Introduction
Venous thromboembolism (VTE), which includes deep
venous thrombosis (DVT) and pulmonary embolism (PE), exerts a
considerable disease burden with long-term complications and
morbidity (1). Patients with
cancer are at high risk of developing VTE. Results of a previous
study indicated that the 12-month cumulative incidence of VTE
following cancer diagnosis was 3%, which is 9-fold higher than that
in the general population (2). VTE
risk in patients with cancer increases by 3-fold overall and 6-fold
in those receiving chemotherapy or targeted therapy (2). Certain patients with cancer exhibit
abnormalities in each component of Virchow's triad contributing to
thrombosis, due to patient-, tumor- and treatment-associated risk
factors (3,4). This can result in malignancy with
activation of the coagulation system and prothrombotic states that
are exacerbated by chemotherapy, hormone therapy or surgery
(4-6).
Cancer-associated VTE is associated with poor survival, need for
hospitalization and potential delay or interruption of anticancer
therapy (3). VTE has become the
second most common cause of death in patients with cancer (7).
To the best of our knowledge, a study performed in
1999 was the first to report that the rate of VTE in patients with
cancer was only 0.6% (8). However,
the incidence of cancer-associated VTE ranged from 1.3-22.6% in
subsequent studies (2,9-13).
Cancer-associated VTE has exhibited a sharp rise in 1-year
cumulative incidence between 1997 and 2017(2), which varies widely in different
studies depending on cancer types, the follow-up duration and the
detection method of thrombotic events. These increased rates may be
due to the aggressiveness of anticancer therapies, greater
awareness of the issue, improved diagnostic imaging techniques or
improved cancer survival rates. Although the prevalence of
cancer-associated VTE has previously been described (2,10,14),
research focusing on VTE in patients with cancer in China is
lacking (11). Furthermore,
clinical practice guidelines for VTE treatment among cancer
patients are continually updated. The American Society of Clinical
Oncology (ASCO) first published a guideline focused on the topics
in 2007, with updates in 2013, 2015 and 2019(15). The 2019 ASCO clinical practice
guideline re-affirmed that most hospitalized patients with cancer
required thromboprophylaxis throughout hospitalization and
suggested that rivaroxaban and edoxaban were options for VTE
treatment (15). Research
detailing current anticoagulation therapy strategies and
implementation among patients with cancer-associated VTE is
important, however, further investigations are required (3). The present study was performed to
investigate and analyze the prevalence, characteristics and
anticoagulation therapy status of cancer-associated VTE in a
clinical setting, to provide a reference for its prevention and
treatment.
Materials and methods
Patients and study design
The present retrospective cohort study was conducted
at The First Affiliated Hospital of Guangxi Medical University
(Nanning, China). Written informed consent was waived due to no
identifiable patient data and the retrospective nature of the
study. The Ethical Review Committee of The First Affiliated
Hospital of Guangxi Medical University (Nanning, China; approval
no. KY-E-059) approved the present study. The present study focused
on hospitalized patients with major solid tumors that were admitted
to the hospital from January 2020 to December 2020. Inclusion
criteria were as follows: Inpatients aged ≥18 years with a
diagnosis of primary lung, ovarian, breast, gastric, colorectal,
cervical or pancreatic cancer. Patients with double primary cancer
were excluded. Patients were identified according to the
International Classification of Diseases, 10th Revision, Clinical
Modification (ICD-10-CM) guidelines (16). Patients who received
