Introduction
Preeclampsia (PE) refers to new-onset hypertension
after 20 weeks of pregnancy that is accompanied by proteinuria,
headaches, vertigo, nausea, vomiting, epigastric discomfort
(1). PE is a severe obstetric
emergency worldwide, with an annual incidence rate of 2-8%, and is
the leading cause of increased morbidity and mortality rates in
pregnant women and newborns (2).
Therefore, understanding the mechanisms underlying the onset of PE
remains an urgent priority for obstetricians. Insufficient invasion
of extravillous trophoblasts (EVTs) was suggested to be the leading
cause of PE, which results in reduced anchorage of EVTs to the
endometrium and decreased vascular remodeling. The insufficiently
remodeled blood vessel clusters at the maternal-fetal interface and
is unable to withstand the huge volume of blood in the later
trimester, leading to high blood pressure and the vascular
endothelial cells subsequently releasing various inflammatory
factors, which induces the clinical symptoms of hypertension and
eclampsia (3,4).
MicroRNAs (miRNAs/miRs) are non-coding RNAs that
have been revealed to serve regulatory roles in various
pathological or physiological processes (5-8),
including placental development (9). Several miRNAs have been found to
modulate trophoblast cells. For example, miR-145-5p facilitated PE
development by affecting the proliferation and invasion of
trophoblast cells (10); miR-218-5p
was shown to promote trophoblast invasion and endovascular EVT
differentiation by regulating the miR-218-5p/TGFβ2 signaling
pathway (11); and the expression
levels of miR-221-3p were found to be downregulated in PE and
promoted trophoblast proliferation, invasion and migration, at
least partly, by targeting thrombospondin 2(12). However, how miRNAs affect
trophoblast cell biological behaviors requires further
investigations.
The present study revealed that miR-372-3p was
associated with the occurrence of PE and that it suppressed
trophoblast cell migration, invasion and epithelial-mesenchymal
transition (EMT) by regulating twist1. These findings may provide a
potential novel biomarker or therapeutic target for PE.
Materials and methods
Patient studies
A total of 59 pregnant (maternal age shown in
Table I) women who had undergone a
cesarean section at the Obstetrics Department of The First Hospital
of China Medical University (Shenyang, China) were recruited for
this study between October 2018 and October 2020. A diagnosis of PE
with severe features defined using the following criteria: i)
Systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110
mmHg; and ii) 24 h urine protein >300 mg and protein/creatinine
≥0.3. In the case of negative urine protein, the following new
manifestations are met: i) Thrombocytopenia, platelet count
<100x109/l; ii) renal insufficiency, serum creatinine
>97 µmol/l or 2 times higher compared with the upper limit of
normal, excluding other kidney diseases; iii) impaired liver
function, transaminase is 2 times higher compared with the upper
limit of normal; iv) pulmonary edema; and v) new headache that
common medication does not relieve one other causes or blurred
vision have been ruled out according to the diagnostic criteria of
The American College of Obstetricians and Gynecologists (1) was used as the inclusion criterium to
avoid the influence of non-placental factors. The following
exclusion criteria were used for recruitment: i) Twin pregnancy;
and ii) diagnosis of gestational diabetes, renal disease, chronic
hypertension, acute or chronic hepatitis, hyperthyroidism or
hypothyroidism. Patients with hypertension, proteinuria, twin
pregnancy, diagnosis of gestational diabetes, renal disease,
chronic hypertension, acute or chronic hepatitis, hyperthyroidism
and hypothyroidism were excluded. According to the gestational age,
the 59 patients were divided into early-onset PE (EOPE; ≤34 weeks),
late-onset PE (LOPE; >34 weeks), pre-term delivery (ENP) and
full-term delivery (NP tissues) groups. Control groups were set
according to gestational age. The method of placenta collection in
the NP group was the same as that in the PE group. Patients in the
NP group were recruited to try to ensure that the gestational age
was similar to that in the PE group. The clinicopathological data
of each patient are shown in Table
I. Samples were obtained from the maternal surface of the
placental tissue from each patient, and once the blood had been
thoroughly washed off, the samples were stored in liquid nitrogen
(-196˚C) within 15 min of surgery and then stored at -80˚C. The
Ethics Committee of The First Hospital of China Medical University
approved the present study (approval no. AF-SOP-07-1.1-01), and all
patients participating in the study signed informed consent forms
prior to participation.
 | Table IClinical characteristics of patients
in the present study. |
Table I
Clinical characteristics of patients
in the present study.
