Introduction
Coronary artery disease (CAD) is the most common
cardiovascular disease and has a notable impact on global health
(1). ST-segment elevation
myocardial infarction (STEMI) is one of the most acute
manifestations of CAD, which is typically characterized by acute
onset and high mortality (2). The
recanalization of infarct-associated arteries or culprit vessels
and reestablishing myocardial perfusion is the primary treatment
for STEMI (3). Percutaneous
coronary intervention (PCI) is the most effective and widely used
method for reopening occluded vessels (3). With the application of PCI, mortality
of STEMI significantly decreased (4). However, a review revealed that
certain patients may experience slow blood flow or no reflow
following PCI, decreasing the benefits of PCI (5). It has been reported that the
incidence of slow blood flow or no reflow after PCI in patients
with STEMI is ~30%, which leads to an increase in infarct size,
heart failure and mortality rate (6). Slow blood flow and no reflow
following PCI in patients with STEMI are independent risk factors
for short-time prognosis and long-time major adverse cardiovascular
events (MACEs) (6).
High thrombus burden, prolonged reperfusion time,
stent diameter and post-stent expansion are all potential factors
verified to affect the incidence of slow blood flow and no reflow
after PCI (7). Therefore, adequate
anticoagulation before and during PCI is key for the prevention of
slow blood flow and no reflow. However, anticoagulation may
increase the risk of bleeding. How to properly balance decreased
slow blood flow and no reflow and the potential increased risk of
bleeding is an urgent cardiovascular problem for treatment of acute
STEMI.
Recombinant human prourokinase (Pro-UK) is a
fibrin-specific plasminogen activator that shares structural
similarities with tissue plasminogen activator but functions via a
different mechanism (8). Studies
show that Pro-UK presents with fewer hemorrhagic complications and
lower re-occlusion rate in patients with acute STEMI compared with
conventional drugs (9,10). In addition, certain prospective
study found that Pro-UK decreases MACEs whereas a retrospective
study revealed that Pro-UK does not affect MACEs (11). To date, Pro-UK is not a frequent
agent applied to patients for acute STEMI due to lack of evidence.
To the best of our knowledge, there are limited studies
investigating the efficacy and safety of Pro-UK in patients with
acute STEMI (11,12). Therefore, further investigations
are needed to assess intracoronary administration of Pro-UK and
non-Pro-UK treatment in patients with acute STEMI.
Since Pro-UK is a coronary thrombolytic drug from
China, most clinical trials on Pro-UK are led by Chinese scholars
or conducted in China. In the present study, a meta-analysis of
randomized controlled trials (RCTs) from China was performed to
compare the safety and efficacy between Pro-UK and non-Pro-UK for
treatment of acute STEMI. This analysis aimed to provide novel
evidence-based medical information for the intracoronary
application of Pro-UK in patients with acute STEMI.
Patients and methods
Search strategy
Studies published before June 2022 were retrieved
from the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/) and
China National Knowledge Infrastructure (CNKI) (https://www.cnki.net/). The terms ‘STEMI’ and ‘PCI’ or
‘Percutaneous coronary intervention’ and ‘Prourokinase’ or ‘Pro-UK’
were used as the key search words.
Selection criteria
Studies were included if the following criteria were
met: i) RCT; ii) study subjects were patients with acute STEMI;
iii) patients with acute STEMI received Pro-UK intracoronary
therapy and iv) efficacy evaluation indicators included at least
recanalization indicators, bleeding and MACEs. By contrast, studies
were excluded if the following criteria were met: i) Non-RCT; ii)
duplicate publication; iii) follow-up <30 days; iv) ongoing or
unpublished study; v) the study did not contain the original data
or statistical analysis could not be performed and vi)
observational or cohort study.
Quality assessment
The included RCTs were assessed using the method of
Jadad which is recommended by the Cochrane Library (13). The quality of RCTs was evaluated
based on the following components: i) Randomized method; ii)
allocation concealment; iii) blinding of participant personnel and
outcome assessors; iv) complete outcome data; v) free of selective
outcome reporting; and vi) clear causes for loss or quitting of the
follow-up.
Data extraction
The data utilized in the present study were
extracted by two independent authors (GF and DG) and not blinded.
The information regarding first author, publication date, study
design, baseline characteristics and endpoints was noted. The study
method described in this article refers to previously published
research by Fan et al (14). The endpoints included MACE,
bleeding, ST-segment resolution (STR), corrected thrombolysis in
myocardial infarction (TIMI) frame count (CTFC), TIMI grade 3
(TIMI-3), TIMI myocardial perfusion grade (TMPG), left ventricular
ejection fraction LVEF, left ventricular end-diastolic diameter
(LVEDd) and cardiac troponin I (cTnI). During extraction, a third
reviewer was used to resolve any disagreement between the two
authors.
Statistical analysis
The data were analyzed using Review Manager 5.3
software (Cochrane). Continuous effective outcomes are presented as
standardized mean difference (SMD) while dichotomous effective
outcomes were analyzed using risk ratio (RR). Continuous data were
mean with SD in this study. The 95% CI was also calculated. The
heterogeneity across studies was analyzed using Q-test. Values of
P>0.10 and I2<50% were considered to indicate no
significant heterogeneity and the pooled outcomes were estimated
using the Mantel-Haenszel fixed-effects model. P≤0.10 and
I2≥50% were considered to indicate significant
heterogeneity and the pooled analyses were estimated using the
Mantel-Haenszel random-effects model. P<0.05 was considered to
indicate a statistically significant difference.
Results
Included studies
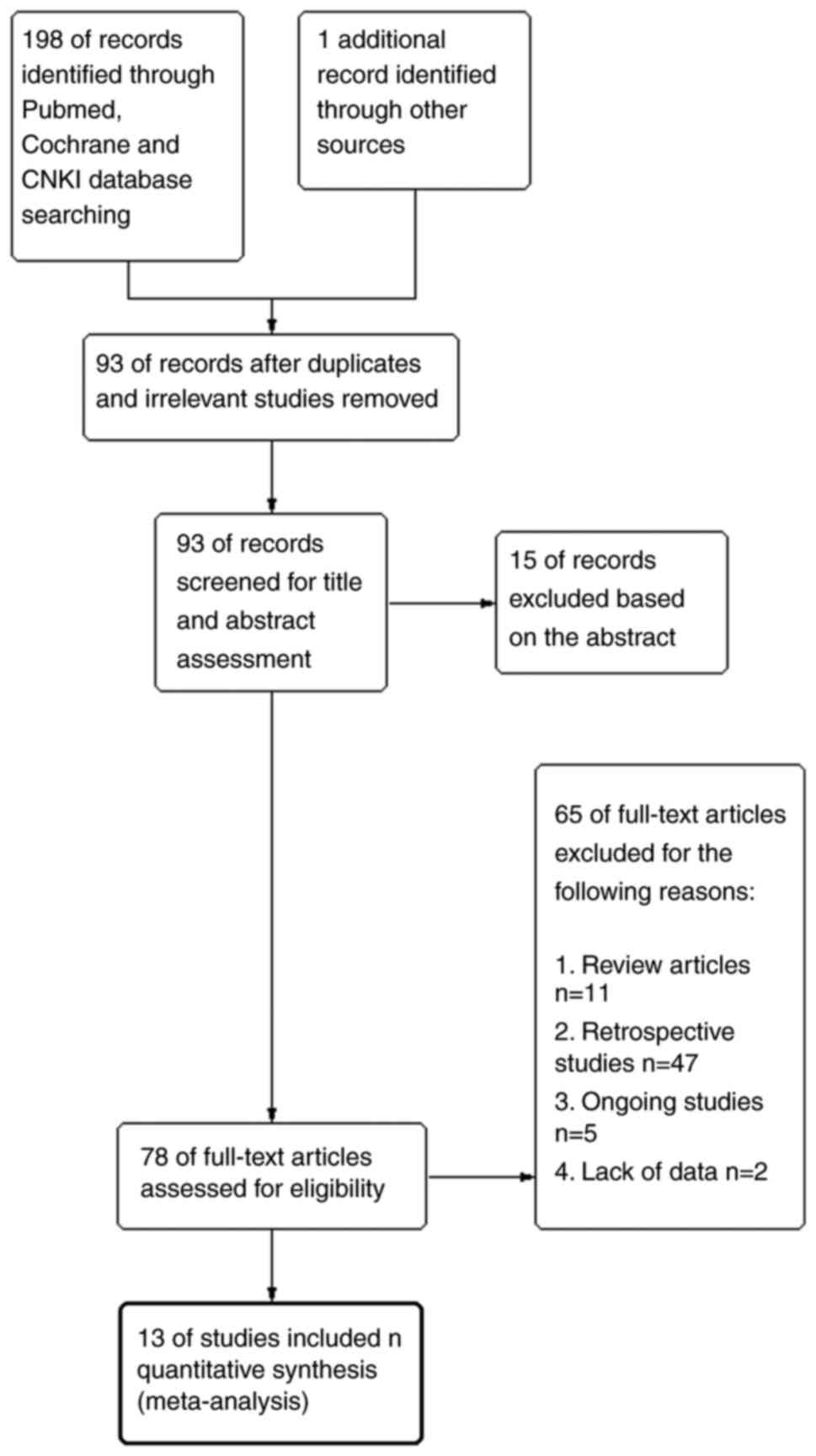
Studies were screened from PubMed (n=21), Cochrane
Library (n=14) and CNKI (n=164) databases. After scanning the
publications, 106 of 199 studies were excluded because of
irrelevant or duplicate records. After further reading, 15 of the
remaining 93 studies were excluded based on the abstract. Among the
remaining 78 papers, 11 were review articles, 47 were retrospective
studies, five were ongoing studies and two studies were excluded
owing to lack of data. Finally, a total of 13 studies comprising
1,797 patients were included in this meta-analysis, including 897
patients who received Pro-UK and 900 patients who were in the
control group. The procedure for use in the study is presented in
Fig. 1.
