Introduction
Cachexia is a complex disorder accompanied by
chronic syndromes. It is characterized by extreme loss of body
weight, metabolic disturbance and weakness (1). Aberrant metabolism includes
neurohormonal dysregulation, energy expenditure and catabolism
increase (2). Patients with
cancer, including those with lung, colon, pancreas and stomach
cancer, and melanoma usually exhibit cachexia (3). Chemotherapy and radiotherapy are two
of the major contributive factors to cachexia (4). Moreover, patients with certain
chronic and infectious diseases, including acquired immune
deficiency syndrome, tuberculosis and sepsis, also experience
cachexia (5). In total, cachexia
occurs in 50-80% of patients in the late stages of cancer, which
severely affects the survival time and quality of life of the
patients, reduces the sensitivity of the treatment and increases
the incidence of complications (6,7).
Inflammatory factors and metabolic abnormalities such as energy
expenditure increase, fat breakdown and decreased protein synthesis
serve important roles in the process of cachexia (5,8). The
clinical management of cachexia is challenging due to the
complexity of multifactorial metabolic dysregulation.
Adipose tissue is generally regarded as a lipid
depot for energy stores; however, recent studies have reported that
it also acts as a secretory organ that contributes to adjusting the
body composition through the regulation of energy homeostasis
(9,10). Loss of fat is one of the main
features of cancer cachexia and occurs earlier compared with muscle
wasting in cachexia. Murphy et al (11) reported that accelerated loss of
adipose tissue begins 7 months before mortality, and the average
rate of adipocyte loss reaches 29% at 2 months prior to mortality
in patients with colorectal and lung cancer. Liu et al
(12) also demonstrated that
accelerated loss of adipose tissue is significantly associated with
increased cancer morbidity and mortality. The mechanism of the loss
of adipose tissue in cancer cachexia is considered to be due to
increased lipolysis (13). High
levels of glycerol or fatty acids from lipolysis have been reported
in cachectic patients with cancer (14). Lipolysis in adipocytes is activated
in patients with cancer, which results in a decline in cellular
volume and synthesis of de novo lipogenesis (15,16),
which may also contribute to adipose wasting. Furthermore, browning
of white adipose tissue (WAT) is able to facilitate lipid
mobilization and increases energy expenditure, which eventually
leads to fat mass reduction (2).
A progesterone-based drug, megestrol acetate (MA),
which has been used for cachexia treatment and approved by the US
Food and Drug Administration, is able to improve the loss of
appetite and increase the body weight of patients (17-19).
However, the therapeutic mechanisms of MA for anorexia and cachexia
are not well clarified. It has been reported that MA decreases the
synthesis and release of cytokines, including IL-1, IL-6 and TNF,
and relieves the symptoms of cachexia syndrome (20). Furthermore, a double-blind,
placebo-controlled randomized clinical trial suggested that the
effects of MA on anorexia and cachexia are similar to those of
glucocorticosteroids (21). This
may be because MA is a glucocorticoid with weak androgenic
activity, which partly contributes to the augment in body weight
(22). However, adverse effects
induced by MA treatment, including thromboembolic events, edema and
adrenal suppression, have been reported (23). Nomegestrol acetate (NOMAc), a
19-norprogesterone derivative, has been used for contraception and
treatment of menstrual disorders. It has higher progesterone
activity compared with medroxyprogesterone but no androgenic or
glucocorticoid properties (23,24).
In a preliminary experiment, the results
demonstrated that NOMAc was able to increase body weight in a rat
model of endometriosis (data not shown). Therefore, it was
hypothesized that NOMAc could serve a role in cisplatin-induced
cachexia. The present study established a rat model of cachexia
using cisplatin and then assessed whether administrating NOMAc
could alleviate the adipose atrophy induced by cisplatin. The
effects of NOMAc were compared with those of MA. Furthermore, the
effect of NOMAc on the genes responsible for the modulation of
adipose degradation and synthesis in cisplatin-induced cachexia was
evaluated.
Materials and methods
Chemicals and reagents
Cisplatin was purchased from Qilu Pharmaceutical
Co., Ltd. and dissolved in 0.9% NaCl solution. NOMAc was kindly
provided by Lijiang Yinghua Biochemical and Pharmaceutical Co.,
Ltd. MA was purchased from Qingdao GuoHai Biological Pharmaceutical
Co., Ltd. MA and NOMAc were dissolved in 0.5% sodium
carboxymethylcellulose (CMC-Na). Rat TNFα/Tumor Necrosis Factor
ELISA Kit PicoKine® (cat. no. EK0526) and Rat
IL-6/Interleukin-6 ELISA Kit PicoKine® (cat. no. EK0412)
kits were purchased from Boster Biological Technology. Adipose
tissue protein extraction kit (cat. no. HR0049) was purchased from
Beijing Biolab Technology Co., Ltd. Anti-adipose triglyceride
lipase (ATGL; cat. no. sc-365278) and peroxidase-conjugated goat
anti-mouse IgGκ (cat. no. sc-516102) antibodies were purchased from
Santa Cruz Biotechnology, Inc. Peroxisome proliferator activated
receptor γ (PPARγ; cat. no. abs125245) and sterol regulatory
element binding protein-1 (SREBP-1; cat. no. abs131802) antibodies
were purchased from Absin Bioscience, Inc. Fatty acid synthase
(FASN; cat. no. 3180S), GAPDH (cat. no. 2118s) and
peroxidase-conjugated goat anti-rabbit IgG (cat. no. 7074) were
purchased from Cell Signaling Technology, Inc. Hormone-sensitive
lipase (HSL; cat. no. ab45422) antibodies were purchased from
Abcam. BCA Protein Assay kit (cat. no. C503021) was purchased from
Sangon Biotech Co., Ltd. Pierce™ ECL Western Blotting
Substrate (cat. no. 32106) was purchased from Thermo Fisher
Scientific, Inc.
Animals
A total of 125 male Sprague-Dawley rats (body
weight, 180±10 g; age, 7-8 weeks) were purchased from Sino-British
Experiment Animal (Shanghai Lab Animal Research Center). Animals
were housed at a rate of two animals per cage at a temperature of
22±2˚C and 60% humidity with a 12/12 h light/dark cycle and free
access to sterilized food and water in specific pathogen free
conditions.
Establishment of a rat model of
cachexia
A total of 36 male rats were divided into four
groups randomly and received interventions via peritoneal injection
as follows: Rats in the control group were treated with 0.9% NaCl
solution, while the other three groups of rats were treated with 1,
2 or 3 mg/kg cisplatin. The rats were weighed once daily at the
same time each day. When their body weight declined by 5%
administration of cisplatin was ceased, otherwise the treatment was
ended after 5 days. The optimal dose for establishing a rat model
of cachexia was evaluated in terms of a decline in the body weight
of the animals without observation of major adverse effects. The
specific major adverse effects where the experiment would be
terminated and the rats would be euthanized were severe diarrhea or
ulceration on the limb. If no severely abnormal phenomenon were
observed, the rats were euthanized at the end of experiment. All
rats were sacrificed by exsanguination of 6-7 ml under anesthesia
using 3% pentobarbital sodium solution (30 mg/kg) by
intraperitoneal injection on the day after the last administration
with cisplatin or 0.9% NaCl solution. Mortality of the rats was
confirmed after exsanguination by the absence of heartbeat and
respiration for 1-2 min.
