Introduction
Osteosarcoma is a common high-grade malignant bone
tumor in children and adolescents. In recent years, due to the
continuous progress of neoadjuvant chemotherapy and other
comprehensive treatment strategies, the survival rate of
osteosarcoma has increased to 60-70% (1). However, due to the constraints of
distant metastasis and chemotherapy resistance, it remains
difficult to further improve the survival rate (2). Currently, a large number of studies
aim to identify biomarkers to provide evidence for chemotherapy
resistance and prognosis.
Long non-coding RNAs (lncRNAs) contain >200
nucleic acid sequences and are not translated into functional
proteins (3). lncRNAs have been
increasingly explored, and several studies have reported that these
RNAs are associated with the migration and invasion of
osteosarcoma, including the lncRNAs EPIC1 and NHG1 (4,5).
Circular RNA (circRNA) is synthesized by the reverse splicing of an
mRNA precursor, resulting in a circular, rather than a linear
structure (6). Through the rapid
development of high-throughput RNA sequencing technology, more
circRNAs related to osteosarcoma have been discovered. Notably
circMYO10 has been reported to be associated with chromatin
remodeling in osteosarcoma cells, and circTADA2A may be associated
with the proliferation and migration of osteosarcoma (7,8).
Through high-throughput screening of the whole transcriptome, our
previous study revealed that the expression levels of lncRNA
OIP5-AS1, hsa_circ_0081001, lncRNA ODRUL and hsa_circ_0004674 were
significantly increased in osteosarcoma, and were closely
associated with the proliferation, metastasis and drug resistance
of the disease (9-12).
Upregulation of these RNA indicators may promote tumor
proliferation and increase chemotherapy resistance, and may have a
tumor-promoting role in osteosarcoma. Some studies have reported
similar conclusions. For example, regulation of the
OIP5-AS1/microRNA (miR)-223/CDK14 axis has been shown to have a
significant influence on tumorigenesis, which is closely associated
with the poor prognosis of patients with osteosarcoma (13). In addition, lncRNA OIP5-AS1 can
cause cisplatin resistance in osteosarcoma (14). The present study further explored
the clinical significance of these RNAs and their potential for
clinical application. The expression levels of these four
non-coding RNAs were initially detected in samples. Results of
hsa_circ_0081001 and lncRNA ODRUL showed no statistical
significance in chemotherapy sensitivity. So the data of
hsa_circ_0081001 and lncRNA ODRUL was not referred. The results of
lncRNA OIP5-AS1 and hsa_circ_0004674 in pilot study was showed in
Fig. 1, Fig. 2 and Fig. 3. Therefore, the present study
concentrated on the combined use of lncRNA OIP5-AS1 and
hsa_circ_0004674 in a clinical setting.
Although previous studies have investigated the
expression and mechanism of lncRNA OIP5-AS1 and hsa_circ_0004674 in
osteosarcoma cell lines and tissues (9,12),
and demonstrated their potential as biomarkers, adequate clinical
data have not been obtained. The present study analyzed the serum
and tissues of patients with osteosarcoma to explore the
association between the two types of non-coding RNAs and the
patient data regarding chemotherapy resistance (tumor necrosis rate
<90%), distant metastasis and prognosis.
Materials and methods
Patients and specimens
Tumor specimens were collected from patients with
primary osteosarcoma who were treated at Shanghai Tenth People's
Hospital (Shanghai, China) between June 2014 and December 2019. The
age of patients was between 5 and 32 years old, with an average age
of 15.25 years. The study received ethical approval (approval no.
SHSY-IEC-4.1/21-300/01) from the Institutional Review Board of
Shanghai Tenth People's Hospital Affiliated to Tongji University.
All patients/guardians participating in the present study provided
written informed consent. For children, the study was explained in
simple language and written informed consent was obtained from
their guardian. Enrolled patients were diagnosed with osteosarcoma,
and pathologists determined that the pathological subtype was
conventional osteosarcoma. The conventional osteosarcoma is the
most frequent variant, which arises from the medullary bone
(15). All patients received two
cycles of preoperative chemotherapy and at least four cycles of
postoperative chemotherapy. The dominating chemotherapy protocol
was IOR/OS-4 (high-dose methotrexate, 8-12 g/m2/day;
cisplatin, 100 mg/m2/day; Adriamycin, 30
mg/m2/day; and high-dose ifosfamide, 2-3
g/m2/day) (16). In
addition, the inclusion criteria also included patients that had
not received any treatment before and who cooperated with regular
follow-up every month. Exclusion criteria were as follows: i) The
patient had an infectious disease; ii) patients with difficulties
in venous blood collection; iii) the patient was >3 years old or
>60 years old; and iv) patients with other serious physical or
mental diseases. The total number of conventional osteosarcoma
cases was 28. Patients were excluded who were lost to follow-up
(n=3) or who died from serious postoperative complications (n=1).
Considering that incomplete surgical resection also affects the
prognosis, patients with local tumor recurrence were also excluded
(n=4). Finally, 20 patients (Table
I) were included in the present study. Postoperative follow-up
was performed every 2-3 months. Tumors and adjacent (normal) tissue
were collected and placed in liquid nitrogen for immediate storage
at -196˚C. Chemotherapy response (tumor necrosis) was graded based
on the amount of tumor necrosis in the resected specimen. Tumor
specimens with a necrosis rate of <90% were defined as
chemotherapy resistant (17) and
the results of the necrosis rate were evaluated by pathologists.
The surgical margin in the bone tissue was 3 cm outside the tumor,
and 5 cm in soft tissue. Adjacent tissues were taken from outside
the surgical margin. Pathological results indicated that all
surgical margins were negative. The preoperative serum samples
refer to the 5 ml venous blood samples taken before chemotherapy.
For comparison, serum samples were collected from 8 patients with
bone fractures who had not yet undergone surgery (Table II). These patients had no ailment
other than bone fractures and were range from 13 to 31 years old.
Their average age was 21.125 years old. After the blood samples
were collected, they were centrifuged at 194 x g for 5 min at 25˚C.
