Introduction
A keloid is a hyperplastic skin disease
characterized by excessive fibroblast proliferation, excessive
deposition of extracellular matrix (ECM) and inflammatory cell
infiltration (1). Keloids result
in excessive fibrosis of local tissues caused by the abnormal
healing of the skin following injury or inflammation (2). They can be accompanied by pain,
hypersensitivity and itching, which can significantly affect the
quality of life of patients (3).
Their formation is mainly caused by changes in the regulation of
growth factors, abnormal conversion of collagen, genetics, immune
dysfunction and sebum reaction (4). At present, surgical resection is the
main treatment for keloids; however, treatment efficiency is low
and relapse is common (5).
Alternative treatments include steroid injections, radiotherapy,
and laser, silicone and stress therapy (6). Although keloids benign dermal tumors,
they have high recurrence rates (7). Keloid fibroblasts are considered the
key effector cells for keloid formation (8). Studies have found that fibroblast
dysfunction, including abnormal proliferation, invasive migration
and invasion, and excessive ECM production, is involved in keloid
formation (9). Therefore,
identifying treatments or drugs that can inhibit fibroblast
dysfunction may be an effective way to treat keloids.
Isorhamnetin (IH) is a type of flavonoid widely
found in sea buckthorn, ginkgo biloba and sophora japonica
(10). Recent studies have shown
that IH has biological activities, including cardiovascular and
cerebrovascular protective, anti-tumor, anti-inflammatory,
antioxidant activities, and is also associated with obesity
prevention (11). IH can inhibit
the migration and invasion of non-small-cell lung cancer cells by
inhibiting the Akt/ERK-mediated epithelial-mesenchymal transition
(EMT) process (12). Another study
showed that IH alleviates bleomycin-induced endoplasmic reticulum
stress and PERK signal activation to play a protective role in
bleomycin-induced pulmonary fibrosis in mice (13). In addition, Yang et al
(14) found that IH suppressed
hepatic stellate cell activation and prevented hepatic fibrosis by
inhibiting the TGF-β/Smad signaling pathway and alleviating
oxidative stress. However, the biological roles of IH in keloid
progression remain unclear. Therefore, identifying the roles of IH
in keloid fibroblasts and the potential underlying mechanisms is
crucial.
Materials and methods
Bioinformatics analysis
The TargetNet database (http://targetnet.scbdd.com/calcnet/calc_text/) was
used to predict the binding between IH and sphingosine-1-phosphate
receptor-1 (S1PR1) in keloids. In addition, the structure of IH was
obtained from the Zinc (http://zinc.docking.org) and PubChem databases
(https://pubchem.ncbi.nlm.nih.gov), and
protein structure was obtained from the PDB database (http://www.rcsb.org). AutoDockTools 1.5.6 software was
used to process proteins and Discovery Studio 4.5 software was used
to visualize the molecular docking results to obtain 2D and 3D
images.
Cell culture and treatment
Keloid fibroblasts (ATCC®CRL-1762™) were
obtained from the American Type Culture Collection and cultivated
in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS and 1% penicillin/streptomycin in a humidified
atmosphere with 5% CO2 at 37˚C. The cells were then
treated with different doses of IH (20, 50 and 100 µM) for 24, 48
or 72 h. IH (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA)
and was adjusted to final concentrations using complete culture
medium. The final DMSO concentration was <0.05% in all
experiments (i.e., a non-cytotoxic range).
Cell transfection
To knock down S1PR1, specific siRNA targeting S1PR1
(siRNA-S1PR1-1: 5'-GCUAUUCAUUAGAUAGUAAUU-3';
siRNA-S1PR1-2:5'-GCAUUGUCAAGCUCCUAAAGG-3') and corresponding
control siRNA (siRNA-NC: 5'-UUCUCCGAACGUGUCACGU-3'; both
synthesized by Gene Pharma) were used. To overexpress S1PR1, the
pc-DNA3.1 vector containing the whole length of S1PR1 (Ov-S1PR1)
and the empty vector (Ov-NC; both synthesized by Gene Pharma) were
used. These recombinants were transfected into keloid fibroblasts
using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Following 48 h of transfection, cells were harvested for subsequent
experiments.
Cell counting kit-8 (CCK-8) assay
Keloid fibroblasts were treated with different doses
of IH (20, 50 and 100 µM) with or without Ov-S1PR1, seeded into
96-well plates at a density of 5x104 cells/ml and
cultured in DMEM with 10% FBS for 24, 48 and 72 h. Next, 10 µl
WST-8 (Beyotime Institute of Biotechnology) was added to each well
followed by incubation at 37˚C for 2 h. The absorbance value was
measured at a wavelength of 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
The treated keloid fibroblasts were fixed in 4%
polyoxymethylene and permeabilized with 0.5% Triton-X100. Following
blocking with 10% BSA in PBS for 1 h at room temperature, they were
incubated with primary antibodies against proliferating cell
nuclear antigen (PCNA;1:100, ab29, Abcam Inc., Cambridge, UK) and
smooth muscle α-actin (α-SMA; 1:2,000, ab7817, Abcam Inc.,
Cambridge, UK) at 4˚C overnight. Next, Goat Anti-Mouse IgG H&L
(Alexa Fluor® 488) secondary antibody (1:400, ab150113, Abcam Inc.,
Cambridge, UK) was added and incubated for 1 h at room temperature.
The cells were subsequently counter-stained with DAPI and examined
under a fluorescence microscope (Nikon Eclipse 80i; Nikon
Corporation).
Wound healing assay
Transfected cells were seeded into a 6-well plate
and cultured to 80-90% confluence. A 20-µl tip was used to make a
straight scratch. The cells were then washed three times and
cultured with serum-free medium. After a 24-h incubation, the area
occupied by migrated cells in the scratch was evaluated. Five
randomly selected fields were analyzed in each well. The migration
rate was calculated based on the following formula: (wound width at
0 h-wound width at 24 h)/wound width at 0 h x 100%. Five fields per
well were randomly selected for analysis.
Transwell assay
An invasion assay was performed to examine tumor
invasion using a Transwell chamber (6.5 mm in diameter, 8 µm
pore-size, Corning, Inc.). The Transwell chambers (Corning, Inc.)
were first coated with 0.1 ml Matrigel (Nippon Becton Dickinson) at
37˚C for 1 h. The cells were then collected and suspended at a
final concentration of 2x105 cells/ml in serum-free
DMEM. Cell suspensions were then loaded onto the upper compartment,
and medium with 10% fetal bovine serum was added in the lower
compartment. Following incubation for 24 h, the non-invaded cells
on the upper face of the Transwell membrane were wiped off with a
cotton swab. The invaded cells on the lower face were fixed with
100% methanol, stained with hematoxylin and eosin, and counted
under a microscope.
