Introduction
Gastric esophageal cancer can be categorized into
gastric cancer, esophageal cancer and esophagogastric junction
cancer. Gastric esophageal cancer is a common tumour with high
morbidity and mortality worldwide. The 2020 Global Cancer
Statistics show that the incidence and mortality rates of
esophageal cancer were 3.1 and 5.5%, respectively. The incidence
(5.6%) and mortality (7.7%) rates of gastric cancer ranked fifth
and fourth, respectively, among all cancers (1). Gastric esophageal cancer is insidious
and aggressive. Most patients have advanced to the local stage at
the time of diagnosis and have thus lost the best opportunity for
surgery (2). For locally advanced
or metastatic cancers of the esophagus, stomach, or esophagogastric
junction that are unresectable, it is generally accepted that
comprehensive treatment including chemotherapy, targeted therapy
and other systemic antitumour drugs should be adopted. Despite
comprehensive treatment regimens, patients still have a high rate
of metastasis and recurrence, which is one of the leading causes of
death, and the current five-year survival rate for gastric
esophageal cancer is between 15 and 25% (3,4).
In recent years, immune checkpoint inhibitors have
become a new therapeutic method for improving the survival of
patients with malignant tumours after molecular targeted therapy.
Immune checkpoint inhibitors mainly include PD-1 (programmed death
receptor 1), PD-L1 (programmed death receptor ligand 1) and CTLA-4
(cytotoxic T lymphocyte-associated protein 4). T lymphocytes play
an important role in cancer immune monitoring, but cancer cells can
evade tumour reactive T lymphocyte hyperplasia, leading to the
occurrence and development of tumours. Immune checkpoint inhibitors
mainly block the chain of action of tumour cells by acting on the
immune checkpoint on the body's lymphocytes or tumour surface to
achieve antitumour effects. CTLA-4 is an immunosuppressive molecule
expressed on the surface of regulatory T cells (TreGs), activated
CD4+ T cells and depletion-like T cells. CD80/CD86 in
antigen-presenting cells (APCs) activates the immune response by
binding to the costimulatory receptor CD28, whereas CTLA-4 competes
with CD28 to bind CD80/CD86 with greater affinity, resulting in
CD28 shedding from APCs. Thus, its ability to further mediate
immune activation, induce immune tolerance and produce
depletion-like T cells is limited (5). The use of anti-CTLA-4 mab can block
the above inhibition, restore the immunoactivation signal mediated
by the binding of CD28 to CD80/CD86, and stimulate the activation
and proliferation of tumour-specific T cells in lymph nodes. Since
CTLA-4-expressing Tregs and depletion-like T cells are also present
in the tumour microenvironment, anti-CTLA-4 monoclonal antibodies
can also play a role locally in the tumour (5-6). In
addition, whole-human immunoglobulin G1 and the toxicity of
IgG1-mediated cytotoxicity (ADCC) can be influenced by the
combination of ipilimumab and CTLA-4 on Treg cells. Macrophages in
the tumour microenvironment are induced to clear Tregs with high
expression of CTLA-4, and CD8+ effector T cells with low expression
of CTLA-4 are retained to improve the efficiency of the antitumour
immune response (7). Anti-ctla-4
mab also stimulates Th1-like CD4+ T-cell expansion during adaptive
immune response initiation and the early stage and promotes memory
T-cell formation and migration to tumour tissue (8).
On the lymphocyte surface of the body, the
combination of programmed cell death protein-1 (PD-1) with
programmed cell death-ligand 1 (PD-L1) and programmed cell
death-ligand 2 (PD-L2) on the surface of tumour cells can inhibit
lymphocyte function, decrease the antitumour immune response,
increase the incidence of tumour immune escape, and then lead to a
decrease in the ability of the immune system to clear the tumour.
The use of anti-PD-1 or PD-L1 monoantibodies can block this
inhibitory signal and enhance CD8+ effector T-cell proliferation
and the local tumour immune response. Other types of immune cells
(eg. dendritic cells and B cells) can also be inhibited by the
PD-1/PD-L1 pathway, so anti-PD-1 or PD-L1 monoantibodies can
simultaneously produce non-T-cell-dependent antitumour effects
(9-11).
In recent years, a number of clinical studies on
ICIs have shown that they have a good antitumour effect in the
treatment of melanoma, NSCLC, pancreatic cancer and other systemic
tumours (12-14).
At present, according to domestic and foreign guidelines, immune
checkpoint inhibitors combined with chemotherapy have become the
new standard of first-line therapy for advanced metastatic gastric
esophageal cancer. Surgery combined with neoadjuvant
chemotherapy-radiotherapy or concurrent chemoradiotherapy is the
standard of care for patients with locally advanced esophageal
squamous cell carcinoma. However, some locally advanced patients
cannot be treated because of complications and other reasons.
Several studies, such as KEYNOTE-590, ORIENT-15, and CHECKMATE-648,
included patients with inoperable locally advanced esophageal
squamous cell carcinoma who were not candidates for radical surgery
or radical concurrent chemotherapy. However, the spatial and
temporal heterogeneity of gastric esophageal cancer is strong, and
the tumour microenvironment is complex. There are differences in
epidemiological characteristics, clinicopathological
characteristics, biological behaviour, treatment mode and drug
selection between Eastern and Western populations of gastric
esophageal cancer (15).
Therefore, some patients do not benefit from immunotherapy. The
selection of markers for predicting tumour immunotherapy can
predict the efficacy of immunotherapy. A common immunotherapy
sensitivity biomarker is programmed death ligand 1 (PD-L1).
Patients with PD-L1 CPS ≥10 have a predictive role for clinical
guidance (16). Additionally, the
mechanism of immune resistance should be further explored to
provide guidance for the follow-up treatment of patients to prolong
the overall survival of patients.
Based on the above background, this meta-analysis
systematically evaluated the efficacy and safety of first-line
immunotherapy combined with chemotherapy compared with conventional
chemotherapy for unresectable locally advanced or metastatic
gastric esophageal cancer. The study aimed to provide an
evidence-based reference for clinical medication.
Subjects and methods
Literature search strategy
The PubMed, Embase and Cochrane Library electronic
databases were searched. The search terms included ‘esophagogastric
junction carcinoma’, ‘esophageal cancer’, ‘gastric cancer’, ‘PD-1’,
‘immunotherapy’, ‘RCT’, etc. The databases were searched from
inception to June 2022. Subject words and free words were used for
retrieval. In addition, the reference lists of the included studies
were manually searched to identify eligible articles. Detailed
search strategies are presented in Table SI. Our study has registered in
Prospero and the ID is CRD42022351575.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Randomized controlled trials (RCTs) published in English; ii)
inoperable locally advanced stage or metastatic gastric esophageal
cancer patients of any race, nationality, gender and age; iii)
patients in the experimental group were treated with
immunotherapy-based regimens (including nivolumab + ipilimumab,
nivolumab + FP/SOX, toripalimab + TP, etc.), while patients in the
control group were treated with chemotherapy alone (including SOX,
FP, CAPOX, TP or DP); and iv) outcome indicators included overall
survival (OS) and progression-free survival (PFS) in the total
population, PD-L1 CPS ≥10 and PD-L1 <10, objective response rate
(ORR), disease control rate (DCR), adverse event (AEs) and adverse
event grade ≥3.
The exclusion criteria were as follows: i) Duplicate
literature, case reports, editorials or review literature, etc.;
ii) non-first-line therapy literature for patients with inoperable
locally advanced stage or metastatic gastric esophageal cancer;
iii) literature with missing primary data; and iv) non-English
articles.
Study outcome
The primary outcomes of interest of this study were
a meta-analysis of the efficacy and safety of first-line
immunotherapy combined with chemotherapy in patients with
unresectable locally advanced or metastatic gastroesophageal
cancer. The secondary outcomes of interest of this study were the
OS and PFS of patients with PD-L1 expression level (CPS ≥10, CPS
<10). In addition, Asian and non-Asian populations were
analysed.