The serum (supernatant fraction) was then collected into a 1.5-ml
Eppendorf (EP) tube and stored at -20˚C.
 | Table IPatient information. |
Table I
Patient information.
| Patient no. | Sex | Age, years | Tumor location | Progression-free
survival, months | Lung metastasis | Chemotherapy
resistance | Expression of
hsa_circ_0004674 | Expression of lncRNA
OIP5-AS1 |
|---|
| 1 | Male | 12 | Proximal fibula | 26 | Yes | No | Low | High |
| 2 | Male | 14 | Proximal tibia | 4 | Yes | No | High | High |
| 3 | Female | 8 | Proximal tibia | 12 | Yes | Yes | High | High |
| 4 | Male | 18 | Distal femur | 2 | Yes | No | High | High |
| 5 | Male | 20 | Scapula | 11 | Yes | Yes | High | High |
| 6 | Female | 5 | Distal femur | 12 | Yes | Yes | High | High |
| 7 | Male | 11 | Proximal tibia | 28 | Yes | No | High | Low |
| 8 | Male | 14 | Proximal tibia | 18 | Yes | No | High | Low |
| 9 | Male | 17 | Distal femur | 48 | Yes | Yes | High | High |
| 10 | Female | 6 | Distal femur | 4 | Yes | No | High | Low |
| 11 | Female | 28 | Distal femur | 13 | Yes | Yes | High | High |
| 12 | Male | 14 | Distal femur | 14 | Yes | Yes | Low | High |
| 13 | Male | 18 | Proximal tibia | 36 | No | No | Low | Low |
| 14 | Male | 17 | Distal femur | 9 | Yes | Yes | Low | Low |
| 15 | Male | 16 | Proximal tibia | 60 | No | No | Low | Low |
| 16 | Female | 15 | Distal femur | 30 | No | Yes | Low | Low |
| 17 | Male | 13 | Distal tibia | 22 | No | No | Low | Low |
| 18 | Male | 32 | Distal femur | 30 | No | No | Low | Low |
| 19 | Female | 17 | Proximal
humerus | 32 | Yes | Yes | Low | Low |
| 20 | Female | 10 | Proximal
fibula | 26 | No | No | Low | Low |
 | Table IIPatient information of the control
group. |
Table II
Patient information of the control
group.
| Patients | Sex | Age, years | Site of
fracture |
|---|
| 1 | Male | 23 | Femur |
| 2 | Female | 29 | Femur |
| 3 | Female | 18 | Humerus |
| 4 | Male | 13 | Ulna and
radius |
| 5 | Male | 23 | Clavicle |
| 6 | Male | 17 | Radius |
| 7 | Female | 15 | Tibia |
| 8 | Male | 31 | Tibia and
fibula |
RNA extraction
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to mung tissues with a volume of ~3 ml,
which were ground using a grinding rod for full lysis.
Subsequently, 200 µl chloroform was added to the lysate, thoroughly
vortexed and centrifuged at 14,548 x g for 10 min at 4˚C. After
centrifugation, the solution was divided into three layers, and the
upper layer was aspirated into a new 1.5-ml EP tube. Subsequently,
500 µl isopropanol was added to the aspirated supernatant,
thoroughly vortexed and centrifuged at 14,548 x g for 20 min at
4˚C. Afterward, the supernatant was removed to retain the
precipitate. Prechilled ethanol (80%) was added to wash the