anticoagulation therapy for cancer-associated VTE were followed up
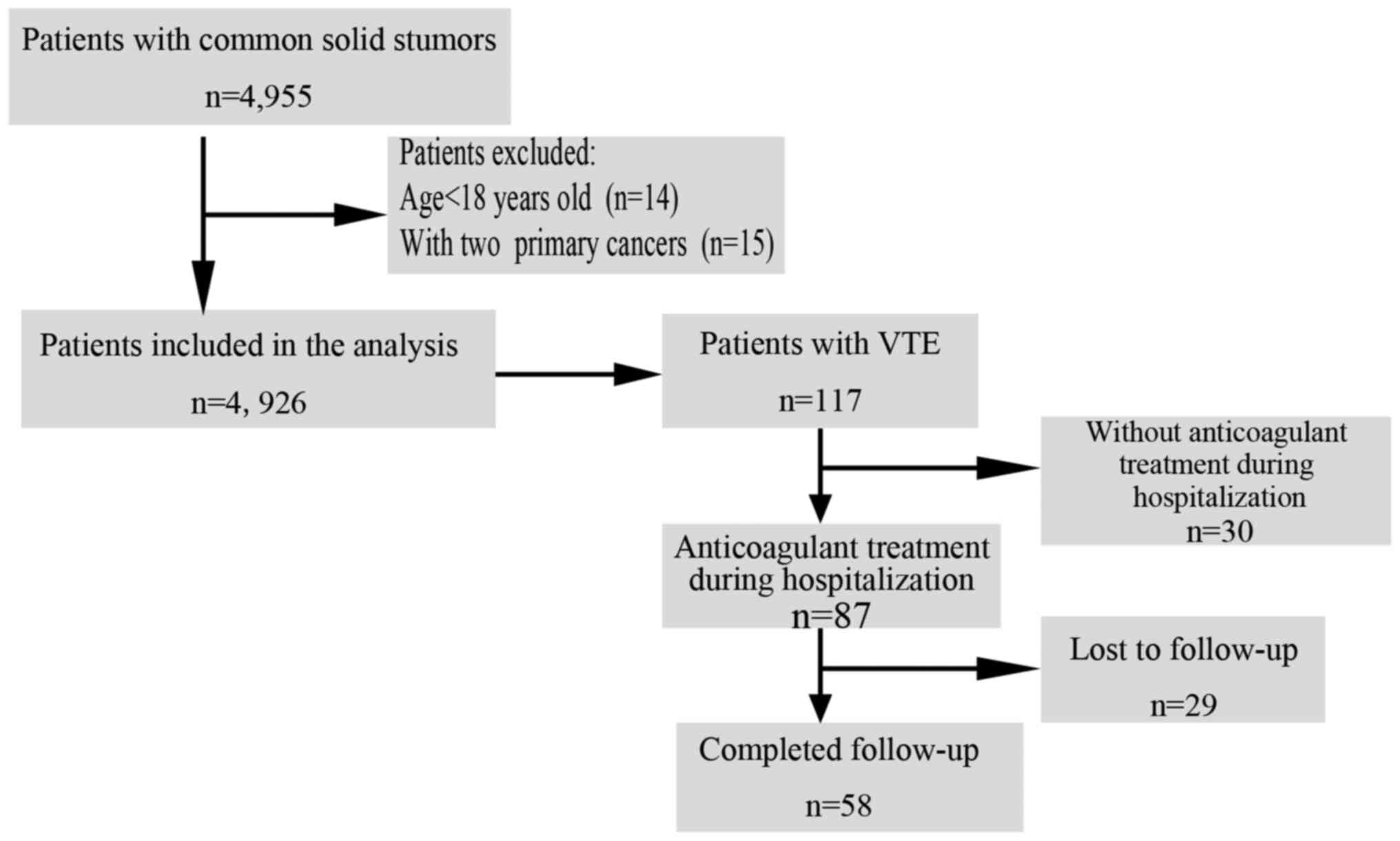
for 1 year. The study flow chart is displayed in Fig. 1.
Medical records primarily included baseline
demographic information such as sex, age, ethnicity, educational
background and marital status. Disease-associated information
(diagnosis, diagnosis time, tumor staging, comorbidities and
symptoms, among others) and treatment-associated information
(treatment regimens, treatment initiation time, termination time
and hemorrhagic complications) were also available. Data were
abstracted from the medical database by medical records data
specialists and reviewed by the first authors of the current study.
The follow-up information was mainly collected through the review
of medical records and/or contacting the patients via telephone
communication. DVT included any thromboses in a deep leg/calf vein,
pelvic vein, vena cava or upper extremity. DVT was diagnosed based
on ultrasonographic results. PE was diagnosed via computerized
tomographic pulmonary angiography. Both new and existing VTE in
combination, as well as recurrent VTEs, were included in the
analysis. In case of suspicious VTE symptoms, such as limb
swelling, pain, chest pain, shortness of breath, dyspnea and
others, the patient underwent diagnostic tests for VTE.
Additionally, the screening decision of VTE was also based on the
disease status and the clinical experience of physicians. Patients
with multiple hospitalizations were regarded as one case and
underwent comprehensive analysis during the observational year.
Cancer stage was established through clinical evaluation, imaging
and the Union for International Cancer Control TNM cancer
classification (17). Patients who
had completed all planned cancer treatments for 6 months and
experienced recurrence were defined as new cancer cases and were
handled as stage IV cases in the present study.
Statistical analysis
Data were annotated with an anonymous medical code
and reviewed by the first authors of the present study. All data
were subsequently analyzed using SPSS 17.0 software (SPSS, Inc.).
The demographic and clinical characteristics of the patients were
summarized and statistically analyzed. Continuous variables are
presented as the mean ± standard deviation, and categorical
variables are presented as counts and percentages. The prevalence
of cancer-associated VTE was calculated using the number of
positive cases vs. the total number of patients. Categorical
variables were compared using either χ2 or Fisher's
exact tests. P<0.05 was considered to indicate a statistically
significant difference. All variables with P<0.05 following
univariate analyses were examined for a second time using a
logistic regression model.
Results
A total of 4,926 patients with solid tumors (mean
age, 55.86±11.97 years; range, 19-92 years) were eligible for
analysis, of which 40.9% were male (2,016/4,926) and 59.1% were
female (2,910/4,926). Lung cancer (45.4%; 2,237/4,926) was the most
common cancer type, followed by breast (21.9%; 1,078/4,926),
colorectal (12.7%; 628/4,926), gastric (9.8%; 482/4,926), cervical
(6.0%; 296/4,926), ovarian (3.2%; 156/4,926) and pancreatic cancer
(1.0%; 49/4,926). There were statistically significant differences
(P<0.05) in VTE rates between different wards, cancer types and
cancer stages, as well as between the presence and absence of
hypertension. The characteristics of the study population are
displayed in Table I. Multivariate
analysis identified hypertension [odds ratio (OR), 1.661; 95% CI,
1.031-2.674; P=0.037)] and cancer stage (OR, 1.266; 95% CI,
1.079-1.486; P=0.004) as independent risk factors for
cancer-associated VTE.
 | Table IBaseline characteristics of the study
population and associated prevalence of VTE (n=4,926). |
Table I
Baseline characteristics of the study
population and associated prevalence of VTE (n=4,926).
