Introduction
Diabetes mellitus (DM) is a common disease worldwide
(1). According to the 10th edition
of the IDF Diabetes Atlas published in 2021, >537 million adults
aged 20-79 years are living with diabetes and a continuous increase
in DM prevalence is predicted (2).
This disease is characterized by uncontrolled and elevated blood
glucose levels due to inadequate insulin secretion by the β cells
of the pancreatic islets of Langerhans (3).
The pancreas is an organ in mammals that is
comprised of α and β cells, and DM is associated with dysfunction
of the β cells (3). DM can be
categorized into type 1 diabetes (T1DM) and type 2 diabetes (T2DM)
(3-5).
T1DM is an autoimmune disorder that accounts for 5-10% of cases of
diabetes and involves the destruction of β cells in the pancreas
(5). This destruction results in a
deficiency of insulin, a peptide hormone, which leads to
insufficient glucose uptake by cells and increased blood glucose
levels (6,7). T2DM is a progressive metabolic
disorder that involves insulin resistance (8-10).
In the early stage of T1DM, insulin is normally secreted by β
cells, but the abnormal and increased demand for insulin in the β
cells results in insulin failure (11,12).
There are numerous therapies for T1DM and T2DM, but none are able
to cure the disease. Moreover, the complications of diabetes can be
serious, although there are therapeutic agents that can be used to
address these complications. For example, diabetic retinopathy is a
complication that may result in a significant reduction in visual
acuity (13), and results from
neovascularization in the retina and cornea (14,15).
In clinical studies (16-19),
DM has been shown to reduce tear film quality and impair corneal
sensation, and diabetic patients may experience discomfort,
particularly dry eye disease (DED). According to a previous study,
diabetic patients suffer autonomic dysfunction caused by chronic
inflammation, which can manifest as DED (19).
DED is a common ocular disease characterized by
itching, discomfort, pain and impaired tear production (20,21).
DED has various causes, such as computer use, contact lenses,
infection, age and environmental damage (22). The eyes of mammals comprise the
cornea, iris, lens, retina, choroid, sclera, conjunctiva and ocular
adnexa, including the lacrimal gland, lacrimal canaliculus,
lacrimal sac and nasolacrimal duct (23). Increased angiogenesis is observed
in the cornea and lacrimal gland of patients with DED, which is
comparable with that in diabetic retinopathy (24,25).
As DED progresses, the cornea becomes thinner and the ocular
surface becomes more sensitive to the external environment
(26). In this manner, ocular
cells can be damaged and lose their function (27). The cells of the lacrimal gland that
secrete the aqueous tear fluid may also be damaged (28). Current therapies for DED include
oily eye drops, preservative-free drops, anti-inflammatory
treatments and surgery (28-30).
Reactive oxygen species (ROS) and nicotinamide
adenine dinucleotide phosphate (NADPH) oxidase contribute to
diabetes complications (31-33),
and NADPH oxidase (NOX) species also play important roles in the
generation of ROS in diabetes (34,35).
NOX species are enzymes that have the capacity to transport
electrons across the plasma membrane and to generate superoxide and
other downstream ROS (34). NOX
species include NOX1-5 and dual oxidase 1/2(36). ROS are upregulated due to sunlight,
microbial antigens, pollutants, hormone and age, and are
downregulated by various antioxidant proteins (37,38).
However, if the signaling pathways associated with the antioxidant
defense are disrupted, ROS levels increase and damage occurs to the
tear lipid layer as well as epithelial and goblet cells in ocular
surface, which can induce vascular endotheliopathy, corneal
neuropathy and reduced tear secretion (39,40).
As oxidative stress upregulates ROS and NOX,
antioxidant molecules such as carnosine and hyaluronic acid are
potential treatments for the reduction of ROS and NOX levels
(41,42). Previous studies of the pan-NOX
inhibitor APX-115A have demonstrated its potential as a therapeutic
agent for diabetes in mouse models. Based on this, the present
study aimed to investigate the potential of APX-115A as a therapy
for dry eye syndrome due to diabetes as an alternative to other
models involving an impaired evaporative tear film (43-45).
Moreover, it has been reported that the inhibition of ROS by
treatment with APX-115A in Epstein Barr virus-infected ARPE-19
cells induces caspase-dependent apoptosis by activating the ERK-JNK
pathway (46). In the present
study, the effects of APX-115A on NOX2 expression were evaluated in
a diabetic rat model (47). NOX2
is the phagocyte NADPH oxidase that is secreted by the cornea and
lacrimal gland and is activated during phagocytosis to produce
superoxide (48), while NOX4 is a
phagocyte-type oxidase that modulates the ROS level in diabetes
(49). Therefore, the present
study investigated whether DED induced by diabetes can be relieved
by the pan-NOX inhibitor, APX-115A, and evaluated the efficacy of
APX-115A as a downregulator of ROS levels in a diabetic rat model
(45).
Materials and methods
Reagents
Streptozotocin (STZ) and 0.1 M citrate buffer were
obtained from Sigma-Aldrich (Merck KGaA) and BioPrince
(Tech&Innovation), respectively. Primary antibodies against
NOX2 were obtained as a gift from Professor Bae of Ewha Woman's
University (Seoul, South Korea). APX-115A, a pan-NADPH oxidase
inhibitor, was synthesized and provided by AptaBio Therapeutics
Incorporation.
Animals and eye drop treatment
Male Sprague Dawley rats (6 weeks of age, >200 g)
were purchased from Orient Bio Inc. The animal study was granted
and approved by the Institutional Animal Care and Use Committee of
Inje University College of Medicine (Busan, Korea; approval no.
2016-11), and all procedures were conducted in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. All rats were kept on a 12-h alternating
light/dark cycle at 22±2˚C and 55-60% humidity. All animals had
free access to sterile food and sterile tap water during this
study. For the study, the rats were divided into four groups as
follows: Normal saline (n=7), normal rats treated with normal
saline; normal APX-115A (n=7), normal rats treated with APX-115A;
diabetic saline (n=7), diabetic rats treated with normal saline;
and diabetic APX-115A (n=7), diabetic rats treated with APX-115A.
Diabetes was induced by the intraperitoneal injection of 60 mg/kg
STZ in a citrate buffer (100 mM sodium citrate and 100 mM citric
acid, pH 4.5) in a single dose after the rats had fasted for 6 h
(50). The body weights and blood
glucose levels of the rats were measured once a week for 6 weeks.
APX-115A was dissolved in normal saline at a concentration of 10
mg/ml and 20 µl was applied to the rat eyeballs three times each
day (at 9:00, 13:00 and 18:00) for 1 month.
