Introduction
Ischemic stroke is one of the most disabling and
fatal cerebrovascular diseases (1). In addition, >70% of survivors
suffer from neurological dysfunction following a stroke (2). Improving neurological dysfunction
after stroke has become an urgent medical and social issue. Neural
stem/progenitor cells (NSPCs) are a group of pluripotent nerve
cells with regenerative ability in the central nervous system (CNS)
(3,4). NSPCs can self-renew through
symmetrical division, or can generate neuronal progenitors and
glial progenitors through asymmetric division, and can then
differentiate into mature neurons, oligodendrocytes and astrocytes
to maintain the homeostasis of CNS cell components (5-7).
Therefore, it is important to promote the proliferation and
differentiation of NSPCs to improve recovery after ischemic
stroke.
Following CNS injury, NSPCs located in the
subventricular zone and subgranular zone regions have been shown to
proliferate, migrate and differentiate into neurons and glial
cells, and serve therapeutic effects through nutritional support,
regulation of inflammatory responses, directional differentiation
for the replacement of neurons, reconstruction of neural circuits
and functions, and release of paracrine nerve growth factors
(8-10).
These findings suggest that endogenous stem cells can repair CNS
tissue damage. Notably, stem cell-based treatment of neurological
diseases may be divided into exogenous stem cell transplantation
and activation of endogenous neural stem cells (5).
Artesunate (ART), a water-soluble derivative of
artemisinin with low toxicity (11), is an antimalarial drug that can
easily penetrate the blood-brain barrier (12). ART has been demonstrated to have a
potential role in cancer therapy, prevention of organ damage and
dysfunction in hemorrhagic shock and trauma, regulation of immune
and inflammatory responses, regulation of neurotransmission and
treatment of type I diabetes (13-16).
Artemisinin is insoluble in water, thus its application is severely
inhibited. To overcome this issue, the water-soluble derivatives
artelinic acid and ART have been synthesized. Compared with ART,
artemisinic acid has greater embryonic toxicity, neurotoxicity and
nephrotoxicity (17). Moreover,
ART can reach a high concentration in the brain; even if the level
of ART drops significantly within 1 h in other tissues, it can
still be detected in the brain, fat, intestines and serum (18). Therefore, ART may have a strong
advantage in the treatment of neurological diseases. Dang et
al (19) indicated that ART
prolonged the survival of MRL/lpr mice, ameliorated symptoms of
lupus nephritis and decreased the levels of pathogenic cytokines
through activating the JAK-2/STAT-3 signaling pathway. Therefore,
the present study investigated the effects of ART treatment on
NSPCs following oxygen-glucose deprivation (OGD), in order to
clarify the role of ART in ischemic stroke.
Materials and methods
Cell culture and establishment of an
OGD model
A frozen aliquot of the third passage of NSPCs was
donated by the Department of Neurosurgery and Key Laboratory of
Neurotrauma, Southwest Hospital, Army Military Medical University
(Chongqing, China) and was used for further experiments (20). As previously described, primary
NSPCs were isolated from adult male C57BL/6 mice (age, 6-8 weeks;
weight, 18-22 g) (20). Cells were
cultured in Dulbecco's Modified Eagle's Medium (DMEM; HyClone;
Cytiva) supplemented with 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2.
Cells in the logarithmic growth phase were subjected to
trypsinization and subculture with 0.25% trypsin (HyClone; Cytiva).
The OGD model was established as previously described (20). Cells were incubated with
glucose-free and FBS-free Earle's BSS buffer (Thermo Fisher
Scientific, Inc.) at 37˚C for 8 h with 94% N2, 5%
CO2 and 1% O2. Different concentrations of
ART (0.25, 0.5, 1, 2 and 4 µmol/l) and IL-6 (2 ng/ml) were added to
medium to treat NSPCs for 48 h after ODG.
Cell counting kit-8 (CCK-8) assay
The viability of NSPCs was measured using the CCK-8
kit (Beyotime Institute of Biotechnology). CCK-8 reagent (10 µl)
was added to the cultured NSPCs (1x103 cells per well)
after OGD, followed by incubation at 37˚C for 2 h. The absorbance
was determined at 450 nm using a microplate reader (BioTek
Instruments, Inc.). The content of formazan dye was directly
proportional to the number of live cells.
Flow cytometry
NSPCs (1x106) were harvested and washed
three times with PBS. The apoptosis levels were measured using the
Annexin V-FITC apoptosis detection kit (MilliporeSigma) according
to the manufacturer' instructions. The cell suspension (190 µl) was
incubated with 5 µl Annexin V-FITC and 5 µl propidium iodide.
Subsequently, cells were incubated in the dark for 20 min at room
temperature. Apoptotic cells were subsequently analyzed using a
flow cytometer (BD Biosciences). The total apoptosis rate (early +
late) was measured by ModFit LT 5.0 (Verity Software House) in this
study.
Reverse transcription-quantitative PCR
(qPCR)
Total RNA was extracted from NSPCs using RNAiso Plus
(Takara Bio, Inc.) and the A260/A280 ratio was calculated for RNA
quantification. Total RNA was reverse transcribed into cDNA
according to manufacturer's protocol (37˚C for 15 min and 85˚C for
5 sec) using the PrimeScript™ RT Master Mix (Takara Bio,
Inc.). Subsequently, the SYBR Premix Ex Taq™ kit
(Shanghai Yihui Biotechnology Co., Ltd.) was used to perform qPCR.
The primer pairs for qPCR were designed using Primer Premier 6.0
software (PREMIER Biosoft), according to each gene sequence. The
sequences were as follows: PCNA forward,
5'-CACCTTAGCACTAGTATTCGAAGCAC-3' and reverse,
5'-CACCCGACGGCATCTTTATTAC-3'; GAPDH forward,
5'-GACATCAAGAAGGTGGTGAAGC-3' and reverse,
5'-GAAGGTGGAAGAGTGGGAGTT-3'. The following thermocycling conditions
were used for qPCR: Initial denaturation at 94˚C for 30 sec; 40
cycles at 94˚C for 15 sec and 60˚C for 60 sec. PCNA mRNA expression
levels were quantified using the 2-∆∆Cq method and
normalized to the internal reference gene GAPDH (21).