Western blot analysis
The total proteins were extracted from cells using
RIPA buffer (Auragene). The protein concentration was determined
using Detergent Compatible Bradford Protein Assay Kit (Beyotime
Institute of Biotechnology). Proteins (20-30 µg) were resolved
using 10% PAGE (Bio-Rad Laboratories, Inc.), transferred to a
nitrocellulose membrane (Pall Life Sciences) at 25 V for 30 min,
and blocked for 1 h in 10% non-fat milk in 1x Tris-buffered
saline/0.1% (v/v) Tween 20 at room temperature. The membranes were
incubated at 4˚C overnight with the following primary antibodies:
Collagen I (1:1,000, ab138492, Abcam), α-SMA (1:1,000, ab7817,
Abcam), fibronectin (FN; 1:1,000, ab2413, Abcam), S1PR1 (1:1,000,
55133-1-AP, Proteintech), p-PI3K (1:1,000, ab191606, Abcam), PI3K
(1:1,000, ab182651, Abcam), p-AKT (1:1,000, ab192623, Abcam), AKT
(1:1,000, ab179463, Abcam), and GAPDH (1:2,500, ab9485, Abcam).
Next, the membranes were washed with PBST four times and then
incubated with the HRP-conjugated goat anti-rabbit or mouse
secondary antibodies (cat. nos. sc-2004 or sc-2005; 1:5,000; Santa
Cruz Biotechnology) for 1 h at room temperature. Finally, the
protein bands were visualized using an ECL detection system
(Beyotime Institute of Biotechnology) and quantified using ImageJ
software 1.49 (National Institutes of Health).
Statistical analysis
Statistical analysis was conducted using SPSS 22.0
software (IBM Corp). Data are presented as the mean ± standard
deviation of three independent experiments. Differences among
multiple groups were analyzed using one-way ANOVA with a post-hoc
Bonferroni multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
IH inhibits the proliferation,
migration, invasion and fibrosis of keloid fibroblasts
To investigate the roles of IH in keloid
progression, the effects of IH on keloid fibroblast proliferation
were first detected. As shown in Fig.
1A, cell proliferation was markedly suppressed following
treatment with IH, as compared with the control cells in a
dose-dependent manner. In addition, the protein levels of PCNA was
reduced in IH-treated cells (Fig.
1B). In addition, 20-100 µM IH reduced the cell migration rate,
when compared with the control cells (Fig. 1C and D). Transwell assay results indicated that
the invasive ability was restrained by the different doses of IH
(Fig. 1E and F). Western blot analysis showed that IH
treatment specifically decreased the protein levels of collagen I,
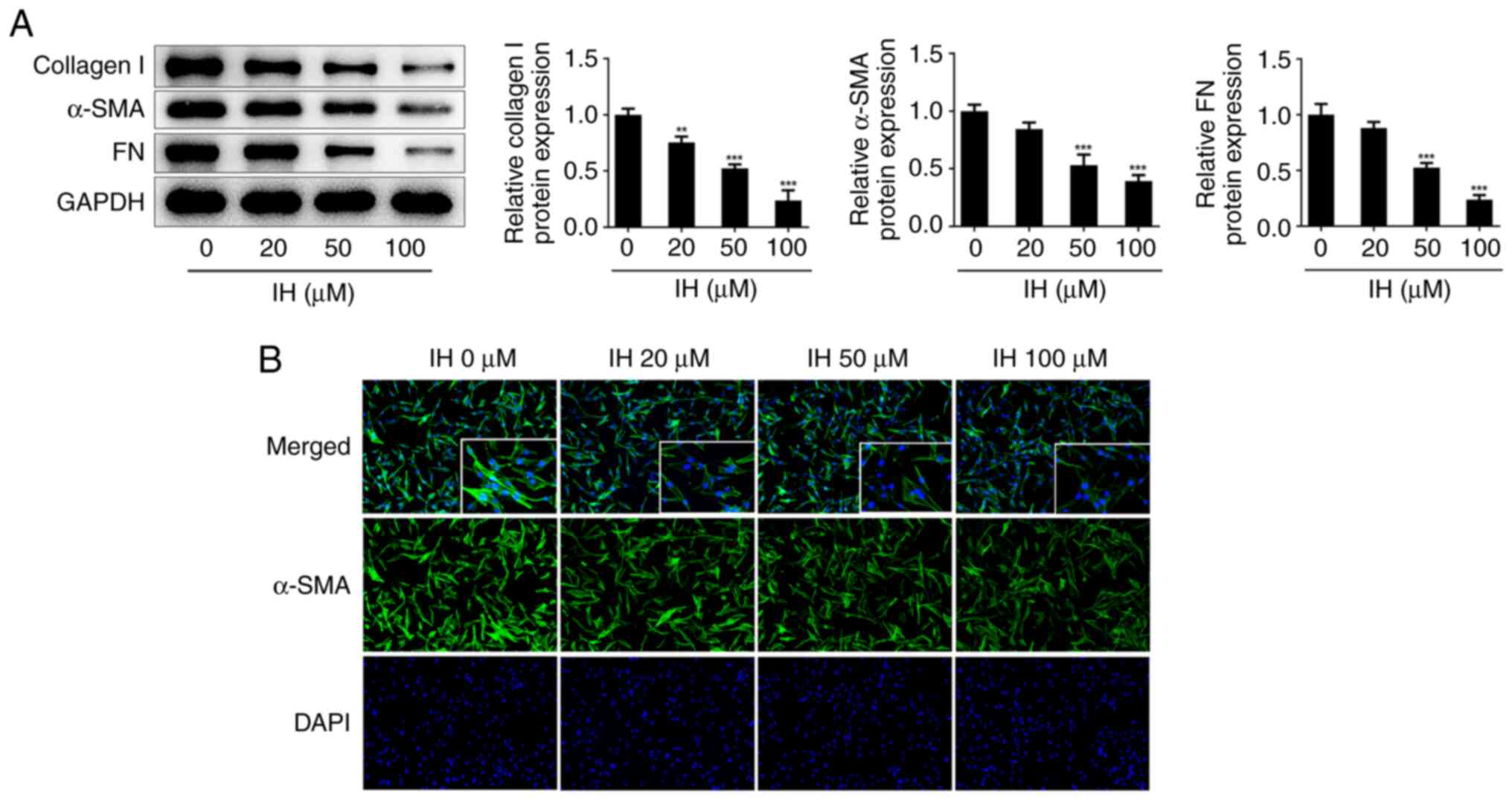
α-SMA and FN (Fig. 2A). Consistent
with these results, the immunofluorescence staining data revealed
that the number of positive cells in IH groups were significantly
decreased (Fig. 2B).