| Characteristic | Total, n (%) | Without VTE, n
(%) | VTE, n (%) | P-value |
χ2-value |
|---|
| Sex | | | | | |
|
Female | 2,910 (59.1) | 2,851 (98.0) | 59 (2.0) | 0.054 | 3.706 |
|
Male | 2,016 (40.9) | 1,958 (97.1) | 58 (2.9) | | |
| Age range,
years | | | | | |
|
18-30 | 88 (1.8) | 87 (98.9) | 1 (1.1) | 0.360 | 4.352 |
|
31-40 | 433 (8.8) | 423 (97.7) | 10 (2.3) | | |
|
41-50 | 976 (19.8) | 956 (98.0) | 20 (2.0) | | |
|
51-60 | 1,626 (33.0) | 1,593 (98.0) | 33 (2.0) | | |
|
>60 | 1,803 (36.6) | 1,750 (97.1) | 53 (2.9) | | |
| Ethnicity | | | | | |
|
Han | 3,067 (62.3) | 2,993 (97.6) | 74 (2.4) | 0.735a | 1.274 |
|
Zhuang
minority | 1,694 (34.4) | 1,657 (97.8) | 37 (2.2) | | |
|
Yao
minority | 85 (1.7) | 82 (96.5) | 3 (3.5) | | |
|
Other
minorities | 80 (1.6) | 77 (96.3) | 3 (3.8) | | |
| Wards | | | | | |
|
Respiratory
Medicine | 1,106 (22.5) | 1,048 (94.8) | 58 (5.2) | <0.001 | 89.832 |
|
Gynecology | 242 (4.9) | 233 (96.3) | 9 (3.7) | | |
|
Colorectal
Surgery | 350 (7.1) | 349 (99.7) | 1 (0.3) | | |
|
Digestive
and Liver Diseases | 924 (18.8) | 917 (99.2) | 7 (0.8) | | |
|
Oncology | 1,207 (24.5) | 1,179 (97.7) | 28 (2.3) | | |
|
Radiation
Oncology | 241 (4.9) | 241 (100.0) | 0 (0.0) | | |
|
Thoracic
Surgery | 670 (13.6) | 668 (99.7) | 2 (0.3) | | |
|
Others | 186 (3.8) | 174 (93.5) | 12 (6.5) | | |
| Cancer types | | | | | |
|
Lung | 2,237 (45.4) | 2,164 (96.7) | 73 (3.3) | <0.001 | 46.162 |
|
Ovarian | 156 (3.2) | 147 (94.2) | 9 (5.8) | | |
|
Breast | 1,078 (21.9) | 1,068 (99.1) | 10 (0.9) | | |
|
Colorectal | 628 (12.7) | 623 (99.2) | 5 (0.8) | | |
|
Gastric | 482 (9.8) | 471 (97.7) | 11 (2.3) | | |
|
Cervical | 296 (6.0) | 292 (98.6) | 4 (1.4) | | |
|
Pancreatic | 49 (1.0) | 44 (89.9) | 5 (10.2) | | |
| Diabetes
comorbidity | | | | | |
|
Yes | 291 (5.9) | 287 (98.6) | 4 (1.4) | 0.248 | 1.335 |
|
No | 4,635 (94.1) | 4,522 (97.6) | 113 (2.4) | | |
| Hypertension
comorbidity | | | | | |
|
Yes | 4,188 (85.0) | 712 (96.5) | 26 (3.5) | 0.026 | 4.933 |
|
No | 738 (15.0) | 4,097 (97.8) | 91 (2.2) | | |
| Cancer stage | | | | | |
|
Stage I | 901 (18.5) | 891 (98.9) | 10 (1.1) | <0.001 | 34.604 |
|
Stage
II | 723 (14.8) | 718 (99.3) | 5 (0.7) | | |
|
Stage
III | 836 (17.1) | 819 (98.0) | 17 (2.0) | | |
|
Stage
IV | 1,386 (28.4) | 1,329 (95.9) | 57 (4.1) | | |
|
Undefined | 1,031 (21.1) | 1,008 (97.8) | 23 (2.2) | | |
A total of 117 (2.4%; 117/4,926) patients with solid
tumors were diagnosed with cancer-associated VTE. In the population
analyzed in the present study, PE and DVT occurred with 0.9%
(43/4,926) and 2.0% (100/4,926) prevalence, respectively. Among the
different cancer types, patients with pancreatic cancer exhibited
the highest prevalence of VTE (10.2%; 5/49), followed by patients
with ovarian (5.8%; 9/156), lung (3.3%; 73/2,237), gastric (2.3%;
11/482), cervical (1.4%; 4/296), breast (0.9%, 10/1,078) and
colorectal cancer (0.8%, 5/628). Moreover, the prevalence of
cancer-associated VTE were significantly different between the
cancer types (P<0.001). The prevalence of PE and DVT also varied
significantly between different cancer types (data not shown).
Results of the present study demonstrated that PE was the main
burden for patients with pancreatic cancer and lung cancer, while
DVT was the main burden for patients with pancreatic cancer and
ovarian cancer. Both PE and DVT occurred in various cancer stages
with a significant difference in prevalence (P<0.001; data not
shown). Lower extremity DVT occurred in 1.3% (62/4,926) of all
cancer cases, accounting for 62.0% of all DVT diagnoses and 53.0%
of all VTE diagnoses in the present study. DVT accompanied PE in
58.1% of all PE cases. Lower extremity DVTs accompanied by PE
accounted for 53.5% of all PE cases. A total of 25.0% (26/104) of
all DVTs were associated with a central venous catheter.
