Introduction
Lung cancer is currently the leading cause of
cancer-related mortality worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for the majority of all lung cancer cases. The 5-year
survival rate of patients with NSCLC is ~15% (2). Moreover, most patients with NSCLC are
at the advanced/metastatic stage when diagnosed, and the 5-year
survival rate is only 5.5% (3).
However, the 5-year survival rate of patients with early-stage
NSCLC is ~60% (4,5). Therefore, the early detection and
diagnosis of lung cancer are essential for the improvement of the
overall survival rate. However, the techniques for early diagnosis
still face challenges.
Recently, lung cancer-related autoantibodies (AABs)
have been reported as biomarkers with high specificity, good
stability and non-invasion have been identified and have become a
focus of research in the early diagnosis of high-risk lung cancer
cohorts (6,7). Notably, the positive results of lung
cancer-related AABs are observed before lung cancer is clinically
diagnosed. Patient cohorts with lung cancer have been screened
using computed tomography (CT) at the Mayo Clinic, and studies have
reported that lung cancer-related AABs can provide a valuable
warning up to 5 years before the clinical diagnosis of lung cancer
(8). It has also been reported
that lung cancer-related AABs have serve an important role in
giving early warnings up to 4 years before the clinical diagnosis
of pulmonary carcinoma in 200,000 high-risk postmenopausal women
(9). The sensitivities and
specificities of a panel consisting of four lung cancer-related
autoantibodies (4-AABs), namely tumor protein 53 (p53), melanoma
antigen 1 (MAGEA1), protein gene product 9.5 (PGP9.5) and
sex-determining region Y-box 2 (SOX2), as a diagnostic tool for
monitoring 458 patients at high risk of lung cancers were reported
to be 71.8 and 89%, respectively (10). A panel of seven lung cancer-related
autoantibodies (7-AABs) in peripheral circulating blood, including
p53, PGP9.5, SOX2, cancer/testis G antigen 7 (GAGE7), ATP-dependent
RNA helicase 4-5 (GBU4-5), cancer-associated antigen (CAGE) and
MAGEA1 was reported to be able to distinguish malignant nodules
from benign nodules and healthy controls (HCs), with a sensitivity
of 56.53% and a specificity of 91.60% (11). The specificity of 7-AABs can be
further increased to 95.80% when combined with CT (11). Another large-scale prospective
study consisting of 1,915 Chinese individuals recruited from five
research centers reported that the panel of 7-AABs has
substantially higher sensitivity and specificity for the early
diagnosis of lung cancer compared with non-specific tumor markers,
such as carcinoembryonic antigen (CEA), neuron-specific enolase and
cytokeratin fragment antigen 21-1 (CYFRA21-1) (12). Therefore, as a specific tumor
marker, the lung cancer-related AAB panel possesses marked clinical
application value in the diagnosis of pulmonary carcinoma. However,
the means to improve its sensitivity remains largely unsolved.
A previous study reported that heat shock protein
90a (HSP90a), which maintains the stability of various protein
molecules, is closely related to the occurrence and development of
malignant tumors (13). The cutoff
value of HSP90a is 50 ng/ml, which can accurately distinguish
malignant and benign tumors in multiple tissues, such as the liver,
lung, pancreas and breast (14).
However, HSP90a alone has limitations in early lung cancer
diagnosis due to its non-specificity. Therefore, the aim of the
present study was to combine the 7-AABs with HSP90a to improve
their diagnostic value in early-stage lung cancer.
In the present study, the level of each lung
cancer-related AAB in patients with malignant lung nodules (MLNs)
or benign lung nodules (BLNs), as well as HCs, was investigated.
The sensitivity, specificity, positive predictive value (PPV),
negative predictive value (NPV) and relative risk (RR) of the
7-AABs with combinations of the 7-AABs and other non-specific tumor
markers, such as HSP90a, CEA, CYFRA21-1, carbohydrate antigen 199
(CA199) or carbohydrate antigen 125 (CA125) were assessed.
Furthermore, the diagnostic efficiency of the aforementioned
markers in patients with different sizes, types and stages of lung
nodules was evaluated.
Patients and methods
Patients
A total of 217 individuals who were in good health,
with lung nodules (ground-glass opacity or solid nodule) firstly
diagnosed using high-resolution CT (HRCT) scans during routine
examinations were enrolled as patients. Patients were recruited
from October 2020 to August 2021 in The First Affiliated Hospital
of Wannan Medical College (Wuhu, China). There were 78 males and
139 females, and the average age was 54.5±1.3 (mean ± standard
deviation) years. The exclusion criteria for patients were as
follows: Chronic obstructive pulmonary disease (including chronic
bronchitis, pneumonectomies and pulmonary heart disease), pulmonary
fibrosis, or a history of treated or untreated pulmonary
malignancy. A total of 30 individuals who were in good health
without underlying lung diseases, nor other major diseases screened
for in the routine examinations were selected as healthy controls.
The present study was approved by the Ethics Review Committee of
the First Affiliated Hospital of Wannan Medical College (approval
no. 202076; Wuhu, China) and the experimental procedures were in
agreement with The Declaration of Helsinki. Written informed
consent was obtained from all participants.
Laboratory measurements
The serum concentrations of 7-AABs were quantified
using the Seven Autoantibodies Detection Kit (ELISA) (cat. no.
20210106. CancerProbe Biotechnology Co., Ltd.). The concentration
of HSP90a in the blood was assessed using the Human Heat Shock
Protein HSP90-α ELISA kit (cat. no. HUEB0886; Assay Genie Co.,
Ltd.). CEA, CYFRA21-1, CA199 and CA125 were assessed using a
Multiple Tumor Markers Detection Kit (cat. no. LP120280; Shanghai
Tellgen Life Science Co., Ltd.) and were quantified using a Tesmi
F4000 automated flow fluorescence immune analyzer (Shanghai Tellgen
Life Science Co., Ltd.).
Hematoxylin and eosin (H&E)
staining
Tissue samples from the patients with lung nodules
were immersed and fixed using 4% formaldehyde at room temperature
(~20˚C) for 24 h, embedded in paraffin, and then sectioned at 3-5
µm thickness. Sections were baked at 70˚C for 1 h and dewaxed using
xylene, before rehydration in a 100, 95, 85 and 75% ethanol series
for 5 min at a time. Then, the sections were transferred into
distilled water for 5 min. Next, the sections were stained using
H&E at room temperature for 5 min, followed by dehydration with
absolute ethyl alcohol at room temperature for 5 min and sealed
using neutral gum (cat. no. N116470, Shanghai Aladdin Biochemical
Technology Co., Ltd.) for assessment. Following the World Health
Organization Classification of Tumours (5th Edition)-Thoracic
Tumours (15) guidelines,
microscopic assessment of the sections was then performed by
experienced pathologists.