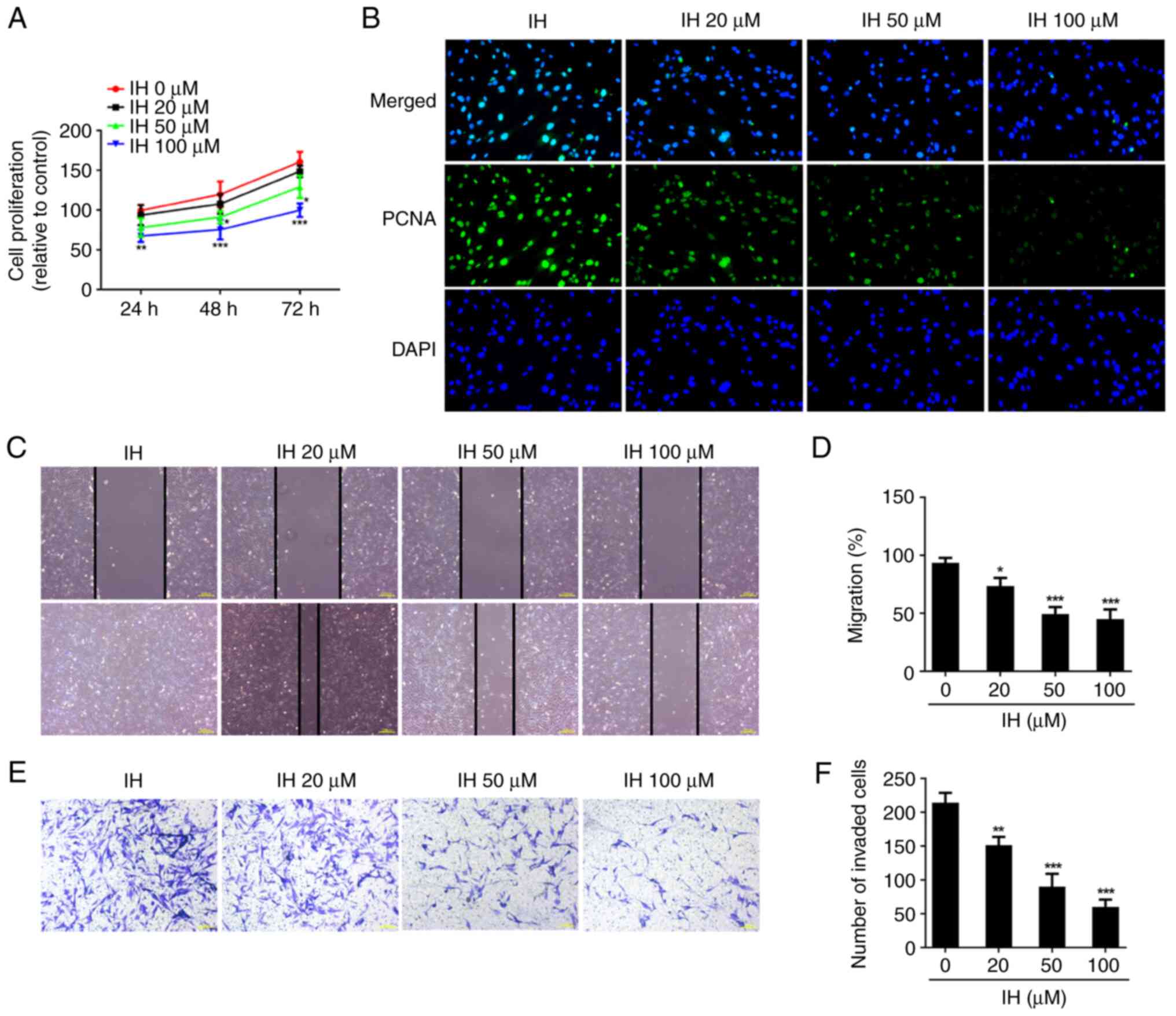 | Figure 1IH inhibits the proliferation,
migration, invasion of keloid fibroblasts. (A) CCK-8 assay was used
to assess cell proliferation. (B) Immunofluorescence was used to
detect the expression of PCNA. Original magnification, x400. (C and
D) The migration were evaluated by wound healing assay. Original
magnification, x40. (E and F) The invasiveness were evaluated by
Transwell assay. Original magnification, x100. Results are the mean
± SD. *P<0.05, **P<0.01,
***P<0.001 vs. IH 0 µM. IH, isorhamnetin; PCNA,
proliferating cell nuclear antigen; DAPI,
4',6-diamidino-2-phenylindole. |
IH suppresses S1PR1/PI3K/AKT signaling
in keloid fibroblasts
Autodock software was used to dock IH with S1PR1
protein at the molecular level, and the results showed that the
binding free energy of the active ingredient and S1PR1 was -8.5
kcal/mol (Fig. 3A); the molecular
docking diagram is shown in Fig.
3B. Next, western blot analysis was used to detect the
expression of the S1PR1/PI3K/AKT pathway. The results revealed that
IH reduced the protein levels of S1PR1, p-PI3K and p-AKT in a
dose-dependent manner in keloid fibroblasts (Fig. 3C).
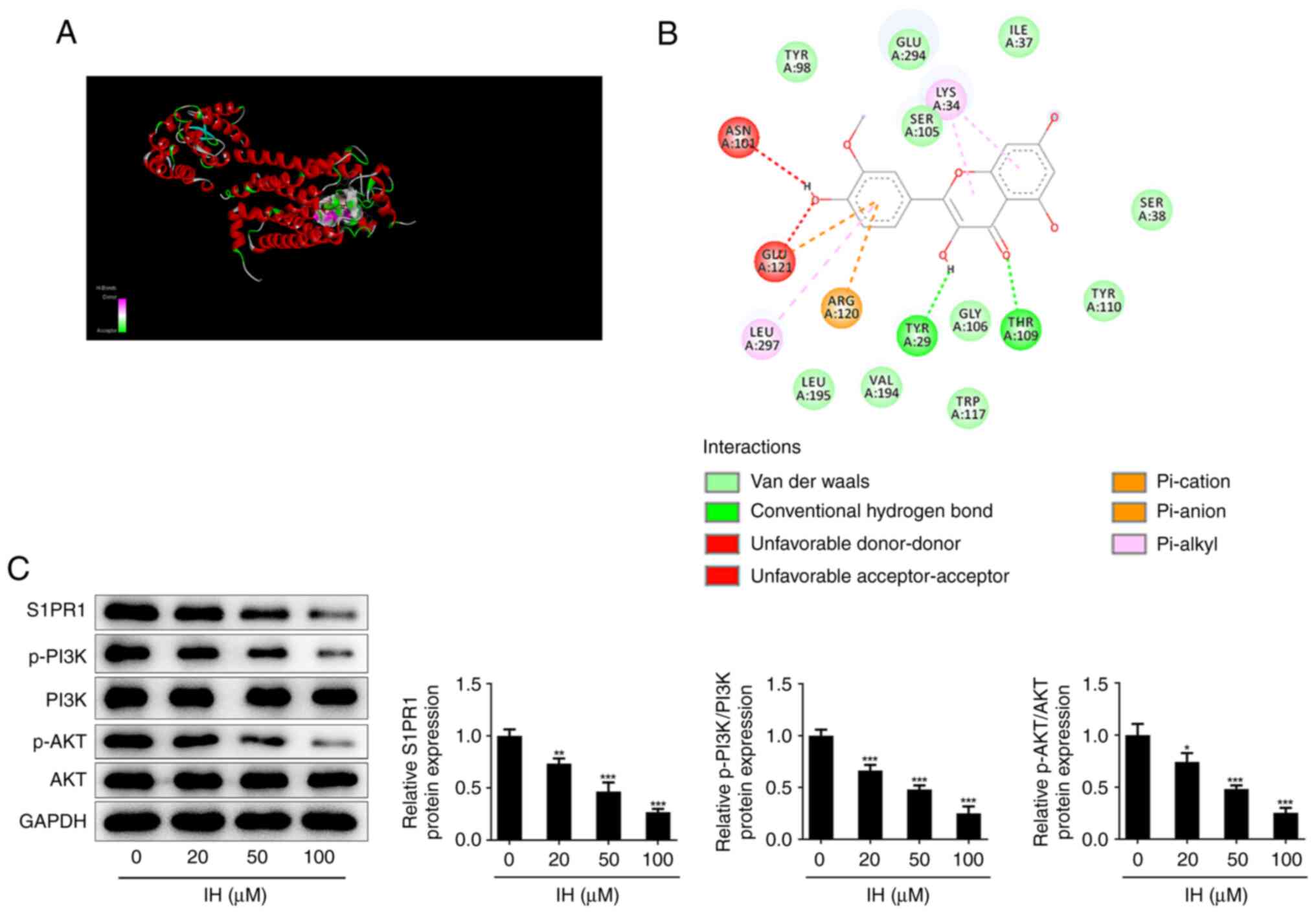 | Figure 3IH suppresses the expression of
S1PR1/PI3K/AKT signaling in keloid fibroblasts. Molecular docking
(A) 3D and (B) 2D diagram of the binding of IH and S1PR1. (C)
Western blot assay was performed to detect the protein level of