Quality assessment and baseline
characteristics
The primary characteristics of the included studies
are illustrated in Table I.
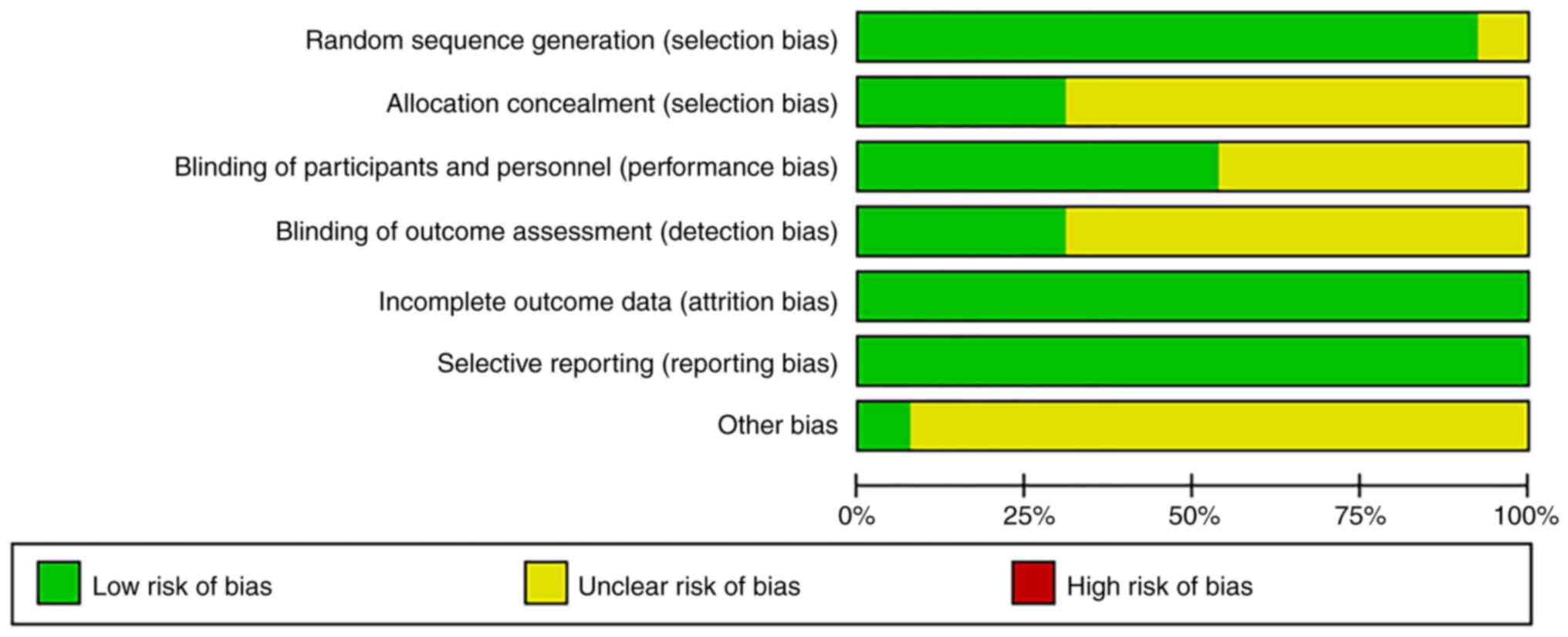
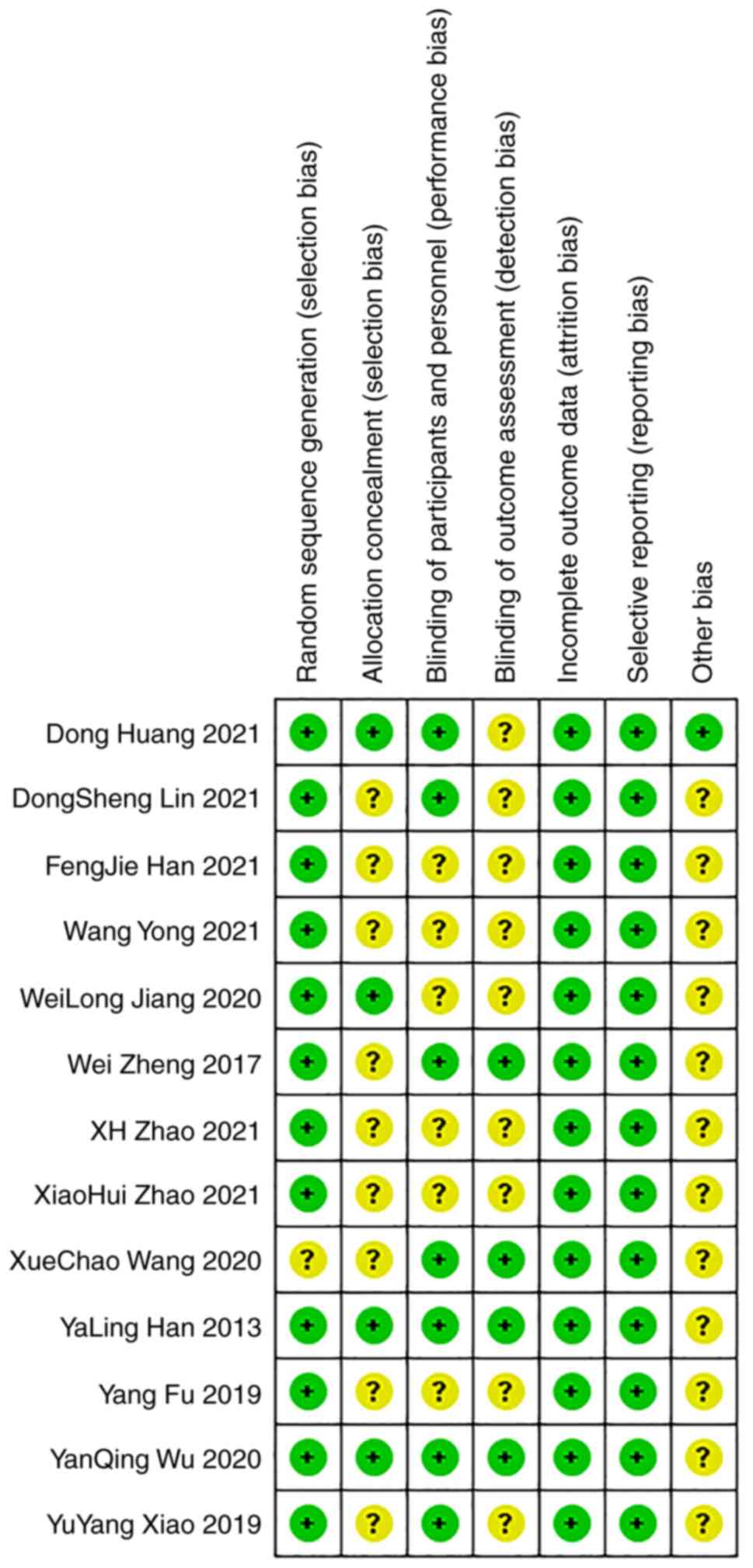
Patient age ranged from 49.0 to 64.9 years. The bias condition of
selected studies is illustrated in Fig. 2 and bias summary is indicated in
Fig. 3. The quality and grading of
the included articles is presented in Table II. The selected reports were RCTs
from China. The Jadad scoring of the included studies ranged from 5
to 7, which indicated high quality.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | N | Age, years (mean ±
SD) | Endpoint | |
|---|
| First author,
year | Setting | Journal | Pro-UK | Control | Pro-UK | Control | Primary | Secondary | Control
therapy | Follow-up days | (Refs.) |
|---|
| Wu et al,
2020 | Single-center | BMC Cardiovascular
Disorders | 25 | 25 | 59.5±14.4 | 61.0±12.6 | Coronary
physiological indexes |
Angiographic/reperfusion assessment;
infarct size; cardiac function | Saline | 90 | (15) |
| Jiang et al,
2020 | Single-center | Coronary artery
Disease | 125 | 135 | 53.9±6.6 | 55.1±6.8 | Infarct size;
reperfusion assessment; | Cardiac function;
MACEs; Hemorrhagic complications | Saline | 180 | (16) |
| Fu et al,
2019 | Single-center | Catheter
Cardiovascular Intervention | 20 | 19 | 62.6±11.1 | 63.2±11.2 | TIMI flow grade;
CTFC | MACEs; Bleeding;
Electrocardiogram features and myocardial necrosis markers | Thrombus
aspiration | 90 | (17) |
| Huang et al,
2021 | Multi-center | Frontiers in
cardiovascular medicine | 111 | 117 | 59.4±10.1 | 58.5±9.9 | CTFC | TIMI flow grade;
MACEs; Myocardial necrosis markers | Saline | 30 | (18) |
| Geng et al,
2018 | Single-center | Journal of
international Cardiology | 118 | 112 | 53.5±11.4 | 55.2±10.4 | Markers of infarct
size and myocardial reperfusion | Indicators of
cardiac functions; MACEs; bleeding | Saline | 180 | (19) |
| Xiao et al,
2019 | Single-center | Coronary artery
Disease | 33 | 38 | 62.1±15.8 | 64.9±13 | TMPG and IMR
values | Cardiac functions;
MACEs | Thrombus
aspiration | 90 | (20) |
| Wang et al,
2020 | Single-center | Coronary artery
Disease | 92 | 90 | 61.1±11.3 | 58.8±11 | Incidence of
restored myocardial reperfusion | TIMI flow grade;
MACEs; CTFC | Saline | 180 | (21) |
| Lin et al,
2021 | Single-center | Journal of Clinical
Cardiology (China) | 36 | 40 | 65.2±11.2 | 52.4±11.7 | Incidence of
restored myocardial reperfusion; CTFC | Cardiac functions;
MACEs | Tirofiban | 365 | (22) |
| Wang et al,
