Introduction
Chronic kidney disease (CKD) is a rapidly increasing
global health burden and an important risk factor for
cerebrovascular diseases (1).
Idiopathic cerebral haemorrhage (ICH) is the second most common
subtype of stroke, and usually results in severe disability or
mortality, particularly in elderly Asian males (2). CKD is an independent risk factor for
ICH, with ICH being one of the factors leading to a higher
mortality rate of stroke among patients with CKD (3). Spontaneous brainstem haemorrhage
(SBH) is the most lethal type of ICH, with a poor prognosis. SBH is
one of the common neurosurgery diseases, which may usually manifest
with acute and critical onset, poor prognosis and high mortality
rate. Its incidence rate is ~10% of cerebral hemorrhage, but the
fatality rate is ~65%, depending on the site and amount of the
cerebral haemorrhage (4). Though
there are differences in its morbidity, the neurological prognosis
of the majority of patients is poor. Since surgery is not the
recommended treatment strategy for SBH at present, conservative
treatment is usually adopted. With the continuous understanding of
the disease, as well as the development of imaging technology and
the improvement of the diagnosis and treatment, the survival rate
of patients with SBH has been continuously improved, and a few
patients can be cured after treatment (4). However, the specific treatment method
remains controversial worldwide (4).
The present study reports the case of an elderly
male patient with uraemia who suffered from ICH at night in
hospital, during the induction of haemodialysis (HD). On the basis
of neurosurgery guidance, an active treatment approach was adopted
for controlling the blood and intracranial pressures of the
patient, and to continue regular HD. The condition of the patient
improved, and the limbs showed no impairment of sensation, with
normal movement lastly.
Case report
A 67-year-old Han Chinese male patient was admitted
to Nephrology Department of Baoding First Central Hospital
(Baoding, China) on 25th May 2022. The patient had been diagnosed
with intermittent bilateral lower limb oedema 9 years before, and
was experiencing chest tightness for the past 1 month. He was
diagnosed with coronary atherosclerotic heart disease 4 years
before, and with hypertension 2 years ago, with a maximum blood
pressure (BP) of 190/100 mmHg; however, his BP was not monitored
regularly. When he was diagnosed with bilateral lower extremity
oedema 9 years before in our hospital, he had a urine protein level
of 3+ (normal range, negative), 24-h urine protein level of 9.1 g
(normal range, <150 mg), serum creatinine level of 89 µmol/l
(normal range, 46.2-78.3), cholesterol level of 13.3 mmol/l (normal
range, 3.2-5.2), triglyceride level of 2.40 mmol/l (normal range,
0.25-1.71), albumin level of 23.37 g/l (normal range, 40-55) and
total protein level of 49.9 g/l (normal range, 60-85). The patient
was diagnosed with nephrotic syndrome but refused to undergo renal
biopsy. Methylprednisolone (40 mg once daily) and compounded
cyclophosphamide (50 mg twice daily) were recommended. His
condition improved following the above treatment and he was
subsequently discharged. However, he was not followed up regularly
as an outpatient. He was diagnosed with elevated creatinine levels
2 years ago and received oral herbal medications for around 1 year,
but the specific name, dosage and frequency were unknown. Without
regular re-evaluation of renal function. The patient developed
chest tightness with weakness and shortness of breath 10 days prior
to presentation to the hospital, which worsened after activity, and
was admitted to our hospital for further treatment.
Physical examination at admission revealed the
following findings: BP of 165/108 mmHg, anaemic appearance, heart
rate of 105 beats/min, and facial and bilateral lower limb oedema.