S1PR1, p-PI3K, PI3K, AKT and p-AKT. Results are the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001 vs. IH (0 µM). IH, isorhamnetin; S1PR1,
sphingosine-1-phosphate receptor-1; p-PI3K, phosphorylated
phosphatidylinositol-3-kinase; PI3K, phosphatidylinositol-3-kinase;
p-AKT, phosphorylated protein kinase B; AKT, protein kinase B. |
S1PR1 silencing suppresses keloid
fibroblast proliferation, migration, invasion and fibrosis
To explore the biological roles of S1PR1 in keloid
fibroblasts following IH treatment, cells were transfected with
siRNA-S1PR1-1/2 to silence S1PR1. Western blot analysis showed that
siRNA-S1PR1-1 exhibited a better transfection efficiency. Thus,
siRNA-S1PR1-1 (termed siRNA-S1PR1) was selected for the following
assays (Fig. 4A). The CCK-8 assay
results showed that S1PR1 silencing suppressed cell proliferation
compared with the negative control (Fig. 4B). Similarly, immunofluorescence
revealed that the PCNA level was significantly decreased following
transfection with siRNA-S1PR1 (Fig.
4C). In addition, wound healing and Transwell assays showed
that keloid fibroblast migration and invasion were decreased
following S1PR1 silencing (Fig.
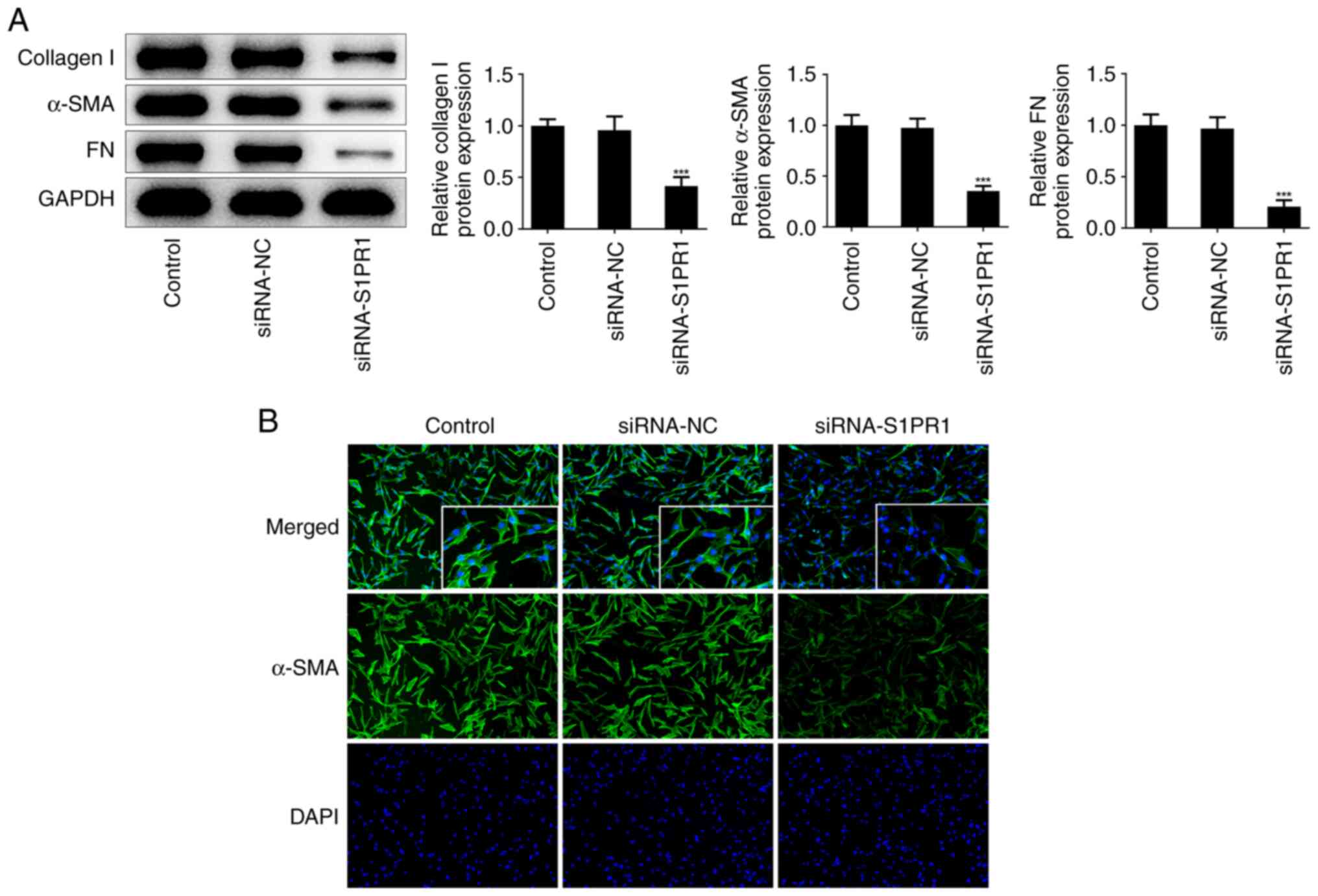
4D-G). Furthermore, a reduction in the levels of collagen I,
α-SMA and FN was observed in cells transfected with siRNA-S1PR1
(Fig. 5A), which was consistent
with the immunofluorescence results (Fig. 5B).
