Introduction
Spinal cord injury (SCI) is a highly debilitating
neurological trauma caused by traumatic injury or disease (1). The induced damage causes a series of
pathological changes, including hemorrhage, inflammation, edema and
fibrous scar formation, markedly impair neuron regeneration and
functional recovery following SCI. Moreover, the self-recovery
capacity of the central nervous system is inherently poor (2,3).
Thus, majority of patients with SCI suffer from lifelong
disabilities. Thus, its clinical treatment remains a worldwide
challenge (4).
Previous studies using rodent models have
demonstrated that cell transplantation is a promising repair
strategy for SCI. Generally, the transplanted healthy cells can
replace damaged cells, and simultaneously secrete a variety of
neurotrophic factors required for axon remyelination and neuronal
regeneration (5,6). However, directly injecting the cell
suspension into the cavities usually cannot achieve ideal
therapeutic efficacy. A vast number of cells are lost during the
injection and the harsh microenvironment of the injured spinal cord
contributes to the necrosis or apoptosis of 80% of the transplanted
cells (7,8). Co-transplantation with a
biocompatible scaffold provides available solutions for this issue.
The seed cells are encapsulated in the scaffold and the scaffold
creates a suitable local environment to enhance cell retention and
the survival rate (9-11).
Currently, the combination of biomaterial with cell transplantation
is under intense investigation in the scope of SCI.
Schwann cells (SCs) are glial cells in the
peripheral nervous system. They play a notable role in Wallerian
degeneration and the regeneration of the injured peripheral nerve
(12,13). Following peripheral nerve injury,
the resting SCs are activated, proliferate and migrate to the
injury site, and co-phagocytose damaged axonal myelin with
macrophages. Furthermore, activated SCs (ASCs) secrete numerous
nutritional factors, cell-adhesion molecules and extracellular
matrix (ECM) to promote and guide neuronal regeneration (14). In SCI research, SC transplantation
therapy has exhibited immense potential. Accumulating evidence has
indicated that the transplantation of SCs inhibits inflammation,
reduces cyst formation and promotes axon elongation. In addition,
motor and functional recovery have also been observed (15,16).
SCs are considered promising candidate cells for SCI repair.
However, single SC therapy is also associated with difficulties as
aforementioned.
Gelatin methacryloyl (GelMA) hydrogel is a
photosensitive hydrogel. It is mainly processed from the native ECM
which confers its favorable biocompatibility (17). Furthermore, GelMA hydrogel
possesses highly adjustable physiochemical properties and an inner
three-dimensional structure, which render it an effective option as
a vehicle for cell delivery (18,19).
To date, certain researchers have co-transplanted GelMA hydrogels
with various cells to repair the injured spinal cord, including
bone marrow-derived mesenchymal cells (BMSC), neural stem cells
(NSC) and induced pluripotent stem cells (iPSCs), and the results
have been satisfactory (20,21).
However, the therapeutic efficacy of ASC-loaded GelMA hydrogel for
SCI has not yet been evaluated, at least to the best of our
knowledge.
The present study transplanted ASC-loaded GelMA
hydrogels into rat spinal-transected segments. Through behavioral
and histological analyses, the present study aimed to evaluate the
therapeutic efficacy of ASC-loaded GelMA hydrogel in SCI
repair.
Materials and methods
Ethics
Sprague-Dawley (SD) female rats (8-weeks old,
180-250 g body weight) used in this experiment were obtained from
SPF (Beijing) Biotechnology Co., Ltd. [permission number: SCX
(JING) 2019-0010]. The rats were group-housed 2-3 per cage and
maintained with a 12-h light/dark cycle (light at 7 a.m.) under a
temperature (22-23˚C) and humidity (55-65%)-controlled environment.
They were given free access to food and water and acclimated for at
least 7 days before the surgery for the adaption to the
environment. All procedures involving animals complied with the
Guiding Principles for the Care and Use of Vertebrate Animals in
Research and Training. The experiments were approved (approval no.
MDL20210810-01) by the Animal Ethical and Welfare Committee of The
Tianjin Medical University General Hospital (Tianjin, China).
Isolation and identification of
ASCs
The isolation, culture and identification of ASCs
have been previously described (22). Briefly, an 8-week-old SD rat was
anesthetized with an intraperitoneal injection of pentobarbital
sodium (30 mg/kg). To change SCs into ASCs, following anesthesia,
the bilateral sciatic nerves were ligated for one week. Then the
rat was sacrificed with intraperitoneal injection of excess
pentobarbital (300 mg/kg) and the bilateral sciatic nerves distal
to the ligation spot were isolated. After removing the endoneurium,
the remaining nerve tissues were cut into 0.5-1 mm3
fragments. The nerve tissues were digested with 0.05% collagenase
(cat. no. C8176; MilliporeSigma) for 45 min at 37˚C and
subsequently replaced with culture medium comprised of Dulbecco's
modified Eagle's medium (DMEM; cat. no. C11330500BT; Gibco; Thermo
Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS; cat. no.
10100147; Gibco; Thermo Fisher Scientific, Inc.). The mixture was
transferred to a 15-ml centrifuge tube. Following centrifugation
(200 x g, 4˚C, 5 min), the supernatant was discarded and the
sediment was resuspended with DMEM supplemented with 10% FBS and 1%
antibiotic solution (penicillin, streptomycin) (cat. no. 15070063;
Gibco; Thermo Fisher Scientific, Inc.). The cell suspension was
placed into a 25-ml culture flask and incubated at 37˚C and 5%
CO2. The culture medium was changed every 3 days. After
1 week, the ASCs reached a confluency of 90% and the identity was
confirmed by immunostaining of S-100 (1:250; cat. no. ab109384;
Abcam). After nuclei staining with 5 µg/ml DAPI for 10 mi at room
temperature, cells were observed under a fluorescence inverted
phase contrast microscope (Olympus Corporation).
Preparation and evaluation of
ASC-GelMA hydrogels
The freeze-dried GelMA powder was purchased from
MilliporeSigma. The hydrogel was obtained by dissolving 3% (W/V)
lyophilized powder and 0.5% (W/V) lithium
phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) into
phosphate-buffered saline (PBS), followed by exposure to 405 nm UV
irradiation for 15 sec.
The ASCs were trypsinized, centrifuged (200 x g,
4˚C, 5 min), and then resuspended in 3% (w/v) GelMA solution at the
concentration of 1x107/ml. Under the exposure of 405 nm
UV irradiation for 15 sec, the cell-seeded GelMA hydrogel was
obtained. The hydrogel was then transferred into a six-well plate
in which ASC basal medium was added followed by culture at 37˚C
with 5% CO2. Because the gel reflected the light and
ASCs were encapsulated in the gels, the cells could be hardly
observed under the light microscope. Scanning electron microscopy
(SEM, S-3400; Hitachi, Ltd.) was then used to observe the
microstructure of the GelMA hydrogel with or without seeded cells.
After 1, 3 and 5 days of in vitro culture, the
biocompatibility of the GelMA hydrogel with ASCs was evaluated
using Calcein-AM/ethidium staining according to the manufacturer's
procedure (cat. no. BL3224; Invitrogen; Thermo Fisher Scientific,
Inc.).
