1. Introduction
Poor long-term glycemic control is a hallmark of
diabetes mellitus, which increases the risk of complications,
including microvascular complications, such as nerve damage,
diabetic nephropathy and renal failure, as well as macrovascular
complications, such as coronary heart disease, stroke and
peripheral arterial disease (1).
Glycated hemoglobin (Hb)A1c (HbA1c) is the product of the
non-enzymatic interaction between the N-terminal valine of the Hb β
chain and glucose, and it can be used to assess glycemic control
over a period of 2-3 months. Furthermore, since its successful
standardization, HbA1c is currently used to monitor long-term
glycemic control, make treatment decisions and evaluate the risk of
developing complications (2,3).
Since 2010, HbA1c has been used in the diagnosis of diabetes.
Therefore, a glycated hemoglobin value of >6.5% (48 mmol/mol),
is indicative of diabetes (4).
HbA1c levels between 5.7-6.4% (39-46 mmol/mol) indicate that an
individual is at a high risk of developing diabetes (4). The reference range for HbA1c is 4-6%
(20-42 mmol/mol) (2). The National
Glycohemoglobin Standardization Program (NGSP) units or mmol/mol
are different ways with which to express HbA1c [International
Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
units]. The formula used to express the association between % NGSP
HbA1c and IFCC mmol/mol is the following: NGSP=(0.09148 IFCC) +
2.152(5). However, HbA1c values
may be unreliable in particular circumstances. Therefore, the
present review article aimed to summarize and discuss the common
obstacles encountered in determining HbA1c values, as well as their
consequences.
2. Data collection methods
For the purposes of the present review article, a
search of the literature was performed using the PubMed Embase, Web
of Science, Cochrane Library and CNKI databases using the following
search terms: HbA1c methods; HbA1c interferences; HbA1c
interpretation; hemoglobin A, glycosylated; glycated hemoglobin;
hemoglobin variant; fetal hemoglobin; Hb derivatives; carbamylated
hemoglobin; acetylated hemoglobin; aspirin; vitamins C and E;
dapsone; sulfasalazine; HIV; iron deficiency anemia. Language was
limited primarily to English.
3. Biochemistry of hemoglobin A1c
Hb is a tetramer composed of four (two pairs) globin
peptide chains. The common globin peptide chains are termed α, β, δ
and γ. Comprising two α and two β (α2β2) chains, HbA is the most
abundant Hb in adults, accounting for 95-98% of all Hb types. HbF
(α2γ2), which is the most dominant form of Hb found in fetuses, and
HbA2 (α2δ2), accounting for ~2% of total Hb in adults, are the
other forms of normal Hb. In the presence of glucose, amino acids
interact with Hb, which in turn undergoes a non-enzymatic glycation
reaction, a process known as ‘glycation’. There are two steps in
this process. The first step is the reaction of the aldehyde group
of glucose with the NH2 group of the amino acid to form
a Schiff base or aldodiamine, also known as ‘labile HbA1c’ or LA1c.
In the second step, LA1c undergoes the Amadori rearrangement to
form 1-amino-1-deoxyfructose, which contains a more stable and
irreversible ketoamine bond, namely HbA1c. In HbA1c, valine at the
N-terminus of the β chain of Hb is linked to amino-1-deoxyfructose
via a ketoamine linkage. However, the glycation process can also
occur between the N-terminal valine of the α-polypeptide chain and
the ε-amino group of the lysine side chain on the globin peptide
chain (6-8).
It is worth noting that HbA1c does not contain LA1c or α-globin
subunits glycated on the N-terminal valine and α- or β-globin
subunits glycated on lysine side chains. HbA0 refers to
non-glycated Hb or glycated at positions other than the N-terminus
of the β chain. In addition, HbA is also glycated to form HbA1a1,
containing fructose-1,6-bisphosphate, HbA1a2 containing
glucose-6-phosphate and HbA1b containing a pyruvate at the
N-terminal valine (9). Therefore,
the concepts of ‘glycated Hb’ and ‘HbA1c’ need to be clarified.
4. HbA1c detection methods
The most common method for detecting HbA1c levels is
cation/ion-exchange chromatography (IEC). During this method, Hb
molecules are separated based on charge differences. Therefore,
each positively charged ion in the sample interacts with a
negatively charged column, where positively charged Hb molecules
travel slower than negatively charged ones. Eventually, each
component of HbA is eluted at different time points due to charge
differences. Glycated Hb gains an extra negative charge when
glucose attaches to the N-terminal valine of the chain, causing it
to be accelerated in the cation exchange resin and to be eluted
earlier. To measure the concentration of Hb, the area beneath each
peak of the chromatogram is calculated and compared with the
standardized chromatogram using a spectrometer. Currently, IEC
systems can already generate high-resolution separation curves that
can distinguish HbA1c from LA1c and other common variants, such as
Hb S, C and D (10,11).
The boronate affinity chromatography (BAC) method is
often used as a reference method in several studies, since it can
reveal the presence of Hb variants with minimal analytical
interference. BAC is based on the ability of the cis-diol
group of glycosylated Hb to interact and bind with
m-aminophenylboronic acid immobilized on the carrier, in an
alkaline environment (pH >8.0). While other non-glycated Hb
species pass through, the trapped glycated Hb molecules are
released from the filter using an acid reagent. Therefore, Hb can
be divided into two parts, namely the glycated and non-glycated
forms. Finally, the total glycated Hb is converted to %HbA1c
according to an empirical formula. However, as BAC only recognizes
the presence of total glycated Hb, it is unable to detect the
existence of hemoglobin variations (12).
During capillary electrophoresis (CE), which is used
to separate proteins, different protein molecules are encouraged to
move from the anode to the cathode through the capillaries by an
electric field produced using a high-voltage power source. Using
CE, Hb variants are divided based on their rate of diffusion, which
is defined by their charge and mass. Therefore, positively charged
substances migrate through the capillaries more rapidly than
neutral and negatively charged ones. The disadvantage of this
method is that for high efficiency operation, parallel capillaries
are required. Furthermore, the consistency of the results across
all capillaries remains a challenge (12,13).
Immunoassay is an immunoturbidimetric inhibitory
assay that uses antibodies precisely binding to HbA1c by
recognizing the N-terminal glycosylated amino acids. Polyhapten
lectins, synthetic molecules with different HbA1 epitopes,
agglutinate with anti-HbA1c antibodies to create insoluble
antibody-polyhapten complexes. Therefore, a considerable light
scattering is developed via the antibody-polyhapten complexes in
the absence of HbA1c. A soluble antigen-antibody combination is
generated when HbA1c is combined with its corresponding anti-HbA1c
antibody, thus reducing light scattering. Increased %HbA1c
indicates attenuated agglutination reactions. The HbA1c value is
then calculated by dividing the amount of total Hb. In addition,
chemical spectroscopic analysis is used to determine the quantity
of total Hb. However, the aforementioned chemical procedures,
immunoassay and enzymatic analysis, require two independent tests,
namely HbA1c and total Hb assays, which may negatively affect the
analytical quality (11,12).
The enzymatic method is based on the
protease-mediated release of the N-terminal glycosylated valine of
the HbA1c molecule from the blood sample and red blood cell (RBC)
lysate of the patient. Glycosylated valine is oxidized by fructosyl
valine oxidases to generate hydrogen peroxide, which is in turn
used to quantify HbA1c levels. Therefore, the total Hb
concentration is simultaneously determined using an optical method.
Hb variations have no effect on the enzymatic approach in terms of
analysis (12,14).