| | Tissue | | P-value |
|---|
| Variable | EOPE, n=18 | LOPE, n=13 | ENP, n=13 | NP, n=15 | F-value | P-value | EOPE vs. LOPE
tissues | EOPE vs. ENP
tissues | LOPE vs. NP
tissues |
|---|
| Maternal age,
years | 29.72±4.47 | 30.54±3.36 | 27.38±3.20 | 31.93±4.23 | 3.24 | <0.05 | 0.57 | 0.11 | 0.35 |
| Gestational age,
weeks | 31.60±1.55 | 37.26±0.99 | 32.01±2.77 | 38.94±0.57 | 71.21 | <0.05 | <0.05 | 0.48 | 0.63 |
| Body mass index,
kg/m2 | 29.76±2.55 | 31.23±3.57 | 29.55±3.29 | 28.47±4.51 | 1.45 | 0.24 | 0.26 | 0.87 | <0.05 |
| Systolic blood
pressure, mmHg | 172.28±14.70 | 168.38±11.99 | 118.77±19.55 | 113.07±7.63 | 75.95 | <0.05 | 0.45 | <0.05 | <0.05 |
| Diastolic blood
pressure, mmHg | 114.83±13.01 | 106.15±10.42 | 78.31±15.61 | 70.47±8.92 | 47.27 | <0.05 | 0.06 | <0.05 | <0.05 |
| 24-h proteinuria
quantification, g/24 | 12.73±9.31 | 7.61±6.36 | - | - | 17.26 | <0.05 | <0.05 | - | - |
| Proteinuria level,
mg/dl | 954.82±765.41 | 629.74 ±596.06 | - | - | 13.73 | <0.05 | 0.09 | - | - |
| Urine, ml/24 h |
1,605.00±705.88 |
1,338.46±386.30 |
1,080.77±149.36 |
1,260.00±289.83 | 3.50 | <0.05 | 0.12 | <0.05 | 0.66 |
| PLT,
109/l | 163.22±55.72 | 174.85±60.04 | 221.77±39.01 | 219.67±49.62 | 5.14 | <0.05 | 0.54 | <0.05 | <0.05 |
| AST, U/l | 36.06±24.80 | 26.85±18.58 | 20.77±10.43 | 14.53±4.60 | 4.66 | <0.05 | 0.15 | <0.05 | 0.06 |
| ALT, U/l | 28.72±22.37 | 21.08±13.91 | 18.23±10.30 | 12.40±3.42 | 3.40 | <0.05 | 0.17 | 0.06 | 0.13 |
| Creatinine,
mg/dl | 0.86±0.31 | 0.75±0.25 | 0.58±0.08 | 0.62±0.08 | 5.64 | <0.05 | 0.17 | <0.05 | 0.10 |
| Birth weight,
g |
1,607.78±389.86 |
2,826.15±392.21 |
2,413.85±294.29 |
3,619.33±366.30 | 85.71 | <0.05 | <0.05 | <0.05 | <0.05 |
| Birth length,
cm | 37.39±3.42 | 46.38±1.94 | 40.92±6.34 | 51.00±1.36 | 41.67 | <0.05 | <0.05 | <0.05 | <0.05 |
| Apgar score, 1
min | 8.28±1.07 | 9.62±0.51 | 9.31±0.63 | 10.00±0.00 | 18.18 | <0.05 | <0.05 | <0.05 | 0.16 |
Cell lines and culture
The human EVT cell line (HTR-8/SVneo cells) was
provided by Dr Charles Graham (College of Life Sciences, Queen's
University, Kingston, ON, Canada). The cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; Cytiva) and maintained at 37˚C
with 5% CO2.
Cell transfection
miR-372-3p inhibitor
(5'-GCUCAAAUGUCGCAGCACUUUUU-3'), inhibitor-negative control
(inhibitor-NC; 5'-UUCUCCGAACGUGUCACGUTT-3'), miR-372-3p mimic
(5'-AAAGUGCUGCGACAUUUGAGCGUGCUCAAAUGUCGCAGCACUUUUU-3') and mimic-NC
(5'-ACGUGACACGUUCGGAGAATT-3') (Mass/concentration, 250 µl) were
purchased from Guangzhou RiboBio Co., Ltd. Short hairpin (sh)RNA
targeting twist family bHLH transcription factor 1 (twist1;
sh-twist1), sh-NC, twist1 overexpression plasmid and empty vector
(twist1-NC) were purchased from Beijing Syngentech Co., Ltd. Cells
were seeded into 6-well plates at a density of 2x105
cells/well at 37˚C for 48 h and were subsequently transfected with
each oligonucleotide or plasmid (250 µl) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol
(temperature and duration of transfection, 37˚C).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The purity and concentration of the RNA was analyzed using a
NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.)
at an optical density of 260/280 nm. Total RNA was reverse
transcribed into cDNA using random primers from the Transcriptor
First Strand cDNA Synthesis kit (Roche Applied Science). RT kit was
used according to the manufacturer's protocol. qPCR was
subsequently performed on a ViiA 7 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). SYBR GREEN mastermix
(Takara Bio, Inc.) fluorophore used for the qPCR. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of 95˚C for
10 sec, 60˚C for 60 sec and 95˚C for 15 sec. Primer sequences:
MiR-372-3p forward, 5'-TTTCACGACGCTGTAAACTCGCA-3' and reverse,
5'-GTGCAGGGTCCGAGGT-3'; twist1 forward, 5'-TGAATGCATTTAGACACCG-3',
and reverse, 5'-AGAGGAAGTCGATGTACCT-3'; GAPDH forward,
5'-GGGAAACTGTGGCGTGAT-3', and reverse, 5'-GAGTGGGTGTCGCTGTTGA-3';
U6 forward, 5'-AACGCTTCACGAATTTGCGT-3', and reverse,
5'-CTCGCTTCGGCAGCACA-3'. The relative expression levels were
quantified using the 2-∆ΔCq method (13), using GAPDH or U6 as the controls to
normalize the expression levels of mRNAs and miRNAs,
respectively.
Transwell assays
HTR-8/SVneo cells were cultured in six-well plates
at a density of 1x105 cells/well. After transfection,
the cells were plated into the upper chambers of Transwell plates
(pore size, 8-µm; Corning, Inc.), which were precoated (37˚C; 2 h)
with 50 µl Matrigel or without (BD Biosciences) for the invasion
and migration assays, respectively. The lower chambers were filled
with RPMI-1640 medium supplemented with 10% FBS. The cells were
cultured for 24 or 48 h for the migration or invasion assays,
respectively, at 37˚C. Following the incubation, the cells
remaining in the upper chambers were removed with cotton swabs, and
the cells in the lower chambers were fixed with methanol (4%; 25
min) and stained with crystal violet (0.4%; 5 min) at 37˚C. Stained
cells were visualized (magnification, x200) using a light
microscope. The experiment was independently repeated three
times.
Wound healing assay
HTR-8/SVneo cells were cultured in six-well plates
at a density of 1x105 cells/well. Cells were evenly
distributed (70% confluency). An artificial wound was subsequently
made in the cell monolayer by scratching the cells with a 200-µl
pipette tip and non-adherent cells were removed by three washes
with PBS. Serum-free RPMI-1640 medium was added to each well and
cells were incubated for 48 h at 37˚C with 5% CO2.
Images of the wound were obtained at 0 and 48 h at the same
position. Cell migration rate=(0 h scratch width-scratch width
after 48 h)/0 h scratch width x100%. The images were captured using
light microscope. The experiment was independently repeated three
times.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Proteins were
quantified using the BCA method. Total protein (10 µl per lane) was
quantified and then separated by 10% SDS-PAGE. The separated
proteins were subsequently transferred onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.) and blocked using
5% (M/V) skimmed milk powder prepared with TBST buffer for 1 h at
4˚C (0.1% tween). The membranes were then incubated overnight at
4˚C with the following primary antibodies: Anti-E-cadherin
(1:1,000; cat. no. 14472S; Cell Signaling Technology, Inc.),
anti-vimentin (1:1,000; cat. no. 5741S; Cell Signaling Technology,
Inc.), anti-N-cadherin (1:1,500; cat. no. 13116S; Cell Signaling
Technology, Inc.), anti-twist1 (1:1,000; cat. no. 69366S; Cell
Signaling Technology, Inc.) and anti-GAPDH (1:1,000; cat. no.