Tear volume measurements
Reduced tear volume is one of the main
characteristics of DE syndrome (21). To determine whether APX-115A
prevents reduced tear volume in STZ-induced diabetic rats, tear
volume was measured using a phenol red thread (PRT) tear test with
Zone-Quick (Menicon Co., Ltd.) (51). The thread was placed into the
palpebral conjunctiva for 15 sec, and then the entire length of the
red portion of the thread was measured. Tear volume was measured
once a week for 1 month. All rats were sacrificed humanely at 6
weeks after STZ injection using a CO2 chamber with a
CO2 fill rate of 60% chamber volume/min (chamber volume,
15.73 liters; flow rate, 9.44 l/min). The lacrimal glands and
eyeballs were collected and the lacrimal glands were weighed.
Hematoxylin and eosin (H&E)
staining
The eyeballs and lacrimal gland tissues were fixed
in 4% neutral formaldehyde solution at 4˚C overnight and formed
into paraffin-embedded blocks using a Tissue Processor (TP 1020;
Leica Microsystems, Inc.) according to a programming worksheet
(alcohol and xylene series; starting in 70% alcohol for 1 h and
ending in xylene for 2 h) and embedding system (EG1150 model;
embedding temperature, 65˚C). The blocks were sectioned into 7-µm
cross sections and subjected to H&E staining using an
Autostainer (Autostainer RSt model; Vision BioSystems, Inc.)
according to the programming worksheet (briefly staining with alum
hematoxylin for 20 min, followed by washing steps, and staining
with 1% eosin Y for 5 min). The corneal thickness was measured
using Image viewing software (Japan NDP.view2; Hamamatsu Photonics
K.K.) after slide images were digitized with a Nanozoomer Virtual
Microscope (Hamamatsu Photonics K.K.).
Immunohistochemistry
After deparaffinization (briefly, sections were
treated with xylene for 2 h, and then rehydration processing was
performed with a descending alcohol series at room temperature) and
antigen blocking using antigen retrieval solution (cat. no. S2369;
Dako; Agilent Technologies, Inc.) for 30 min at room temperature
according to the manufacturer's instructions, the samples were
incubated with anti-NOX2 (1:200 dilution) antibodies at 37˚C for
1.5 h. After washing three times with TBS, the samples were
incubated with peroxidase-conjugated goat anti-mouse/rabbit IgG
antibody (1:500 dilution; K5007 EnVision Detection System; Dako;
Agilent Technologies, Inc.) at 37˚C for 1 h. DAB was used to
visualize the target NOX2, while Meyer's hematoxylin was used for 5
min at room temperature (Dako; Agilent Technologies, Inc.) to
visualize the nucleus. The images were digitized with a Hamamatsu
Nanozoomer Virtual Microscope (Hamamatsu Photonics K.K.) and
visualized at high resolution with the aid of NDP View2 software
(Hamamatsu Photonics K.K.).
Statistical analysis
DAB-positive areas (NOX2-positive areas) were
measured using ImageJ 1.53 software (National Institutes of Health)
and percentages of area are expressed as the mean ± standard error
of the mean. For all comparisons among multiple groups, data were
analyzed using a two-way ANOVA with Bonferroni post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
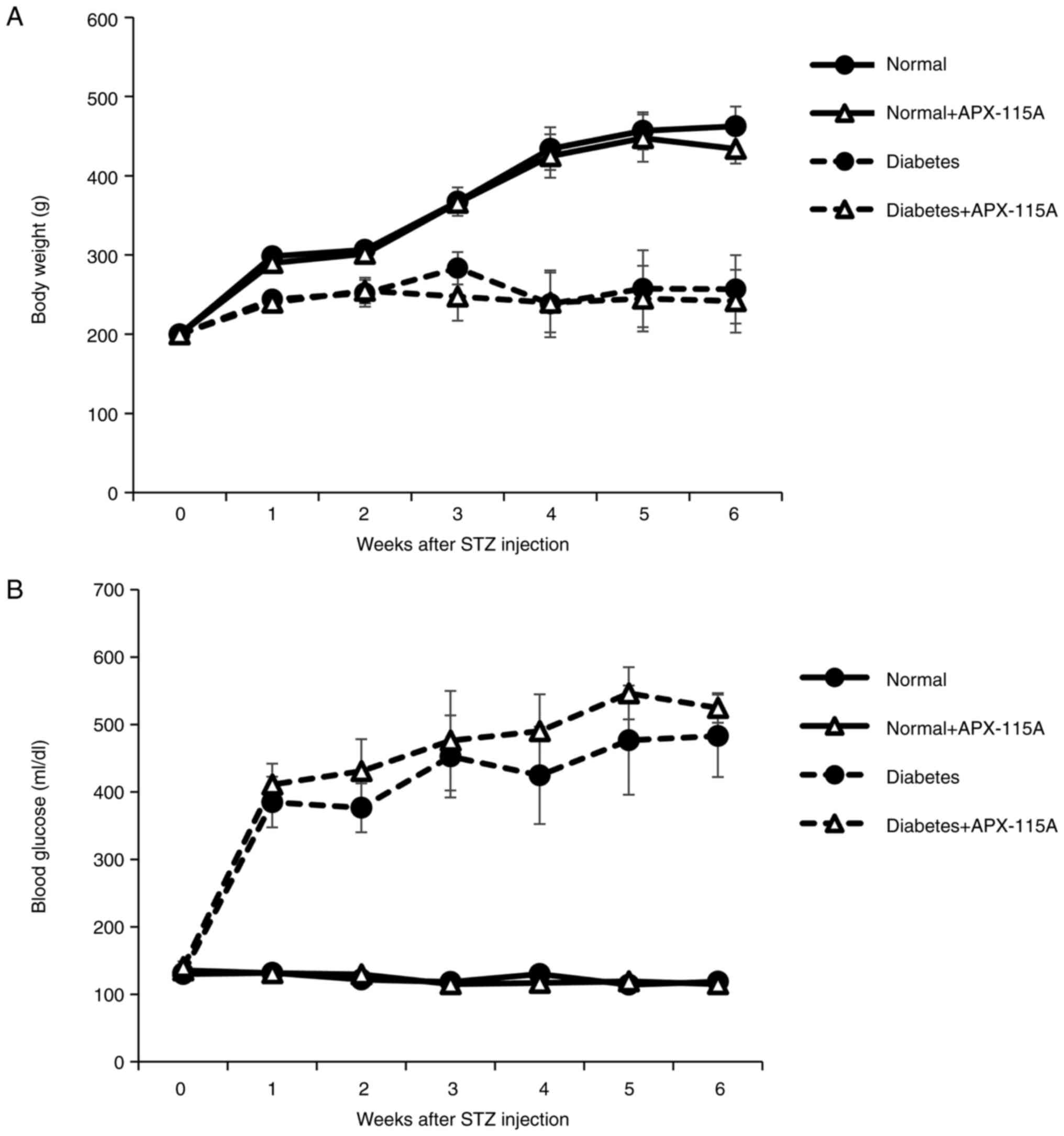
APX-115A has no effect on body weight
or blood glucose level
To determine whether APX-115A affected body weight
and blood glucose in an STZ-induced diabetic model, rats were
induced to develop DM using STZ in a citrate buffer by
intraperitoneal injection (50).