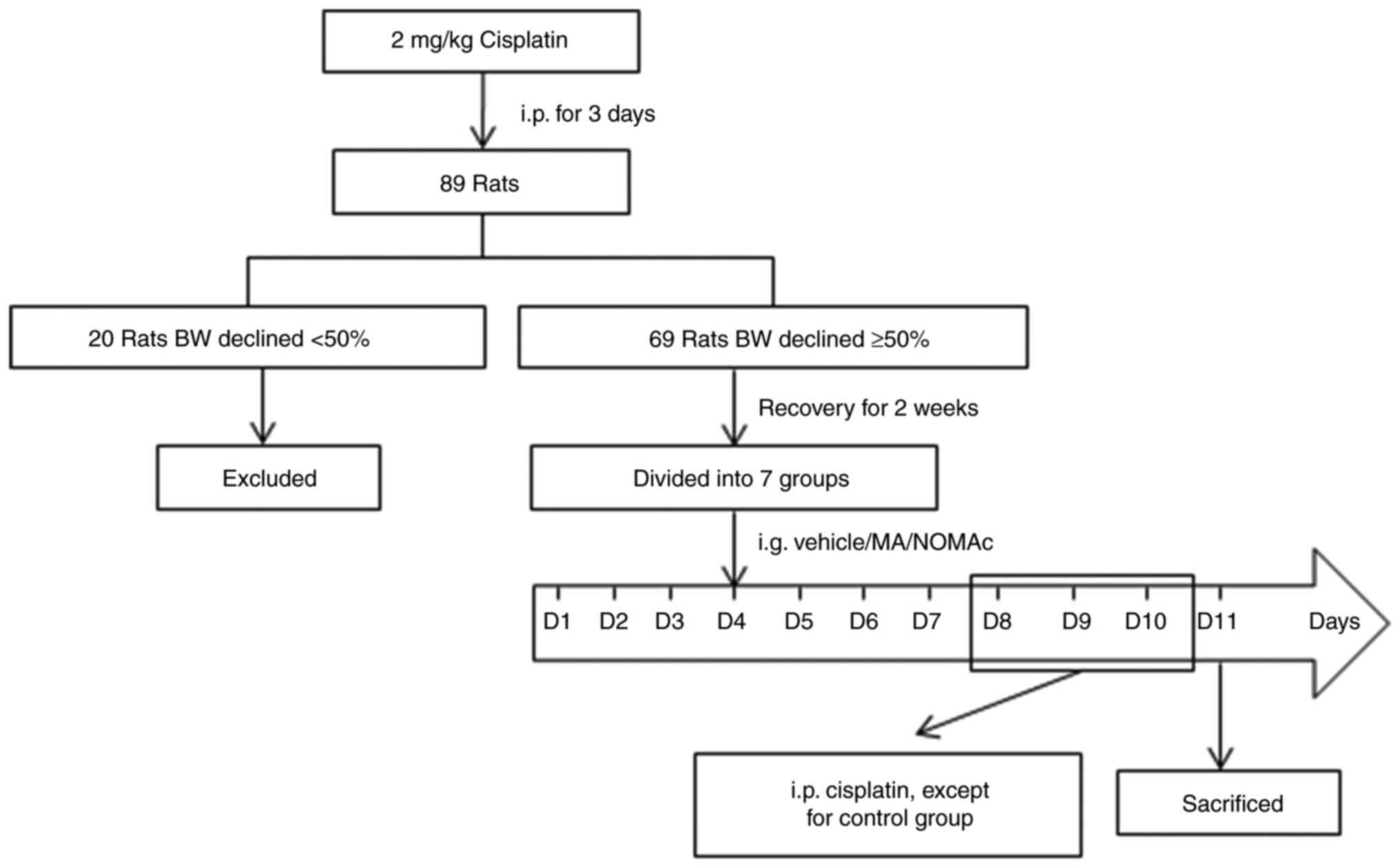
Screening of cachectic rats and NOMAc
administration
A total of 89 animals were used to establish a rat
model of cachexia. The animals were treated with 2 mg/kg cisplatin
for 3 days to screen the rat's response to cisplatin and the
inclusion criteria were that the body weight of the rats declined
>5%. The 21 rats who had not responded to cisplatin were
euthanized using exsanguination of 6-7 ml under anesthesia using 3%
pentobarbital sodium solution (30 mg/kg) by intraperitoneal
injection and mortality was confirmed for 1-2 min after
exsanguination by respiratory and cardiac arrest. In total, 69 rats
that responded to cisplatin were retained, allowed to recover for 2
weeks and then randomly divided into 7 groups (n=8-11). The range
of group size came from multiple batches of repetition and dose
selection. The grouped animals were administered the corresponding
treatment via gavage once daily for 11 consecutive days from day 1
(D1) to D11. Rats in the control and model groups received 0.5%
CMC-Na, rats in the MA groups received 5 or 10 mg/kg MA and rats in
the NOMAc groups received 2.5, 5 or 10 mg/kg NOMAc. Rats in the
model and drug treatment groups were intraperitoneally injected
with 2 mg/kg cisplatin once daily to induce cachexia 30 min after
treatment with 0.5% CMC-Na, MA or NOMAc from D8 to D10. The
treatment schedule for the groups is presented in Fig. 1. During cisplatin injection, the
body weights of the animals were recorded daily and changes were
described as increment in body weight at 24, 48 and 72 h after
cisplatin injection against prior to treatment. For example, the
body weights of the rats at D9, D10 and D11 respectively minus
those at D8. The food intakes were calculated by weighing the
leftover food daily. The average food intake of each rat daily was
calculated by the remaining forage divided by the number of the
animals. On D11, 2 h after the last administration of MA and NOMAc,
all 69 rats were anesthetized using 3% pentobarbital sodium
solution (30 mg/kg) by intraperitoneal injection and euthanized
using exsanguination, collecting 5 ml blood from the abdominal
aorta. Rat mortality was confirmed after exsanguination by the
absence of respiratory and cardiac arrest within 2 min.
Measurement of serum TNF-α and IL-6
levels using ELISA
Serum collected from the rats was separated by
centrifuging at 2,095 x g at 4˚C for 15 min. The serum levels of
TNF-α and IL-6 were assessed using ELISA according to the
manufacturer's protocol of the aforementioned kits (cat. no. EK0526
and EK0412).
Hematoxylin and eosin (H&E)
staining
Inguinal white adipose tissues (iWATs) and
epididymal white adipose tissue (eWATs) were collected and immersed
in specific fixative for adipose tissue (cat. no. G1119; Wuhan
Servicebio Technology Co., Ltd.) for 48 h at room temperature,
embedded in paraffin and cut into 5-µm sections. Next, H&E
staining was performed at room temperature by immersing the slides
into 0.25% eosin alcohol solution for 1 min and 0.2% hematoxylin
staining solution for 5 min and then the morphology of adipocytes
was assessed using a Leica DM3000 light microscope (Leica
Microsystems GmbH). The size and perimeters of adipocytes were
evaluated using Image-Pro Plus 6.0 software (version 6.0.0.260;
Media Cybernetics, Inc.).
Western blotting
Total proteins were extracted from the iWAT and eWAT
of the 7 groups of rats using adipose tissue protein extraction kit
(cat. no. HR0049). The protein concentration was assessed using a
BCA protein assay kit. The protein extracts (80 µg per lane) were
subjected to 10% SDS-PAGE and transferred to PVDF membranes. The
membranes were blocked with 5% milk for 1 h at room temperature and
incubated overnight at 4˚C with antibodies against ATGL, HSL,
PPARγ, FASN, SREBP-1 or GAPDH at a dilution of 1:1,000. Next, the
membrane was washed for 30 min with TBS-Tween-20 (0.1%) solution
and incubated with peroxidase-conjugated secondary antibodies at a
dilution of 1:3,000 for 1 h at room temperature. The bands were
visualized using an ECL kit. Images were obtained and
semi-quantified analysis of the blots was performed using a
ChemiDoc XRS+ Imaging System (version 4.0, Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
mean. Comparisons were performed using one-way ANOVA followed by
Dunnett's multiple comparison test using GraphPad Prism software
version 7 (GraphPad Prism software Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Evaluation of the optimal dose for
establishing a rat model of cachexia
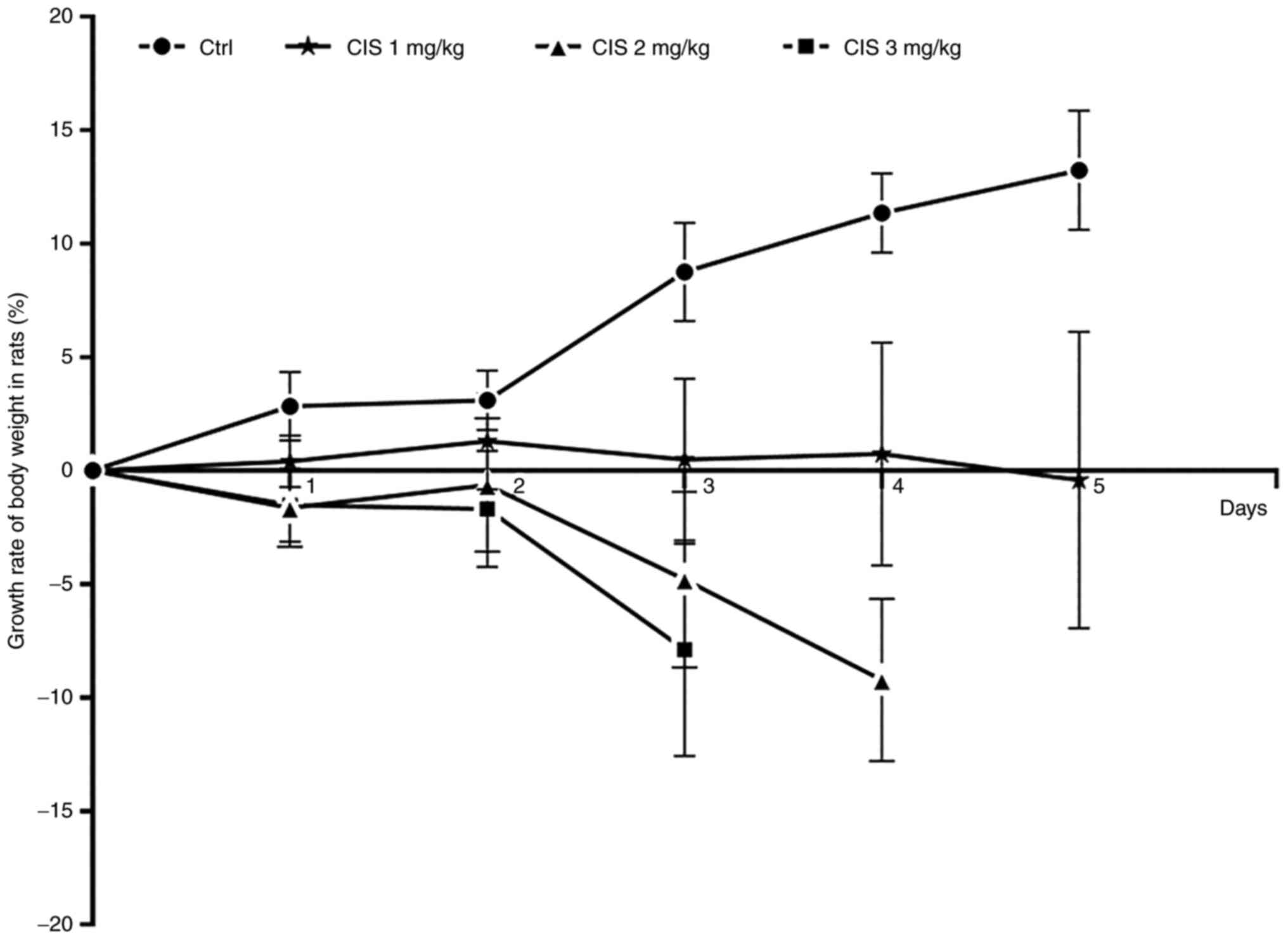
The ability of cisplatin to induce cachexia in rats
at doses of 1, 2 and 3 mg/kg via intraperitoneal injection for ≤5
days was evaluated. When the rats received cisplatin at 1 mg/kg for
5 consecutive days, their body weight did not decline. The rats
received cisplatin at 2 mg/kg for 3 consecutive days, their body
weight decreased by 9.32% on the fourth day (Fig. 2). When the rats received 3 mg/kg
cisplatin for 3 consecutive days, their body weight decreased by
7.88% on the fourth day (Fig. 2).