5174S). Following the primary antibody incubation, the membranes
were washed with TBST and incubated with HRP-conjugated secondary
antibodies goat anti-rabbit (1:1,000; cat. no. GTX213110-01;
GeneTex) and anti-mouse antibodies (1:1,000; cat: GTX213111-01;
GeneTex) for 2 h at 4˚C. Protein bands were visualized using ECL
reagent (Beyotime Institute of Biotechnology). GAPDH was used as
the internal loading control.
Dual luciferase reporter assay
A dual luciferase reporter assay (Luciferase
detection kit; cat. no. E1910; Promega Corporation) was performed
to determine the interaction between miR-372-3p and twist1. The
entire sequence of twist1 containing the wild-type (WT)
3'-untranslated region (UTR) with the predicted target site of
miR-372-3p (LUC-twist1-WT) or a mutant-type (Mut) 3'-UTR target
site (LUC-twist1-Mut) was subcloned into the pmirGLO vector
(Syngentech Co., Ltd.). Then, 293T cells (National Collection of
Authenticated Cell Cultures) were seeded into 24-well plates at a
density of 5x105 cells/well and co-transfected with
either LUC-twist1-Mut or -WT vectors and either miR-372-3p mimic or
mimic-NC using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Following 48 h of transfection, the
relative firefly luciferase activity was measured and normalized to
Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.), and data are presented as the mean ± SD. Each
experiment was repeated three times independently. Statistical
differences between three or more groups were determined using a
one-way ANOVA followed by a Bonferroni's post hoc test. Statistical
differences between two groups were determined using an unpaired
Student's t-test. Pearson's correlation analysis was performed to
analyze the correlation between two groups. Bioinformatics analysis
used the following databases: RNA22 v2 (https://cm.jefferson.edu); RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-372-3p expression levels are
upregulated in PE tissues
RT-qPCR was performed to analyze the expression
levels of miR-372-3p in PE tissues. The expression levels of
miR-372-3p were upregulated in PE tissues compared with placenta
tissues from women who had a full-term delivery (NP tissues)
(Fig. 1A). According to the
gestational age, PE tissues were divided into EOPE (≤34 weeks) and
LOPE (>34 weeks) tissues, which have differential pathogeneses.
As shown in Table I, there were
statistically significant differences in clinical characteristics
such as systolic blood pressure, systolic blood pressure, 24-h
urine protein, ALT, AST and creatinine between the preeclampsia
group (EOPE, LOPE) and the control group (ENP, NP). EOPE is
considered to originate from complications in the placenta, such as
trophoblast dysfunction and insufficient spiral artery remodeling
(14,15). By contrast, LOPE is considered to be
of maternal origin and can arise from metabolic disorders and
endothelial cell dysfunction (16,17).
RT-qPCR results revealed that the expression levels of miR-372-3p
were upregulated in EOPE tissues compared with the pre-term
delivery (ENP) and NP groups (Fig.
1B). In addition, the expression levels of miR-372-3p were
upregulated in the LOPE group compared with the NP group, which
suggested that miR-372-3p may be closely associated with PE.
Moreover, miR-372-3p expression levels were upregulated in the EOPE
group compared with the LOPE group, which suggested that miR-372-3p
may be associated with trophoblast function. Overall, the results
suggested that miR-372-3p may serve a regulatory role over
trophoblast behaviors.
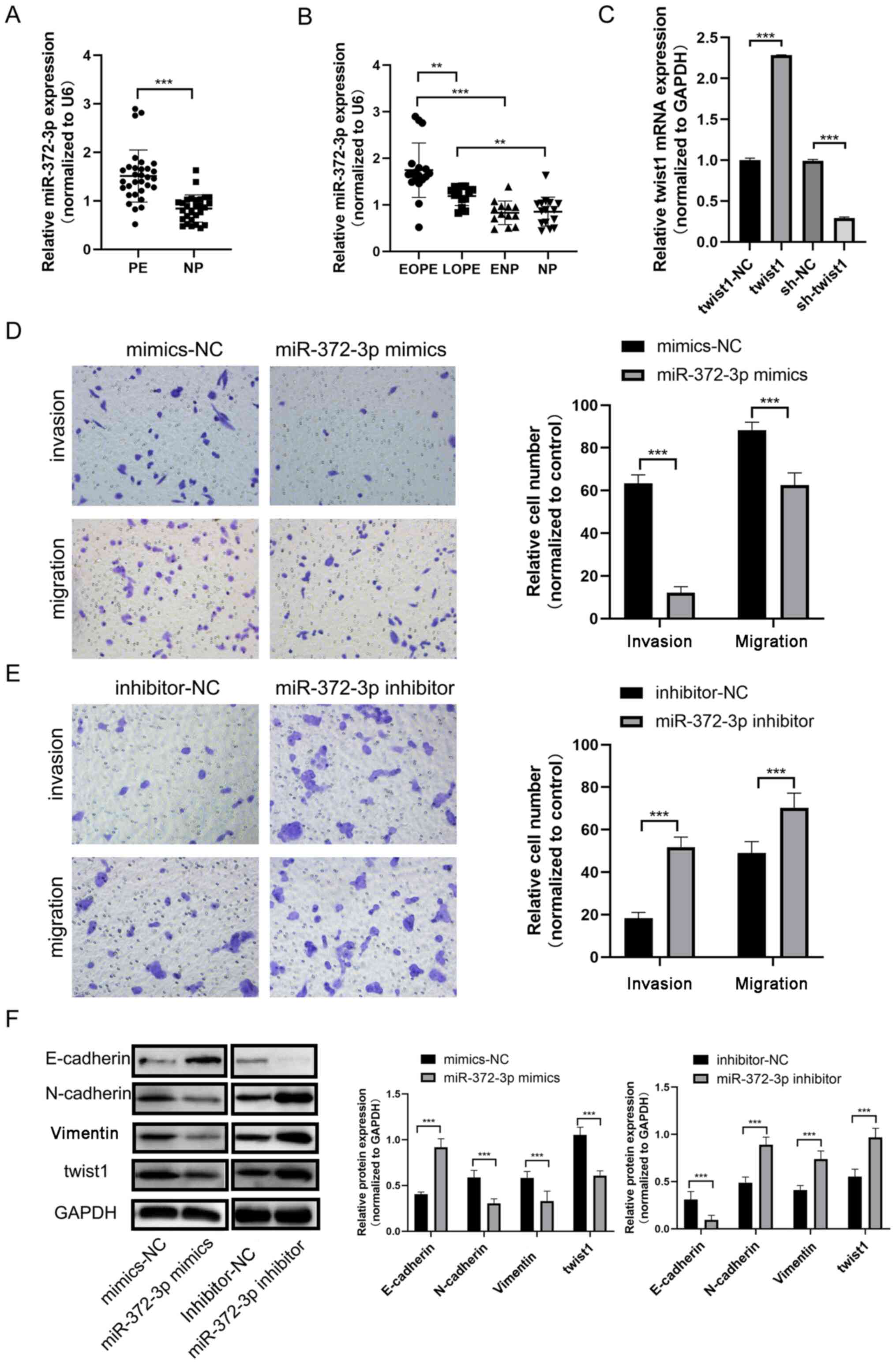 | Figure 1miR-372-3p expression levels in PE.
