Introduction
Idiopathic sudden sensorineural hearing loss
(ISSNHL) is defined as sensorineural hearing loss of ≥30 dB,
including at least three consecutive audiometric frequencies,
started within 72 h, and is generally unilateral without any known
aetiology (1). It affects 5-20
patients in 100,000 population (2). SSNHL is an otological emergency with
unknown etiopathology. Infection, vascular pathology, blood
disorders, ototoxic drugs and immune and metabolic disorders are
common causes (3).
α-1-acid glycoprotein (AGP) is an acute-phase
reactant that acts against inflammation in the body. AGP levels are
also higher in acute inflammation, such as viral infections, which
is hypothesized to be the cause of ISSNHL (4). AGP increases in response to systemic
injuries, inflammation, infection or tissue damage (5). AGP has immunomodulatory activity and
regulates neutrophil migration and superoxide generation (6).
Galectin-3 is considered to be a multifunctional
oncogenic protein responsible for cell adhesion, angiogenesis,
proliferation and apoptosis (7).
It is also responsible for ischemia and vascular damage (8).
As the etiopathology of ISSNHL is unclear, its
treatment is controversial. Systemic or intratympanic steroids,
vasodilators or hyperbaric oxygen treatment can be used. Systemic
corticosteroids are generally the first choice, although they have
adverse effects (9). such as acne,
weight gain, fatty liver, atherosclerosis, peptic ulcer,
hypertension, diabetes mellitus, Cushingoid appearance, adrenal
insufficiency, osteonecrosis, myopathy, impaired wound hearing,
increased susceptibility to infections, atherosclerosis,
osteoporosis, glaucoma, cataracts, pancreatitis, emotional
lability, psychosis, pseudotumor cerebri can be seen (10).
The present study evaluated serum AGP and galectin-3
levels in patients with ISSNHL and aimed to investigate the
correlation between these markers and severity of disease and
response to treatment.
Materials and methods
Patient selection
Patients (29 female, 26 male; aged between 21 to 78)
diagnosed with ISSNHL between January 2018 and January 2020 in Ufuk
University Ridvan Ege Hospital, Ankara, Turkey were included in the
present study. ISSNHL was defined as a unilateral hearing loss ≥30
dB for at least three consecutive frequencies occurring within
three days. Patients were hospitalized and temporal bone magnetic
resonance imaging was performed (data not shown). Disorders such as
vestibular schwannoma, cerebrovascular disease and intracerebral
malignancies were criteria for exclusion from the study. Patients
with head trauma, a history of surgery, use of ototoxic drugs and
chronic disease such as diabetes, hypertension, autoimmune or liver
diseases, renal failure or cancer were also excluded. Zinc is
essential in systemic inflammation because it is an
anti-inflammatory and antioxidant element (11). Serum zinc levels were also measured
and patients with low zinc levels (<10 µmol/l) were
excluded.
The control group (25 female, 22 male; aged between
26-65 ages) consisted of healthy volunteers without trauma,
surgery, other chronic disease or drug users who had no otological
complaints or ear pathologies between 18 and 65 years of age
between January 2018 and December 2020 in Ufuk University, Ridvan
Ege Hospital.
The local ethics committee of Ufuk University,
Ankara, Turkey, approved the present study (approval no.
30042015-6). Written Informed consent was obtained from all
patients and healthy volunteers.
Audiological examination
All participants underwent detailed otological and
audiological examination. Audiological examinations were in an
isolated, quiet chamber performed using the same clinical
audiometer (Interacoustics AC-33 Clinical Audiometer; Assens).
Patient audiograms were divided into three groups:
i) Downward sloping (falling curves) including sensorineural
hearing loss at high frequencies; ii) ‘U-shaped’ or ‘cookie bite’,
including sensorineural hearing loss in mid-frequencies and iii)
all the frequencies >90 dB, defined as profound loss. Pure tone
average (PTA) was calculated using the mean value of the
frequencies of 250, 500, 1,000, 2,000, 3,000, 4,000, 6,000 and
8,000 Hz. Speech discrimination score (SDS) was measured by
correctly identifying monosyllabic words from 50 selected words.