Immunofluorescence staining
NSPCs at 60-80% confluence were fixed in ice-cold
acetone (Beyotime Institute of Biotechnology) at room temperature
for 10 min, then washed three times with PBS and blocked with 5%
normal goat serum (Boster Biological Technology) at room
temperature for 1 h. Subsequently, cells were incubated with
anti-PCNA (1:100 dilution; cat. no. ab18197; Abcam) and anti-DCX
(1:400; cat. no. ab18723; Abcam) primary antibodies at 4˚C
overnight. Following primary antibody incubation, the cells were
washed with PBS and incubated with a fluorescence-labeled secondary
antibody (1:100 dilution; cat. no. BA1032; Boster Biological
Technology) at 37˚C for 1 h. A group only treated with secondary
antibody was used as a negative control. The nuclei were
counterstained with DAPI (Beyotime Institute of Biotechnology). The
stained cells were observed under a Leica fluorescence DMLB
microscope (Leica Microsystems GmbH), and the images were captured
using a CCD camera and analyzed using Image-Pro Plus v6.0
(Cool-SNAP-Pro; Media Cybernetics, Inc.).
Western blot analysis
Total protein was extracted from NSPCs using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Total protein
was quantified using a BCA assay and proteins (40 µg per lane) were
separated by SDS-PAGE on 10% gels. Subsequently, the proteins were
transferred onto a PVDF membrane (MilliporeSigma). Then the
membranes were blocked with skimmed milk (Shanghai Yifan
Biotechnology Co., Ltd.) at room temperature for 2 h and incubated
at 4˚C for 24 h with primary antibodies against cleaved-caspase-3
(1:1,000 dilution; 19 kDa; cat. no. ab214430; Abcam), DCX (1:1,000;
45 kDa; cat. no. ab18723; Abcam), phosphorylated (p)-JAK-2
(1:3,000; 120 kDa; cat. no. ab32101; Abcam), JAK-2 (1:3,000; 130
kDa; cat. no. ab108596; Abcam), p-STAT-3 (1:3,000; 98 kDa; cat. no.
ab32143; Abcam), STAT-3 (1:1,000; 88 kDa; cat. no. ab68153; Abcam)
and GAPDH (1:5,000; 36 kDa; cat. no. ab8245; Abcam). Following
primary antibody incubation, the membrane was incubated with
HRP-conjugated IgG secondary antibody (1:4,000; cat. no. 7074; Cell
Signaling Technology, Inc.) at room temperature for 2 h. The
protein bands were visualized using an enhanced chemiluminescence
kit (cat. no. P1060-25; Applygen Technologies, Inc.). The
expression levels were normalized to GAPDH using ImageJ version