precipitate, and the precipitate was centrifuged at 14,548 x g for
2 min at 4˚C. After removing the supernatant, the mixture was
placed at room temperature until the precipitate became
transparent. Next, 30 µl RNase-free water was added to dissolve the
precipitate. The previous step was repeated. A whole blood total
RNA extraction kit (Aidlab Biotechnologies, Ltd.) was used for
serum RNA extraction, and the extraction method was performed
according to the manufacturer's instructions.
Reverse transcription-quantitative
PCR
A PrimeScript™ RT Master Mix RT kit
(Takara Biotechnology Co., Ltd.) was used to generate cDNA from RNA
according to the manufacturer's protocols. Specify a sample volume
of 20 µl and edit the protocol parameters to match those shown
below: i) Pre-denaturation at 95˚C for 30 sec; ii) cycling stage,
95˚C for 5 sec, then 60˚C for 10 sec, repeat this step 39 times;
iii) melt curve stage. The expression levels of hsa_circ_0004674
and lncRNA OIP5-AS1 were detected using a TB green kit (Takara
Biotechnology Co., Ltd.). The primer sequences are presented in
Table III, and GAPDH was used as
the internal reference. The 2-ΔΔCq method was used for
quantification (18); ΔCq is the
difference in Cq values between the gene of interest and the
endogenous control.
 | Table IIIPCR primer sequences. |
Table III
PCR primer sequences.
| Gene | Accession
number | Oligo sequence,
5'-3' |
|---|
|
hsa_circ_0004674 | NM_001391981 | F: GTTGACCAAGC
AAGCTTCCAG |
| | | R: GGTACTTGCAG
GTTTTACTGGG |
| lncRNA
OIP5-AS1 | NR_026757 | F: TGCGAAGATGG
CGGAGTAAG |
| | | R: TAGTTCCTCTC
CTCTGGCCG |
| GAPDH | NM_001289745 | F: TCTCTGCTCCT
CCTGTTCGA |
| | | R: GCGCCCAATA
CGACCAAATC |
Statistical analysis
Statistical analyses were performed and diagrams
were generated using SPSS 22.0 statistical software package (IBM
Corporation). Paired and unpaired Student's t-tests were performed
to assess associations between expression and clinicopathological
parameters. The detection of expression levels of serum alkaline
phosphatase (ALP) is a necessary test for patients when they are
admitted to hospital, and ALP data were obtained from the hospital
laboratory reports. The alkaline phosphatase test is performed on
fully automated analyzers which are based on the principle of
photometry. It was noted that most of the tumor cells in the tissue
samples were necrotized after preoperative chemotherapy; therefore,
preoperative serum instead of surgically obtained tumor specimens
were used when judging chemotherapy sensitivity. Receiver operating
characteristic (ROC) curves of hsa_circ_0004674, lncRNA OIP5-AS1
and ALP were plotted to discuss their differences. Progression-free