Animal experiments. Design and spinal
cord surgery
A total of 80 SD rats were used in the experiments
and they were randomly divided into four groups as follows: The
sham group (n=20), the SCI group (n=20), the ASC group (n=20) and
the GelMA/ASC group (n=20).
Spinal cord hemi-transection and
scaffold transplantation
Firstly, the healthy adult SD rats were deeply
anesthetized with an intraperitoneal injection of pentobarbital (30
mg/kg). With the back shaved and the skin disinfected, a midline
incision over the spinous process of ~2 cm was made to expose
T9-T11. The paravertebral muscles and the surrounding connective
tissue were also cut using micro scissors. The T9-T10 laminectomy
was performed. For the latter three groups (SCI, ASC and GelMA/ASC
groups), the right lateral hemisection at T10 was performed to
create a gap of ~2 mm in length. For the ASC group, the ASC
suspension with a cell density of 1x107/ml was injected;
for the GelMA/ASCs group, the prepared cell-loaded scaffold was
transplanted. The incision was then closed and the rat bladders
were manually expressed twice a day until the urinary function
recovered. Antibiotics (penicillin, 40,000 µ/kg/day) were also
administered to prevent post-operative infection.
Behavioral assessment
The Basso-Beattie-Bresnahan (BBB) scoring system was
used to evaluate hindlimb motor function. This is an open-field
locomotor evaluation test scoring 21 points, with 0 indicating no
movement of hindlimbs and 21 indicating normal levels (23). Prior to the evaluation, the rat was
separately placed in an open field with a non-slippery surface and
allowed to move freely for 5 min. Two independent observers blinded
to the grouping of the rats scored the rat's motor performance
according to the BBB scale. The test lasted 4 min and once the rat
stopped moving for 1 min, it was placed in the center of the open
field again for a re-test.
The inclined plate test was also used for a
behavioral assessment. The rat was placed on a plate covered with a
6-mm-thick rubber pad. According to the previous study, the body
axis of the rats was perpendicular to the orientation of the plate
(24). The plate was then lifted
5˚ every 30 sec and the maximum degree at which the rat could
maintain its balance was recorded. Each rat was tested three times
and the mean value was the final angle for the individual rat. The
behavioral tests were performed from week 1 to week 6 after surgery
until all the rats were sacrificed.
Sample harvesting and histological
analysis
At 7 and 42 days after surgery, 3 rats were randomly
selected and deeply anesthetized with an intraperitoneal injection
of pentobarbital (30 mg/kg). Following heart perfusion with 150 ml
pre-cooled PBS containing 4% paraformaldehyde, ~10 mm spinal cord
containing the injury site was dissected. It was then post-fixed
overnight with 4% paraformaldehyde. The specimen was then embedded
in paraffin and sliced into 5-µm-thick sections.
The samples collected at 42 days were stained with
hematoxylin and eosin (H&E) to evaluate the lesion volume.
Slices were stained with hematoxylin for 10 min and with eosin for
2 min at room temperature. The images were observed under an
optical microscope and the volume was measured using ImageJ
software (1.49 V; National Institutes of Health).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) staining and immunochemistry
TUNEL assay kit (cat. no. C1086; Beyotime Institute
of Biotechnology) staining was used to evaluate the number of
apoptotic cells. The paraffin-embedded sections were stained
according to the instructions of the manufacturer. The
TUNEL-positive cells with green fluorescence were observed under a
fluorescence microscope and six fields of view for each section
were randomly selected for subsequent calculation using ImageJ
software.
The streptavidin-biotin-peroxidase-complex method
was used to detect Bcl-2 expression using immunochemistry. The
paraffin-embedded sections were sequentially subjected to standard
dewaxing, hydration, antigen retrieval and rinsing, followed by
incubation with endogenous peroxidase blockers for 10 min, and
sealing in serum for 20 min. After discarding the serum, the
primary antibody Bcl-2 (1:250; cat. no. ab196495; Abcam) was added
followed by incubation for 24 h at 4˚C. The following day, the
sections were rinsed and a horseradish peroxidase (HRP)-conjugated
goat anti-rabbit secondary antibody (1:500; cat. no. ab6721; Abcam)
was added, followed by incubation for 30 min at 37˚C. After rinsing
thoroughly, the streptomycin-biotin-peroxidase solution was added
in a dropwise manner, reacting with the specimen for 20 min
followed by diaminobenzidine (DAB) color development. Finally, the
nuclei were counterstained with hematoxylin for 2 min at room
temperature. Images were observed under an optical microscope and
the Bcl-2 positive cells were counted using ImageJ software.
Western blot analysis
The tissue samples collected at day 7 after surgery
were minced using eye scissors on ice and homogenized in 300 µl
lysis buffer (cat. no. ST505; Beyotime Institute of Biotechnology)
for 30 min. Homogenates were then centrifuged at 15,000 x g for 10
min at 4˚C and the supernatant was collected to quantify the
protein concentration using a bicinchoninic acid (BCA) kit (cat.
no. A53225; Thermo Fisher Scientific, Inc.) as per the
manufacturer's instructions. Subsequently, equal amounts of protein
(40 µg per lane) were separated by SDS-PAGE (10% of acrylamide) gel
electrophoresis and transferred onto PVDF membrane. The membrane
was blocked with 5% bovine serum albumin (BSA; cat. no. 9998; Cell
Signaling Technology, Inc.) for 2 h at room temperature and then
incubated with primary antibodies at 4˚C overnight. The following
day, after three rinses with PBS, the membranes were incubated with
the HRP-conjugated goat anti-rabit (cat. no. 7074) and horse
anti-mouse (cat. no. 7076) secondary antibodies (1:1,000; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The bands
were visualized in an enhanced chemiluminescence system (ChemiDox
XRS; Bio-Rad Laboratories, Inc.) and images of the target proteins
were semi-quantified using ImageJ software. The primary antibodies
used were as follows: Anti-Bcl-2 (1:1,000; cat. no. ab196495;
Abcam), anti-caspase-3 (1:1,000; cat. no. ab32351; Abcam), anti-p38
(1:1,000; cat. no. 8690; Cell Signaling Technology, Inc.),
anti-p-p38 (1:1,000; cat. no. 4511; Cell Signaling Technology,
Inc.), anti-ERK1/2 (1:1,000; cat. no. 9194; Cell Signaling
Technology, Inc.), anti-p-ERK1/2 (1:1,000; cat. no. 4370; Cell
Signaling Technology, Inc.), anti-JNK1/2 (1:1,000; cat. no. 3708;
Cell Signaling Technology, Inc.), anti-p-JNK1/2 (1:1,000, cat. no.
9255, Cell Signaling Technology, Inc.), and anti-GAPDH (1:1,000,
cat. no. AF0006, Beyotime Institute of Biotechnology).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8 software (GraphPad Software, Inc.). The comparison
among multiple groups was performed by one-way ANOVA followed by
Tukey's post hoc test. Significant differences in BBB locomotion
evaluation were determined by the Kruskal-Wallis test with Dunn's
multiple comparison. The data are presented as the mean ± standard
deviation (SD) or median (IQR). P<0.05 was considered to
indicate a statistically significant difference.