PTA and SDS were calculated before and after treatment in the study
group.
Hearing thresholds were recorded at the onset of
disease and at 30 and 180 days of treatment. Improvement was
defined as >10 dB improvement in PTA or 15% improvement in SDS.
No recovery was described as <10 dB improvement in PTA (1). PTA improvement was calculated by
subtracting the post-treatment PTA score from the pre-treatment PTA
score of the same patient.
Treatment strategy
All patients were treated with prednisone
(Precort-Liyo, Koçak Farma IlaC ve Kimya Sanayi A.S.) at a dose of
1 mg/kg/day (max dose, 60 mg) and the doses were gradually
decreased (20 mg/3 days for 2 weeks.
Laboratory measurements
Blood samples (10 ml) were collected on the first
day of hospitalization. Complete blood count, including white blood
cell count, haemoglobin level, platelet count was measured with
autoanalyzer (Abbott Cell-Dyn Ruby; Serial no. 54507BG; Abbott
Diagnostics, Illinois, USA). Electrolyte and other biochemical
parameters were analyzed with autoanalyzer (Abbott Architect c8000,
Abbott Diagnostics). The Architect cSystems ICT (Integrated Chip
Technology) is used for the quantitation of sodium, potassium and
chloride in human serum. Erythrocyte sedimentation rate was
analyzed with Cystat erythrocyte sedimentation rate analyzer (ESR
20-4-100). C-reactive protein level was measured with
immunoturbidimetric assay (Abbott Architect c8000, Abbott
Diagnostics).
Serum samples were obtained after blood samples were
centrifuged at 2,500 g for 12 min in room temperature and stored at
-80˚C. Serum galectin-3 levels were analysed with an autoanalyzer
(Architect, G6-6005/R05, B5P03T, Abbott Pharmaceutical Co. Ltd.)
using chemiluminescent microparticle immunoassay principles. The
assay is based on two specific monoclonal antibodies 87B5 and
M3/38. In the first step, galectin-3 in the sample and M3/38
anti-galectin-3 coated microparticles were combined. After washing,
87B5 anti-galectin-3 conjugate was added. Following another wash,
pre-trigger and trigger solutions were added to result in
chemiluminescent reaction. The amount of galectin-3 in the sample
was detected by the Architect i System optics.
AGP levels were analysed using a QUANTIA A-1-AGP kit
(Abbott Architect c 8000; Abbott Pharmaceutical Co. Ltd.).
Intra-assay and inter-assay coefficient variation values were
defined as 1.0 and 1.2%, respectively.
Statistical analysis
Data analysis was performed using IBM SPSS version
21.0 (IBM Corp.). The distribution of variables was determined
using the Kolmogorov-Smirnov test. All data are presented as the
mean ± standard deviation. Mann-Whitney and Kruskal-Wallis H tests
were used to evaluate the differences between groups. The
χ2 test was used for categorical variables. The degree
of association between continuous variables was calculated using
Spearman's correlation coefficient. Receiver operating
characteristic (ROC) curve analysis was used to determine the
cut-off values for galectin. P<0.05 was considered to indicate a
statistically significant difference. The effect size was
calculated as 0.6 and the actual power was 0.90 using GPower 3.1
(G*Power version 3.1.9.6, Universitat Kiel).
Results
A total of 55 patients diagnosed with ISSNHL [29
(52.7%) female and 26 (47.3%) male] were included in the study
group. The mean age of the study group was 46.76±17.68 years
(range, 21-78 years; data not shown). The control group consisted
of 47 volunteers [25 (53.2%) female and 22 (46.8%) male; mean age,
43.95±12.96 years (range, 26-65 years)]. There was no significant
difference between the study and control groups regarding sex and
age (P=0.96 and P=0.38, respectively).