(A) RT-qPCR was performed to analyze miR-372-3p expression levels
in PE and NP tissues. (B) RT-qPCR was performed to determine
miR-372-3p expression among EOPE, LOPE, ENP and NP. (C) RT-qPCR was
performed to verify the transfection efficiency of the miR-372-3p
mimic and inhibitor into HTR-8/Svneo cells. Transwell assays were
performed to evaluate HTR-8/Svneo cell migration and invasion
following transfection with the (D) miR-372-3p mimic or (E)
miR-372-3p inhibitor (magnification, x200). (F) Western blotting
was used to analyze the expression levels of epithelial-mesenchymal
transition-related proteins. Data are presented as the mean ± SD.
**P<0.01 and ***P<0.001. miR, microRNA;
RT-qPCR, reverse transcription-quantitative PCR; PE, preeclampsia;
EOPE, early-onset PE; LOPE, late-onset PE; NP tissues, placenta
tissues from women who had a full-term delivery; PE tissues,
placenta tissues from patients with PE; NC, negative control;
twist1, twist family bHLH transcription factor 1; ENP, placenta
tissues from women who had a premature delivery. |
miR-372-3p regulates trophoblast cell
migration, invasion and EMT
To investigate the function of miR-372-3p in
trophoblast cells, miR-372-3p was successfully overexpressed or
knocked down by transfection of a miR-372-4p mimic or inhibitor
into HTR-8/SVneo cells, respectively (Fig. 1C). The results of the Transwell and
Matrigel assays revealed that the number of migratory and invasive
cells, respectively, in the miR-372-3p mimic group was
significantly decreased compared with the mimic-NC group, which
suggested that miR-372-3p may suppress HTR-8/SVneo cell migration
and invasion (Fig. 1D). The
opposite results were observed following transfection with the
miR-372-3p inhibitor (Fig. 1E).
Western blotting was used to analyze the expression levels of
several EMT-related proteins to determine if miR-372-3p could
modulate EMT in trophoblast cells. The results demonstrated that
the expression levels of E-cadherin were upregulated, whereas the
expression levels of N-cadherin and vimentin were downregulated
following the overexpression of miR-372-3p. The trends in the
expression levels of these proteins were the opposite in
miR-372-3p-knockdown cells (Fig.
1F). These results suggested that miR-372-3p may suppress
trophoblast cell migration, invasion and EMT.
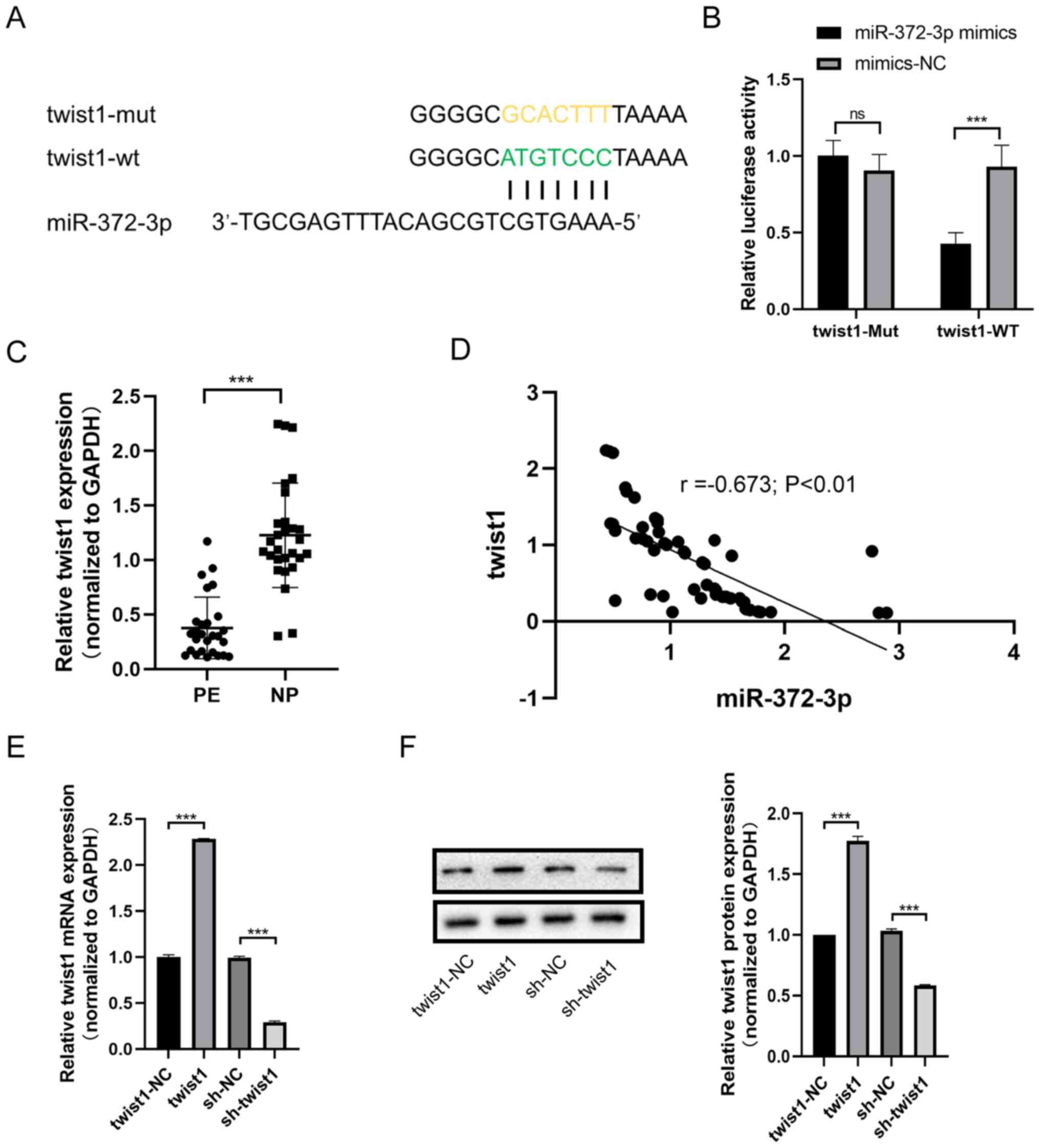
miR-372-3p targets twist1
According to the competing endogenous RNA (ceRNA)
theory, circular RNA can compete with mRNA to bind miRNA, thereby
affecting the expression of mRNA (18-20).