Results
Culture and identification of
ASCs
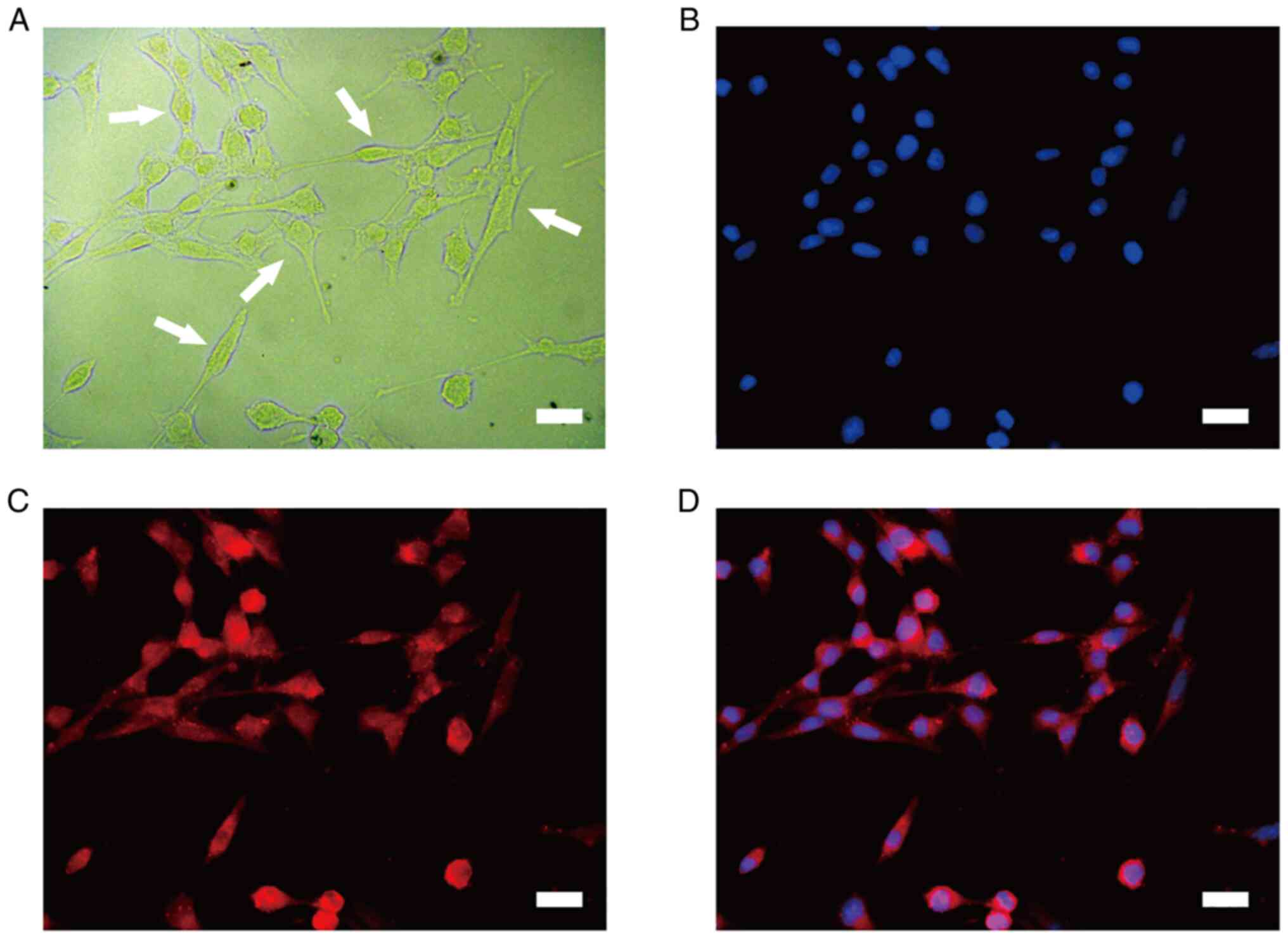
At 10 days post-isolation of the ASCs, the ASCs
proliferated and covered the T75 culture flask (Fig. 1A). The isolated cells expressed
strong red fluorescence of the S100 SC marker after immunostaining
(Fig. 1C). A previous study has
demonstrated that ASCs exhibit a greater proliferative and adhesive
ability than normal SCs (NSCs) (24). Thus, ASCs were used in the
following experiments.
Encapsulation of ASCs in GelMA
hydrogel
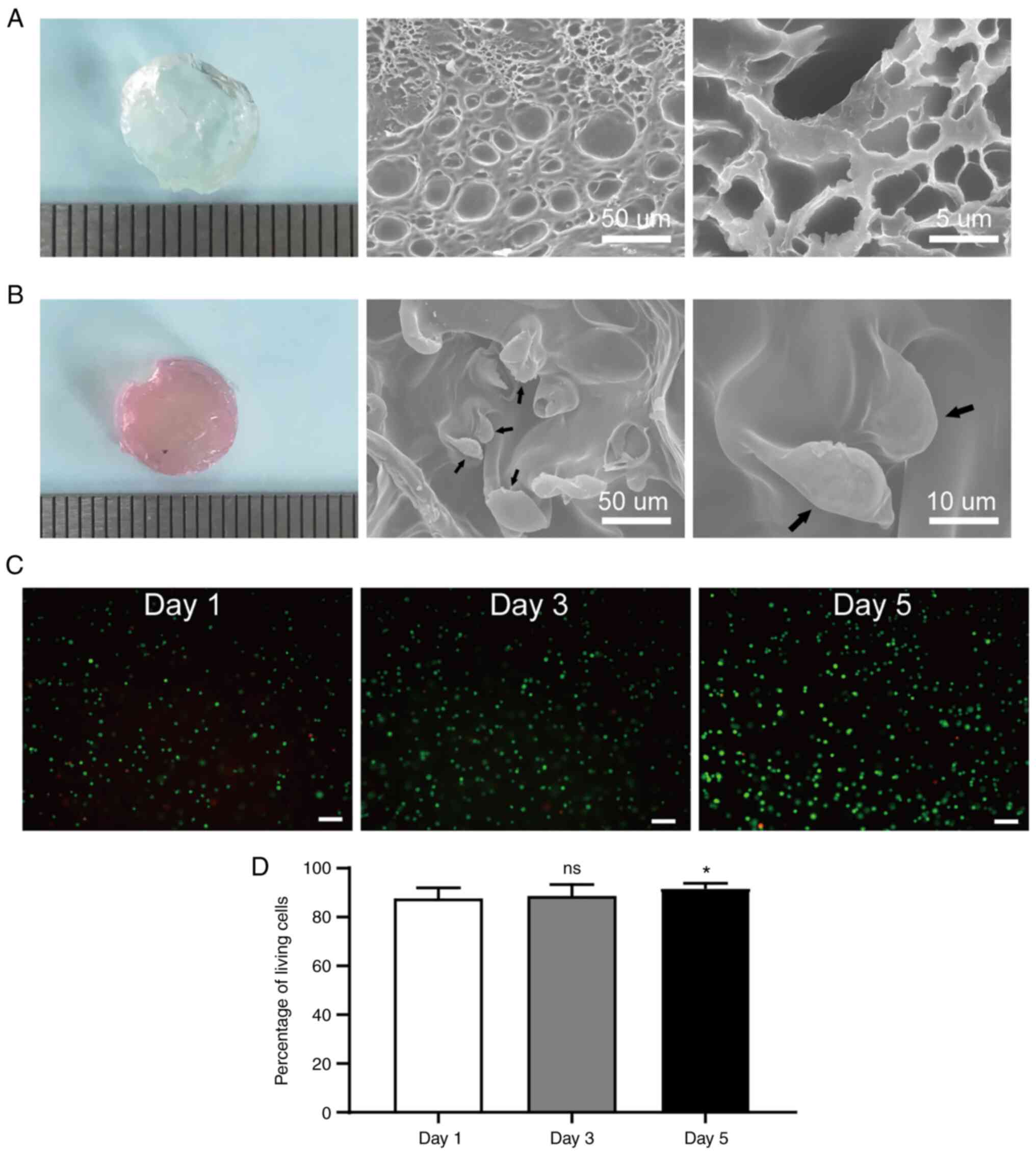
The SEM results displayed the 3D porous
microstructure of the GelMA hydrogel, which was essential for
oxygen and nutrient exchange, as well as for promoting cell
survival, proliferation and migration (Fig. 2A). Moreover, it was also found that
the ASCs were well encapsulated in the GelMA hydrogel (Fig. 2B).