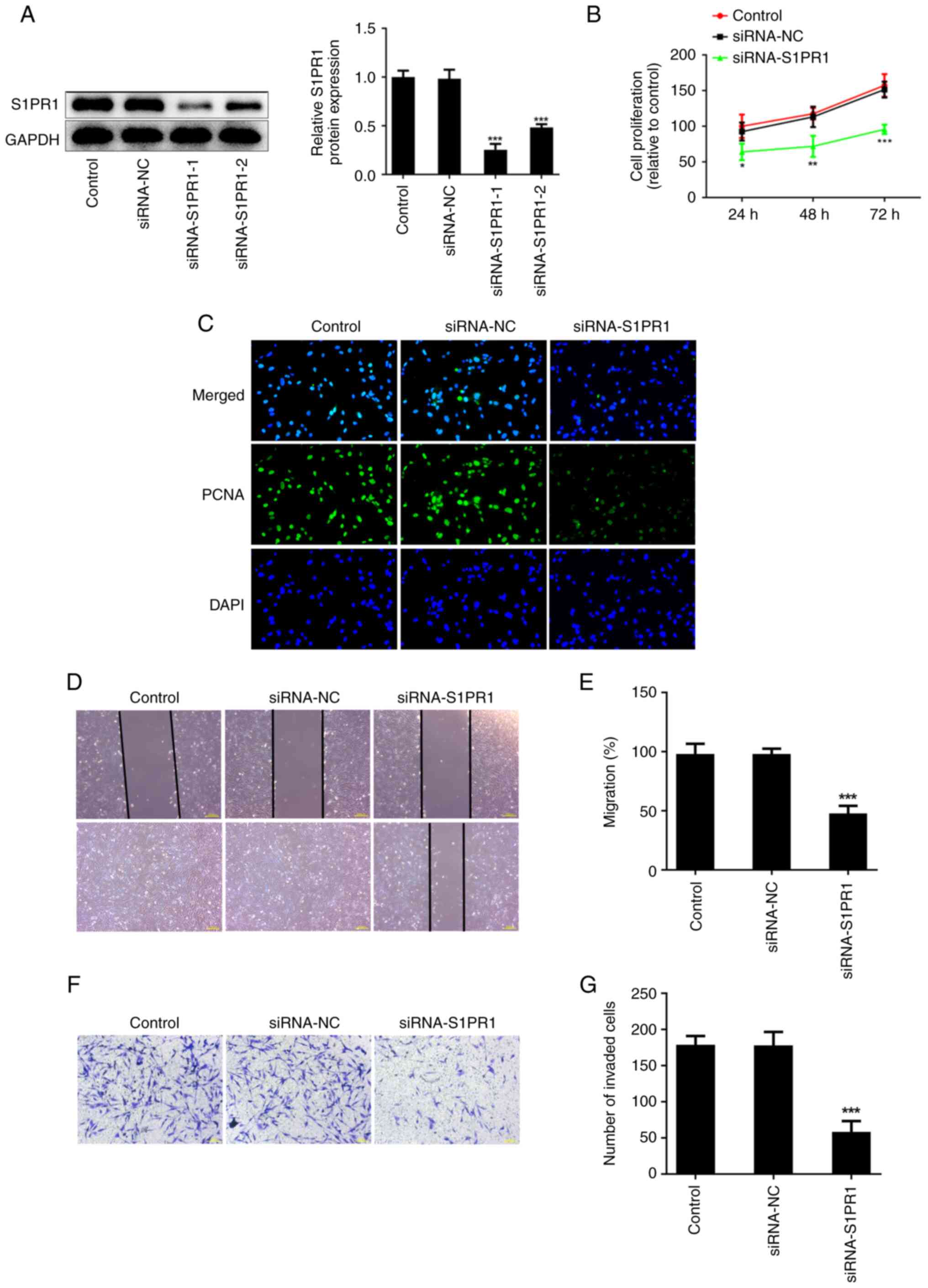 | Figure 4S1PR1 silencing restrains keloid
fibroblasts proliferation, migration and invasion. (A) Western blot
assay was performed to detect the protein level of S1PR1. (B) CCK-8
assay was used to assess cell proliferation. (C) Immunofluorescence
was used to detect the expression of PCNA. Original magnification,
x400. (D and E) The migration were evaluated by wound healing
assay. Original magnification, x40. (F and G) The invasiveness were
evaluated by Transwell assay. Original magnification, x100. Results
are the mean ± SD. *P<0.05, **P<0.01,
***P<0.001 vs. siRNA-NC. S1PR1,
sphingosine-1-phosphate receptor-1; PCNA, proliferating cell
nuclear antigen; DAPI, 4',6-diamidino-2-phenylindole. |
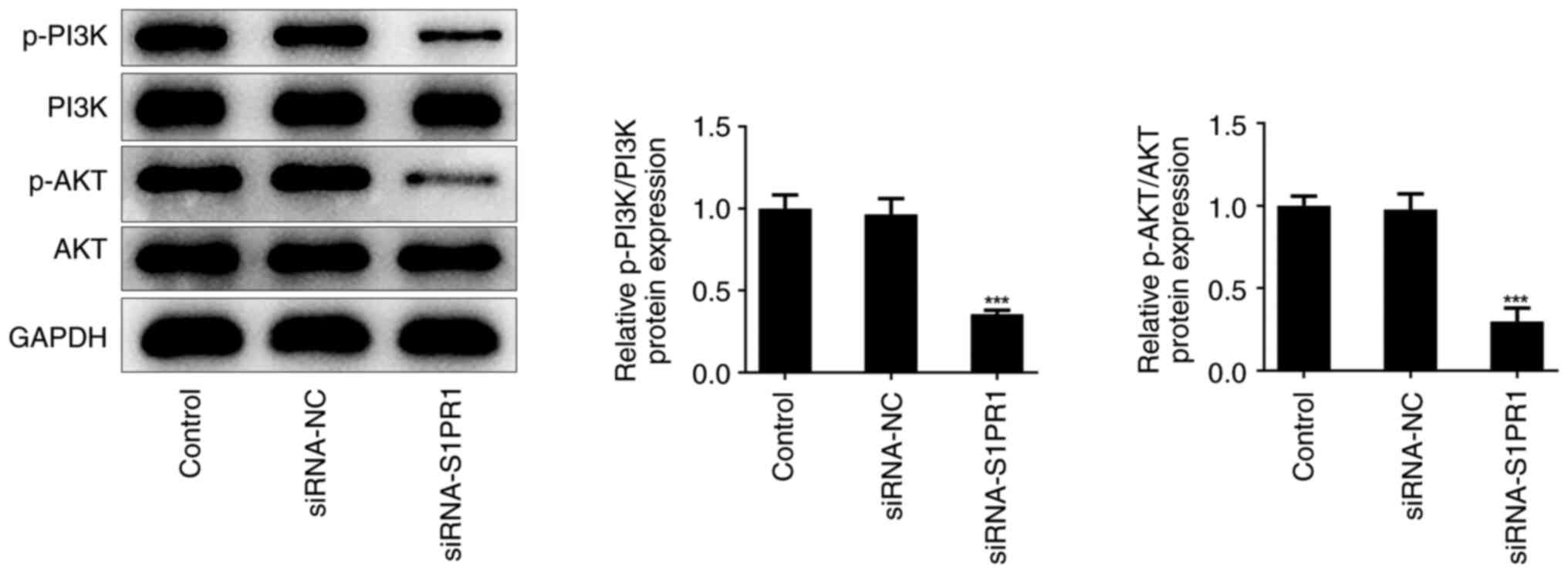
S1PR1 knockdown inhibits the PI3K/AKT
pathway
Next, the association between S1PR1 and the PI3K/AKT
pathway was explored in IH-treated keloid fibroblasts. Western blot
analysis showed that the levels of p-PI3K and p-AKT were markedly
reduced, but the total levels of PI3K and AKT did not change
following S1PR1 silencing, which indicated that S1PR1 may regulate
the expression of the PI3K/AKT pathway in keloid fibroblasts
(Fig. 6).