5. Interfering factors
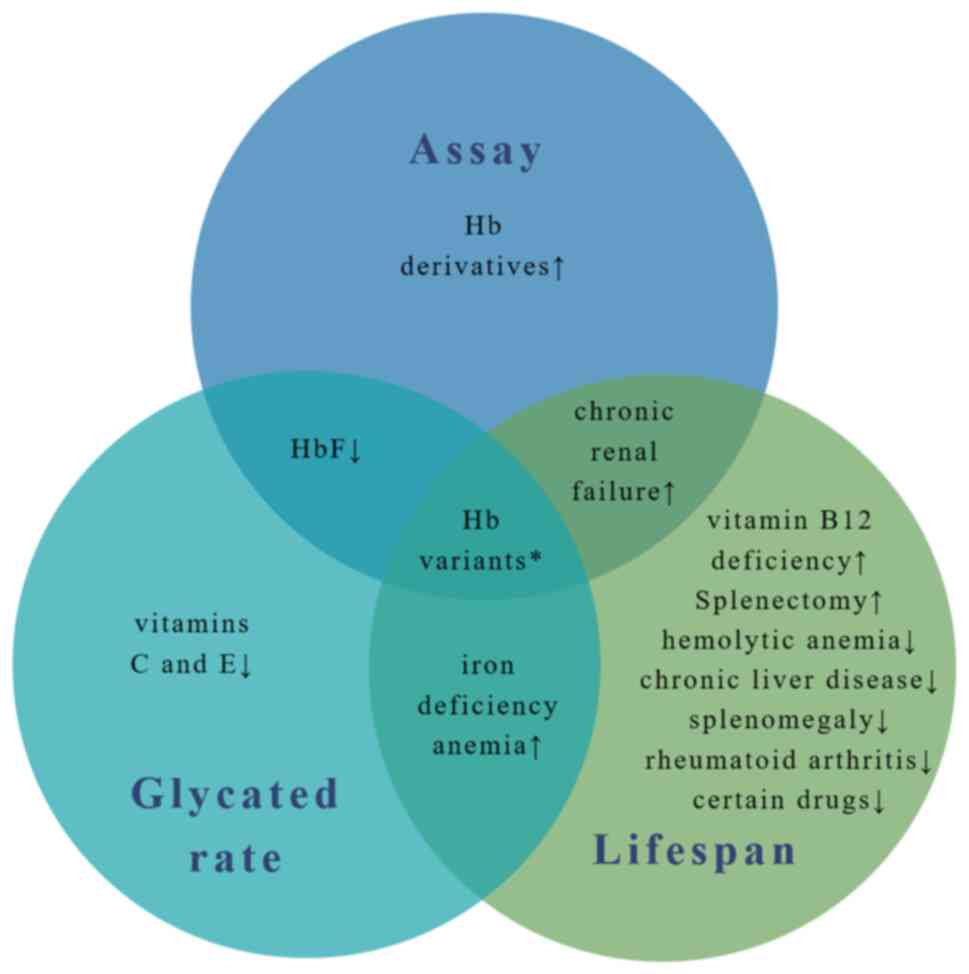
HbA1c assays are mainly affected by three factors:
i) Methodological-specific interference, commonly associated with
the effect of several Hb variants, HbF and Hb derivatives, on the
detection method (15-19);
ii) biochemical effects: For example, an inconsistent glycation
rate of Hb variants and HbA can lead to biased results,
particularly when affinity chromatography is used (20); and iii) abnormal results due to
changes in the life cycle of RBCs. For instance, hemolytic anemia
can result in a reduced RBC lifespan, while iron deficiency anemia
can cause the opposite effect (21-23).
Such factors are summarized in Fig.
1.
Hb variants
Hb variants are a group of prevailing inherited
genetic defects caused by point mutations in the globin gene,
eventually resulting in amino acid substitution (24). Previous studies have demonstrated
that Hb variations can result in HbA1c values that do not
correspond to blood glucose levels in the same patient, while the
degree of interference is dependent on the method used and the
specificity of the variation (15-17,25).
Therefore, for each variant, this interference can be divided into
method-specific, where some, but not all HbA1c determination
methods are affected and variant-specific, where HbA1c levels are
affected by an altered erythrocyte lifespan and glycation rate.
Method-specific interference of Hb variant.
The charge and mass of Hb can change when an amino acid at a
particular location on the globin peptide chain is altered.
Therefore, detection methods based on the physical properties of
Hb, such as IEC and CE, are vulnerable to interference, since
variants interfere with the elution of the peak of interest, thus
resulting in false to glycated Hb values and even invalid HbA1c
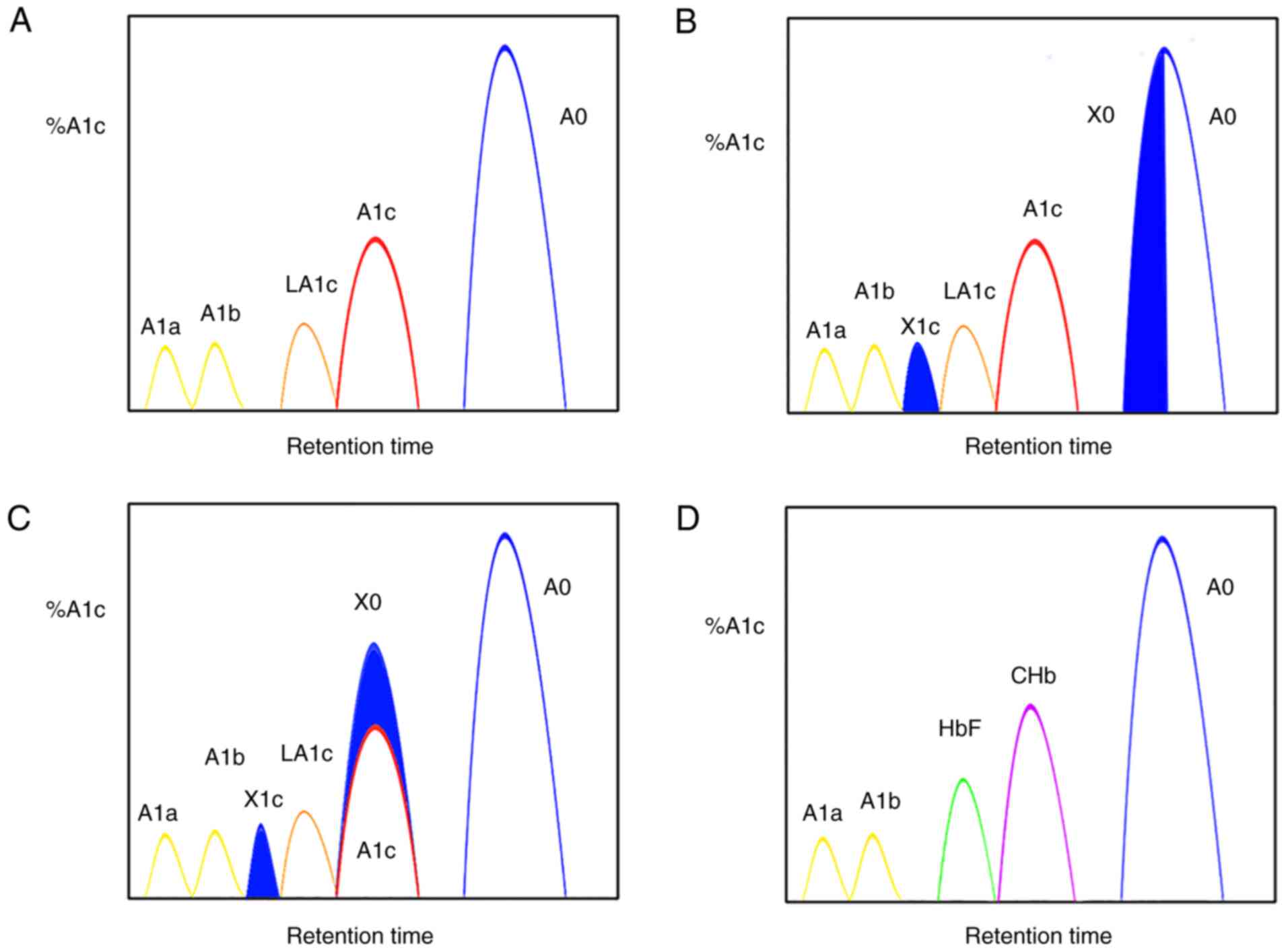
measurements. For the IEC method, the %A1c is measured by excluding
the A1c peak area, divided by the area sum of A1a, A1b, LA1c, A1c,
A0 and HbX (both HbX1c and unmodified HbX) from the calculation.