To further assess the viability of the ASCs
encapsulated in the GelMA hydrogel, live/dead staining was
performed. The green fluorescence indicated that the majority of
encapsulated ASCs were alive and that the cells proliferated from
days 1 to 5, as indicated by a gradual increase in green labels
(Fig. 2C). Overall, the percentage
of alive ASCs was >85% which indicated the favorable
biocompatibility of the GelMA hydrogel (Fig. 2D).
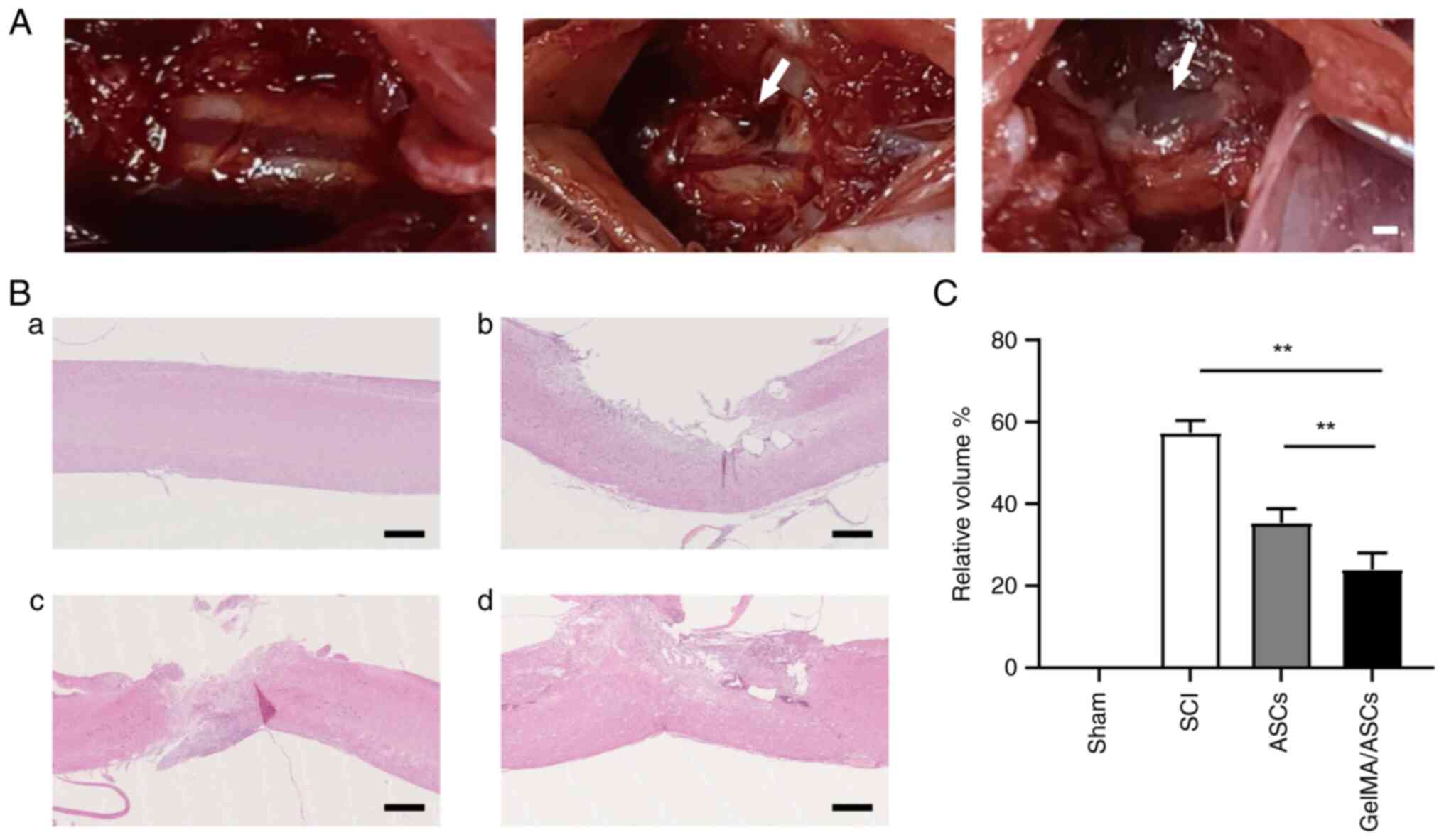
Morphology of the lesion site, as
revealed by H&E staining
Following 48 h of co-culture, the ASC-loaded GelMA
hydrogel was transplanted into the hemisection area of the rat
spinal cord (Fig. 3A). To
determine whether the ASC-loaded GelMA hydrogel could repair the
injured spinal cord, H&E staining was performed on the samples
collected on day 42 following surgery (Fig. 3B). In the SCI group, H&E
staining revealed large cavities and a disordered structure at the
injury site. By contrast, the GelMA/ASC groups exhibited an evident
decrease in volume and an improvement of tissue integrity
(P<0.01). Moreover, compared with the ASC group, the cavities in
the GelMA/ASC group were smaller, which demonstrated the improved
tissue repair ability of ASCs when encapsulated in the GelMA
hydrogel (Fig. 3C).
