Introduction
Atherosclerosis is a critical pathological process
that may result in the stenosis of the artery, eventually leading
to cerebral-cardiovascular diseases, such as coronary artery
disease and ischemic stroke, two notorious diseases associated with
high mortality rate worldwide (1-3).
Currently, the treatment approaches for the above
cerebral-cardiovascular diseases, such as thrombolysis,
thrombectomy and percutaneous coronary intervention, have greatly
improved the clinical outcomes of patients (4-7).
However, fundamental management strategies to prevent or even
reverse the process of atherosclerosis are still lacking.
Therefore, exploring potential treatment targets for
atherosclerosis is of great importance.
It has been reported that the dysregulation of
vascular smooth muscle cells (VSMCs) is critically involved in the
pathogenesis and progression of atherosclerosis (8). It is generally considered that the
abnormal proliferation or invasion of VSMCs can promote the
formation of atherosclerotic lesions, as well as elevate lipid
accumulation, another key event involved in the progression of
atherosclerosis (9). A previous
study also demonstrated that the switching of VSMCs from a
contractile phenotype towards a synthetic phenotype could enhance
inflammation, thus also contributing to atherosclerosis (10). Additionally, the apoptosis of VSMCs
at the late stage of atherosclerosis could facilitate the rupture
of atherosclerotic lesion (11).
Therefore, inhibiting the abnormal cellular functions of VSMCs
could be a potential strategy for managing atherosclerosis.
Mucosa-associated lymphoid tissue lymphoma
translocation protein 1 (MALT1) is part of the caspase recruitment
domain recruited membrane associated protein 3/B-cell lymphoma
10/MALT1 (CBM) signaling complex that regulates the activation of
the nuclear factor-κB (NF-κB) signaling pathway involved in several
diseases and more particularly in allergy and cancer (12-14).
However, it has become gradually accepted that MALT1 may be
involved in other pathological processes. Therefore, a previous
study showed that MALT1 could activate the NF-κB signaling pathway
to elevate inflammation in the vasculature (14). In addition, the aforementioned
study also revealed that CBM complex-deficient mice could not
develop atherosclerosis following stimulation with angiotensin,
thus supporting that MALT1 could be involved in atherosclerosis
(14). However, whether MALT1
could modulate the cellular functions of VSMCs to regulate
atherosclerosis remains unknown.
Therefore, the current study aimed to evaluate the
effect of MALT1 on proatherogenic VSMC proliferation, apoptosis,
invasion and phenotype switching, as well as its potential
underlying mechanism of action.
Materials and methods
Cell culture
Human primary VSMCs were purchased from Bluefcell
Bio and maintained in DMEM supplemented with 10% FBS (both from
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin solution (Sangon Biotech Co. Ltd.) at 37˚C
and 5% CO2. The use of VSMCs was approved by the Ethics
Committee of Affiliated Hospital of Inner Mongolia Medical
University with approval number KY (2020015).
VSMC activation with oxidized
low-density lipoprotein (oxLDL)
VSMCs at a density of 2x104 cells/well
were seeded into a 6- or 96-well plate. Following incubation for 24
h at 37˚C, VSMCs were stimulated with 0, 25, 50, 100 or 200 µg/ml
oxLDL (Beijing Solarbio Science & Technology Co., Ltd.)
(15). At 24 h after stimulation
at 37˚C, further experiments, including reverse
transcription-quantitative (RT-q) PCR, western blot analysis and
cell viability, cell apoptosis and cell invasion assays were
performed.
MALT1 regulation experiment
The lentivirus overexpressing MALT1 (Lv-MALT1) or
knocking down MALT1 (Lv-anti-MALT1) and the empty lentivirus
(lentivirus containing empty vector, Vector) were obtained from
Shanghai GenePharma Co., Ltd. The frame of vectors overexpressing
MALT1 or knocking down MALT1 are shown in Fig. S1. Briefly, VSMCs were seeded into
culture plates and were then transfected with the above
lentiviruses using 6 µg/ml polybrene (cat. no. 40804ES76; Shanghai
Yeasen Biotechnology Co., Ltd.). Untransfected VSMCs served as the
control group. Following incubation for 72 h at 37˚C (16), VSMCs were activated with 100 µg/ml
oxLDL for 24 h and were then collected for RT-qPCR, western
blotting, cell viability, cell apoptosis and cell invasion
assays.
VSMC treatment with phorbol
12-myristate 13-acetate (PMA)
PMA (1 µM; MedChemExpress) (17), a NF-κB activator, was adopted to
evaluate the MALT1-mediated regulation of the NF-κB signaling
pathway. Briefly, VSMCs were transfected with Lv-anti-MALT1 or
empty lentivirus for 72 h, as previously described. Subsequently,
VSMCs were divided into the following four groups: Vector group,
where cells were transfected with empty lentivirus and were not
treated with PMA; Lv-anti-MALT1 group, where cells were transfected
with MALT1 knockdown lentivirus and were not treated with PMA; PMA
group, where VSMCs were transfected with empty lentivirus and
treated with PMA; and Lv-anti-MALT1 + PMA group, where cells were
transfected with Lv-anti-MALT1, followed by treatment with PMA.
Untransfected and untreated VSMCs served as the control group.
VSMCs in all groups were cultured in medium supplemented with 100
µg/ml oxLDL. Following treatment for 24 h at 37˚C, cells were
harvested for RT-qPCR, western blot, cell viability, cell apoptosis
and cell invasion assays.
RT-qPCR
The mRNA expression levels of MALT1 in VSMCs were
assessed using RT-qPCR. Briefly, total RNA was extracted from
1x106 VSMCs using a Trizol (Beyotime Institute of
Biotechnology) and RT-PCR and qPCR amplification were performed
using the RT reagent kit (Takara Bio, Inc.) and qPCR mix kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocols, respectively. For qPCR, the following
thermocycling conditions were performed: One cycle of 95˚C for 5
min, followed by 40 cycles at 95˚C for 2 min and 61˚C for 20 sec.
qPCR was performed in triplicate. The relative expression levels of
MALT1 were analyzed using the 2-ΔΔCq method (18). The primer sequences used were as
follows: For MALT1, forward, 5'-TCTTGGCTGGACAGTTTGTGA-3' and
reverse, 5'-GCTCTCTGGGATGTCGCAA-3'; and for GAPDH, forward,
5'-GAGTCCACTGGCGTCTTCAC-3' and reverse,
5'-ATCTTGAGGCTGTTGTCATACTTCT-3'.
Western blot analysis
Total proteins were extracted from VSMCs using a
RIPA reagent (Shanghai Yeasen Biotechnology Co., Ltd.) and
quantified with a BCA kit (Mlbio). Subsequently, the 20 µg protein
extracts were separated by SDS-PAGE on 4-20% precast gels (Beyotime
Institute of Biotechnology) and were then transferred onto
nitrocellulose membranes (MilliporeSigma). The membranes were then
blocked with 5% BSA (Beyotime Institute of Biotechnology) for 1 h
at 37˚C, followed first by incubation with primary antibodies at
4˚C overnight and then with the corresponding secondary antibody at
37˚C for 1 h. The protein bands were visualized using an ECL
reagent (UNIV). Densitometry was performed using Image J (version
1.8.0; National Institutes of Health). The antibodies used for
western blot analysis were all purchased from Affinity Biosciences
and were as follows: Anti-MALT1 (dilution, 1:1,000; cat. no.