survival (PFS) was calculated using the Kaplan-Meier survival
analysis and was evaluated by the log-rank test and Breslow test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
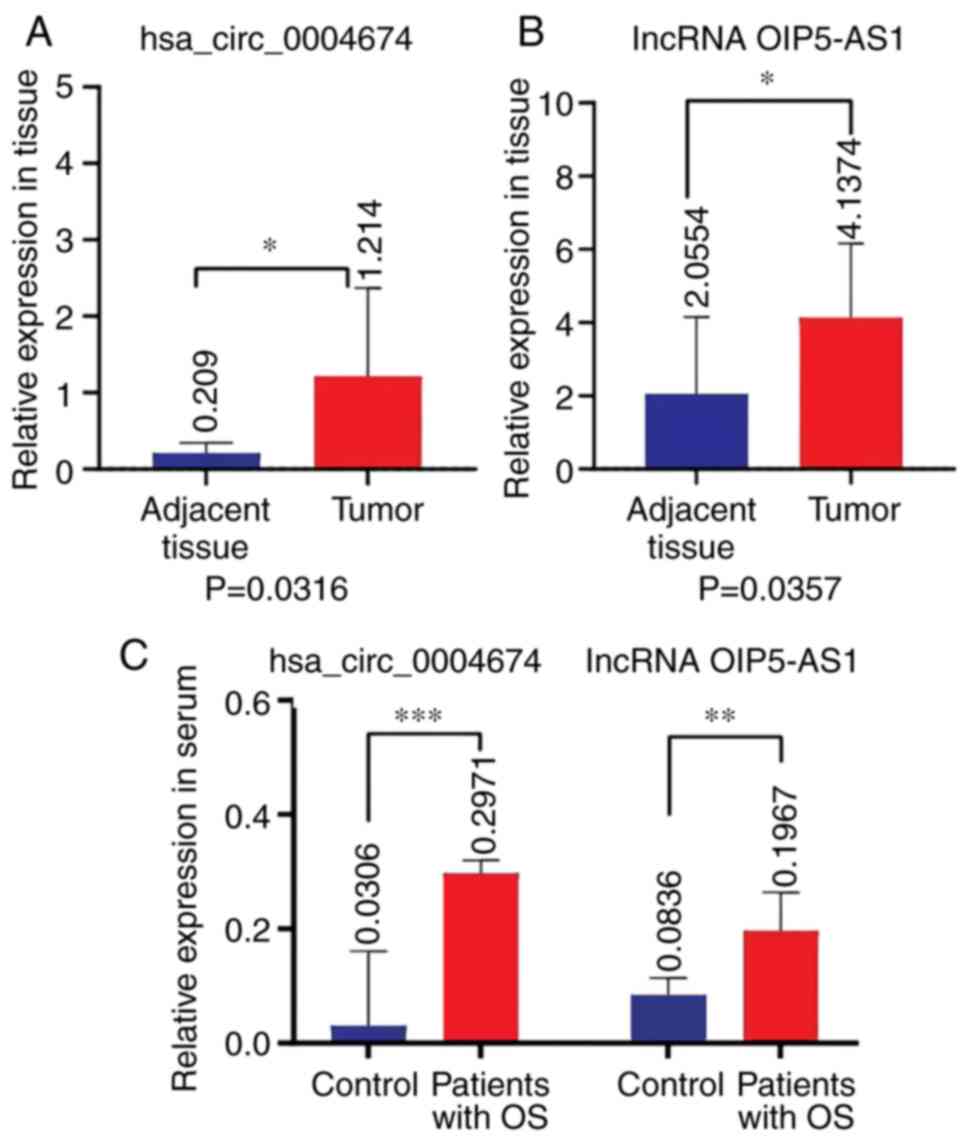
Expression levels of hsa_circ_0004674
and lncRNA OIP5-AS1 are significantly increased in the tumor
tissues and serum of patients with osteosarcoma
The tumor and adjacent tissues of each patient
(n=20) were obtained during surgery. The analysis of expression
showed that the levels of hsa_circ_0004674 and lncRNA OIP5-AS1 were
significantly higher in tumor tissues than those in adjacent
tissues (P=0.0316 and 0.0357; Fig.
1A and B). These results
indicated that these two non-coding RNAs were highly expressed in
tumor tissues from patients with osteosarcoma. Serum samples from
patients with bone fractures and preoperative serum samples from
patients with osteosarcoma were then assessed. The results revealed
that the expression levels of hsa_circ_0004674 and lncRNA OIP5-AS1
were significantly elevated in the serum of patients with
osteosarcoma (Fig. 1C).
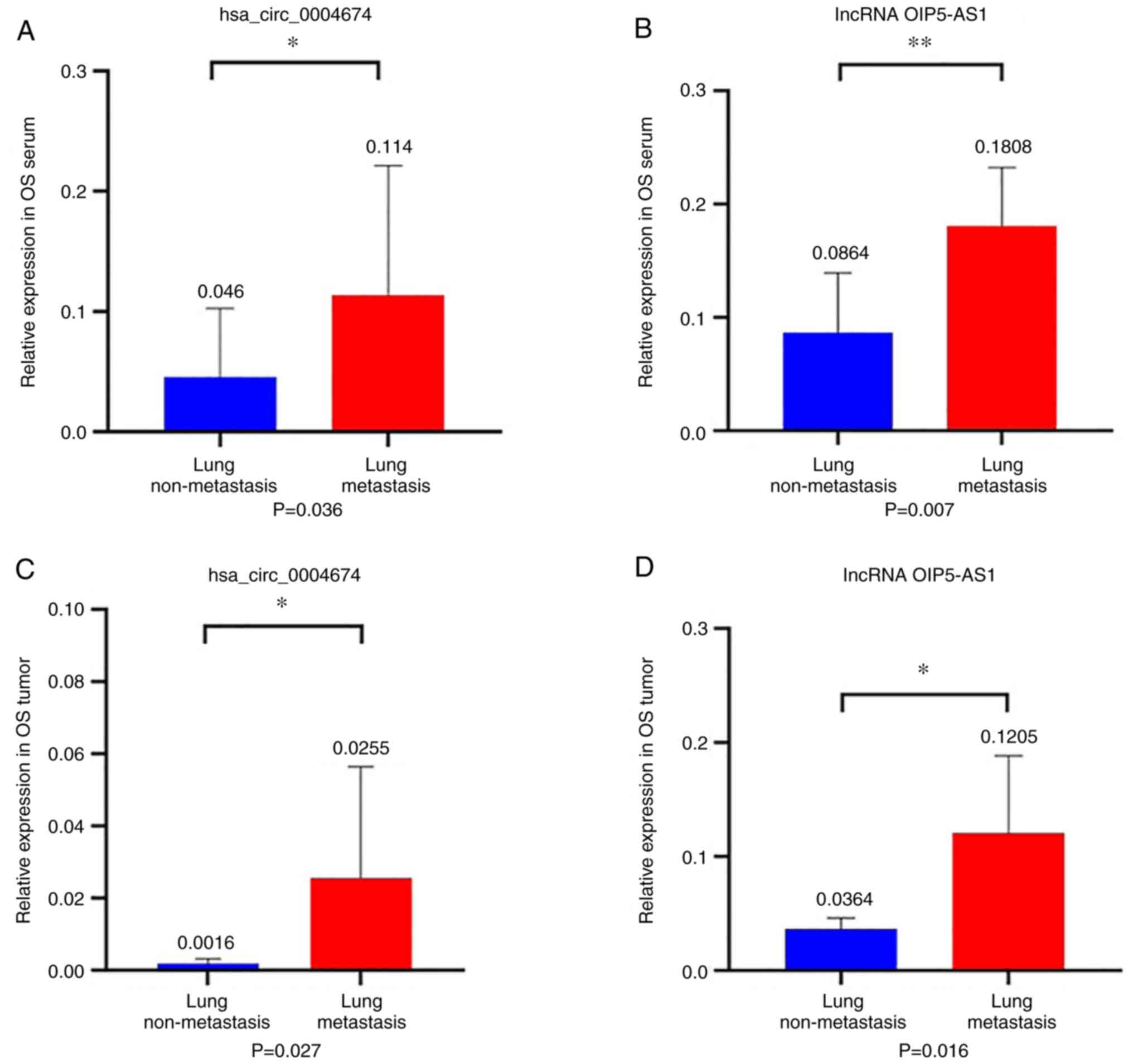
Expression levels of hsa_circ_0004674
and lncRNA OIP5-AS1 are significantly increased in the tumor
tissues and serum of patients with osteosarcoma and lung
metastasis
The expression levels of two non-coding RNAs in the
tumor tissues and serum of patients with osteosarcoma and lung
metastasis were compared. The results revealed that the expression
levels of hsa_circ_0004674 and lncRNA OIP5-AS1 were increased in
the tumor tissues of patients with lung metastasis compared with
the levels in patients without metastasis (P=0.036 and 0.007;
Fig. 2A and B). The expression levels of
hsa_circ_0004674 and lncRNA OIP5-AS1 were also significantly
increased in the serum of patients with lung metastasis compared
with the levels in patients without metastasis (P=0.027 and 0.016;
Fig. 2C and D). These findings indicated that the
non-coding RNAs hsa_circ_0004674 and lncRNA OIP5-AS1 were
significantly associated with lung metastasis in patients with
osteosarcoma.
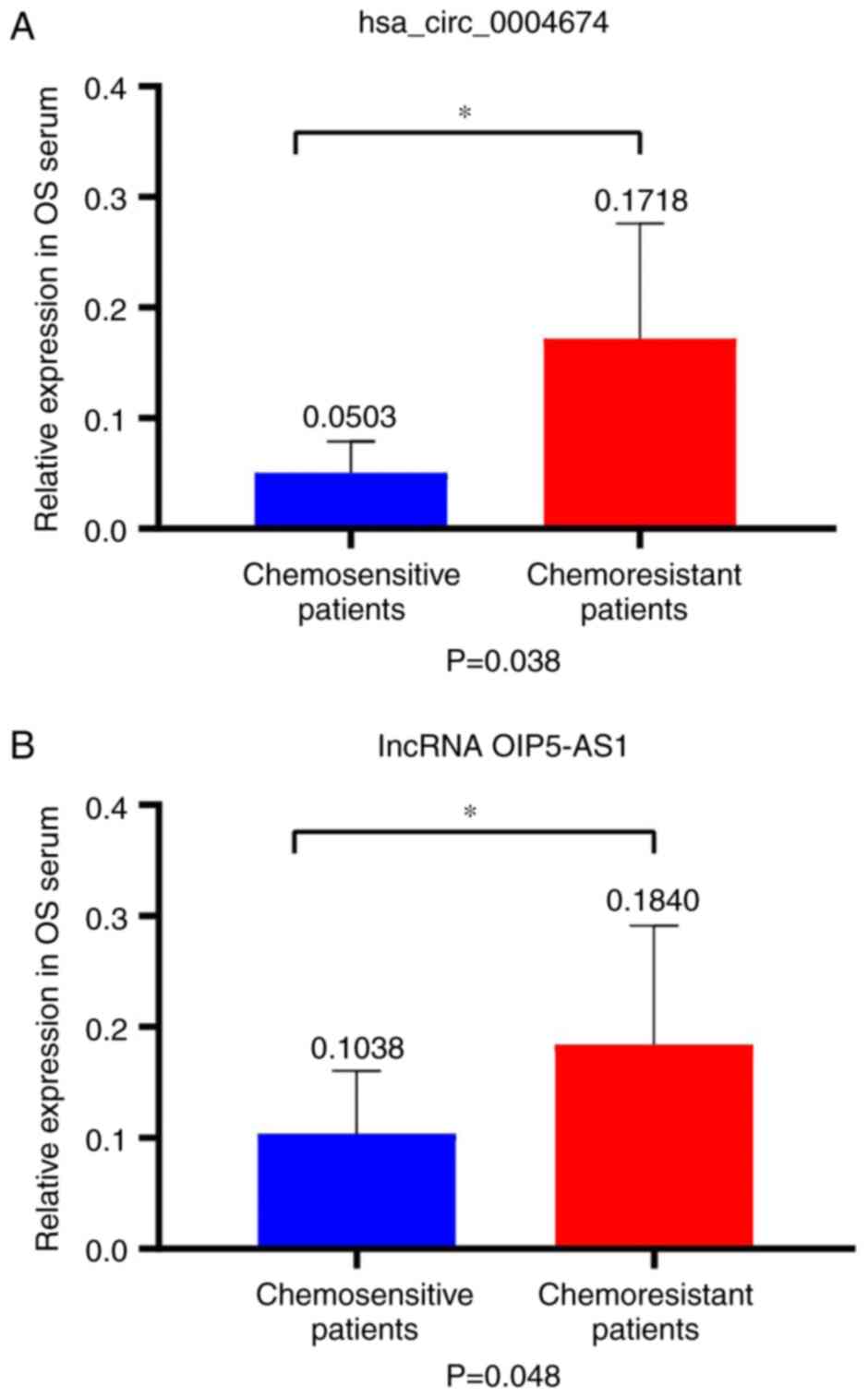
hsa_circ_0004674 and lncRNA OIP5-AS1
expression is associated with the sensitivity of patients with
osteosarcoma to chemotherapy
The serum samples used for these experiments were
collected from the patients with osteosarcoma before treatment. The
results revealed that there was a significant increase in the
expression of the two non-coding RNAs in the serum from
chemoresistant patients, and suggested that the expression levels
of these two non-coding RNAs could provide evidence for determining
whether patients were sensitive to chemotherapy ((P=0.038 and
0.048; Fig. 3A and B).