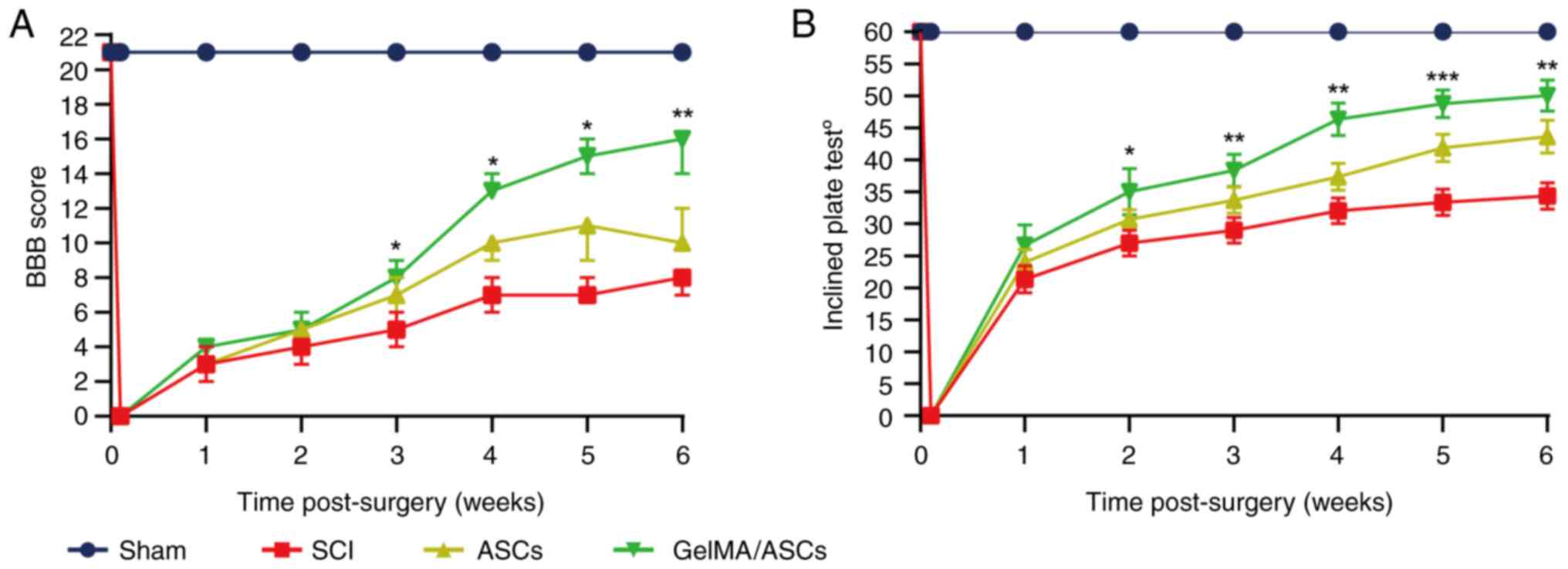
Evaluation of locomotor function
The ability of the ASC/GelMA hydrogel to repair SCI
was also assessed by examining the recovery of hindlimb locomotor
function. Thus, the BBB scoring system and inclined plate test were
applied to evaluate the motor functional recovery of the rats with
SCI from weeks 1 to 6 following surgery. Higher BBB scores or
inclined angles represent an improved motor function and vice
versa. In the beginning, the rats with SCI exhibited a
significant decrease in both BBB scores and inclined angles,
suggesting the loss of motor function following SCI. Subsequently,
there were overall upward trends in both BBB scores and inclined
angles for rats with SCI, suggesting the gradual recovery of their
motor function. The GelMA/ASC groups presented significantly higher
scores (P<0.05) and inclined angles (P<0.01) than the SCI
group from 3 weeks post-injury onward up to the final evaluation
(Fig. 4A and B). In particular, the GelMA/ASC group
exhibited an improved therapeutic efficacy than single ASC
treatment from the second week after surgery to the time of
sacrifice.
Transplantation of GelMA/ASCs inhibits
cell apoptosis following SCI
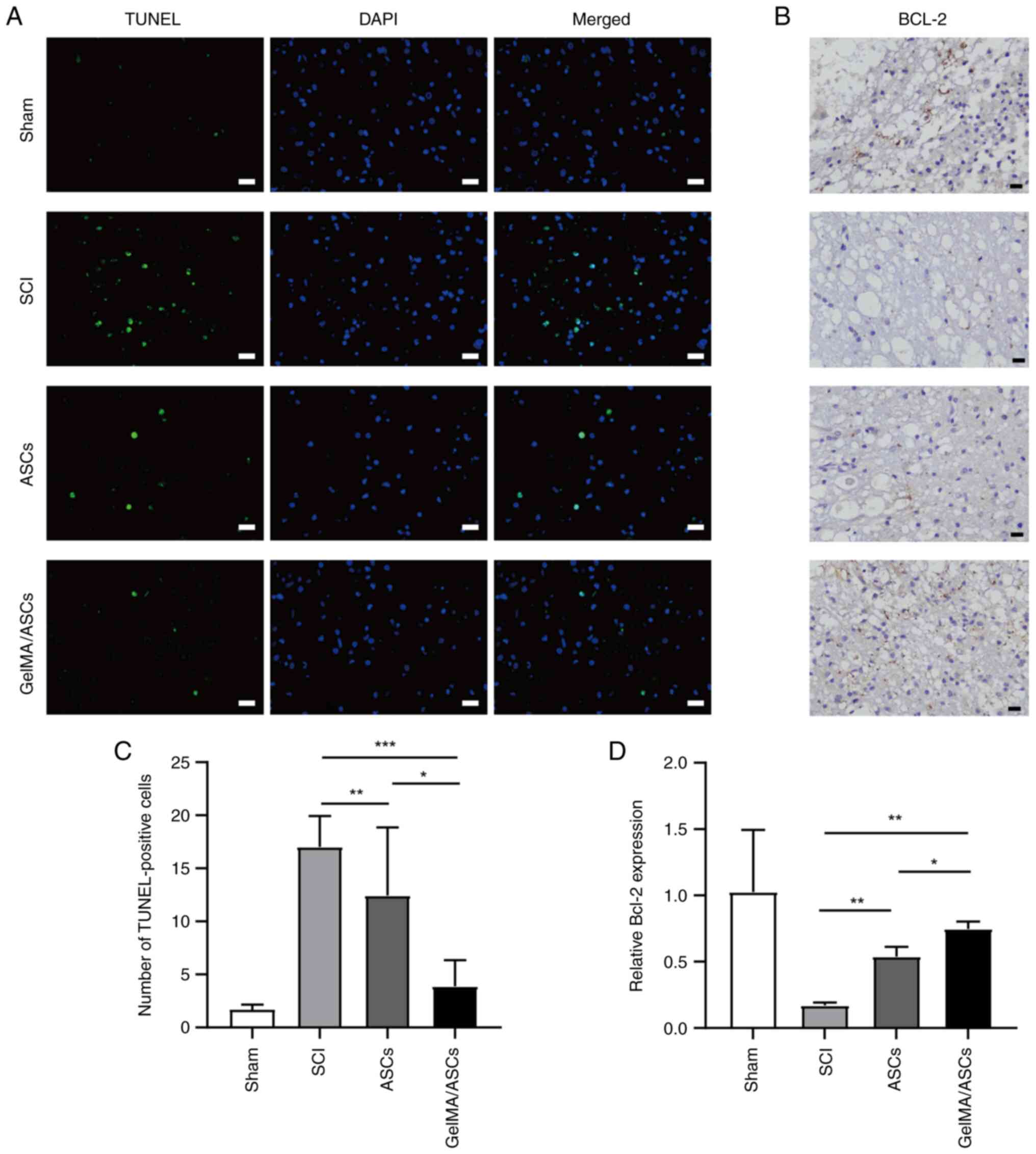
To investigate cell apoptosis, TUNEL staining and
Bcl-2 immunochemistry were performed on the 7th day. For TUNEL
staining, compared with the sham group, the number of apoptotic
cells in the SCI group was increased (Fig. 5A). The ASC (P<0.01) and
GelMA/ASC (P<0.001) groups both exhibited a significantly
decreased number of apoptotic cells compared with the SCI group,
and GelMA/ASC treatment was found to be more advantageous as
compared with single ASC treatment in inhibiting cell apoptosis
(P<0.05) (Fig. 5C).
