(39). Consequently, the
phagocytosis of HCV by macrophages contributes to viral clearance.
In our previous research, the phagocytic function of M2 macrophages
was revealed to be more prominent than that of M1 macrophages, and
phagocytic activity of both M1 and M2 macrophages was downregulated
by HCVc (16). The results of the
present study are in line with the aforementioned observations. The
phagocytic activity of all three subtypes of M2 macrophages was
downregulated by HCVc; however, the blockade of TLR2 signaling
restored the macrophage polarization and the phagocytic activity of
all the types of M2 macrophages. These findings suggest a mechanism
by which HCV can escape phagocytosis by macrophages.
In conclusion, the present study showed that
monocyte polarization toward M2 macrophage subtypes (M2a, M2b and
M2c) may be impaired in patients with CHC via the interaction of
HCVc with TLR2, resulting in a decline in phagocytosis. The present
study provided a novel perspective regarding the mechanism by which
HCV develops into a chronic persistent infection due to HCVc. It
may be proposed that blocking the binding of HCVc to TLR2 could be
a therapeutic strategy against HCV infection.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Natural Science
Foundation of Shandong Province (grant no. ZR2017BH056) and the
Breeding Programs of National Natural Science Foundation of Jining
Medical University (grant no. JNYXYZRPY2016-3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ contributed to the conception and design of the
study. SZ performed the experiments and drafted the article. XD
contributed to the acquisition of the blood samples and operation
of the experiments. MS and LK contributed to the implementation of
the study and the acquisition of the data. DW contributed to data
analysis. SZ and XD confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Gastroenterology of
Shandong First Medical University. All patients enrolled in the
present study provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Association for the Study of the
Liver, Electronic address: simpleeasloffice@easloffice.eu,
Clinical Practice Guidelines Panel. Chair, EASL Governing Board
representative, Panel members: EASL recommendations on treatment of
hepatitis C: Final update of the series. J Hepatol. 73:1170–1218.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ciesek S and Manns MP: Hepatitis in 2010:
The dawn of a new era in HCV therapy. Nat Rev Gastroenterol
Hepatol. 8:69–71. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marascio N, Quirino A, Barreca GS, Galati
L, Costa C, Pisani V, Mazzitelli M, Matera G, Liberto MC, Focà A
and Torti C: . Discussion on critical points for a tailored therapy
to cure hepatitis C virus infection. Clin Mol Hepatol. 25:30–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wijaya RS, Read SA, Selvamani SP, Schibeci
S, Azardaryany MK, Ong A, van der Poorten D, Lin R, Douglas MW,
George J and Ahlenstiel G: Hepatitis C virus (HCV) eradication with
interferon-free direct-acting antiviral-based therapy results in
KLRG1+ HCV-specific memory natural killer cells. J Infect Dis.
223:1183–1195. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sung PS and Shin EC: Immunological
mechanisms for hepatocellular carcinoma risk after direct-acting
antiviral treatment of hepatitis C virus infection. J Clin Med.
10(221)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lauer GM: Immune responses to hepatitis C
virus (HCV) infection and the prospects for an effective HCV
vaccine or immunotherapies. J Infect Dis. 207 (Suppl 1):S7–S12.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mohammadzadeh S, Roohvand F, Ehsani P,
Salmanian AH and Ajdary S: Canola oilseed- and Escherichia coli-
derived hepatitis C virus (HCV) core proteins adjuvanted with oil
bodies, induced robust Th1-oriented immune responses in immunized
mice. APMIS. 128:593–602. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guilliams M, Mildner A and Yona S:
Developmental and functional heterogeneity of monocytes. Immunity.
49:595–613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao X, Yakala GK, van den Hil FE, Cochrane
A, Mummery CL and Orlova VV: Differentiation and functional
comparison of monocytes and macrophages from hiPSCs with peripheral
blood derivatives. Stem Cell Reports. 12:1282–1297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Boyette LB, Macedo C, Hadi K, Elinoff BD,
Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG and
Metes DM: Phenotype, function, and differentiation potential of
human monocyte subsets. PLoS One. 12(e0176460)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rios FJ, Touyz RM and Montezano AC:
Isolation and differentiation of human macrophages. Methods Mol
Biol. 1527:311–320. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koh YC, Yang GL, Lai CS, Weerawatanakorn M
and Pan MH: Chemopreventive effects of phytochemicals and medicines
on M1/M2 polarized macrophage role in inflammation-related
diseases. Int J Mol Sci. 19(2208)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol.
877(173090)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mily A, Kalsum S, Loreti MG, Rekha RS,
Muvva JR, Lourda M and Brighenti S: Polarization of M1 and M2 human
monocyte-derived cells and analysis with flow cytometry upon
mycobacterium tuberculosis infection. J Vis Exp. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Zhang Q, Wang Y, Zhai N, Song H, Li H,
Yang Y, Li T, Guo X, Chi B, Niu J, et al: HCV core protein inhibits
polarization and activity of both M1 and M2 macrophages through the
TLR2 signaling pathway. Sci Rep. 6(36160)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chinese Society of Hepatology, Chinese
Medical Association, Wei L, Chinese Society of Infectious Diseases,
Chinese Medical Association, Hou JL. The guideline of prevention
and treatment for hepatitis C: A 2015 update. Zhonghua Gan Zang
Bing Za Zhi. 23:906–923. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Ohlsson SM, Linge CP, Gullstrand B, Lood
C, Johansson A, Ohlsson S, Lundqvist A, Bengtsson AA, Carlsson F
and Hellmark T: Serum from patients with systemic vasculitis
induces alternatively activated macrophage M2c polarization. Clin
Immunol. 152:10–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Graham DB, Stephenson LM, Lam SK, Brim K,
Lee HM, Bautista J, Gilfillan S, Akilesh S, Fujikawa K and Swat W:
An ITAM-signaling pathway controls cross-presentation of
particulate but not soluble antigens in dendritic cells. J Exp Med.