Statistical analysis
Data were statistically analyzed using SPSS version
22.0 (IBM Corp.). After the Shapiro-Wilk test was performed,
comparison of the levels of each AAB (namely, p53, PGP9.5, SOX2,
GAGE7, GBU4-5, MAGEA1 and CAGE) and HSP90a among the three groups
was performed using the Kruskal-Wallis test followed by Dunn's test
for non-parametric data. Comparison of the diagnostic efficiency of
7-AABs for discrimination between MLNs and BLNs in different groups
was performed using the χ2 test. Clinical performance was presented
in terms of sensitivity (the percentage of true positives) and
specificity (the percentage of true negatives). PPV (the
probability of MLNs given a positive test result), NPV (the
probability of BLNs given a negative test result) and RR (the
proportion of cases with a positive outcome in the two groups) were
also calculated. McNemar's test was used for comparison of the
sensitivity between 7-AABs + HSP90a and other groups (such as
7-AABs, 7-AABs + CEA, 7-AABs + CYFRA21-1, 7-AABs + CA199 and 7-AABs
+ CA125). For comparison of the NPV between 7-AABs + HSP90a and
other groups, the marginal regression model based on the
generalized estimation equation was used as follows: Model,
g[P(D=1|Z, X=0)]=αN + βNZ, where g represented the contiguous
function, Z represented the indicator variable and X represented
the diagnostic result. Null hypothesis (H0) β=0 was used to check
the difference between tests. When g took the natural logarithmic
function, eβ represented an estimate of the relative
NPV. The diagnostic efficiencies of 7-AABs, 7-AABs + HSP90a, 7-AABs
+ CEA, 7-AABs + CYFRA21-1, 7-AABs + CA199 and 7-AABs + CA125 were
assessed using receiver operating characteristic (ROC) curves. All
tests were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference. The data were presented as
mean ± standard deviation, number of subjects (n) and percentage
(%). Three replicate experiments were performed for each
measurement.
Results
Clinical characteristics of the
patients
A total of 217 patients with lung nodules and 30 HCs
were enrolled in the present study. Table I summarizes the clinical features
of these subjects. A total of 159 patients with MLNs including 158
NSCLC cases (41.8% were cases with adenocarcinoma in situ;
57.6% were cases with invasive adenocarcinoma and 0.6% were cases
with squamous cell carcinoma) and one case with small cell lung
cancer (SCLC) and, 58 patients with BLNs were confirmed by
pathological examination (Fig. 1).
According to the histopathological results, the 159 patients were
divided into one of four stages: Stage 0, 66 patients (41.5%);
stage I, 91 patients (57.2%); stage II, 1 patient (0.6%); and stage
III, 1 patient (0.6%).
 | Table IClinical characteristics of patients
with lung nodules and HCs. |
Table I
Clinical characteristics of patients
with lung nodules and HCs.
| Clinical
characteristic | Lung nodule | MLN | BLN | HC |
|---|
| Number of
patients | 217 | 159 | 58 | 30 |
| Age, years | 54.5±11.3 | 54.5±11.7 | 54.5±10.1 | 53.2±9.1 |
| Sex (M/F), n | 78/139 | 50/109 | 28/30 | 11/19 |
| NSCLC, n | 158 | 158 | - | - |
| Adenocarcinoma
in situ, n (%) | 66 (41.8) | 66 (41.8) | - | - |
| Invasive
adenocarcinoma, n (%) | 91 (57.6) | 91 (57.6) | - | - |
| Squamous cell
carcinoma, n (%) | 1 (0.6) | 1 (0.6) | - | - |
| SCLC, n | 1 | 1 | - | - |
| Stage, n (%) | | | | |
|
0 | 66 (41.5) | 66 (41.5) | - | - |
|
I | 91 (57.2) | 91 (57.2) | - | - |
|
II | 1 (0.6) | 1 (0.6) | - | - |
|
III | 1 (0.6) | 1 (0.6) | - | - |
| Size, n (%) | 217 | 159 | 58 | |
|
<8
mm | 68 (31.3) | 42 (26.4) | 26 (44.8) | - |
|
8-20 mm | 149 (68.7) | 117 (73.6) | 32 (55.2) | - |
| Type, n (%) | 217 | 159 | 58 | |
|
Pure
GGO | 58 (26.7) | 45 (28.3) | 13 (22.4) | - |
|
Mix GGO | 97 (44.7) | 85 (53.5) | 12 (20.7) | - |
|
Solid
nodule | 62 (28.6) | 29 (18.2) | 33 (56.9) | - |
Of the 217 enrolled patients, 68 (31.3%) and 149
(68.7%) patients, with lung nodules ≤8 mm and 8-20 mm,
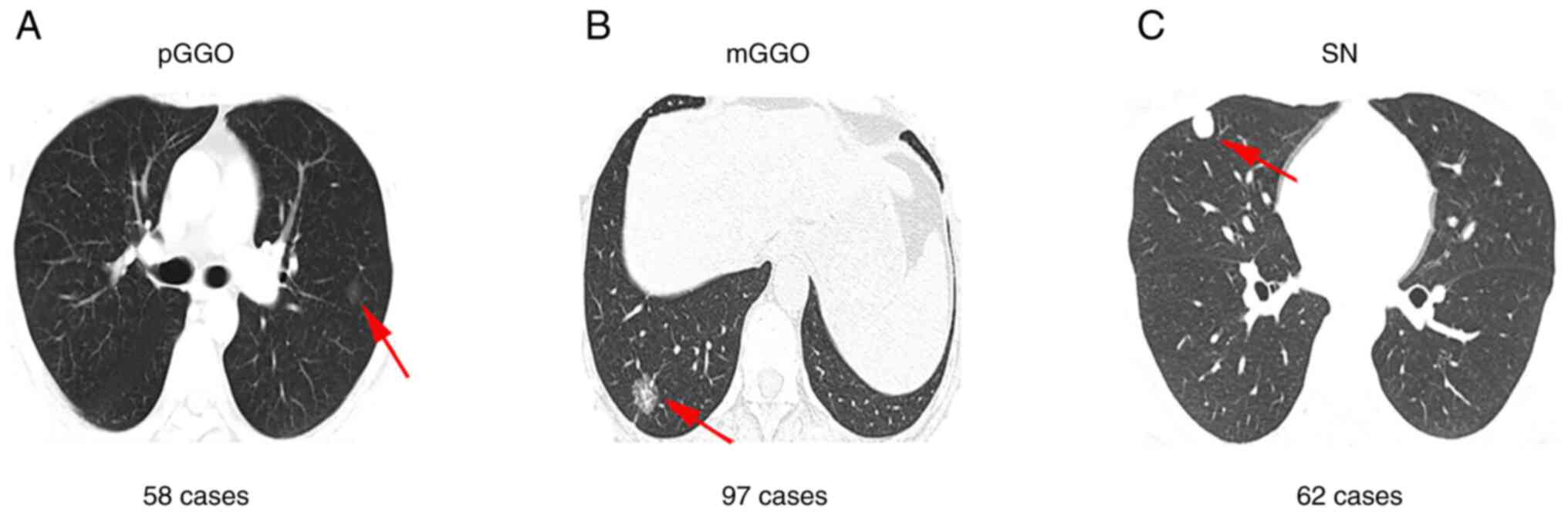
respectively, were evaluated using HRCT. Moreover, 58 (26.7%), 97
(44.7%) and 62 (28.6%) patients with pure ground-glass opacity
(pGGO), mix ground-glass opacity (mGGO) and solid nodule (SN),
respectively, were also evaluated using HRCT (Fig. 2). A total of 159 patients with MLNs
and 58 patients with BLNs were classified and were presented in
Table I.