Ultrasonography showed that both kidneys were shrunken with diffuse
lesions. The results of major laboratory tests conducted at the
hospital are shown in Table I. In
view of the condition that the patient had successively suffered
from multiple chronic diseases, his clinical diagnoses at admission
included the following: i) Chronic renal failure, uraemia and renal
anaemia; ii) coronary heart disease; iii) heart failure; and iv)
hypertensive disease (grade 3, high risk of coronary heart
disease). On 11th May 2022, the patient underwent HD via right
internal jugular vein catheterisation, and received treatment as
follows: Induced hemodialysis, lowering BP, improving heart
function and correcting anaemia and electrolyte disorders. His
condition gradually improved.
 | Table IResults of laboratory examination
change in hospital. |
Table I
Results of laboratory examination
change in hospital.
| Investigation | Normal range | Day 1 | Day 3 | Day 7 | Day 14 |
|---|
| Hb (g/l) | 110.0-150.0 | 63.5 | 68.3 | 78.4 | 85.9 |
| Plt
(x109/l) | 125.0-350.0 | 342.9 | 295.4 | 312.4 | 288.2 |
| Alb (g/l) | 40.0-55.0 | 35.1 | 32.7 | 35.4 | 38.1 |
| Urea (mmol/l) | 2.6-7.5 | 38.1 | 18.3 | 24.9 | 15.7 |
| CREA (µmol/l) | 41.0-73.0 | 1,076.7 | 665.7 | 485.2 | 514.3 |
| Serum sodium
(mmol/l) | 137.0-145.0 | 134.7 | 139.6 | 142.3 | 139.6 |
| Serum potassium
(mmol/l) | 3.5-5.3 | 5.9 | 4.8 | 5.1 | 4.7 |
| Serum calcium
(mmol/l) | 2.1-2.5 | 1.8 | 1.9 | 2.0 | 2.1 |
| Serum phosphorus
(mmol/l) | 0.8-1.5 | 2.1 | 1.7 | 1.4 | 1.3 |
| BNP (pg/ml) | 0.0-100.0 | 4,185.2 | 2,645.9 | 1,856.7 | 1,223.6 |
| dimer (mg/l) | 0.0-0.5 | 2.1 | 1.6 | 1.2 | 0.9 |
| CRP (mg/l) | 0.0-10.0 | 10.3 | 9.3 | 8.5 | 7.2 |
| Fbg (g/l) | 1.8-3.5 | 4.6 | 4.2 | 3.9 | 3.4 |
| LVEF (%) | 45.0-55.0 | 38.0 | 42.0 | 45.0 | 51.0 |
On 14th May 2022, the patient had a sudden onset of
weakness and numbness in the right limb during sleep at night,
which was accompanied by blurred and double vision, without