The laboratory findings of the participants are
shown in Table I. Galectin-3
levels were 16.80±4.55 ng/ml in the study group and 15.15±3.74
ng/ml in the control group (P=0.05).
 | Table ILaboratory findings of participants in
the control and study groups. |
Table I
Laboratory findings of participants in
the control and study groups.
| Characteristic | Control group (n=47;
mean ± SD) | Study group (n=55;
mean ± SD) | P-value |
|---|
| White blood cell,
1x103/µl | 6.9±0.9 | 7.1±1.1 | ns |
| Haemoglobin,
g/dl | 14.2±1.4 | 14.1±1.5 | ns |
| Platelet,
103/µl | 232±35.2 | 245±46 | ns |
| C-reactive protein,
mg/dl | 2.8±2.3 | 3.4±2.8 | ns |
| E Erythrocyte
sedimentation rate, mm/h | 2.2±1.2 | 1.9±1 | ns |
| α-1-acid
glycoprotein, ng/ml | 67.01±21.6 | 64.1±25.1 | 0.087 |
| Galectin-3,
ng/ml | 15.2±3.7 | 16.80±4.6 | 0.050 |
Audiological tests were all performed in Ufuk
University, Ridvan Ege Hospital. The audiological scores are shown
in Table II. ISSNHL significantly
increased PTA and decreased SDS. According to audiogram graphic
configurations, 14 patients (25.5%) had a downward sloping
audiogram. Of these, eight patients (57.1%) responded positively to
medical treatment. U-shaped hearing loss was identified in 27
patients (49.1%), 12 (44.4%) of whom recovered following medical
treatment. A total of 14 patients (25.5%) had profound hearing loss
and only four (28.6%) recovered following medical treatment. In
summary, out of 55 patients, 24 (43.6%) showed recovery, whereas 31
(56.4%) showed no improvement in hearing loss. There was no
significant difference in response to treatment regarding the three
different audiogram configurations (P=0.3). In addition, galectin-3
and AGP levels did not differ significantly between audiogram
configurations (P=0.836 and P=0.402; respectively).
 | Table IIPure tone average and speech
discrimination score of the control and study groups. |
Table II
Pure tone average and speech
discrimination score of the control and study groups.
| | Study group (n=55;
mean ± SD) | |
|---|
| Characteristic | Control group (n=47;
mean ± SD) | Unaffected side (side
without hearing loss) | Affected side (side
with hearing loss) | P-valuea |
|---|
| Pure tone average,
dB | 21.3±5.7 | 25.0±13.7 | 60.4±21.7 | <0.001 |
| Speech discrimination
score, % | 95.0±3.7 | 92.4±7.7 | 49.7±27.9 | <0.001 |
Serum galectin-3 levels were significantly
correlated with PTA improvement in the study group (r=-0.35;
P=0.010). There was no correlation between AGP and PTA score
(P=0.301; Table III).
 | Table IIICorrelations between galectin-3,
α-1-acid glycoprotein levels and improvement in pure tone average
scores in patients with idiopathic sudden sensorineural hearing
loss. |
Table III
Correlations between galectin-3,
α-1-acid glycoprotein levels and improvement in pure tone average
scores in patients with idiopathic sudden sensorineural hearing
loss.