1.49 software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 (IBM Corp.). All experiments were repeated at least
three times. Data are presented as the mean ± standard deviation.
One-way analysis of variance was used for multiple comparisons
followed by Bonferroni correction or Student-Newman-Keuls post-hoc
test. A P<0.05 was considered to indicate a significant
difference.
Results
ART promotes the proliferation of
NSPCs
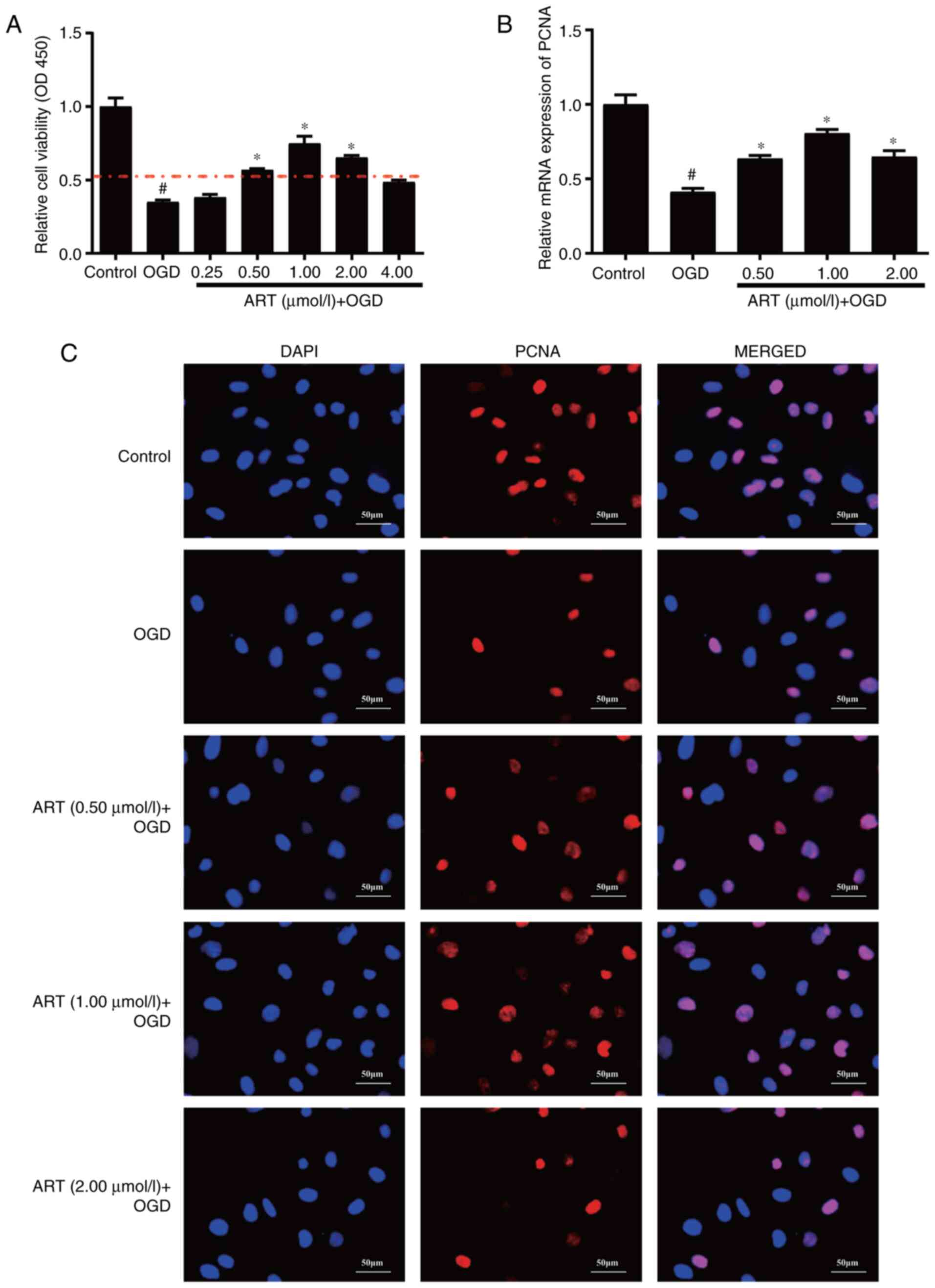
To explore the effect of ART on the viability of
NSPCs, different concentrations of ART (0.25, 0.5, 1, 2 and 4
µmol/l) were used to treat NSPCs for 48 h after ODG. The CCK-8
assay indicated that the viability of NSPCs after ODG was
significantly decreased, whereas ART promoted the viability of
NSPCs after OGD at concentrations of 0.5, 1 and 2 µmol/l (Fig. 1A). Therefore, these concentrations
were used for further experiments. Moreover, ODG significantly
inhibited the mRNA expression levels of PCNA in NSPCs compared with
those in the control group (Fig.
1B), whereas ART treatment following ODG significantly
increased the mRNA expression levels of PCNA in NSPCs compared with
those in the ODG group (Fig. 1B).
Furthermore, immunofluorescence staining indicated that ART
enhanced the fluorescence intensity of PCNA in NSPCs after ODG
(Fig. 1C). Taken together, these
data indicated that ART promoted the proliferation of NSPCs after
OGD.
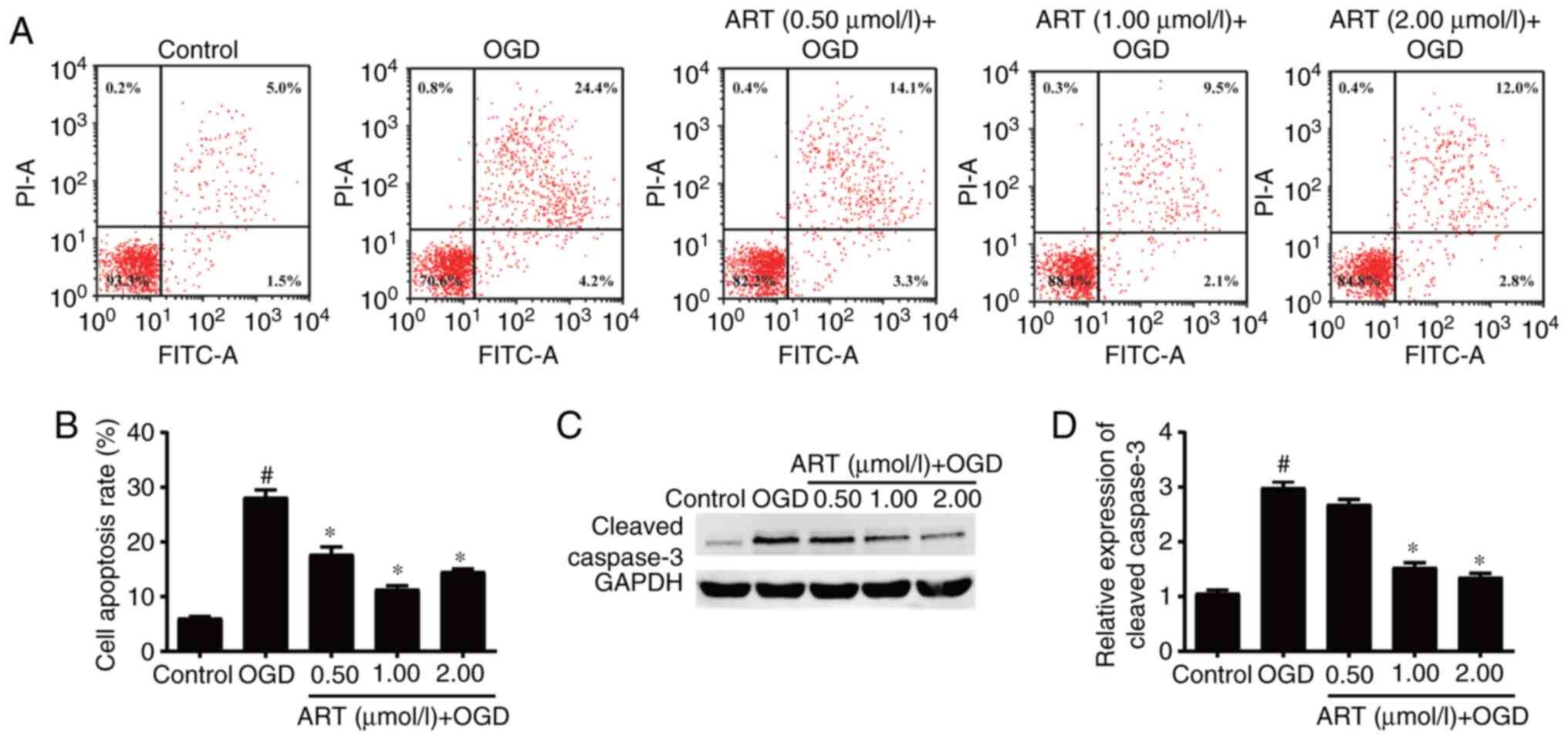
ART inhibits apoptosis of NSPCs
The proliferation and apoptosis of NSPCs have an
important role in repairing nerve function after ischemic stroke
(7). Flow cytometry suggested that
OGD promoted the apoptosis of NSPCs compared with that in the
control group, whereas treatment with ART decreased the rate of
apoptosis of NSPCs after OGD compared with that in the OGD group
(Fig. 2A and B). Western blot analysis demonstrated
that OGD significantly increased the expression levels of
cleaved-caspase-3 in NSPCs compared with those in the control
group, whereas ART treatment at concentrations of 1 and 2 µmol/l
significantly decreased the protein expression levels of
cleaved-caspase-3 in NSPCs after ODG compared with those in the ODG
group (Fig. 2C and D). These results demonstrated that ART
may inhibit the OGD-induced apoptosis of NSPCs.