2021 | Single-center | Evolution and
analysis of drug-use in hospitals of China | 30 | 30 | 62.3±9.4 | 61.4±11.5 | TIMI flow
grade | Cardiac function;
MACEs | Sodium
nitroprusside | 30 | (23) |
| Zhao et al,
2021 | Single-center | Medical Science
Journal of central south China | 50 | 50 | 49.6±3.5 | 49.9±3.9 | TIMI flow
grade | MACEs;
Bleeding | Alteplase | 180 | (24) |
| Han et al,
2021 | Single-center | Chinese journal
crit care medicine | 60 | 60 | 64.7±5.9 | 62.9±6.6 | TIMI flow grade;
CTFC; TMPG; Incidence of restored myocardial reperfusion | Cardiac functions;
MACEs | Sodium
nitroprusside | 180 | (25) |
| Han et al,
2013 | Single-center | Cardiovascular
Therapeutics | 100 | 97 | 56.8.7±9.8 | 57.1±8.9 | TIMI flow
grade | MACEs;
Bleeding | Anti-platelet | 365 | (26) |
| Zhao et al,
2021 | Single-center | PJCCPVD | 92 | 92 | 61.9±8.2 | 62.9±8.2 | TIMI flow
grade | Myocardial necrosis
markers; MACEs | Tirofiban | 60 | (27) |
 | Table IIQuality of included studies. |
Table II
Quality of included studies.
| First author,
year | Randomized
method | Allocation
concealment | Blinding | Complete outcome
data | Free of selective
outcome reporting | Clear cause for
loss or quitting of follow-up | Jadad score | (Refs.) |
|---|
| Wu et al,
2020 | Yes | Yes | Single-blind | Yes | Yes | Yes | 7 | (15) |
| Jiang et al,
2020 | Yes | Yes | Unclear | Yes | Yes | Yes | 6 | (16) |
| Fu et al,
2019 | Yes | Unclear | Unclear | Yes | Yes | Yes | 6 | (17) |
| Dong et al,
2021 | Yes | Yes | Single-blind | Yes | Yes | Yes | 7 | (18) |
| Wei et al,
2018 | Unclear | Unclear | Single-blind | Yes | Yes | Yes | 6 | (19) |
| Xiao et al,
2019 | Yes | Unclear | Single-blind | Yes | Yes | Yes | 7 | (20) |
| Wang et al,
2020 | No | Yes | Single-blind | Yes | Yes | Yes | 6 | (21) |
| Lin et al,
2021 | Yes | Unclear | Single-blind | Yes | Yes | Yes | 6 | (22) |
| Wang et al,
2021 | Yes | Unclear | Unclear | Yes | Yes | Yes | 6 | (23) |
| Zhao et al,
2021 | Unclear | Unclear | Unclear | Yes | Yes | Yes | 5 | (24) |
| Han et al,
2021 | Yes | No | Unclear | Yes | Yes | Yes | 6 | (25) |
| Han et al,
2013 | Yes | Yes | Single-blind | Yes | Yes | Yes | 7 | (26) |
| Zhao et al,
2021 | Yes | Unclear | Unclear | Yes | Yes | Yes | 6 | (27) |
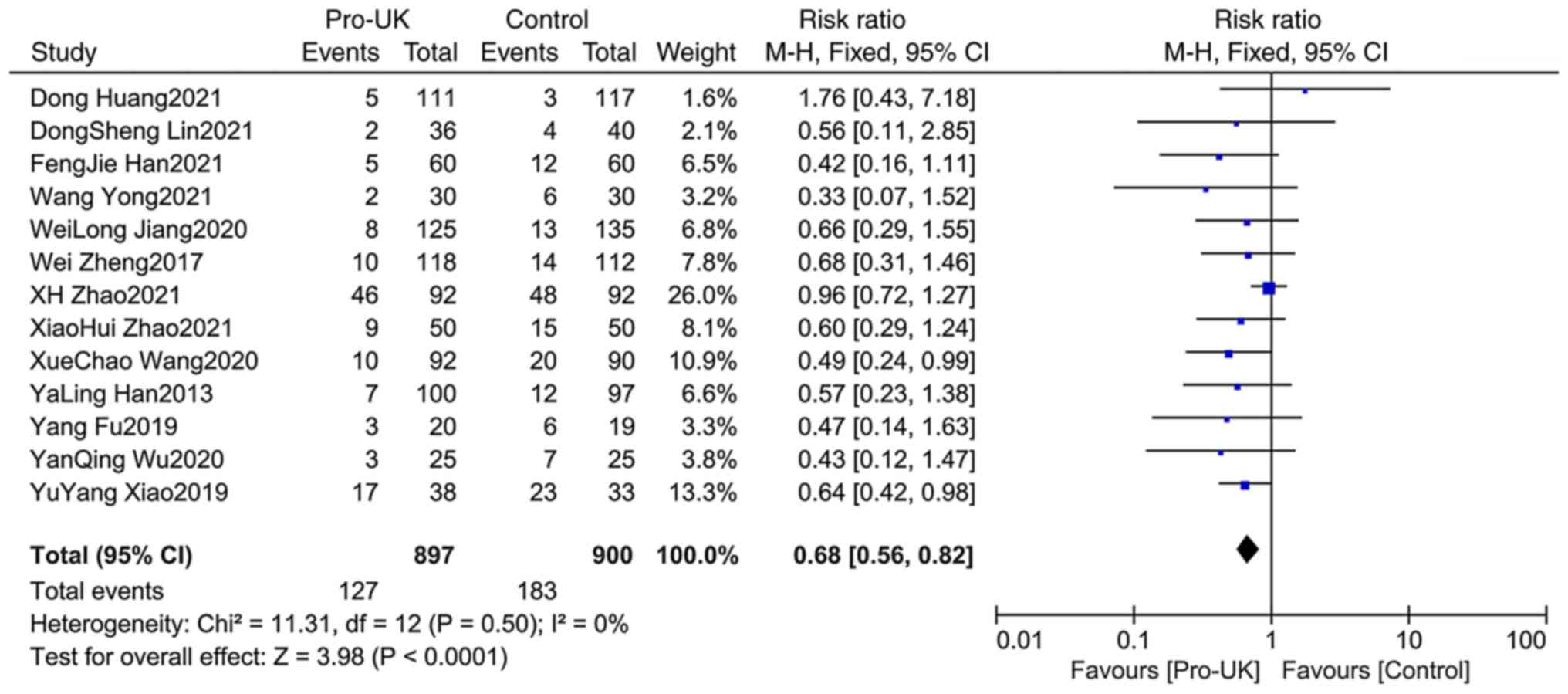
Comparison of MACEs between
groups
A total of 13 studies comprising 1,797 patients
reported MACEs. There was no significant heterogeneity between
studies (P=0.50; I2=0%). The effect size of the pooled
RRs was calculated using the Mantel-Haenszel fixed effects model.