Additionally, 25 patients exhibited dual diagnoses of both PE and
DVT. Notably, this overlap created a disparity between the sums of
all PE and DVT cases compared with the total number of VTEs. A
summary of all VTEs experienced among patients with different
cancer types is displayed in Table
II.
 | Table IISummary of VTE in different cancer
types. |
Table II
Summary of VTE in different cancer
types.
| Cancer type | Total, n (%) | Total VTE, n
(%) | PE with or without
DVT, n (%) | PE with DVT, n
(%) | PE with lower
extremity-DVT, n (%) | DVT, n (%) | Lower extremity-
DVT, n (%) |
|---|
| Lung | 2,237 (45.4) | 73 (3.3) | 36 (1.6) | 21 (0.9) | 21 (0.9) | 59 (2.6) | 45 (2.0) |
| Ovarian | 156 (3.2) | 9 (5.8) | 2 (1.3) | 2 (1.3) | 1 (0.6) | 9 (5.8) | 7 (4.5) |
| Breast | 1,078 (21.9) | 10 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (0.9) | 1 (0.1) |
| Colorectal | 628 (12.7) | 5 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.8) | 1 (0.2) |
| Gastric | 482 (9.8) | 11 (2.3) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 10 (2.1) | 4 (0.8) |
| Cervical | 296 (6.0) | 4 (1.4) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 4 (1.4) | 3 (1.0) |
| Pancreatic | 49 (1.0) | 5 (10.2) | 3 (6.1) | 1 (2.0) | 0 (0.0) | 3 (6.1) | 1 (2.0) |
| Total | 4,926 | 117 (2.4) | 43 (0.9) | 25 (0.5) | 23 (0.5) | 100 (2.0) | 62 (1.3) |
Cancer-associated VTE was dominated by symptomatic
VTE (59.8%; 70/117), with the most common symptoms including chest
tightness, shortness of breath in patients with symptomatic PE and
swelling in patients with symptomatic DVT. Most cases of
cancer-associated VTE (63.2%; 74/117) developed 30 days before or
after cancer diagnosis. Out of the 117 patients with
cancer-associated VTE, 87 (74.4%) received anticoagulant treatment
with or without adjuvant treatment, 52 received heparin treatment,
44 received rivaroxaban treatment and 6 received rivaroxaban
combined with aescuven forte during hospitalization. After
discharge, patients with VTE predominantly received direct oral
anticoagulant (DOAC) treatment, such as rivaroxaban (57.3%;
67/117). The median duration of anticoagulant treatment was 79 days
(range 3-420 days). The anticoagulants associated with bleeding
events were rivaroxaban (4.2%; 3/72) and enoxaparin (1.9%; 1/54). A
total of 62.1% (36/58) of patients received anticoagulant treatment
for <90 days. The main reason for discontinuing anticoagulant
treatment was the judgement of physicians (51.7%; 30/58), followed
by the disappearance of the thrombus (24.1%; 14/58) and patient
decisions (22.4%; 13/58). In patients receiving anticoagulant
treatment until thrombolysis was complete, the mean time needed to
complete thrombolysis was 233.14±112.40 days. The clinical
characteristics of patients with VTE are shown in Table III.
 | Table IIIClinical characteristics of VTE
cases. |
Table III
Clinical characteristics of VTE
cases.
| Characteristic | n | % |
|---|
| Type of VTE
(n=117) | | |
|
Symptomatic | 70 | 59.8 |
|
Asymptomatic | 47 | 40.2 |
| Time of VTE
diagnosis (n=117) | | |
|
Before
cancer diagnosis, days | | |
|
1-30 | 29 | 24.8 |
|
31-180 | 9 | 7.7 |
|
>180 | 0 | 0 |
|
After cancer
diagnosis, days | | |
|
0-30 | 45 | 38.5 |
|
31-180 | 18 | 15.4 |
|
>180 | 16 | 13.7 |
| VTE therapy during
hospitalization (n=117) | | |
|
Without
anticoagulant treatment | 30 | 25.6 |
|
Anticoagulant
treatment | 87 | 74.4 |
|
Heparin | 52 | 44.4 |
|
Warfarin | 1 | 0.9 |
|
Direct
oral anticoagulant (Rivaroxaban) | 44 | 37.6 |
|
Rivaroxaban
combined with aescuven forte | 6 | 5.1 |
|
Thrombolysis | 3 | 2.6 |
|
Inferior
vena cava filter use | 8 | 6.8 |
| VTE therapy after
discharge (n=117) | | |
|
Without
anticoagulant treatment | 40 | 34.2 |
|
Anticoagulant
treatment | 77 | 65.8 |
|
Heparin | 12 | 10.3 |
|
Direct
oral anticoagulant (Rivaroxaban) | 67 | 57.3 |
| Anticoagulant
treatment (n=87) | | |
|
Lost to
follow-up | 29 | 33.3 |
|
Completed
follow-up | 58 | 66.7 |
|
1-14
days | 13 | 22.4 |
|
15-30
days | 8 | 13.8 |
|
31-90
days | 15 | 25.9 |
|
91-180
days | 11 | 19.0 |
|
181-365
days | 10 | 17.2 |
|
>365
days | 1 | 1.7 |
| Reasons for
discontinued anticoagulant treatment (n=58) | | |
|
Disappearance
of the thrombus | 14 | 24.1 |
|
Physician's
other judgment | 30 | 51.7 |
|
Patient's
decision | 13 | 22.4 |
|
Patient
death | 1 | 1.7 |
Discussion
Cancer and cancer-associated treatment methods are
well-established VTE risk factors (3). The present study focused on patients
with lung, ovarian, breast, colorectal, gastric, cervical and
pancreatic cancer to determine the prevalence, characteristics and
anticoagulation therapy strategies for cancer-associated VTE in a
clinical setting. The overall prevalence of cancer-associated VTE
was 2.4% and this was significantly different among cancer types.