DF6867), anti-α-smooth muscle actin (SMA; dilution, 1:1,000; cat.
no. AF1032), anti-osteopontin (OPN; dilution, 1:1,000; cat. no.
AF0227), anti-phosphorylated (p)-IκBα (dilution, 1:500; cat. no.
AF2002), anti-IκBα (dilution, 1:500; cat. no. af5002), anti-p-p65
(dilution, 1:500; cat. no. AF2006), anti-p65 (dilution, 1:500; cat.
no. AF5006), anti-GAPDH (dilution, 1:5,000; cat. no. AF7021) and
HRP conjugated goat-anti rabbit secondary antibody (dilution,
1:10,000; cat. no. S0001).
Cell viability assay
The viability of VSMCs was assessed using a Cell
Counting Kit-8 (CCK-8; MilliporeSigma). Briefly, VSMCs were seeded
into 96-well culture plates (Wuxi NEST Biotechnology Co., Ltd.) and
were then treated for 24 h as previously described. Subsequently,
cells were supplemented with CCK-8 reagent for 2 h and the optical
density was measured using a microplate reader (BioTek Instruments,
Inc.).
Cell apoptosis assay
The apoptosis of VSMCs was assessed using a TUNEL
apoptosis kit (Beyotime Institute of Biotechnology). Briefly,
following treatment, VSMCs were fixed with 4% paraformaldehyde
(Beyotime Institute of Biotechnology) for 10 min at room
temperature, followed by incubation with TUNEL reagent for 1 h at
37˚C. Finally, VSMCs were stained with DAPI (5 mg/l, Sangon Biotech
Co. Ltd.) for 10 min at room temperature.
Cell invasion assay
The invasion ability of VSMCs was evaluated using
Transwell assays. Briefly, treated VSMCs were seeded into the upper
chamber, which was precoated in Matrigel at 37˚C for 1 h (Corning,
Inc.), while the lower chamber was supplemented with complete
medium. Following incubation for 24 h at 37˚C, cells were stained