The results revealed that the Pro-UK group presented a
significantly lower incidence of MACEs compared with that in the
control group (RR, 0.68; 95% CI, 0.56-0.82; P<0.0001; Fig. 4).
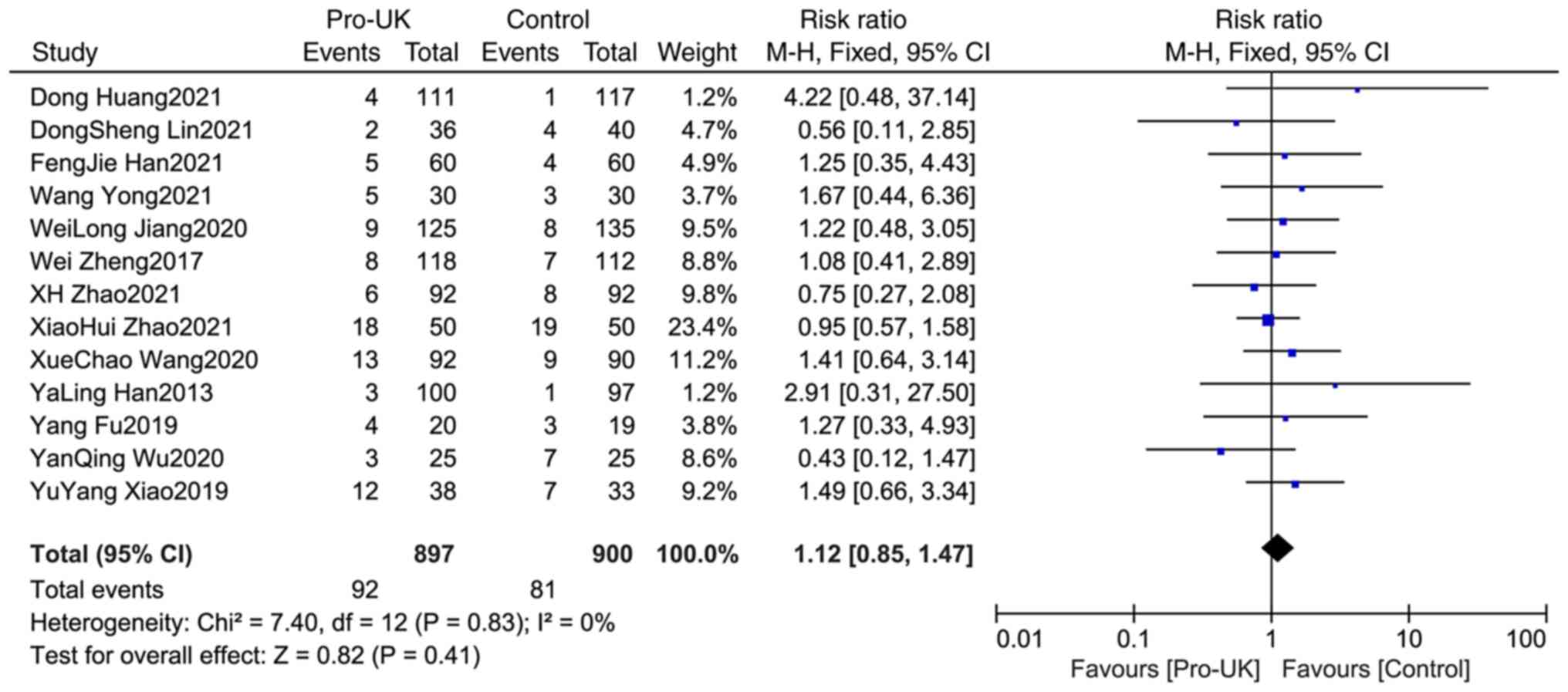
Comparison of bleeding between two
groups
A total of 13 studies reported bleeding, including
897 patients who received Pro-UK and 900 patients who in the
control group. There was no significant heterogeneity between
studies (P=0.83; I2=0%). The results showed that there
was no significant difference in bleeding incidence between the two
groups (RR, 1.12; 95% CI, 0.85-1.47; P=0.41; Fig. 5).
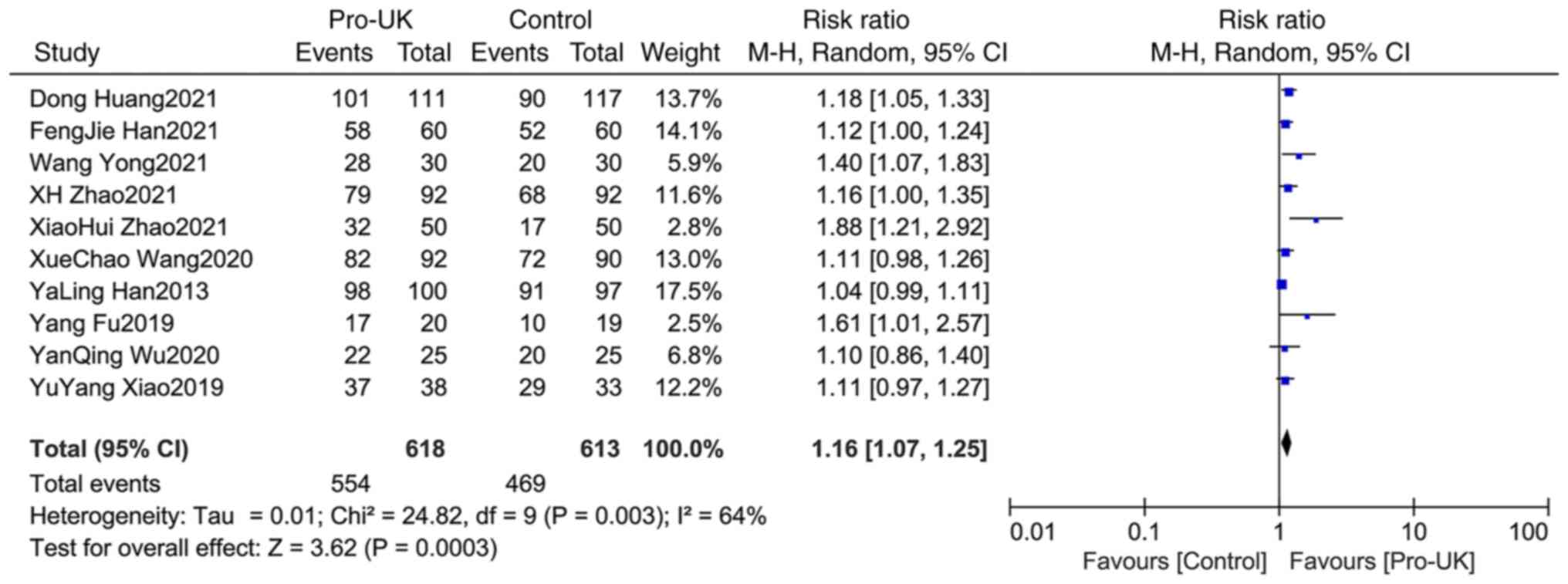
Comparison of TIMI-3 between
groups
A total of 10 studies comprising 1,301 patients
reported TIMI-3, including 618 patients who received Pro-UK and 613
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.003; I2=64%). The
effect size of the pooled RRs was estimated using the random
effects model. The Pro-UK group presented a significantly increased
TIMI-3 rate compared with that in the control group (RR, 1.16; 95%
CI, 1.07-1.25; P=0.0003; Fig.