204:2889–2897. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ablin J, Verbovetski I, Trahtemberg U,
Metzger S and Mevorach D: Quinidine and procainamide inhibit murine
macrophage uptake of apoptotic and necrotic cells: A novel
contributing mechanism of drug-induced-lupus. Apoptosis.
10:1009–1018. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tu Z, Hamalainen-Laanaya HK, Nishitani C,
Kuroki Y, Crispe IN and Orloff MS: HCV core and NS3 proteins
manipulate human blood-derived dendritic cell development and
promote Th 17 differentiation. Int Immunol. 24:97–106.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oishi Y and Manabe I: Macrophages in
inflammation, repair and regeneration. Int Immunol. 30:511–528.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wolf AA, Yáñez A, Barman PK and Goodridge
HS: The ontogeny of monocyte subsets. Front Immunol.
10(1642)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fletcher P, Hamilton RF Jr, Rhoderick JF,
Pestka JJ and Holian A: Docosahexaenoic acid impacts macrophage
phenotype subsets and phagolysosomal membrane permeability with
particle exposure. J Toxicol Environ Health A. 84:152–172.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rhee I: Diverse macrophages polarization
in tumor microenvironment. Arch Pharm Res. 39:1588–1596.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ohama H, Asai A, Ito I, Suzuki S,
Kobayashi M, Higuchi K and Suzuki F: M2b macrophage elimination and
improved resistance of mice with chronic alcohol consumption to
opportunistic infections. Am J Pathol. 185:420–431. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Carta T, Razzuoli E, Fruscione F, Zinellu
S, Meloni D, Anfossi A, Chessa B, Dei Giudici S, Graham SP, Oggiano
A and Franzoni G: Comparative phenotypic and functional analyses of
the effects of IL-10 or TGF-β on porcine macrophages. Animals
(Basel). 11(1098)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lurier EB, Dalton D, Dampier W, Raman P,
Nassiri S, Ferraro NM, Rajagopalan R, Sarmady M and Spiller KL:
Transcriptome analysis of IL-10-stimulated (M2c) macrophages by
next-generation sequencing. Immunobiology. 222:847–856.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao SX, Li WC, Fu N, Kong LB, Zhang QS,
Han F, Ren WG, Cui P, Du JH, Wang BY, et al: CD14+
monocytes and CD163+ macrophages correlate with the
severity of liver fibrosis in patients with chronic hepatitis C.
Exp Ther Med. 20(228)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Biswas SK, Chittezhath M, Shalova IN and
Lim JY: Macrophage polarization and plasticity in health and
disease. Immunol Res. 53:11–24. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Knowles LM, Kagiri D, Bernard M, Schwarz
EC, Eichler H and Pilch J: Macrophage polarization is deregulated
in haemophilia. Thromb Haemost. 119:234–245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yao Z, Song X, Cao S, Liang W, Lu W, Yang
L, Zhang Z and Wei L: Role of the exogenous HCV core protein in the
interaction of human hepatocyte proliferation and macrophage
sub-populations. PLoS One. 9(e108278)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gutierrez JA, Lawitz EJ and Poordad F:
Interferon-free, direct-acting antiviral therapy for chronic
hepatitis C. J Viral Hepat. 22:861–870. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Samrat SK, Vedi S, Singh S, Li W, Kumar R
and Agrawal B: Immunization with recombinant adenoviral vectors
expressing HCV core or F proteins leads to T cells with reduced
effector molecules granzyme B and IFN-γ: A potential new strategy
for immune evasion in HCV infection. Viral Immunol. 28:309–324.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhai N, Chi X, Li T, Song H, Li H, Jin X,
Crispe IN, Su L, Niu J and Tu Z: Hepatitis C virus core protein
triggers expansion and activation of CD4(+)CD25(+) regulatory T
cells in chronic hepatitis C patients. Cell Mol Immunol.
12:743–749. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tu Z, Pierce RH, Kurtis J, Kuroki Y,
Crispe IN and Orloff MS: Hepatitis C virus core protein subverts
the antiviral activities of human Kupffer cells. Gastroenterology.
138:305–314. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhai N, Li H, Song H, Yang Y, Cui A, Li T,
Niu J, Crispe IN, Su L and Tu Z: Hepatitis C virus induces
MDSCs-like monocytes through TLR2/PI3K/AKT/STAT3 signaling. PLoS
One. 12(e0170516)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu Y, Wang W, Zou Z, Hu Z, Fan Q and
Xiong J: Hepatitis C virus entry macrophages/monocytes mainly
depends on the phagocytosis of macrophages. Dig Dis Sci.
64:1226–1237. 2019.PubMed/NCBI View Article : Google Scholar
|