The present study investigated the underlying regulatory mechanism
of miR-372-3p on trophoblast cell behavior. Bioinformatics analysis
predicted that the 3'-UTR of twist1 was targeted by miR-372-3p
(Fig. 2A). A dual luciferase
reporter assay was used to validate the binding relationship
between miR-372-3p and twist1. The relative luciferase activity of
the Twist1-WT vector was significantly decreased when
co-transfected with the miR-372-3p mimic compared with the mimic-NC
(Fig. 2B). However, the relative
luciferase activity of the Twist1-Mut vector was unchanged between
cells co-transfected with the miR-372-3p mimic or mimic-NC.
(Fig. 2B). RT-qPCR was performed to
analyze the expression levels of twist1 in PE and NP tissues, and
the results revealed that the expression levels of twist1 were
lower in PE tissues compared with those in NP tissues (Fig. 2C). Moreover, the expression levels
of miR-372-3p and twist1 were found to be negatively correlated in
the tissues including PE and NP (r=-0.673; P<0.01; Fig. 2D).
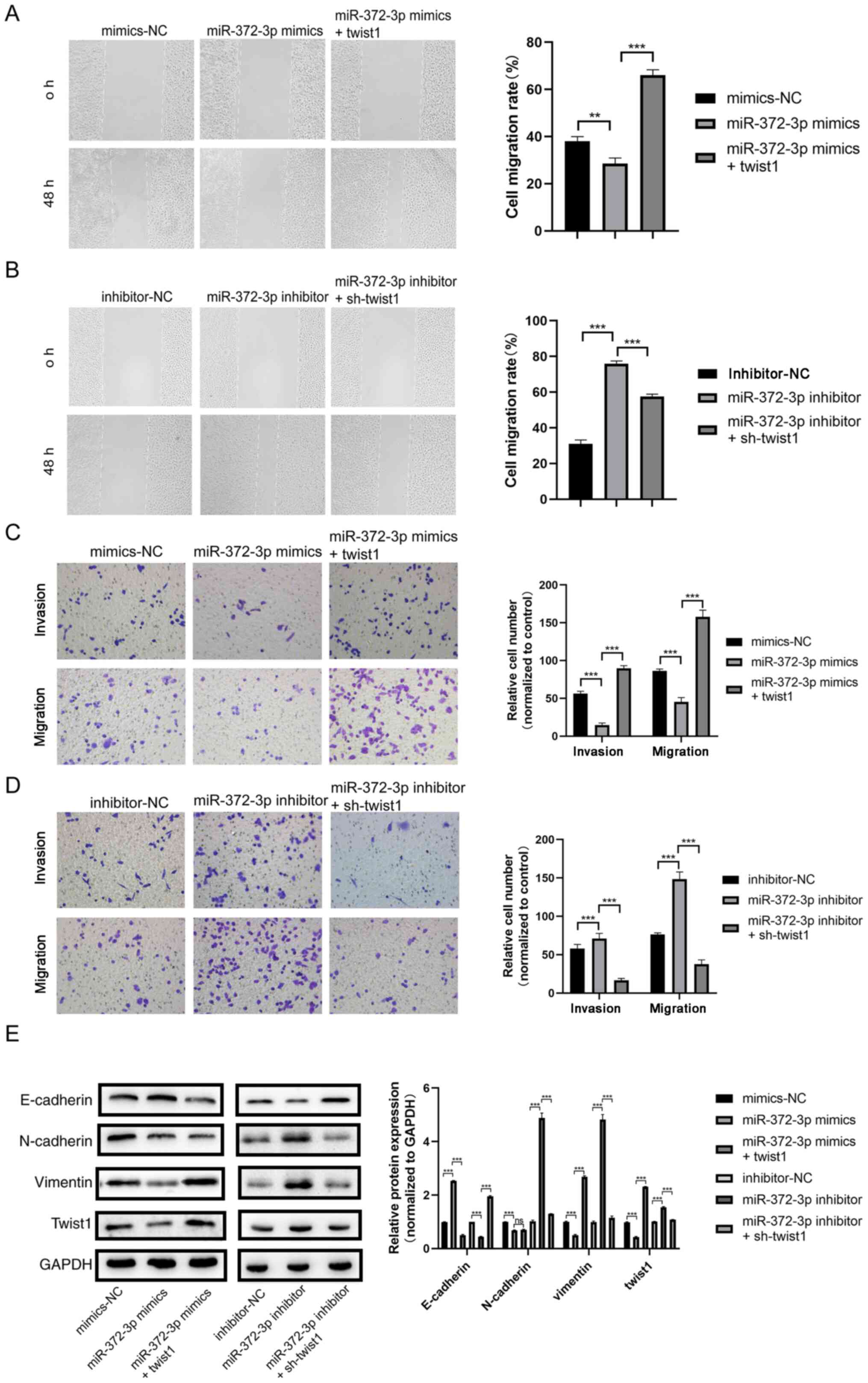
miR-372-3p suppresses trophoblast cell
migration, invasion and EMT by targeting twist1
Rescue experiments were performed by co-transfecting
cells with miR-372-3p mimic and twist1 overexpression vector to
determine their effects on trophoblast cell behaviors. As shown in
Fig. 2E and F, twist1 was successfully overexpressed or
knocked down at both the mRNA and protein levels (Fig. 2E and F). The wound healing assay results showed
that the cell migration rate was decreased in the miR-372-3p mimic
group compared with the mimic-NC group, whereas migration was
increased in the miR-372-3p mimic + twist1 group compared with the
miR-372-3p mimic group (Fig. 3A).