| Variable | r-value | P-value |
|---|
| Galectin, ng/ml | -0.350 | 0.010 |
| α-1-acid
glycoprotein, mg/dl | -0.142 | 0.301 |
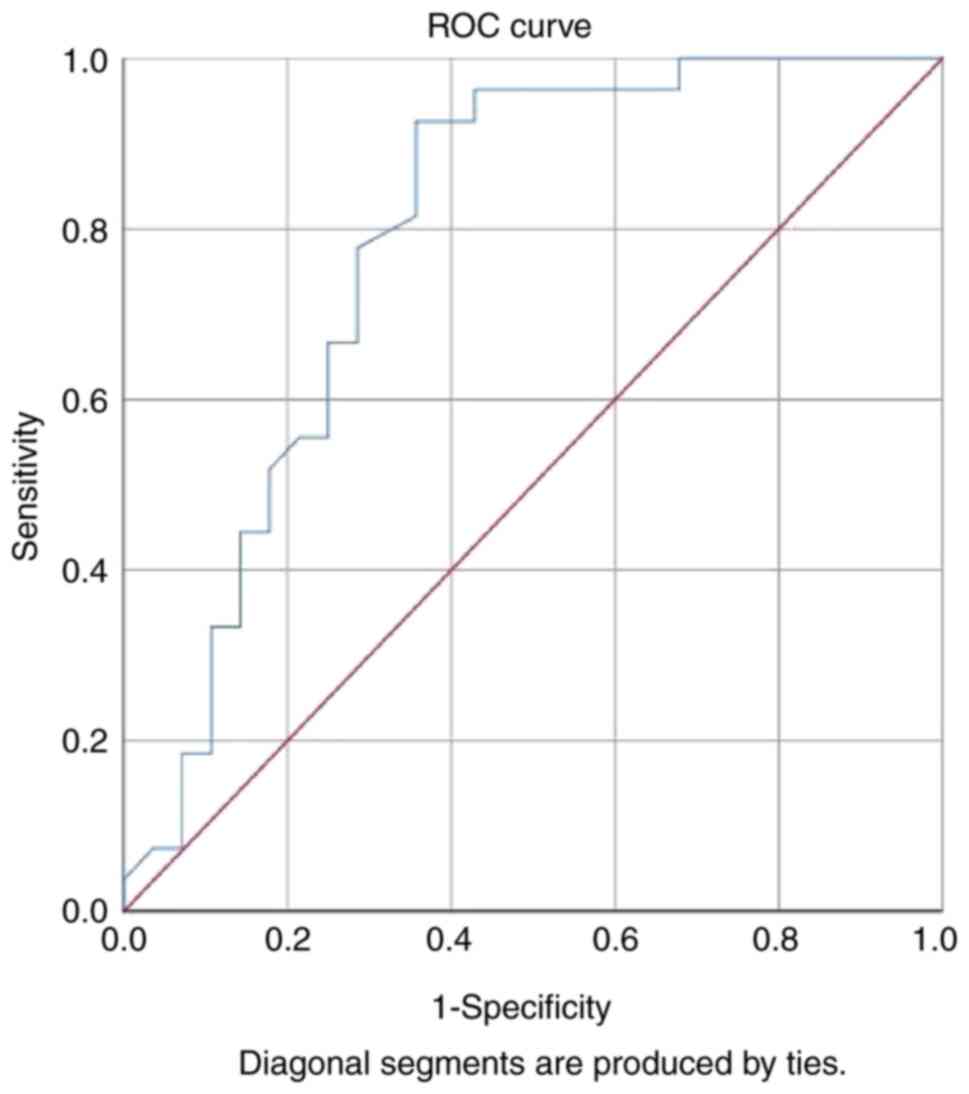
Based on ROC analysis, the cut-off level for
galectin-3 for the prediction of treatment success was 14.95 ng/ml.
Galectin-3 levels >14.95 ng/ml indicated poor improvement in PTA
scores in patients with ISSNHL. The area under the ROC curve was
0.79 (P<0.001), with a sensitivity of 93% (95% CI, 0.77-0.98)
and a specificity of 64% (95% CI, 0.46-0.79; Fig. 1).
Discussion
The present study showed that galectin-3 levels were
elevated in patients with ISSNHL and that serum galectin-3 levels
may help predict the prognosis of ISSNHL treatment. High galectin-3
levels were associated with poor improvement in PTA scores in
patients with ISSNHL following treatment. By contrast, AGP levels
showed no difference in patients with ISSNHL compared with the
control group and no association with PTA scores after
treatment.
The etiopathogenesis of ISSNHL remains unclear.