After the induction of DM, APX-115A was applied to the rat eyes in
drop form three times a day for 1 month. The body weights and blood
glucose levels of the rats were measured once a week for 1 month
(Fig. 1). The results showed
reduced body weights (Fig. 1A) and
increased blood glucose levels (Fig.
1B) in the diabetic rats compared with the normal rats. No
differences in body weight or blood glucose level were observed
between the diabetic controls and the diabetic rats treated with
APX-115A. These results demonstrate that APX-115A treatment had no
effect on the body weights and blood glucose levels of the
rats.
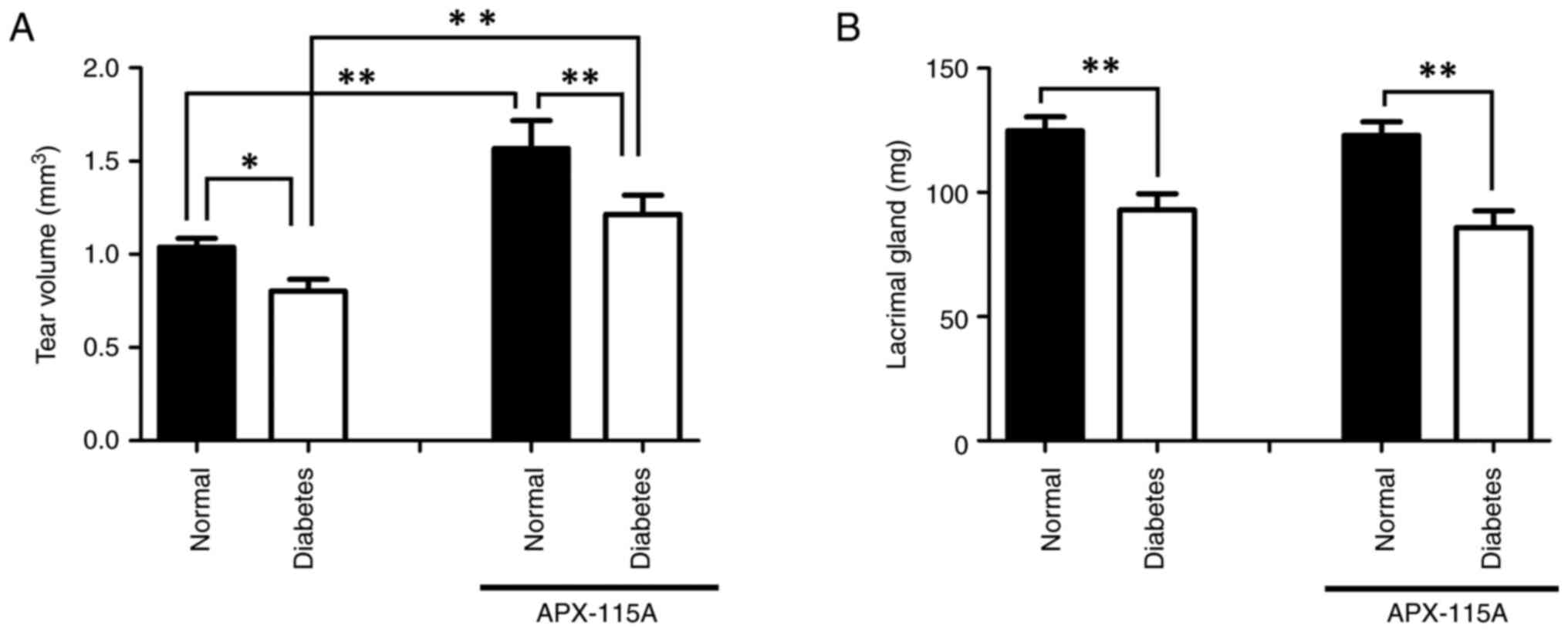
APX-115A prevents reduced tear volume
in STZ-induced diabetic rat models
Reduced tear volume is one of the main
characteristics of DE syndrome (21). To determine whether APX-115A
affects the reduced tear volume in STZ-induced diabetic rats, tear
volume was measured using a PRT tear test. Tear volume was reduced
in the diabetic control group compared with the normal control
group; however, there was a significant increase in tear volume in
the normal and diabetic rats treated with APX-115A compared with
the respective saline-treated controls (Fig. 2A). The weights of the lacrimal
glands were decreased in both diabetic groups compared with the
respective saline-treated controls, and APX-115A treatment
exhibited no significant effect (Fig.
2B). These results indicate that APX-115A administration
increased the tear volume of the rats but had no effect on lacrimal
gland size.
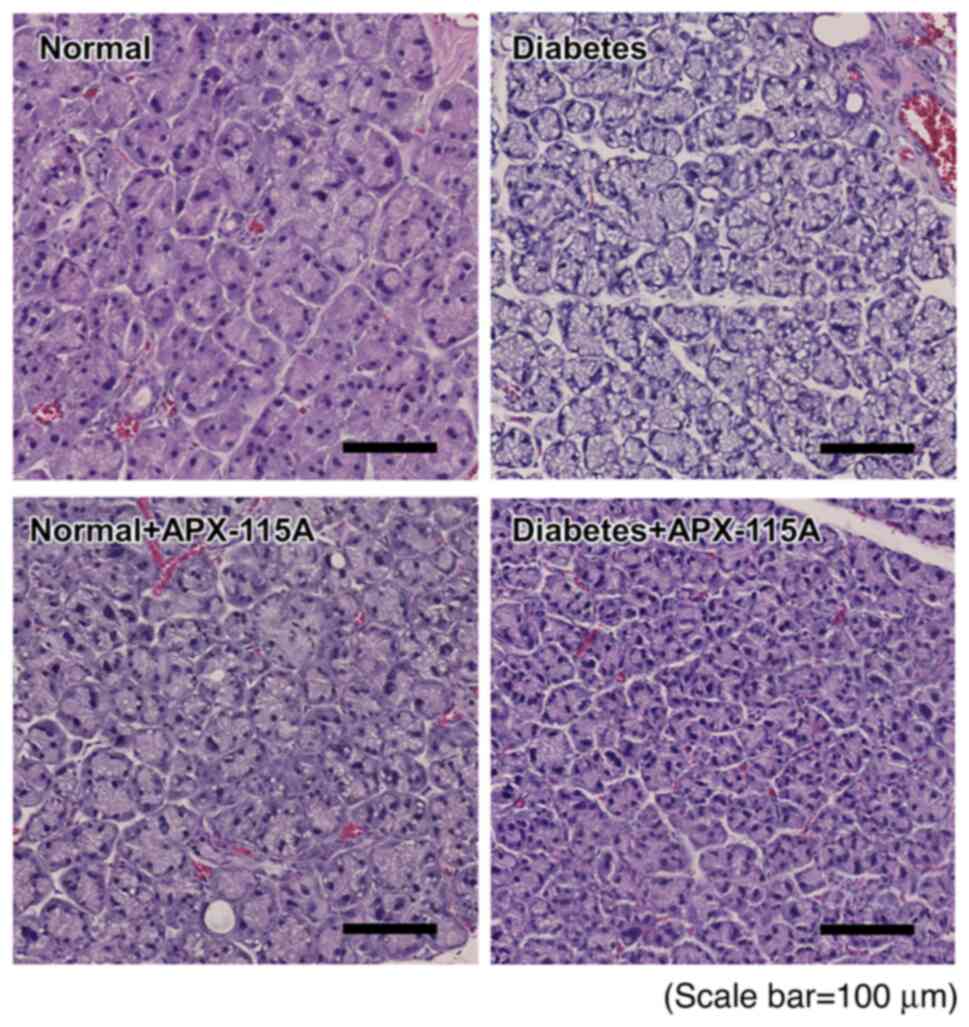
APX-115A restores morphological
changes in lacrimal glands induced by DM
To investigate how ocular mucosal inflammation is
affected by DED (52,53), the morphologies of the lacrimal
glands were examined using H&E staining. The lacrimal glands of
the diabetic controls contained numerous vacuoles and exhibited
partial acinar atrophy, but these morphological changes were
ameliorated in the diabetic rats treated with APX-115A (Fig. 3). These results indicate that
APX-115A inhibited the lacrimal gland from forming vacuoles and
undergoing acinar atrophy.