Combination of hsa_circ_0004674 and
lncRNA OIP5-AS1 is better than ALP in predicting chemotherapy
sensitivity and metastasis
The expression levels of hsa_circ_0004674, lncRNA
OIP5-AS1 and ALP were analyzed from the serum. The associations
between the expression levels of these markers and metastasis or
chemotherapy sensitivity were assessed, and receiver operating
characteristic (ROC) curves were plotted. The two non-coding RNAs
were more sensitive than ALP in predicting chemosensitivity.
Compared with a single index, the combination of hsa_circ_0004674
and lncRNA OIP5-AS1 had better specificity in predicting
chemosensitivity (Fig. 4A;
Table IV). In terms of predicting
metastasis, the area under the curve (AUC) value of
hsa_circ_0004674 was lower than that of ALP, which may indicate
that ALP is better in reflecting tumor metastasis. However, AUC
values close to 0.9 also indicated a good specificity of
hsa_circ_0004674 in predicting metastasis. The AUC values of
hsa_circ_0004674, lncRNA OIP5-AS1 and ALP were similar, and all of
them had good predictive function in metastasis. However, the
combination of hsa_circ_0004674 and lncRNA OIP5-AS1 had the best
predictive value (Fig. 4B;
Table V).
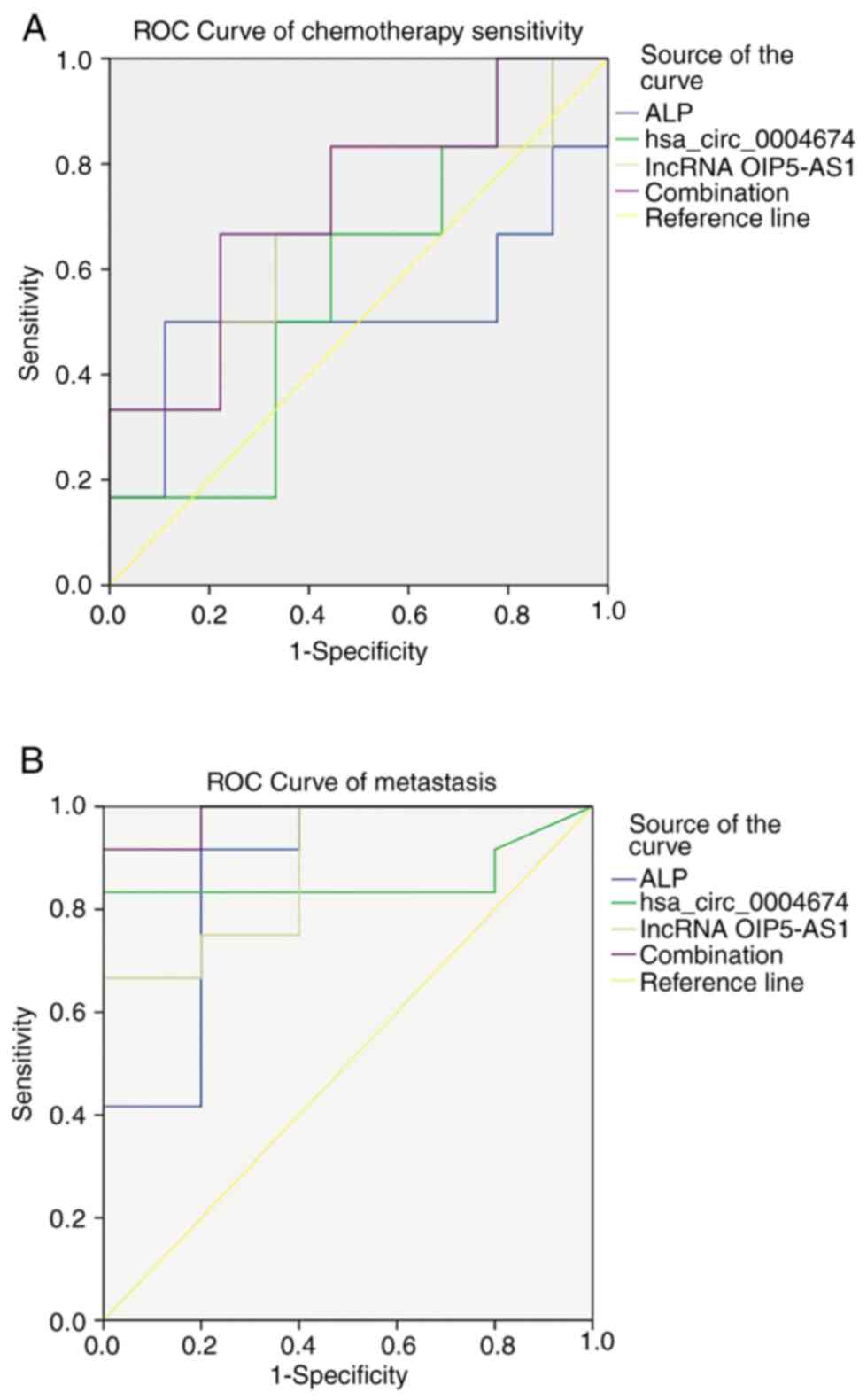 | Figure 4ROC curve of chemosensitivity and
metastasis. (A) ROC curve analysis of hsa_circ_0004674, lncRNA
OIP5-AS1, ALP, and the combination of hsa_circ_0004674 and lncRNA
OIP5-AS1 for sensitivity to chemotherapy. A tumor necrosis rate
≥90% was considered a good response to chemotherapy. The
combination of two non-coding RNAs had the best predictive value,
while ALP had little value in this respect. (B) ROC curve analysis
of hsa_circ_0004674, lncRNA OIP5-AS1, ALP, and the combination of
hsa_circ_0004674 and lncRNA OIP5-AS1 for metastasis. All potential
markers had great value in predicting metastasis. The AUC of the
combination of the two non-coding RNAs was much larger than that of
the others. AUC, area under the curve; lncRNA, long non-coding RNA;
ROC, receiver operating characteristic. |
 | Table IVAUC values for chemotherapy
sensitivity. |
Table IV
AUC values for chemotherapy
sensitivity.