6).
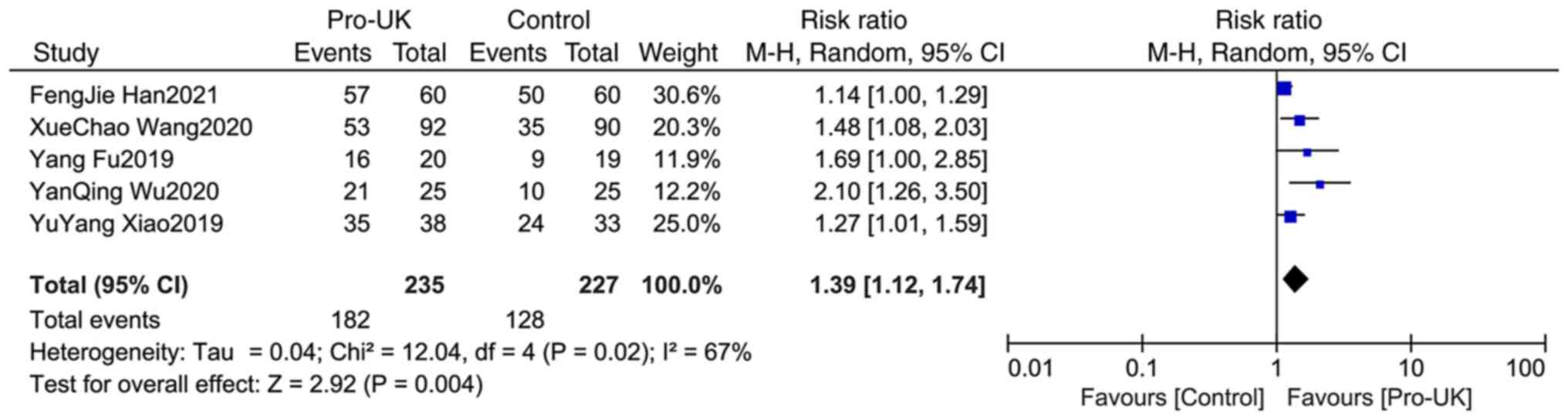
Comparison of TMPG-3 between
groups
A total of five studies comprising 462 patients
reported TMPG-3, including 235 patients who received Pro-UK and 227
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.02; I2=67%). The
effect size of the pooled RRs was calculated using the random
effects model. The Pro-UK group presented a significantly increased
TMPG-3 rate compared with that in the control group (RR, 1.39; 95%
CI, 1.12-1.74; P=0.004; Fig.
7).
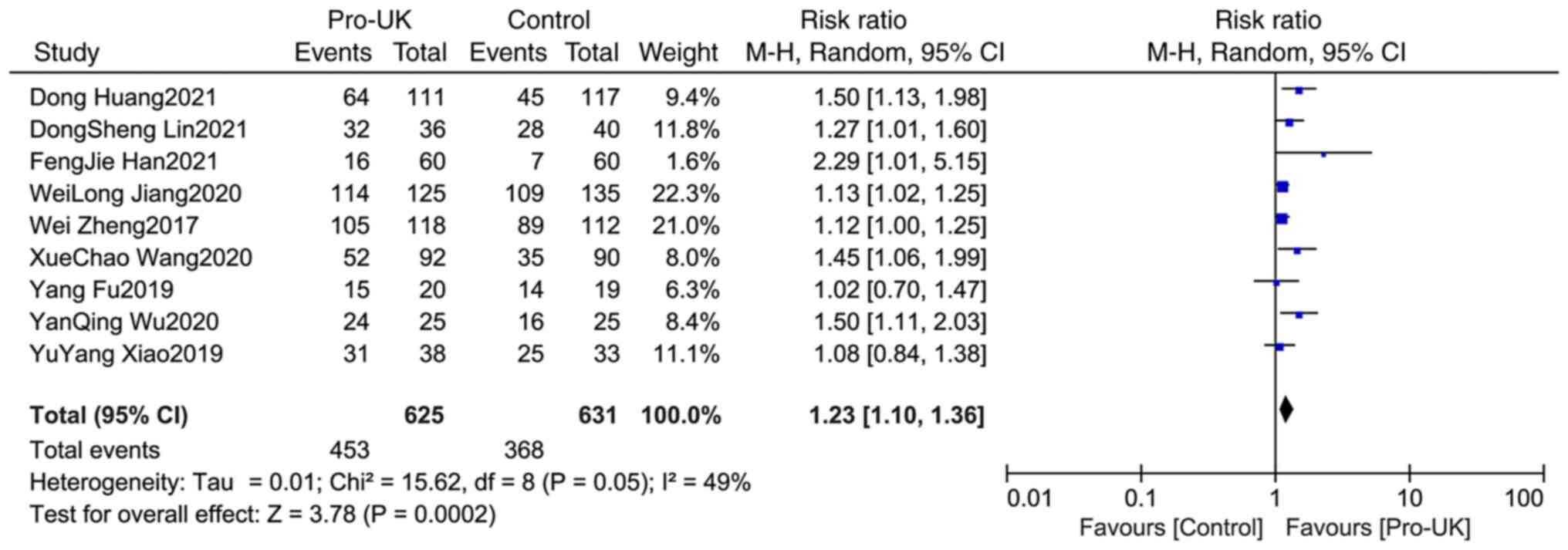
Comparison of STR between two
groups
A total of nine studies comprising 1,256 patients
reported STR, including 625 patients who received Pro-UK and 631
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.05; I2=49%). The
effect size of the pooled RRs was estimated using the
Mantel-Haenszel random effects model. The Pro-UK group presented a
significantly increased STR rate compared with that in the control
group (RR, 1.23; 95% CI, 1.10-1.36; P=0.0002; Fig. 8).