Data extraction
Two investigators independently screened the
literature according to the inclusion and exclusion criteria. In
case of disagreement, the third investigator was consulted and
settled. The following data were extracted: experiment name, first
author and publication year, country, number of patients, PD-L1
expression level (CPS ≥10, CPS <10), efficacy (OS, PFS, ORR,
DCR) and safety (AEs, grade ≥3 AEs).
Outcome index
According to the curative effect evaluation criteria
of solid tumours RECIST1.1 (Response Evaluation Criteria In Solid
Tumours version 1.1) (17), the
curative effect was divided into complete response (CR), partial
response (PR), progressive disease (PD) and stable disease (SD).
Objective response rate (ORR)=(CR cases + PR cases)/total cases
x100%, disease control rate (DCR)=(CR cases + PR cases + SD
cases)/total cases x100%.
Quality assessment
The quality of the included RCTs was evaluated using
the risk of bias assessment tools recommended in the Cochrane
Manual for Systematic Reviewers 5.1.0, including random sequence
generation, allocation hiding, blinding of subjects and
investigators, blinded evaluation of study outcomes, integrity of
outcome data, selective reporting of study results, and other
sources of bias. Each item was categorized as low risk of bias,
high risk of bias, or unclear risk of bias (18).
Statistical analysis
RevMan 5.3 software was used for meta-analysis.
Enumeration data were expressed as the risk ratio (RR), hazard
ratio (HR) and 95% confidence interval (CI). The chi-square test
and I2 value were used to analyse the heterogeneity
among studies. If there was no statistical heterogeneity among
studies (P>0.1, I2<50%), the fixed effects model
was used. In contrast, if there was statistical heterogeneity among
the studies, a leave-one-out sensitivity analysis was performed to
explore the possible sources of heterogeneity. After analysing the
sources of heterogeneity and excluding heterogeneity, a random
effects model was used for analysis. OS and PFS were subgroup
analysed according to the expression of PD-L1. P<0.05 was
considered to indicate a statistically significant difference.
Funnel plots were used to analyse publication bias.
Results
Search results and
characteristics
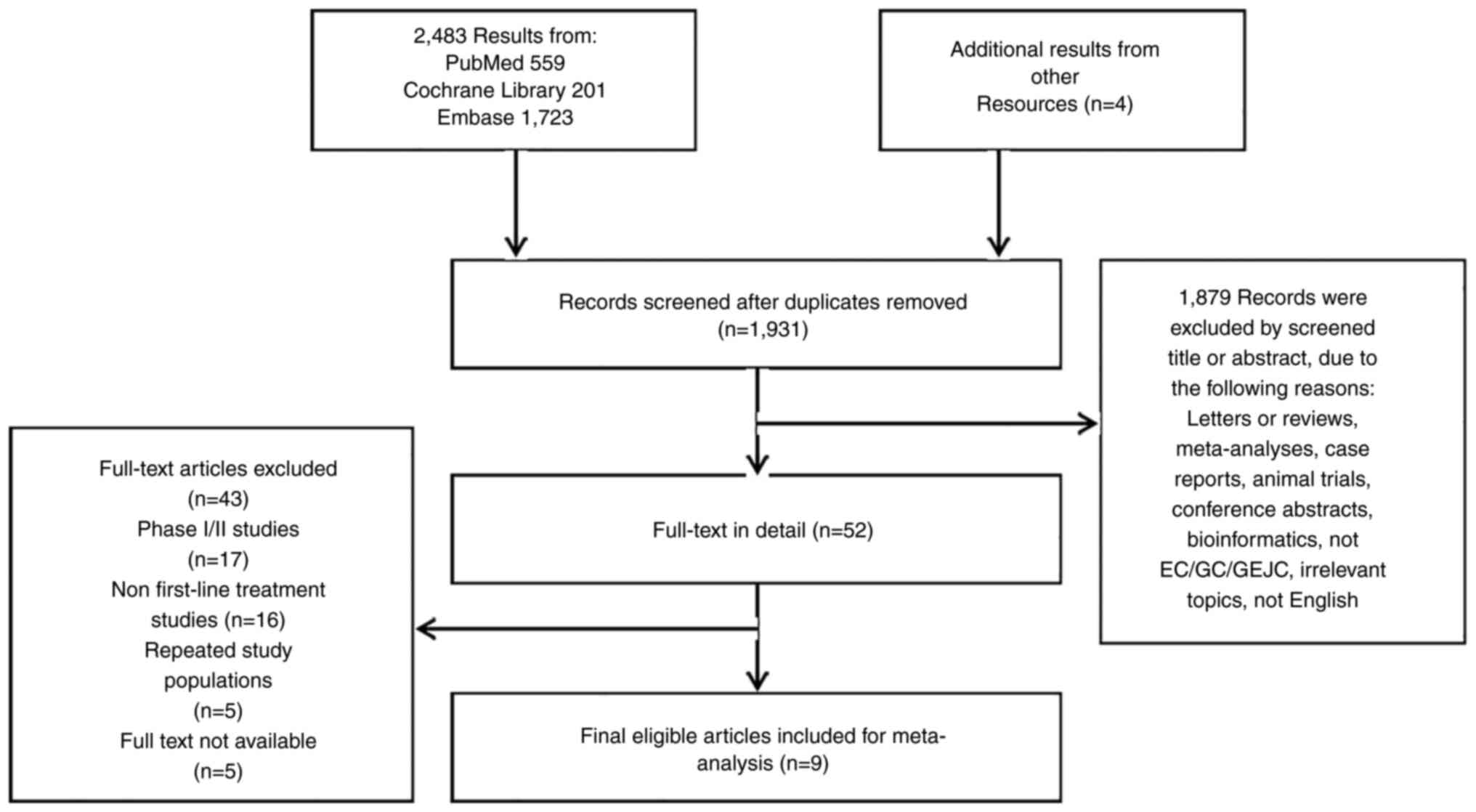
The literature selection process of this study is
detailed in the flow chart (Fig.
1). Through data retrieval and manual retrieval, a total of
2487 articles were identified. After reading the title, abstract
and full text, letters or reviews, meta-analyses, cases, animal
trials, conference abstracts, bioinformatics and other related
articles were deleted. A total of nine phase III randomized
controlled trials (RCTs) were included (19-27),
although KEYNOTE-062 and CHECKMATE- 648 had two evaluable
experimental treatment groups. All included papers were published
between 2020 and 2022. A total of 6,820 patients with gastric
esophageal cancer were involved in the included studies: 3,798
patients in the experimental group and 3,152 patients in the
control group. Asian populations and non-Asian populations were
included in the study. Four of the studies focused only on EC,
while the others focused on GC/GEJC or EC/GEJC. Detailed basic
characteristics are shown in Table
I.
 | Table IThe characteristics of included
studies. |
Table I
The characteristics of included
studies.
| First author,
year | Clinical trial | Study design | Ethnicity | N, P/C | Line of
therapy | Histology | Arms, P vs. C | ORR %, P/C | DCR %, P/C | PFS, HR (95%
CI) | OS, HR (95%CI) | ≥Grade 3 AEs %,
P/C | Any grade AEs %,
P/C | (Refs.) |
|---|
| Luo, 2021 | ESCORT-1st | RCT/III | Asian | 298/298 | 1st | EC | Camrelizumab+ CT
vs. CT alone | 72.1/62.1 | 91.3/88.9 | 0.56
(0.46-0.68) | 0.70
(0.56-0.88) | 63.4/67.7 | 99.3/97 | (21) |
| Sun, 2021 | KEYNOTE-590 | RCT/III | Asian and non-
Asian | 373/376 | 1st | EC/GEJC | Pembrolizumab +CT
vs. CT alone | 45/29.3 | NA | 0.56
(0.46-0.68) | 0.73
(0.62-0.86) | 86/83 | 100/99 | (23) |
| Janjigian,
2021 | KEYNOTE-811 | RCT/III | Asian and non-
Asian | 133/131 | 1st | EC | Pembrolizumab
+trastuzumab+ CT vs. placebo+ trastuzumab+CT | 74.4/51.9 | 96.2/89.3 | NA | NA | 57.1/57.4 | 97.2/98.1 | (24) |
| Shitara, 2020 | KEYNOTE-062 | RCT/III | Asian and non-
Asian | 256 (P1)/ 257 (P2)/
250 (C) | 1st | GC/GEJC | Pembrolizumab (P1)
vs. Pembrolizumab +CT (P2) vs. CT (C) | NA | NA | NA | NA | 16.9 (P1)/ 73.2
(P2)/ 69.3 (C) | 95.3(P1)/ 97.6
(P2)/ 98.4 (C) | (20) |
| Janjigian,
2021 | CHECKMATE- 649 | RCT/III | Asian and non-
Asian | 789/792 | 1st | GC/GEJC/ EC | Nivolumab+CT vs.