The opposite trends were observed in cells co-transfected with the
miR-372-3p inhibitor and sh-twist1 (Fig. 3B). The Transwell and Matrigel assay
results revealed that the number of migratory and invasive cells,
respectively, in the miR-372-3p mimic group was decreased compared
with the mimic-NC group, whereas cell invasion and migration were
increased in the miR-372-3p mimic + twist1 group compared with the
miR-372-3p mimic group (Fig. 3C);
the opposite trends were observed in cells co-transfected with the
miR-372-3p inhibitor and sh-twist1 (Fig. 3D). These results suggested that
twist1 may reverse the effects of miR-372-3p on trophoblast cell
migration and invasion.
The results of the western blotting analysis
demonstrated that the expression levels of E-cadherin were
upregulated following the transfection with the miR-372-3p mimic
compared with those in the mimic-NC group, whereas these effects
were reversed following the co-transfection with the miR-372-3p
mimic and twist1 (Fig. 3E). The
expression levels of N-cadherin, vimentin and twist1 in the
miR-372-3p mimic group was lower than the co-transfection group,
which indicated that twist1 reverse miR-372-3p-modulated EMT in
trophoblasts (Fig. 3E). The
expression of E-cadherin in the miR-372-3p inhibitor group was
significantly lower compared with that of the miR-372-3p inhibitor
+ sh-twist1 group, while N-cadherin, vimentin and twist1 expression
levels in the miR-372-3p inhibitor group were higher compared the
co-transfection group (Fig.
3E).
Discussion
PE progression is a long and complicated process
that can be divided into six stages: i) The mother first develops
immune intolerance to the paternal genes of the embryo between the
fertilization and implantation stages of the embryo; ii) abnormal
placenta formation occurs and trophoblast invasion into the uterine
spiral artery is decreased during the 8-18th weeks of pregnancy,
which is an important period of placental development; iii) the
stress response is activated; iv) various placental-derived injury
factors are released into the maternal blood circulation during the
second half of the pregnancy; v) clinical symptoms, such as
hypertension, which enable the clinical diagnosis of PE, appear;
and vi) an acute illness is exacerbated in half of the patients,
atherosclerosis in the spiral artery rapidly develops, placental
perfusion is further reduced and spiral artery thrombosis and even
placental infarction are induced. Owing to the early occurrence and
long duration of the second stage of this process, the trophoblasts
invading the spiral artery from the 8th week participate in the
formation of the placenta. This stage is considered to be the key
step for triggering the development of PE and can subsequently
activate signaling molecules to create a cascading amplification
effect, causing continuous damage at the maternal-fetal interface.
Therefore, this stage is the most crucial period for studying the
pathophysiological changes of trophoblasts and for identifying
biomarkers for the early prediction and diagnosis of PE.
During the process of placental implantation in
early pregnancy, extraembryonic trophoblast cells differentiate
into syncytiotrophoblast cells and extravillous cytotrophoblast
cells (3). Syncytiotrophoblasts are
trophoblasts with a secretory phenotype, which are distributed on
the surface of the placenta and secrete cytokines to maintain the
normal development of the placenta. The proximal extravillous
cytotrophoblasts near the fetal side acquire a proliferative
phenotype, whereas the distal extravillous cytotrophoblasts acquire
a migratory and invasive phenotype (3,21). In
more detail, the morphology of these trophoblasts changes from a
rounded epithelial cell to a more elongated, mesenchymal phenotype,
with enhanced migratory and invasive abilities that enables the
placenta to firmly anchor onto the decidua (4). This cellular morphology transformation
is also called trophoblast EMT (4).
Several EMT-related proteins, such as E-cadherin, N-cadherin,
vimentin and twist1, were discovered to be differentially expressed
during the transition (4,21).
miRNAs are short non-coding RNAs that are highly
conserved throughout evolution (22,23).
Owing to their long half-life and high stability in extracellular
fluid, such as serum, plasma and urine, miRNAs are suggested to be
more suitable diagnostic biomarkers than mRNAs (24,25).
Previous studies have reported that miRNAs served roles in numerous
mechanisms in various diseases, such as prostate cancer, ovarian
cancer and Alzheimer's (26-28).
In PE, several miRNAs were found to be differentially expressed in
the circulation, decidual-derived mesenchymal stem cells, amniotic
fluid and placenta (29-31).
Thus, whether miRNAs can regulate PE progression should be studied
in further depth in the future.
It has been proposed that some protein-coding
transcripts can act as endogenous miRNA sponges, which are also
known as ceRNAs (20). ceRNAs
communicate with and co-regulate each other by competing for the
binding to shared miRNAs, thereby sequestering miRNA availability
(20). Co-transfection of
miR-372-3p (mimics/inhibitors) and twist1 (mimics/inhibitors)
demonstrated that twist1 could partially reverse the effects of
miR-372-3p (mimics/inhibitors) on trophoblast cells, which
suggested that miR-372-3p may suppress trophoblast migration,
invasion and EMT (E-cadherin, N-cadherin, vimentin and twist1) by
targeting twist1. Previous studies have reported that miR-372-3p
serves important roles in numerous types of disease, for example
colorectal cancer, lung squamous cell carcinoma, osteosarcoma,
hepatocellular carcinoma (8,32-36).