The effect of Hb variants on IEC is mainly due to the inability of
IEC to completely separate the HbA from HbA1c variants. Therefore,
when the peak of HbA1c cannot be completely separated from that of
HbX, a proportion of HbX value is calculated into that of HbA1c,
thus resulting in falsely elevated HbA1c values (Fig. 2C). Conversely, when HbX is
calculated into the denominator of the formula, a spuriously
reduced HbA1c value is obtained (Fig.
2B). However, the IEC-generated interference can vary in
different systems and is mainly dependent on the position of the
elution peaks of HbX, HbA, HbX1c and HbA1c, and whether or not a
clear separation occurs. IEC, the most commonly used method for
HbA1c detection, is also susceptible to interference from Hb
variants. From another perspective, IEC exhibits more advantages
compared with other methods, since it is easier to identify
abnormal Hb by examining the chromatogram. Therefore, it is of
utmost importance for the laboratory to routinely inspect the
chromatogram prior to issuing the report. In terms of CE, the
reported HbA1c is derived from the ratio (A1c)/(A0+A1c), where A1c
indicates HbA glycated at the N-terminal valine of the β chain and
A0 the not glycated one. Consistent with the IEC method, any
mutations that can cause changes in charge and mass, can
potentially co-migrate with A1c or A0, thus resulting in erroneous
results or even invalid HbA1c measurements. However, CE runs
considerably longer and is more capable of distinguishing between
HbX and HbA, as well as between HbX1c and HbA1c, compared with IEC,
when dealing with the majority of variants. Yun et al
(16) suggested that the changes
in the glycosylation rate can be estimated by comparing the results
of the CE method with those obtained using immunoassay or BAC.
Since immunoassays rely on antibodies that specifically recognize
and bind to the first 4-10 amino acids of the N-terminal of the β
chain, the use of this technique can be limited when bases at this
site are mutated. However, the main disadvantage of the immunoassay
is its inability to distinguish between HbA and HbX (26). By using an immunoassay, %HbA1c is
calculated by dividing A1c + X1c by total HbA + HbX. Therefore,
when HbA is absent or erythrocyte biology is altered, using an
immunoassay may lead to errors in clinically reported HbA1 levels.
This issue is discussed in further detail below. Ideally, repeated
analyses using methods based on different analytical principles
need to be performed, since the effect of a particular Hb variation
on HbA1c readings could be associated with the method sensitivity.
The benefits and challenges of each method are summarized in
Table I.
 | Table IThe advantages and challenges for
each Hb determination method. |
Table I
The advantages and challenges for
each Hb determination method.
| Method | Principle | Advantages | Challenges |
|---|
| IE-HPLC | Separates Hb
species based on charge | Ability to detect
the most Hb variants | Susceptible to
interference from Hb variants, Hb derivatives and HbF |
| Boronate
affinity | Glycohemoglobin
binds affinity resin while non-glycated hemoglobin pass through the
column | Strong
anti-interference ability from Hb variant and Hb adducts | Interfered by rare
Hb variants; unable to detect Hb variants; measures total glycated
Hb; HbF at higher levels |
| Capillary
electrophoresis | Separates Hb
species based on charge and mass | High
chromatographic resolution and resulting ability to detect many Hb
variants | Throughput;
consistency of results across all capillaries |
| Immunoassay | Uses an antibody
targeted against the glycated N-terminus of the β chain | No analytical
interference from the most common Hb variants | Susceptible to
interference from rare Hb variants; unable to detect Hb variants;
HbF at higher levels; two independent tests may affect analytical
quality |
| Enzymatic | Uses an enzyme that
specifically cleaves the N-terminal valine | No analytical
interference from the Hb variants | Unable to detect Hb
variants; two independent tests may affect analytical quality |
Variant-specific erythrocyte lifespan
changes. Interpretation of HbA1c values in terms of glycemic
control is affected by a combination of factors. For example, a
HbA1c value of 7%, generally corresponds to an average plasma
glucose value of 154 mg/dl in the majority of individuals (27). However, for individuals with a
shorter RBC lifespan or higher glycation rates, a HbA1c value of 7%
may be associated with different average glucose levels. A normal
RBC has a lifespan of ~100-115 days on average, although the range
is much wider, ~70-140 days (28).
It has been reported that the levels of A1c are most commonly
affected by the levels of blood glucose over the past 30 days,
accounting for ~50% of the total A1c levels. However, only 10% of
A1c levels are influenced by blood glucose levels from the past
90-120 days (29). When the
glycation phase of RBCs changes, and more specifically their
lifespan, HbA1c can no longer accurately represent glycemic
control. HbS (β6Glu>Val) and HbC (β6 Glu>Lys) are the most
common Hb variants (30,31). It has been demonstrated that ~75%
of patients with sickle cell disease are found in Sub-Saharan
Africa (32). Additionally,
significant prevalence rates have been also recorded in the Middle
East, India and the Mediterranean area (33). In West Africa, the prevalence of
HbC has reached 40-50%, while that in Benin, the United States and
North Africa is estimated to 20, 3 and 1-10%, respectively
(31). Since heterozygous forms of
both variants do not cause hemolytic disease, they cannot,
therefore, affect glycosylation. Sickle cell disease, and more
particularly its homozygous clinically severe condition (HbSS),
where the lifetime of RBCs is decreased to <20 days, is
accompanied by severe hemolysis (12). Therefore, HbA1c values in those
patients need to be interpreted with caution, taking into
consideration factors, such as anemia, an enhanced RBC turnover,
increased blood transfusion needs and increased HbF levels, which
may all have a negative impact on HbA1c as a long-term glycemic
control indicator. Additionally, the heterozygous type of the
disease, HbSC, exhibits the same confounding issues as HbSS when it
comes to determining HbA1c values. However, HbSC causes less severe
anemia compared with sickle cell disease. In a large cohort study
published by Lacy et al (34) in 2017, African-Americans with
sickle trait had a decrease of 0.3% in HbA1c values compared to
those without the sickle trait. In addition, patients with the
sickle trait had a decrease of 0.29% in HbA1c levels at the same
fasting blood glucose levels.
Variant-specific glycation rate alterations.
The biological question is whether the glycation rates of HbA and
Hb variations are equivalent. When they are not equal, the results
of the method used to measure total glycated Hb (affinity
chromatography) may be biased. Therefore, the interpretation of
glycemic control based on the glycated Hb levels results may be
incorrect. Although none of the first codon variants in the
β-globin gene can cause any major clinical condition, such
mutations are of interest due to their potential interference with
co-translational modifications, such as acetylation at the same
site during β-globin synthesis. The type of N-terminal amino acid
can determine the degree of acetylation. Therefore, valine can
substantially inhibit this process, resulting in the slight
acetylation of α- and β-globin. However, it has been reported that
the N-terminal glycine of γ-globin is less inhibitory, thus leading
to ~15% acetylation (35). A
previous study demonstrated that in Hb Raleigh (β1Val>Ala), the
N-terminal amino acid of its β chain could be substituted to
produce acetylated alanine, thus producing a large amount of
acetylated Hb, which could not be glycosylated normally (36). It was hypothesized that the
glycation process occurred close to the N-terminal valine and the
140th amino acid in the β chain (37). Mutants located near valine at the
N-terminus of the β chain could cause changes in the glycation
rate, such as reduced Hb Görwihl (β5Pro→Ala) levels, thus
suggesting attenuated glycation reaction (38). Hb Himeji (β140Ala→Asp), a rare
variant occurring on the β chain, is characterized by an enhanced
glycation (37). In heterozygous
carriers, HbA1c values determined by immunoassay or BAC have been
found to be significantly higher compared with those determined
using cation exchange chromatography (37). Mutations in this region can either
increase or decrease glycosylation. For example, a previous study
demonstrated that individuals with Hb Sagami (β139Asn→Lys)
exhibited low HbA1c levels, as assessed using immunoassay, thus
indicating a reduction in the glycation response (39). Glycosylation rates can even have an
effect on HbA1c measurements of more common variants, such as those
associated with sickle traits. Therefore, the glycosylation rate of
βS appears to be higher than that of βA.