Infection, vascular pathologies and systemic and metabolic disease
have been reported to play a role in the etiopathogenesis of
ISSNHL(2). The present analysis
aimed to investigate inflammatory and vascular/ischemia biomarkers,
namely galectin-3 and AGP, in ISSNHL and determine their potential
predictive role for recovery after medical treatment.
The cochlea is vulnerable because few branches of
the internal auditory artery support its vascularization.
Therefore, hypoxia-induced vascular disorder can lead to cochlear
damage and hearing loss (12).
However, to the best of our knowledge, the association between
sensorineural hearing loss and cardiovascular disorder has not been
identified. A previous study investigated ISSNHL with
ischemia-modified albumin (IMA) and found no significant
relationship between ISSNHL and IMA levels (13).
Local ischemia serves a role in ISSNHL (14). Kim et al (15) found that vascular structures play
an essential role in ISSNHL aetiology.
The present study used galectin-3 levels as
indicators of ischemia and AGP values for inflammation. Only
galectin-3 levels were increased in patients with ISSNHL and were
also predictive of prognosis following treatment. When the
association of serum galectin-3 levels with several types of
ischemic disorder is considered (8), an ischemic aetiology may be
considered as the etiopathogenesis of ISSNHL in patients with high
galectin-3 levels and this may also explain the unresponsiveness of
the patients to steroids. Patients with high galectin-3 levels may
be evaluated for potential ischemic aetiology and anti-aggregant
and anticoagulant therapy may be started to treat ISSNHL (8). Therefore, it would be a reasonable
option to avoid the side effects of systemic corticosteroids and
start anticoagulant therapy in patients with high galectin-3
levels.
AGP is an anti-inflammatory and immunoregulatory
mediator that is synthesized in hepatocytes. It is hypothesized to
be involved in the extravasation of leukocytes. AGP has
anti-inflammatory effects against infection, inflammation,
neoplasms and tissue damage (5).
In addition, AGP is frequently investigated in blood especially in
defining sepsis-associated mortality. Therefore, AGP is used to
monitor the outcome of inflammation and infection (5). AGP can also be increased by drugs,
such as phenobarbitone and rifampicin, burns and pregnancy
(16). Thus, patients with chronic
disease or drug use were excluded from the present study to search
for a correlation with ISSNHL. Here, AGP was assessed as an
inflammatory parameter; however, a significant association between
this marker and ISSNHL was not found.
The galectin family consists of 14 members essential
for binding proteins. The phosphorylation of galectin-3 is key for
protein and carbohydrate interactions. Phosphorylated galectin-3
activates the mitogen-activated protein kinase pathway, thus
affecting the anti-apoptotic process (17). Galectin-3 functions in cell
proliferation, differentiation, adhesion, apoptosis and malignant
transformation (18-22).
Bertocchi et al (23)
reported that serum galectin-3 levels serve a role in ischemic
reperfusion injuries, particularly in the kidneys. A recent study
also demonstrated that serum galectin-3 levels are associated with
cardiovascular events, especially in patients with type 2 diabetes
(24). Galectin-3 was chosen in
the present study for these reasons and because of its broad range
of actions, such as induction of oxidative stress, tissue damage
and ischemia, which are believed to be the most common causes of
ISSNHL (8,12).
Elevated galectin-3 levels are hypothesized to be
related to atherosclerosis and morbidity associated with acute
ischemic stroke (25-27).
The increased levels of galectin-3 in ISSNHL and its
association with non-responsiveness to steroids should be studied
further to determine the potential effect of an ischemic aetiology
on ISSNHL and different treatment strategies may be planned
according to the cause of ischemia.
To the best of our knowledge, the present study is
the first to assess serum galectin-3 and AGP levels in patients
with ISSNHL. However, the primary limitation of this study is the
small sample size. More accurate results for galectin levels could
be obtained with more participants. In addition, the ischemic
aetiology of ISSNHL should be investigated in more detail to
determine treatment strategy.