with crystal violet (0.1%, Sangon Biotech Co. Ltd.) at room
temperature for 20 min.
Statistical analysis
The differences among multiple groups were compared
with one-way ANOVA followed by Dunnett's or Tukey's multiple
comparisons test using GraphPad Prism 8.0 (Dotmatics). P<0.05
was considered to indicate a statistically significant
difference.
Results
OxLDL upregulates MALT1, enhances cell
viability and inhibits apoptosis in VSMCs
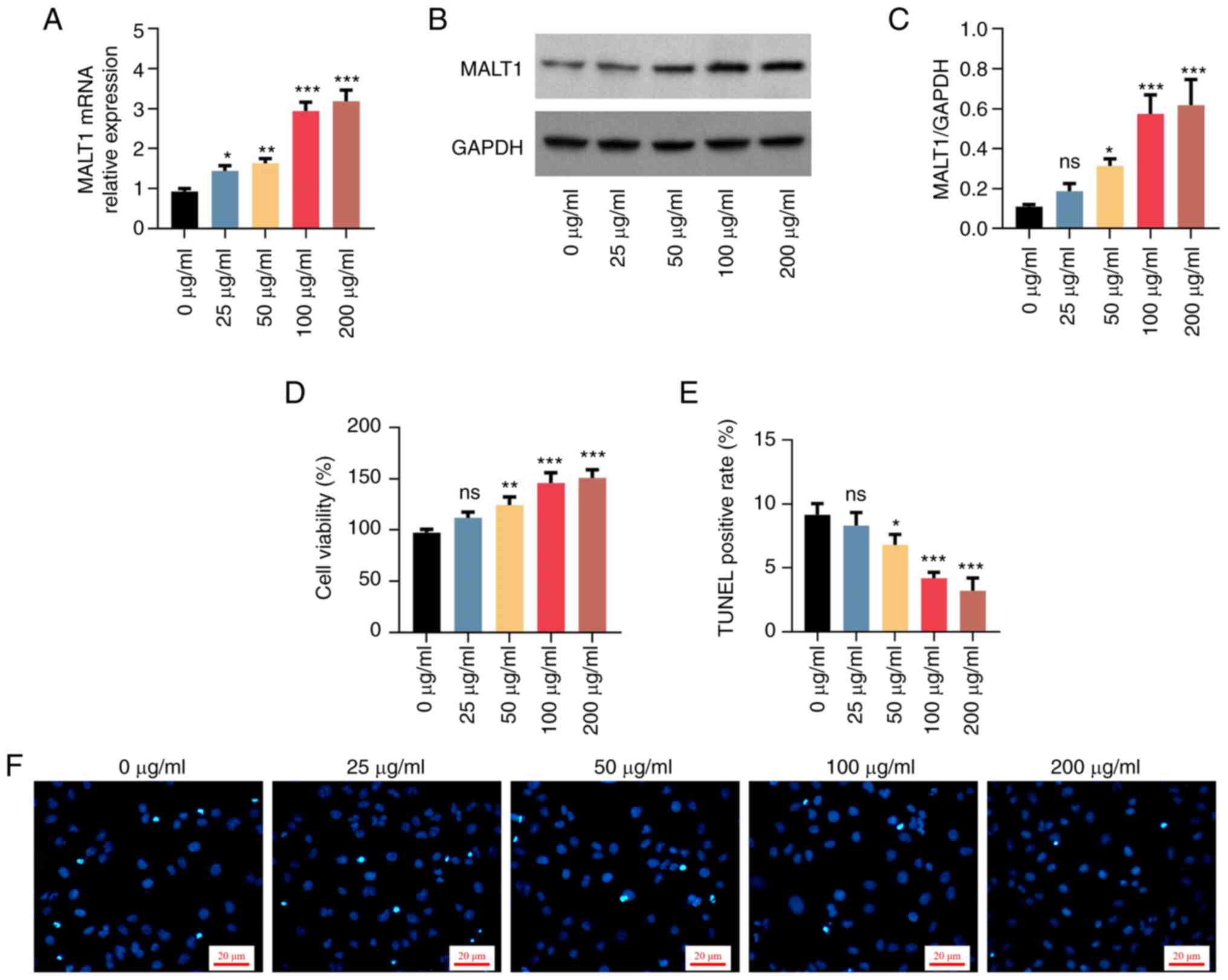
The mRNA expression levels of MALT1 were notably
elevated in a dose-dependent manner following VSMC treatment with
25-200 µg/ml oxLDL (all P<0.05; Fig. 1A). Consistently, the protein
expression levels of MALT1 were also significantly increased in a
dose-dependent manner in VSMCs treated with 50-200 µg/ml oxLDL (all
P<0.05; Fig. 1B and C). However, no statistical significance
was observed in the 25 µg/ml oxLDL treatment group. In addition,
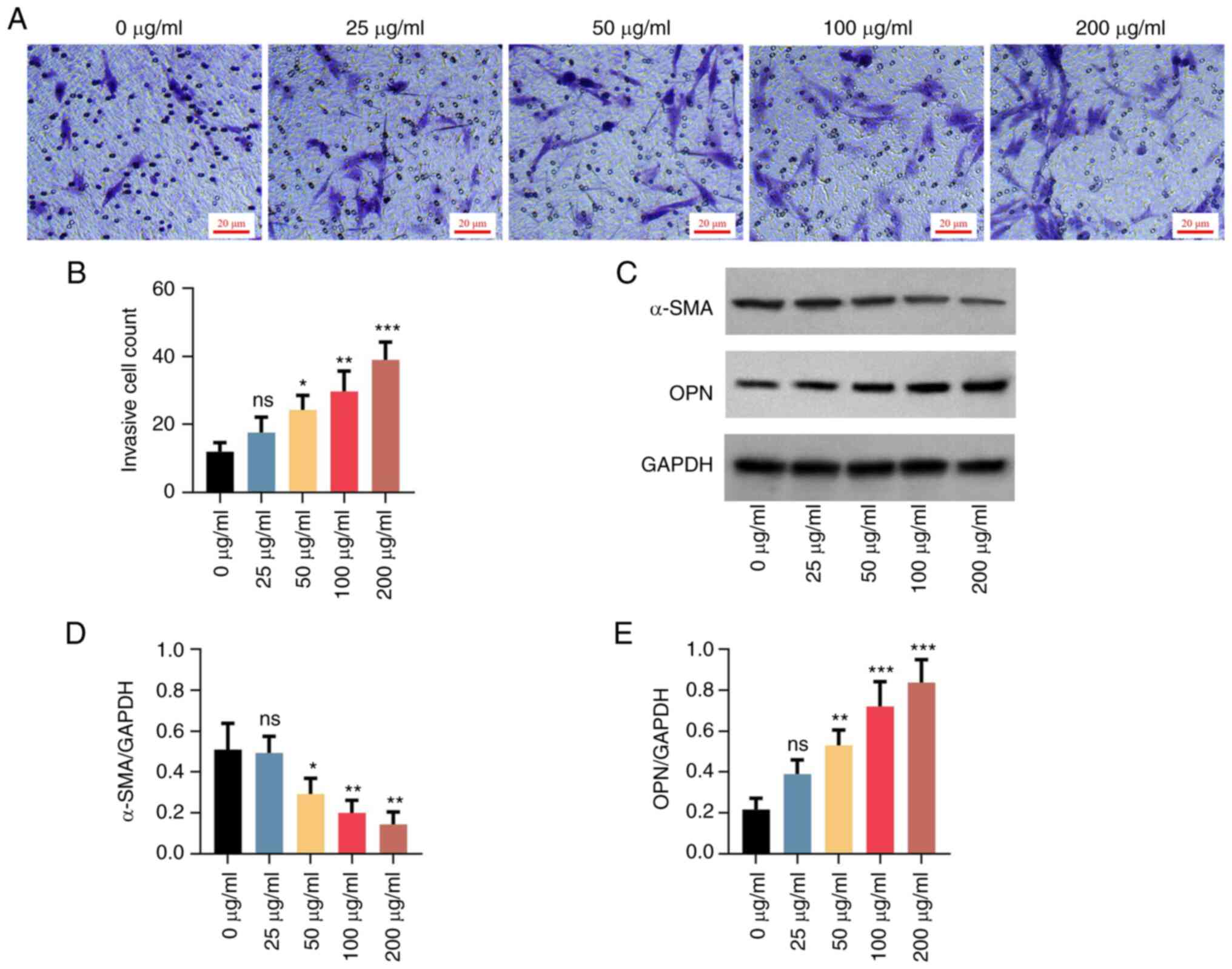
oxLDL enhanced cell proliferation (Fig. 1D) suppressed cell apoptosis
(Fig. 1E and F). It enhanced invasion (Fig. 2A and B), downregulated α-SMA and upregulated
OPN (Fig. 2C-E) in a
dose-dependent manner. However, again, no statistical significance
was observed in the 25 µg/ml oxLDL treatment group. The effect of
oxLDL on cell proliferation, apoptosis, invasion and varying α-SMA
and OPN levels showed a dose-dependent manner between 0 and 100
µg/ml, but it reached a plateau between 100 and 200 µg/ml.
Therefore, a lower dose at the plateau was chosen for the following
experiment (which is common practice). The above data supported the
successful establishment of the proatherogenic VSMC model.