However, Kabytaev et al (40) concluded that the clinical
interpretation was relatively unaffected by the tiny net difference
between HbAS (sickle phenotype) and uncharacterized total
glycosylation. A summary of the altered glycosylation rates
presented in mutants located in the first 10 amino acids of the β
chain and at amino acid positions 139-140 is presented in Table II (41-44).
 | Table IIHb mutations relative to the altered
glycosylation rates for the first 10 amino acids of the β chain and
at amino acid positions 139-140. |
Table II
Hb mutations relative to the altered
glycosylation rates for the first 10 amino acids of the β chain and
at amino acid positions 139-140.
| Name | Mutation | Clinical
significance | Glycation rate |
|---|
| Hb Niigata | β1Val>Leu | Clinically
silent | ↓ |
| Hb South
Florida | β1Val>Met | Heterozygote
clinically silent | ↓ |
| Hb Raleigh | β1Val>Ala | Heterozygote
clinically silent | ↓ |
| Hb Görwihl | β5Pro>Ala | Heterozygote
clinically silent | ↓ |
| Hb Tyne | β5Pro>Ser | Heterozygote
clinically silent | ↓ |
| Hb
Aix-les-Bains | β5Pro>Leu | Heterozygote
clinically silent | ↓ |
| Hb Sagami | β139Asn>Ly | produced
β-thalassemia carrier phenotype when combined with
β+-thalassemia allele | ↓ |
| Hb Himeji | β140Ala>Asp | Heterozygote
clinically silent | ↑ |
HbF
Elevated HbF can cause related problems. Certain
thalassemias and genetic persistence of fetal Hb (HPFH) can lead to
elevated HbF. Increased HbF levels can be also caused by
hydroxyurea, a medication commonly used to treat sickle cell
disease and several hematological malignancies (45). In the IEC measurement method, the
HbF and A1c peaks are eluted adjacently. Therefore, high HbF may
overlap and distort the shape of the A1c peak, thus resulting in
falsely high values (Fig. 2D).
Elevated HbF can also cause other effects in immunoassay and BAC
(10,18). This interference could be caused by
the slower rate of glycation compared with that of HbA. HbF lacks
the β chain and encompasses glycine instead of valine at the
N-terminus of the γ chain. HbF can only be glycosylated on the
lysine residue at the N-terminus of α chain. A previous study
showed that the rate of glycosylation at the N-terminus of the α
chain was indeed 8-10 times lower compared with that of the β
chain's N-terminus (6). Therefore,
for the boronate affinity assay, the glycation fraction in
individuals with elevated HbF levels would be lower compared with
those without increased HbF value due to the lower degree of
glycation of HbF. During immunoassay, HbA1c antibodies cannot bind
with the glycated portion of HbF and, therefore, total Hb
measurements also include HbF value, eventually leading to a
significant reduction in the measured HbA1c. From a clinical
perspective, in patients with HbF levels of <20%, the use of
immunoassays or BAC could reduce HbA1c levels by 1-2% (18). The Diabetes Control and
Complications Trial clearly showed that a 1% reduction in HbA1c
levels was equivalent to a reduction in the risk of diabetes
complications by approximately 30% (2). A spurious reduction of 1-2% in HbA1c
value could result to undertreatment of hyperglycemia, which in
turn could lead to a significantly increased risk of
complications.
Hb derivatives
When separation techniques based on charge
differences are utilized, HbA1c measurements may also be affected
by the chemical changes of Hb, which may physically and chemically
imitate HbA1c, thus leading to an incorrect assessment of HbA1c.
Carbamylated Hb is more common in uremic patients and is the most
common derivative (46). This is
due to the decomposition of urea nitrogen into ammonia and cyanate
in the body. In turn, cyanate is protonated to form isocyanic acid,
which combines with the α and ε amino groups of proteins to form
carbamoyl moieties (46,47). Valine at the N-terminus of the Hb β
chain reacts specifically with isocyanic acid to form stable
carbamyl-Hb (CHb). The isoelectric points of CHb and HbA1c are
similar. Therefore, the peak times of both CHb and HbA1c are close
in the IEC system based on the detection principle of Hb species
with different charges. When CHb reaches a certain concentration,
the peak time of LA1c/CHb is delayed or the peak shape increases,
thus resulting in the overlap of the LA1c/CHb peak with that of
HbA1c. The overlap increases with the enhanced CHb concentration
(Fig. 2D). If the peak is large,
depending on the system, it can lead to biased results in the HbA1c
levels (either increase or decrease) (19,48).
In vitro, the carbamylation of Hb at concentrations up to
5.4% can result in erroneous readings in glycated Hb levels, when
different cation-exchange techniques are used (19). However, studies evaluating the
in vivo effects of carbamoyl Hb have revealed several
differences, ranging from insignificant to significant (49-51).
The studies by Little et al (50) and Dolscheid-Pommerich et al
(51) demonstrated that the
effects of CHb were statistically, yet not clinically significant.
However, the assessment of HbA1c in this population should be
always performed using the same measuring technique to ensure
longitudinal comparability and produce comparable readings.
Furthermore, the association between chronic kidney disease (CKD)
and A1c is complex. Therefore, previous studies have demonstrated
that patients with CKD exhibit lower levels of erythropoietin,
possible increased glycation and enhanced carbamylated Hb levels,
while dialysis in such patients may shorten the RBC lifespan and
decrease A1c levels (50-53).
The study by Little et al (50), comparing the levels of glycated
albumin (GA) with HbA1c in patients with chronic renal failure,
demonstrated that the levels of HbA1c in such patients were
decreased by ~1.5% compared with those of GA. Additionally, the
results of a clinical trial revealed that the treatment of 15
individuals with type 2 diabetes mellitus (T2DM) and CKD (3B/4)
with erythropoietin resulted in a clinically meaningful decrease of
~0.7% in HbA1C readings (52).
Therefore, HbA1c testing should be interpreted cautiously in
patients with renal failure. Long-term aspirin use can also induce
the acetylation of Hb, thus resulting in falsely elevated levels of
HbA1c, due to its interference with some of the assays used
(54,55). More specifically, aspirin promotes
the acetylation of Hb to change its surface charge. In turn, this
acetylated product can co-migrate with A1c, eventually leading to a
falsely elevated HbA1c value (56). Although the exposure of normal Hb
to aspirin in vitro results in falsely high acetylated Hb
values, as verified using different cation-exchange methods, the
results of in vivo studies have contradicted this finding
(56,57). Camargo et al (56) demonstrated that treatment with
lower doses of aspirin (200 mg/day) for 4 months did not result in
a clinically relevant increase in HbA1c levels. In clinical
practice, the effect of aspirin on HbA1c levels could not be
palpable until long-term and high-dose therapy (55). However, it has been reported that
the BAC method is not affected by carbamylated and acetylated Hb
(50,57).
Drugs
High doses of vitamins C and E have also been shown
to be associated with decreased A1c levels mediated by the
inhibition of Hb glycosylation. Vitamin C can form ionic bonds with
several biomolecules. In turn, ionic interactions of ascorbate and
its free radical with proteins can affect the reactivity of
molecular complexes via altering the local redox potentials and
charge transfer reactions. It has been suggested that the potential
for non-enzymatic glycosylation is reduced when vitamin C reacts
directly with the glucose-binding site (lysine residue) (58). A previous study revealed that
vitamin E supplements could prevent the glycosylation of Hb by
blocking glycation in the early stages of the Maillard reaction or
by partially preventing the development of advanced glycosylation
end-products (59). However, the
inhibition of Hb glycosylation by vitamins C and E is clinically
controversial. An in vitro study demonstrated that vitamins
C and E could prevent the development of protein glycosylation
(60). However, the outcomes of
in vivo experiments are contradictory with the
aforementioned finding. While Ceriello et al (61) demonstrated the inverse association
between vitamin E consumption and glycated Hb levels, a later
meta-analysis (59) was unable to
reveal a discernible difference. However, further subgroup analysis
revealed that following vitamin E supplementation, HbA1c was
noticeably reduced in patients with T2DM in the group with low
vitamin E levels. However, the small datasets used in this subgroup
analysis, suggested that further research should be conducted to
support this conclusion (59).