consciousness or limb movement disorder, and his BP was 186/105
mmHg. Physical examination showed normal muscle strength of the
upper right limb and grade IV muscle strength of the lower right
limb. Bilateral pathological signs were absent. Urgent cranial
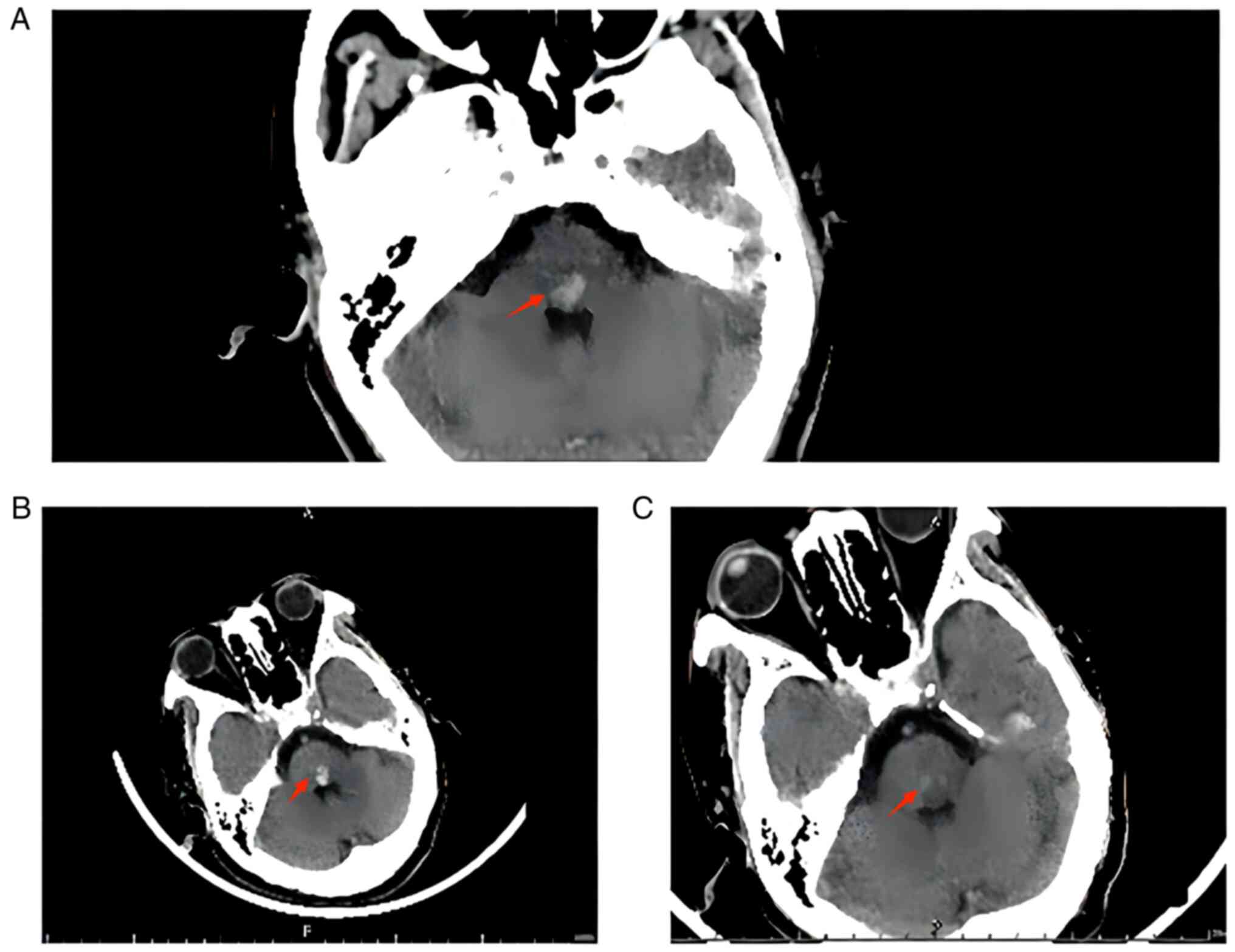
computed tomography (CT) (Fig. 1A)
showed high-density brainstem shadow, and brainstem haemorrhage was
considered. The neurosurgery department was therefore consulted,
and the patient was suspected to have a brainstem haemorrhage of
<5 ml. Since the patient was conscious and did not have any limb
movement disorder, surgery was contraindicated; instead, bed rest
and symptomatic antihypertensive treatment were recommended. The
patient was treated with absolute bed rest, active BP monitoring to
decrease BP, intravenous infusion of glycerol fructose and
tranexamic acid, regular HD three times a week, and anticoagulation
with citrate during HD.
On 17th May, re-evaluation via cranial CT (Fig. 1B) showed that the area of brainstem
high density was slightly larger than that observed in the previous
scan. In addition, the patient experienced more discomfort, which
was accompanied by intermittent drowsiness. However, he did not
have any limb movement disorder, and he received intravenous
infusion of human serum albumin and diuretic therapy. On 21st May,
his condition improved, since the weakness and numbness on the
right side were significantly reduced, and he did not have double
vision. However, the patient refused to undergo cranial CT owing to
personal reasons. Glycerol fructose infusion was discontinued, but
the rest of the treatment continued to be the same. On 27th May,
cranial CT (Fig. 1C) showed high
density in the brainstem, which was significantly lower than that
previously observed. Although the patient had numbness in the right
limb, he did not have other symptoms of discomfort, and could walk
normally.