The reactivity of each AAB and HSP90a
in patients and HCs
The levels of 7-AABs, namely p53, PGP9.5, SOX2,
GAGE7, GBU4-5, MAGEA1 and CAGE in serum samples and plasma HSP90a
levels in 217 patients and 30 HCs were assessed using ELISA. The
levels of p53, PGP9.5, SOX2, GAGE7, MAGEA1 and HSP90a in the MLN
group were significantly elevated compared with those in the HC
group (Fig. 3). However, there
were no significant differences in the levels of GBU4-5 and CAGE
among the three groups. These data suggested that the levels of
7-AABs and HSP90a from patients with MLNs showed marked differences
compared with patients with BLNs and HCs.
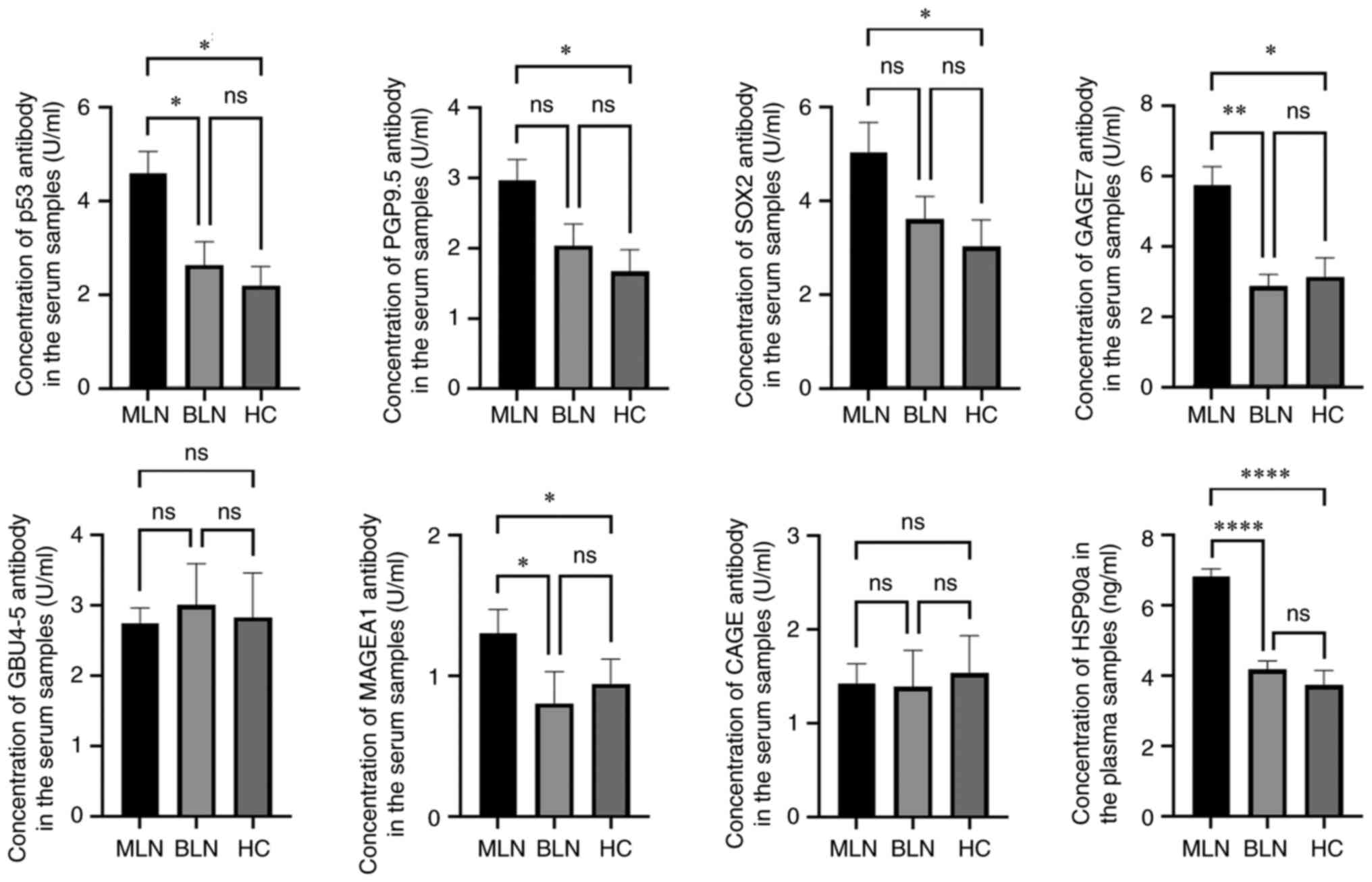 | Figure 3Levels of each autoantibody and
HSP90a in the MLN, BLN and HC groups. *P<0.05,
**P<0.01 and ****P<0.0001. After the
normality test was performed, Kruskal-Wallis test followed by
Dunn's test was used to analyze the data. MLN, malignant lung
nodules; BLN, benign lung nodules; HC, healthy control; p53, Tumor
protein 53; MAGEA1, melanoma antigen 1; PGP9.5, protein gene
product 9.5; SOX2, sex-determining region Y-box 2; GAGE7,
cancer/testis G antigen 7; GBU4-5, ATP-dependent RNA helicase 4-5;
CAGE, cancer-associated antigen; HSP90a, heat shock protein 90a;
ns, not significant. |
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules
The diagnostic values of 7-AABs alone or in
combination with non-specific tumor markers for patients with lung
nodules were evaluated. It was found that although the 7-AABs
demonstrated a high specificity (77.6%; 95% CI, 64.4-87.1%) and PPV
(84.0%; 95% CI, 73.8-90.8%) in patients with lung nodules, a low
sensitivity (42.8%; 95% CI, 35.0-50.9%) and NPV (33.1%; 95% CI,
25.4-41.7%) were also demonstrated. Moreover, 7-AABs reflected a
1.3-fold increase in RR of MLNs, which demonstrated a good
diagnostic efficiency (AUC=0.612, P=0.012). However, statistical
analysis indicated that 7-AABs + HSP90a exhibited an elevated
sensitivity (87.4%; 95% CI, 81.0-92.0%; P<0.0001) and NPV
(66.1%; 95% CI, 52.5-77.6%; P<0.0001) compared with 7-AABs alone
or in combination with other non-specific tumor markers, such as
CEA, CYFRA21-1, CA199 or CA125. Furthermore, a positive result for
7-AABs + HSP90a reflected a 2.6-fold increase in RR for MLNs, which
was significantly higher compared with 7-AABs alone or other
combinations (Table II). ROC
curves of 7-AABs + HSP90a (AUC=0.842; P<0.0001) also
demonstrated that such a combination possessed significantly
improved diagnostic efficiency for the discrimination between MLNs
and BLNs compared with 7-AABs (AUC=0.612; P=0.012), 7-AABs + CEA
(AUC=0.612; P=0.012), 7-AABs + CYFRA21-1 (AUC=0.612; P=0.012),
7-AABs + CA199 (AUC=0.612; P=0.012) and 7-AABs + CA125 (AUC=0.674;
P<0.0001) (Fig. 4A), which
indicated that 7-AABs + HSP90a exhibited better diagnostic
efficiency for patients with pulmonary nodules.