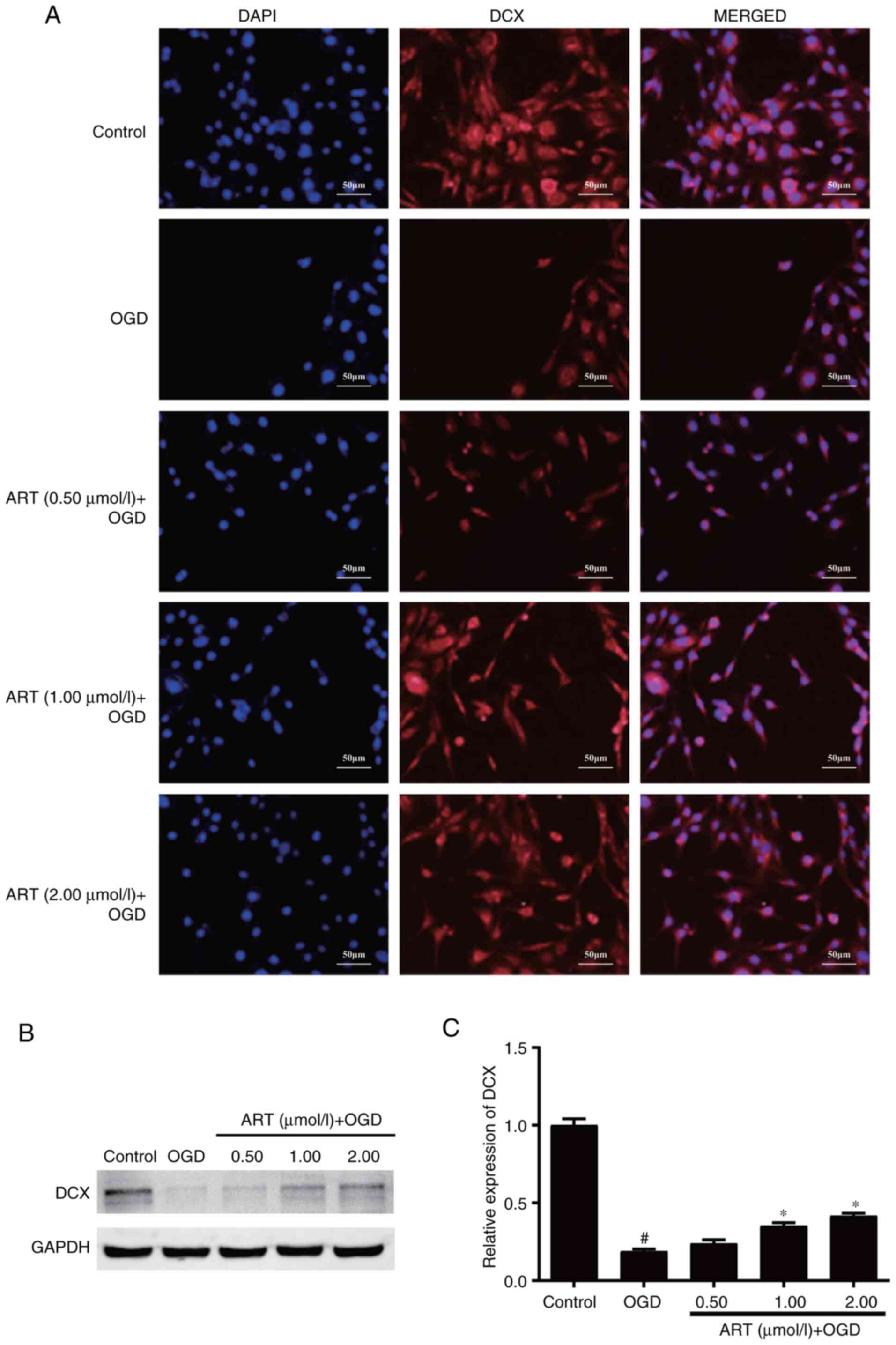
ART enhances the differentiation of
NSPCs
NSPCs are capable of self-renewal, and are able to
differentiate into neurons, oligodendrocytes and astrocytes
(3). NSPCs may be the key to cell
replacement therapy following CNS damage. Immunofluorescence
staining indicated that the fluorescence intensity of DCX was
decreased in NSPCs following OGD compared with that in the control
group, whereas ART treatment following OGD could partially restore
the fluorescence intensity of DCX in NSPCs compared with that in
the OGD group (Fig. 3A). Western
blot analysis demonstrated that OGD significantly decreased the
expression levels of DCX in NSPCs compared with those in the
control group, whereas treatment with ART at 1 and 2 µmol/l
following OGD significantly enhanced the expression levels of DCX
compared with those in the OGD group (Fig. 3B and C). The present data indicated that ART
may restore the differentiation of NSPCs impaired by OGD.