During the injection, no aberrant symptoms were observed in the
rats treated with 1 or 2 mg/kg cisplatin; however, adverse effects
including mild diarrhea and ulceration of limbs were observed in
the rats treated with 3 mg/kg cisplatin at one day after
withdrawal; however, the side effects slowly disappeared after
cisplatin withdrawal and no rats were euthanized due to the side
effects of cisplatin. These results suggested that 2 mg/kg
cisplatin injected for 3 consecutive days was the optimal dose to
establish chemotherapy-induced cachexia in rats (Fig. 2).
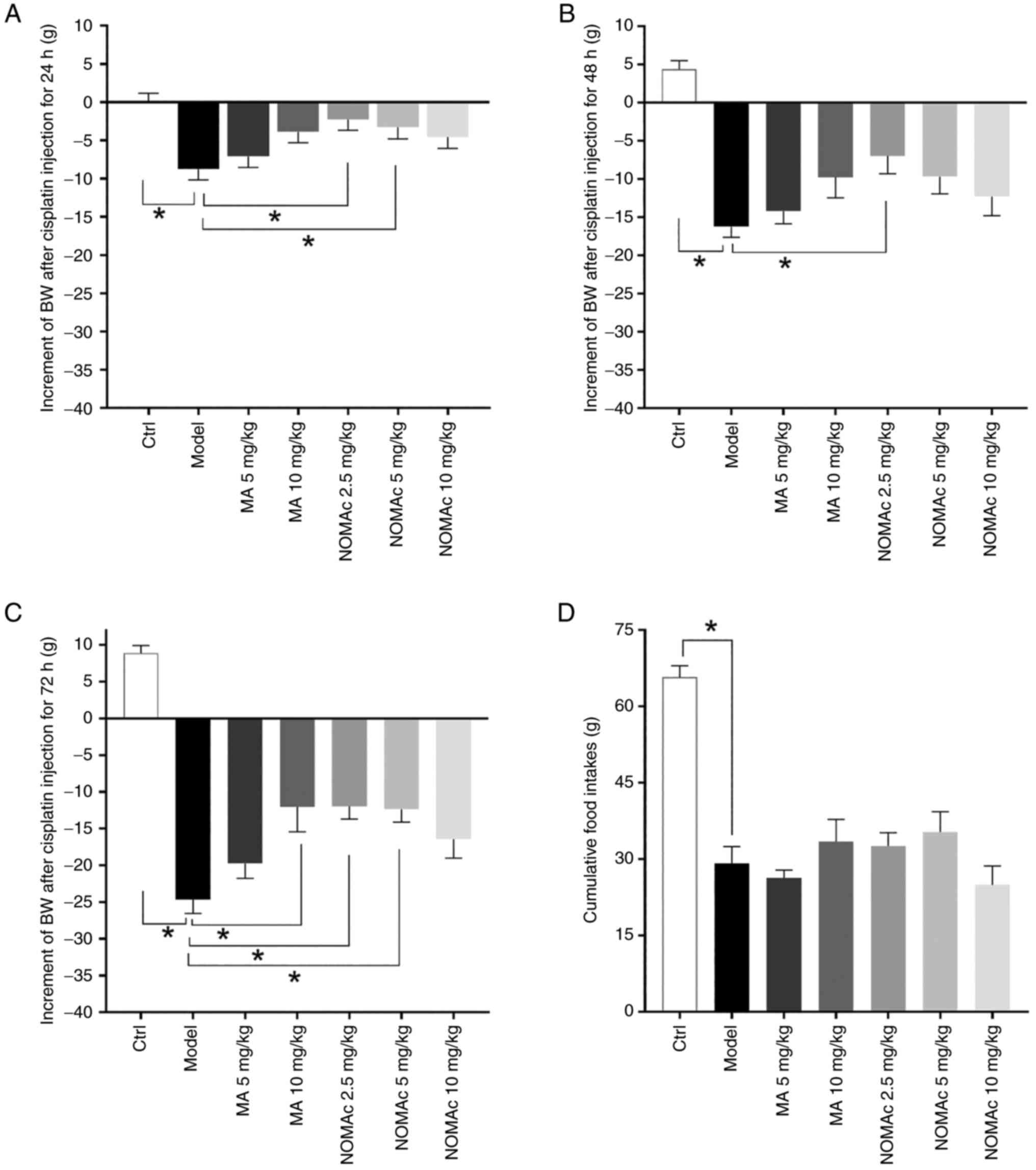
NOMAc alleviates the loss of body
weight induced by cisplatin in cachectic rats
A total of 69 of the rats were selected out for
responding to cisplatin and were used in subsequent experiments.
Cisplatin (2 mg/kg) was administered to the rats via injection for
3 consecutive days to induce cachexia after 7 days of pretreatment
with MA or NOMAc. In the experiment which established the model of
cachexia rats using 2 mg/kg/day cisplatin injection and treatment
of NOMAc, there were no significant side effects, including limb
ulceration and diarrhea observed and no rats were euthanized due to
side effects of cisplatin. In all groups, the body weights of the
rats demonstrated no significant differences with those before the
administration of cisplatin (data not presented). Cisplatin
produced a progressive decrease in the body weight of model rats
compared with that of the control group at 24, 48 and 72 h after
first injection (Fig. 3A-C). In
rats pretreated with 2.5, 5 mg/kg NOMAc, the body weight of the
rats declined significantly less compared with that of the model
group 24 h after first administration of cisplatin (Fig. 3A). Rats pretreated with 2.5 mg/kg
NOMAc demonstrated significantly reduced loss in body weight
compared with the model group 48 h after first administration of
cisplatin (Fig. 3B). The mean body
weight loss of rats pretreated with 10 mg/kg MA or 2.5 and 5 mg/kg
NOMAc were significantly lower compared with those of the model
group 72 h after first administration of cisplatin (Fig. 3C). During cisplatin injection, no
significant differences were demonstrated for the loss of body
weight of rats in the 5 mg/kg MA or 10 mg/kg NOMAc treatment group
compared with that of rats in the model group (Fig. 3A-C).
Cumulative food intakes were evaluated. The food
consumption in the model group was significantly decreased compared
with that in the control group (Fig.
3D). The rats in the 10 mg/kg MA and 2.5 and 5 mg/kg NOMAc
treatment groups appeared to eat more compared with those in the
model group, but the difference was not statistically significant
(Fig. 3D).