To the best of our knowledge, the present study is
first to show galectin-3 levels are higher in patients with ISSNHL
compared with those in healthy controls. In addition, the poor
response of patients with high galectin-3 levels supports a
potential ischemic cause. As AGP did not differ between the groups,
anticoagulant therapy may be useful in patients with high levels of
galectin-3. Further studies are needed to assess the role of
galectin-3 levels in ISSNHL, particularly in selecting treatment
strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HA and TC confirm the authenticity of all the raw
data. MMB wrote the manuscript and constructed figures. HA
performed experiments and data analysis. HA and TC designed the
study. TC collected data. MMB and HA performed the literature
review and interpreted data. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the local ethics
committee of Ufuk University, Ankara (approval no. 30042015-6).
Patients provided written informed consent to participate in the
study.
Patients consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr. Mustafa Mert Basaran: ORCID ID:
0000-0001-8927-3512;
Dr. Hande Arslan: ORCID ID: 0000-0003-0344-2712;
Dr. Tuba Candar: ORCID ID: 0000-0002-3922-5915
References
|
1
|
Stachler RJ, Chandrasekhar SS, Archer SM,
Rosenfeld RM, Schwartz SR, Barrs DM, Brown SR, Fife TD, Ford P,
Ganiats TG, et al: Clinical practice guideline: Sudden hearing
loss. Otolaryngol Head Neck Surg. 146:1–35. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mirian C and Ovesen T: Intratympanic vs.
systemic corticosteroids in first-line treatment of idiopathic
sudden sensorineural hearing loss. JAMA Otolaryngol Head Neck Surg.
146:421–428. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chau JK, Lin JR, Atashband S, Irvine RA
and Westerberg BD: Systematic review of the evidence for the
etiology of adult sudden sensorineural hearing loss. Laryngoscope.
120:1011–1021. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pucher B, Sobieska M, Grzegorowski M and
Szydlowski J: Acute phase protein reaction in children suffering
from pseudocroups. Mediators Inflamm. 26:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsiao SY, Lai YR, Kung CT, Tsai NW, Su CM,
Huang CC, Wang HC, Cheng BC, Su YJ, Lin WC, et al: α 1-acid
glycoprotein concentration as an outcome predictor in adult
patients with sepsis. Biomed Res Int. 2019(3174896)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hochepied T, Berger FG, Baumann H and
Libert C: α 1-acid glycoprotein: An acute phase protein with
inflammatory and immunomodulating properties. Cytokine Growth
Factor Rev. 14:25–34. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fukumori T, Kanayama HO and Raz A: The
role of galectin-3 in cancer drug resistance. Drug Resist Updat.
10:101–108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mo D, Tian W, Zhang HN, Feng YD, Sun Y,
Quan W, Hao XW, Wang XY, Liu XX, Li C, et al: Cardioprotective
effects of galectin-3 inhibition against ischemia/reperfusion
injury. Eur J Pharmacol. 863(172701)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kitoh R, Nishio SY and Usami SI: Treatment
algorithm for idiopathic sudden sensorineural hearing loss based on
epidemiologic surveys of a large Japanese cohort. Acta Otolaryngol.
140:32–39. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alan IS and Alan B: Side effects of
glucocorticoids. In: Pharmacokinetics and adverse effects of
drugs-mechanisms and risks factors. Malangu N (ed). Intechopen,
London, 2017.
|
|
11
|
Jarosz M, Olbert M, Wyszogrodzka G,
Mlyniec K and Librowski T: Antioxidant and anti-inflammatory
effects of zinc. Zinc-dependent NF-κB signaling.