The main cancer types affected by VTE were pancreatic, ovarian and
lung cancer. The incidence rates observed in the present study were
higher than those obtained in a previous study, which indicated
that the incidence rate of VTE was 1.79% in patients with solid
tumors and 4.49% in patients with ovarian cancer, 4.42% in patients
with pancreatic cancer and 2.57% in patients with lung cancer.
Results of this previous study also indicated that patients were at
a relatively high risk of developing VTEs (11) The results of the present study are
comparable to those obtained by Mulder et al (2), who revealed that the 12-month
cumulative incidence of VTE following cancer diagnosis is 3% and
that pancreatic cancer (4.4%) exhibits the highest 6-month
cumulative incidence of VTE across cancer types. A previous study
demonstrated an overall VTE rate of 4.1%, including DVTs (3.4%) and
PEs (1.1%), in which pancreatic (8.1%), ovarian (5.6%), lung (5.1%)
and stomach (4.9%) cancers were also predominant cancers burdened
with VTE (18). An updated
literature review on cancer-associated VTE indicated that patients
with pancreatic, brain, lung and ovarian cancer were at the
greatest risk of VTE development, with a lower risk observed in
patients with breast cancer (5).
Results of the present study demonstrated that cancer-associated
VTE is common in the clinical setting. The prevalence of
cancer-associated VTE varies widely in different studies depending
on the study design, cancer types, follow-up time and detection
method for thrombotic events. However, results of previous studies
have demonstrated that patients with pancreatic, ovarian, lung and
gastric cancer exhibit a higher risk of VTE than other cancers
(2,5,9,18).
This may be associated with the occurrence of cancer metastasis or
advanced cancer stages. The characteristics of VTE in patients with
cancer in China remain unclear; thus, further investigations of
cancer-associated VTE are required.
Although previous studies have identified the
association between medical comorbidities and a higher risk of VTE
in patients with cancer, statistically significant differences in
VTE development were only observed among patients with cancer with
and without renal failure, respiratory disease, obesity or
infection comorbidities (5,19,20).
The present study found that patients with cancer and hypertension
exhibited a higher risk of VTE development (OR, 1.661) compared
with patients without hypertension. Hypertension as a
cardiovascular risk factor associated with thrombotic disease was
previously examined in a study of myeloproliferative neoplasm
(21). We hypothesized that the
presence of hypertension may induce endothelial dysfunction. Thus,
physicians should control and review vascular comorbidities in
patients with cancer.
Cancer stage (OR,1.266) was also an independent risk
factor for cancer-associated VTE in the present study. Cancer stage
and metastasis have previously been recognized as high-risk factors
for VTE (2,5,10,11,19,20,22).
A review on cancer-associated thrombosis has indicated that cancer
stage, rather than cancer type, was the dominant risk factor for
VTE (6). Stage IV cancer with
metastatic spread and increased tumor burden contributes to
increasing thromboembolic risk, which in turn accelerates tumor
aggressiveness and poor prognosis by forming fibrin microclots that
protect circulating tumor cells from shear stress and natural
killer cell-mediated attacks (23,24).
Thus, VTE, metastatic spread and cancer aggressiveness interact to
promote cancer development, therefore the prevention and treatment
of cancer-associated VTE are crucial for a favorable outcome.
Overall, DVT prevalence was higher than PE
prevalence in patients with cancer-associated VTE in the present
study (2.0% vs. 0.9%), which is consistent with the results of
previous studies, showing that DVT was more common than PE
(10,12,14).
However, Cohen et al (13)
showed that PE (53.7%) was more prevalent than DVT (46.3%) in
patients with active cancer, a notable difference from the findings
of the present study. We hypothesize that the prevalence of PE may
be higher than the data (0.9%) we found. Since some patients may
refuse diagnostic imaging detection, PE may be undiagnosed. In
addition, only those patients with suspicious PE symptoms would
undergo diagnostic imaging tests to confirm the presence or absence
of PE, allowing certain PE cases to go undiagnosed. The prevalence
of PE and DVT was also markedly different among different cancer
types observed in the present study. PE predominantly occurred in
patients with pancreatic and lung cancer, while DVT mainly occurred
in patients with pancreatic and ovarian cancer. Pancreatic cancer
is commonly detected at advanced stages and is accompanied by
increased mucin expression and tumor cell-derived coagulation
factor and cytokine secretion, which facilitate both PE and DVT
development (25). Khorana et
al (26) indicated that PE was
most frequent in patients with lung cancer, followed by gastric
cancer. A previous study investigated patients with solid
malignancies complicated with PE and demonstrated that patients
with lung cancer exhibited a higher incidence of PE than patients
with other solid tumors (27).