MALT1 positively regulates cell
viability, invasion and phenotype switching and negatively
regulates apoptosis in proatherogenic VSMCs
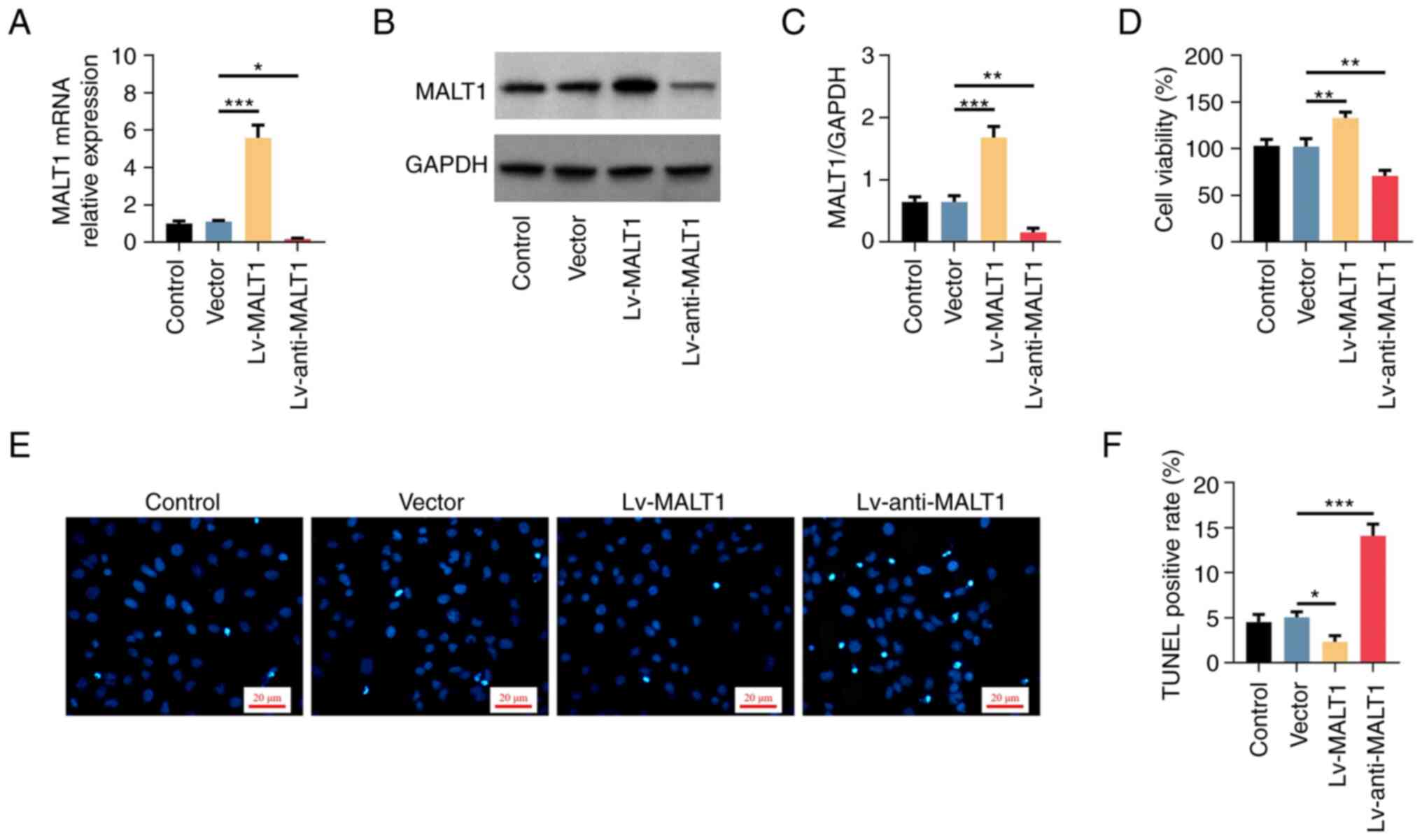
Subsequently, to evaluate the effect of MALT1 on the
cellular functions of proatherogenic VSMCs, the expression of MALT1
was modulated in VSMCs transfected with the corresponding
lentivirus. The results showed that the mRNA (Fig. 3A) and protein (Fig. 3B and C) expression levels of MALT1 were
increased in VSMCs transfected with Lv-MALT1 (both P<0.001)
compared with the Vector group. By contrast, MALT1 was
downregulated in cells transfected with Lv-anti-MALT1 (both
P<0.05) compared with the Vector group, thus suggesting that the
transduction of VSMCs with lentiviral particles was successful.
Furthermore, compared with the Vector group, the viability of
proatherogenic VSMCs was elevated and reduced by MALT1
overexpression and knockdown, respectively (both P<0.01;
Fig. 3D). Additionally, apoptosis
assessment by TUNEL assay revealed that the number of apoptotic
cells was decreased in the Lv-MALT1 group and increased in the
Lv-anti-MALT1 group compared with the Vector group (Fig. 3E). Consistently, semi-quantified
analysis confirmed that the changes in the number of apoptotic
VSMCs were statistically significant compared with the Vector group
(P<0.05, for Lv-MALT1; P<0.001, for Lv-anti-MALT1; Fig. 3F).
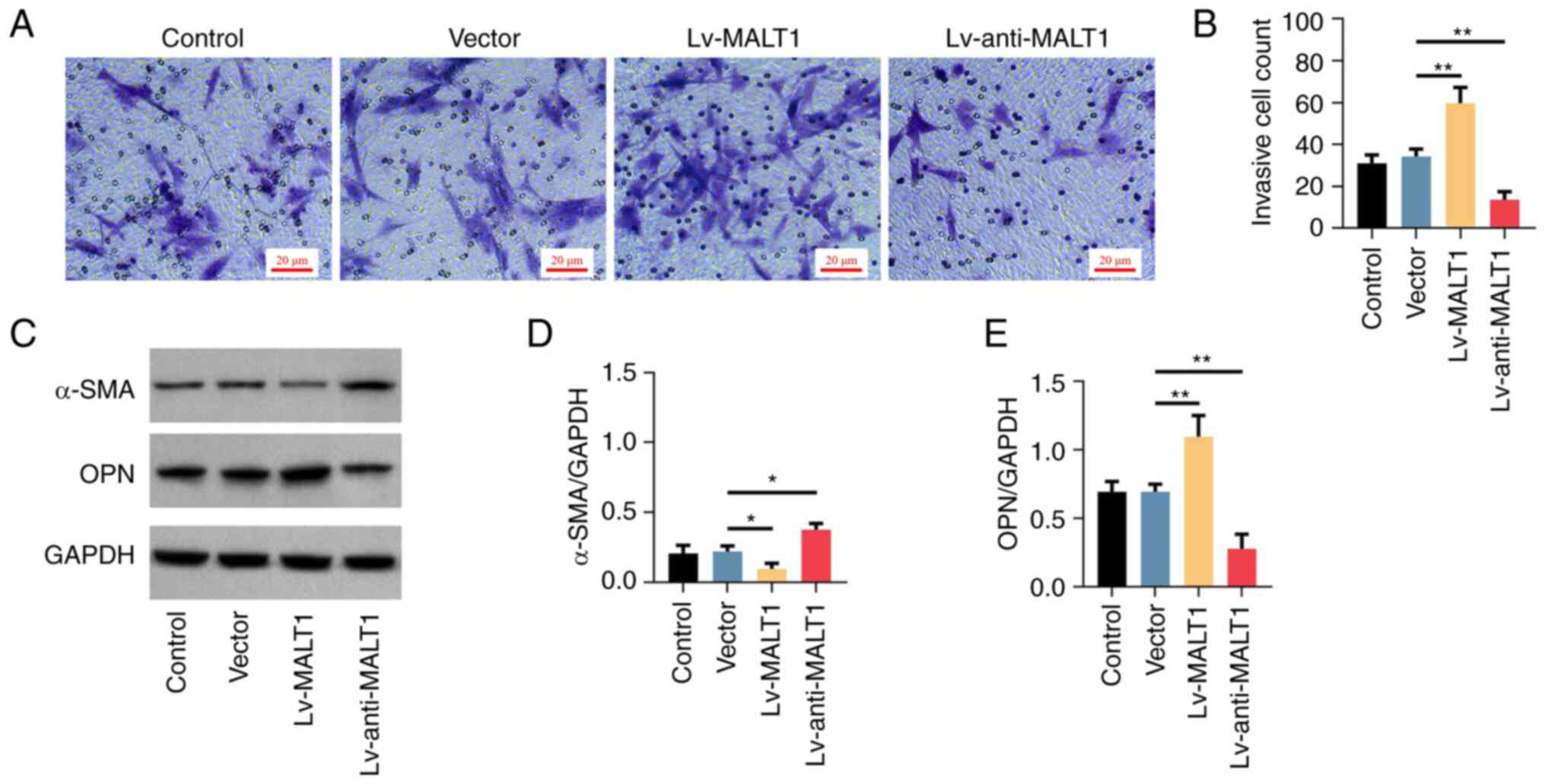
Transwell assay and crystal violet staining showed
that the invasion ability of proatherogenic VSMCs was enhanced by
MALT1 overexpression and reduced by MALT1 knockdown compared with
the Vector group (Fig. 4A).