IH suppresses keloid fibroblast
proliferation, metastasis and fibrosis by targeting the
S1PR1/PI3K/AKT pathway
To verify the role of the S1PR1/PI3K/AKT pathway in
IH-treated keloid fibroblasts, S1PR1 was overexpressed, and western
blot analysis was used to determine transfection efficiency
(Fig. 7A). S1PR1 overexpression
noticeably enhanced the cell proliferation rate and the level of
PCNA in IH-exposed cells (Fig. 7B
and C). In addition, the cell
migration and invasion rates were increased following S1PR1
overexpression (Fig. 7D-G). In
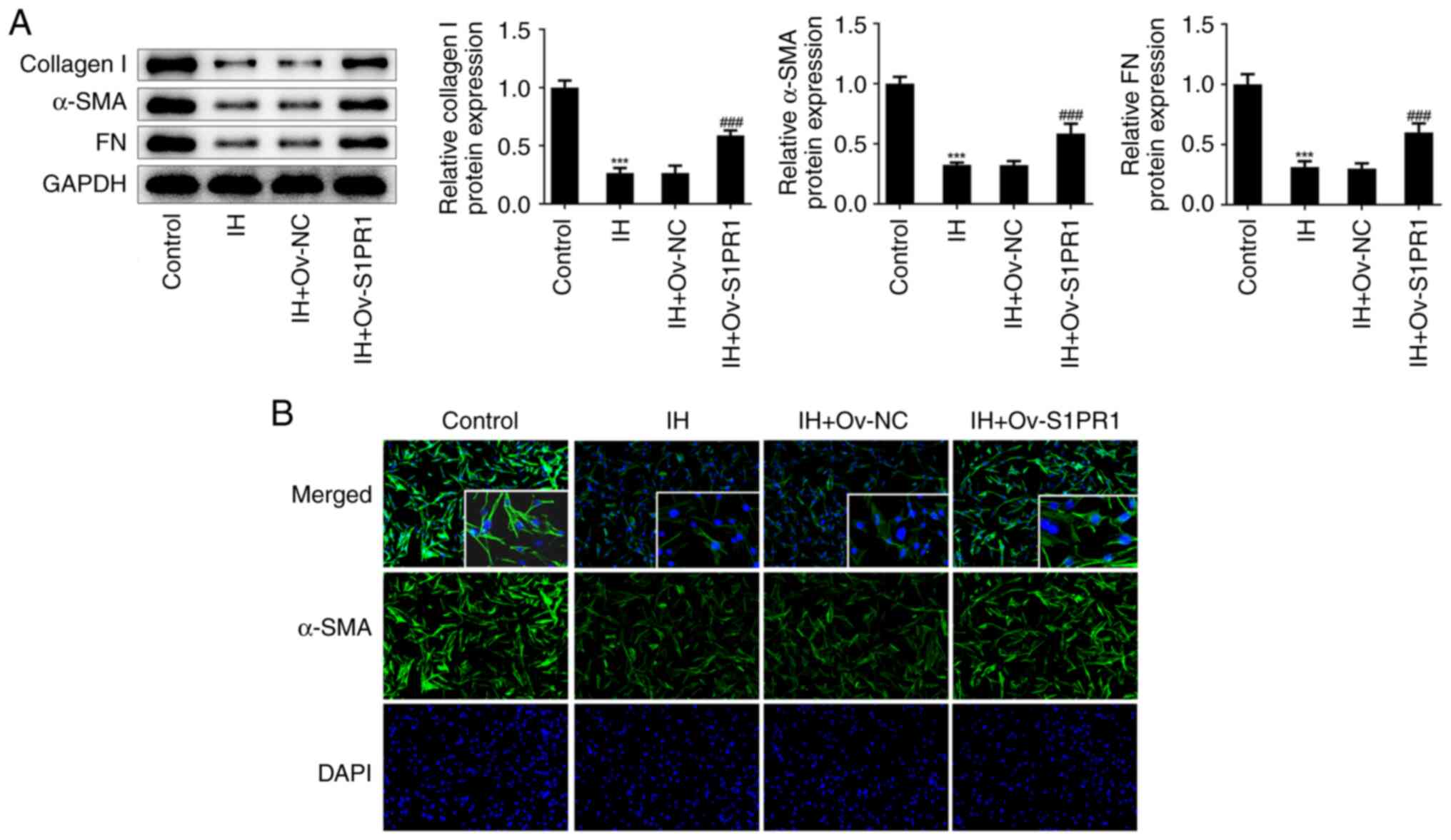
addition, S1PR1 upregulation reversed the effects of IH on the
protein levels of collagen I, α-SMA and FN in keloid fibroblasts
(Fig. 8A). Immunofluorescence
revealed that the relative fluorescence intensity of α-SMA was
elevated in cells with S1PR1 overexpression (Fig. 8B).
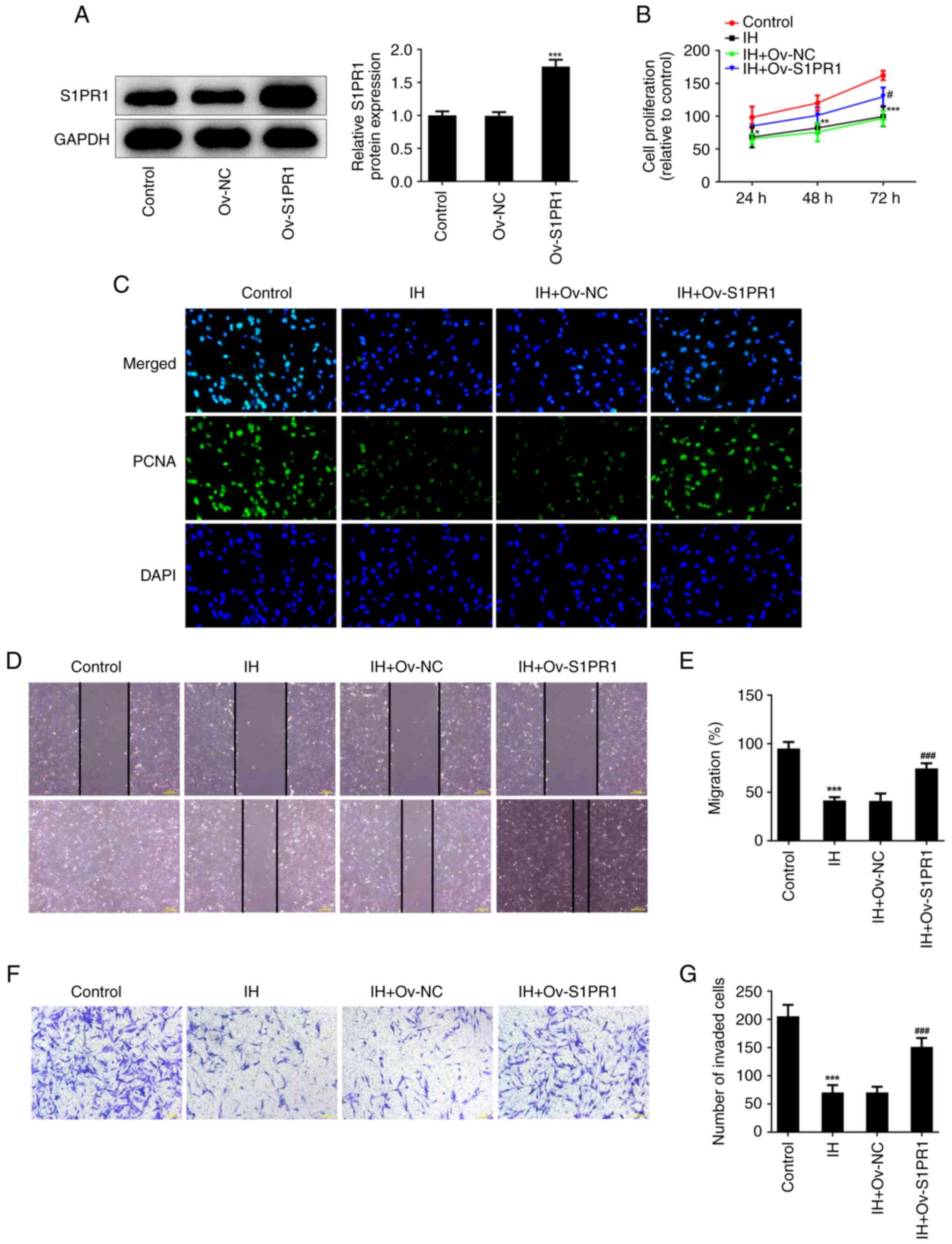 | Figure 7IH suppresses keloid fibroblasts
proliferation and metastasis by targeting S1PR1/PI3K/AKT pathway.