However, the specific underlying mechanisms require further
study.
In the present study, lower extremity DVTs accounted
for 53.0% (62/117) of all VTEs and 53.5% (23/43) of PEs. This
differs from a previous study demonstrating that 87.3% of DVTs
without PE and upper extremity DVTs (47.2%) are more common than
lower extremity DVTs in patients with cancer (11).
Central venous catheters were the major factor
contributing to upper extremity DVTs. Central venous
catheter-associated DVTs comprised 25.0% of total DVTs in the
present study, which was notably lower than the percentage (74.9%)
reported by Peng et al (11). These differences may be due to
different central venous catheter applications and clinical
practices. Additionally, the majority of PEs developed from lower
extremity DVTs in the present study. PE (often developed from lower
extremity DVTs) is the most dangerous form of VTE and can be fatal
if left undiagnosed or untreated (28). Therefore, the prevention of VTE,
particularly among patients with cancer and lower extremity DVTs,
should not be overlooked. CT pulmonary angiography is widely
available for PE testing, but is not adequate because some patients
give up CTPA screening (1).
Ultrasound surveillance and systematic anticoagulation therapy have
also been evaluated for the improved management of lower extremity
DVTs (29). Moreover, certain
physical interventions, including leg or upper body exercises,
intermittent pneumatic compression and compression stockings for
lower extremity DVT prevention, have also been applied in the
clinical setting (30-32).
This highlights the importance of lower extremity DVT prevention.
Further studies are required in patients with cancer.
In the present study, the majority of
cancer-associated VTEs (63.2%) developed 30 days before or after
cancer diagnosis. Comparable with the results of a previous study,
the risk of VTE was the highest in the first 6 months following
cancer diagnosis (11). Reasons
for higher risk of cancer-associated thrombosis in the months
surrounding cancer diagnosis are likely associated with higher
tumor burden and intensive therapy (including surgery, radiation
and systemic therapy). Moreover, cancer-associated VTE was
dominated by symptomatic VTE (59.8%), which is consistent with the
results of a previous study, highlighting that the majority of
patients with lung cancer were symptomatic for VTE (33). The most common symptoms of VTE were
chest tightness, shortness of breath and swelling. Thus, healthcare
professionals should be familiar with the characteristics of VTE
for earlier identification, evaluation, diagnosis and
treatment.
Results of the present study displayed that 74.4%
(87/117) and 65.8% (77/117) of patients with cancer-associated VTE
received anticoagulant treatment during hospitalization and after
discharge, respectively. A DOAC, such as rivaroxaban, was widely
used during hospitalization, particularly following discharge.
Rivaroxaban use was consistent with the updated clinical practice
guidelines (15,34), which indicated that DOACs, such as
rivaroxaban, have been added as options for VTE treatment among
patients with cancer. The present finding is also similar to that
of a previous study, indicating that 57.6% of patients with
cancer-associated VTE received oral anticoagulants (33). However, a recent study showed that
only 25.0% of patients with cancer-associated VTE received
anticoagulation therapy, and low-molecular-weight heparin (LMWH)
remained the most widely used anticoagulant drug among patients
with advanced cancer (14). The
difference may be attributed to the patients with advanced cancer
having an increased risk of bleeding because of rivaroxaban use
(14). Moreover, the results of
the present study demonstrated that the anticoagulants associated
with bleeding events were rivaroxaban (4.2%) and enoxaparin (1.9%).
We hypothesize that rivaroxaban exerts a higher rate of bleeding. A
meta-analysis including three randomized controlled trials and
1,739 patients with cancer-associated VTE indicated that DOACs
exerted a greater reduction in VTE recurrence and an increased
incidence of major bleeding compared with dalteparin (35). Results of another previous study
demonstrated that rivaroxaban exerted significantly higher
clinically relevant non-major bleeding than dalteparin (13% vs. 4%)
(36). In conclusion, DOACs have
been recommended as adequate and preferable options to LMWH for
cancer-associated VTE therapy (15). Additional clinical trials
addressing anticoagulation therapy strategies and their efficacy
and safety are still needed.
Furthermore, guidelines recommend long-term
anticoagulation therapy lasting at least 6 months for improved
efficacy (15). The median
duration of anticoagulant treatment was only 79 days in the present
study. A total of 62.1% of patients received anticoagulant
treatment for <90 days. Anticoagulation therapy strategies in
daily clinical practice do not follow current guideline
recommendations. The main reason for discontinuing anticoagulant
treatment was the judgement of physicians (51.7%), followed by the
disappearance of the thrombus (24.1%) and patient decisions
(22.4%). We hypothesize that inadequate knowledge may contribute to
discontinuing anticoagulant treatment. Moreover, high costs due to
the long-term use of anticoagulants and the inconvenience due to
subcutaneous injection of LMWH for patients may be barriers to
continuing anticoagulant treatment. Therefore, long-term
anticoagulation therapy remains challenging due to balancing the
benefits and risks of bleeding as well as the inconvenience of
LMWH.