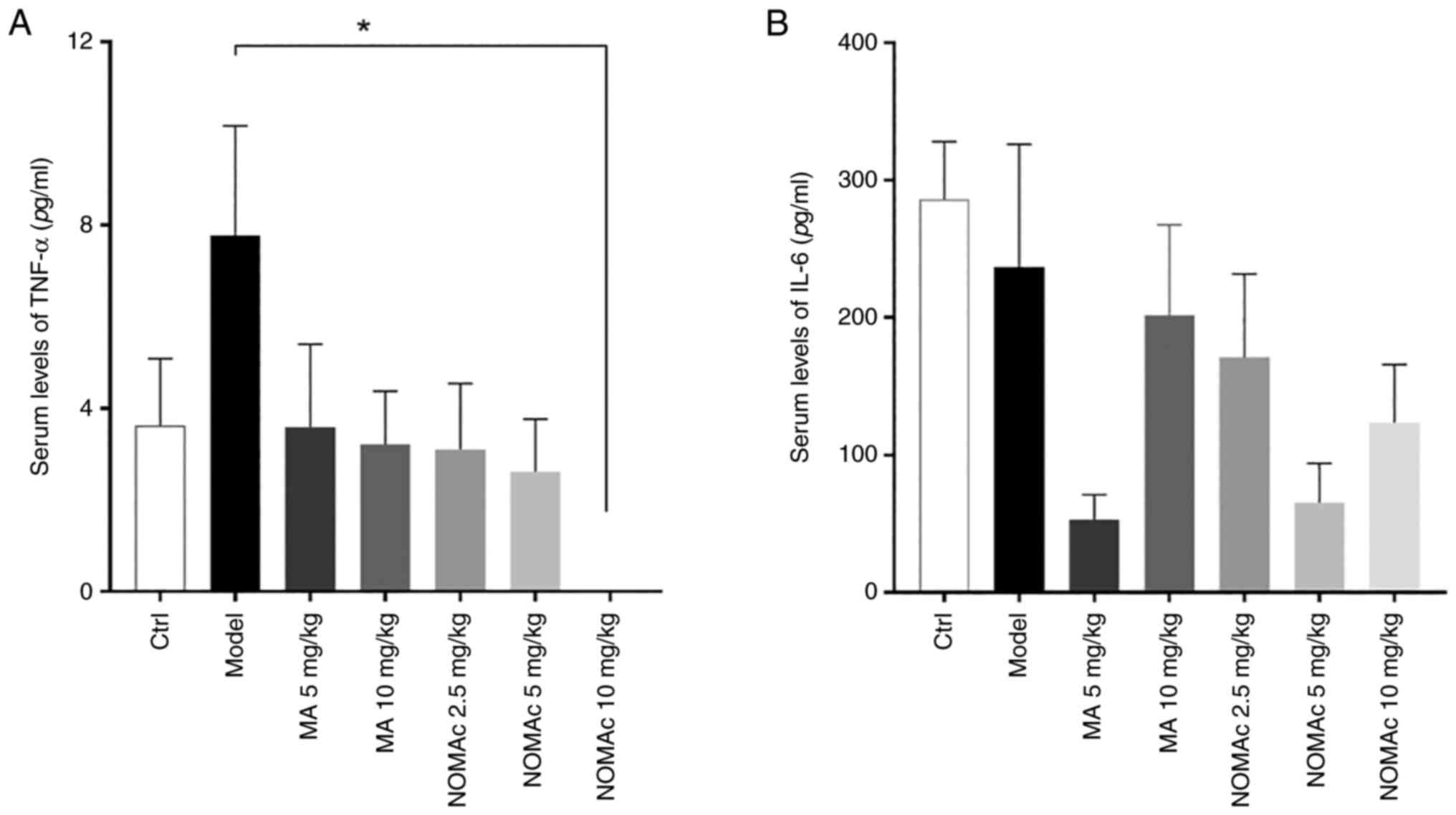
NOMAc decreases the serum levels of
TNF-α and IL-6 in cisplatin-induced cachectic rats
To evaluate the effects of NOMAc on biomarkers
associated with inflammatory cytokines and cachexia in
cisplatin-induced rats, the serum levels of TNF-α and IL-6 were
quantified using ELISA. The levels of serum TNF-α in the model
group were 2.15-fold higher compared with those in the control
group; however, this was not significantly different (Fig. 4A). With the dosage of MA and NOMAc
increasing, the serum levels of TNF-α were decreased in all groups
of MA and NOMAc, but the difference was statistically significant
between 10 mg/kg NOMAc-treated rats and those in the model group
(Fig. 4A).
The levels of serum IL-6 were also evaluated and
there was no apparent difference between the control and model
groups. The levels of IL-6 in 5 mg/kg MA-treated rats decreased by
77.8% compared with the model group, but this was not statistically
significant. The serum levels of IL-6 were decreased by 27.8, 72.8
and 47.8% in the groups subjected to 2.5, 5 and 10 mg/kg NOMAc
treatment, respectively, compared with those in the model group;
however, these results were not statistically significant (Fig. 4B).
Effects of NOMAc on the sizes of
adipocytes in iWAT and eWAT in cisplatin-induced cachectic
rats
To assess the effect of NOMAc on adipose tissue in
the cisplatin-induced cachexia model rats, the morphological
changes of adipocytes in iWAT and eWAT were evaluated.
Morphologically, eWAT was characterized by increased blood vessels
and a richer blood supply compared with iWAT. The sizes and
perimeters of adipocytes were assessed using Image-Pro Plus 6
software. Increased numbers of shrunken adipocytes of iWAT were
observed in the rats of the model group compared with those in the
control group, while MA and NOMAc treatments reduced the atrophy of
adipocytes induced by cisplatin (Fig.
5A). The sizes of adipocytes in 5 mg/kg NOMAc treated rats
increased 9.74% and declined 3.85% in 10 mg/kg NOMAc group compared
with the same dosage of MA group (Fig.
5A). The cell perimeter of iWAT cells was reduced in model rats
compared with that in control rats. NOMAc and MA increased the cell
perimeter; however, no significant difference was observed compared
with the model group (Fig. 5A).
The sizes of adipocytes in eWAT were also evaluated, but no
significant difference was demonstrated among all the tested
groups. No significant difference was demonstrated among all the
tested groups for the cell perimeters sizes of adipocytes in eWAT
(Fig. 5B).
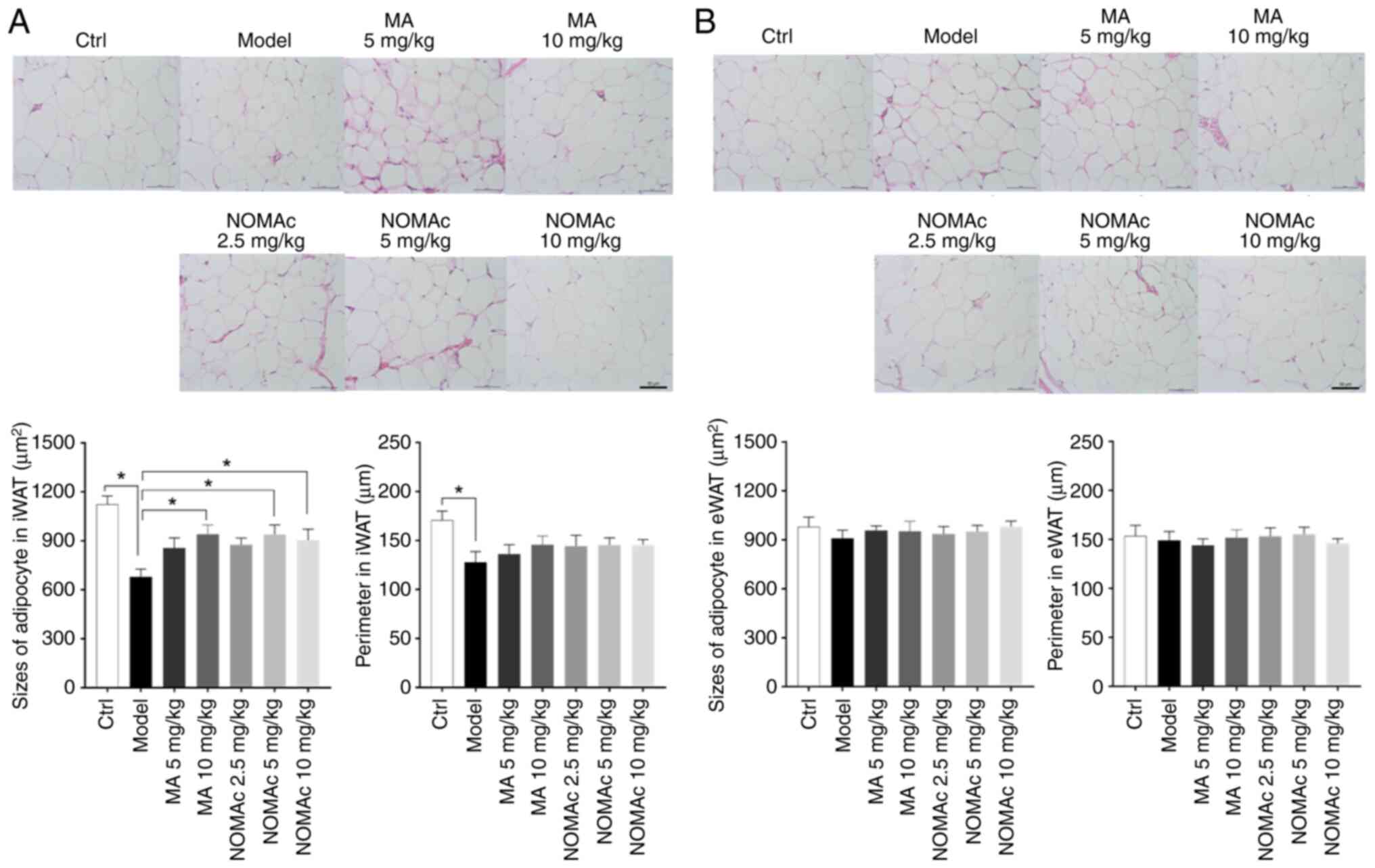 | Figure 5Morphological characteristics of
adipocytes in rats. (A) H&E staining of adipocytes in iWAT
(magnification, x20), the sizes of adipocyte and perimeters in
iWAT. (B) H&E staining of adipocytes in eWAT (magnification,
x20), the sizes of adipocyte and perimeters in eWAT. Arrows
indicate blood vessels. *P<0.05. Ctrl, control group;
MA, megestrol acetate; NOMAc, nomegestrol acetate; iWAT, inguinal
white adipose tissue; eWAT, epididymal white adipose tissue;
H&E, hematoxylin and eosin. |