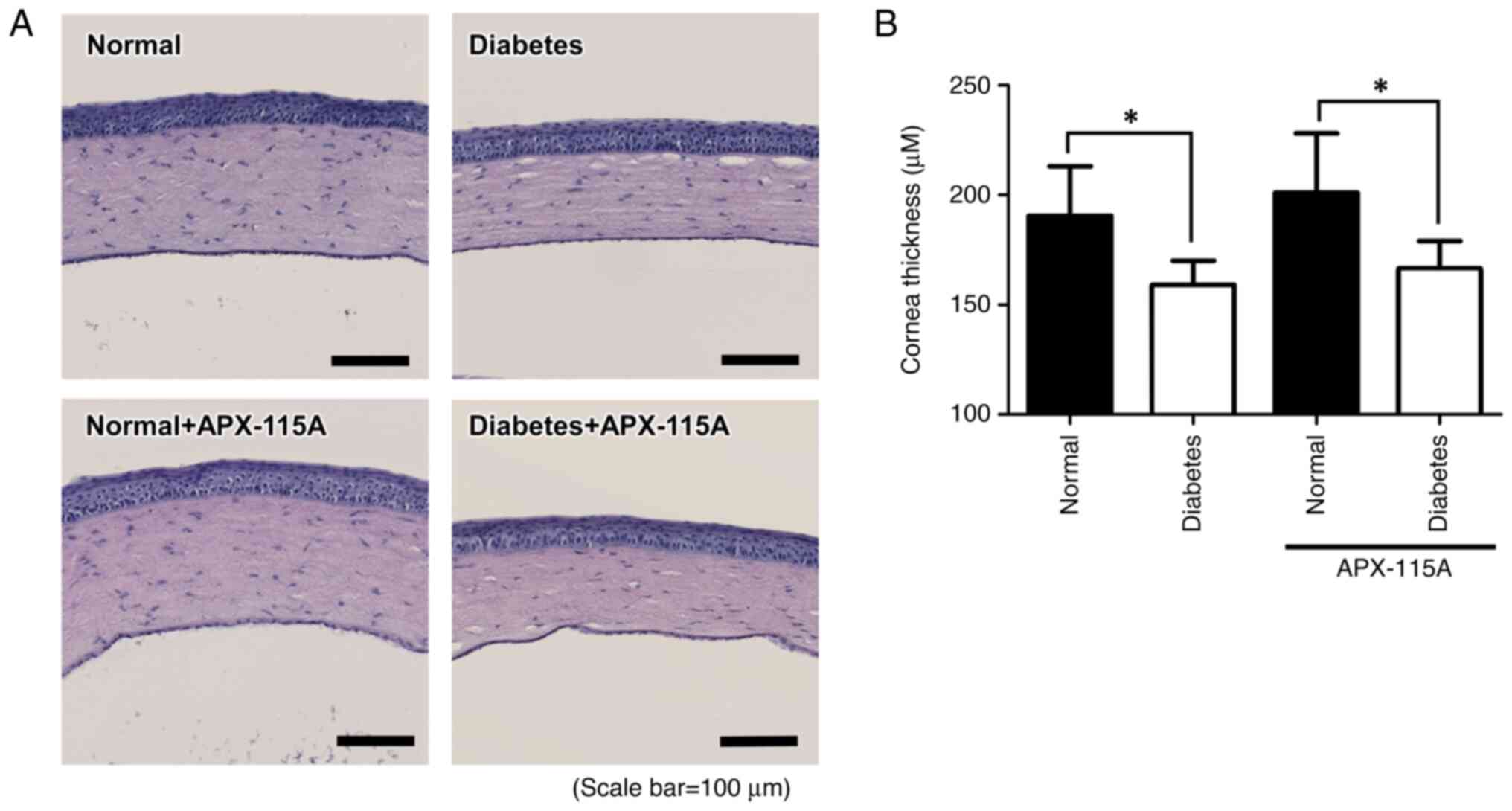
APX-115A does not recover corneal
thickness
Corneal thickness is significantly decreased in DED
(54). To determine whether
treatment with APX-115A affected the corneal thickness in
STZ-induced diabetic rats, sectioned eyeball tissues were examined
by H&E staining. The corneal stromal layers in the STZ-induced
diabetic rats were decreased compared with those in the respective
normal rats. APX-115A treatment did not attenuate the reduction in
corneal thickness (Fig. 4). These
results revealed that the corneal thickness of the eyeballs from
rats with DM-associated DED was reduced, and APX-115A had no effect
on corneal thickness.