 | Figure 4Diagnostic efficiency of 7-AABs alone
or in combination with non-specific tumor markers. (A) Diagnostic
efficiency was assessed using the ROC curve for patients with lung
nodules. The results of 7-AABs, 7-AABs + CEA, 7-AABs + CYFRA21-1
and 7-AABs + CA199 were very similar to the ROC analysis using the
overlapped data. (B) Diagnostic efficiency was assessed using the
ROC curve for patients with lung nodules of different sizes. The
results of 7-AABs, 7-AABs + CEA, 7-AABs + CYFRA21-1, 7-AABs + CA199
and 7-AABs + CA125 were similar to the ROC analysis using the
overlapped data in the <8 mm group. The results of 7-AABs,
7-AABs + CEA, 7-AABs + CYFRA21-1 and 7-AABs + CA199 were very
similar to the ROC analysis using the overlapped data in the 8-20
mm group. (C) Diagnostic efficiency was assessed using the ROC
curve for patients with lung nodules of different types. The
results of 7-AABs, 7-AABs + CEA, 7-AABs + CYFRA21-1, 7-AABs + CA199
and 7-AABs + CA125 were similar to the ROC analysis using the
overlapped data in the pGGO and mGGO groups. The results of 7-AABs
alone or in combination with non-specific tumor markers were
identical to the ROC analysis using the overlapped data in the SN
group. 7-AABs, seven lung cancer-related autoantibodies; ROC,
receiver operating characteristic; CEA, carcinoembryonic antigen;
CYFRA21-1, cytokeratin fragment antigen 21-1; CA199, carbohydrate
antigen 199; CA125, carbohydrate antigen 125; pGGO, pure
ground-glass opacity; mGGO, mix ground-glass opacity; SN, solid
nodule; HSP90a, heat shock protein 90a. |
 | Table IIDiagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules. |
Table II
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules.
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
|---|
| Specificity, % | 77.6 | 67.2 | 70.7 | 72.4 | 75.9 | 77.6 |
| Sensitivity, % | 42.8 | 87.4a | 45.9 | 49.1 | 44.0 | 47.8 |
| PPV, % | 84.0 | 88.0 | 81.1 | 83.0 | 83.3 | 85.4 |
| NPV, % | 33.1 | 66.1a | 32.3 | 34.1 | 33.1 | 35.2 |
| RR (95% CI) | 1.3 (1.1-1.5) | 2.6 (1.8-3.7) | 1.2 (1.0-1.4) | 1.3 (1.1-1.5) | 1.2 (1.1-1.5) | 1.3 (1.1-1.5) |
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules of different sizes
The diagnostic values of 7-AABs alone or in
combination with non-specific tumor markers for patients with
pulmonary nodules of 8-20 mm or <8 mm was assessed. The results
demonstrated a better diagnostic value of 7-AABs for discrimination
between MLNs and BLNs in the 8-20 mm group (χ2=5.4,
P=0.02) compared with the <8 mm group (χ2=2.9,
P=0.09). Furthermore, 7-AABs + HSP90a demonstrated an elevated
sensitivity (all P<0.0001) and NPV (all P<0.0001) compared
with 7-AABs alone or in combination with other non-specific tumor
markers, such as CEA, CYFRA21-1, CA199 and CA125, in both the 8-20
mm and <8 mm groups. A positive result for 7-AABs + HSP90a
indicated a 2.2-fold and 4.0-fold increase in the RR of MLNs in the
8-20 mm and <8 mm groups, respectively (Table III). ROC curves of 7-AABs +
HSP90a (AUC=0.868, P<0.0001; AUC=0.784, P<0.0001) also
demonstrated that such a combination possessed markedly improved
diagnostic efficiency for the discrimination between MLNs and BLNs
compared with 7-AABs (AUC=0.595, P=0.099; AUC=0.630, P=0.074),
7-AABs + CEA (AUC=0.595, P=0.099; AUC=0.630, P=0.074), 7-AABs +
CYFRA21-1 (AUC=0.595, P=0.099; AUC=0.630, P=0.074), 7-AABs + CA199
(AUC=0.595, P=0.099; AUC=0.630, P=0.074) and 7-AABs +
CA125 (AUC=0.696, P=0.001; AUC=0.630, P=0.074) in the
8-20 mm and <8 mm groups, respectively (Fig. 4B). These data demonstrated that
7-AABs + HSP90a exhibited improved diagnostic values for patients
with small pulmonary nodules (<20 mm).
 | Table IIIDiagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules of different sizes. |
Table III
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules of different sizes.