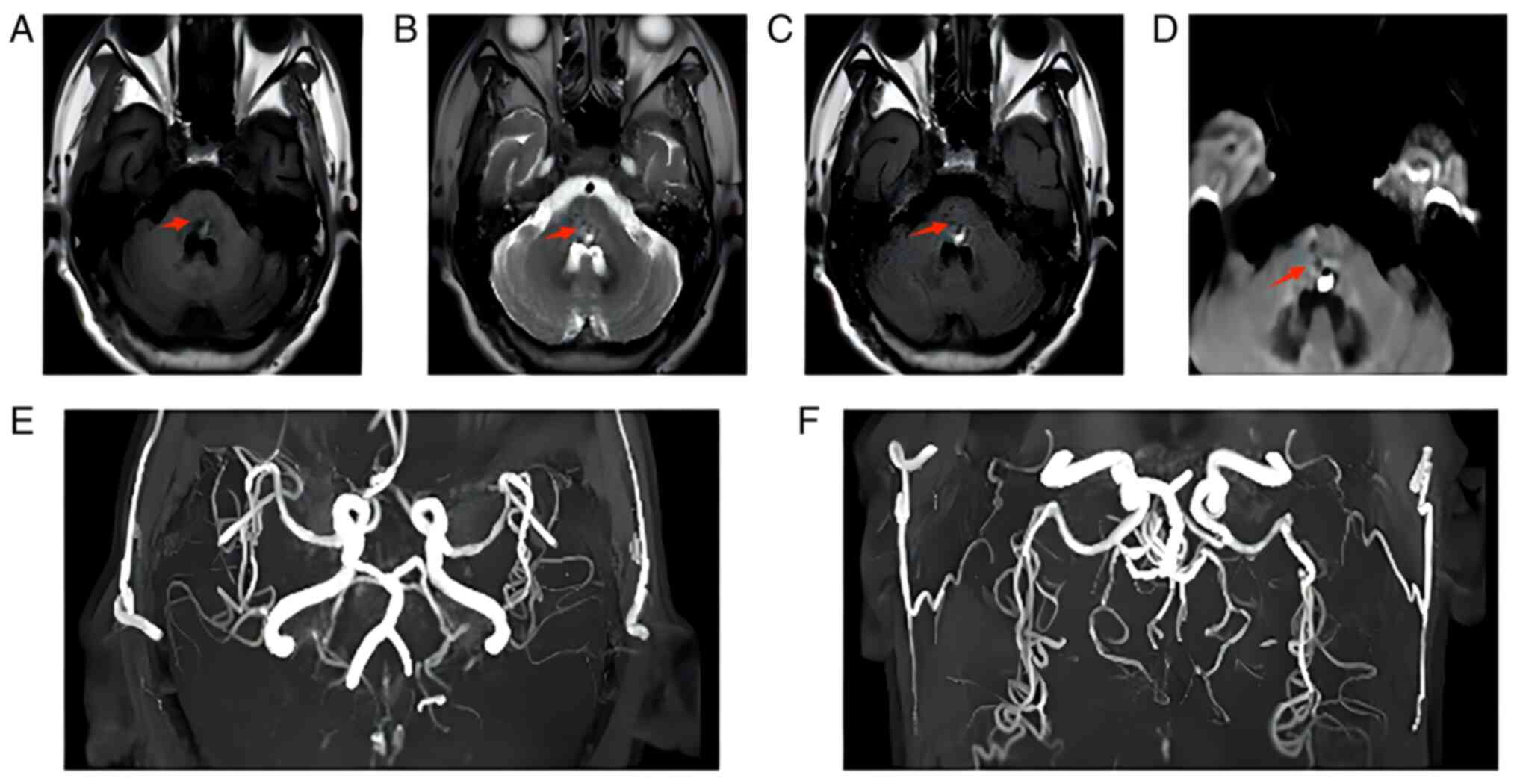
On 31st May, cranial magnetic resonance imaging and
magnetic resonance angiography (Fig.