The present study exhibits several limitations that
should be acknowledged. The results of the present study relied on
data obtained from a single medical center, which may be
insufficient to estimate the prevalence of cancer-associated VTE
for a more generalized population. Because of the retrospective
nature of the present study, there may be cases that missed
screening and went underdiagnosed, leading to the underestimation
of the prevalence of VTE. In addition, the prevalence of
cancer-associated VTE was not analyzed in relation to different
chemotherapy regimens. Moreover, although the effects and bleeding
complications of anticoagulation therapy were assessed in the
present study, VTE recurrence was not measured due to the 1-year
follow-up limit. Further research is required using prospective
multicenter cohorts to detect new possible trends and assess the
variety of treatment regimens with a longer follow-up, to further
explore more operational management strategies for
cancer-associated VTE and also assess the prognosis between
patients with VTE and those without VTE.
At present, cancer-associated VTE is a common
occurrence. Notably, the prevalence of cancer-associated VTE, PE
(with or without DVT) and DVT is significantly different among
different cancer types. Patients with cancer and hypertension
comorbidity, as well as patients with stage IV cancer, are at
higher risk for the development of cancer-associated VTE. Moreover,
the majority of cancer-associated VTE cases develop 30 days before
or after cancer diagnosis. DOACs, such as rivaroxaban, have been
widely used for anticoagulation therapy. However, more studies
investigating the efficacy and safety of anticoagulant drugs are
still needed. At present, compliance with long-term anticoagulant
treatment is not adequate. The present study results highlighted
that further VTE screening among patients with cancer is required,
along with standardized anticoagulation therapy, long-term
medication monitoring and patient follow-up.
Acknowledgements
Not applicable.
Funding
Funding: This work was partially supported by the National
Natural Science Foundation of China (grant no. 81860032), Climbing
Project of Nursing Clinical Research (grant no. YYZS2020025,
YYZS2020031) and Self-Funded Plan Projects of Guangxi Health
Commission (grant no. Z20190398).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and YH designed the present study, applied for
the ethics committee approval, provided general supervision, and
revised and finalized the manuscript. HZ, FL and YL participated in
the study design, reviewed the data analysis, drafted and revised
the manuscript. BF contributed to the study design, drafted and
revised the manuscript, and was involved in language editing and
picture processing. TL and TD participated in the study design,
collection of data, data analysis and writing of the manuscript. HZ
and YH assessed the raw data and confirm the authenticity of all
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethical Review Committee of the First Affiliated
Hospital of Guangxi Medical University approved the present study
(Nanning, China; approval no. KY-E-059). Written informed consent
was waived due to no identifiable patient data and the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Nisio M, van Es N and Büller HR: Deep
vein thrombosis and pulmonary embolism. Lancet. 388:3060–3073.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mulder FI, Horváth-Puhó E, van Es N, van
Laarhoven HWM, Pedersen L, Moik F, Ay C, Büller HR and Sørensen HT:
Venous thromboembolism in cancer patients: A population-based
cohort study. Blood. 137:1959–1969. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khorana AA, Mackman N, Falanga A, Pabinger
I, Noble S, Ageno W, Moik F and Lee AYY: Cancer-associated venous
thromboembolism. Nat Rev Dis Primers. 8(11)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Falanga A, Russo L, Milesi V and Vignoli
A: Mechanisms and risk factors of thrombosis in cancer. Crit Rev
Oncol Hematol. 118:79–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mahajan A, Brunson A, White R and Wun T:
The epidemiology of cancer-associated venous thromboembolism: An
update. Semin Thromb Hemost. 45:321–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fernandes CJ, Morinaga LTK, Alves JLJ,
Castro MA, Calderaro D, Jardim CVP and Souza R: Cancer-associated
thrombosis: The when, how and why. Eur Respir Rev.
28(180119)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weitz JI, Haas S, Ageno W, Goldhaber SZ,
Turpie AGG, Goto S, Angchaisuksiri P, Nielsen JD, Kayani G, Farjat
AE, et al: Cancer associated thrombosis in everyday practice:
Perspectives from GARFIELD-VTE. J Thromb Thrombolysis. 50:267–277.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Levitan N, Dowlati A, Remick SC, Tahsildar
HI, Sivinski LD, Beyth R and Rimm AA: Rates of initial and
recurrent thromboembolic disease among patients with malignancy
versus those without malignancy. Risk analysis using Medicare
claims data. Medicine (Baltimore). 78:285–291. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kenmotsu H, Notsu A, Mori K, Omori S,
Tsushima T, Satake Y, Miki Y, Abe M, Ogiku M, Nakamura T, et al:
Cumulative incidence of venous thromboembolism in patients with
advanced cancer in prospective observational study. Cancer Med.
10:895–904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohashi Y, Ikeda M, Kunitoh H, Sasako M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: Venous thromboembolism in cancer patients: Report of baseline
data from the multicentre, prospective cancer-VTE registry. Jpn J
Clin Oncol. 50:1246–1253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Peng M, Yang S, Li G, Zhang T, Qin X, Shi
C, Chang J, Chen M, Chen C, Li B, et al: Solid tumor complicated
with venous thromboembolism: A 10-year retrospective
cross-sectional study. Clin Appl Thromb Hemost.
27(1076029620975484)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bao Y, Wan X, Fu J and Wu B: The risk of
venous thromboembolism in cancer patients receiving chemotherapy: A
meta-analysis with systematic review. Ann Transl Med.
9(277)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cohen AT, Katholing A, Rietbrock S, Bamber
L and Martinez C: Epidemiology of first and recurrent venous
thromboembolism in patients with active cancer. A population-based
cohort study. Thromb Haemost. 117:57–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gurizzan C, Roca E, Faggiano A, Paoli D,
Dinatolo E, Masini G, Tomasi C, De Palma G, Metra M, Berruti A and
Faggiano P: Rate of venous thromboembolism and atrial fibrillation
in a real-world case series of advanced cancer patients: The CaTEV
study. J Cardiovasc Med (Hagerstown). 22:444–452. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW,
et al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J Clin Oncol.