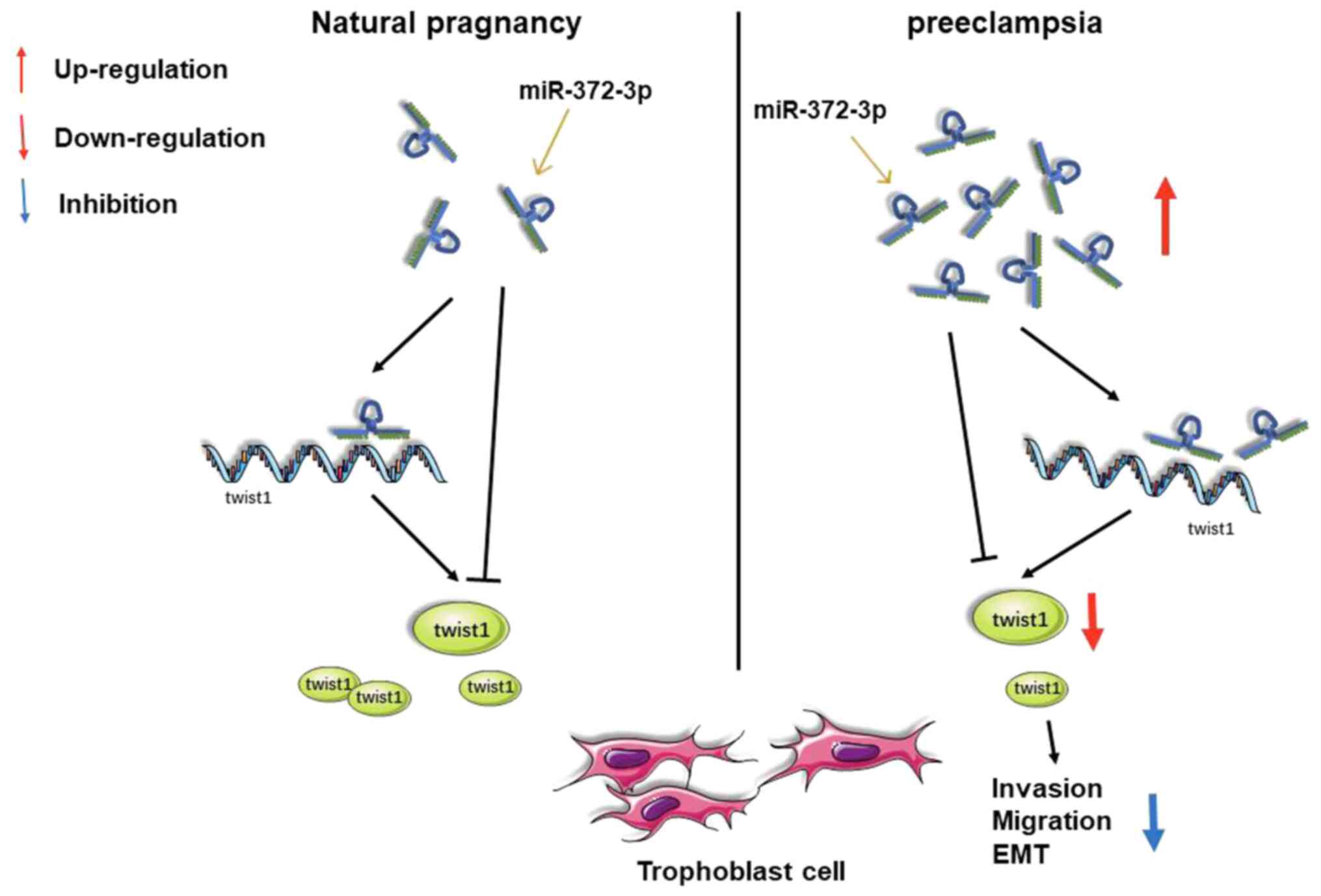
In conclusion, to the best of our knowledge, the
present study was the first to identify the role of miR-372-3p in
PE. miR-372-3p was revealed to suppress trophoblast cell invasion,
migration and EMT via inhibiting twist1 (Fig. 4). In future studies, a larger sample
size will be used, and the expression levels of miR-372-3p in the
peripheral blood and placenta from women of different gestational
weeks will be investigated to verify the role of miR-372-3p in
PE.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The National Natural
Science Foundation (grant no. 81871173).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived and designed the experiments, performed
the experiments, analyzed the data and wrote the manuscript; JW and
HC collected the clinical data and sample tissues, and participated
in drafting the manuscript or revising it critically for important
content; DL collected the clinical data and sample tissues and
participated in the design of experimental ideas. TM designed the
study and supervised the progression of the project as the
corresponding author. All authors have read and approved the final
manuscript. ZL and TM confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The Ethics Committee of The First Hospital of China
Medical University approved the present study (approval no.
AF-SOP-07-1.1-01), and all patients participating in the study
signed informed consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. Gestational
hypertension and preeclampsia: ACOG practice bulletin, number 222.
Obstet Gynecol. 135:e237–e260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shen XY, Zheng LL, Huang J, Kong HF, Chang
YJ, Wang F and Xin H: CircTRNC18 inhibits trophoblast cell
migration and epithelial-mesenchymal transition by regulating
miR-762/Grhl2 pathway in pre-eclampsia. RNA Biol. 16:1565–1573.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
E Davies J, Pollheimer J, Yong HE,
Kokkinos MI, Kalionis B, Knofler M and Murthi P:
Epithelial-mesenchymal transition during extravillous trophoblast
differentiation. Cell Adh Migr. 10:310–321. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
DaSilva-Arnold SC, Zamudio S, Al-Khan A,
Alvarez-Perez J, Mannion C, Koenig C, Luke D, Perez AM, Petroff M,
Alvarez M, et al: Human trophoblast epithelial-mesenchymal
transition in abnormally invasive placenta. Biol Reprod.