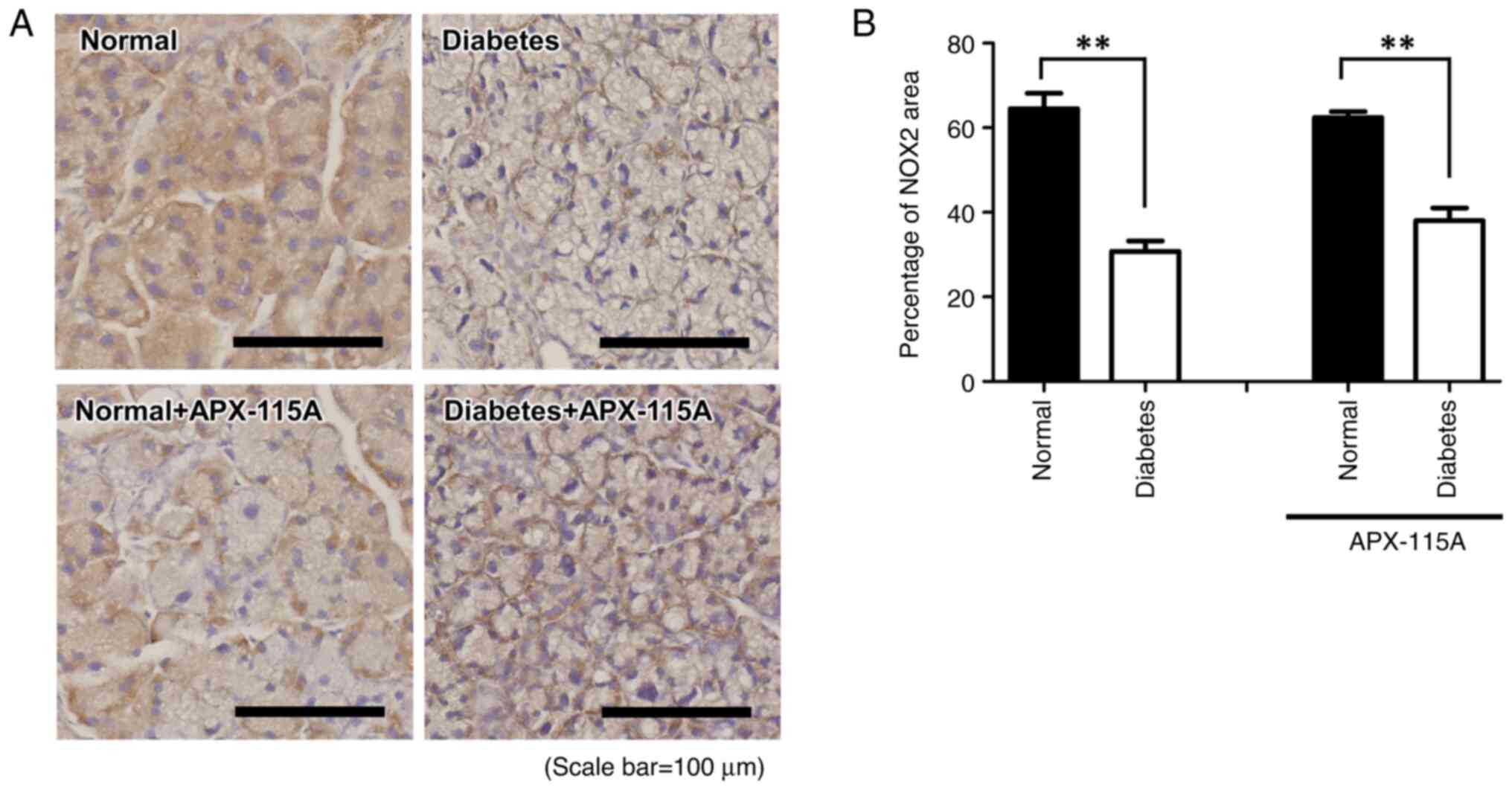
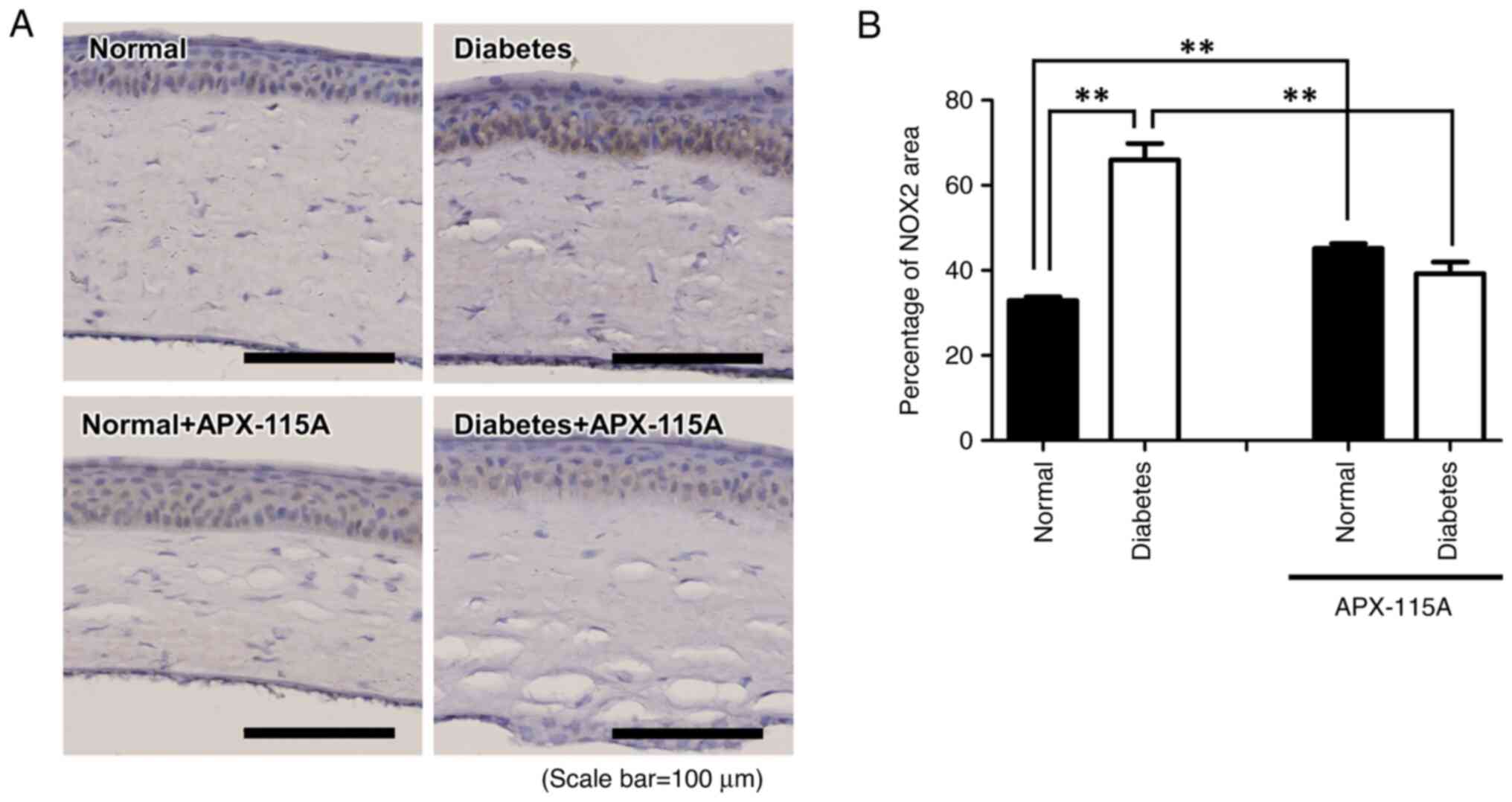
APX-115A reduces corneal NOX2
levels
To evaluate whether APX-115A reduced NADPH oxidase
levels and thereby ameliorated ocular inflammation, sectioned
lacrimal gland and eyeball tissues were subjected to NOX2
immunochemistry (Figs. 5 and
6). In the lacrimal gland, the
level of NOX2 was significantly lower in the diabetic controls
compared with the normal controls, and no significant difference
was detected between the diabetic controls and the diabetic rats
treated with APX-115A (Fig. 5). In
contrast with the results for the lacrimal gland, the level of NOX2
was increased in the epithelial layer of the cornea in diabetic
rats compared with the normal controls, and APX-115A treatment
significantly attenuated the diabetes-induced increase in NOX2
level (Fig. 6).