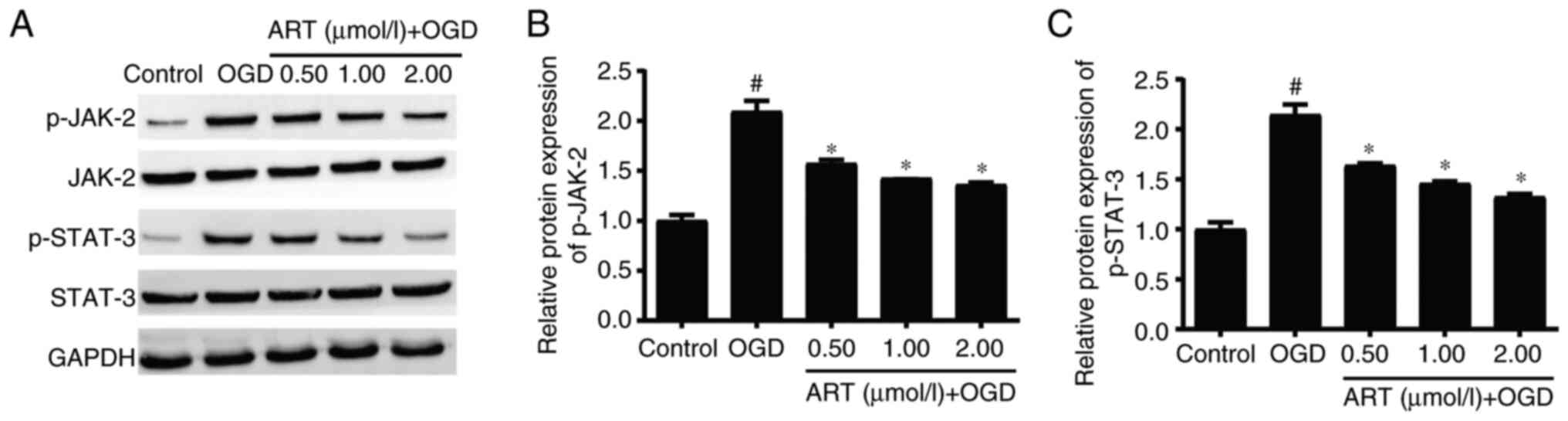
ART inhibits the JAK-2/STAT-3
signaling pathway in NSPCs
Activation of the JAK-2/STAT-3 signaling pathway is
involved in the pathophysiological process of ischemic stroke and
affects the proliferation and differentiation of astrocytes, which
is of great significance in the recovery of neurological function
after ischemic stroke (22).
Western blot analysis indicated that OGD significantly increased
the expression levels of p-JAK-2 and p-STAT-3 in NSPCs compared
with those in the control group, whereas ART treatment
significantly downregulated the expression levels of p-JAK-2 and
p-STAT-3 in NSPCs after OGD compared with those in the OGD group
(Fig. 4A-C). These findings
suggested that ART may regulate the biological functions of NSPCs
through the JAK-2/STAT-3 signaling pathway.
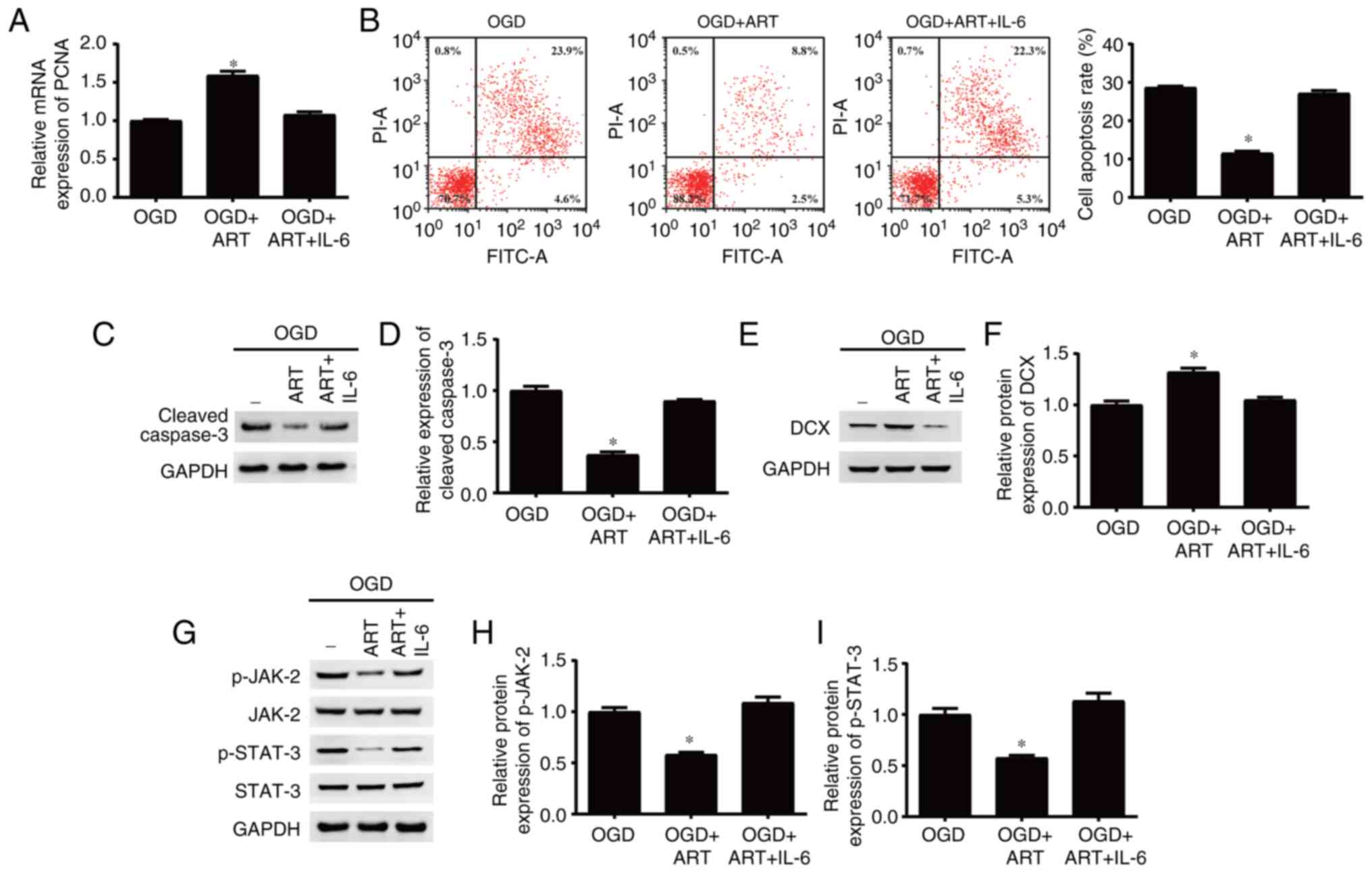
ART regulates proliferation, apoptosis
and differentiation of NSPCs after OGD through the JAK-2/STAT-3
signaling pathway
Previous studies have indicated that ART is able to
inhibit the JAK-2/STAT-3 signaling pathway in different cells
(23,24). In the present study, IL-6 was used
to activate the JAK-2/STAT-3 signaling pathway. The results
indicated that activation of the JAK-2/STAT-3 signaling pathway and
treatment with ART reversed the effects of ART treatments on the
proliferation (Fig. 5A), apoptosis
(Fig. 5B-D) and differentiation
(Fig. 5E and F) of NSPCs after OGD. Moreover,
activation of the JAK-2/STAT-3 signaling pathway inhibited by ART
may be reactivated by IL-6 in NSPCs after OGD (Fig. 5G-I). In summary, the present
results indicated that ART may regulate proliferation, apoptosis
and differentiation of NSPCs after OGD via inhibition of the
JAK-2/STAT-3 signaling pathway.