CT | 60/45 | NA | 0.77
(0.68-0.87) | 0.8
(0.68-0.94) | 59.6/44 | 94.4/88.5 | (22) |
| Doki, 2022 | CHECKMATE- 648 | RCT/III | Asian and non-
Asian | 321 (P1)/ 325 (P2)/
324 (C) | 1st | EC | Nivolumab+
ipilimumab (P1) vs. Nivolumab+ CT (P2) vs. CT (C) | 28 (P1)/ 47 (P2)/
27 (C) | 59.4 (P1)/ 79.4
(P2)/ 72.5 (C) | P1 vs..C:1.26
(1.04-1.52) P2 vs.C:0.81 (0.64-1.04) | P1 vs. C:0.78
(0.62-0.98) P2 vs. C:0.74 (0.58-0.96) | 32 (P1)/ 47 (P2)/
36 (C) | 80 (P1)/ 96 (P2)/
90 (C) | (19) |
| Kang, 2022 | ATTRACTION- 4 | RCT/III | Asian | 362/362 | 1st | GC/GEJC | Nivolumab+ CT vs.
CT | 57/48 | NA | 0.68
(0.51-0.90) | 0.90
(0.71-1.08) | 20/16 | 98/97 | (25) |
| Lu, 2022 | ORIENT-15 | RCT/III | Asian and non-
Asian | 327/332 | 1st | EC | Sintilimab+CT vs.
CT | 66/45 | 90/84 | 0.56
(0.46-0.68) | 0.63
(0.51-0.78) | 60/55 | 98.2/98.2 | (26) |
| Wang, 2022 | JUPITER-06 | RCT/III | Asian | 257/257 | 1st | EC | Toripalimab+ CT vs.
CT | 69.3/52.1 | 89.1/82.1 | 0.58
(0.46-0.74) | 0.58
(0.43-0.78) | 73.2/70 | 99.2/99.2 | (27) |
Efficacy outcomes of the
immunotherapy-based regimens
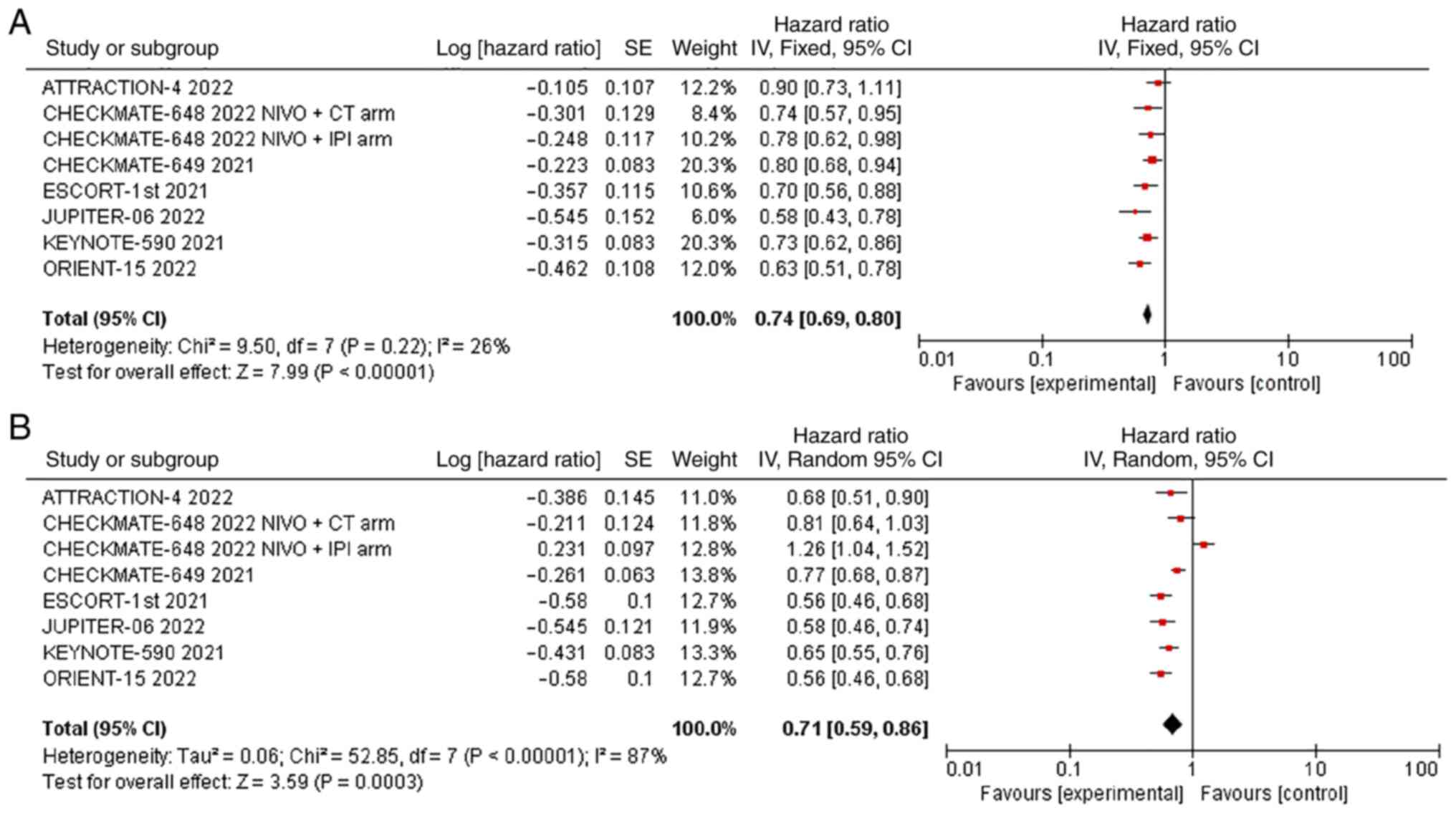
OS was reported in all 7 studies, and there was no
significant statistical heterogeneity (P=0.22, I2=26%).
Therefore, we used the fixed effects model for meta-analysis, and
the results showed that the OS of the experimental group
(immunotherapy-based regimens) was significantly longer than that
of the control group (chemotherapy alone) [HR=0.74; 95% CI
(0.69-0.80); P<0.00001] (Fig.
2A).
With respect to PFS, all 7 studies reported PFS,
with significant statistical heterogeneity among studies
(P<0.00001, I2=87%). Therefore, we used the random
effects model for meta-analysis, and the results showed that the
PFS of the experimental group (immunotherapy as the main regimen)
was significantly longer than that of the control group
(chemotherapy alone) [HR=0.71; 95% CI (0.59, 0.86); P=0.0003]
(Fig. 2B). A leave-one-out
sensitivity analysis revealed that the source of heterogeneity in
the examination of PFS was the CHECKMATE-648 2022 NIVO + IPI group.