2A-F) showed dominant mixed-signal shadow in the cerebral
bridge with a low signal. The left middle cerebral artery was
slightly narrowed, and the posterior cerebral artery was stiffened
bilaterally, which was considered cerebral arteriosclerosis. The
patient still had numbness in the right limb and was then treated
with rehabilitation acupuncture, which was the stimulation of
specific acupuncture points along the skin of the body using thin
needles. It gradually improved his numbness in the right limb, and
subsequently he received regular HD.
Discussion
CKD and stroke are closely related, and the
prevalence and mortality of stroke are higher among patients with
CKD, particularly in those with uraemia (5). The specific pathogenic mechanism of
stroke can be attributed to both traditional and non-traditional
factors. The former include hypertension, diabetes mellitus,
carotid artery disease, heart failure and dyslipidaemia, while the
latter include proteinuria, uraemic toxins, anaemia and mineral
bone disease (1). In patients with
uraemia, the levels of toxins such as urea, creatinine and
guanidine normally increase, which may affect the adhesion and
production of platelets. In addition, the number of anticoagulant
substances decreases, and the anticoagulant effect is weakened,
which may result to bleeding. Furthermore, HD causes pathological
alterations in haemodynamics in patients with stroke undergoing HD,
resulting in cerebral underperfusion, enhanced atherosclerosis and
greater BP fluctuations, which further enhance the risk of stroke,
particularly during the early stages of HD (6).
SBH has the worst prognosis of all cerebral
haemorrhages and lacks uniform diagnostic criteria (7). The clinical diagnosis is mainly based
on a history of hypertension, clinical manifestations and imaging,
and the exclusion of structural cerebrovascular lesions and
haemorrhagic brain tumor, which may be diagnosed as hypertensive
brainstem haemorrhage. Since hypertensive brainstem haemorrhage
mainly occurs in the cerebral bridge, it is also called primary
cerebral bridge haemorrhage (7).
The present elderly male patient, who had a 9-year
history of nephrotic syndrome, a 4-year history of coronary artery
disease and a 2-year history of hypertension, was admitted to the
hospital with uraemia combined with heart failure, and was treated
with conventional HD. During the initial phase of HD, the patient
suddenly experienced SBH at night during sleep, with a significant
increase in BP. SBH may have occurred due to traditional factors
such as hypertension, heart failure and hyperlipidaemia, or to
non-traditional factors such as proteinuria, uraemic toxins or
anaemia. In addition, it may be associated with the pathological
alterations in haemodynamics caused by HD. Sudden onset of SBH
during night-time sleep is considered to be associated with a
significant increase in nocturnal BP. Clinical guidelines recommend
24-h ambulatory BP monitoring in patients with CKD to detect
nocturnal hypertension and to help clinicians to treat it with
antihypertensive therapy (8,9).
The incidence of SBH is low, accounting for 5-10% of
all ICH cases. SBH is characterised by acute onset, rapid
deterioration and a high mortality rate (56-61.2%); hence, it is
the worst type of haemorrhagic stroke. The mortality rate of SBH
varies greatly due to differences in the bleeding site and
quantity; however, the prognosis of the majority of patients
remains markedly poor (4,10). The treatment of SBH is usually
conservative or surgical (craniotomy, puncture or drainage), which
remains controversial (11,12).
The majority of patients with SBH who are hospitalised choose
conservative medical treatment due to unstable vital signs, high
surgical and complications, high costs, and lack of large-sample,
high-level, evidence-based assessment of surgical efficacy
(13). However, irrespectively of
the treatment approach, the neurological recovery of patients is
usually poor.
In the present case study, the patient was conscious
during the onset of SBH, and CT revealed <5 ml cranial bleeding.