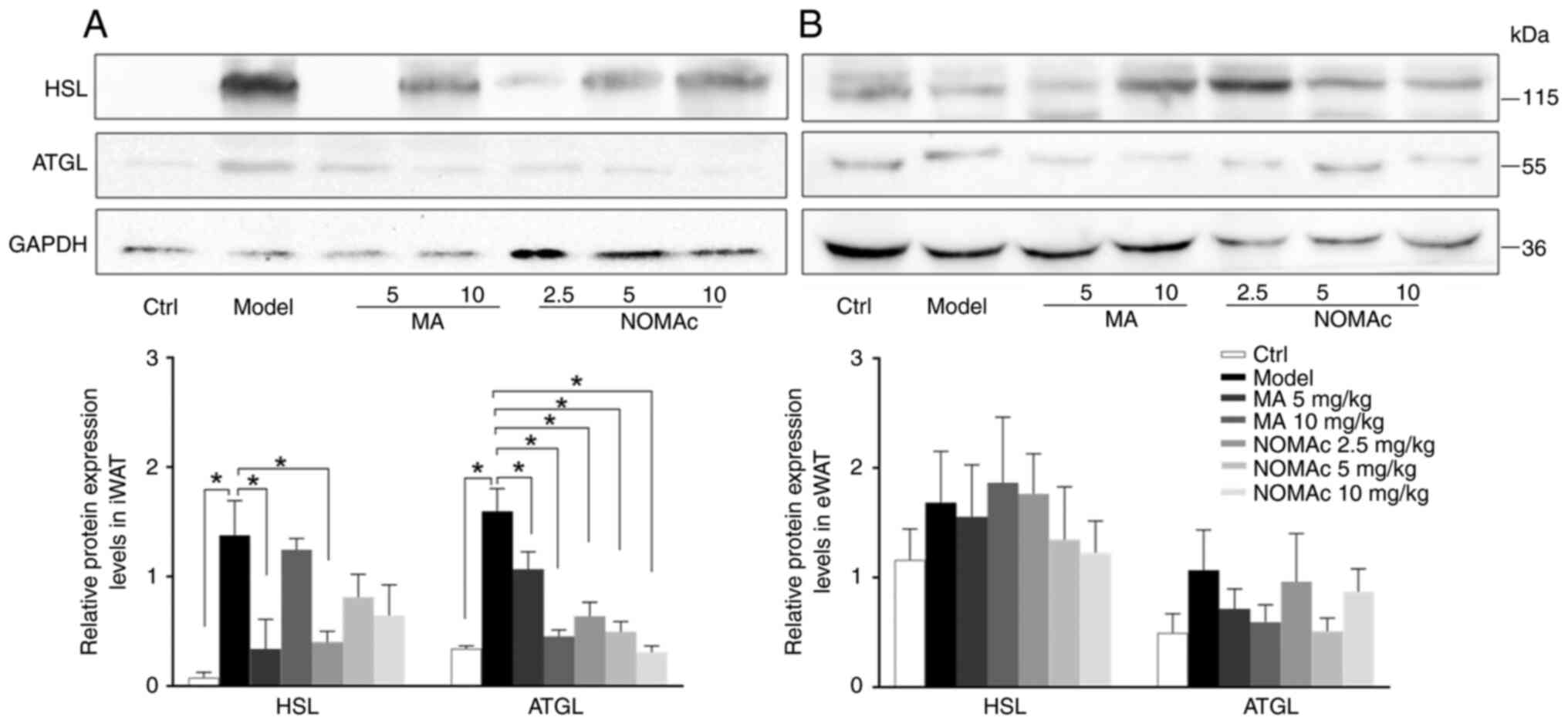
Effects of NOMAc on the protein
expression levels of lipolysis-related genes in iWAT and eWAT in
cisplatin-induced cachectic rats
Biomarkers of lipolysis were assayed to further
evaluate the effects of NOMAc on cisplatin-induced rats with
cachexia. Cisplatin markedly increased the protein expression
levels of HSL and ATGL in iWAT compared with those in the model
group (Fig. 6A). The protein
expression levels of HSL in the 5 mg/kg MA and 2.5 mg/kg NOMAc
treatment groups significantly decreased compared with those in the
model group (Fig. 6A); however, 10
mg/kg MA and 5 and 10 mg/kg NOMAc did not influence the protein
expression levels of HSL compared with the model group (Fig. 6A). The protein expression levels of
ATGL were significantly suppressed by all groups of MA and NOMAc
compared with those in the model group (Fig. 6A).
Changes in the protein expression levels of HSL and
ATGL in eWAT were evaluated. Cisplatin markedly enhanced the
protein expression levels of HSL and ATGL in eWAT compared with
those in the control group, but it was not statistically
significant. Treatment with 5 and 10 mg/kg NOMAc as well as 5 mg/kg
MA decreased the levels of HSL and ATGL in eWAT compared with those
in the model group; however, no significant difference was
demonstrated (Fig. 6B).
Effects of NOMAc on the protein
expression of lipid synthesis-related genes in iWAT and eWAT in
cisplatin-induced cachectic rats
The protein expression levels of lipid
synthesis-related genes, including PPARγ, FASN and SREBP-1, were
semi-quantified using western blotting. The protein expression
levels of FASN in iWAT adipocytes in the model group were
significantly decreased by 50% compared with those in the control
group (Fig. 7A). Compared with
those in the model group, the protein expression levels of FASN of
the 10 mg/kg NOMAc groups were significantly increased (Fig. 7A). Despite the increasing trend
observed, no significant difference in the protein expression level
of FASN was demonstrated in the 5 and 10 mg/kg MA or 2.5 and 10
mg/kg NOMAc treatment groups compared with that in the model group
(Fig. 7A). No significant
difference was observed in the protein expression levels of SREBP-1
or PPARγ between iWAT adipocytes in the model and control groups
(Fig. 7A). Compared with that of
the rats in the model group, 5 or 10 mg/kg MA had no significant
effect on the protein expression levels of SREBP-1 (Fig. 7A). After administering 2.5 and 10
mg/kg NOMAc to the rats, the protein expression levels of SREBP-1
were markedly higher than those in the rats of the model group;
however, the result was not statistically significant (Fig. 7A). NOMAc at 5 mg/kg significantly
increased the protein expression level of SREBP-1 compared with the
model group (Fig. 7A). Compared
with those in the model group, the protein expression levels of
PPARγ were significantly increased in the rats subjected to 5 and
10 mg/kg NOMAc treatment (Fig.
7A); however, no significant difference was observed in the
groups subjected to 5 or 10 mg/kg MA or 2.5 mg/kg NOMAc treatment
compared with the model group (Fig.
7A).