The forest plot was redrawn without this study, and the
heterogeneity was significantly reduced (Fig. S1).
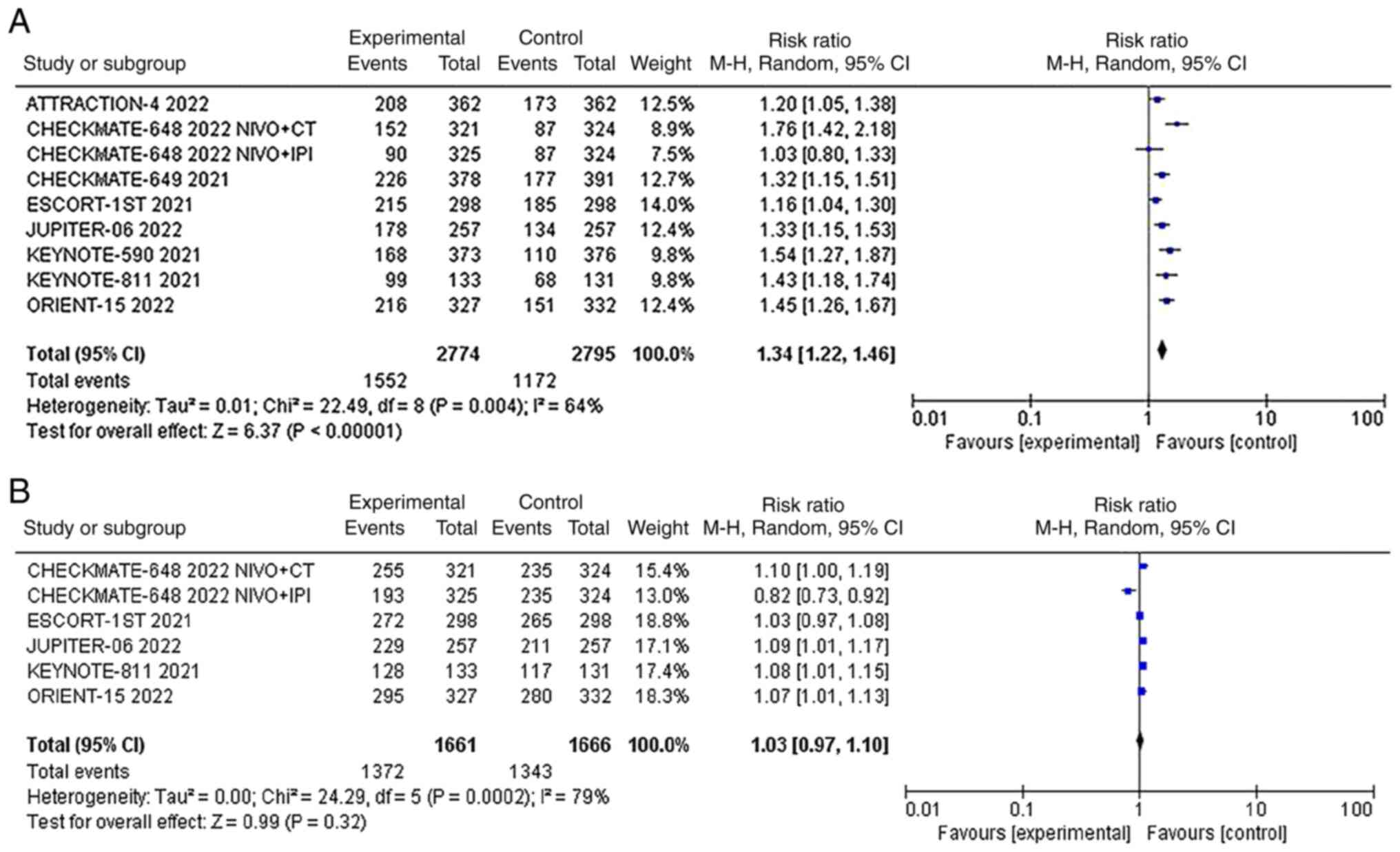
The ORR was reported in all 8 studies, and there was
significant statistical heterogeneity among the studies
(P<0.00001, I2=64%). Therefore, we used the random
effects model for meta-analysis, and the results showed that the
ORR of the two groups of patients was compared. The ORR of the
experimental group (immunotherapy as the main regimen) was
significantly higher than that of the control group (chemotherapy
alone) [RR=1.34; 95% CI (1.22, 1.46); P=0.004] (Fig. 3A).
Five studies presented data on DCR, and there was
significant statistical heterogeneity among the studies (P=0.0002,
I2=79%). Therefore, we used the random effects model for
meta-analysis, and the results showed that there was no significant
difference in DCR between the two groups [RR=1.03; 95% CI (0.97,
1.10); P=0.32] (Fig. 3B). A
leave‑one‑out sensitivity analysis showed that the source of
heterogeneity was CHECKMATE-648 NIVO + IPI arm (Fig. S2).
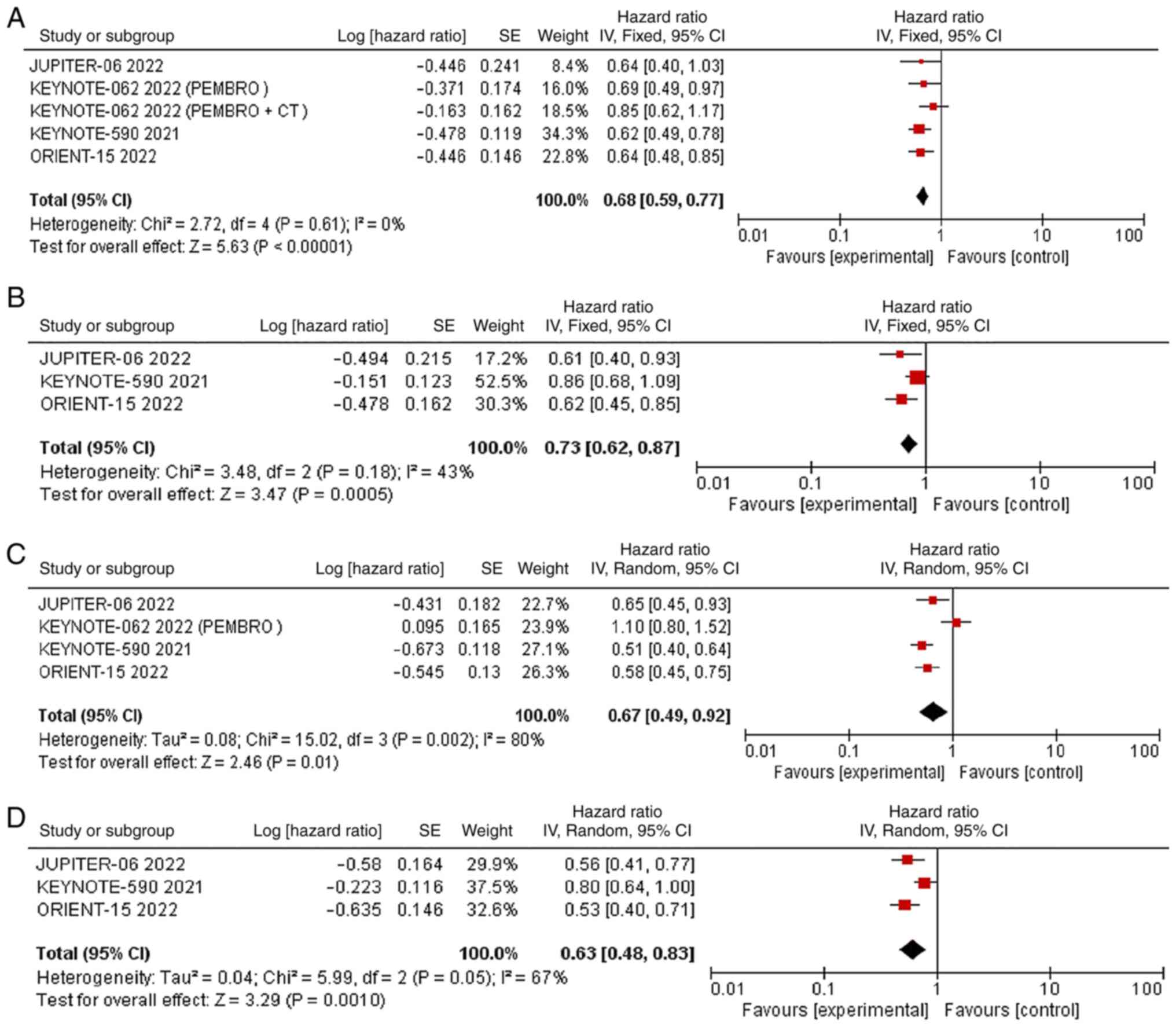
Subgroup analysis of PD-L1 expression
state with PFS and OS
Subgroup analysis was performed based on PD-L1 CPS
≥10 and PD-L1 CPS <10. JUPITER-06, KEYNOTE-590, KEYNOTE-062 and
ORIENT-15 reported the OS data of the outcome subgroups. With
respect to the OS of patients with PD-L1 CPS ≥10, there was no
significant statistical heterogeneity (P=0.61, I2=0%).