Semi-quantified analysis verified the aforementioned effects (both
P<0.01; Fig. 4B). Furthermore,
the protein expression levels of VSMC phenotype markers were
evaluated by western blot analysis (Fig. 4C). Therefore, the results
demonstrated that the expression of the contractile phenotype
marker, α-SMA, was inhibited by MALT1 overexpression and elevated
by MALT1 knockdown (both P<0.05; Fig. 4D). However, the opposite effects
were observed in the protein expression levels of the synthetic
phenotype marker OPN (both P<0.01; Fig. 4E), thus indicating that the
phenotype of proatherogenic VSMCs was regulated by the expression
of MALT1.
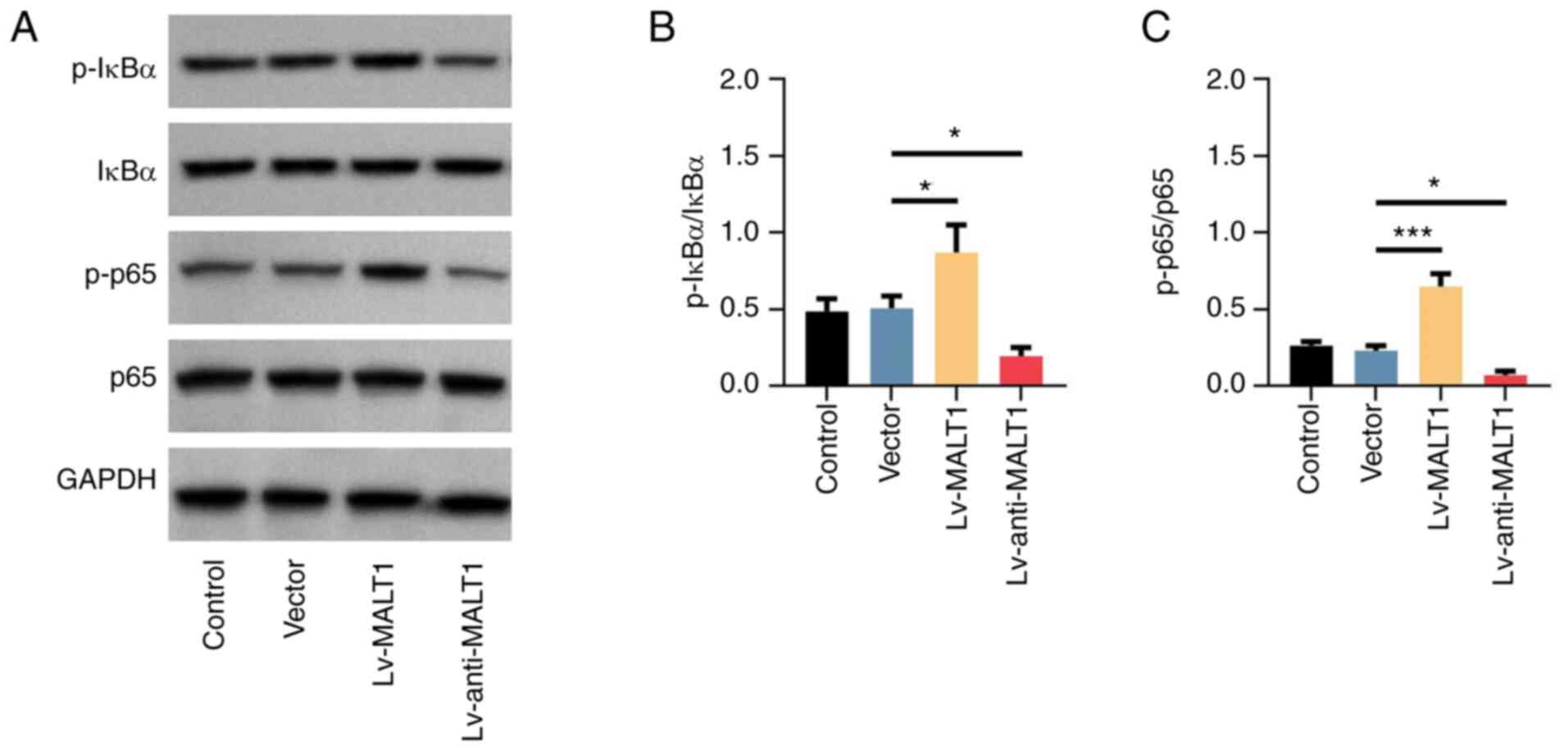
MALT1 activates the NF-κB signaling
pathway in proatherogenic VSMCs
Subsequently, the activation status of the NF-κB
signaling pathway, a potential downstream pathway of MALT1, was
detected in proatherogenic VSMCs using western blot analysis
(Fig. 5A). The data revealed that
compared with the Vector group, p-IκBα was upregulated by MALT1
overexpression and downregulated by MALT1 knockdown (both
P<0.05; Fig. 5B). Additionally,
the protein expression levels of p-p65 were increased (P<0.001)
and reduced (P<0.05) by MALT1 overexpression and knockdown,
respectively (Fig. 5C).