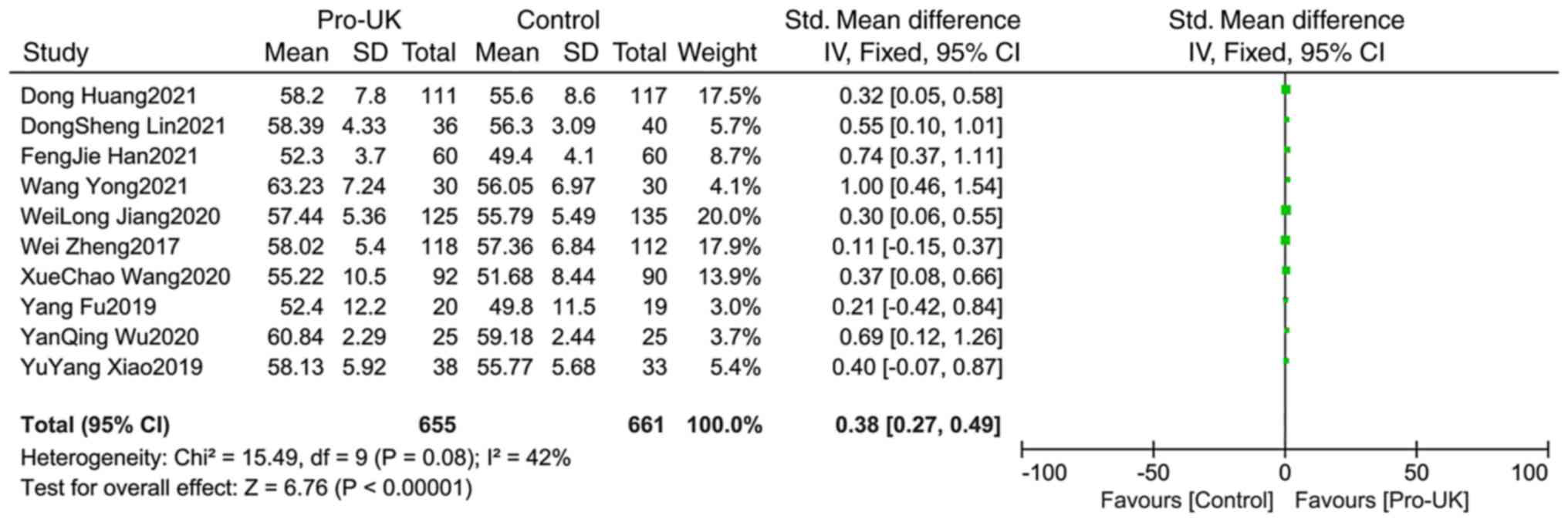
Comparison of LVEF between groups
A total of 10 studies comprising 1,316 patients
reported LVEF, including 655 patients who received Pro-UK and 661
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.08; I2=42%). The
effect size of the pooled SMD was estimated using the
Mantel-Haenszel fixed effects model. The Pro-UK group presented a
significantly higher LVEF compared with the control group (SMD:
0.38, 95% CI: 0.27-0.49, P<0.00001, Fig. 9).
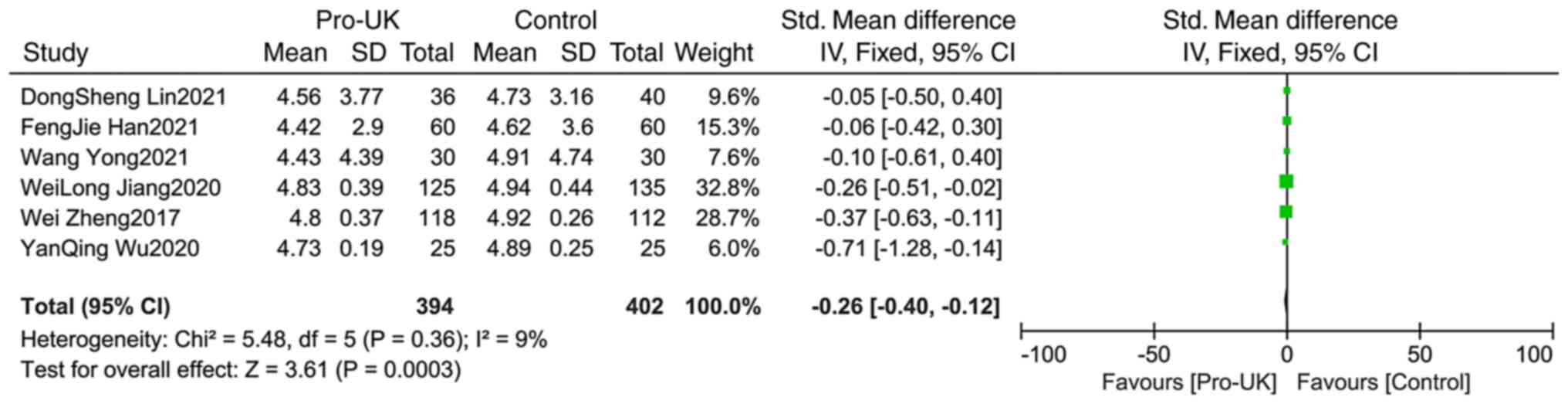
Comparison of LVEDd between
groups
A total of six studies comprising 796 patients
reported LVEDd, including 304 patients who received Pro-UK and 402
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.36; I2=9%). The
effect size of pooled SMD was estimated using the fixed effects
model. The Pro-UK group presented a significantly decreased LVEDd
compared with that in the control group (SMD, -0.26; 95% CI, -0.40
– -0.12; P=0.0003; Fig. 10).
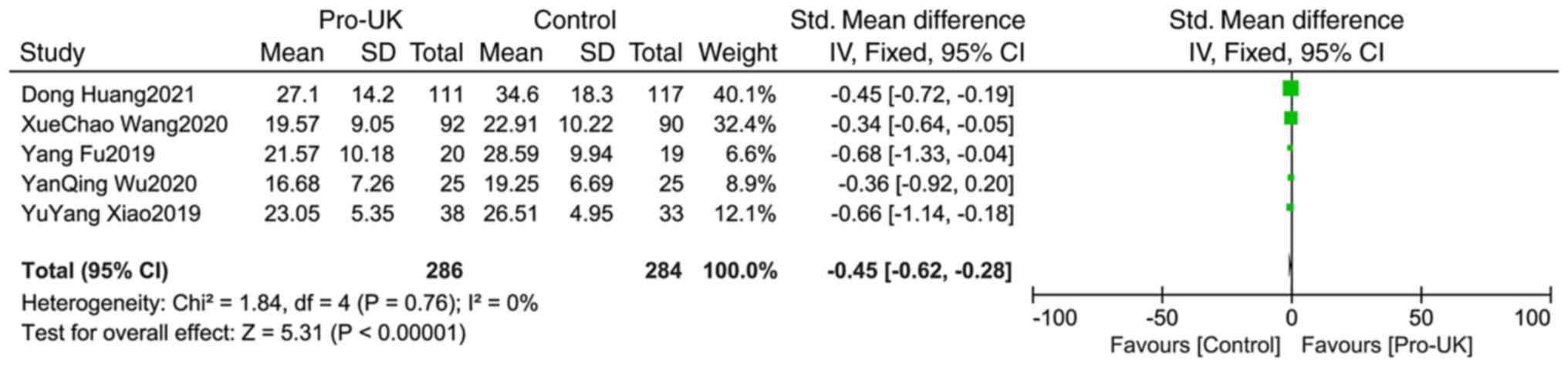
Comparison of CTFC between groups
A total of five studies comprising 570 patients
reported CTFC, including 286 patients who received Pro-UK and 284
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.76; I2=0%). The
effect size of pooled SMD was estimated using Mantel-Haenszel fixed
effects model. The Pro-UK group presented a significantly decreased
CTFC compared with that in the Control group (SMD, -0.45; 95% CI,
-0.62 – -0.28, P<0.00001; Fig.
11).
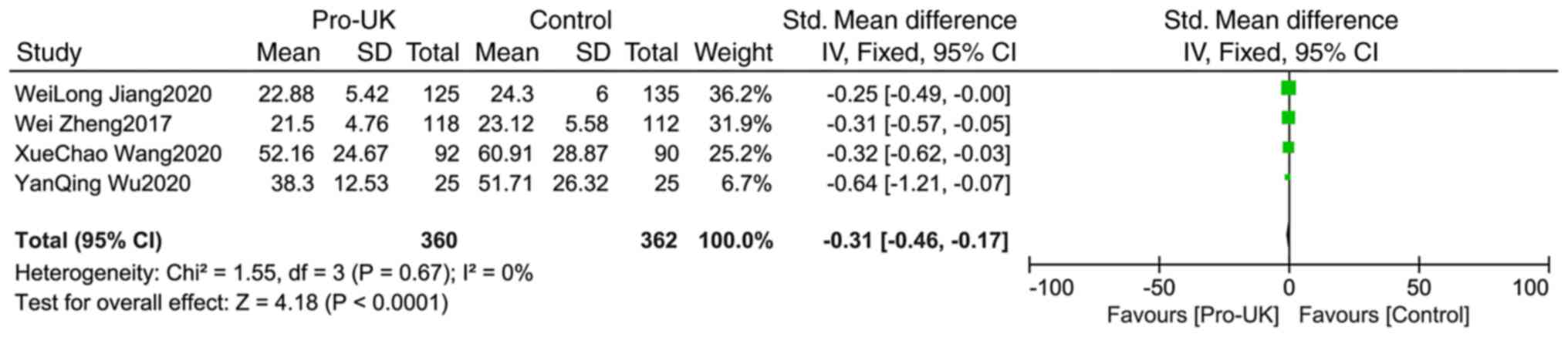
Comparison of cTnI between groups
A total of four studies comprising 722 patients
reported cTnI, including 360 patients who received Pro-UK and 362
patients who were in the control group. There was no significant
heterogeneity between studies (P=0.67; I2=0%). The
effect size of pooled SMD was estimated using the fixed effects
model. The Pro-UK group presented a significantly decreased cTnI
level compared with that in the control group (SMD, -0.31; 95% CI,
-0.46 – -0.17; P<0.0001; Fig.