The Bcl-2 positive cells exhibited a brown-stained
cytoplasm. In the sham group, a great number of Bcl-2 positive
cells appeared, while in the SCI group, only a small number of
cells were Bcl-2-immunoreactive (Fig.
5B). In the ASC group, there were more Bcl-2-positive cells
than SCI group (P<0.01); however, this number was lower than
that in the GelMA/ASC group (P<0.05) (Fig. 5D).
p38 MAPK is involved in the decreased
cell apoptosis following transplantation treatment
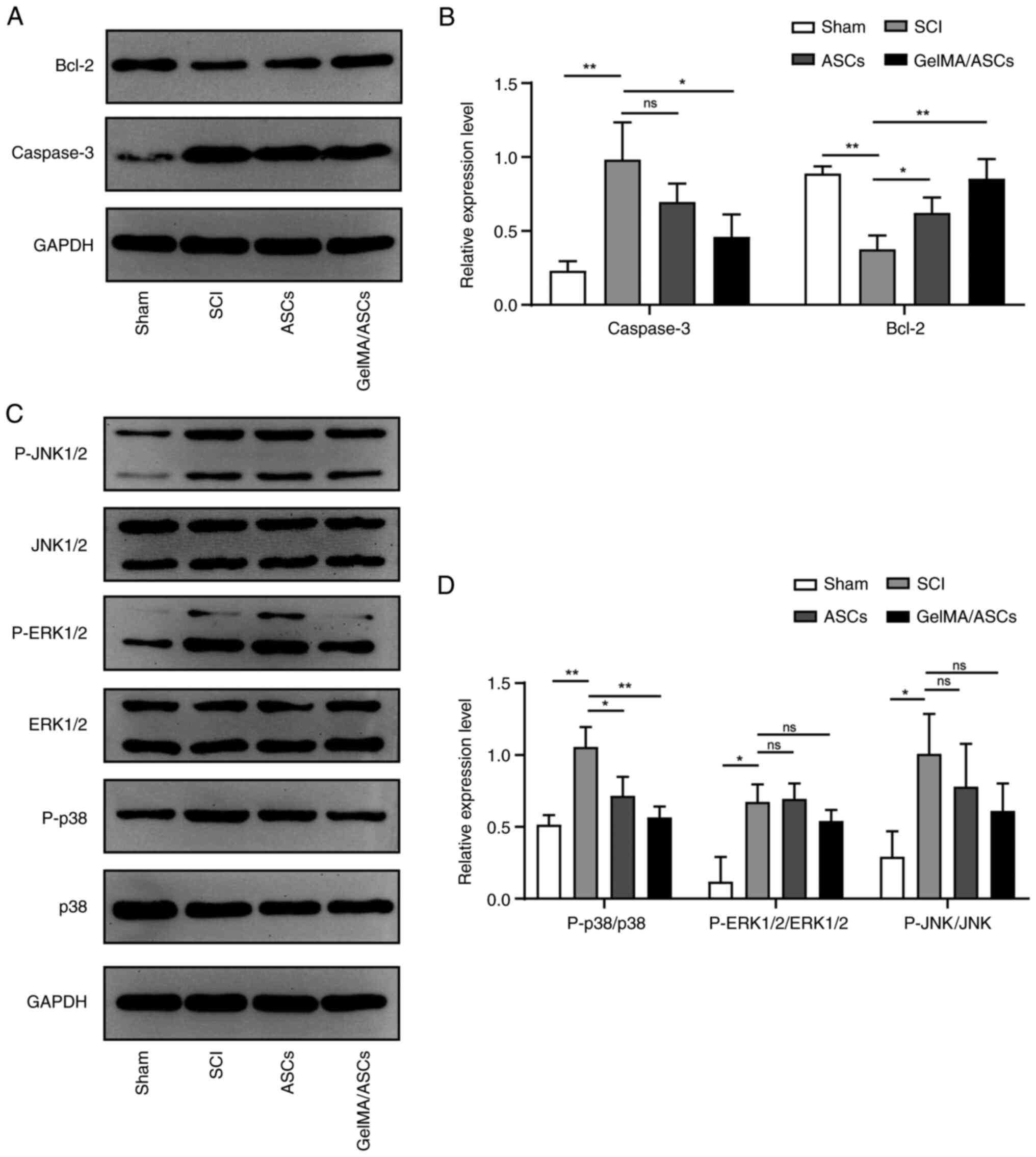
The levels of apoptosis-related proteins, caspase-3
and Bcl-2, were further verified using western blot analysis. The
results of quantitative analysis demonstrated that, following SCI,
the expression of the pro-apoptotic protein, caspase-3,
significantly increased (P<0.01), indicating a higher rate of
cell death, while GelMA/ASC transplantation decreased the protein
level of caspase-3 (P<0.05). The expression of the
anti-apoptotic protein, Bcl-2, was consistent with the results of
immunochemistry. Compared with the SCI group, the protein
expression of Bcl-2 in the ASC (P<0.05) and GelMA/ASC group
(P<0.01) was significantly increased (Fig. 6A and B).
To examine the mechanisms of GelMA/ASCs in
inhibiting cell apoptosis following SCI, three important components
of the MAPK family were detected, including p38, ERK1/2 and JNK1/2.
It was found that the activity of the MAPK family was activated
following SCI compared with that of the sham group (p38: P<0.01;
ERK1/2 and JNK1/2: P<0.05) (Fig.
6C and D). However,
transplantation treatment decreased the phosphorylation level of
p38 signaling (ASCs: P<0.05; GelMA/ASCs: P<0.01), while that
of ERK1/2 and JNK1/2 signaling were relatively unaltered (Fig. 6C and D). Collectively, the results indicated
that GelMA/ASC treatment decreased cell apoptosis following SCI and
that these effects may be mediated via the p38 MAPK pathway.
Discussion
Numerous studies have confirmed the effectiveness of
SC transplantation in the treatment of SCI. However, SCs are known
for having two different states and the majority of studies have
focused on their normal states (NSCs). Studies on the
transplantation of ASCs in the treatment of SCI are limited.
Compared with NSCs, ASCs have a higher cell proliferative and
adhesive ability, and can synthesize and secrete various
neurotrophic factors (25). Marcol
et al (26) used ASCs to
repair focal injury in the spinal cords of rats. In particular,
they found the transplanted ASCs could elicit the body's
self-repair by inducing endogenous SCs in the nerve root to migrate
to the lesion site (26).