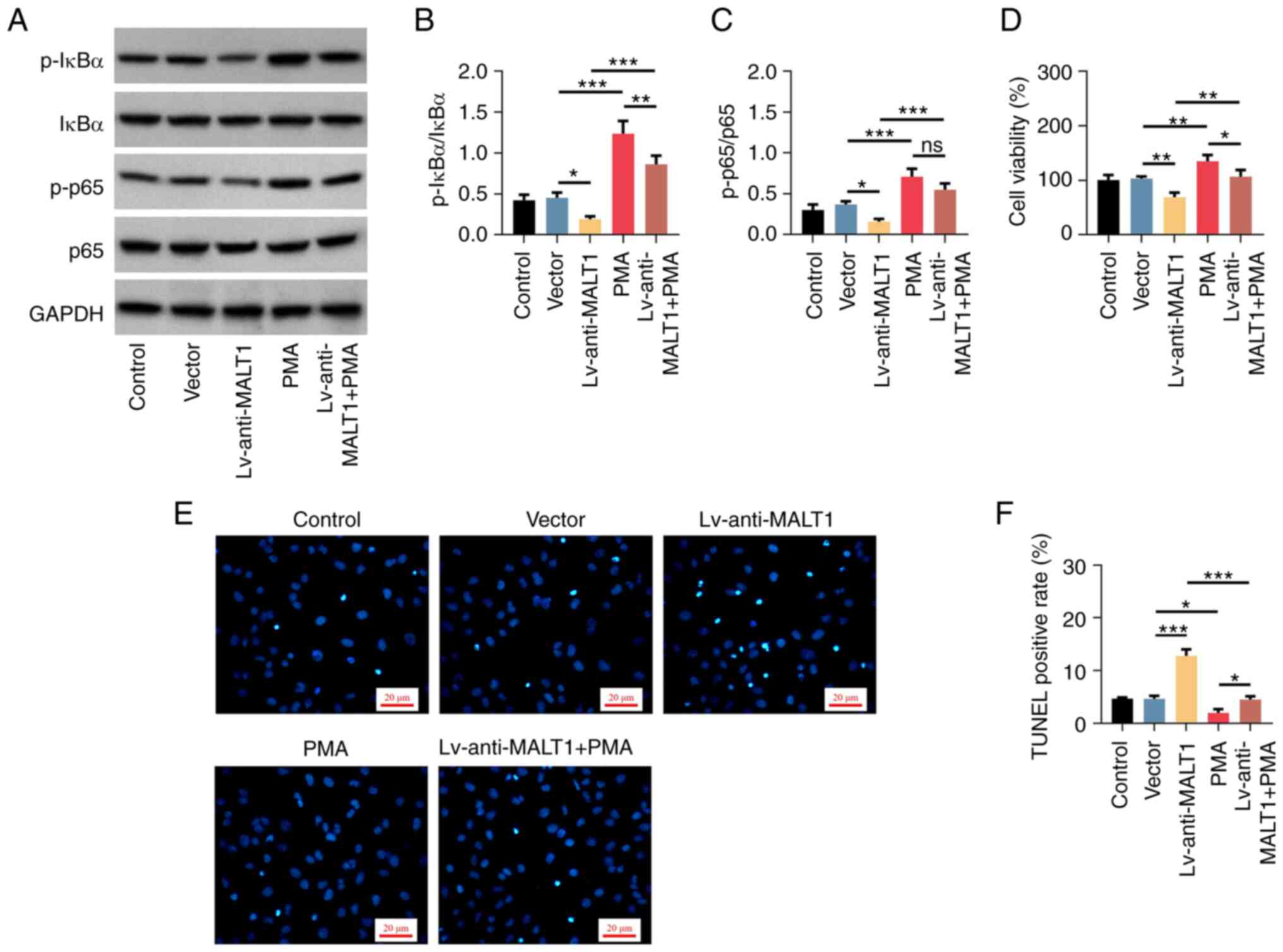
MALT1 regulates the cellular functions
of proatherogenic VSMCs via the NF-κB signaling pathway
To reveal the association between MALT1 and NF-κB
signaling in the cellular functions of proatherogenic VSMCs,
MALT1-depleted VSMCs and MALT1-containg VSMCs were treated with
PMA, a NF-κB pathway activator. Western blot analysis showed that
cell treatment with PMA upregulated both p-IκBα and p-p65, compared
with the Vector group (both P<0.001; Fig. 6A). Furthermore, treatment of
MALT1-depleted proatherogenic VSMCs with PMA increased the levels
of p-IκBα and p-p65, which were reduced by MALT1 knockdown (both
P<0.001; Fig. 6B and C). Regarding the cellular functions of
proatherogenic VSMCs, PMA promoted cell viability (P<0.01;
Fig. 6D), suppressed cell
apoptosis (P<0.05; Fig. 6E and
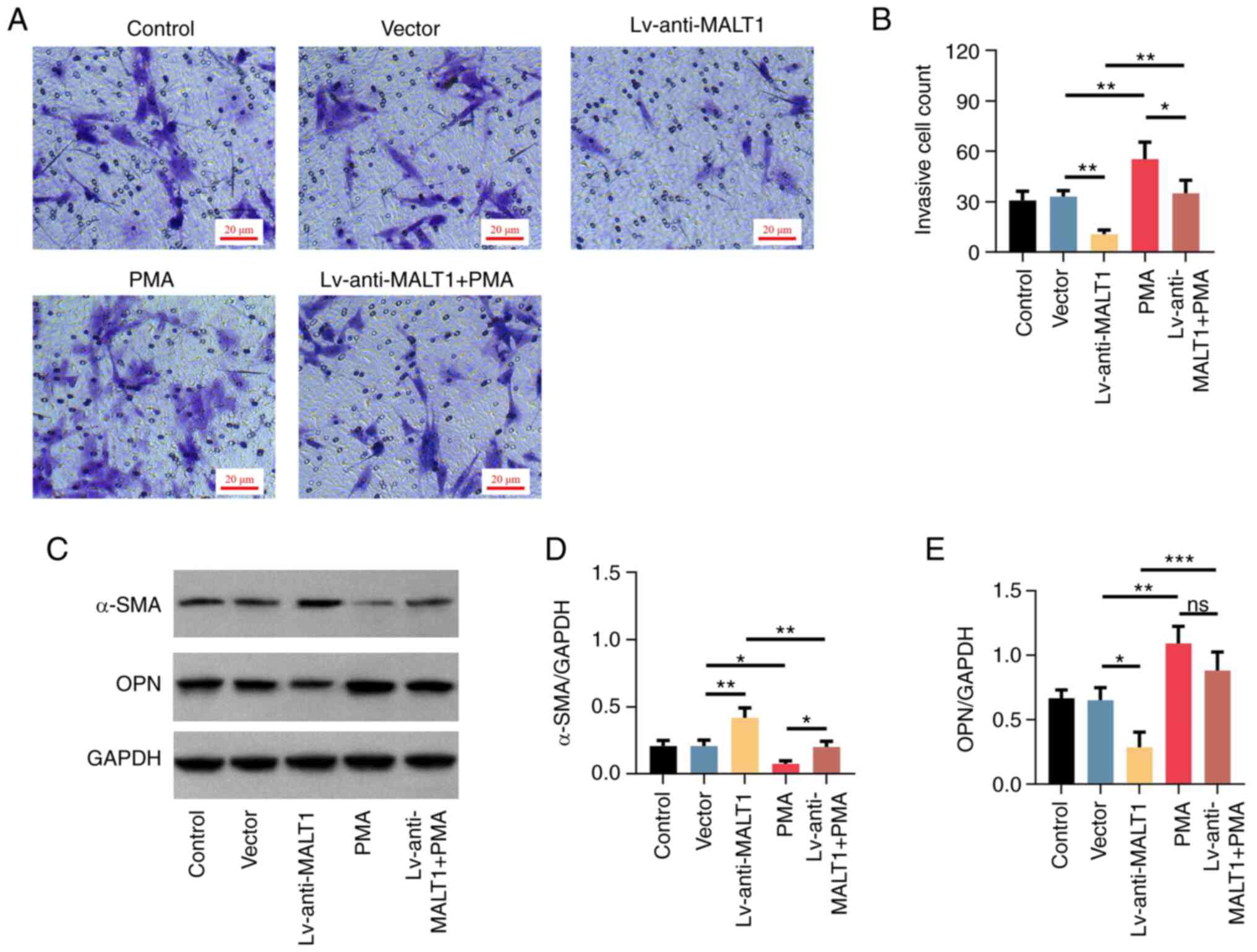
F), increased cell invasion
(P<0.01; Fig. 7A and B), downregulated α-SMA and upregulated
OPN (both P<0.05; Fig. 7C-E)
compared with the Vector treatment group. Furthermore, additional
treatment with PMA further reduced the viability of VSMCs, that had
been elevated by MALT1 knockdown (P<0.01; Fig. 6D). In terms of apoptosis,
additional PMA treatment suppressed the MALT1 knockdown-mediated
enhanced cell apoptosis (P<0.001; Fig. 6E and F). Furthermore, additional treatment of
VSMCs with PMA promoted the cell invasion, that was inhibited by
MALT1 knockdown (both P<0.01; Fig.