Therefore, we used the fixed effects model for meta-analysis.
Meta-analysis showed that the OS of patients with PD-L1 CPS ≥10 in
the experimental group was significantly longer than that in the
control group [HR=0.68; 95% CI (0.59, 0.77); P<0.00001]
(Fig. 4A). For the OS of patients
with PD-L1 CPS <10, there was no significant statistical
heterogeneity (P=0.18, I2=43%). Therefore, we used the
fixed effects model for meta-analysis, The OS of patients with
PD-L1 CPS <10 in the experimental group was also longer than
that in the control group [HR=0.73; 95% CI (0.62, 0.87); P=0.0005]
(Fig. 4B). We also showed OS no
statistical difference between CPS ≥10 and CPS <10 groups
(P=0.46) (Fig. S3). The HR for OS
in patients with PD-L1 CPS ≥10 and CPS <10 groups were
respectively 0.68 (95% CI 0.59-0.77; P<0.01) and 0.73 (95% CI
0.62-0.87; P<0.01) .
With respect to PFS of patients with PD-L1 CPS ≥10,
all 4 studies reported, with significant statistical heterogeneity
among studies (P=0.002, I2=80%). Therefore, we used the
random effects model for meta-analysis. The results of subgroup
analysis showed that the PFS of patients with PD-L1 CPS ≥10 in the
experimental group was significantly longer than that in the
control group [HR=0.67; 95% CI (0.49, 0.92); P=0.01] (Fig. 4C). A leave‑one‑out sensitivity
analysis showed that the source of heterogeneity was KEYNOTE‑062
PEMBRO arm (Fig. S4). The PFS of
patients with PD-L1 CPS <10 was reported in all 3 studies, with
significant statistical heterogeneity among studies (P=0.05,
I2=67%). Therefore, we used the random effects model for
meta-analysis. The PFS of patients with PD-L1 CPS <10 in the two
groups was also prolonged in the experimental group compared with
the control group [HR=0.63; 95% CI (0.48, 0.83); P=0.001] (Fig. 4D). PD-L1 CPS <10 had
heterogeneity, which was found by a leave-one-out sensitivity
analysis, and the source of heterogeneity was KEYNOTE-590 (Fig. S5). We also showed PFS no
statistical difference between CPS ≥10 and CPS <10 groups
(P=0.002) (Fig. S6). The HR for
OS in patients with PD-L1 CPS ≥10 and CPS <10 groups were
respectively 0.67 (95% CI 0.49-0.92; P=0.01) and 0.63 (95% CI
0.48-0.83; P<0.01).
Subgroup analysis of Asian and
non-Asian with OS
Subgroup analysis was performed based on Asian and
non-Asian ethnicity. CHECKMATE-648, CHECKMATE-649, KEYNOTE-590 and
ORIENT-15 reported the OS data of the outcome subgroups. there was
no significant statistical heterogeneity (P=0.57,
I2=0%). Therefore, we used the fixed effects model for
meta-analysis, and the results showed that in the Asian population,
the OS of the experimental group (immunotherapy-based regimens) was
significantly longer than that of the control group (chemotherapy
alone) [HR=0.71; 95% CI (0.64-0.78); P<0.00001]. In the
non-Asian population, the OS of the experimental group
(immunotherapy-based regimens) was also significantly longer than
that of the control group (chemotherapy alone) [HR=0.78; 95% CI
(0.70-0.87); P<0.00001] (Fig.
S7).
Safety evaluation of anti-PD-1/PD-L1
immunotherapy
The overall incidence of adverse events was reported
in all 9 studies, and there was moderate statistical heterogeneity
(P<0.01, I2=79%). Therefore, we used the random
effects model for meta-analysis, and the results showed that there
was no difference in the overall incidence of adverse events
between the experimental group and the control group [RR=1; 95% CI
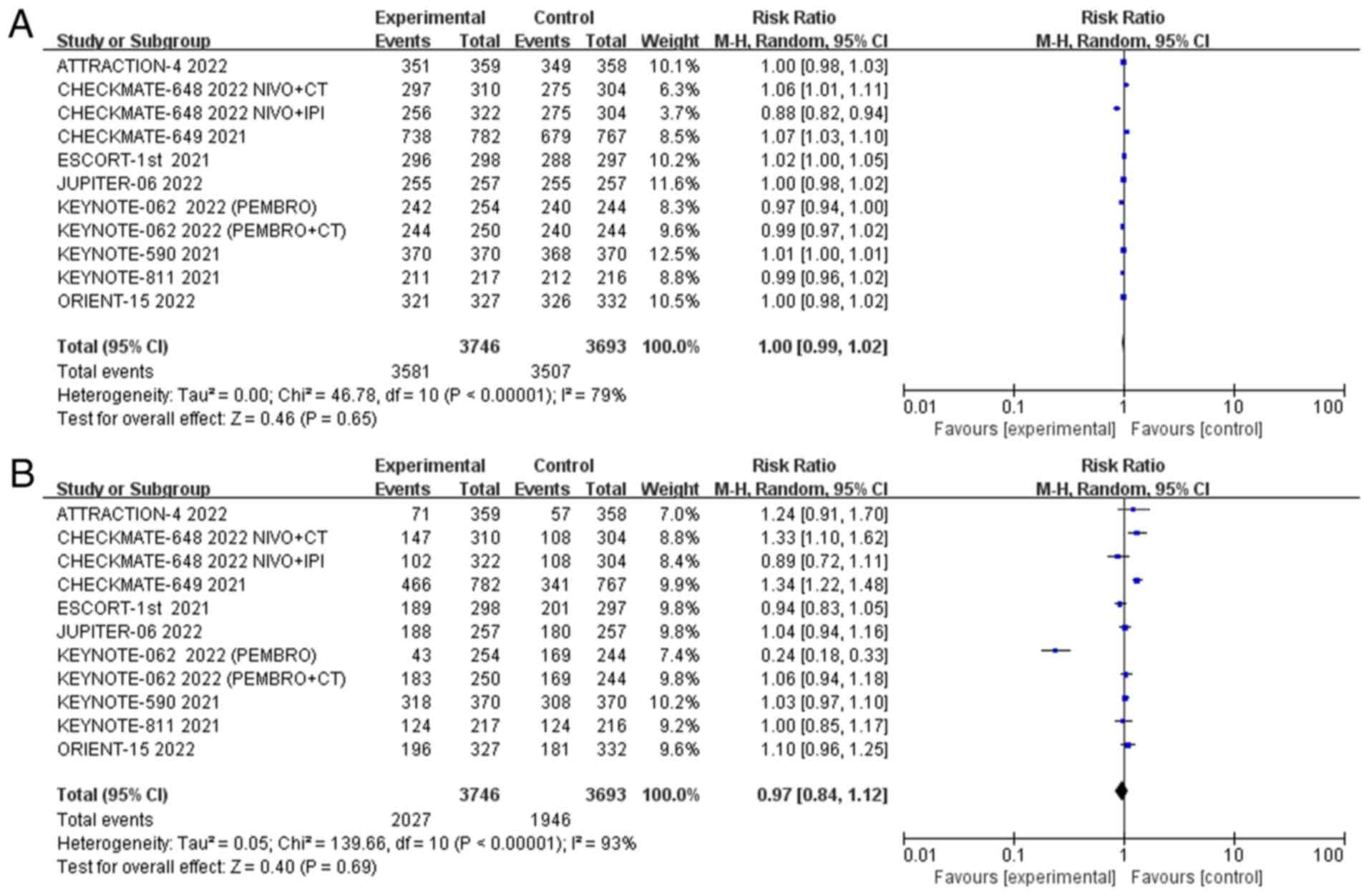
(0.99, 1.02); P=0.65] (Fig. 5A).