Discussion
Ischemic stroke is a common cause of disability and
death worldwide (25); notably,
the repair and reconstruction of the CNS after stroke are very
important (26). Following an
ischemic stroke, NSPCs can accelerate proliferation and
differentiation, migrate to the surrounding infarct area,
differentiate into mature neurons or glial cells, and become part
of the neuronal circuit (27).
However, the proliferation of endogenous NSPCs caused by trauma or
ischemia is not enough to induce nerve repair, which may lead to
permanent disability in patients following a stroke (28). Zhu et al (29) reported that niche-dependent
regulation of NSPC proliferation occurred following adult hypoxic
ischemia injury via the novel RBM3/IMP2/IGF2 signaling pathway.
Knotek et al (30)
demonstrated that Wnt signaling regulated the biological function
of NSPCs and could be considered useful in ischemic stroke therapy.
Gan et al (31) indicated
that long noncoding RNA H19 promoted NSPCs proliferation and
differentiation via the p53 signaling pathway. During an ischemic
stroke, due to damage to the blood-brain barrier, excitotoxicity
and neuroinflammation destroy the cellular microenvironment to a
great extent (32). The cell
microenvironment presents an evident pathological environment in
the damaged brain tissue, which is not conducive to the survival,
neurogenesis and differentiation of endogenous NSPCs (33).
Recent studies have shown that ART has
anti-neuritis, antioxidant and blood-brain barrier-protective
functions, and multipotency in promoting neurogenesis (34,35).
Liu et al (36) reported
that ART inhibited neutrophil infiltration, microglial activation
and inflammatory cytokines by suppressing the NF-κB pathway in the
distal middle cerebral artery occlusion mouse model. Zhang et
al (20) demonstrated that ART
promoted NSPCs proliferation and reduced ischemia-reperfusion
injury via the PI3K/Akt/FOXO-3a/p27 signaling pathway in ischemic
stroke. The present study indicated that ART significantly promoted
the proliferation of NSPCs after OGD, inhibited the apoptosis of
NSPCs, and promoted the expression of PCNA and DCX. The present
results indicated that ART may be considered a potential
therapeutic drug for ischemic stroke.
JAK-2/STAT-3 is an important signaling pathway in
the JAK/STAT family (37).
JAK/STAT serves an important role in cell proliferation,
differentiation and angiogenesis (38,39).
After ischemic stroke, the expression of p-STAT-3 has been reported
to increase significantly, and to participate in processes such as
neuroinflammation and angiogenesis (40). Cheng et al (22) reported that endothelin-1 could
promote the proliferation and differentiation of neural progenitor
cells through the JAK-2/STAT-3 signaling pathway after transient
middle cerebral artery occlusion. Furthermore, microRNA-101 has
been shown to inhibit the apoptosis of neuronal cells and reduce
ischemic brain injury through suppressing the JAK-2/STAT-3
signaling pathway in ischemic stroke (41). In addition, the JAK-2/STAT-3
signaling pathway has been shown to be activated in ischemic
stroke, which may be closely related to cell apoptosis,
angiogenesis, inflammatory response and oxidative stress in the
pathogenesis of ischemic stroke (42). However, it remains unclear as to
whether ART regulates the JAK-2/STAT-3 signaling pathway in
ischemic stroke. In the present study, OGD significantly increased
the expression levels of p-JAK-2 and p-STAT-3 in NSPCs, whereas ART
treatment significantly reduced the impact of OGD on the p-JAK-2
and p-STAT-3 expression in NSPCs. These results suggested that ART
may regulate the biological functions of NSPCs through the
JAK-2/STAT-3 signaling pathway.
IL-6 is an important inflammatory factor that
promotes the phosphorylation of STAT-3 protein through
JAK-2(43). The IL-6/JAK-2/STAT-3
signaling pathway has been reported to be involved in various
diseases, including tumors, ulcerative colitis, hyperuricemic
nephropathy and chronic mild stress (44-47).