12).
Discussion
Altogether, 13 RCTs were included in the present
meta-analysis. The pooled data estimations revealed that
intracoronary Pro-UK administration was associated with decreased
MACEs, LVEDd, CTFC and cTnI levels in patients with acute STEMI.
Additionally, there were increased TIMI-3, TMPG-3, STR rate and
LVEF levels in the Pro-UK group compared with those in the control
group. No significant difference was identified regarding the
safety indexes (bleeding) between groups.
The primary aim for the treatment of acute STEMI is
to restore effective perfusion of the myocardium and minimize
ischemic damage. PCI is the first option to reopen
infarct-associated arteries and restore coronary blood flow
(28). Stent implantation in
patients with acute STEMI is beneficial. However, the incidence of
slow blood flow or no reflow after PCI in patients with STEMI is
~30%, which usually leads to worse prognosis (29). Patients with STEMI and slow blood
flow or no reflow have higher MACE occurrence rate compared with
those with optimal flow (30).
Delayed reperfusion, high thrombosis burden, glucose levels and
stent diameter are factors that contribute to slow blood flow or no
reflow following PCI (31).
High-burden thrombosis is the most important risk
factor for no reflow or slow blood flow phenomena following PCI
(32). Thrombus aspiration (TA) is
a common method used for treating intracoronary thrombus, but TA
cannot completely remove the thrombus and no reflow rate is still
high following emergency PCI (33). Moreover, TA may cause local
micro-thrombosis, which leads to no reflow or slowed blood flow and
affects myocardial perfusion, increasing the risk of recurrent
myocardial infarction, cardiogenic shock and malignant arrhythmia
(33). Evidence-based study have
suggested that TA is not associated with a decrease in long-term
mortality or clinical outcomes in patients with STEMI (34). Currently, drugs such as tirofiban,
sodium nitroprusside, nicorandil and diltiazem are widely used to
decrease coronary thrombus burden. However, the incidence of no
reflow after PCI is still high, which affects the prognosis of
acute STEMI (35,36).
Recombinant human Pro-UK is the precursor of
urokinase. The activated plasminogen combines with the thrombus Y/E
tablet segment. Pro-UK quickly reacts with kininase and selectively
activates plasminogen in thrombus fibrin, but it does not activate
free plasminogen. Therefore, Pro-UK may decrease or avoid
cytotoxicity, coagulation system allergy and systemic hemorrhage
and other adverse events (37).
In the current study, 13 relatively high-quality
RCTs were included. The present results revealed that intracoronary
administration of Pro-UK therapy was associated with a lower
incidence of MACEs (RR 0.68; 95% CI, 0.56-0.82; P<0.0001), lower
LVEDd (SMD, -0.26; 95% CI, -0.40 – -0.12; P=0.0003), CTFC (SMD,
-0.45; 95% CI, -0.62 – -0.28; P<0.00001) and cTnI (SMD, -0.31;
95% CI, -0.46 – -0.17; P<0.0001) in treating patients with acute
STEMI. Furthermore, Pro-UK treatment had higher TIMI-3 (RR, 1.16;
95% CI, 1.07-1.25; P=0.0003), TMPG-3 (RR, 1.39; 95% CI, 1.12-1.74;
P=0.004), STR (RR, 1.23; 95% CI, 1.10-1.36; P=0.0002) and LVEF
(SMD, 0.38; 95% CI, 0.27 – -0.49; P<0.00001). Bleeding incidence
(RR, 1.12; 95% CI, 0.85-1.47; P=0.41) was comparable between
groups. Based on the present meta-analysis, intracoronary
administration of Pro-UK during PCI in treatment of patients with
acute STEMI should be recommended in clinical practice.
Certain limitations of the present meta-analysis
should be mentioned. First, the meta-analysis was based on
published RCTs and some large-scale ongoing trials were not
included. Second, the analysis was performed on the trial level,
not on the patient level. Third, there was only one multicenter
trial in our meta-analysis that evaluated the Pro-UK effect.
Additionally, the follow-up duration in studies was not uniform.
Use of additional databases (such as Web of Science (https://www.webofscience.com/) and European Molecular
Biology Organization (http://www.embo.org/) is required to validate the
present results.
Intracoronary administration of Pro-UK not only
decreases MACE, LVEDd, and cTnI levels, but also increases TIMI-3,
TMPG-3, STR, and LVEF levels in patients with acute STEMI. Pro-UK
is safe and effective to combine with PCI in treating patients with
acute STEMI. However, more large-scale multicenter RCTs comparing
Pro-UK and non-Pro-UK studies are needed to confirm this
conclusion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
GF and DG conceived and designed the study. GF, XW
and WJ carried out literature search, study selection and quality
assessment. WJ and HZ performed data extraction. GF and HZ confirm
the authenticity of all the raw data. GF, XW, DG and WJ performed
statistical analysis. GF wrote the manuscript. GF and DG
interpreted the data and revised the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing
interests.
References
|
1
|
Ralapanawa U and Sivakanesan R:
Epidemiology and the magnitude of coronary artery disease and acute
coronary syndrome: A narrative review. J Epidemiol Glob Health.
11:169–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Poudel I, Tejpal C, Rashid H and Jahan N:
Major adverse cardiovascular events: An inevitable outcome of
ST-elevation myocardial infarction? A Literature Review. Cureus.
11(e5280)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vogel B, Mehta SR and Mehran R:
Reperfusion strategies in acute myocardial infarction and
multivessel disease. Nat Rev Cardiol. 14:665–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ottani F, Limbruno U, Latini R, Misuraca L
and Galvani M: Reperfusion in STEMI patients: Still a role for
cardioprotection? Minerva Cardioangiol. 66:452–463. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muller O, Trana C and Eeckhout E:
Myocardial no-reflow treatment. Curr Vasc Pharmacol. 11:278–275.
2013.PubMed/NCBI
|
|
6
|
Soeda T, Higuma T, Abe N, Yamada M,
Yokoyama H, Shibutani S, Ong DS, Vergallo R, Minami Y, Lee H, et
al: Morphological predictors for no Reflow phenomenon after primary
percutaneous coronary intervention in patients with ST-segment
elevation myocardial infarction caused by plaque rupture. Eur Heart
J Cardiovasc Imaging. 18:103–110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Movahed MR and Butman SM: The pathogenesis
and treatment of no-reflow occurring during percutaneous coronary
intervention. Cardiovasc Revasc Med. 9(443)2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gurewich V: Fibrinolysis: A misunderstood
natural defense whose therapeutic potential is unknown. Cardiovasc
Drugs Ther. 33:749–753. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu Y, Yang Y, Li Y and Peng X: Comparison
of efficacy and safety of recombinant human prourokinase and
alteplase in the treatment of STEMI and analysis of influencing
factors of efficacy. Evid Based Complement Alternat Med.