Likewise, conflicting information on vitamin C intake and glycated
Hb levels can be found in the literature (56,62,63).
Additionally, other drugs, such as dapsone, sulfasalazine,
antiretrovirals and ribavirin can increase the hemolysis rate, thus
reducing glycated Hb levels (64-68).
In a retrospective review of 49 individuals with T2DM and Hansen's
illness, 35 patients (71%) had HbA1c readings lower than the mean
blood glucose levels (65). At the
same fasting blood glucose concentration, the HbA1c discordant
group had a significantly lower hemoglobin A1c value (mean HbA1c,
4.4±1.8%) compared with the HbA1c consistent group (mean HbA1c,
7.9±2.1%). During the first 3 months of dapsone therapy, the HbA1c
levels decreased considerably (65).
Illness-related factors
Particular pathologies can alter the lifespan of
RBCs, thus affecting the HbA1c levels. The average lifetime of RBC
increases when erythropoiesis is suppressed, due to the lack of
iron and vitamin B12, leading to high HbA1c levels (21-23,69).
Kim et al (70)
demonstrated that women with an iron deficiency without anemia
(n=1,150) exhibited a small increase in HbA1c levels (<5.5% to
≥5.5%), independent of fasting glucose levels. In another study,
comparing the use of both HbA1c and fasting blood glucose as
diagnostic criteria, Attard et al (71) demonstrated that males with an iron
deficiency alone or with iron deficiency anemia (IDA) exhibited a
greater relative risk of developing pre-diabetes. However, other
studies have yielded inconclusive results. According to a
meta-analysis, IDA and iron deficiency had no effect on the HbA1c
levels (72). In addition,
patients with IDA have been found to have a higher glycation rate,
which may be due to the higher malondialdehyde levels, a lipid
peroxidation metabolite, observed in this population, thus
enhancing Hb glycation (73,74).
Additionally, it has been reported that splenectomy can promote RBC
survival, thus enhancing glycated Hb levels (75). Conversely, a decrease in the mean
age of RBCs can reduce glycated Hb levels. However, in the absence
of fibrosis and splenomegaly, this can be observed in chronic liver
disease, although the cause remains unknown (76). Furthermore, splenomegaly and
rheumatoid arthritis can also increase the rate of hemolysis, thus
resulting in a decrease in glycated Hb value (77).
Age and race
It has been reported that HbA1c levels can be
affected by age and race. Previous studies have indicated that
after the age of 30, the glycated Hb value can be increased by
~0.1% every 10 years (78,79). The effects of race on HbA1c values
are controversial, however. Accumulating evidence has suggested
that differences in HbA1c levels can be observed among different
races. Therefore, several studies have demonstrated that African
Americans and Hispanics have higher glycated Hb levels compared
with Caucasians at the same blood glucose levels (79-81).
Additionally, Selvin et al (82) demonstrated that the mean HbA1c
value of African Americans was 0.4% higher compared with that of
non-Hispanic whites. However, race did not alter the association
between HbA1c concentrations and adverse cardiovascular outcomes or
mortality (82).
6. Overview
In summary, various factors influence the
determination of HbA1c values. These are discussed below and are
summarized in Table III.
 | Table IIISummary of the influence of various
interference factors on the determination of HbA1c values. |
Table III
Summary of the influence of various
interference factors on the determination of HbA1c values.
| Author, year of
publication | Variable | Influencing
factor | Effect upon
HbA1c | Comment | (Refs.) |
|---|
| Lacy et al,
2017 | Sickle trait | L | Possible decrease
of 0.3% | Used only to assess
long-term glycemic control and should use the same measurement
technique to ensure longitudinal comparability | (34) |
| Harris et
al, 2021 | Sickle disease | L | Unable to utilize
due to ↓↓ RBC lifespan clinically | Consider the use of
additional biomarkers | (9) |
| Rohlfing et
al, 2008 | ↑↑ HbF to
15-30% | M&G | ↓ Hb A1c of 1-2%
and/or distorted chromatogram | Consider the use of
additional biomarkers | (18) |
| Little et
al, 2013; Dolscheid-Pommerich et al, 2015 | CHb (when eGFR
<11 ml/min or BUN >80 mg/dl) | M | Statistically but
not clinically significant bias in HbA1c | Other issues with
CKD need to be considered (see below) | (50,51) |
| Little et
al, 2013; Ng et al, 2010 | CKD (stages
4-5) | M&L | Possible decrease
of 0.7-1.5% after treatment | Stages 4-5 do not
use HbA1c | (50,52) |
| Xu et al,
2014 | Vitamin E (>400
mg/day) | G | Possible decrease
of 0.35% | Insufficient
evidence at present, further research required | (59) |
| Basavaraj et
al, 2022 | Dapsone | L | Clinically
significant reduction in HbA1 | Consider the use of
additional biomarkers | (65) |
| Kim et al,
2010 | Iron
deficiency | L&G | Statistically, but
not clinically significant increase in HbA1c | Consider the use of
additional biomarkers until red cell indices become stable | (70) |
i) The effect of rare Hb variations, often observed
in various national populations, on HbA1c measurement varies, as
the prevalence and types of Hb variants also vary across different
nations. A study on 114 patients with rare Hb variants, common in
the Korean population revealed that the proportion of
‘unacceptable’ HbA1c results (relative bias, >±7%) in samples
significantly varied depending on the assay used. More
specifically, the rates recorded were 16% for CE (17/109 patients),
7% for immunoassay (8/114 patients), 51% for IEC1 (58/114
patients), 80% for IEC2 (108/114 patients) and 89% for IEC3 (66/74
patients). CE and IEC3 assays yielded no results, in 5 and 40
samples with Hb variations, respectively. However, IEC revealed a
considerable deviation compared with immunoassay and CE (16).
ii) Another study demonstrated that
African-Americans with sickle phenotype exhibited somewhat
decreased the HbA1c readings, while having an apparently normal RBC
lifetime (34). As shown in
Table II, the variations with
different glycation rates were mostly clustered at positions 1, 5,
139 and 140. Therefore, greater caution should be taken for the
changes in the glycation rate in the presence of variations with
alterations in these two locations.
iii) The association between CKD and A1c is complex
(50-53).
HbA1c can be still used to track therapy in patients with stage 1-3
CKD. However, various biomarkers need to be employed in patients
suffering from stage 4/5 CKD or in those treated with
erythropoietin (50,52).
iv) Vitamins C and E can block Hb glycation over
time, thus reducing A1c levels. However, this result is not always
observed (59-63).
In addition, it has been reported that acetylated Hb does not
interfere with the measurement of HbA1c under the conventional
dosage of aspirin (56). Other
drugs, such as sulfasalazine, antiretroviral drugs and ribavirin
have been less studied in recent years (66-68).
Therefore, caution should be exercised when applying HbA1c to this
population.
v) The threshold value of HbA1c for the diagnosis of
hyperglycemia associated with T2DM in patients with IDA remains
debatable. Misinterpretation may result in misdiagnosis or
under-diagnosis, thus having severe implications (83).
7. Conclusions and future perspectives
Over the past 30 years, there has been a marked
increase in the prevalence of diabetes worldwide. Diabetes affects
~420 million individuals worldwide, accounting for >6% of the
world's population (84). The
self-monitoring of blood glucose and the measurement of HbA1c
levels are essential for the management of diabetes. Glycated Hb
testing has become straightforward and convenient, since it does
not require overnight fasting or the ingestion of a standard
glucose dose and it can be performed at any time of the day.
However, the ease of measuring HbA1c belies its biochemical
complexity. It is widely accepted that HbA1c is a hematological
parameter whose interpretation is affected by numerous factors,
including methodology, clinical biochemistry and hematological
factors. Any sequence that affects the globin polypeptide chain,
the biochemical properties of erythrocytes and their lifespan may
interfere with HbA1c values. Therefore, the more accurate recording
of HbA1c levels could enhance the effective management of diabetes.