Since the patient had uraemia, hypertension and coronary disease,
surgery was relatively contraindicated, and conservative treatment
was considered more suitable instead. Tranexamic acid may be safely
and effectively administrated for acute spontaneous intracerebral
hemorrhage (14). On the other
hand, hyponatremia (Na <135 mmol/l) may be easily overlooked in
certain occasions, and it has been shown to correlate with worse
outcome in various studies on patients with SBH (15). Thus, it is necessary to rectify
hyponatremia, but not too rapidly, as otherwise it may cause
demyelination of the pons and aggravate nervous system damage.
Therefore, serum sodium levels should be monitored, and the
treatment should be adjusted accordingly.
Moreover, intravenous dehydrating agents are often
used to correct plasma osmotic level and reduce intracranial
pressure during the acute period in patients with intracerebral
hemorrhage. Those dehydrating agents generally include albumin,
dexamethasone, mannitol and glycerin fructose. Mannitol and
glycerol fructose are the most commonly used in clinical practice.
Intravenous infusion of mannitol has a rapid effect and a
relatively short drug action time, but it is not suitable for
patients with renal insufficiency. By contrast, glycerin fructose
has a slower effect, but its drug action time is longer, which is
particularly suitable for patients with renal insufficiency.
Intravenous albumin or dexamethasone can also be applied to
increase clinical efficacy apart from the ones mentioned above. In
brief, the specific type and dosage of drugs should be selected
according to the patient's condition, including the quantity and
location of intracranial hemorrhage, and the patient's state of
consciousness (13).
The present patient clinically manifested with
hypoalbuminemia, hyperkalemia, hypocalcemia and hyperphosphatemia
(Table I) before dialysis, which
is the common presentation in patients with uraemia. The
aforementioned laboratory abnormalities were significantly improved
after inducing hemodialysis. However, the patient suffered from SBH
at sleep. Although the patient showed early-stage exacerbation, his
condition gradually improved after symptomatic treatment, and
numbness in the limbs at a later stage was relieved via
acupuncture. Finally, the patient recovered completely without
sequelae of limb movement or sensory impairment. Therefore,
conservative medical treatment appears to be more appropriate for
patients with SBH without impaired consciousness at the onset and
with less bleeding (4,16).
In conclusion, to the best of our knowledge, this is
the first case that reported sudden onset of SBH in a patient with
uraemia during the induction of HD. Although this patient still
need to be improved on his treatment in acute stage of SBH, the
present study may have clinical guidance for other patients similar
to him. Conservative treatment is suitable for mild cases of SBH,
but not severe ones. Since the onset of the SBH is sudden, and the
therapeutic effects and prognosis are usually poor, prevention is
especially important. It is of great clinical importance to
actively screen the risk factors of SBH in patients with CKD, and
to promptly control hypertension, hyperglycaemia and
hyperlipidaemia. Hence, systematic evaluation of the aforementioned
factors can help clinicians to select an appropriate treatment
strategy and predict prognosis (17). SBH may cause serious and extensive
damage of neurological function, so it is unlikely to be
fundamentally improved through one prescription, which requires
persistent exploration and the integration of multidisciplinary
technologies (16). In the future,
more delicate internal- medicine and surgical techniques will be
applied to reduce the damage of those patients and enhance
recovery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the study. YG acquired the
data. RJ, XL and YG analyzed and interpreted the data and drafted
the manuscript. XL and RJ confirm the authenticity of all the raw
data. All authors critically revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The treatment of this patient was conducted in
accordance with established clinical practice standards (1,7)
following his fully informed written consent. The patient's privacy
was respected in this case report by eliminating any personal
identifiers, in compliance with the Declaration of Helsinki.
Patient consent for publication
Written consent was obtained for the publication of
the patient's data and images in the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelly DM, Ademi Z, Doehner W, Lip GYH,
Mark P, Toyoda K, Wong CX, Sarnak M, Cheung M, Herzog CA, et al:
Chronic kidney disease and cerebrovascular disease: Consensus and
guidance from a KDIGO controversies conference. Stroke.