In the present study, IL-6 was used to activate the JAK-2/STAT-3
signaling pathway. The present results indicated that the
activation of the JAK-2/STAT-3 signaling pathway and treatment with
ART could reverse the effects of ART on the proliferation,
apoptosis and differentiation of NSPCs after OGD. Moreover,
activation of the JAK-2/STAT-3 signaling pathway inhibited by ART
could be reactivated by IL-6 in NSPCs after OGD. Therefore, these
findings suggested that ART regulated the proliferation, apoptosis
and differentiation of NSPCs after OGD by inhibiting the
JAK-2/STAT-3 signaling pathway. Preliminary studies on cell
migration (data not shown) indicated that ART had little effect on
the migration of NSPCs; therefore, NSPCs migration should be
further explored in future studies.
In conclusion, the present study demonstrated that
ART could promote the proliferation and differentiation of NSPCs,
and reduced the apoptosis of NSPCs by inhibiting the JAK-2/STAT-3
signaling pathway. Therefore, ART may be considered a promising
therapeutic option for the effective treatment of ischemic
stroke.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW designed the experiments. YL and YB performed the
experiments. YL and FW analyzed the data. YL, YB and FW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Conway J and Friedman BW: Aspirin after
acute ischemic stroke. Am Fam Physician. 102(Online)2020.PubMed/NCBI
|
|
3
|
Boese AC, Le QE, Pham D, Hamblin MH and
Lee JP: Neural stem cell therapy for subacute and chronic ischemic
stroke. Stem Cell Res Ther. 9(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang M, Liang X, Cheng M, Yang L, Liu H,
Wang X, Sai N and Zhang X: Homocysteine enhances neural stem cell
autophagy in in vivo and in vitro model of ischemic stroke. Cell
Death Dis. 10(561)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bernstock JD, Peruzzotti-Jametti L, Ye D,
Gessler FA, Maric D, Vicario N, Lee YJ, Pluchino S and Hallenbeck
JM: Neural stem cell transplantation in ischemic stroke: A role for
preconditioning and cellular engineering. J Cereb Blood Flow Metab.
37:2314–2319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Othman FA and Tan SC: Preconditioning
strategies to enhance neural stem cell-based therapy for ischemic
stroke. Brain Sci. 10(893)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang GL, Zhu ZH and Wang YZ: Neural stem
cell transplantation therapy for brain ischemic stroke: Review and
perspectives. World J Stem Cells. 11:817–830. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bernstock JD, Peruzzotti-Jametti L,
Leonardi T, Vicario N, Ye D, Lee YJ, Maric D, Johnson KR, Mou Y,
Van Den Bosch A, et al: SUMOylation promotes survival and
integration of neural stem cell grafts in ischemic stroke.
Ebiomedicine. 42:214–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Spellicy SE, Kaiser EE, Bowler MM,
Jurgielewicz BJ, Webb RL, West FD and Stice SL: Neural stem cell
extracellular vesicles disrupt midline shift predictive outcomes in
porcine ischemic stroke model. Transl Stroke Res. 11:776–788.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Webb RL, Kaiser EE, Jurgielewicz BJ,
Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West
FD and Stice SL: Human neural stem cell extracellular vesicles
improve recovery in a porcine model of ischemic stroke. Stroke.
49:1248–1256. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kong Z, Liu R and Cheng Y: Artesunate
alleviates liver fibrosis by regulating ferroptosis signaling
pathway. Biomed Pharmacother. 109:2043–2053. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Adebayo JO, Tijjani H, Adegunloye AP,
Ishola AA, Balogun EA and Malomo SO: Enhancing the antimalarial
activity of artesunate. Parasitol Res. 119:2749–2764.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Frosch AEP: Artesunate versus quinine:
Keeping our options open. Clin Infect Dis. 70:288–289.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang F, Zhou JY, Zhang D, Liu MH and Chen
YG: Artesunate induces apoptosis and autophagy in HCT116 colon
cancer cells, and autophagy inhibition enhances the
artesunate-induced apoptosis. Int J Mol Med. 42:1295–1304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH,
Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al: Artesunate
synergizes with sorafenib to induce ferroptosis in hepatocellular
carcinoma. Acta Pharmacol Sin. 42:301–310. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Persaud S, Eid S, Swiderski N, Serris I
and Cho H: Preparations of rectal suppositories containing
artesunate. Pharmaceutics. 12(222)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Walimbwa SI, Lamorde M, Waitt C, Kaboggoza
J, Else L, Byakika-Kibwika P, Amara A, Gini J, Winterberg M, Chiong
J, et al: Drug interactions between dolutegravir and
artemether-lumefantrine or artesunate-amodiaquine. Antimicrob
Agents Chemother. 63:e01310–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song L, Ge T, Li Z, Sun J, Li G, Sun Y,
Fang L, Ma YJ and Garred P: Artesunate: A natural product-based
immunomodulator involved in human complement. Biomed Pharmacother.
136(111234)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dang WZ, Li H, Jiang B, Nandakumar KS, Liu
KF, Liu LX, Yu XC, Tan HJ and Zhou C: Therapeutic effects of
artesunate on lupus-prone MRL/lpr mice are dependent on T
follicular helper cell differentiation and activation of JAK2-STAT3
signaling pathway. Phytomedicine. 62(152965)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang K, Yang Y, Ge H, Wang J, Chen X, Lei
X, Zhong J, Zhang C, Xian J, Lu Y, et al: Artesunate promotes the
proliferation of neural stem/progenitor cells and alleviates
Ischemia-reperfusion Injury through
PI3K/Akt/FOXO-3a/p27kip1 signaling pathway. Aging
(Albany NY). 12:8029–8048. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng X, Yeung PKK, Zhong K, Zilundu PLM,
Zhou L and Chung SK: Astrocytic endothelin-1 overexpression
promotes neural progenitor cells proliferation and differentiation
into astrocytes via the Jak2/Stat3 pathway after stroke. J
Neuroinflamm. 16(227)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ilamathi M, Prabu PC, Ayyappa KA and
Sivaramakrishnan V: Artesunate obliterates experimental
hepatocellular carcinoma in rats through suppression of
IL-6-JAK-STAT signalling. Biomed Pharmacother. 82:72–79.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ilamathi M, Santhosh S and
Sivaramakrishnan V: Artesunate as an anti-cancer agent targets
Stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top
Med Chem. 16:2453–2463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Randolph SA: Ischemic stroke. Workplace
Health Saf. 64(444)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Inatomi Y, Nakajima M, Yonehara T and Ando
Y: Ipsilateral hemiparesis in ischemic stroke patients. Acta Neurol
Scand. 136:31–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sinden JD, Hicks C, Stroemer P,
Vishnubhatla I and Corteling R: Human neural stem cell therapy for
chronic ischemic stroke: Charting progress from laboratory to
patients. Stem Cells Dev. 26:933–947. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu X, Wang X, Zeng S and Tuo X: Protective
effects of primary neural stem cell treatment in ischemic stroke
models. Exp Ther Med. 16:2219–2228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu X, Yan J, Bregere C, Zelmer A, Goerne
T, Kapfhammer JP, Guzman R and Wellmann S: RBM3 promotes
neurogenesis in a niche-dependent manner via IMP2-IGF2 signaling
pathway after hypoxic-ischemic brain injury. Nat Commun.