Discussion
DM is a common disease and has an increasing
incidence (2). This disease is
associated with numerous complications, including diabetic
retinopathy, diabetic kidney disease and diabetic neuropathy.
Treatments for these complications are limited, which has prompted
research aiming to address this deficiency. DED syndrome is a
complication of DM, which is associated with dysfunction of the
corneal layers and lacrimal glands. Due to various causes (mainly
dysfunction of meibomian glands, aging, ocular and general
diseases, including DM, contact lens wear and adverse environment
exposure), the lipid layer and aqueous layer of the tear film lose
thickness, and the epithelial layer forms new blood vessels
(55-57).
APX-115 is a pan-NOX inhibitor that has been
investigated as a treatment for diabetic complications (45,58).
The present study investigated the efficacy of APX-115A as a
treatment for DED in diabetic rats. The STZ-induced diabetic rat is
a good model for diabetes because it demonstrates various symptoms
in a short period of time, including hyperglycemia and reduced body
weight. Moreover, the STZ-induced diabetic rat model has been used
in numerous studies such as those investigating diabetic
retinopathy and DM pathogeneses, including endoplasmic reticulum
stress and oxidative stress (59-61).
APX-115A had no effects on blood glucose level and body weight in
the present study, which is in accordance with a previous study
(58). However, APX-115A increased
the tear volume compared with that in the saline-treated controls.
Previous studies have shown that ROS inhibitors such as APX-115A
can restore tear secretion to a normal level (45,62-64).
Furthermore, pathological alterations were detected in the cornea
and lacrimal glands of the diabetic rat models. The relationship
between changes in cornea thickness and diabetic dry eye remains
unclear, but studies have demonstrated that reduced corneal
epithelial thickness is a diabetic complication (65,66).
However, in the present study, the change in corneal thickness in
diabetic rats was not reversed, although the intracellular vacuoles
and acinar atrophy were ameliorated by APX-115A treatment in the
diabetic rats. These observations indicate that APX-115A can
prevent DED at an early stage.
Oxidative stress, such as that produced by NOX and
ROS, plays a vital role in diabetic complications. NOX2 is an NADPH
oxidase isoform (47). Increased
expression of NOX2 has been observed in cells with a high ROS level
(46). In the present study, NOX2
levels in sectioned corneal and lacrimal gland tissues were
evaluated using immunohistochemistry. Positive effects of APX-115A
on NOX2 were noted, as the level of NOX2 in the corneal epithelium
of APX-115A-treated diabetic rats was decreased compared with that
in the saline-treated diabetic group. The expression of NOX2 in the
lacrimal gland was particularly reduced in the proximity of blood
vessels. Although the corneas were directly treated with APX-115A
using an eye drop method, it is challenging to apply APX-115A
directly to the lacrimal gland. This may explain why the NOX2
expression level in the cornea was strongly regulated while that in
the lacrimal gland was weakly regulated in diabetic rats treated
with APX-115A. Moreover, it has previously been shown that
meibomian glands, which are important for maintenance of the tear
film and are associated with the condition and integrity of the
ocular surface (67), are
influenced by APX-115A eye drops, which protect the tear film
structure and attenuate DED (68).
The conjunctiva is an important physical barrier for protecting
against ocular inflammation (69).
Morphological changes of the conjunctiva have been demonstrated due
to dry eye in diabetic rats (70),
but APX-115A has little effect on the conjunctiva. Further studies
are required to evaluate the effects of APX-115A on conjunctival
inflammation in diabetic dry eye.
In summary, the present study showed that APX-115A
protects against the early stages of DED due to diabetes by
inhibiting NOX2 expression in the cornea and attenuating
morphological changes of the lacrimal glands.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a 2019 Inje
University research grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
SYH, SHM and DYH were responsible for
conceptualization, and for writing, reviewing and editing the
manuscript. MHN and DKL were responsible for data collection. MHN
and YSK performed the formal analysis. SYH acquired funding. MHN
performed the experiments. SHM and DYH were responsible for study
design. MHN wrote the original draft of the manuscript. HYK
performed statistical analysis and revised the manuscript. MHN and
DYH confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were granted and approved by
the Institutional Animal Care and Use Committee of Inje University
College of Medicine (approval number: 2016-11), and all procedures
were conducted in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
SHM is an employee of Aptabio Therapeutics Inc.,
Republic of Korea, which is developing APX-115A. The remaining
authors declare that they have no competing interests.
References
|
1
|
Amos AF, McCarty DJ and Zimmet P: The
rising global burden of diabetes and its complications: Estimates
and projections to the year 2010. Diabet Med. 14 (Suppl 5):S1–S85.
1997.PubMed/NCBI
|
|
2
|
Ogurtsova K, Guariguata L, Barengo NC,
Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ and Magliano DJ:
IDF diabetes Atlas: Global estimates of undiagnosed diabetes in
adults for 2021. Diabetes Res Clin Pract.
183(109118)2022.PubMed/NCBI View Article : Google Scholar
|
|
|
Lovic D, Piperidou A, Zografou I, Grassos
H, Pittaras A and Manolis A: The growing epidemic of diabetes
mellitus. Curr Vasc Pharmacol. 18:104–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen C, Cohrs CM, Stertmann J, Bozsak R
and Speier S: Human beta cell mass and function in diabetes: Recent
advances in knowledge and technologies to understand disease
pathogenesis. Mol Metab. 6:943–957. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Memon B and Abdelalim EM: Stem cell
therapy for diabetes: Beta cells versus pancreatic progenitors.
Cells. 9(283)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cnop M, Welsh N, Jonas JC, Jörns A, Lensen
S and Eizirik DL: Mechanisms of pancreatic beta-cell death in type
1 and type 2 diabetes: Many differences, few similarities.
Diabetes. 54 (Suppl 2):S97–S107. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jean-Baptiste VSE, Xia CQ, Clare-Salzler
MJ and Horwitz MS: Type 1 diabetes and type 1 interferonopathies:
Localization of a type 1 common thread of virus infection in the
pancreas. EBioMedicine. 22:10–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tomita T: Apoptosis of pancreatic β-cells
in Type 1 diabetes. Bosn J Basic Med Sci. 17:183–193.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Al-Mrabech A: β-Cell dysfunction, hepatic
lipid metabolism, and cardiovascular health in type 2 diabetes: New
directions of research and novel therapeutic strategies.