| Variable | AUC |
|---|
| ALP | 0.519 |
|
hsa_circ_0004674 | 0.574 |
| lncRNA
OIP5-AS1 | 0.685 |
| Combination | 0.722 |
 | Table VAUC values for metastasis. |
Table V
AUC values for metastasis.
| Variable | AUC |
|---|
| ALP | 0.867 |
|
hsa_circ_0004674 | 0.858 |
| lncRNA
OIP5-AS1 | 0.883 |
| Combination | 0.983 |
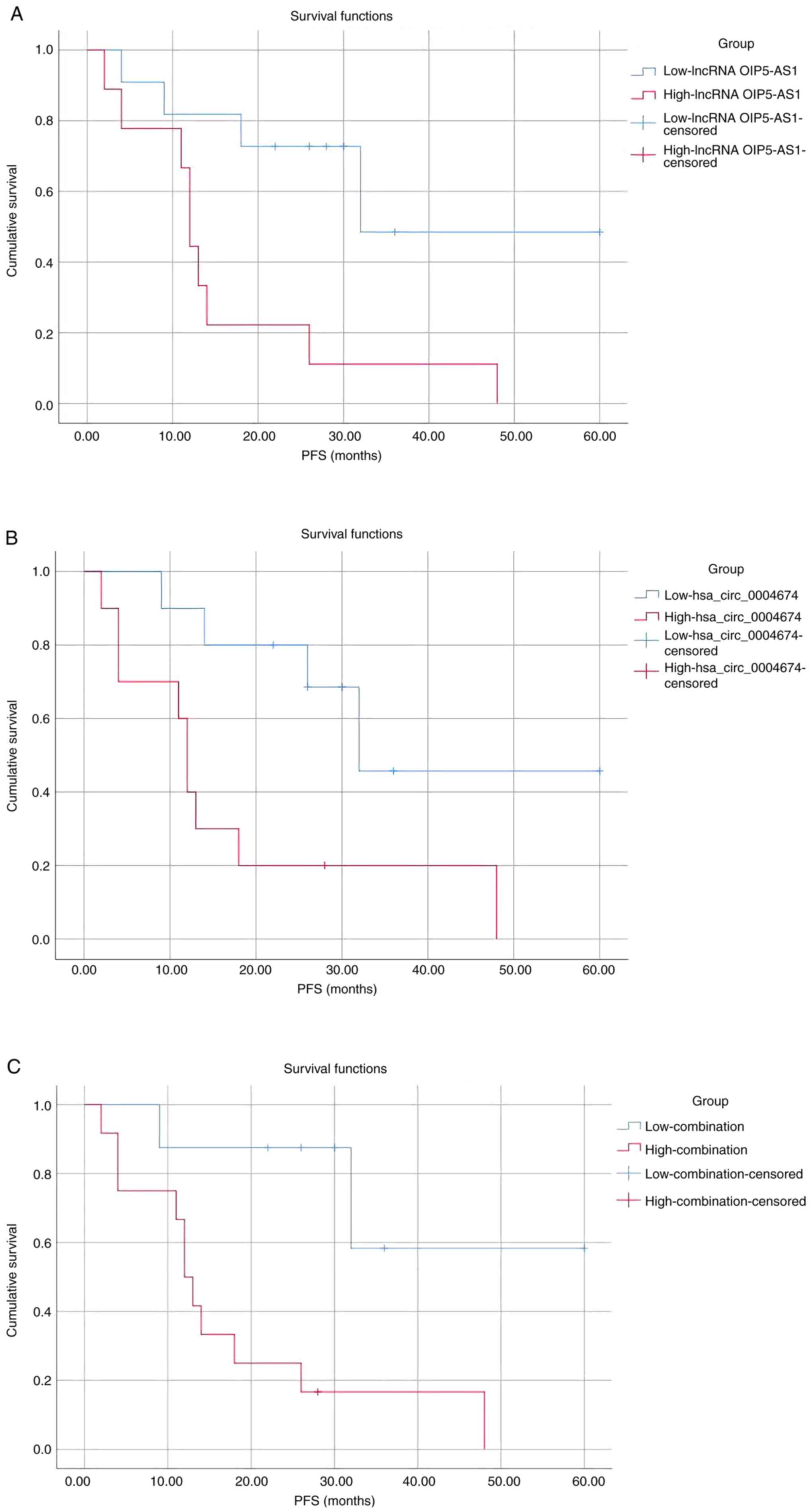
Combination of hsa_circ_0004674 and
lncRNA OIP5-AS1 as clinical biomarkers to predict the prognosis of
patients
Based on the aforementioned results, it was
hypothesized that the high expression of the two non-coding RNAs
may indicate a poor prognosis for patients. The mean values of RNA
expression in serum from patients with fractures (control) were
considered to be normal compared with those from patients with
tumors. Subsequently, the association between the expression levels
of lncRNA OIP5-AS1 and patient prognosis was assessed (Fig. 5A). Patients with lower expression
than mean expression in control individual were assigned to the low
expression group, whereas the remaining patients (higher expression
than mean expression in control individual) were assigned to the
high expression group. The PFS of all patients was assessed and
survival curves were generated. The PFS time of all patients ranged
between 1 and 60 months, with a mean average of 27.95 months.
Excluding deaths from other causes and loss to follow-up, 20
patients were successfully assessed, 13 of whom developed
postoperative tumor recurrence or metastasis. A total of 5 patients
died during the assessment period, and the remaining patients
survived until the end of the study. As shown in Fig. 5A, patients with low serum lncRNA
OIP5-AS1 expression had a better prognosis than those with high
serum expression, and this difference was significant according to
the log-rank test (P=0.009) and the Breslow test (P=0.02). The mean
PFS time in the low expression and high expression groups was
39.667 and 15.778 months, respectively.
The same method was used to generate the survival
curve according to the expression levels of hsa_circ_0004674
(Fig. 5B). A total of 10 out of
the 20 patients were assigned to the high expression group, as
their expression levels were greater than those in the control
group, and there were 10 patients in the low expression group. The
mean PFS time was 40.014 months in the low expression group and
17.200 months in the high expression group, and the difference was
significant according to the log-rank test (P=0.015) and the
Breslow test (P=0.012).
Although each single indicator was shown to have
prognostic potential, a new strategy of combining two RNAs for
prognosis was adopted in the present study. Since the AUC value of
the combination was the largest (Fig.