10(3983)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Knotek T, Janeckova L, Kriska J, Korinek V
and Anderova M: Glia and neural stem and progenitor cells of the
healthy and ischemic brain: The workplace for the Wnt signaling
pathway. Genes (Basel). 11(804)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gan L, Liao S, Tong Y, Li W, Peng W and
Deng S: Long noncoding RNA H19 mediates neural stem/progenitor
cells proliferation, differentiation and apoptosis through the p53
signaling pathway after ischemic stroke. Biochem Biophys Res
Commun. 597:8–15. 2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
32
|
Lin R, Lang M, Heinsinger N, Stricsek G,
Zhang J, Iozzo R, Rosenwasser R and Iacovitti L: Stepwise
impairment of neural stem cell proliferation and neurogenesis
concomitant with disruption of blood-brain barrier in recurrent
ischemic stroke. Neurobiol Dis. 115:49–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pandamooz S, Jurek B, Salehi MS, Mostaghel
M, Miyan JA, Dianatpour M and Borhani-Haghighi A: The
implementation of preconditioned epidermal neural crest stem cells
to combat ischemic stroke. Comment on Othman, F.A.; Tan, S.C.
Preconditioning strategies to enhance neural stem cell-based
therapy for ischemic stroke. Brain Sci. 2020, 10, 893. Brain Sci.
11(653)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Heller L, Roepe PD and de Dios AC:
Artesunate activation by heme in an aqueous medium. Inorganica Chim
Acta. 496(119029)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yao X, Zhao CR, Yin H, Wang K and Gao JJ:
Synergistic antitumor activity of sorafenib and artesunate in
hepatocellular carcinoma cells. Acta Pharmacol Sin. 41:1609–1620.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu Y, Dang W, Zhang S, Wang L and Zhang
X: Artesunate attenuates inflammatory injury and inhibits the NF-κB
pathway in a mouse model of cerebral ischemia. J Int Med Res.
49(3000605211053549)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang C, Liu J, Yuan C, Ji Q, Chen D, Zhao
H, Jiang W, Ma K and Liu L: JAK2/STAT3 is associated with the
inflammatory process in periapical granuloma. Int J Clin Exp
Pathol. 12:190–197. 2019.PubMed/NCBI
|
|
38
|
Park SY, Lee CJ, Choi JH, Kim JH, Kim JW,
Kim JY and Nam JS: The JAK2/STAT3/CCND2 axis promotes colorectal
cancer stem cell persistence and radioresistance. J Exp Clin Cancer
Res. 38(399)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu W, Fu J, Gu Y, Wei Y, Ma P and Wu J:
JAK2/STAT3 regulates estrogen-related senescence of bone marrow
stem cells. J Endocrinol. 245:141–153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hou Y, Wang K, Wan W, Cheng Y, Pu X and Ye
X: Resveratrol provides neuroprotection by regulating the
JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis.
5:245–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo X, Shen X and Yong Z: MiR-101 protects
against the cerebral I/R injury through regulating JAK2/STAT3
signaling pathway. Neuropsychiatr Dis Treat. 17:2791–2802.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhong Y, Yin B, Ye Y, Dekhel OYAT, Xiong
X, Jian Z and Gu L: The bidirectional role of the JAK2/STAT3
signaling pathway and related mechanisms in cerebral
ischemia-reperfusion injury. Exp Neurol. 341(113690)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
El-Sherbiny M, El-Sayed RM, Helal MA,
Ibrahiem AT, Elmahdi HS, Eladl MA, Bilay SE, Alshahrani AM, Tawfik
MK, Hamed ZE, et al: Nifuroxazide mitigates angiogenesis in
Ehlrich's solid carcinoma: Molecular docking, bioinformatic and
experimental studies on inhibition of Il-6/JAK2/STAT3 signaling.
Molecules. 26(6858)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qian R, Sibei T, Fan G, Wang B, Yang L, Ma
L and Fu P: Natural flavonol fisetin attenuated hyperuricemic
nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3
signaling. Phytomedicine. 87(153552)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guan X, Wang Q, Liu M, Sun A and Li X:
Possible involvement of the IL-6/JAK2/STAT3 pathway in the
hypothalamus in depressive-like behavior of rats exposed to chronic
mild stress. Neuropsychobiology. 80:279–287. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhao X, Ma W, Li X, Li H, Li J, Li H and
He F: ANXA1 enhances tumor proliferation and migration by
regulating epithelial-mesenchymal transition and IL-6/JAK2/STAT3
pathway in papillary thyroid carcinoma. J Cancer. 12:1295–1306.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang
Y and Li R: Gegen Qinlian decoction relieved DSS-induced ulcerative
colitis in mice by modulating Th17/Treg cell homeostasis via
suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine.
84(153519)2021.PubMed/NCBI View Article : Google Scholar
|