Biomedicines. 9(226)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu H and Mahato RI: Mesenchymal stem
cell-based therapy for type 1 diabetes. Discov Med. 17:139–143.
2014.PubMed/NCBI
|
|
10
|
Hopkins M, Beaulieu K and Finlayson G:
Psychobiology of appetite and food reward in adults with type 1 and
type 2 diabetes: Is there a role for exercise? Can J Diabetes.
44:768–774. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zand H, Morshedzadeh N and Naghashian F:
Signaling pathways linking inflammation to insulin resistance.
Diabetes Metab Syndr. 11 (Suppl 1):S307–S309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu H, Du X, Xu J, Zhang Y, Tian Y, Liu G,
Wang X, Ma M, Du W, Liu Y, et al: Pancreatic β cell microRNA-26a
alleviates type 2 diabetes by improving peripheral insulin
sensitivity and preserving β cell function. PLoS Biol.
18(e3000603)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rossino MG and Casini G: Nutraceuticals
for the treatment of diabetic retinopathy. Nutrients.
11(771)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chaurasia SS, Lim RR, Parikh BH, Wey YS,
Tun BB, Wong TY, Luu CD, Agrawal R, Ghosh A, Mortellaro A, et al:
The NLRP3 inflammasome may contribute to pathologic
neovascularization in the advanced stages of diabetic retinopathy.
Sci Rep. 8(2847)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shih KC, Lam KSL and Tong L: A systematic
review on the impact of diabetes mellitus on the ocular surface.
Nutr Diabetes. 7(e251)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kang JH and Shin SY: What is the meaning
of hs-CRP and HbA1c in patients with dry eye syndrome in diabetes?
J Korea Soc Comput Inform. 25:158–190. 2020.
|
|
17
|
Nadeem H, Malik TG, Mazhar A and Ali A:
Association of dry eye disease with diabetic retinopathy. J Coll
Physicians Surg Pak. 30:493–497. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Almohammed BA, Alnafeesah AA, Aldharman
SS, Alensi MH, Mahjari AA, Albalawi FA, Amer KA, Alkhathami GH and
Al Taisan AA: Prevalence and severity of dry eye disease symptoms
among diabetics: A nationwide survey. Cureus.
14(e30981)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zagon IS, Sassani JW and McLaughlin PJ:
Sex differences in diabetic ocular surface complications and
dysregulation of the OGF-OGFr pathway. J Diabetes Clin Res.
4:20–24. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gomes JAP and Santo RM: The impact of dry
eye disease treatment on patient satisfaction and quality of life:
A review. Ocul Surf. 17:9–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Johnson ME and Murphy PJ: Changes in the
tear film and ocular surface from dry eye syndrome. Prog Retin Eye
Res. 23:449–474. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chiva A: Electrophoresis of tear proteins
as a new diagnostic tool for two high risk groups for dry eye:
Computer users and contact lens wearers. J Med Life. 4:228–233.
2011.PubMed/NCBI
|
|
23
|
Kaplan HJ: Anatomy and function of the
eye. Chem Immunol Allergy. 92:4–10. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dartt DA: Neural regulation of lacrimal
gland secretory processes: Relevance in dry eye diseases. Prog
Retin Eye Res. 28:155–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chu EPF, Cho CHH, Lee WJ, Lee IT, Cheng
IF, Kuo TC, Chen RY, Sheu WHH and Shen CH: Generation of three
induced pluripotent stem cell lines from type 2 diabetic patients
with ocular complications. Stem Cell Res. 49(102109)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rüfer F and Erb C: Influence of dry eye
syndrome on glaucoma diagnostic procedures. Ophthalmologe.
109:1082–1086. 2012.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
27
|
Stern ME, Beuerman RW, Fox RI, Gao J,
Mircheff AK and Pflugfelder SC: The pathology of dry eye: The
interaction between the ocular surface and lacrimal glands. Cornea.
17:584–589. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goto E, Shimazaki J, Monden Y, Takano Y,
Yagi Y, Shimmura S and Tsubota K: Low-concentration homogenized
castor oil eye drops for noninflamed obstructive meibomian gland
dysfunction. Ophthalmology. 109:2030–2035. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ribeiro MVMR, Barbosa FT, Ribeiro LEF,
Sousa-Rodrigues CF and Ribeiro EAN: Effectiveness of using
preservative-free artificial tears versus preserved lubricants for
the treatment of dry eyes: A systematic review. Arq Bras Oftalmol.
82:436–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Toda I: Dry eye after LASIK. Invest
Ophthalmol Vis Sci. 59:DES109–DES115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jha JC, Banal C, Chow BS, Cooper ME and
Jandeleit-Dahm K: Diabetes and kidney disease: Role of oxidative
stress. Antioxid Redox Signal. 25:657–684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lee YC, Bae CS and Ahn TH: Chlorogenic
acid attenuates pro-inflammatory response in the blood of
streptozotocin-induced diabetic rats. Lab Anim Re.
38(37)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li S, Deng J, Sun D, Chen S, Yao X, Wang
N, Ahang J, Gu Q, Zhang S, Wang J, et al: FBXW7 alleviates
hyperglycemia-induced endothelial oxidative stress injury via ROS
and PARP inhibition. Redox Biol. 58(102530)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rojas M, Zhang W, Xu Z, Lemtalsi T,
Chandler P, Toque HA, Caldwell RW and Caldwell RB: Requirement of
NOX2 expression in both retina and bone marrow for diabetes-induced
retinal vascular injury. PLoS One. 8(e84357)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H,
Jiang G, Lu J, Antony S and Doroshow JH: NADPH oxidases and cancer.
Clin Sci (Lond). 128:863–875. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Venditti P and Di Meo S: Thyroid
hormone-induced oxidative stress. Cell Mol Life Sci. 63:414–434.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D'Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and age-related diseases. Oxid Med Cell Longev.
2016(3565127)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Seen S and Tong L: Dry eye disease and
oxidative stress. Acta Ophthalmol. 96:e412–e420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dogru M, Kojima T, Simsek C and Tsubota K:
Potential role of oxidative stress in ocular surface inflammation
and dry eye disease. Invest Ophthalmol Vis Sci. 59:DES163–DES168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fresta CG, Fidilio A, Lazzarino G, Musso
N, Grasso M, Merlo S, Amorini AM, Bucolo C, Tavazzi B, Lazzarino G,
et al: Modulation of pro-oxidant and pro-inflammatory activities of
M1 macrophages by the natural dipeptide carnosine. Int J Mol Sci.
21(776)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lee JE, Kim SY, Lee HK, Chung TY, Kim JY,
Choi CY, Chung SH, Kim DH, Kim KW, Chung JK, et al: A randomized
multicenter evaluation of the efficacy of 0.15% hyaluronic acid
versus 0.05% cyclosporine A in dry eye syndrome. Sci Rep.