Laboratories need to be aware of common HbA1c assay interferences,
that should be taken into consideration when glycated Hb testing
does not match clinical perceptions or other metabolic indicators.
For cases suspected of possible interference, and particularly for
factors involving both methodological interference and altered RBC
properties, HbA1c levels need to be determined using instruments
with different analytical principles to obtain more useful clinical
information. Clinicians need to be advised of the limitations of
HbA1c testing used in patients, particularly as regards analytical
interference or changes in RBC properties, in order to help them
better understand HbA1c. For any situation that can cause an
abnormal lifespan of RBCs, the American Diabetes Association (ADA)
has recommended the use of glucose criteria for the diagnosis of
diabetes (85). When the
interpretation of HbA1c is negatively affected by factors affecting
erythrocyte lifespan and their glycation rate, alternative
non-Hb-based tests, such as GA and serum fructosamine should be
used to assess long-term blood glucose levels. However, clinicians
need to be aware that GA and serum fructosamine can only assess
plasma glucose levels of the previous 2 weeks (86).
The present review article summarized the
interference of glycated hemoglobin detection in terms of
methodology, glycation rate, and erythrocyte lifespan. To better
grasp the principle of diverse interference factors, the
biochemical concept and typical HbA1c detection methods were
briefly described at the beginning of the manuscript. The
methodological component explains the aspects influencing HbA1c
detection, whereas the RBC biological properties (glycation rate
and erythrocyte lifespan) summarize the factors influencing
glycated hemoglobin interpretation. However, unlike Campbell et
al (87), the authors consider
that certain factors (such as hemoglobinopathies, HbF, IDA and
chronic renal disease) can have numerous effects. In
hemoglobinopathies, for example, there may be changes in the
erythrocyte lifespan and/or glycation rate, while the globin
peptide chain varies. Rather than merely categorizing
hemoglobinopathies based on RBC longevity, as Campbell et al
(87). A summary of the factors
influencing HbA1c values is illustrated in Fig. 1. In addition, hemoglobinopathies
are a common glycated hemoglobin interference factor, a large part
of which are asymptomatic. They frequently appear when
chromatograms and/or HbA1c levels are abnormal (16,88).
Therefore, the present review mainly discussed the interference of
hemoglobinopathies in an effort to remind laboratories to consider
hemoglobinopathies when they meet abnormal HbA1c readings,
disparities between blood glucose and HbA1c levels, and abnormal
chromatograms. The present review also summarized the changes in
the glycation rate caused by rare hemoglobinopathies at some loci,
which are rare in the literature.
Acknowledgements
The authors would like to thank Dr Hongjin Shi
(Department of Urology, Kunming Medical University, Kunming, China)
for providing valuable comments on the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZC and TZ were involved in the conception of the
study and in data interpretation, as well as in the writing and
critical revision of the manuscript. LS and MJ wrote the
manuscript. BM and XB were involved in the conception of the study,
and in the design of the figures. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Emerging Risk Factors Collaboration.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio
E, Ingelsson E, Lawlor DA, Selvin E, et al: Diabetes mellitus,
fasting blood glucose concentration, and risk of vascular disease:
A collaborative meta-analysis of 102 prospective studies. Lancet.
375:2215–2222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Diabetes Control and Complications Trial
Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford
O, Davis M, Rand L and Siebert C: The effect of intensive treatment
of diabetes on the development and progression of long-term
complications in insulin-dependent diabetes mellitus. N Engl J Med.
329:977–986. 1993.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Intensive blood-glucose control with
sulphonylureas or insulin compared with conventional treatment and
risk of complications in patients with type 2 diabetes (UKPDS 33).
UK prospective diabetes study (UKPDS) group. Lancet. 352:837–853.
1998.PubMed/NCBI
|
|
4
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2021. Diabetes Care. 44 (Suppl 1):S15–S33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weykamp C, John WG, Mosca A, Hoshino T,
Little R, Jeppsson JO, Goodall I, Miedema K, Myers G, Reinauer H,
et al: The IFCC reference measurement system for HbA1c: A 6-year
progress report. Clin Chem. 54:240–248. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bunn HF: Evaluation of glycosylated
hemoglobin diabetic patients. Diabetes. 30:613–617. 1981.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mossine VV and Mawhinney TP:
1-Amino-1-deoxy-D-fructose (‘fructosamine’) and its derivatives.
Adv Carbohydr Chem Biochem. 64:291–402. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shapiro R, McManus MJ, Zalut C and Bunn
HF: Sites of nonenzymatic glycosylation of human hemoglobin A. J
Biol Chem. 255:3120–3127. 1980.PubMed/NCBI
|
|
9
|
Harris NS, Weaver KD, Beal SG and Winter
WE: The interaction between Hb A1C and selected genetic factors in
the African American population in the USA. J Appl Lab Med.
6:167–179. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weykamp C: HbA1c: A review of analytical
and clinical aspects. Ann Lab Med. 33:393–400. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ang SH, Thevarajah M, Alias Y and Khor SM:
Current aspects in hemoglobin A1c detection: A review. Clin Chim
Acta. 439:202–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rhea JM and Molinaro R: Pathology
consultation on HbA(1c) methods and interferences. Am J Clin
Pathol. 141:5–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jaisson S, Leroy N, Meurice J, Guillard E
and Gillery P: First evaluation of Capillarys 2 Flex
Piercing® (Sebia) as a new analyzer for HbA1c assay by
capillary electrophoresis. Clin Chem Lab Med. 50:1769–1775.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Teodoro-Morrison T, Janssen MJ, Mols J,
Hendrickx BH, Velmans MH, Lotz J, Lackner K, Lennartz L, Armbruster
D, Maine G and Yip PM: Evaluation of a next generation direct whole
blood enzymatic assay for hemoglobin A1c on the ARCHITECT c8000
chemistry system. Clin Chem Lab Med. 53:125–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Little RR, Rohlfing CL, Hanson S, Connolly
S, Higgins T, Weykamp CW, D'Costa M, Luzzi V, Owen WE and Roberts
WL: Effects of hemoglobin (Hb) E and HbD traits on measurements of
glycated Hb (HbA1c) by 23 methods. Clin Chem. 54:1277–1282.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yun YM, Ji M, Ko DH, Chun S, Kwon GC, Lee
K, Song SH, Seong MW, Park SS and Song J: Hb variants in Korea:
Effect on HbA1c using five routine methods. Clin Chem Lab Med.
55:1234–1242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu A, Chen W, Xia Y, Zhou Y and Ji L:
Effects of common hemoglobin variants on HbA1c measurements in
China: Results for α- and β-globin variants measured by six
methods. Clin Chem Lab Med. 56:1353–1361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rohlfing CL, Connolly SM, England JD,
Hanson SE, Moellering CM, Bachelder JR and Little RR: The effect of
elevated fetal hemoglobin on hemoglobin A1c results: Five common
hemoglobin A1c methods compared with the IFCC reference method. Am
J Clin Pathol. 129:811–814. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chachou A, Randoux C, Millart H, Chanard J
and Gillery P: Influence of in vivo hemoglobin carbamylation on
HbA1c measurements by various methods. Clin Chem Lab Med.
38:321–326. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weykamp C, Kemna E, Leppink S and
Siebelder C: Glycation rate of haemoglobins S, C, D, E, J and G,
and analytical interference on the measurement of HbA1c with
affinity chromatography and capillary electrophoresis. Clin Chem
Lab Med. 53:e207–e210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Coban E, Ozdogan M and Timuragaoglu A:
Effect of iron deficiency anemia on the levels of hemoglobin A1c in
nondiabetic patients. Acta Haematol. 112:126–128. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koga M, Morita S, Saito H, Mukai M and
Kasayama S: Association of erythrocyte indices with glycated
haemoglobin in pre-menopausal women. Diabet Med. 24:843–847.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Son JI, Rhee SY, Woo JT, Hwang JK, Chin
SO, Chon S, Oh S, Kim SW and Kim YS: Hemoglobin a1c may be an
inadequate diagnostic tool for diabetes mellitus in anemic
subjects. Diabetes Metab J. 37:343–348. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thom CS, Dickson CF, Gell DA and Weiss MJ:
Hemoglobin variants: Biochemical properties and clinical
correlates. Cold Spring Harb Perspect Med.