52:e328–e346. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wyld M and Webster AC: Chronic kidney
disease is a risk factor for stroke. J Stroke Cerebrovasc Dis.
30(105730)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen D, Tang Y, Nie H, Zhang P, Wang W,
Dong Q, Wu G, Xue M, Tang Y, Liu W, et al: Primary brainstem
hemorrhage: A review of prognostic factors and surgical management.
Front Neurol. 12(727962)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hanna RM, Ferrey A, Rhee CM and
Kalantar-Zadeh K: Renal-cerebral pathophysiology: The interplay
between chronic kidney disease and cerebrovascular disease. J
Stroke Cerebrovasc Dis. 30(105461)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aono T, Shinya Y, Miyawaki S, Sugiyama T,
Kumagai I, Takenobu A, Shin M, Saito N and Teraoka A: Changes in
the risk of stroke in dialysis patients: A retrospective analysis
over the last 40 years. Toxins (Basel). 13(350)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cerebrovascular disease group of
Neurosurgery branch in Chinese Medical Association, cerebrovascular
surgery group of neurosurgeon branch in Chinese Medical Doctor
Association. Consensus of diagnosis and treatment of primary
brainstem hemorrhage in Chinese neurosurgery. National Med J China.
102:1068–1075. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheung AK, Chang TI, Cushman WC, Furth SL,
Hou FF, Ix JH, Knoll GA, Muntner P, Pecoits-Filho R, Sarnak MJ, et
al: Executive summary of the KDIGO 2021 clinical practice guideline
for the management of blood pressure in chronic kidney disease.
Kidney Int. 99:559–569. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mayeda L and Rivara MB: Nighttime
hypertension in chronic kidney disease-are we in the dark without
ambulatory blood pressure monitoring? JAMA Netw Open.
5(e2214469)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo X, Ma L, Li H, Qi X, Wei Y, Duan Z, Xu
J, Wang C, You C and Tian M: Brainstem iron overload and injury in
a rat model of brainstem hemorrhage. J Stroke Cerebrovasc Dis.
29(104956)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang S, Chen T, Han B and Zhu W: A
retrospective study of puncture and drainage for primary brainstem
hemorrhage with the assistance of a surgical robot. Neurologist.
16:2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
12
|
Li Y, Wu DX, Liu JF, Li H, Wang JW, Li YX,
Guo H, Liu W, Ji L, Chen LY, et al: Analysis of the curative effect
and influencing factors of stereotactic aspiration in the treatment
of primary brainstem haemorrhage. J Clin Neurosci. 89:122–127.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chao Y and Tao CY: History, present and
future of diagnosis and treatment of primary brainstem hemorrhage.
Chin J Contemp Neurol Neurosurg. 21:71–75. 2021.
|
|
14
|
Pszczolkowski S, Sprigg N, Woodhouse LJ,
Gallagher R, Swienton D, Law ZK, Casado AM, Roberts I, Werring DJ,
Al-Shahi Salman R, et al: Effect of tranexamic acid administration
on remote cerebral ischemic lesions in acute spontaneous
intracerebral hemorrhage: A substudy of a randomized clinical
trial. JAMA Neurol. 79:468–477. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hannon MJ and Thompson CJ: Neurosurgical
hyponatremia. J Clin Med. 3:1084–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ge HF, Zhang CY, Y Z, Li J, Chen YH, Yu J,
et al: Analysis of independent risk factors in deaths of patients
with primary brainstem hemorrhage. Chin Neurosurg J. 35:588–891.
2019.
|
|
17
|
Zhang SL, Huang JS, Luo WW, Wang XL, Wu D,
Wei LF, et al: Clinical characteristics and prognostic analysis of
hypertensive brain stem hemorrhage. Chin J Neuroimmunol Neurol.
27:317–321. 2020.
|