The incidence of grade 3 or higher adverse events was reported in 9
studies, with high statistical heterogeneity among studies
(P<0.01, I2=93%). Therefore, we used the random
effects model for meta-analysis, and the results showed that there
was no difference in the incidence of grade 3 or higher adverse
events between trial patients and the control group [RR=0.97; 95%
CI (0.84, 1.12); P=0.69] (Fig.
5B). Grade ≥3 adverse events had a high heterogeneity of 93%.
Sensitivity analysis showed that the source of heterogeneity was
KEYNOTE-062 PEMBRO arm, and the degree of heterogeneity was reduced
to 77% after deletion of this study (Fig. S8).
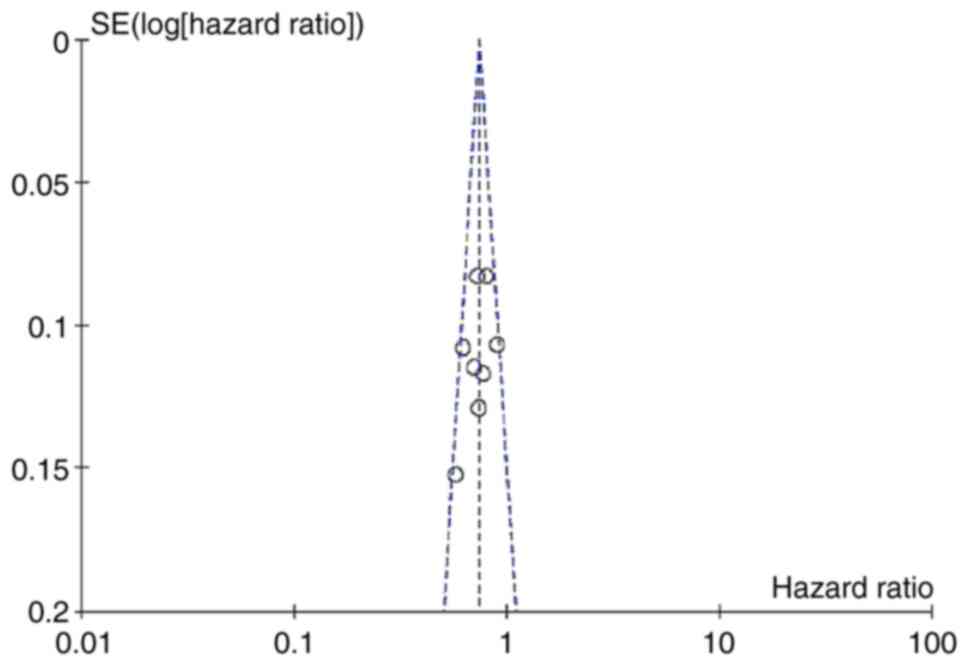
Publication bias
To detect publication bias, PFS and OS were used to
draw an inverted funnel plot, as shown in Fig. 6. The scattered points of each study
were basically symmetric and evenly distributed in the inverted
funnel plot, suggesting that there was little possibility of
publication bias in our meta-analysis.
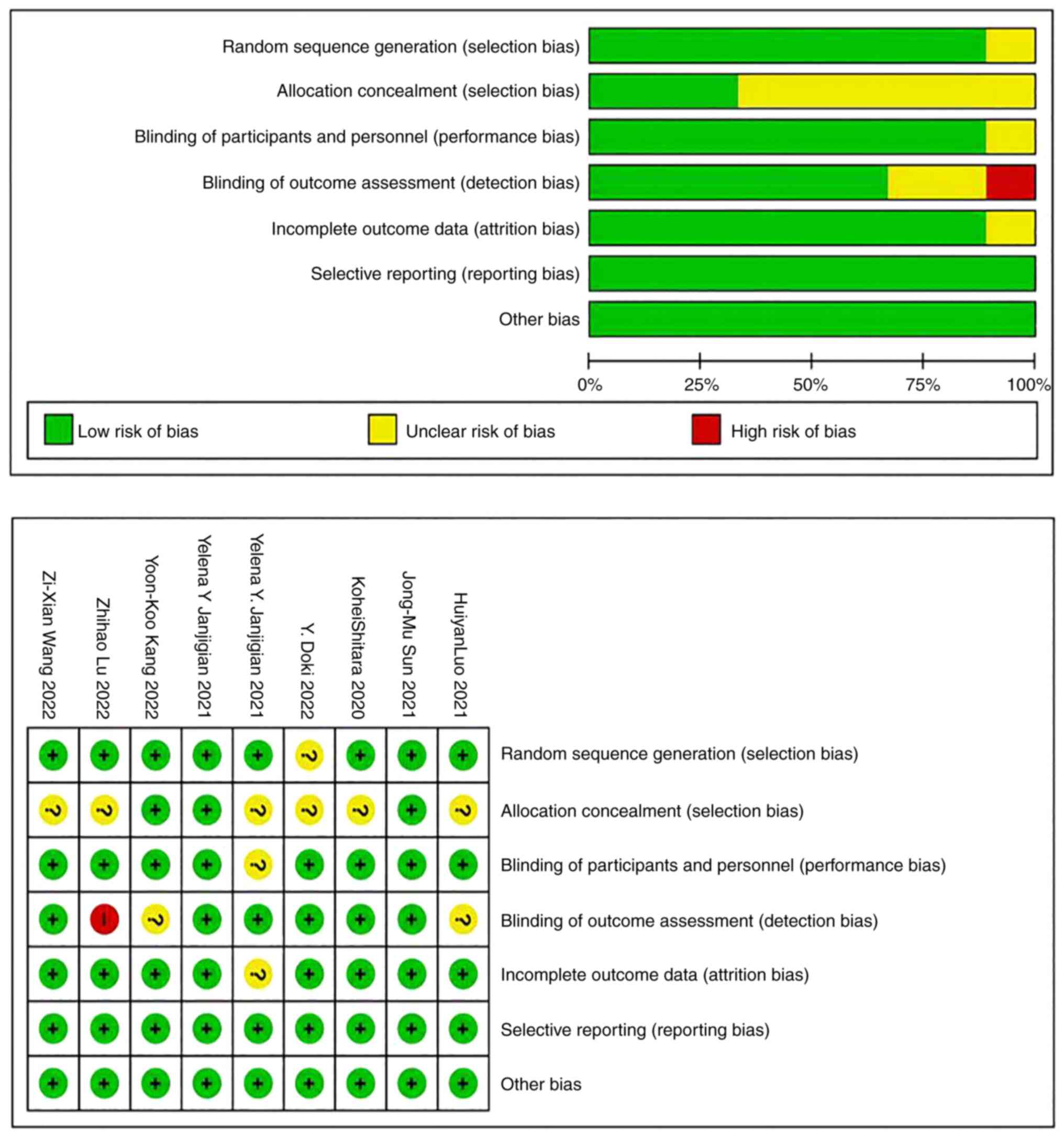
Assessment of study quality
The quality of the 9 RCTs included was evaluated by
the risk of bias assessment tool recommended by the Cochrane
Systematic Reviewers Manual 5.1.0. The results showed that the
quality of the articles was high, and the overall risk of bias was
low (Fig. 7).