4), this new strategy should be able to reflect the prognosis
more accurately. Highly expressed lncRNA OIP5-AS1 and hsa_ circ_
0004674 are considered to be risk factors. Therefore, only when the
two indicators are both lower compared with the mean expression in
control individuals can the patient be included in the low
expression group. If any one of the two indicators is higher
compared with the mean expression of the control group, the patient
will be assigned to the high expression group Under this new
strategy, there were 12 patients in the high expression group and 8
patients in the low expression group. A survival curve was then
plotted according to PFS (Fig.
5C). The mean PFS time was 45.46 months in the low expression
group and 17.67 months in the high expression group. The results
showed that by comparing the expression levels of both lncRNA
OIP5-AS1 and hsa_ circ_ 0004674 between patients and the healthy
subjects, the survival time could be more effectively predicted
compared with analyzing a single indicator (log-rank test P=0.006;
Breslow test P=0.012).
The results of the three survival curves indicated
that combination of the two RNAs had the smallest P-value, thus
suggesting that the combination of hsa_circ_0004674 and lncRNA
OIP5-AS1 was a better tool than either of them alone in predicting
patient prognosis and survival.
Discussion
Currently, surgical resection of primary lesions
combined with neoadjuvant chemotherapy is the main treatment for
osteosarcoma, and neoadjuvant chemotherapy has greatly improved the
survival time of patients in recent years. However, once metastasis
occurs, the 5-year survival rate of patients is <20% (19). Chemotherapy serves an important
role in preventing metastasis (20), and patients who are resistant to
chemotherapy are more inclined to have a poor prognosis due to
metastasis. To date, there are no reliable biomarkers to reflect
the postoperative chemotherapy or distant metastasis of
osteosarcoma. Effective biomarkers can assist doctors in optimizing
patients' therapeutic schedules. In addition, biomarkers serve an
important role in the development of new drugs. Researchers can
therefore select patients to participate in clinical trials
according to the results of the biomarkers, which in turn could
accelerate the research and development process (21).
Non-coding RNAs have remained a popular topic in the
research of osteosarcoma biomarkers in recent years. The most
studied non-coding RNAs include lncRNAs, circRNAs and microRNAs.
lncRNAs and circRNAs are closely associated with the metastasis and
drug resistance of osteosarcoma (22). Multiple studies have reported
correlations in vitro (23,24).
There have also been some reports on the expression levels of
single non-coding RNAs in patients with osteosarcoma. However, due
to the individual differences of patients with osteosarcoma and the
complexity of the disease, it is likely that a single non-coding
RNA would not be effective in predicting prognosis.
The present study analyzed two non-coding RNAs that
were significantly upregulated in the serum and tumor tissues of
patients with osteosarcoma. Before treatment, the expression levels
of hsa_circ_0004674 and lncRNA OIP5-AS1 in the serum may provide
strong evidence regarding the sensitivity of patients with
osteosarcoma to chemotherapy. If the expression levels of
hsa_circ_0004674 or lncRNA OIP5-AS1 in the serum are high, the risk
of metastasis and a poor response to chemotherapy may also be
increased. In addition, the expression levels of these two RNAs may
be useful when estimating the prognosis of patients after surgery.
In addition, through the expression of hsa_circ_0004674 and lncRNA
OIP5-AS1, the possibility of metastasis in patients can be
predicted. The present results agreed with those of previous
studies. lncRNA OIP5-AS1 plays a key role in doxorubicin resistance
by sponging miR-200b-3p to upregulate the expression of
FN1(9). In addition, it has been
suggested that hsa_circ_0004674 may mediate chemotherapy resistance
by regulating the circRNA/miR-490-3p/ABCC2 or circRNA/miR-1254/EGFR
axes (12). The present study
indicated that the possibility of metastasis or cellular response
to chemotherapy may be predicted by monitoring the expression
levels of both RNAs; however, some problems need to be solved
before clinical application. First, an interval value should be set
for healthy individuals, which will require a large control sample
that covers all population characteristics. Clinically, patient
conditions may change, for example, from chemotherapy sensitivity
to chemotherapy resistance. Therefore, it must be determined as to
whether the change in the expression levels of the two RNAs is
consistent with the changes in clinical characteristics, as well as
the specific statistical significance. Finally, the benefit of a
combined biomarker must be determined in patients. We plan to
address these concerns in future research. However, the current
results suggested that these two RNAs are feasible and potential
biomarkers. Compared with traditional single biomarkers, the
combination of multiple serum RNA indicators has higher sensitivity
and specificity for predicting disease progression (25). Elevated ALP has been observed in
most patients with osteosarcoma. A number of studies (26,27)
have explored the relationship between ALP and clinical prognosis,
and the association between ALP levels and survival outcomes has
been shown to be significant. The increase in ALP is associated
with shorter survival times, increased incidence of lung metastasis
and a poor chemotherapeutic response (26). The present study suggested that the
combination of hsa_circ_0004674 and lncRNA OIP5-AS1 was a better
biomarker than ALP with regard to predicting patient prognosis and
survival.
Notably, the present study has some limitations.
First, the sample size of the present study was small, and samples
from the same individual at different treatment stages were
lacking. This limited the further exploration of the association
between changes in expression levels and disease progression. In
the future, we aim to explore the association between serum changes
at different time periods post-surgery and clinical progression. A
follow-up study of this cohort is still ongoing. Second, the
insufficient follow-up time in some patients did not fully
demonstrate the potential of the combined use of these RNAs to
predict long-term survival outcomes. There remain some unresolved
problems, and it could not be ruled out whether there were other
factors, such as inflammation and chemotherapy drugs, that may have
led to false-positive results (28). As the subject of this study was
conventional osteosarcoma, the results cannot be used to assess
non-conventional osteosarcoma at present. The molecular mechanism
would be the focus of follow-up research to try to solve this
problem step by step and screen out biomarkers of osteosarcoma.
In conclusion, in the present study, two non-coding
RNA markers were identified, and their clinical value in predicting
the chemosensitivity and distant metastasis of conventional
osteosarcoma was elucidated. The AUC values suggested that the
combined use of hsa_circ_0004674 and lncRNA OIP5-AS1 was more
valuable than the use of the conventional ALP marker or any one of
the non-coding RNA markers alone. Through the comprehensive use of
two non-coding RNA markers, the survival time of patients could be
effectively estimated in the clinic. The present study provides a
basis for further exploration of the role of these two non-coding
RNAs in the diagnosis and treatment of osteosarcoma.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by grants from the National
Natural Science Foundation of China (grant nos. 81872174, 82072963
and 82103513), the Program of Shanghai Sailing (grant no.