12(18737)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee SR, An EJ, Kim J and Bae YS: Function
of NADPH oxidases in diabetic nephropathy and development of nox
inhibitors. Bio Ther (Seoul). 28:25–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cha JJ, Min HS, Kim KT, Kim JE, Ghee JY,
Kim HW, Lee JE, Han JY, Lee G, Ha HJ, et al: APX-115, a
first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db
mice from renal injury. Lab Invest. 97:419–431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kwon G, Uddin MJ, Lee G, Jiang S, Cho A,
Lee JH, Lee SR, Bae YS, Mon SH, Lee SJ, et al: A novel pan-Nox
inhibitor, APX-115, protects kidney injury in
streptozotocin-induced diabetic mice: Possible role of peroxisomal
and mitochondrial biogenesis. Oncotarget. 8:74217–74232.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hong SW, Noh MH, Kin YS, Jin DH, Moon SH,
Yang JW and Hur DY: APX-115A, a pan-NADPH oxidase inhibitor,
induces caspase-dependent cell death by suppressing NOX4-ROS
signaling in EBV-infected retinal epithelial cells. Curr Eye Res.
45:1136–1143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Steinhorn B, Sartoretto JL, Sorrentino A,
Romero N, Kalwa H, Abel ED and Michel T: Insulin-dependent
metabolic and inotropic responses in the heart are modulated by
hydrogen peroxide from NADPH-oxidase isoforms NOX2 and NOX4. Free
Radic Biol Med. 113:16–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chan EC, van Wijngaarden P, Chan E, Ngo D,
Wang JH, Peshavariya HM, Dusting GJ and Liu GS: NADPH oxidase 2
plays a role in experimental corneal neovascularization. Clin Sci
(Lond). 130:683–696. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu
Z, Liu K and Sun H: Catalpol ameliorates hepatic insulin resistance
in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway.
Pharmacol Res. 130:466–480. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Furman BL: Streptozotocin-induced diabetic
models in mice and rats. Curr Protoc Pharmacol. 70:5.47.1–5.47.20.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tomlinson A, Blades KJ and Pearce EI: What
does the phenol red thread test actually measure? Optom Vis Sci.
78:142–146. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Obata H: Anatomy and histopathology of the
human lacrimal gland. Cornea. 25 (10 Suppl 1):S82–S89.
2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ji YW, Mittal SK, Hwang HS, Chang EJ, Lee
JH, Seo Y, Yeo A, Noh H, Lee HS, Chaihan SK and Lee HK: Lacrimal
gland-derived IL-22 regulates IL-17-mediated ocular mucosal
inflammation. Mucosal Immunol. 10:1202–1210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu Z and Pflugfelder SC: Corneal
thickness is reduced in dry eye. Cornea. 18:403–407.
1999.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Arita R, Fukuoka S and Morishige N:
Functional morphology of the lipid layer of the tear film. Cornea.
36 (Suppl 1):S60–S66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Georgiev GA, Eftimov P and Yokoi N:
Structure-function relationship of tear film lipid layer: A
contemporary perspective. Exp Eye Res. 163:17–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gokul A, Wang MTM and Craig JP: Tear lipid
supplement prophylaxis against dry eye in adverse environments.
Cont Lens Anterior Eye. 41:97–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee ES, Kim HM, Lee SH, Ha KB, Bae YS, Lee
SJ, Moon SH, Lee EY, Lee JH and Chung CH: APX-115, a pan-NADPH
oxidase inhibitor, protects development of diabetic nephropathy in
podocyte specific NOX5 transgenic mice. Free Radic Biol Med.
161:92–101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Assiri AMA, El-Beeh ME, Amin AH and
Ramadan MF: Ameliorative impact of Morus alba leaves' aqueous
extract against embryonic ophthalmic tissue malformation in
streptozotocin-induced diabetic rats. Biomed Pharmacother.
95:1072–1081. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sadikan MZ, Masir NAA, Iezhitsa I and
Agarwal R: Antioxidant and anti-apoptotic effects of
tocotrienol-rich fraction against streptozotocin-induced diabetic
retinopathy in rats. Biomed Pharmacother.
153(113533)2022.PubMed/NCBI View Article : Google Scholar
|
|
|
Wu K, Zhou K, Zhao M, Xiang L, Mei T, Xu
W, Shang B, Liu X, Lai Y, Lin M, et al: TCF7L2 promotes ER stress
signaling in diabetic retinopathy. Exp Eye Res.
221(109142)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Agardh E, Hultberg B and Agardh C: Effects
of inhibition of glycation and oxidative stress on the development
of cataract and retinal vessel abnormalities in diabetic rats. Curr
Eye Res. 21:543–549. 2000.PubMed/NCBI
|
|
62
|
Bucol C, Fidilio A, Platania CBM, Geraci
F, Lazzara F and Drago F: Antioxidant and osmoprotecting activity
of taurine in dry eye models. J Ocul Pharmacol Ther. 34:188–194.
2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Choi W, Lee JB, Cui L, Li Y, Li Z, Choi
JS, Lee HS and Yoon KC: Therapeutic efficacy of topically applied
antioxidant medicinal plant extracts in a mouse model of
experimental dry eye. Oxid Med Cell Longev.
2016(4727415)2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lee HS, Choi JH, Cui L, Li Y, Yang JM, Yun
JJ, Jung JE, Choi W and Yoon KC: Anti-inflammatory and
antioxidative effects of Camellia japonica on human corneal
epithelial cells and experimental dry eye: In vivo and in vitro
study. Invest Ophthalmol Vis Sci. 58:1196–1207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pont C, Ascaso FJ, Grzybowski A and Huerva
V: Corneal endothelial cell density during diabetes mellitus and
ocular diabetes complications treatment. J Fr Ophtalmol.
43:794–798. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hong SC, Yu HS, Kim JW, Lee EH, Pan CH,
Hong KW and Kim JC: Protective effect of Tisochrysis lutea on dry
eye syndrome via NF-κB inhibition. Sci Rep.
12(19576)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yoo YS, Na KS, Kim DY, Yang SW and Joo CK:
Morphological evaluation for diagnosis of dry eye related to
meibomian gland dysfunction. Exp Eye Res. 163:72–77.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Steven P, Augustin AJ, Geerling G,
Kaercher T, Kretz F, Kunert K, Menzel-Severing J, Schrage N,
Schrems W, Krösser S, et al: Semifluorinated alkane eye drops for
treatment of dry eye disease due to meibomian gland disease. J Ocul
Pharmacol Ther. 33:678–685. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li L, Li Y, Zhu X, Wu B, Tang Z, Wen H,
Yuan J, Zheng Q and Chen W: Conjunctiva resident γδ T cells
expressed high level of IL-17A and promoted the severity of dry
eye. Invest Ophthalmol Vis Sci. 63(13)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Han SB, Yang HK and Hyon JY: Influence of
diabetes mellitus on anterior segment of the eye. Clin Interv
Aging. 14:53–63. 2018.PubMed/NCBI View Article : Google Scholar
|