Inflammopharmacology. 25:11–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim JY, Hong JY and Kim DK: Association of
sudden sensorineural hearing loss with risk of cardiovascular
disease. JAMA Otolaryngol Head Neck Surg. 144:129–135.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cirik AA, Evcimik MF, Ulfer G, Yigitbasi T
and Aktas O: Ischemia-modified albumin levels in patients with
idiopathic sudden sensorineural hearing loss. Turkish J Ear Nose
Throat. 29:21–27. 2019.
|
|
14
|
Rudack C, Langer C, Stoll W, Rust S and
Walter M: Vascular risk factors in sudden hearing loss. Thromb
Haemost. 95:454–461. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim C, Sohn JH, Jang MU, Hong SK, Lee JS,
Kim HJ, Choi HC and Lee JH: Ischemia as a potential etiologic
factor in idiopathic sudden sensorineural hearing loss: Analysis of
posterior circulation arteries. Hear Res. 331:144–151.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Radaković M, Borozan S, Djelić N, Ivanović
S, Miladinović DĆ, Ristanić M, Spremo-Potparević B and Stanimirović
Z: Nitroso-oxidative stress, acute phase response, and cytogenetic
damage in wistar rats treated with adrenaline. Oxid Med Cell
Longev. 2018(1805354)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takenaka Y, Fukumori T, Yoshii T, Oka N,
Inohara H, Kim HR, Bresalier RS and Raz A: Nuclear export of
phosphorylated galectin-3 regulates its antiapoptotic activity in
response to chemotherapeutic drugs. Mol Cell Biol. 24:4395–4406.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mazurek N, Conklin J, Byrd JC, Raz A and
Bresalier RS: Phosphorylation of the beta-galactoside-binding
protein galectin-3 modulates binding to its ligands. J Biol Chem.
275:36311–36315. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Califice S, Castronovo V, Bracke M and van
den Brûle F: Dual activities of galectin-3 in human prostate
cancer: Tumor suppression of nuclear galectin-3 vs tumor promotion
of cytoplasmic galectin-3. Oncogene. 23:7527–7536. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sanjuán X, Fernández PL, Castells A,
Castronovo V, van den Brule F, Liu FT, Cardesa A and Campo E:
Differential expression of galectin 3 and galectin 1 in colorectal
cancer progression. Gastroenterology. 113:1906–1915.
1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pacis RA, Pilat MJ, Pienta KJ, Wojno K,
Raz A, Hogan V and Cooper CR: Decreased galectin-3 expression in
prostate cancer. Prostate. 44:118–123. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu XC, el-Naggar AK and Lotan R:
Differential expression of galectin-1 and galectin-3 in thyroid
tumors. Potential diagnostic implications. Am J Pathol.
147:815–822. 1995.PubMed/NCBI
|
|
23
|
Bertocchi APF, Campanhole G, Wang PH,
Gonçalves GM, Damião MJ, Cenedeze MA, Beraldo FC, de Paula Antunes
Teixeira V, Dos Reis MA, Mazzali M, et al: A role for galectin-3 in
renal tissue damage triggered by ischemia and reperfusion injury.
Transpl Int. 21:999–1007. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lorenzo-Almorós A, Pello A, Aceña Á,
Martínez-Milla J, González-Lorenzo Ó, Tarín N, Cristóbal C,
Blanco-Colio LM, Martín-Ventura JL, Huelmos A, et al: Galectin-3 is
associated with cardiovascular events in post-acute coronary
syndrome patients with type-2 diabetes. J Clin Med.
9(1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang A, Zhong C, Zhu Z, Xu T, Peng Y, Xu
T, Peng H, Chen CS, Wang J, Ju Z, et al: Serum galectin-3 and poor
outcomes among patients with acute ischemic stroke. Stroke.
49:211–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Doverhag C, Hedtjärn M, Poirier F, Mallard
C, Hagberg H, Karlsson A and Sävman K: Galectin-3 contributes to
neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 38:36–46.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhuang JJ, Zhou L, Zheng YH and Ding YS:
The serum galectin-3 levels are associated with the severity and
prognosis of ischemic stroke. Aging. 13:7454–7464. 2021.PubMed/NCBI View Article : Google Scholar
|