| A, tumor size <8
mm |
|---|
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
|---|
| Specificity, % | 73.1 | 61.5 | 73.1 | 69.2 | 73.1 | 73.1 |
| Sensitivity, % | 47.6 | 90.5a | 50.0 | 52.4 | 47.6 | 47.6 |
| PPV, % | 74.1 | 79.2 | 75.0 | 73.3 | 74.1 | 74.1 |
| NPV, % | 46.3 | 80.0a | 47.5 | 47.4 | 46.3 | 46.3 |
| RR (95% CI) | 1.4 (1.0-1.4) | 4.0 (1.6-9.6) | 1.4 (1.0-2.1) | 1.4 (1.0-2.0) | 1.4 (1.0-2.0) | 1.4 (1.0-2.0) |
| B, tumor size 8-20
mm |
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
| Specificity, % | 81.3 | 71.9 | 68.8 | 75.0 | 78.1 | 81.3 |
| Sensitivity, % | 41.0 | 86.3a | 44.4 | 47.9 | 42.7 | 47.9 |
| PPV, % | 88.9 | 91.8 | 83.9 | 87.5 | 87.7 | 90.3 |
| NPV, % | 27.4 | 60.0a | 25.3 | 28.2 | 27.2 | 29.9 |
| RR (95% CI) | 1.2 (1.0-1.4) | 2.2 (1.5-3.3) | 1.1 (1.0-1.3) | 1.2(1.0-1.4) | 1.2 (1.0-1.4) | 1.3 (1.1-1.5) |
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
lung nodules of different types
The diagnostic values of 7-AABs alone or in
combination with non-specific tumor markers for patients with pGGO,
mGGO or SN were evaluated. Statistical analysis indicated that a
better diagnostic value of 7-AABs for discrimination between MLNs
and BLNs was demonstrated in the pGGO group (χ2=3.9,
P=0.048) compared with the mGGO (χ2=1.2, P=0.27) and SN
(χ2=2.1, P=0.15) groups. It was noteworthy that 7-AABs +
HSP90a exhibited an increased sensitivity and NPV compared with
7-AABs alone or in combination with other non-specific tumor
markers, such as CEA, CYFRA21-1, CA199 and CA125, in the three
groups. A positive result for 7-AABs + HSP90a indicated a 3.3-fold,
1.5-fold and 5.5-fold increase in the RR for MLNs in the pGGO, mGGO
and SN groups, respectively (Table
IV). ROC curves of 7-AABs + HSP90a (AUC=0.925, P<0.0001;
AUC=0.836, P<0.0001) also indicated that such a combination
possessed improved diagnostic efficiency for discrimination between
MLNs and BLNs compared with 7-AABs (AUC=0.803, P=0.001; AUC=0.724,
P=0.012), 7-AABs + CEA (AUC=0.803, P=0.001; AUC=0.724, P=0.012),
7-AABs + CYFRA21-1 (AUC=0.803, P=0.001; AUC=0.724, P=0.012), 7-AABs
+ CA199 (AUC=0.803, P=0.001; AUC=0.724, P=0.012) and 7-AABs + CA125
(AUC=0.803, P=0.001; AUC=0.724, P=0.012) in the pGGO and mGGO
groups; however, no significance was detected in the SN group
(AUC=0.5, P=1.0) (Fig. 4C). The
above results demonstrated the improved diagnostic efficiency of
7-AABs + HSP90a for patients with lung nodules of different types,
especially pGGO and mGGO.
 | Table IVDiagnostic values 7-AABs alone or in
combination with non-specific tumor markers for patients with lung
nodules of different types. |
Table IV
Diagnostic values 7-AABs alone or in
combination with non-specific tumor markers for patients with lung
nodules of different types.
| A, pure
ground-glass opacity type lung nodules |
|---|
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
|---|
| Specificity, % | 84.6 | 76.9 | 84.6 | 84.6 | 84.6 | 84.6 |
| Sensitivity, % | 51.1 | 91.1a | 51.1 | 57.8 | 51.1 | 53.3 |
| PPV, % | 92.0 | 93.2 | 92.0 | 92.9 | 92.0 | 92.3 |
| NPV, % | 33.3 | 71.4a | 33.3 | 36.7 | 36.7 | 36.7 |
| RR (95% CI) | 1.4 (1.1-1.8) | 3.3 (1.4-7.5) | 1.4 (1.1-1.8) | 1.5 (1.1-2.0) | 1.4 (1.1-1.8) | 1.4 (1.1-1.9) |
| B, mix ground-glass
opacity type lung nodules |
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
| Specificity, % | 83.3 | 66.7 | 75.0 | 75.0 | 83.3 | 83.3 |
| Sensitivity, % | 37.6 | 84.7a | 41.2 | 42.4 | 38.8 | 43.5 |
| PPV, % | 94.1 | 94.7 | 92.1 | 92.3 | 94.3 | 94.9 |
| NPV, % | 15.9 | 38.1a | 15.5 | 15.5 | 16.1 | 16.1 |
| RR (95% CI) | 1.1 (1.0-1.3) | 1.5 (1.1-2.1) | 1.1 (1.0-1.3) | 1.1 (1.0-1.3) | 1.1 (1.0-1.3) | 1.1 (1.0-1.3) |
| C, solid type lung
nodules |
| Diagnostic
value | 7-AABs | 7-AABs +
HSP90a | 7-AABs + CEA | 7-AABs +
CYFRA21-1 | 7-AABs + CA199 | 7-AABs + CA125 |
| Specificity, % | 72.7 | 63.6 | 63.6 | 66.7 | 69.7 | 72.7 |
| Sensitivity, % | 44.8 | 89.7a | 51.7 | 55.2 | 48.3 | 51.7 |
| PPV, % | 59.1 | 68.4 | 55.6 | 59.3 | 58.3 | 62.5 |
| NPV, % | 60.0 | 87.5b | 60.0 | 62.9 | 60.5 | 63.2 |
| RR (95% CI) | 1.5 (0.9-2.5) | 5.5 (1.9-16.1) | 1.4 (0.8-2.4) | 1.6 (0.9-2.7) | 1.5 (0.9-2.5) | 1.7 (1.0-2.9) |
Discussion
Early-stage NSCLC has a notably better prognosis
compared with advanced-stage NSCLC, and can usually be treated
radically with relatively benign outcomes (16). It has been reported that lung
cancer-related AABs have high specificity and PPV in diagnosing
early-stage lung cancer. However, the relatively low sensitivity
and NPV greatly limit their wider application in clinical practice
(11,12,17).
7-AABs consisted of seven antibodies against p53, MAGEA1, GAGE7,
CAGE, GBU4-5, SOX2 and PGP9.5. p53 was first reported as a tumor
suppressor, and has been reported to be associated with
tumorigenesis, regulation and apoptosis (18,19).
It is an important biological indicator for the evaluation of tumor
biological behavior and the screening of high-risk patients with
lung cancer (20-22).