(A) Western blot assay was performed to detect the protein level of
S1PR1. ***P<0.001 vs. Ov-NC. (B) CCK-8 assay was used
to assess cell proliferation. (C) Immunofluorescence was used to
detect the expression of PCNA. Original magnification, x400. (D and
E) The migration were evaluated by wound healing assay. Original
magnification, x40. (F and G) The invasiveness were evaluated by
Transwell assay. Original magnification, x100. Results are the mean
± SD. *P<0.05, **P<0.01,
***P<0.001 vs. Control; #P<0.05,
###P<0.001 vs. IH + Ov-NC. IH, isorhamnetin; S1PR1,
sphingosine-1-phosphate receptor-1; PCNA, proliferating cell
nuclear antigen; DAPI, 4',6-diamidino-2-phenylindole. |
Discussion
A keloid is a skin lesion secondary to connective
tissue hyperplasia following trauma (15). Currently, the development of
keloids is considered to be associated with the accumulation of ECM
collagen and excessive fibroblast proliferation (16). The fibrosis pathway and
fibrosis-related cytokines play a promoting role in keloid
development (17). At present, the
etiology of keloids is unknown, and despite the existence of
several treatment methods, relapse is common, which has a great
impact on the work and life of patients. Thus, developing more
effective and feasible strategies for keloid treatment is critical.
The aim of the present study was to explore the effects of IH in
keloids and investigate whether IH can attenuate keloid fibroblast
proliferation, invasion, migration and fibrosis by inhibiting S1PR1
signaling.
IH is a flavonoid isolated from sea buckthorn, which
has anti-inflammatory, antioxidant and antitumor activities
(18-20).
A previous study showed that IH improved liver injury markers,
reduced collagen deposition and decreased gene expression of
fibrogenic markers in mice with nonalcoholic steatohepatitis
(21). Liu et al (22) reported that IH protected against
liver fibrosis by suppressing ECM formation and autophagy through
the inhibition of the TGF-β1-mediated Smad3 and p38 MAPK signaling
pathways. In addition, Luo et al (12) found that IH suppressed migration
and invasion through the inhibition of Akt/ERK-mediated EMT in
human non-small-cell lung cancer cells. Another study showed that
IH suppressed cell proliferation and metastasis, triggered
apoptosis and arrested the G2/M phase through the inactivation of
the PI3K/AKT signaling (23). In
the present study, the effects of IH on proliferation, metastasis
and fibrosis in keloid fibroblasts was detected, and it was found
that IH can inhibit cell proliferation, migration, invasion and
fibrosis in a dose-dependent manner.
S1P can regulate multiple physiological and
pathological processes (24). It
has been shown that the S1P/S1PR1 signaling could enhance
tumorigenesis and stimulate the growth, angiogenesis, expansion,
survival and metastasis of cancer cells (25). In addition, S1P influences
different aspects of fibrosis modulating the recruitment of
inflammatory cells, as well as cell proliferation, migration and
transdifferentiation into myofibroblasts, the cell type mainly
involved in fibrosis development (26). Jung et al (27) reported that S1P-induced signal
transduction was associated with increased collagen synthesis in
the keloid tissue through the S1PR-mediated signaling pathways, and
the mRNA and protein levels of S1PR1 were increased in keloid
tissues. Using the TargetNet database, it was found that IH targets
S1PR1, and molecular docking analysis verified the docking of IH
and S1PR1. Next, S1PR1 was silenced in keloid fibroblasts and the
results showed that S1PR1 silencing significantly repressed keloid
fibroblast proliferation, metastasis and fibrosis. In addition,
S1PR1 overexpression reversed the effects of IH on keloid
fibroblasts, which indicated that IH may regulate keloid
fibroblasts by targeting S1PR1.
The PI3K/AKT pathway was also found to be associated
with keloid progression (28). A
previous study showed that the PI3K/AKT pathway promoted keloid
formation (27). Xin et al
(29) revealed that CD26 may be
closely associated with proliferation and invasion in keloids
through the IGF-1-induced PI3K/AKT/mTOR pathway. Meanwhile, Liu
et al (30) found that the
S1P/S1PR1 axis activated the AKT/nitric oxide synthase 3 signaling
pathway to regulate pressure overload-induced cardiac remodeling.
Rostami et al (31)
revealed that S1PR1 signaling can trigger various signaling
pathways involved in carcinogenesis, including the activation of
PI3K/AKT. In the present study, IH treatment reduced the
phosphorylation of PI3K and AKT in the PI3K/AKT pathway. In
addition, S1PR1 silencing decreased the levels of p-PI3K and p-AKT
in keloid fibroblasts, suggesting that the S1PR1-mediated PI3K/AKT
pathway may be involved in the regulation of IH in keloids. In
conclusion, the results of the present study suggested that IH
suppressed cell proliferation, migration and invasion, and
inhibited fibrosis in keloid fibroblasts. These protective effects
may be dependent on the regulation of the S1PR1-mediated PI3K/AKT
pathway, which may provide a novel insight into keloid pathogenesis
and develop a therapeutic strategy for patients with keloids.
However, we preliminarily explored the combination of IH and S1PR1
and the effects of IH on expressions of S1PR1/PI3K/AKT signaling,
but how IH affects specifically S1PR1/PI3K/AKT signaling needs to
be further explored.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XP and XC designed the study, and drafted and
revised the manuscript. HL and WH analyzed the data and searched
the literature. XP, XC, HL, WH, LZ and TJ performed the
experiments. All authors read and approved the final manuscript. XP
and XC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Unahabhokha T, Sucontphunt A, Nimmannit U,
Chanvorachote P, Yongsanguanchai N and Pongrakhananon V: Molecular
signalings in keloid disease and current therapeutic approaches
from natural based compounds. Pharm Biol. 53:457–463.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hawash AA, Ingrasci G, Nouri K and
Yosipovitch G: Pruritus in keloid scars: Mechanisms and treatments.