3(a011858)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dessi M, Pieri M, Pignalosa S, Martino FG
and Zenobi R: Performances of capillary electrophoresis and HPLC
methods in HbA1c determination: Diagnostic accuracy in HbS and
HbD-Iran variants' presence. J Clin Lab Anal. 29:57–60.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Suo M, Wen D, Wang W, Zhang D, Xu S, Wang
X and Hu T: False measurement of glycated hemoglobin in patients
without hemoglobin A. Biosci Rep. 39(BSR20180128)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nathan DM, Kuenen J, Borg R, Zheng H,
Schoenfeld D and Heine RJ: A1c-Derived Average Glucose Study Group.
Translating the A1C assay into estimated average glucose values.
Diabetes Care. 31:1473–1478. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Franco RS: Measurement of red cell
lifespan and aging. Transfus Med Hemother. 39:302–307.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Goldstein DE, Little RR, Lorenz RA, Malone
JI, Nathan D, Peterson CM and Sacks DB: Tests of glycemia in
diabetes. Diabetes Care. 27:1761–1773. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Piel FB, Howes RE, Patil AP, Nyangiri OA,
Gething PW, Bhatt S, Williams TN, Weatherall DJ and Hay SI: The
distribution of haemoglobin C and its prevalence in newborns in
Africa. Sci Rep. 3(1671)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ouzzif Z, El Maataoui A, Oukhedda N,
Messaoudi N, Mikdam M, Abdellatifi M and Doghmi K: Hemoglobinosis C
in Morocco : A report of 111 cas. Tunis Med. 95:229–233.
2017.PubMed/NCBI
|
|
32
|
Kato GJ, Piel FB, Reid CD, Gaston MH,
Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA,
Weatherall DJ, Costa FF and Vichinsky EP: Sickle cell disease. Nat
Rev Dis Primers. 4(18010)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Piel FB, Steinberg MH and Rees DC: Sickle
cell disease. N Engl J Med. 376:1561–1573. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lacy ME, Wellenius GA, Sumner AE, Correa
A, Carnethon MR, Liem RI, Wilson JG, Sacks DB, Jacobs DR Jr, Carson
AP, et al: Association of sickle cell trait with hemoglobin A1c in
African Americans. JAMA. 317:507–515. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Qiu CC, Kasten-Jolly J and Abraham EC:
Human red cell acetyltransferase. Life Sci. 42:2739–2748.
1988.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen D, Crimmins DL, Hsu FF, Lindberg FP
and Scott MG: Hemoglobin Raleigh as the cause of a falsely
increased hemoglobin A1C in an automated ion-exchange HPLC method.
Clin Chem. 44:1296–1301. 1998.PubMed/NCBI
|
|
37
|
Koga M, Inada S, Shimizu S, Hatazaki M,
Umayahara Y and Nishihara E: Aldimine formation reaction, the first
step of the maillard early-phase reaction, might be enhanced in
variant Hemoglobin, Hb Himeji. Ann Clin Lab Sci. 45:643–649.
2015.PubMed/NCBI
|
|
38
|
Bissé E, Schauber C, Zorn N, Epting T,
Eigel A, Van Dorsselaer A, Wieland H, Kister J and Kiger L:
Hemoglobin Görwihl [alpha2beta(2)5(A2)Pro->Ala], an
electrophoretically silent variant with impaired glycation. Clin
Chem. 49:137–143. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nakanishi T, Miyazaki A, Kishikawa M,
Shimizu A, Aoki K and Kikuchi M: Hb Sagami [beta
139(H17)Asn->Thr]: A new hemoglobin variant not detected by
isoelectrofocusing and propan-2-ol test, was detected by
electrospray ionization mass spectrometry. J Mass Spectrom.
33:565–569. 1998.
|
|
40
|
Kabytaev K, Connolly S, Rohlfing CL, Sacks
DB, Stoyanov AV and Little RR: Higher degree of glycation of
hemoglobin S compared to hemoglobin A measured by mass
spectrometry: Potential impact on HbA1c testing. Clin Chim Acta.
458:40–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Watanabe T, Kato K, Yamada D, Midorikawa
S, Sato W, Shiga M, Otsuka Y, Miura M, Harano K and Harano T: A
nondiabetic case of hemoglobin variant (Hb Niigata) with
inappropriately high and low HbA1c titers detected by different
methods. Clin Chem. 44:1562–1564. 1998.PubMed/NCBI
|
|
42
|
Shah SC, Malone JI, Boissel JP and Kasper
TJ: Hemoglobin South Florida. New variant with normal
electrophoretic pattern mistaken for glycosylated hemoglobin.
Diabetes. 35:1073–1076. 1986.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Langdown JV, Williamson D, Beresford CH,
Gibb I, Taylor R and Deacon-Smith R: A new beta chain variant, Hb
Tyne [beta 5(A2)Pro->Ser]. Hemoglobin. 18:333–336.
1994.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Joly P, Garcia C, Lacan P, Couprie N and
Francina A: Two new hemoglobin variants: Hb Aix-Les-Bains
[β5(A2)Pro→Leu; HBB:c.17 C>T] and Hb Dubai [α122(H5)His→Leu
(α2); HBA2:c.368 A>T]. Hemoglobin. 35:147–151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Karsegard J, Wicky J, Mensi N, Caulfield A
and Philippe J: Spurious glycohemoglobin values associated with
hydroxyurea treatment. Diabetes Care. 20:1211–1212. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bry L, Chen PC and Sacks DB: Effects of
hemoglobin variants and chemically modified derivatives on assays
for glycohemoglobin. Clin Chem. 47:153–163. 2001.PubMed/NCBI
|
|
47
|
Stim J, Shaykh M, Anwar F, Ansari A,
Arruda JA and Dunea G: Factors determining hemoglobin carbamylation
in renal failure. Kidney Int. 48:1605–1610. 1995.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jaisson S, Leroy N, Guillard E, Desmons A
and Gillery P: Analytical performances of the D-100TM hemoglobin
testing system (Bio-Rad) for HbA1c assay. Clin Chem Lab Med.
53:1473–1479. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Weykamp CW, Miedema K, de Haan T and
Doelman CJ: Carbamylated hemoglobin interference in glycohemoglobin
assays. Clin Chem. 45:438–440. 1999.PubMed/NCBI
|
|
50
|
Little RR, Rohlfing CL, Tennill AL, Hanson
SE, Connolly S, Higgins T, Wiedmeyer CE, Weykamp CW, Krause R and
Roberts W: Measurement of Hba(1C) in patients with chronic renal
failure. Clin Chim Acta. 418:73–76. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dolscheid-Pommerich RC, Kirchner S, Weigel
C, Eichhorn L, Conrad R, Stoffel-Wagner B and Zur B: Impact of
carbamylation on three different methods, HPLC, capillary
electrophoresis and TINIA of measuring HbA1c levels in patients
with kidney disease. Diabetes Res Clin Pract. 108:15–22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ng JM, Cooke M, Bhandari S, Atkin SL and
Kilpatrick ES: The effect of iron and erythropoietin treatment on
the A1C of patients with diabetes and chronic kidney disease.
Diabetes Care. 33:2310–2313. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kilpatrick ES and Atkin SL: Using
haemoglobin A(1c) to diagnose type 2 diabetes or to identify people
at high risk of diabetes. BMJ. 348(g2867)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bridges KR, Schmidt GJ, Jensen M, Cerami A
and Bunn HF: The acetylation of hemoglobin by aspirin. In vitro and
in vivo. J Clin Invest. 56:201–207. 1975.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Unnikrishnan R, Anjana RM and Mohan V:
Drugs affecting HbA1c levels. Indian J Endocrinol Metab.