2021(6702965)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen T, Wang Q, Liu G, Jin Q, Liu C, Gao
L, Chen Y and Guo J: Safety and efficacy of intracoronary
thrombolytic therapy via a new infusion catheter in patients with
ST-segment elevation myocardial infarction with large thrombus
burden: A pilot study. Coron Artery Dis. 32:205–210.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yao Z, Li W, Cheng L, Cao M, Pang Z and Li
Y: Comparison of the effect of recombinant human pro-urokinase and
tirofiban on myocardial blood flow perfusion in ST elevation
myocardial infarction patients receiving primary percutaneous
coronary intervention: A one-center retrospective observational
study. Medicine (Baltimore). 98(e16143)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao J, Wang WJ, Liu YH and Luo DL:
Efficacy analysis of half-dose recombinant human prourokinase
thromboiysis compined with early PCI in 48 patients with
ST-segment-elevation myocardial infarction (STEMI). Asian J Surg:
Oct 6, 2022 (Epub ahead of print).
|
|
13
|
Beitland S, Sandven I, Kjærvik LK, Sandset
PM, Sunde K and Eken T: Thromboprophylaxis with low molecular
weight heparin versus unfractionated heparin in intensive care
patients: A systematic review with meta-analysis and trial
sequential analysis. Intensive Care Med. 41:1209–1219.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fan G, Zhang YW, Lin L, Chen M, Wei J and
Diao J: Optimal reperfusion strategy in patients with acute STEMI
and multivessel disease-an updated meta-analysis. Herz. 45:272–279.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Y, Fu X, Feng Q, Gu X, Hao G, Fan W and
Jiang Y: Efficacy and safety of intracoronary prourokinase during
percutaneous coronary intervention in treating ST-segment elevation
myocardial infarction patients: A randomized, controlled study. BMC
Cardiovasc Disord. 20(308)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang W, Xiong X, Du X, Ma H, Li W and
Cheng F: Safety and efficacy study of prourokinase injection during
primary percutaneous coronary intervention in acute ST-segment
elevation myocardial infarction. Coron Artery Dis. 32:25–30.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fu Y, Gu XS, Hao GZ, Jiang YF, Fan WZ, Fan
YM, Wei QM, Fu XH and Li YJ: Comparison of myocardial
microcirculatory perfusion after catheter-administered
intracoronary thrombolysis with anisodamine versus standard
thrombus aspiration in patients with ST-elevation myocardial
infarction. Catheter Cardiovasc Interv. 93:839–845. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang D, Qian J, Liu Z, Xu Y, Zhao X, Qiao
Z, Fang W, Jiang L, Hu W, Shen C, et al: Effects of Intracoronary
Pro-urokinase or tirofiban on coronary flow during primary
percutaneous coronary intervention for acute myocardial infarction:
A Multi-center, placebo-controlled, single-blind, randomized
clinical trial. Front Cardiovasc Med. 8(710994)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Geng W, Zhang Q, Liu J, Tian X, Zhen L,
Song D, Yang Y, Meng H, Wang Y and Chen J: A randomized study of
prourokinase during primary percutaneous coronary intervention in
acute ST-segment elevation myocardial infarction. J Interv Cardiol.
31:136–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xiao Y, Fu X, Wang Y, Fan Y, Wu Y, Wang W
and Zhang Q: Effects of different strategies on high thrombus
burden in patients with ST-segment elevation myocardial infarction
undergoing primary percutaneous coronary catheterization. Coron
Artery Dis. 30:555–563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang X, Liu H, Wu H, Xiao Y, Bai S, Li X,
Li X, Zhang L, Chen T, Li H, et al: Safety and efficacy of
intracoronary prourokinase administration in patients with high
thrombus burden. Coron Artery Dis. 31:493–499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin D, Fu G, Chun HZ, Lixia M and Luohui
and Zhaofei W: Effect of low-dose recombinant human prourokinase
and tirofiban on myocardial perfusion in STEMI patients with
primary PCI. J Clin Cardiol (China). 37:215–219. 2021.(In
Chinese).
|
|
23
|
Yong W, Gao J, Hu J, Wang Y, Qing H and
Pei L: Improvement of combined application of sodium nitroprusside
and recombinant human prourokinase in coronary artery on no-reflow
in elderly patients with acute STEMI during emergency PCI.
Evolution Analysis Drug-Use Hosp China. 21:19–22. 2021.(In
Chinese).
|
|
24
|
Zhao X, Lu S, Jie C, Yang X and Huizhe W:
The evaluation of recombinant human prourokinase versus alteplase
in the treatment of acute STEMI. Med Sci J Central South China.
49:72–77. 2021.(In Chinese).
|
|
25
|
Han F, Haijun Z, Zhongming W, Cuiting Q,
Hui Z, Hui J, Jing L, Qingqing Z and Yanxia Z: Effect of
recombinant human prourokinase combined with sodium nitroprusside
and tirofiban by intracoronary injection on the efficacy of PCI in
STEMI patients with high thrombus load. Chin J Crit Care Med.
41:1028–1034. 2021.(In Chinese).
|
|
26
|
Han YL, Liu JN, Jing QM, Ma YY, Jiang TM,
Pu K, Zhao RP, Zhao X, Liu HW, Xu K, et al: The efficacy and safety
of pharmacoinvasive therapy with prourokinase for acute ST-segment
elevation myocardial infarction patients with expected long
percutaneous coronary intervention-related delay. Cardiovasc Ther.
31:285–290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao X, Shuangdong L, Jie C, Yang X and
Huizhe W: Preventive effect of recombinant human prourokinase on no
reflow phenomenon in patients with STEMI after PCI. J Prac
Cardiovas Dis. 29:105–109. 2021.(In Chinese).
|
|
28
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: A quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Morishima I, Sone T, Okumura K, Tsuboi H,
Kondo J, Mukawa H, Matsui H, Toki Y, Ito T and Hayakawa T:
Angiographic no-reflow phenomenon as a predictor of adverse
long-term outcome in patients treated with percutaneous
transluminal coronary angioplasty for first acute myocardial
infarction. J Am Coll Cardiol. 36:1202–1209. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ito H, Okamura A, Iwakura K, Masuyama T,
Hori M, Takiuchi S, Negoro S, Nakatsuchi Y, Taniyama Y, Higashino
Y, et al: Myocardial perfusion patterns related to thrombolysis in
myocardial infarction perfusion grades after coronary angioplasty
in patients with acute anterior wall myocardial infarction.
Circulation. 93:1993–1999. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dong-Bao L, Qi H, Zhi L, Shan W and
Wei-Ying J: Predictors and long-term prognosis of angiographic
slow/no-reflow phenomenon during emergency percutaneous coronary
intervention for ST-elevated acute myocardial infarction. Clin
Cardiol. 33:E7–E12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar R, Khan KA, Shah JA, Ammar A, Kumar
D, Khowaja S, Sial JA, Kazmi S, Murtaza M and Karim M:
Quantification of thrombus burden as an independent predictor of
intra-procedural No-reflow in patients with St-segment elevation
myocardial infarction undergoing primary percutaneous coronary
revascularization. J Ayub Med Coll Abbottabad. 34:288–294.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Scarpone M, Cenko E and Manfrini O:
Coronary No-reflow phenomenon in clinical practice. Curr Pharm Des.
24:2927–2933. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Elgendy AY, Elgendy IY, Mahmoud AN and
Bavry AA: Long-term outcomes with aspiration thrombectomy for
patients undergoing primary percutaneous coronary intervention: A
meta-analysis of randomized trials. Clin Cardiol. 40:534–541.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Z, Li W, Wu W, Xie Q, Li J, Zhang W
and Zhang Y: Myocardial reperfusion with tirofiban injection via
aspiration catheter: Efficacy and safety in STEMI patients with
large thrombus burden. Herz. 45:280–287. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang L, Qi X and Jia X: Effect of
different methods of administration of diltiazem on clinical
efficacy in patients with acute ST-segment elevation myocardial
infarction. Med Sci Monit. 24:6544–6550. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao L, Zhao Z, Chen X, Li J, Liu J and Li
G: Group of Prourokinase Phase IV Clinical Trials Investigators.
Safety and efficacy of prourokinase injection in patients with
ST-elevation myocardial infarction: Phase IV clinical trials of the
prourokinase phase study. Heart Vessels. 33:507–512.
2018.PubMed/NCBI View Article : Google Scholar
|