7A and B). Finally, additional
treatment with PMA restored the MALT1 knockdown-mediated high
levels of α-SMA and low levels of OPN in proatherogenic VSMCs (both
P<0.01; Fig. 7C-E). The
aforementioned findings suggested that the NF-κB signaling pathway
was essential for regulating the MALT1-triggered cellular functions
of proatherogenic VSMCs.
Discussion
Currently, several factors have been identified to
be closely associated with the risk of atherosclerosis, including
hyperlipidemia, diabetes mellitus, smoking, increasing age and
biological sex (19,20). Among the mentioned risk factors,
hyperlipidemia is considered to be the most critical one (21). It has been reported that oxLDL, one
of the major members of lipidemia, is a main culprit of
atherosclerosis and is involved in the formation, progression and
rupture of atherosclerotic lesions (22). In addition, oxLDL is widely used in
preclinical studies to mimic atherosclerotic conditions (23-25).
In the current study, oxLDL was also used to establish a
proatherogenic VSMC model. Treatment of VSMCs with oxLDL promoted
cell viability, cell invasion and synthetic phenotype and
suppressed cell apoptosis in a dose-dependent manner. Additionally,
in the current study, treatment of proatherogenic VSMCs with oxLDL
increased the mRNA and protein expression levels of MALT1 in a
dose-dependent manner. A previous study showed that MALT1 was
upregulated in patients with acute ischemic stroke compared with
healthy subjects (26). The above
finding was partly in line with the results of the present study.
Together with the previous study, these data suggested that MALT1
could be associated with atherosclerosis. This may be due to the
fact that oxLDL could interact with the members of the CRAMA
protein family, thus upregulating MALT1(27).
It has been suggested that MALT1 is a potential
regulator of atherosclerosis. For example, previous studies
demonstrated that MALT1 could positively regulate the
differentiation of T helper 17 (Th17) cells, a vital class of
immune cells involved in promoting atherosclerosis progression
(28,29). Additionally, MALT1 could also
activate NF-κB signaling, which in turn induced inflammation to
positively regulate atherosclerosis (13,30).
Furthermore, another study revealed that angiotensin could not
promote the development of atherosclerosis in mice deficient in CBM
complex (14). However, whether
MALT1 can directly regulate the dysregulation of proatherogenic
VSMCs remains to be elucidated. The results of the current study
showed that MALT1 overexpression enhanced the dysregulation of
proatherogenic VSMCs, as supported by the increased cell
proliferation, invasion and synthetic phenotype and reduced cell
apoptosis. However, MALT1 knockdown exerted the opposite effects.
The aforementioned findings could be due to: i) MALT1 could
activate downstream signaling pathways, such as the NF-κB and Janus
kinase pathways to modulate the cellular functions of
proatherogenic VSMCs (13,31); ii) MALT1 could promote the
pathogenesis of atherosclerosis via activating the G
protein-coupled type 1 receptor for angiotensin II via the CBM
complex (14); and iii) MALT1
could promote the differentiation of Th17 cells, thus inducing the
secretion of interleukin-17, which in turn could further promote
the pathogenesis of atherosclerosis (28,32).
The NF-κB pathway is a vital signaling pathway
involved in the regulation of multiple cellular functions, such as
cell survival, immune response and inflammation, thus participating
in the onset of several diseases, including cancer, autoimmune
diseases and center nervous system diseases (33,34).
Notably, it has been reported that the NF-κB pathway is critically
involved in atherosclerosis (35-37)
and it is the primary downstream target of MALT1 (13,14).
Therefore, the current study further investigated whether the NF-κB
signaling pathway was essential for the MALT1-mediated modulation
of proatherogenic VSMC dysregulation. First, the results revealed
that MALT1 could positively regulate the NF-κB pathway in
proatherogenic VSMCs, which was in agreement with a previous study
(14). Second, the data suggested
that the activation of NF-κB signaling could hamper the effect of
MALT1 knockdown on attenuating the dysregulation of proatherogenic
VSMCs. Taken together, the above results indicated that MALT1 could
exaggerate the dysregulation of proatherogenic VSMCs via activation
of the NF-κB signaling pathway. It was therefore hypothesized that
the high levels of MALT1 could activate the CBM complex, thus
promoting the activation of the NF-κB pathway (38). Furthermore, the NF-κB pathway was
involved in the functional alteration of VSMCs (39). However, the above findings should
be further verified in vivo. Additionally, whether MALT1
could facilitate atherosclerosis via other processes, such as lipid
accumulation, inflammation and foam cell formation, should be
further evaluated. Cell images at a lower magnification could
provide an alternative view on cell apoptosis and invasion.
Collectively, the results of the present study
suggested that MALT1 could increase cell growth, invasion and
synthetic phenotype switching via activating NF-κB signaling in
proatherogenic VSMCs. The aforementioned findings could provide the
basis for the development of MALT1-based treatment approaches for
atherosclerosis. However, further validation experiments are
needed.
Supplementary Material
Frame illustration of vectors
overexpression or knocking down MALT1. MALT1, mucosa-associated
lymphoid tissue lymphoma translocation protein 1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LB contributed to the conception and design of the
study. HZ contributed to data acquisition, analysis and
interpretation of data. LB and HZ confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The use of VSMCs was approved by the Ethics
Committee of Affiliated Hospital of Inner Mongolia Medical
University [approval number KY (2020015)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bjorkegren JLM and Lusis AJ:
Atherosclerosis: Recent developments. Cell. 185:1630–1645.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dawson LP, Lum M, Nerleker N, Nicholls SJ
and Layland J: Coronary atherosclerotic plaque regression: JACC
State-of-the-Art review. J Am Coll Cardiol. 79:66–82.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mendelson SJ and Prabhakaran S: Diagnosis
and management of transient ischemic attack and acute ischemic
stroke: A review. JAMA. 325:1088–1098. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Seiffge DJ, Wilson D and Wu TY:
Administering thrombolysis for acute ischemic stroke in patients
taking direct oral anticoagulants: To treat or how to treat. JAMA
Neurol. 78:515–516. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiong Y, Wakhloo AK and Fisher M: Advances
in acute ischemic stroke therapy. Circ Res. 130:1230–1251.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhatt DL, Lopes RD and Harrington RA:
Diagnosis and treatment of acute coronary syndromes: A review.