Discussion
Gastric esophageal cancer is an aggressive tumour
that significantly affects cancer-related mortality worldwide. For
patients with locally advanced esophageal squamous cell carcinoma
who are not eligible for surgery combined with neoadjuvant
chemotherapy-radiotherapy or concurrent chemoradiotherapy,
immuno/chemotherapy as the first line of the gold standard
treatment options. In addition, most gastric esophageal cancers are
advanced at the time of diagnosis and cannot be surgically
resected, resulting in poor prognosis and short survival. Recently,
in the treatment of gastric esophageal cancers, immunotherapy, as a
new treatment regimen, has the advantages of prolonging the
survival time of patients and reducing the incidence of adverse
reactions compared with conventional chemotherapy (4). However, due to the short time of
approval of immunocombination therapy for the first-line treatment
of advanced gastric esophageal cancer, its unique immune-related
adverse events and high price, its clinical application is
limited.
This meta-analysis of 9 articles that examined
anti-PD-1/PD-L1 immunotherapy as a first-line treatment for
advanced gastric esophageal cancer and systematically evaluated the
efficacy and safety of this treatment. Additionally, the programmed
death ligand 1 binding positive score (CPS) subgroup was also
analysed to identify the characteristics of patients with immune
benefit and provide a decision-making basis for clinical
practice.
The results of this study show that combined
immunotherapy can prolong PFS and OS and reduce the risk of disease
recurrence and death in patients with gastric oesophageal cancer
compared with conventional first-line chemotherapy. Moreover,
combined immunotherapy does not increase toxicity compared with
chemotherapy alone.
Therefore, biomarkers such as PD-L1 expression may
have a potential predictive role. In previous studies of
gastroesophageal adenocarcinoma, increasing the CPS threshold from
1 to 5 or 10 maximizes the therapeutic index of immunotherapy
(28).
Therefore, PD-L1 detection as a biomarker needs to
be further considered to determine whether it is related to tumour
heterogeneity, the interval between biopsy and treatment,
antibodies and staining methods, cut-off value definition,
inconsistent immunohistochemical evaluation criteria and other
factors. However, some studies have found that patients with
microsatellite instability (MSI) and EBV positivity have a better
response to immunotherapy. Second, gastric cancer is also
correlated with tumour mutation burden (TMB), ctDNA mutation burden
and other factors, which may become potential biomarkers for
immunotherapy (16). The results
of this meta-analysis also showed that immune checkpoint inhibitors
in the treatment of advanced gastric oesophageal cancer prolonged
the overall survival of patients in the PD-l1 (+) subgroup.
Asian and non-Asian populations were divided into
two groups for meta-analysis. Based on the above subgroup analysis,
there was no heterogeneity between the two groups
(I2=0%, P>0.1), meaning that the efficacy of gastric
oesophageal cancer was not affected in Asian and non-Asian
populations, and the risk of death was reduced in both Asian and
non-Asian populations. Among them, there was no intra-group
heterogeneity in the Asian population, and the results of 5 studies
were combined. The fixed-effect model was selected to combine the
effect size HR, which was 71% and significant (z=6.63,
P=0.00001<0.05), meaning that immunotreatment-based regimens
significantly reduced the risk of death in the Asian population,
only 71 percent of that of chemotherapy alone. Secondly, there was
no intra-group heterogeneity in the non-Asian population, and the
results of four studies were combined. The fixed-effect model
combined the effect size HR, which was 78% and significant (z=4.58,
P=0.00001<0.05), meaning that immunotreatment-based regimens
significantly reduced the risk of death in the non-Asian
population, to only 78% of that of chemotherapy alone. Zhang et
al (29) showed that Asian and
Western patients have similar responses to systemic therapy in
unresectable gastric or gastroesophageal adenocarcinoma. However,
first-line immunotherapy in Asian populations showed better OS in
unresectable gastric or gastroesophageal adenocarcinoma than in
Western populations. This was considered to be related to
inconsistencies in enrolment studies and treatment lines.
While immune checkpoint inhibitors are widely used,
they can also cause autoimmune or inflammatory responses called
immune-related adverse events (irAEs) (30), which occur in more than 80% of
patients receiving immune checkpoint inhibitors, and the incidence
of grade ≥3 TRAEs is low. Therefore, the safety of immune
checkpoint inhibitor therapy is good. Noori et al (31) showed that first-line ICIs plus
chemotherapy prolonged OS and PFS in patients with advanced
esophageal gastric cancer compared with chemotherapy alone. And the
incidence of AE was higher in the combined treatment group.
Regarding the incidence of adverse reactions, firstly, the
inclusion of this study was inconsistent with that of literature
studies, so this study was included in Phase III randomized
controlled study. Second, when the incidence of grade 3 or higher
adverse events was analysed, the heterogeneity of grade ≥3 adverse
events was high (93%). A leave-one-out sensitivity analysis showed
that the source of heterogeneity was KEYNOTE-062 pembrolizumab
combined with chemotherapy, and the degree of heterogeneity was
reduced to 77% after deletion of this study.
One limitation of this study is that PD-L1 is an
imperfect biomarker of choice for upper gastrointestinal tumours.
There is a lack of standardization of platforms and antibodies for
evaluation (16). Different
studies used different scoring systems, antibodies, and positive
thresholds, making it difficult to combine all available data. The
PD-L1 CPS treatment threshold was dependent on tumour histology and
treatment cycle, and the PD-L1 positive threshold was not
consistent across trials. Although different antibodies were used,
the study with pembrolizumab applied CPS ≥1 and 10, whereas the
study with nivolumab used a cut-off value of 5. The spatial and
temporal heterogeneity of tumours makes the detection of PD-L1
status more difficult. The most reliable biomarkers may require
multiple biopsies and repeated testing during disease progression.
However, it is not clear how to interpret the inconsistent results
(determining treatment based on the lowest or highest CPS). In
addition to PD-L1, upper gastrointestinal tumours should also be
detected for microsatellite high instability (MSI-H) and tumour
mutational burden (TMB). The Food and Drug Administration (FDA) has
approved pembrolizumab for patients with mismatch repair defects
and/or a high tumour mutation burden. Similar to other diseases,
MSI-H status is a strong predictive biomarker of IO response in
upper gastrointestinal tumours (32). Therefore, all patients should be
tested for MSI status or MMR protein expression. Therefore, we
urgently need to continue to optimize the detection of the
biomarker PD-L1 and find new biomarkers for biological prediction.
It is hoped that more in-depth stratified analyses will be
conducted in the future to identify the beneficiary population of
immunotherapy.
There are also the methodological limitations of
their systematic review and meta-analysis. Although statistical
heterogeneity existed between studies, a leave-one-out sensitivity
analysis was used to explore possible sources of heterogeneity.
However, almost all the included studies have the risk of bias in
quality assessment, mainly due to the lack of important outcome
data, which needs to be further discussed and analysed in future
studies, such as the analysis of different cancer types.