20YF1437700), the Climbing Talents Program of Shanghai Tenth
People's Hospital (grant no. 2021SYPDRC021), the Clinical Research
Program of Shanghai Tenth People's Hospital (grant no. YNCR2B002)
and the Youth Cultivation Program of Clinical Research of Shanghai
Tenth People's Hospital (grant no. YNCR2C012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All the authors made a significant contribution to
this manuscript and have read and approved the final manuscript. CZ
and YZ conceptualized the study. ST and KZ designed the
methodology. ST and YZ wrote the original draft preparation, and
reviewed and edited. JH, ST and YZ acquired and analysed the data.
CZ and JH supervised the study. CZ and ST confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The study received ethical approval (approval no.
SHSY-IEC-4.1/21-300/01) from the Institutional Review Board of
Shanghai Tenth People's Hospital Affiliated to Tongji University
(Shanghai, China). All patients/guardians participating in the
present study provided written informed consent. For children, the
study was explained in simple language and written informed consent
was obtained from their guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liao D, Zhong L, Yin J, Zeng C, Wang X,
Huang X, Chen J, Zhang H, Zhang R, Guan XY, et al: Chromosomal
translocation-derived aberrant Rab22a drives metastasis of
osteosarcoma. Nat Cell Biol. 22:868–881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen H, Xu Z and Liu D: Small non-coding
RNA and colorectal cancer. J Cell Mol Med. 23:3050–3057.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao W, Zhang D, Qin P, Zhang J, Cui X,
Gao J, Wang J and Li J: Long non-coding RNA EPIC1 inhibits
viability and invasion of osteosarcoma cells by promoting MEF2D
ubiquitylation. Int J Biol Macromol. 128:566–573. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen B and Huang S: Circular RNA: An
emerging non-coding RNA as a regulator and biomarker in cancer.
Cancer Lett. 418:41–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jin J, Chen A, Qiu W, Chen Y, Li Q, Zhou X
and Jin D: Dysregulated circRNA_100876 suppresses proliferation of
osteosarcoma cancer cells by targeting microRNA-136. J Cell
Biochem. 120:15678–15687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y,
Huang K, Wang G, Wang J, Ma J, et al: Circular RNA circTADA2A
promotes osteosarcoma progression and metastasis by sponging
miR-203a-3p and regulating CREB3 expression. Mol Cancer.
18(73)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kun-Peng Z, Chun-Lin Z, Xiao-Long M and
Lei Z: Fibronectin-1 modulated by the long noncoding RNA
OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of
osteosarcoma cells. J Cell Physiol. 234:6927–6939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kun-Peng Z, Chun-Lin Z, Jian-Ping H and
Lei Z: A novel circulating hsa_circ_0081001 act as a potential
biomarker for diagnosis and prognosis of osteosarcoma. Int J Biol
Sci. 14:1513–1520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu KP, Ma XL and Zhang CL: LncRNA ODRUL
contributes to osteosarcoma progression through the miR-3182/MMP2
axis. Mol Ther. 25:2383–2393. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma XL, Zhan TC, Hu JP, Zhang CL and Zhu
KP: Doxorubicin-induced novel circRNA_0004674 facilitates
osteosarcoma progression and chemoresistance by upregulating MCL1
through miR-142-5p. Cell Death Discov. 7(309)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dai J, Xu L, Hu X, Han G, Jiang H, Sun H,
Zhu G and Tang X: Long noncoding RNA OIP5-AS1 accelerates CDK14
expression to promote osteosarcoma tumorigenesis via targeting
miR-223. Biomed Pharmacother. 106:1441–1447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang
Z, Luo F, Xu J, Zhou Q and Dai F: Long noncoding RNA OIP5-AS1
causes cisplatin resistance in osteosarcoma through inducing the
LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p.
J Cell Biochem. 120:9656–9666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Almeida E, Mascarenhas BA, Cerqueira A and
Medrado AR: Chondroblastic osteosarcoma. Oral Maxillofac Pathol.
18:464–468. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al: Neoadjuvant chemotherapy with high-dose Ifosfamide,
high-dose methotrexate, cisplatin, and doxorubicin for patients
with localized osteosarcoma of the extremity: A joint study by the
Italian and Scandinavian sarcoma groups. J Clin Oncol.
23:8845–8852. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-Year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miwa S, Shirai T, Yamamoto N, Hayashi K,
Takeuchi A, Igarashi K and Tsuchiya H: Current and emerging targets
in immunotherapy for osteosarcoma. J Oncol.
2019(7035045)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bacci G, Mercuri M, Briccoli A, Ferrari S,
Bertoni F, Donati D, Monti C, Zanoni A, Forni C and Manfrini M:
Osteogenic sarcoma of the extremity with detectable lung metastases
at presentation. Results of treatment of 23 patients with
chemotherapy followed by simultaneous resection of primary and
metastatic lesions. Cancer. 79:245–254. 1997.PubMed/NCBI
|
|
21
|
Brooks JD: Translational genomics: The
challenge of developing cancer biomarkers. Genome Res. 22:183–187.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiao H, Zhang F, Zou Y, Li J, Liu Y and
Huang W: The function and mechanism of long non-coding RNA-ATB in
cancers. Front Physiol. 9(321)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Smolle MA, Prinz F, Calin GA and Pichler
M: Current concepts of non-coding RNA regulation of immune
checkpoints in cancer. Mol Aspects Med. 70:117–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17(89)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu R, Zhang C, Hu Z, Li G, Wang C, Yang
C, Huang D, Chen X, Zhang H, Zhuang R, et al: A five-microRNA
signature identified from genome-wide serum microRNA expression
profiling serves as a fingerprint for gastric cancer diagnosis. Eur
J Cancer. 47:784–791. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ren HY, Sun LL, Li HY and Ye ZM:
Prognostic significance of serum alkaline phosphatase level in
osteosarcoma: A meta-analysis of published data. Biomed Res Int.
2015(160835)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Marais LC, Bertie J, Rodseth R, Sartorius
B and Ferreira N: Pre-treatment serum lactate dehydrogenase and
alkaline phosphatase as predictors of metastases in extremity
osteosarcoma. J Bone Oncol. 4:80–84. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yotsukura S and Mamitsuka H: Evaluation of
serum-based cancer biomarkers: A brief review from a clinical and
computational viewpoint. Crit Rev Oncol Hematol. 93:103–115.
2015.PubMed/NCBI View Article : Google Scholar
|