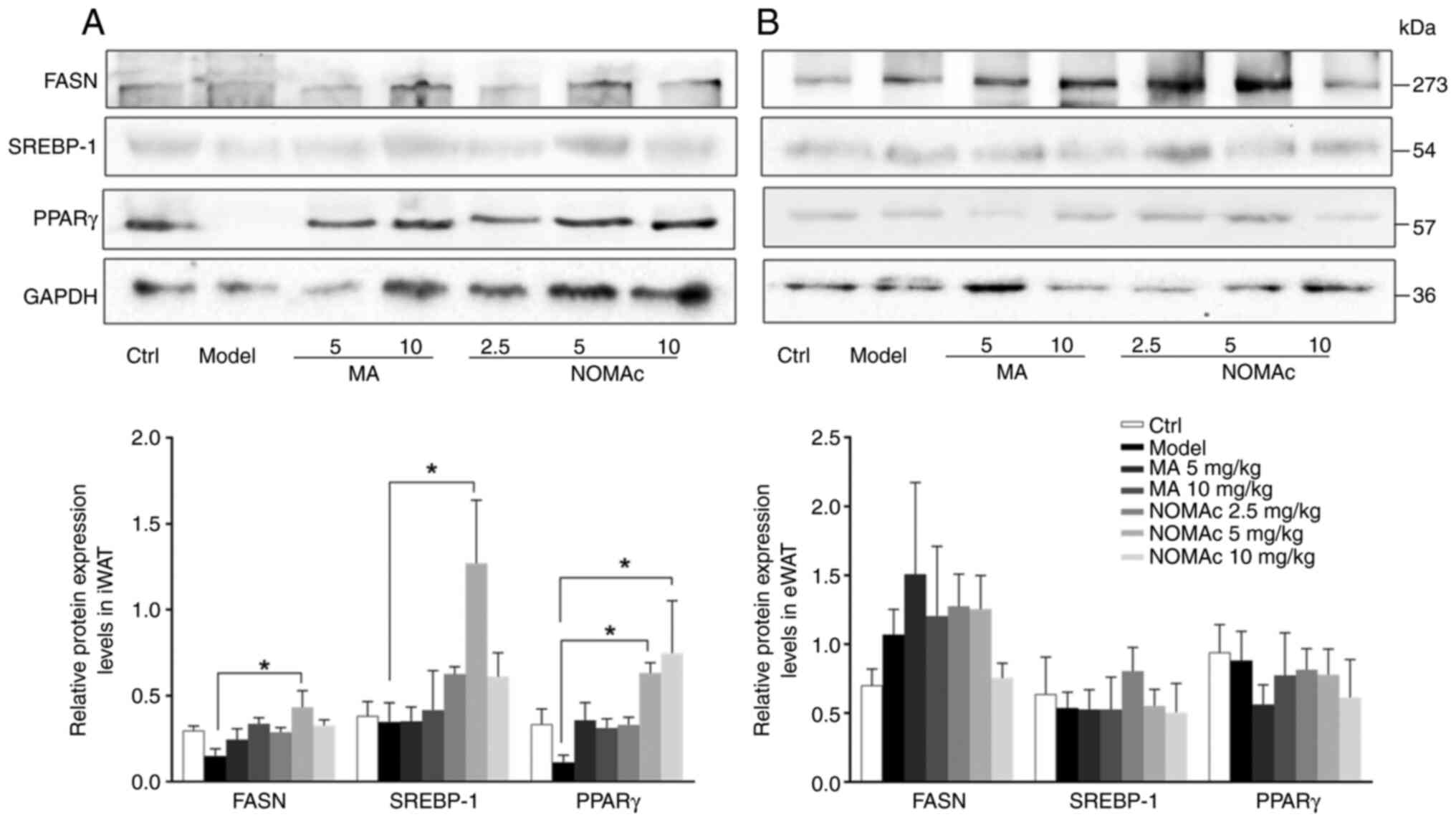 | Figure 7Effects of NOMAC on the protein
expression levels of the lipid synthesis genes PPARγ, FASN and
SREBP-1 in iWAT and eWAT induced by cisplatin. The semi-quantified
protein expression levels of PPARγ, FASN and SREBP-1 in (A) iWAT
and (B) eWAT normalized to GAPDH (n=6). *P<0.05.
Ctrl, control group; MA, megestrol acetate; NOMAc, nomegestrol
acetate; iWAT, inguinal white adipose tissue; eWAT, epididymal
white adipose tissue; PPARγ, peroxisome proliferator activated
receptor γ; FASN, fatty acid synthase; SREBP-1, sterol regulatory
element binding protein-1. |
The protein expression levels of FASN, SREBP-1 and
PPARγ in eWAT adipocytes were also evaluated. Cisplatin did not
significantly change the protein expression levels of these genes
compared with those of the control group and MA or NOMAc treatment
also did not significantly change the protein expression levels of
these genes compared with those of the control group (Fig. 7B).
Discussion
One of the prominent features of cancer cachexia is
weight loss due to adipocyte lipolysis and muscle wasting. The
present study demonstrated that NOMAc exerted protective effects on
cisplatin-induced cachexia by preventing the loss of body weight
and increasing food intake. Furthermore, NOMAc was able to
significantly decrease the serum levels of TNF-α, ameliorate the
atrophy of adipocytes, significantly decrease the protein
expression levels of ATGL and HSL in iWAT in cisplatin-induced
cachectic rats and significantly enhance the protein expression
levels of adipogenesis genes associated with cachexia, including
FASN, SREBP-1 and PPARγ in iWAT. These results suggested that NOMAc
has potential to be used for ameliorating cachexia.
Clinically, cachexia is defined as >5% loss of
body weight or a body mass index <20 kg/m2 with ≥2%
weight loss over 6 months (2).
Establishing models of cachexia require evaluation of the efficacy
of a candidate drug. In preclinical experiments, the methods of
establishing animal models of cachexia include malignant tumor
induction and chemotherapeutic drug injury (25). The presented study established a
cachexia model in rats by injecting cisplatin. Cisplatin is a
potent chemotherapy drug that is effective against a variety of
solid tumors; however, it can cause cachexia during antitumor
therapy due to its adverse effects (26). As a result, cisplatin-induced
animal models of cachexia are considered to be valid models for
identifying potential medication for the treatment of cachexia,
particularly cachexia associated with chemotherapy (27,28).
The role of cisplatin in chemotherapy-induced
cachexia is not well understood. Previously, accumulation of
cisplatin has been detected in adipose tissue (29), which suppresses FASN, stearoyl
coenzyme A desaturase-1 (SCD1) and carnitine palmitoyl
transferase-1 (CPT-1) in WAT to decrease lipogenesis, enhance
lipolysis by interacting with HSL and increasing lipid oxidation by
regulating food intake (28,30).
These previous studies indicated that the effect of cisplatin on
adipose tissue may involve both direct and indirect mechanisms.
Therefore, different doses of cisplatin were administered to the
rats in the present study to assess the optimal dose for
establishing the rat model of cachexia in terms of the clinical
criteria. The results demonstrated that administering 2 mg/kg/day
cisplatin to the rats for 3 consecutive days was the optimal method
for decreasing the body weights of the rats by 5% and subsequent
experiments were performed using this method.
Previous studies report that the reduction of
bodyweight mediated by cisplatin reaches a peak 48-72 h after being
administered and that the effects then return to near baseline
levels after 16 days (31,32). In the present study, rats in which
the body weights declined by <5% after cisplatin injection were
first screened out, since not all animals respond to cisplatin
induction. To eliminate the effects of cisplatin, the rats selected
for use following cisplatin screening were allowed to recover for 2
weeks. Thereafter, the progestins MA and NOMAc were administered
for 7 consecutive days prior to injection of cisplatin into the
rats again. Following cisplatin administration, the rats in the
vehicle-treated model group demonstrated significant weight loss,
while 2.5 and 5 mg/kg NOMAc significantly reduced the loss in body
weight induced by cisplatin. Weight loss in the 2.5 and 5 mg/kg
NOMAc treatment groups was <50% compared with that in the model
group, which was similar to the effects of the 10 mg/kg MA
treatment. These results suggested that NOMAc exerted a protective
effect on weight loss at lower doses than MA in cisplatin-induced
cachexia.
Cisplatin-induced cachexia generally causes anorexia
accompanied by a decline in food intake (33). In the present study, the observed
progressive reduction in body weight was consistent with the
decline in food consumption. While there was no statistical
difference, an increased trend in cumulative food intake was
demonstrated in the rats of the 2.5 and 5 mg/kg NOMAc treatment
groups compared with that demonstrated in the model group. This
indicated that the protective effect of NOMAc against
cisplatin-induced weight loss could partly be attributed to an
increase in food intake.
It is known that there is a link between cachexia
and inflammatory cytokines. TNF-α and IL-6, as pro-inflammatory
cytokines, are able to enhance both systemic and local inflammatory
effects in patients with cancer (34). Increased TNF-α expression levels
are associated with muscle wasting, loss of adipose tissue and
proteolysis in cancer cachexia (35). Sherry et al (36) reported that administering an
anti-TNF-α antibody can attenuate the development of cachexia in
tumor models by preventing the loss of fat, muscle and body weight.
In the present study, it was demonstrated that the serum levels of
TNF-α increased with a progressive decline in body weight in the
model group, which was significantly suppressed by NOMAc treatment.
Furthermore, the IL-6 serum level is also considered to be
correlated with weight loss and reduced survival in patients with
cancer (37). Han et al
(38) reported that IL-6 was able
to enhance the loss in body weight by accelerating the lipolysis of
WAT in patients with cancer and cachexia. In the present study, a
decreasing trend in the levels of serum IL-6 was observed in the
2.5 and 5 mg/kg NOMAc-treated rats; however, it was not
statistically significant. The remarkable decrease in TNF-α levels
contributed to the protective effect of NOMAc against
cisplatin-induced weight loss, but the effects were not observed in
the MA-treated groups.
Previous studies have reported that lipolysis
results in depletion of lipid depots in adipose tissue, which
reduces the sizes of adipocytes and decreases the rate of de
novo lipogenesis (19,22). In the present study, the
morphological changes in adipose tissues were characterized by iWAT
atrophy and a significant reduction in the sizes of adipocytes in
cisplatin-treated model rats; moreover, these alterations could be
significantly reduced by NOMAc treatment. In eWAT, the sizes of the
adipocytes in the model rats did not differ from those in the
control group, which indicated that iWAT was more sensitive to
cisplatin induction compared with eWAT. Moreover, it indicated that
NOMAc could attenuate the atrophy of adipocytes in iWAT but did not
in effect eWAT. It suggested that the short time of cisplatin
treatment could have affected superficial inguinal fat but not
epididymal adipose tissue. Moreover, previous studies have reported
that eWAT has a protective effect on gonadal tissue, and loss of
adipose tissue can cause a decline in fertility (39,40);
therefore, it was hypothesized that the loss of eWAT later compared
with iWAT may be to protect the genitals. In addition, it suggests
that iWAT could be a better indicator compared with eWAT for
evaluating the animal model of cisplatin-induced cachexia and the
effects of NOMAc since the results demonstrated that eWAT was
insensitive to cisplatin and NOMAc. Various genes participate in
the loss of adipose tissue in cancer cachexia. Lipolysis is
regulated through ATGL and HSL. ATGL is the rate-limiting enzyme in
the process of lipolysis, which converts triacylglycerol (TG) to
glycerol and free fatty acids (41,42).