99:409–421. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moldovan L, Batte KE, Trgovcich J, Wisler
J, Marsh CB and Piper M: Methodological challenges in utilizing
miRNAs as circulating biomarkers. J Cell Mol Med. 18:371–390.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cai Z, Li J, Zhuang Q, Zhang X, Yuan A,
Shen L, Kang K, Qu B, Tang Y, Pu J, et al: MiR-125a-5p ameliorates
monocrotaline-induced pulmonary arterial hypertension by targeting
the TGF-β1 and IL-6/STAT3 signaling pathways. Exp Mol Med. 50:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi J, Zhang Y, Jin N, Li Y, Wu S and Xu
L: MicroRNA-221-3p plays an oncogenic role in gastric carcinoma by
inhibiting PTEN expression. Oncol Res. 25:523–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peng H, Pan X, Su Q, Zhu LS and Ma GD:
MiR-372-3p promotes tumor progression by targeting LATS2 in
colorectal cancer. Eur Rev Med Pharmacol Sci. 23:8332–8344.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen DB and Wang W: Human placental
microRNAs and preeclampsia. Biol Reprod. 88(130)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lv Y, Lu X, Li C, Fan Y, Ji X, Long W,
Meng L, Wu L, Wang L, Lv M and Ding H: miR-145-5p promotes
trophoblast cell growth and invasion by targeting FLT1. Life Sci.
239(117008)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brkić J, Dunk C, O'Brien J, Fu G, Nadeem
L, Wang YL, Rosman D, Salem M, Shynlova O, Yougbaré I, et al:
MicroRNA-218-5p promotes endovascular trophoblast differentiation
and spiral artery remodeling. Mol Ther. 26:2189–2205.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang Y, Li H, Ma Y, Zhu X, Zhang S and Li
J: MiR-221-3p is down-regulated in preeclampsia and affects
trophoblast growth, invasion and migration partly via targeting
thrombospondin 2. Biomed Pharmacother. 109:127–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tayyar AT, Karakus R, Eraslan Sahin M,
Topbas NF, Sahin E, Karakus S, Yalcin ET and Tayyar A: Wnt
signaling pathway in early- and late-onset preeclampsia: Evaluation
with Dickkopf-1 and R-Spondin-3 glycoproteins. Arch Gynecol Obstet.
299:1551–1556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
von Dadelszen P, Magee LA and Roberts JM:
Subclassification of preeclampsia. Hypertens Pregnancy. 22:143–148.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Herzog EM, Eggink AJ, Reijnierse A,
Kerkhof MA, de Krijger RR, Roks AJ, Reiss IK, Nigg AL, Eilers PH,
Steegers EA, et al: Impact of early- and late-onset preeclampsia on
features of placental and newborn vascular health. Placenta.
49:72–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cim N, Kurdoglu M, Ege S, Yoruk I, Yaman G
and Yildizhan R: An analysis on the roles of angiogenesis-related
factors including serum vitamin D, soluble endoglin (sEng), soluble
fms-like tyrosine kinase 1 (sFlt1), and vascular endothelial growth
factor (VEGF) in the diagnosis and severity of late-onset
preeclampsia. J Matern Fetal Neonatal Med. 30:1602–1607.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karreth FA and Pandolfi PP: ceRNA
cross-talk in cancer: When ce-bling rivalries go awry. Cancer
Discov. 3:1113–1121. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and
Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to
promote triple-negative breast cancer progression through
regulating CCNE1 expression. Mol Cancer. 18(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee CT, Risom T and Strauss WM:
Evolutionary conservation of microRNA regulatory circuits: An
examination of microRNA gene complexity and conserved
microRNA-target interactions through metazoan phylogeny. DNA Cell
Biol. 26:209–218. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gantier MP, McCoy CE, Rusinova I, Saulep
D, Wang D, Xu D, Irving AT, Behlke MA, Hertzog PJ, Mackay F and
Williams BR: Analysis of microRNA turnover in mammalian cells
following Dicer1 ablation. Nucleic Acids Res. 39:5692–5703.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sayed AS, Xia K, Salma U, Yang T and Peng
J: Diagnosis, prognosis and therapeutic role of circulating miRNAs
in cardiovascular diseases. Heart Lung Circ. 23:503–510.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sur S, Steele R, Shi X and Ray RB:
miRNA-29b inhibits prostate tumor growth and induces apoptosis by
increasing bim expression. Cells. 8(1455)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ghafouri-Fard S, Shoorei H and Taheri M:
miRNA profile in ovarian cancer. Exp Mol Pathol.
113(104381)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hromadnikova I, Kotlabova K, Ivankova K,
Vedmetskaya Y and Krofta L: Profiling of cardiovascular and
cerebrovascular disease associated microRNA expression in umbilical
cord blood in gestational hypertension, preeclampsia and fetal
growth restriction. Int J Cardiol. 249:402–409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao G, Zhou X, Chen S, Miao H, Fan H,
Wang Z, Hu Y and Hou Y: Differential expression of microRNAs in
decidua-derived mesenchymal stem cells from patients with
pre-eclampsia. J Biomed Sci. 21(81)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou C, Zou QY, Li H, Wang RF, Liu AX,
Magness RR and Zheng J: Preeclampsia downregulates MicroRNAs in
fetal endothelial cells: Roles of miR-29a/c-3p in endothelial
function. J Clin Endocrinol Metab. 102:3470–3479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Q, Liu S, Zhao X, Wang Y, Tian D and
Jiang W: MiR-372-3p promotes cell growth and metastasis by
targeting FGF9 in lung squamous cell carcinoma. Cancer Med.
6:1323–1330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Liu JJ, Zhou JH, Chen R and Cen CQ:
LncRNA HULC induces the progression of osteosarcoma by regulating
the miR-372-3p/HMGB1 signalling axis. Mol Med.
26(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fan J, Zhang J, Huang S and Li P: lncRNA
OSER1-AS1 acts as a ceRNA to promote tumorigenesis in
hepatocellular carcinoma by regulating miR-372-3p/Rab23 axis.
Biochem Biophys Res Commun. 521:196–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Soliman MH, Ragheb MA, Elzayat EM, Mohamed
MS, El-Ekiaby N, Abdelaziz AI and Abdel-Wahab AA: MicroRNA-372-3p
predicts response of TACE patients treated with doxorubicin and
enhances chemosensitivity in hepatocellular carcinoma. Anticancer
Agents Med Chem. 21:246–253. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fan X, Huang X, Li Z and Ma X:
MicroRNA-372-3p promotes the epithelial-mesenchymal transition in
breast carcinoma by activating the Wnt pathway. J BUON.
23:1309–1315. 2018.PubMed/NCBI
|