38:496–520. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Centers for Disease Control and
Prevention: ICD-10-CM Browser Tool. https://www.cdc.gov/nchs/icd/icd10cm_browsertool.htm.
Accessed November 20, 2019.
|
|
17
|
Piñeros M, Parkin DM, Ward K, Chokunonga
E, Ervik M, Farrugia H, Gospodarowicz M, O'Sullivan B,
Soerjomataram I, Swaminathan R, et al: Essential TNM: A registry
tool to reduce gaps in cancer staging information. Lancet Oncol.
20:e103–e111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Frequency, risk factors, and trends for
venous thromboembolism among hospitalized cancer patients. Cancer.
110:2339–2346. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abdol Razak NB, Jones G, Bhandari M,
Berndt MC and Metharom P: Cancer-associated thrombosis: An overview
of mechanisms, risk factors, and treatment. Cancers (Basel).
10(380)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakamoto J, Yamashita Y, Morimoto T, Amano
H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, et al:
Cancer-associated venous thromboembolism in the real world-from the
COMMAND VTE registry. Circ J. 83:2271–2281. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Arachchillage DR and Laffan M:
Pathogenesis and management of thrombotic disease in
myeloproliferative neoplasms. Semin Thromb Hemost. 45:604–611.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahlbrecht J, Dickmann B, Ay C, Dunkler D,
Thaler J, Schmidinger M, Quehenberger P, Haitel A, Zielinski C and
Pabinger I: Tumor grade is associated with venous thromboembolism
in patients with cancer: Results from the Vienna cancer and
thrombosis study. J Clin Oncol. 30:3870–3875. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rondon AMR, Kroone C, Kapteijn MY,
Versteeg HH and Buijs JT: Role of tissue factor in tumor
progression and cancer-associated thrombosis. Semin Thromb Hemost.
45:396–412. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Donnellan E and Khorana AA: Cancer and
venous thromboembolic disease: A review. Oncologist. 22:199–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Frere C: Burden of venous thromboembolism
in patients with pancreatic cancer. World J Gastroenterol.
27:2325–2340. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khorana AA, Dalal M, Lin J and Connolly
GC: Incidence and predictors of venous thromboembolism (VTE) among
ambulatory high-risk cancer patients undergoing chemotherapy in the
United States. Cancer. 119:648–655. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma SQ, Lin Y, Ying HY, Shao YJ, Li XY and
Bai CM: Solid malignancies complicated with pulmonary embolism:
clinical analysis of 120 patients. Chin Med J (Engl). 123:29–33.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huisman MV, Barco S, Cannegieter SC, Le
Gal G, Konstantinides SV, Reitsma PH, Rodger M, Vonk Noordegraaf A
and Klok FA: Pulmonary embolism. Nat Rev Dis Primers.
4(18028)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Robert-Ebadi H and Righini M: Management
of distal deep vein thrombosis. Thromb Res. 149:48–55.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Caldwell K, Prior SJ, Kampmann M, Zhao L,
McEvoy S, Goldberg AP and Lal BK: . Upper body exercise increases
lower extremity venous blood flow in deep venous thrombosis. J Vasc
Surg Venous Lymphat Disord. 1:126–133. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shimizu Y, Kamada H, Sakane M, Aikawa S,
Mutsuzaki H, Tanaka K, Mishima H, Ochiai N and Yamazaki M: A novel
apparatus for active leg exercise improves venous flow in the lower
extremity. J Sports Med Phys Fitness. 56:1592–1597. 2016.PubMed/NCBI
|
|
32
|
Shalhoub J, Lawton R, Hudson J, Baker C,
Bradbury A, Dhillon K, Everington T, Gohel MS, Hamady Z, Hunt BJ,
et al: Compression stockings in addition to low-molecular-weight
heparin to prevent venous thromboembolism in surgical inpatients
requiring pharmacoprophylaxis: the GAPS non-inferiority RCT. Health
Technol Assess. 24:1–80. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Suzuki T, Fujino S, Inaba S, Yamamura R,
Katoh H, Noji Y, Yamaguchi M and Aoyama T: Venous thromboembolism
in patents with lung cancer. Clin Appl Thromb Hemost.
26(1076029620977910)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Streiff MB, Holmstrom B, Angelini D,
Ashrani A, Bockenstedt PL, Chesney C, Fanikos J, Fenninger RB,
Fogerty AE, Gao S, et al: NCCN guidelines insights:
cancer-associated venous thromboembolic disease, version 2.2018. J
Natl Compr Canc Netw. 16:1289–1303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fuentes HE, McBane RD II, Wysokinski WE,
Tafur AJ, Loprinzi CL, Murad MH and Riaz IB: Direct oral factor Xa
inhibitors for the treatment of acute cancer-associated venous
thromboembolism: A systematic review and network meta-analysis.
Mayo Clin Proc. 94:2444–2454. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ay C, Beyer-Westendorf J and Pabinger I:
Treatment of cancer-associated venous thromboembolism in the age of
direct oral anticoagulants. Ann Oncol. 30:897–907. 2019.PubMed/NCBI View Article : Google Scholar
|