MAGEA1, GAGE7 and CAGE are members of the cancer-testis antigen
family, which can accelerate tumor formation, resist apoptosis,
promote tumor proliferation and metastasis, and cause cellular and
humoral immune responses (23,24).
Their increased expression in NSCLC has been reported, including
squamous cell carcinoma and adenocarcinoma (6,25).
GBU4-5 is an ATP-dependent DNA helicase, which regulates transposon
methylation and inhibits gene expression in the process of cell
differentiation. The expression of GBU4-5 is increased in lung
cancer (26). SOX2 activates the
expression of oncogenes in lung cancer cells through regulation of
the RAS-MAPK-survivin signaling pathway, which promotes the
occurrence of lung cancer (27,28).
SOX2 also serves a vital role in modulation of the growth and
regulation of lung cancer stem cells, and it has a relatively high
expression level in lung cancer cells compared with those in normal
lung cells (29,30). PGP9.5 can increase the
deubiquitination of functional proteins and regulate the expression
of cell cycle genes (31). The
upregulation of PGP9.5 is associated with lung cancer progression,
and it is highly expressed in both squamous cell carcinoma and
non-squamous cell carcinoma (32).
The combined detection of 7-AABs has a much higher specificity and
accuracy than a single marker, improving the detection rate of lung
cancer (11).
In the present study, 217 patients with different
types of small pulmonary nodules (≤20 mm), such as pGGO, mGGO and
SN, were assessed. The results indicated that p53, PGP9.5, SOX2,
GAGE7 and MAGEA1 protein expression levels were significantly
increased in MLNs compared with BLNs and HCs. The specificity,
sensitivity, PPV and NPV of 7-AABs for discrimination between MLNs
and BLNs were 77.6, 42.8, 84.0 and 33.1%, respectively, which was
similar those values previously reported (11,12).
HSP90a is closely associated with numerous human cancers, and it
has previously been employed for the early screening of certain
tumors, including liver, lung and colorectal cancer, due to its
high sensitivity (33-37).
Chen et al (38) have
reported that HSP90a is frequently over-expressed in human liver
carcinoma cells, and its expression level is negatively correlated
to the prognosis of patients with liver cancer. HSP90a, as a target
of G-Rh2, serves a vital role in liver cancer therapy (38). The inhibition of HSP90a also
enhances anti-tumor immunity (39). In a parallel study comparing
alpha-fetoprotein (AFP), plasma HSP90a demonstrated a significantly
higher diagnostic performance in distinguishing hepatocellular
carcinoma (HCC) from HCs. Moreover, plasma HSP90a exhibited
excellent diagnostic accuracy in discriminating AFP-negative
patients with HCC (sensitivity, 93.9%; specificity, 91.3%) and
AFP-limited liver cancer (sensitivity, 96.6%; specificity, 90.3%)
(40). Furthermore, Zhong et
al (41) reported that HSP90a,
a valuable predictor of early chemotherapy effectiveness in
advanced NSCLC, was closely correlated with tumor remission after
chemotherapy, while HSP90a was not correlated with tumor diameter
and pathological type. Targeting HSP90a suppresses the growth of
lung cancer (42), promotes
apoptosis and inhibits migration (43). A large-sample and multi-center
survey reported a significantly increased level of HSP90a in
early-stage NSCLC. Moreover, when the cutoff of HSP90a was 56.33
ng/ml, the sensitivity was 72%, the specificity was 78% and the
coincidence rate was 75% (44). In
the present study, further evaluation demonstrated that 7-AABs +
HSP90a displayed remarkable advantages compared with 7-AABs alone
or in other combinations. For pulmonary nodules of <8 mm, the
specificity, sensitivity, PPV and NPV of 7-AABs + HSP90a were 61.5,
90.5, 79.2 and 80.0%, respectively, in discrimination between MLNs
and BLNs, which was markedly higher compared with 7-AABs alone or
in other combinations. Furthermore, 7-AABs + HSP90a reflected a
4.0-fold increase in the RR of MLNs, which indicated a better
diagnostic efficiency. Notably, the diagnostic value of 7-AABs +
HSP90a for pulmonary nodules of 8-20 mm was superior to that of
pulmonary nodules of <8 mm. However, the diagnostic value of
7-AABs + HSP90a for pulmonary nodules of >20 mm required further
assessment. For different nodule types, 7-AABs exhibited a good
diagnostic value in patients with pGGO (χ2=3.9, P=0.048)
compared with mGGO (χ2=1.2, P=0.27) and SN
(χ2=2.1, P=0.15). 7-AABs + HSP90a demonstrated a
significantly increased diagnostic efficiency for MLNs in patients
with pGGO, mGGO and SN. In the present study, besides the
significantly increased sensitivity, NPV and RR, it was
demonstrated that the specificity of 7-AABs + HSP90a showed a
slight decrease compared with 7-AABs. However, based on the results
of diagnostic efficiencies assessed using the ROC curve, 7-AABs +
HSP90a exhibited much better diagnostic performance than 7-AABs in
the discrimination of MLNs from BLNs. However, a greater number of
relevant biomarkers require assessment to improve the specificity
in future research. Moreover, the diagnostic values of 7-AABs alone
or in combination with non-specific tumor markers for patients with
stage 0 and I lung cancer was evaluated. However, based on the
χ2 test, the results demonstrated no significant effects
on the discrimination of stage 0 and I lung cancer (Table SI). ROC analysis also demonstrated
no diagnostic value for the use of 7-AABs alone or in combination
with non-specific tumor markers (AUC=0.5, P=1.0) (Fig. S1). Once produced, an antibody can
exist in the peripheral circulating blood for a long time and will
not disappear quickly. Therefore 7-AABs are only used to diagnose
lung cancer instead of evaluating its prognosis. To assess whether
7-AABs (+/- HSP90a) were correlated with age of diagnosis, 159
patients with lung cancer in the present study were divided into
two groups as follows: i) A low-age group (≤50 years old, n=48);
and ii) an advanced-age group (>50 years old, n=111). The
results demonstrated no significant differences in the detection
rate of 7-AABs (+/- HSP90a) in lung cancer between the two groups
(χ2=0.6, P=0.42; χ2=0.8, P=0.38). Moreover,
there were also no significant differences in the detection rate of
7-AABs (+/- HSP90a) in lung cancer between male and female patients
(χ2=0.07, P=0.93; χ2=2.96, P=0.09). As is
well known, histopathological character affects the treatment and
prognosis of patients with lung cancer. Furthermore, the tumor
markers against adenocarcinoma, squamous cell carcinoma and SCLC
are different. In the present study, when two patients with
squamous cell carcinoma (1 case with a solid nodule of 8-20 mm) and
SCLC (1 case with a solid nodule of 8-20 mm) were excluded, 7-AABs
+ HSP90a demonstrated elevated sensitivity (patients with lung
nodule, 88.5 vs. 87.4%; patients with lung nodule of 8-20 mm, 87.8
vs. 86.3%; patients with solid nodule, 96.3 vs. 89.7%).