In conclusion, immunotherapy-based regimens are
superior to standard chemotherapy in the first-line treatment of
advanced gastric oesophageal cancer, with significantly improved
OS, PFS, DCR, and ORR. Furthermore, patients in the PDL1 CPS ≥10
subgroup appeared to benefit more significantly than the total
population. The incidence of adverse reactions in the
immunotherapy-based group was not higher than that in the
chemotherapy-based group. Our results suggest that
immunotherapy-based regimens may be a new choice for first-line
chemotherapy in patients with advanced gastric esophageal cancer.
Our results highlight the need to conduct additional randomized
controlled trials, to further examine PD-L1 CPS treatment
thresholds, and to detect additional biomarkers to identify immune
therapy beneficiary populations.
Supplementary Material
A leave-one-out sensitivity of
progression-free survival in immunotherapy-based regimens compared
with chemotherapy in EC/GC/GEJC. IPI, ipilimumab; NIVO, nivolumab;
CT, chemotherapy; SE, standard error; df, degrees of freedom; CI,
confidence interval.
A leave-one-out sensitivity of disease
control rate in immunotherapy-based regimens compared with
chemotherapy in EC/GC/GEJC. IPI, ipilimumab; NIVO, nivolumab; CT,
chemotherapy; SE, standard error; df, degrees of freedom; CI,
confidence interval; M-H, Mantel-Haenszel.
Subgroup analysis of OS in patients
with PD-L1 CPS ≥10 and CPS <10 groups. CI, confidence interval;
CPS, combined positive score; SE standard error; df, degrees of
freedom; CI, confidence interval; M-H, Mantel-Haenszel.
A leave-one-out sensitivity analysis
of progression-free survival in patients with PD-L1 CPS ≥10. IPI,
ipilimumab; NIVO, nivolumab; CT, chemotherapy; SE, standard error;
df, degrees of freedom; CI, confidence interval; CPS, combined
positive score.
A leave-one-out sensitivity analysis
of progression-free survival in patients with PD-L1 CPS <10. EC,
oesophageal cancer; IPI, ipilimumab; NIVO, nivolumab; CT,
chemotherapy; SE, standard error; df, degrees of freedom; CI,
confidence interval; CPS, combined positive score.
Subgroup analysis of progression-free
survival in patients with PD-L1 CPS ≥10 and CPS <10 groups. CI,
confidence interval; CPS, combined positive score; SE standard
error.
Subgroup analysis of overall survival
in Asian and non-Asian patients. IPI, ipilimumab; NIVO, nivolumab;
CT, chemotherapy; SE, standard error; df, degrees of freedom; CI,
confidence interval.
A leave-one-out sensitivity of the
incidence of grade 3 and above adverse events in the two groups.
IPI, ipilimumab; NIVO, nivolumab; CT, chemotherapy; SE, standard
error; df, degrees of freedom; CI, confidence interval; M-H,
Mantel-Haenszel.
Search strategies.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Medical Science Research
Project of Hebei Province (grant no. 20230971).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JS, HD, XD, RQ and MY designed the study. HD, XD, RQ
and MY reviewed the literature, designed the article structure and
extracted the data. JS, HD, XD and RQ analysed and interpreted the
data results. HD and XD wrote the manuscript. JS and HD revised and
edited key points in the manuscript. XD and RQ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baumgartner R, Taghizadeh H, Jomrich G,
Schoppmann SF, Preusser M and Ilhan-Mutlu A: Utilization and
efficacy of palliative chemotherapy for locally advanced or
metastatic gastroesophageal carcinoma. Anticancer Res. 40:965–975.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van den Ende T, Smyth E, Hulshof MCCM and
van Laarhoven HWM: Chemotherapy and novel targeted therapies for
operable esophageal and gastroesophageal junctional cancer. Best
Pract Res Clin Gastroenterol. 36-37:45–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen K, Wang X, Yang L and Chen Z: The
anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: A
systematic review and meta-analysis and literature review. Cancer
Control. 28(1073274821997430)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seidel JA, Otsuka A and Kabashima K:
Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of
action, efficacy, and limitations. Front Oncol.
8(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vargas FA, Furness AJS, Litchfield K,
Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N,
Roddie C, et al: Fc effector function contributes to the activity
of human anti- CTLA-4 antibodies. Cancer Cell. 33:649–663.e4.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie anti-CTLA-4 and
anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin EM, Gong J, Klempner SJ and Chao J:
Advances in immuno-oncology biomarkers for gastroesophageal cancer:
Programmed death ligand 1, microsatellite instability, and beyond.
World J Gastroenterol. 24:2686–2697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tocheva AS and Mor A: Checkpoint
inhibitors: Applications for autoimmunity. Curr Allergy Asthma Rep.
17(72)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ribas A, Kirkwood JM and Flaherty KT:
Anti-PD-1 antibody treatment for melanoma. Lancet Oncol.
19(e219)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang MY, Jiang XM, Wang BL, Sun Y and Lu
JJ: Combination therapy with PD-1 PD-L1 blockade in non-small cell
lung cancer strategies and mechanisms. Pharmacol Ther.
219(107694)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bear AS, Vonderheide RH and O'Hara MH:
Challenges and opportunities for pancreatic cancer immunotherapy.
Cancer Cell. 38:788–802. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle
S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC,
et al: Signatures of tumour immunity distinguish Asian and
non-Asian gastric adenocarcinomas. Gut. 64:1721–1731.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leone AG, Petrelli F, Ghidini A, Raimondi
A, Smyth EC and Pietrantonio F: Efficacy and activity of PD-1
blockade in patients with advanced esophageal squamous cell
carcinoma: A systematic review and meta-analysis with focus on the
value of PD-L1 combined positive score. ESMO Open.
7(100380)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Watanabe H, Okada M, Kaji Y, Satouchi M,
Sato Y, Yamabe Y, Onaya H, Endo M, Sone M and Arai Y: New response
evaluation criteria in solid tumours-revised RECIST guideline
(version 1.1). Gan To Kagaku Ryoho. 36:2495–2501. 2009.PubMed/NCBI(Article in Japanese).
|
|
18
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal squamous-cell carcinoma.
N Engl J Med. 386:449–462. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer. Jama Oncol. 6:1571–1580. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos B, Bragagnoli A, et al: First-line nivolumab plus
chemotherapy versus chemotherapy alone for advanced gastric,
gastro-oesophageal junction, and oesophageal adenocarcinoma
(CheckMate 649): A randomised, open-label, phase 3 trial. Lancet.
398:27–40. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janjigian YY, Kawazoe A, Yañez P, Li N,
Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al: The
KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive
gastric cancer. Nature. 600:727–730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377(e68714)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng
J, Yang S, Fan Y, Shi J, Zhang X, et al: Toripalimab plus
chemotherapy in treatment-naïve, advanced esophageal squamous cell
carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell.
40:277–288.e3. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lei M, Siemers NO, Pandya D, Chang H,
Sanchez T, Harbison C, Szabo PM, Janjigian Y, Ott PA, Sharma P, et
al: Analyses of PD-L1 and inflammatory gene expression association
with efficacy of nivolumab ± ipilimumab in gastric
cancer/gastroesophageal junction cancer. Clin Cancer Res.
27:3926–3935. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Z, Liu Z and Chen Z: Comparison of
treatment efficacy and survival outcomes between Asian and western
patients with unresectable gastric or gastro-esophageal
adenocarcinoma: A systematic review and meta-analysis. Front Oncol.
12(831207)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noori M, Mahjoubfar A, Azizi S, Fayyaz F
and Rezaei N: Immune checkpoint inhibitors plus chemotherapy versus
chemotherapy alone as first-line therapy for advanced gastric and
esophageal cancers: A systematic review and meta-analysis. Int
Immunopharmacol. 113(109317)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Patel MA, Kratz JD, Lubner SJ, Loconte NK
and Uboha NV: Esophagogastric cancers: Integrating immunotherapy
therapy into current practice. J Clin Oncol. 40:2751–2762.
2022.PubMed/NCBI View Article : Google Scholar
|