Moreover, a previous study by the authors demonstrated that the
clinical application of ASCs in the treatment of patients with SCI
exhibited promising prospects (15). The present study successfully
isolated ASCs, as previously described, and used them to repair the
hemisection injury in the spinal cord of rats. It was found that
ASC treatment attenuated cell apoptosis, rebuilt the disordered
structure of the injured spinal cord, and further improved the
functional recovery of rats with SCI. The present study highlights
the feasibility of using ASCs in the treatment of SCI.
Currently, the use of biological material is making
progress in regenerative medicine. A favorable biomaterial should
be biocompatible and biodegradable with a low immunogenicity and
GelMA hydrogel possesses these properties (27,28).
Moreover, the inner 3D structure and tunable mechanistic
characteristics of the GelMA hydrogel render it effective in
various tissue repair regions (spinal cord, bone and cardio)
(29). The present study
constructed an ASC-GelMA hydrogel composite. The results of SEM and
live/dead staining revealed that the ASCs survived and proliferated
in the GelMA hydrogel. The locomotor test and H&E staining
revealed that the GelMA/ASCs repaired the injured spinal cord and
enhanced functional recovery. Moreover, the post-operative recovery
in the GelMA/ASC group was improved compared with that in the ASC
group, which suggests that the GelMA hydrogel may enhance the
therapeutic efficacy of single ASC treatment. Overall, these
findings indicated that the GelMA/ASC implant is a promising
therapeutic strategy for SCI.
Cell apoptosis is a very common phenomenon following
SCI. It occurs when cells survive from initial trauma, yet endure
sufficient insult, which activates relevant apoptotic pathways.
Generally, in rat SCI, cell apoptosis occurs as early as 4 h
following SCI and peaks on the 7th day (30,31).
In the present study, to examine the anti-apoptotic effects of ASCs
and GelMA/ASCs, the injured spinal cord was collected on the 7th
day following SCI and TUNEL staining was performed. Compared with
the SCI group, the number of TUNEL-positive cells in the treatment
group (the ASC and GelMA/ASC groups) decreased significantly.
Neural survival is the prerequisite of neural regeneration. Thus,
these results may explain why the lesion areas in the treatment
group were reduced. Moreover, the numbers of TUNEL-positive cells
in the GelMA/ASC group were lower than those in the ASC group which
may be attributed to the beneficial effects of the GelMA hydrogel
on the ASCs and injured spinal cord. Furthermore, the Bcl-2 gene
has an anti-apoptotic effect and its overexpression can inhibit the
occurrence of cell apoptosis (32,33).
In the present study, the results of immunochemistry revealed
similar trends as those observed with TUNEL staining. On the whole,
ASCs and GelMA/ASCs may alleviate SCI by attenuating cell
apoptosis.
MAPK is a type of serine/threonine protein kinase
that plays crucial roles in signal transduction from the cell
surface to the nucleus, thereby regulating cell fate
(proliferation, apoptosis and differentiation) (34,35).
There are three important members in the MAPK family, including
p38, ERK1/2 and JNK1/2 and they are known to mediate various
cellular responses in the form of phosphorylation (36). It has been well documented that the
activation of the MAPK pathway is involved in the inflammatory
response, gliosis and apoptosis following SCI (37,38).
In the present study, the protein expression of the MAPK family was
significantly increased following SCI, which is consistent with the
findings of a previous study (24). However, transplantation treatment
could not inhibit the activation of ERK1/2 and JNK1/2 signaling,
while the protein expression of p38 was significantly decreased.
Previous research has demonstrated that p38 MAPK exerts a growth
inhibitory effect (39). Thus, the
findings of the present study indicated that GelMA/ASC implants can
inhibit p38 MAPK pathway activation to reduce cell apoptosis,
thereby protecting the injured spinal cord.
In the present study, ASCs and GelMA hydrogel were
utilized to create a tissue engineering scaffold. The
cell-containing scaffold displayed favorable biocompatibility and
inhibited cell apoptosis, promoting tissue remolding following
transplantation in vivo. Furthermore, this tissue
engineering scaffold promoted motor functional recovery in rats
with SCI. The present study demonstrated that GelMA/ASC scaffolds
may prove to be a clinically effective strategy for the treatment
of SCI in the future. However, several limitations in the present
study need to be acknowledged. Firstly, the present study did not
perform experiments to analyze the comparison between NSCs and
ASCs; however, relevant results were included in previously
published studies (22). Secondly,
the therapeutic efficacy of GelMA/ASCs scaffolds was not further
verified in some larger mammals and the images or videos of rat's
behavior tests were also not recorded timely in this study. Lastly,
certain other pathophysiology changes following SCI, such as
inflammation, angiogenesis and remyelination need to be further
elucidated. Therefore, in the future, the authors aim to focus on
the underlying mechanisms of GelMA/ASCs in the treatment of SCI and
to further verify the therapeutic efficacy of GelMA/ASCs in animal
models of SCI using larger mammals.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81972061,
81871766 and 81902216).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and HY contributed to the study design and data
analysis. HY wrote the manuscript. YL and PY performed the majority
of the experiments. PP, CL and ZX assisted with experiments, data
analysis and interpretation as well as contributing to the critical
revision of the manuscript for intellectual content. DB made
substantial contributions to conception and design as well as
giving final approval of the version to be published. HY, YL and PY
were equal contributors to the study. YL and DB confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
MDL20210810-01) by the Ethics Committee of The Tianjin Medical
University General Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahuja CS, Wilson JR, Nori S, Kotter MRN,
Druschel C, Curt A and Fehlings MG: Traumatic spinal cord injury.
Nat Rev Dis Primers. 3(17018)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou
X, Zhou H, Ning G, Kong X and Feng S: Microenvironment imbalance of
spinal cord injury. Cell Transplant. 27:853–866. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ahuja CS, Nori S, Tetreault L, Wilson J,
Kwon B, Harrop J, Choi D and Fehlings MG: Traumatic spinal cord
injury-repair and regeneration. Neurosurgery. 80 (3S):S9–S22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Courtine G and Sofroniew MV: Spinal cord
repair: Advances in biology and technology. Nat Med. 25:898–908.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Assinck P, Duncan GJ, Hilton BJ, Plemel JR
and Tetzlaff W: Cell transplantation therapy for spinal cord
injury. Nat Neurosci. 20:637–647. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Zhou P, Guan J, Xu P, Zhao J, Zhang C,
Zhang B, Mao Y and Cui W: Cell therapeutic strategies for spinal
cord injury. Adv Wound Care (New Rochelle). 8:585–605.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Piltti KM, Funes GM, Avakian SN, Salibian
AA, Huang KI, Carta K, Kamei N, Flanagan LA, Monuki ES, Uchida N,
et al: Increasing human neural stem cell transplantation dose
alters oligodendroglial and neuronal differentiation after spinal
cord injury. Stem Cell Reports. 8:1534–1548. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nicaise AM, Banda E, Guzzo RM, Russomanno
K, Castro-Borrero W, Willis CM, Johnson KM, Lo AC and Crocker SJ:
iPS-derived neural progenitor cells from PPMS patients reveal
defect in myelin injury response. Exp Neurol. 288:114–121.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu S, Schackel T, Weidner N and
Puttagunta R: Biomaterial-Supported cell transplantation treatments
for spinal cord injury: Challenges and perspectives. Front Cell
Neurosci. 11(430)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marquardt LM, Doulames VM, Wang AT, Dubbin
K, Suhar RA, Kratochvil MJ, Medress ZA, Plant GW and Heilshorn SC:
Designer, injectable gels to prevent transplanted Schwann cell loss
during spinal cord injury therapy. Sci Adv.