16:528–531. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Camargo JL, Stifft J and Gross JL: The
effect of aspirin and vitamins C and E on HbA1c assays. Clin Chim
Acta. 372:206–209. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Weykamp CW, Penders TJ, Siebelder CW,
Muskiet FA and van der Slik W: Interference of carbamylated and
acetylated hemoglobins in assays of glycohemoglobin by HPLC,
electrophoresis, affinity chromatography, and enzyme immunoassay.
Clin Chem. 39:138–142. 1993.PubMed/NCBI
|
|
58
|
Davie SJ, Gould BJ and Yudkin JS: Effect
of vitamin C on glycosylation of proteins. Diabetes. 41:167–173.
1992.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu R, Zhang S, Tao A, Chen G and Zhang M:
Influence of vitamin E supplementation on glycaemic control: A
meta-analysis of randomised controlled trials. PLoS One.
9(e95008)2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jain SK and Palmer M: The effect of oxygen
radicals metabolites and vitamin E on glycosylation of proteins.
Free Radic Biol Med. 22:593–596. 1997.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ceriello A, Giugliano D, Quatraro A,
Donzella C, Dipalo G and Lefebvre PJ: Vitamin E reduction of
protein glycosylation in diabetes. New prospect for prevention of
diabetic complications? Diabetes Care. 14:68–72. 1991.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shoff SM, Mares-Perlman JA, Cruickshanks
KJ, Klein R, Klein BE and Ritter LL: Glycosylated hemoglobin
concentrations and vitamin E, vitamin C, and beta-carotene intake
in diabetic and nondiabetic older adults. Am J Clin Nutr.
58:412–416. 1993.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Weykamp CW, Penders TJ, Baadenhuijsen H,
Muskiet FA, Martina W and van der Slik W: Vitamin C and
glycohemoglobin. Clin Chem. 41:713–716. 1995.PubMed/NCBI
|
|
64
|
Shah AD, Fox RK and Rushakoff RJ: Falsely
decreased HbA1c in a type 2 diabetic patient treated with dapsone.
Endocr Pract. 20:e229–e232. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Basavaraj GS, Gupta RD, Patel B, Jebasingh
F, George AA, Peter D, George L, Paul TV and Thomas N: Erroneous
reduction of HbA1c levels in patients with type 2 diabetes mellitus
on dapsone treatment for Hansen's disease-a single-center
retrospective cohort study. Indian J Dermatol Venereol Leprol.
88:519–522. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mitchell K and Mukhopadhyay B:
Drug-Induced Falsely Low A1C: Report of a case series from a
diabetes clinic. Clin Diabetes. 36:80–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Diop ME, Bastard JP, Meunier N, Thévenet
S, Maachi M, Capeau J, Pialoux G and Vigouroux C: Inappropriately
low glycated hemoglobin values and hemolysis in HIV-infected
patients. AIDS Res Hum Retroviruses. 22:1242–1247. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Robertson M: Artificially low HbA1c
associated with treatment with ribavirin. BMJ.
336(505)2008.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gram-Hansen P, Eriksen J, Mourits-Andersen
T and Olesen L: Glycosylated haemoglobin (HbA1c) in iron- and
vitamin B12 deficiency. J Intern Med. 227:133–136. 1990.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kim C, Bullard KM, Herman WH and Beckles
GL: Association between iron deficiency and A1C Levels among adults
without diabetes in the National Health and Nutrition Examination
Survey, 1999-2006. Diabetes Care. 33:780–785. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Attard SM, Herring AH, Wang H, Howard AG,
Thompson AL, Adair LS, Mayer-Davis EJ and Gordon-Larsen P:
Implications of iron deficiency/anemia on the classification of
diabetes using HbA1c. Nutr Diabetes. 5(e166)2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Cavagnolli G, Pimentel AL, Freitas PA,
Gross JL and Camargo JL: Factors affecting A1C in non-diabetic
individuals: Review and meta-analysis. Clin Chim Acta. 445:107–114.
2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sundaram RC, Selvaraj N, Vijayan G, Bobby
Z, Hamide A and Rattina Dasse N: Increased plasma malondialdehyde
and fructosamine in iron deficiency anemia: Effect of treatment.
Biomed Pharmacother. 61:682–685. 2007.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Guo W, Zhou Q, Jia Y and Xu J: Increased
levels of glycated hemoglobin A1c and iron deficiency Anemia: A
review. Med Sci Monit. 25:8371–8378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Willekens FL, Roerdinkholder-Stoelwinder
B, Groenen-Döpp YA, Bos HJ, Bosman GJ, van den Bos AG, Verkleij AJ
and Were JM: Hemoglobin loss from erythrocytes in vivo results from
spleen-facilitated vesiculation. Blood. 101:747–751.
2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Schnedl WJ, Wallner SJ, Piswanger C,
Krause R and Lipp RW: Glycated hemoglobin and liver disease in
diabetes mellitus. Wien Med Wochenschr. 155:411–415.
2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Bernstein RM, Freedman DB, Liyanage SP and
Dandona P: Glycosylated haemoglobin in rheumatoid arthritis. Ann
Rheum Dis. 41:604–606. 1982.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Pani LN, Korenda L, Meigs JB, Driver C,
Chamany S, Fox CS, Sullivan L, D'Agostino RB and Nathan DM: Effect
of aging on A1C levels in individuals without diabetes: Evidence
from the Framingham Offspring Study and the National Health and
Nutrition Examination Survey 2001-2004. Diabetes Care.
31:1991–1996. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ziemer DC, Kolm P, Weintraub WS, Vaccarino
V, Rhee MK, Twombly JG, Narayan KM, Koch DD and Phillips LS:
Glucose-independent, black-white differences in hemoglobin A1c
levels: A cross-sectional analysis of 2 studies. Ann Intern Med.
152:770–777. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn
SE, Horton ES, Lachin JM, Montez MG, Brenneman T and Barrett-Connor
E: Diabetes Prevention Program Research Group. Differences in A1C
by race and ethnicity among patients with impaired glucose
tolerance in the diabetes prevention program. Diabetes Care.
30:2453–2457. 2007.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Bergenstal RM, Gal RL, Connor CG,
Gubitosi-Klug R, Kruger D, Olson BA, Willi SM, Aleppo G, Weinstock
RS, Wood J, et al: Racial differences in the relationship of
glucose concentrations and hemoglobin A1c Levels. Ann Intern Med.
167:95–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Selvin E, Steffes MW, Zhu H, Matsushita K,
Wagenknecht L, Pankow J, Coresh J and Brancati FL: Glycated
hemoglobin, diabetes, and cardiovascular risk in nondiabetic
adults. N Engl J Med. 362:800–811. 2010.PubMed/NCBI
|
|
83
|
Nakatani R, Murata T, Usui T, Moriyoshi K,
Komeda T, Masuda Y, Kakita-Kobayashi M, Tagami T, Imashuku S, Kono
S, et al: Importance of the average glucose level and estimated
glycated hemoglobin in a diabetic patient with hereditary hemolytic
Anemia and liver cirrhosis. Intern Med. 57:537–543. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International diabetes federation diabetes atlas, 9(th)
edition. Diabetes Res Clin Pract. 157(107843)2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
American Diabetes Association. Standards
of medical care in diabetes-2013. Diabetes Care. 36 (Suppl
1):S11–S66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Bergman M, Abdul-Ghani M, DeFronzo RA,
Manco M, Sesti G, Fiorentino TV, Ceriello A, Rhee M, Phillips LS,
Chung S, et al: Review of methods for detecting glycemic disorders.
Diabetes Res Clin Pract. 165(108233)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Campbell L, Pepper T and Shipman K: HbA1c:
A review of non-glycaemic variables. J Clin Pathol. 72:12–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Xu A, Sun J, Li J, Chen W, Zheng R, Han Z
and Ji L: Hb I: A α-globin chain variant causing unexpected
HbA1c results. J Clin Lab Anal.
33(e22671)2019.PubMed/NCBI View Article : Google Scholar
|