Acta Derm Venereol. 101(adv00582)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mofikoya BO, Adeyemo WL and Abdus-salam
AA: Keloid and hypertrophic scars: A review of recent developments
in pathogenesis and management. Nig Q J Hosp Med. 17:134–139.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ogawa R: Keloid and hypertrophic scars are
the result of chronic inflammation in the reticular dermis. Int J
Mol Sci. 18(606)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qiao XF and Li X: Comparative study of
surgical treatment combined with various methods for treatment of
ear scar. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
31:1341–1343. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Love PB and Kundu RV: Keloids: An update
on medical and surgical treatments. J Drugs Dermatol. 12:403–409.
2013.PubMed/NCBI
|
|
7
|
Lee HJ and Jang YJ: Recent understandings
of biology, prophylaxis and treatment strategies for hypertrophic
scars and keloids. Int J Mol Sci. 19(711)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Q, Wang P, Qin Z, Yang X, Pan B, Nie
F and Bi H: Altered glucose metabolism and cell function in keloid
fibroblasts under hypoxia. Redox Biol. 38(101815)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou P, Shi L, Li Q and Lu D:
Overexpression of RACK1 inhibits collagen synthesis in keloid
fibroblasts via inhibition of transforming growth factor-β1/Smad
signaling pathway. Int J Clin Exp Med. 8:15262–15268.
2015.PubMed/NCBI
|
|
10
|
Rodríguez L, Badimon L, Méndez D, Padró T,
Vilahur G, Peña E, Carrasco B, Vogel H, Palomo I and Fuentes E:
Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation.
Antioxidants (Basel). 10(666)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gong G, Guan YY, Zhang ZL, Rahman K, Wang
SJ, Zhou S, Luan X and Zhang H: Isorhamnetin: A review of
pharmacological effects. Biomed Pharmacother.
128(110301)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo W, Liu Q, Jiang N, Li M and Shi L:
Isorhamnetin inhibited migration and invasion via suppression of
Akt/ERK-mediated epithelial-to-mesenchymal transition (EMT) in A549
human non-small-cell lung cancer cells. Bioscience Reports.
39:2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng Q, Tong M, Ou B, Liu C, Hu C and
Yang Y: Isorhamnetin protects against bleomycin-induced pulmonary
fibrosis by inhibiting endoplasmic reticulum stress and
epithelial-mesenchymal transition. Int J Mol Med. 43:117–126.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang JH, Kim SC, Kim KM, Jang CH, Cho SS,
Kim SJ, Ku SK, Cho IJ and Ki SH: Isorhamnetin attenuates liver
fibrosis by inhibiting TGF-β/Smad signaling and relieving oxidative
stress. Eur J Pharmacol. 783:92–102. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee JY, Yang CC, Chao SC and Wong TW:
Histopathological differential diagnosis of keloid and hypertrophic
scar. Am J Dermatopathol. 26:379–384. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Elsaie ML: Update on management of keloid
and hypertrophic scars: A systemic review. J Cosmet Dermatol.
20:2729–2738. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lim KH, Itinteang T, Davis PF and Tan ST:
Stem cells in keloid lesions: A review. Plast Reconstr Surg Glob
Open. 7(e2228)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Xu Y, Lin Z, Guan L, Chen S and Zhou
L: Isorhamnetin inhibits amplification of influenza A H1N1 virus
inflammation mediated by interferon via the RIG-I/JNK pathway. Ann
Transl Med. 9(1327)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ren X, Han L, Li Y, Zhao H, Zhang Z,
Zhuang Y, Zhong M, Wang Q, Ma W and Wang Y: Isorhamnetin attenuates
TNF-α-induced inflammation, proliferation, and migration in human
bronchial epithelial cells via MAPK and NF-κB pathways. Anat Rec
(Hoboken). 304:901–913. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Hu J, Zhang Y, Jiang X, Zhang H, Gao Z, Li
Y, Fu R, Li L, Li J, Cui H and Gao N: ROS-mediated activation and
mitochondrial translocation of CaMKII contributes to Drp1-dependent
mitochondrial fission and apoptosis in triple-negative breast
cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer
Res. 38(225)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ganbold M, Owada Y, Ozawa Y, Shimamoto Y,
Ferdousi F, Tominaga K, Zheng YW, Ohkohchi N and Isoda H:
Isorhamnetin alleviates steatosis and fibrosis in mice with
nonalcoholic steatohepatitis. Sci Rep. 9(16210)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y,
Han Y, Deng J and Zhou Y: Isorhamnetin Inhibits liver fibrosis by
reducing autophagy and inhibiting extracellular matrix formation
via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediators
Inflamm. 2019(6175091)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhai T, Zhang X, Hei Z, Jin L, Han C, Ko
AT, Yu X and Wang J: Isorhamnetin inhibits human gallbladder cancer
cell proliferation and metastasis via PI3K/AKT signaling pathway
inactivation. Front Pharmacol. 12(628621)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cartier A and Hla T: Sphingosine
1-phosphate: Lipid signaling in pathology and therapy. Science.
366(eaar5551)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anu B, Namitha NN and Harikumar KB: S1PR1
signaling in cancer: A current perspective. Adv Protein Chem Struct
Biol. 125:259–274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Donati C, Cencetti F, Bernacchioni C,
Vannuzzi V and Bruni P: Role of sphingosine 1-phosphate signalling
in tissue fibrosis. Cell Signal. 78(109861)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jung SH, Song YK, Chung H, Ko HM, Lee SH,
Jo DI, Kim B, Lee DH and Kim SH: Association between
sphingosine-1-phosphate-induced signal transduction via
mitogen-activated protein kinase pathways and keloid formation.
Arch Dermatol Res. 311:711–719. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tu T, Huang J, Lin M, Gao Z, Wu X, Zhang
W, Zhou G, Wang W and Liu W: CUDC-907 reverses pathological
phenotype of keloid fibroblasts in vitro and in vivo via dual
inhibition of PI3K/Akt/mTOR signaling and HDAC2. Int J Mol Med.
44:1789–1800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xin Y, Min P, Xu H, Zhang Z and Zhang Y
and Zhang Y: CD26 upregulates proliferation and invasion in keloid
fibroblasts through an IGF-1-induced PI3K/AKT/mTOR pathway. Burns
Trauma. 8(tkaa025)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu X, Wu J, Zhu C, Liu J, Chen X, Zhuang
T, Kuang Y, Wang Y, Hu H, Yu P, et al: Endothelial S1pr1 regulates
pressure overload-induced cardiac remodelling through AKT-eNOS
pathway. J Cell Mol Med. 24:2013–2026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rostami N, Nikkhoo A, Ajjoolabady A, Azizi
G, Hojjat-Farsangi M, Ghalamfarsa G, Yousefi B, Yousefi M and
Jadidi-Niaragh F: S1PR1 as a novel promising therapeutic target in
cancer therapy. Mol Diagn Ther. 23:467–487. 2019.PubMed/NCBI View Article : Google Scholar
|