In the present study, a significant increase in the protein
expression levels of HSL and ATGL was observed in the iWAT of
cisplatin-induced model rats, but this augmentation could be
markedly reduced by NOMAc. Furthermore, NOMAc reduced the protein
expression levels of the aforementioned genes at lower doses
compared with MA, which indicated that NOMAc may have been more
effective compared with MA.
In addition, the protein expression levels of lipid
synthesis-related genes, including FASN, PPARγ and SREBP-1, were
assessed. PPARγ, as a transcription factor, regulates lipid
metabolism when activated by ligands and serves a role in
adipogenesis and the maintenance of mature adipocyte function
(43). Cachexia is able to impair
lipogenesis through reducing the levels of PPARγ (44), as well as through inhibiting the
expression of fatty acid binding protein 4 (aP2), SCD1, CPT-1α and
FASN (28). In the present study,
cisplatin markedly inhibited the expression of PPARγ in iWAT, which
suggested that cisplatin may regulate lipid synthesis by affecting
the transcription factor PPARγ. Previous studies report that
SREBP-1 contributes to the expression of PPARγ and activates PPARγ
through the production of endogenous ligands (45,46).
SREBP-1c, another transcription factor, is a subtype of SREBP-1,
which also regulates the expression of genes involved in lipid
metabolism such as FASN (47). As
a rate-limiting enzyme, FASN controls the de novo conversion
of free fatty acid into TG in adipocyte lipogenesis (46). In the present study, the protein
expression levels of SREBP-1, PPARγ and FASN were significantly
increased in the iWAT of NOMAc-treated rats, which indicated that
NOMAc was not only able to reduce lipolysis but also enhanced
lipogenesis via activation of the transcription factors SREBP-1 and
PPARγ. Lipid metabolism depends on the co-regulation of various
transcriptional factors. Whether NOMAc regulates lipid metabolism
through other transcription factors requires further investigation.
Furthermore, the present study demonstrated that MA has a trend to
promote the protein expression of FASN and PPARγ, but did not
markedly significance which indicated that MA may ameliorate
cachexia via mechanisms other than regulating the expression of
these two genes involved in lipogenesis.
The present study semi-quantified the protein
expression levels of HSL, ATGL, PPARγ, FASN and SREBP-1 in eWAT,
and demonstrated that there were no significant differences among
all groups (Figs. 6B and 7B). This indicated that the genes
associated with lipogenesis and lipolysis in iWAT were more prone
to be affected by cisplatin compared with those in eWAT and that
these genes in iWAT were likely to be regulated by progestins. It
was hypothesized that the reason could be the short time of the
model, which affected superficial inguinal fat but not epididymal
adipose tissue. Furthermore, previous studies have reported that
WAT has a protective effect on gonadal tissue and that loss of
adipose tissue can cause a decline in fertility (39,40);
therefore, it could be suggested that eWAT may be lost after
inguinal fat in order to protect the gonads.
The protein expression levels of two genes
associated with muscle wasting, muscle RING-finger protein-1 and
atrogin-1, in skeletal muscles, were assessed in a preliminary
study and it was demonstrated that neither MA 5 and 10 mg/kg nor
NOMAc 2.5, 5 and 10 mg/kg influenced their protein expression
levels at the tested concentrations (data not shown). These results
were different from those reported by Busquets et al
(47), who observed that both
genes are downregulated when the dose of MA is increased to 100
mg/kg. Future studies should be performed to evaluate the
modulation of progestins in skeletal muscle in cachexia. In the
present study, the level of NOMAc in adipose tissues was not
determined; however, progestins generally exhibit lipophilic
properties (48). Therefore, it
was presumed that NOMAc acted on adipose tissues in a direct
manner, since it was demonstrated that NOMAc treatment not only
improved the appetite of rats but also markedly reduced the protein
expression levels of genes associated with lipid degradation and
increased lipid synthesis. However, a potential indirect mechanism
of action could not be excluded and future investigations are
needed.
In summary, the present study demonstrated that
NOMAc was able to significantly ameliorate the loss of body weight
and reduce the serum level of TNF-α in a rat model of
cisplatin-induced cachexia. In particular, NOMAc attenuated the
atrophy of iWAT by increasing the volume of adipocytes. The
mechanism of NOMAc action not only involved downregulation of the
protein expression levels of key factors of lipolysis, such as HSL
and ATGL, but also enhancement of the protein expression levels of
lipogenesis-associated genes, including SREBP-1, PPARγ and FASN in
iWAT but not in eWAT. Furthermore, NOMAc improved the cachexia at
lower doses compared with MA. These results suggested that NOMAc
was a promising candidate drug for ameliorating cancer cachexia.
The present study therefore provided novel ideas for the
application of progestins in the treatment of cachexia.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Shanghai
Municipal Health Commission (grant no. 20184Y0128) and The Shanghai
Institute for Biomedical and Pharmaceutical Technologies (grant no.
Q2018-8).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ designed the study, performed the animal
experiments and wrote the manuscript. WY and XG performed the
animal experiments. GL and SX performed the experiments to
determine protein expression levels in serum. JZ and BR performed
western blotting. YZ supervised and designed the study, and revised
and approved the manuscript. RZ and YZ confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Ethics Committee of Shanghai Institute for Biomedical and
Pharmaceutical Technologies (approval no. 2019-27).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fearon KCH: Cancer cachexia: Developing
multimodal therapy for a multidimensional problem. Eur J Cancer.
44:1124–1132. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roeland EJ, Bohlke K, Baracos VE, Bruera
E, Del Fabbro E, Dixon S, Fallon M, Herrstedt J, Lau H, Platek M,
et al: Management of cancer cachexia: ASCO guideline. J Clin Oncol.
38:2438–2453. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baracos VE, Martin L, Korc M, Guttridge DC
and Fearon KCH: Cancer-associated cachexia. Nat Rev Dis Primers.
4(17105)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coletti D: Chemotherapy-induced muscle
wasting: An update. Eur J Transl Myol. 28(7587)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siddiqui JA, Pothuraju R, Jain M, Batra SK
and Nasser MW: Advances in cancer cachexia: Intersection between
affected organs, mediators, and pharmacological interventions.
Biochim Biophys Acta Rev Cancer. 1873(188359)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Argilés JM, Busquets S, Stemmler B and
López-Soriano FJ: Cancer cachexia: Understanding the molecular
basis. Nat Rev Cancer. 14:754–762. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Fearon K, Arends J and Baracos V:
Understanding the mechanisms and treatment options in cancer
cachexia. Nat Rev Clin Oncol. 10:90–99. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhu X, Callahan MF, Gruber KA, Szumowski M
and Marks DL: Melanocortin-4 receptor antagonist TCMCB07
ameliorates cancer- and chronic kidney disease-associated cachexia.
J Clin Invest. 130:4921–4934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee MW, Lee M and Oh KJ: Adipose
tissue-derived signatures for obesity and type 2 diabetes:
Adipokines, batokines and MicroRNAs. J Clin Med.
8(854)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chouchani ET and Kajimura S: Metabolic
adaptation and maladaptation in adipose tissue. Nat Metab.
1:189–200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Murphy RA, Wilke MS, Perrine M, Pawlowicz
M, Mourtzakis M, Lieffers JR, Maneshgar M, Bruera E, Clandinin MT,
Baracos VE and Mazurak VC: Loss of adipose tissue and plasma
phospholipids: Relationship to survival in advanced cancer
patients. Clin Nutr. 29:482–487. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu H, Luo J, Guillory B, Chen JA, Zang P,
Yoeli JK, Hernandez Y, Lee II, Anderson B, Storie M, et al: Ghrelin
ameliorates tumor-induced adipose tissue atrophy and inflammation
via Ghrelin receptor-dependent and -independent pathways.