However, due to the limitation in the number of
patients with SCLC, larger pulmonary nodules (>20 mm) or
advanced progression (such as, stage III-IV lung cancer), the
diagnostic values of 7-AABs, alone or in combination with
non-specific tumor markers, were not evaluated in these patients in
the present study. Therefore, these aspects require further
study.
In conclusion, the findings of the present study
demonstrated that 7-AABs + HSP90a had good clinical value for the
diagnosis of early-stage lung cancer, including patients with
different types of small nodules, which suggested its value for
further application in clinical practice.
Supplementary Material
Diagnostic values of 7-AABs alone or
in combination with non-specific tumor markers for patients with
early-stage malignant lung nodules. (A) The specificity,
sensitivity, PPV and NPV of 7-AABs, 7-AABs + HSP90a, 7-AABs + CEA,
7-AABs + CYFRA21-1, 7-AABs + CA199 and 7-AABs + CA125. (B) The RR
of 7-AABs, 7-AABs + HSP90a, 7-AABs + CEA, 7-AABs + CYFRA21-1,
7-AABs + CA199 and 7-AABs + CA125. (C) Diagnostic efficiency was
assessed using the ROC curve. The results of 7-AABs, 7-AABs +
HSP90a, 7-AABs + CEA, 7-AABs + CYFRA21-1, 7-AABs + CA199 and 7-AABs
+ CA125 were identical to the ROC analysis using the overlapped
data. 7-AABs, seven lung cancer-related autoantibodies; ROC,
receiver operating characteristic; CEA, carcinoembryonic antigen;
CYFRA21-1, cytokeratin fragment antigen 21-1; CA199, carbohydrate
antigen 199; CA125, carbohydrate antigen 125; HSP90a, heat shock
protein 90a; PPV, positive predictive value; NPV, negative
predictive value; RR, relative risk; CI, confidence interval.
Clinical findings and evaluation of
7-AABs alone or in combination with non-specific tumor markers for
patients with early-stage malignant lung nodules.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Universities in Anhui Province (grant no. KJ2021A0835
and KJ2021ZD0102), the National College Students' Innovation and
Entrepreneurship Training Program (grant no. 202210368023), and the
Natural Science Foundation of Anhui Province (grant no.
1908085QH325).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC, SZ, WH and GF conceived and designed the study.
QC, SZ, GG, WZ, ZZ, GF and NJ performed the data analysis and
drafted the manuscript. QC, SZ and WH performed data collection.
GG, WZ, GF, NJ and WH analyzed the results. WH and GF edited the
manuscript and confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of The First Affiliated Hospital of Wannan Medical
College (approval no. 202076; Wuhu, China), and the experimental
procedures were in agreement with The Declaration of Helsinki.
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xie E, Lin M, Sun Z, Jin Y, Zhang S, Huang
L, Sun R, Wang F and Pan S: Serum miR-27a is a biomarker for the
prognosis of non-small cell lung cancer patients receiving
chemotherapy. Transl Cancer Res. 10:3458–3469. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 Study. J Clin Oncol. 37:2518–2527.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Albano D, Bilfinger T and Nemesure B: 1-,
3-, and 5-year survival among early-stage lung cancer patients
treated with lobectomy vs SBRT. Lung Cancer (Auckl). 9:65–71.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lutfi W, Schuchert MJ, Dhupar R, Sarkaria
I, Christie NA, Yang CJ, Deng JZ, Luketich JD and Okusanya OT:
Sublobar resection is associated with decreased survival for
patients with early stage large-cell neuroendocrine carcinoma of
the lung. Interact Cardiovasc Thorac Surg. 29:517–524.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luo B, Mao G, Ma H and Chen S: The role of
seven autoantibodies in lung cancer diagnosis. J Thorac Dis.
13:3660–3668. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang X, Liu M, Zhang X, Wang Y and Dai L:
Autoantibodies to tumor-associated antigens in lung cancer
diagnosis. Adv Clin Chem. 103:1–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trudgen K, Khattar NH, Bensadoun E, Arnold
S, Stromberg AJ and Hirschowitz EA: Autoantibody profiling for lung
cancer screening longitudinal retrospective analysis of CT
screening cohorts. PLoS One. 9(e87947)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sullivan FM, Farmer E, Mair FS, Treweek S,
Kendrick D, Jackson C, Robertson C, Briggs A, McCowan C, Bedford L,
et al: Detection in blood of autoantibodies to tumour antigens as a
case-finding method in lung cancer using the
EarlyCDT®-Lung Test (ECLS): Study protocol for a
randomized controlled trial. BMC Cancer. 17(187)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen SS, Li K, Wu J, Peng ZY, Wang ZD,
Wang JC, Xu CW, Zhu CL, Li BC, Ren H, et al: Stem signatures
associated antibodies yield early diagnosis and precise prognosis
predication of patients with non-small cell lung cancer. J Cancer
Res Clin Oncol. 147:223–233. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Du Q, Yu R, Wang H, Yan D, Yuan Q, Ma Y,
Slamon D, Hou D, Wang H and Wang Q: Significance of
tumor-associated autoantibodies in the early diagnosis of lung
cancer. Clin Respir J. 12:2020–2028. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ren S, Zhang S, Jiang T, He Y, Ma Z, Cai
H, Xu X, Li Y, Cai W, Zhou J, et al: Early detection of lung cancer
by using an autoantibody panel in Chinese population.
Oncoimmunology. 7(e1384108)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saini J and Sharma PK: Clinical,
prognostic and therapeutic significance of heat shock proteins in
cancer. Curr Drug Targets. 19:1478–1490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang
Y, Tong M, Chang G and Luo Y: The regulatory mechanism of
Hsp90alpha secretion and its function in tumor malignancy. Proc
Natl Acad Sci U S A. 106:21288–21293. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
WHO Classification of Tumours Editorial
Board. WHO Classification of Tumours. Thoracic Tumours. 5th
edition. IARC Press, Lyon, 2021.
|
|
16
|
Balata H, Fong KM, Hendriks LE, Lam ṀS,
Ostroff JS, Peled N, Wu N and Aggarwal C: Prevention and early
detection for NSCLC: Advances in thoracic oncology 2018. J Thorac
Oncol. 14:1513–1527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang R, Ma L, Li W, Zhou S and Xu S:
Diagnostic value of multiple tumor-associated autoantibodies in
lung cancer. Onco Targets Ther. 12:457–469. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stein Y, Rotter V and Aloni-Grinstein R:
Gain-of-function mutant p53: All the roads lead to tumorigenesis.