6(eaaz1039)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Assunção-Silva RC, Gomes ED, Sousa N,
Silva NA and Salgado AJ: Hydrogels and cell based therapies in
spinal cord injury regeneration. Stem Cells Int.
2015(948040)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jessen KR, Mirsky R and Lloyd AC: Schwann
cells: Development and role in nerve repair. Cold Spring Harb
Perspect Biol. 7(a020487)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feltri ML, Poitelon Y and Previtali SC:
How Schwann cells sort axons: New concepts. Neuroscientist.
22:252–265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hill CE, Moon LD, Wood PM and Bunge MB:
Labeled Schwann cell transplantation: Cell loss, host Schwann cell
replacement, and strategies to enhance survival. Glia. 53:338–343.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen
JT, Zheng YF, Ban DX, Liu T, Li H and Wang P: Transplantation of
autologous activated Schwann cells in the treatment of spinal cord
injury: Six cases, more than five years of follow-up. Cell
Transplant. 21 (Suppl 1):S39–S47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Monje PV, Deng L and Xu XM: Human Schwann
cell transplantation for spinal cord injury: Prospects and
challenges in translational medicine. Front Cell Neurosci.
15(690894)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yue K, Trujillo-de Santiago G, Alvarez MM,
Tamayol A, Annabi N and Khademhosseini A: Synthesis, properties,
and biomedical applications of gelatin methacryloyl (GelMA)
hydrogels. Biomaterials. 73:254–271. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khayat A, Monteiro N, Smith EE, Pagni S,
Zhang W, Khademhosseini A and Yelick PC: GelMA-encapsulated hDPSCs
and HUVECs for dental pulp regeneration. J Dent Res. 96:192–199.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nichol JW, Koshy S, Bae H, Hwang CM,
Yamanlar S and Khademhosseini A: Cell-laden microengineered gelatin
methacrylate hydrogels. Biomaterials. 31:5536–5544. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan L, Liu C, Chen X, Zou Y, Zhou Z, Lin
C, Tan G, Zhou L, Ning C and Wang Q: Directing induced pluripotent
stem cell derived neural stem cell fate with a three-dimensional
biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater
Interfaces. 10:17742–17755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou P, Xu P, Guan J, Zhang C, Chang J,
Yang F, Xiao H, Sun H, Zhang Z, Wang M, et al: Promoting 3D
neuronal differentiation in hydrogel for spinal cord regeneration.
Colloids Surf B Biointerfaces. 194(111214)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shi GD, Cheng X, Zhou XH, Fan BY, Ren YM,
Lin W, Zhang XL, Liu S, Hao Y, Wei ZJ and Feng SQ: iTRAQ-based
proteomics profiling of Schwann cells before and after peripheral
nerve injury. Iran J Basic Med Sci. 21:832–841. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duan HQ, Wu QL, Yao X, Fan BY, Shi HY,
Zhao CX, Zhang Y, Li B, Sun C, Kong XH, et al: Nafamostat mesilate
attenuates inflammation and apoptosis and promotes locomotor
recovery after spinal cord injury. CNS Neurosci Ther. 24:429–438.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou XH, Lin W, Ren YM, Liu S, Fan BY, Wei
ZJ, Shi GD, Cheng X, Hao Y and Feng SQ: Comparison of DNA
methylation in Schwann cells before and after peripheral nerve
injury in rats. Biomed Res Int. 2017(5393268)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Marcol W, Ślusarczyk W, Larysz-Brysz M,
Francuz T, Jędrzejowska-Szypułka H, Łabuzek K and Lewin-Kowalik J:
Grafted activated Schwann cells support survival of injured rat
spinal cord white matter. World Neurosurg. 84:511–519.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Katoh H, Yokota K and Fehlings MG:
Regeneration of spinal cord connectivity through stem cell
transplantation and biomaterial scaffolds. Front Cell Neurosci.
13(248)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Malikmammadov E, Tanir TE, Kiziltay A,
Hasirci V and Hasirci N: PCL and PCL-based materials in biomedical
applications. J Biomater Sci Polym Ed. 29:863–893. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xiao S, Zhao T, Wang J, Wang C, Du J, Ying
L, Lin J, Zhang C, Hu W, Wang L and Xu K: Gelatin methacrylate
(GelMA)-based hydrogels for cell transplantation: An effective
strategy for tissue engineering. Stem Cell Rev Rep. 15:664–679.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li GL, Farooque M and Olsson Y: Changes of
Fas and Fas ligand immunoreactivity after compression trauma to rat
spinal cord. Acta Neuropathol. 100:75–81. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park E, Liu Y and Fehlings MG: Changes in
glial cell white matter AMPA receptor expression after spinal cord
injury and relationship to apoptotic cell death. Exp Neurol.
182:35–48. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen DF and Tonegawa S: Why do mature CNS
neurons of mammals fail to re-establish connections following
injury-functions of bcl-2. Cell Death Differ. 5:816–822.
1998.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang C, Zhang L, Ndong JC, Hettinghouse A,
Sun G, Chen C, Zhang C, Liu R and Liu CJ: Progranulin deficiency
exacerbates spinal cord injury by promoting neuroinflammation and
cell apoptosis in mice. J Neuroinflammation. 16(238)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Crown ED, Gwak YS, Ye Z, Johnson KM and
Hulsebosch CE: Activation of p38 MAP kinase is involved in central
neuropathic pain following spinal cord injury. Exp Neurol.
213:257–267. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kasuya Y, Umezawa H and Hatano M:
Stress-activated protein kinases in spinal cord injury: Focus on
roles of p38. Int J Mol Sci. 19(867)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhan J, He J, Chen M, Luo D and Lin D:
Fasudil promotes BMSC migration via activating the MAPK signaling
pathway and application in a model of spinal cord injury. Stem
Cells Int. 2018(9793845)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen NN, Wei F, Wang L, Cui S, Wan Y and
Liu S: Tumor necrosis factor alpha induces neural stem cell
apoptosis through activating p38 MAPK pathway. Neurochem Res.
41:3052–3062. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qian Z, Chang J, Jiang F, Ge D, Yang L, Li
Y, Chen H and Cao X: Excess administration of miR-340-5p
ameliorates spinal cord injury-induced neuroinflammation and
apoptosis by modulating the P38-MAPK signaling pathway. Brain Behav
Immun. 87:531–542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang
J, Xia K, Liang C, Fang W, Zhou C and Tao H: Escin induces
caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK
signalling pathway in human osteosarcoma cells in vitro and in
vivo. Cell Death Dis. 8(e3113)2017.PubMed/NCBI View Article : Google Scholar
|