Oncotarget. 11:3286–3302. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ding Z, Sun D, Han J, Shen L, Yang F, Sah
S, Sui X and Wu G: Novel noncoding RNA CircPTK2 regulates lipolysis
and adipogenesis in cachexia. Mol Metab. 53(101310)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mantovani G, Macciò A, Esu S, Lai P,
Santona MC, Massa E, Dessì D, Melis GB and Del Giacco GS:
Medroxyprogesterone acetate reduces the in vitro production of
cytokines and serotonin involved in anorexia/cachexia and emesis by
peripheral blood mononuclear cells of cancer patients. Eur J
Cancer. 33:602–607. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Batista ML Jr, Peres SB, McDonald ME,
Alcantara PSM, Olivan M, Otoch JP, Farmer SR and Seelaender M:
Adipose tissue inflammation and cancer cachexia: Possible role of
nuclear transcription factors. Cytokine. 57:9–16. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Argilés JM, Stemmler B, López-Soriano FJ
and Busquets S: Inter-tissue communication in cancer cachexia. Nat
Rev Endocrinol. 15:9–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bruera E, Ernst S, Hagen N, Spachynski K,
Belzile M, Hanson J, Summers N, Brown B, Dulude H and Gallant G:
Effectiveness of megestrol acetate in patients with advanced
cancer: A randomized, double-blind, crossover study. Cancer Prev
Control. 2:74–78. 1998.PubMed/NCBI
|
|
18
|
Greig CA, Johns N, Gray C, MacDonald A,
Stephens NA, Skipworth RJ, Fallon M, Wall L, Fox GM and Fearon KC:
Phase I/II trial of formoterol fumarate combined with megestrol
acetate in cachectic patients with advanced malignancy. Support
Care Cancer. 22:1269–1275. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Ruiz Garcia V, López-Briz E, Carbonell
Sanchis R, Gonzalvez Perales JL and Bort-Martí S: Megestrol acetate
for treatment of anorexia-cachexia syndrome. Cochrane Database Syst
Rev: Mar 28, 2013 (Epub ahead of print).
|
|
20
|
Mantovani G, Macciò A, Lai P, Massa E,
Ghiani M and Santona MC: Cytokine activity in cancer-related
anorexia/cachexia: Role of megestrol acetate and
medroxyprogesterone acetate. Semin Oncol. 25 (Suppl 6):S45–S52.
1998.PubMed/NCBI
|
|
21
|
Loprinzi CL, Kugler JW, Sloan JA,
Mailliard JA, Krook JE, Wilwerding MB, Rowland KM Jr, Camoriano JK,
Novotny PJ and Christensen BJ: Randomized comparison of megestrol
acetate versus dexamethasone versus fluoxymesterone for the
treatment of cancer anorexia/cachexia. J Clin Oncol. 17:3299–3306.
1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
House L, Seminerio MJ, Mirkov S, Ramirez
J, Skor M, Sachleben JR, Isikbay M, Singhal H, Greene GL, Vander
Griend D, et al: Metabolism of megestrol acetate in vitro and the
role of oxidative metabolites. Xenobiotica. 48:973–983.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao C, Zhou JY, Xie SW, Guo XJ, Li GT,
Gong YJ, Yang WJ, Li Z, Zhong RH, Shao HH and Zhu Y: Metformin
enhances nomegestrol acetate suppressing growth of endometrial
cancer cells and may correlate to downregulating mTOR activity in
vitro and in vivo. Int J Mol Sci. 20(3308)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ruan X, Seeger H and Mueck AO: The
pharmacology of nomegestrol acetate. Maturitas. 71:345–353.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Penna F, Busquets S and Argilés JM:
Experimental cancer cachexia: Evolving strategies for getting
closer to the human scenario. Semin Cell Dev Biol. 54:20–27.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Conte E, Camerino GM, Mele A, De Bellis M,
Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, et
al: Growth hormone secretagogues prevent dysregulation of skeletal
muscle calcium homeostasis in a rat model of cisplatin-induced
cachexia. J Cachexia Sarcopenia Muscle. 8:386–404. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirago G, Conte E, Fracasso F, Cormio A,
Fehrentz JA, Martinez J, Musicco C, Camerino GM, Fonzino A, Rizzi
L, et al: Growth hormone secretagogues hexarelin and JMV2894
protect skeletal muscle from mitochondrial damages in a rat model
of cisplatin-induced cachexia. Sci Rep. 7(13017)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garcia JM, Scherer T, Chen JA, Guillory B,
Nassif A, Papusha V, Smiechowska J, Asnicar M, Buettner C and Smith
RG: Inhibition of cisplatin-induced lipid catabolism and weight
loss by ghrelin in male mice. Endocrinology. 154:3118–3129.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vojtek M, Gonçalves-Monteiro S, Pinto E,
Kalivodová S, Almeida A, Marques MPM, Batista de Carvalho ALM,
Martins CB, Mota-Filipe H, Ferreira IMPLVO and Diniz C: Preclinical
pharmacokinetics and biodistribution of anticancer dinuclear
palladium(II)-spermine complex (Pd2Spm) in mice.
Pharmaceuticals (Basel). 14(173)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Conte E, Bresciani E, Rizzi L, Cappellari
O, De Luca A, Torsello A and Liantonio A: Cisplatin-induced
skeletal muscle dysfunction: Mechanisms and counteracting
therapeutic strategies. Int J Mol Sci. 21(1242)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Malik NM, Moore GBT, Smith G, Liu YL,
Sanger GJ and Andrews PLR: Behavioural and hypothalamic molecular
effects of the anti-cancer agent cisplatin in the rat: A model of
chemotherapy-related malaise? Pharmacol Biochem Behav. 83:9–20.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Brierley DI, Harman JR, Giallourou N,
Leishman E, Roashan AE, Mellows BAD, Bradshaw HB, Swann JR, Patel
K, Whalley BJ and Williams CM: Chemotherapy-induced cachexia
dysregulates hypothalamic and systemic lipoamines and is attenuated
by cannabigerol. J Cachexia Sarcopenia Muscle. 10:844–859.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Malik NM, Liu YL, Cole N, Sanger GJ and
Andrews PL: Differential effects of dexamethasone, ondansetron and
a tachykinin NK1 receptor antagonist (GR205171) on
cisplatin-induced changes in behaviour, food intake, pica and
gastric function in rats. Eur J Pharmacol. 555:164–173.
2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bing C and Trayhurn P: New insights into
adipose tissue atrophy in cancer cachexia. Proc Nutr Soc.
68:385–392. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Patel HJ and Patel BM: TNF-α and cancer
cachexia: Molecular insights and clinical implications. Life Sci.
170:56–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sherry BA, Gelin J, Fong Y, Marano M, Wei
H, Cerami A, Lowry SF, Lundholm KG and Moldawer LL:
Anticachectin/tumor necrosis factor-alpha antibodies attenuate
development of cachexia in tumor models. FASEB J. 3:1956–1962.
1989.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fearon KC, Glass DJ and Guttridge DC:
Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell
Metab. 16:153–166. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Han J, Meng QY, Shen L and Wu GH:
Interleukin-6 induces fat loss in cancer cachexia by promoting
white adipose tissue lipolysis and browning. Lipids Health Dis.
17(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chi JY, Wu ZH, Choi CHJ, Nguyen L, Tegegne
S, Ackerman SE, Crane A, Marchildon F, Tessier-Lavigne M and Cohen
P: Three-dimensional adipose tissue imaging reveals regional
variation in beige fat biogenesis and PRDM16-dependent sympathetic
neurite density. Cell Metab. 27:226–236.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Johnson J, Canning J, Kaneko T, Pru JK and
Tilly JL: Germline stem cells and follicular renewal in the
postnatal mammalian ovary. Nature. 428:145–150. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Silvério R, Lira FS, Oyama LM, Oller do
Nascimento CM, Otoch JP, Alcântara PSM, Batista ML Jr and
Seelaender M: Lipases and lipid droplet-associated protein
expression in subcutaneous white adipose tissue of cachectic
patients with cancer. Lipids Health Dis. 16(159)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Das SK, Eder S, Schauer S, Diwoky C,
Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner
M, et al: Adipose triglyceride lipase contributes to
cancer-associated cachexia. Science. 333:233–238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kliewer KL, Ke JY, Tian M, Cole RM,
Andridge RR and Belury MA: Adipose tissue lipolysis and energy
metabolism in early cancer cachexia in mice. Cancer Biol Ther.
16:886–897. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Batista ML Jr, Neves RX, Peres SB,
Yamashita AS, Shida CS, Farmer SR and Seelaender M: Heterogeneous
time-dependent response of adipose tissue during the development of
cancer cachexia. J Endocrinol. 215:363–373. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kim JB, Wright HM, Wright M and Spiegelman
BM: ADD1/SREBP1 activates PPARgamma through the production of
endogenous ligand. Proc Natl Acad Sci USA. 95:4333–4337.
1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang LF, Miao LJ, Wang XN, Huang CC, Qian
YS, Huang X, Wang XL, Jin WZ, Ji GJ, Fu M, et al: CD38 deficiency
suppresses adipogenesis and lipogenesis in adipose tissues through
activating Sirt1/PPARγ signaling pathway. J Cell Mol Med.
22:101–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Busquets S, Serpe R, Sirisi S, Toledo M,
Coutinho J, Martínez R, Orpí M, López-Soriano FJ and Argilés JM:
Megestrol acetate: Its impact on muscle protein metabolism supports
its use in cancer cachexia. Clin Nutr. 29:733–737. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dorai V, Hazard MC, Paris J and Delansorne
R: Lipolytic activity of progesterone and synthetic progestins on
rat parametrial adipocytes in vitro. J Pharmacol Exp Ther.
258:620–625. 1991.PubMed/NCBI
|