Int J Mol Sci. 20(6197)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lacroix M, Riscal R, Arena G, Linares LK
and Le Cam L: Metabolic functions of the tumor suppressor p53:
Implications in normal physiology, metabolic disorders, and cancer.
Mol Metab. 33:2–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nanami T, Hoshino I, Shiratori F, Yajima
S, Oshima Y, Suzuki T, Ito M, Hiwasa T, Kuwajima A and Shimada H:
Presence of serum RalA and serum p53 autoantibodies in 1833
patients with various types of cancers. Int J Clin Oncol. 27:72–76.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang T, Li Y, Zhu R, Song P, Wei Y, Liang
T and Xu G: Transcription factor p53 suppresses tumor growth by
prompting pyroptosis in non-small-cell lung cancer. Oxid Med Cell
Longev. 2019(8746895)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mu Y, Xie F and Sun T: Clinical value of
seven autoantibodies combined detection in the diagnosis of lung
cancer. J Clin Lab Anal. 34(e23349)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meng X, Sun X, Liu Z and He Y: A novel era
of cancer/testis antigen in cancer immunotherapy. Int
Immunopharmacol. 98(107889)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li S, Ma Y, Xiong Y, Zhang P, Wang X, Wang
Y and Yang Y: Five tumor-associated autoantibodies expression
levels in serum predict lung cancer and associate with poor
outcome. Transl Cancer Res. 8:1364–1373. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang H, Luo W, Ni Y, Sun S, Wang C and
Zhang L: The diagnostic efficiency of seven autoantibodies in lung
cancer. Eur J Cancer Prev. 29:315–320. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Jiao Y, Ding CM and Sun WZ: The
role of autoantibody detection in the diagnosis and staging of lung
cancer. Ann Transl Med. 9(1673)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Novak D, Hüser L, Elton JJ, Umansky V,
Altevogt P and Utikal J: SOX2 in development and cancer biology.
Semin Cancer Biol. 67:74–82. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chaudhary S, Islam Z, Mishra V, Rawat S,
Ashraf GM and Kolatkar PR: Sox2: A regulatory factor in
tumorigenesis and metastasis. Curr Protein Pept Sci. 20:495–504.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mollaoglu G, Jones A, Wait SJ,
Mukhopadhyay A, Jeong S, Arya R, Camolotto SA, Mosbruger TL,
Stubben CJ, Conley CJ, et al: The lineage-defining transcription
factors SOX2 and NKX2-1 determine lung cancer cell fate and shape
the tumor immune microenvironment. Immunity. 49:764–779.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schaal CM, Bora-Singhal N, Kumar DM and
Chellappan S: Regulation of Sox2 and stemness by nicotine and
electronic-cigarettes in non-small cell lung cancer. Mol Cancer.
17(149)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fang Y and Shen X: Ubiquitin
carboxyl-terminal hydrolases: Involvement in cancer progression and
clinical implications. Cancer Metastasis Rev. 36:669–682.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen P, Lu W and Chen T: Seven
tumor-associated autoantibodies as a serum biomarker for primary
screening of early-stage non-small cell lung cancer. J Clin Lab
Anal. 35(e24020)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han Y, Zhang Y, Cui L, Li Z, Feng H, Zhang
Y, Sun D and Ren L: Plasma heat shock protein 90alpha as a
biomarker for the diagnosis of liver cancer: In patients with
different clinicopathologic characteristics. World J Surg Oncol.
19(228)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Barghi SM, Yang Y and
Akhavan-Sigari R: Value of HSP90α in lung cancer diagnosis and
recurrence prediction: A cohort study. Oncol Res Treat. 44:583–589.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kasanga M, Liu L, Xue L and Song X: Plasma
heat shock protein 90-alpha have an advantage in diagnosis of
colorectal cancer at early stage. Biomark Med. 12:881–890.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ichikawa T, Shanab O, Nakahata S,
Shimosaki S, Manachai N, Ono M, Iha H, Shimoda K and Morishita K:
Novel PRMT5-mediated arginine methylations of HSP90A are essential
for maintenance of HSP90A function in NDRG2low ATL and various
cancer cells. Biochim Biophys Acta Mol Cell Res.
1867(118615)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao Q, Miao C, Lu Q, Wu W, He Y, Wu S,
Liu H and Lian C: Clinical significance of monitoring circulating
free DNA and plasma heat shock protein 90alpha in patients with
esophageal squamous cell carcinoma. Cancer Manag Res. 13:2223–2234.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen C, Wang YS, Zhang ET, Li GA, Liu WY,
Li Y and Jin YH: (20S) Ginsenoside Rh2 exerts its anti-tumor effect
by disrupting the HSP90A-Cdc37 system in human liver cancer cells.
Int J Mol Sci. 22(13170)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song KH, Oh SJ, Kim S, Cho H, Lee HJ, Song
JS, Chung JY, Cho E, Lee J, Jeon S, et al: HSP90A inhibition
promotes anti-tumor immunity by reversing multi-modal resistance
and stem-like property of immune-refractory tumors. Nat Commun.
11(562)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fu Y, Xu X, Huang D, Cui D, Liu L, Liu J,
He Z, Liu J, Zheng S and Luo Y: Plasma heat shock protein 90alpha
as a biomarker for the diagnosis of liver cancer: An official,
large-scale, and multicenter clinical trial. EBioMedicine.
24:56–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhong B, Shen J, Zhang C, Zhou G, Yu Y,
Qin E, Tang J, Wu D and Liang X: Plasma heat shock protein 90
alpha: A valuable predictor of early chemotherapy effectiveness in
advanced non-small-cell lung cancer. Med Sci Monit.
27(e924778)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ojha R, Nepali K, Chen CH, Chuang KH, Wu
TY, Lin TE, Hsu KC, Chao MW, Lai MJ, Lin MH, et al: Isoindoline
scaffold-based dual inhibitors of HDAC6 and HSP90 suppressing the
growth of lung cancer in vitro and in vivo. Eur J Med Chem.
190(112086)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pan J, Jiang F, Zhou J, Wu D, Sheng Z and
Li M: HSP90: A novel target gene of miRNA-628-3p in A549 cells.
Biomed Res Int. 2018(4149707)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi Y, Liu X, Lou J, Han X, Zhang L, Wang
Q, Li B, Dong M and Zhang Y: Plasma levels of heat shock protein 90
alpha associated with lung cancer development and treatment
responses. Clin Cancer Res. 20:6016–6022. 2014.PubMed/NCBI View Article : Google Scholar
|