JAMA. 327:662–675. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gaudino M and Taggart DP: Percutaneous
coronary intervention vs coronary artery bypass grafting: A
surgical perspective. JAMA Cardiol. 4:505–506. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miano JM, Fisher EA and Majesky MW: Fate
and state of vascular smooth muscle cells in atherosclerosis.
Circulation. 143:2110–2116. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu YX, Yuan PZ, Wu JH and Hu B: Lipid
accumulation and novel insight into vascular smooth muscle cells in
atherosclerosis. J Mol Med (Berl). 99:1511–1526. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang F, Guo X, Xia Y and Mao L: An update
on the phenotypic switching of vascular smooth muscle cells in the
pathogenesis of atherosclerosis. Cell Mol Life Sci.
79(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
DeVore SB and Khurana Hershey GK: The role
of the CBM complex in allergic inflammation and disease. J Allergy
Clin Immunol. 150:1011–1030. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qi T, Luo Y, Cui W, Zhou Y, Ma X, Wang D,
Tian X and Wang Q: Crosstalk between the CBM complex/NF-kappaB and
MAPK/P27 signaling pathways of regulatory T cells contributes to
the tumor microenvironment. Front Cell Dev Biol.
10(911811)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McAllister-Lucas LM, Jin X, Gu S, Siu K,
McDonnell S, Ruland J, Delekta PC, Van Beek M and Lucas PC: The
CARMA3-Bcl10-MALT1 signalosome promotes angiotensin II-dependent
vascular inflammation and atherogenesis. J Biol Chem.
285:25880–25884. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu X, Zheng X, Cheng J, Zhang K and Ma C:
LncRNA TUG1 regulates proliferation and apoptosis by regulating
miR-148b/IGF2 axis in ox-LDL-stimulated VSMC and HUVEC. Life Sci.
243(117287)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang W, Chen L, Shang C, Jin Z, Yao F, Bai
L, Wang R, Zhao S and Liu E: miR-145 inhibits the proliferation and
migration of vascular smooth muscle cells by regulating autophagy.
J Cell Mol Med. 24:6658–6669. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Snow JB, Norton CE, Sands MA, Weise-Cross
L, Yan S, Herbert LM, Sheak JR, Gonzalez Bosc LV, Walker BR, Kanagy
NL, et al: Intermittent hypoxia augments pulmonary vasoconstrictor
reactivity through PKCβ/Mitochondrial oxidant signaling. Am J
Respir Cell Mol Biol. 62:732–746. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meng H, Ruan J, Yan Z, Chen Y, Liu J, Li X
and Meng F: new progress in early diagnosis of atherosclerosis. Int
J Mol Sci. 23(8939)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan J and Watanabe T: Atherosclerosis:
Known and unknown. Pathol Int. 72:151–160. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vekic J, Zeljkovic A, Cicero AFG, Janez A,
Stoian AP, Sonmez A and Rizzo M: Atherosclerosis development and
progression: The role of atherogenic small, dense LDL. Medicina
(Kaunas). 58(299)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin P, Ji HH, Li YJ and Guo SD: Macrophage
plasticity and atherosclerosis therapy. Front Mol Biosci.
8(679797)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang D, Zhou Z and Yuan L: Polydatin
reverses oxidation low lipoprotein (oxLDL)-induced apoptosis of
human umbilical vein endothelial cells via regulating the
miR-26a-5p/BID axis. Eur J Histochem. 66(3505)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang K, Bai X, Mei L, Miao Y and Jin F:
CircRNA_0050486 promotes cell apoptosis and inflammation by
targeting miR-1270 in atherosclerosis. Ann Transl Med.
10(905)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al Mansouri M, Patel PA, Chamberlain J and
Francis S: OxLDL induces IL-1β release from human EC and VSMC via
different caspase-1 dependent mechanisms. Vasc Biol. 4:11–18.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen X, Zhang X, Lan L, Xu G, Li Y and
Huang S: MALT1 positively correlates with Th1 cells, Th17 cells,
and their secreted cytokines and also relates to disease risk,
severity, and prognosis of acute ischemic stroke. J Clin Lab Anal.
35(e23903)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rhoads JP, Lukens JR, Wilhelm AJ, Moore
JL, Mendez-Fernandez Y, Kanneganti TD and Major AS: Oxidized
low-density lipoprotein immune complex priming of the Nlrp3
inflammasome involves TLR and FcγR cooperation and is dependent on
CARD9. J Immunol. 198:2105–2114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Q, Wang Y, Liu Q, Chu Y, Mi R, Jiang
F, Zhao J, Hu K, Luo R, Feng Y, et al: MALT1 regulates Th2 and Th17
differentiation via NF-κB and JNK pathways, as well as correlates
with disease activity and treatment outcome in rheumatoid
arthritis. Front Immunol. 13(913830)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wei S, Sun J, Li Y, Xu K, Wang M and Zhang
Y: Losartan attenuates atherosclerosis in uremic mice by regulating
Treg/Th17 balance via mediating PTEN/PI3K/Akt pathway. Nephron.
146:528–538. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Madonna R and De Caterina R: Relevance of
new drug discovery to reduce NF-κB activation in cardiovascular
disease. Vascul Pharmacol. 57:41–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Knies N, Alankus B, Weilemann A, Tzankov
A, Brunner K, Ruff T, Kremer M, Keller UB, Lenz G and Ruland J:
Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell
proliferation via cooperative NF-κB and JNK activation. Proc Natl
Acad Sci USA. 112:E7230–E7238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Q, Wang Y and Xu D: Research progress
on Th17 and T regulatory cells and their cytokines in regulating
atherosclerosis. Front Cardiovasc Med. 9(929078)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kaltschmidt B, Helweg LP, Greiner JFW and
Kaltschmidt C: NF-κB in neurodegenerative diseases: Recent evidence
from human genetics. Front Mol Neurosci. 15(954541)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang T, Ma C, Zhang Z, Zhang H and Hu H:
NF-κB signaling in inflammation and cancer. MedComm (2020).
2:618–653. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Ding S, Liu J, Han X, Ding W, Liu Z, Zhu
Y, Zhan W, Wan Y, Gai S, Hou J, et al: ICAM-1-related noncoding RNA
accelerates atherosclerosis by amplifying NF-κB signaling. J Mol
Cell Cardiol. 170:75–86. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng Y, Li Y, Ran X, Wang D, Zheng X,
Zhang M, Yu B, Sun Y and Wu J: Mettl14 mediates the inflammatory
response of macrophages in atherosclerosis through the NF-κB/IL-6
signaling pathway. Cell Mol Life Sci. 79(311)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu C, Wu J, Jia H, Lu C, Liu J, Li Y and
Guo M: Oncostatin M promotes the ox-LDL-induced activation of NLRP3
inflammasomes via the NF-κB pathway in THP-1 macrophages and
promotes the progression of atherosclerosis. Ann Transl Med.
10(456)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ruland J and Hartjes L: CARD-BCL-10-MALT1
signalling in protective and pathological immunity. Nat Rev
Immunol. 19:118–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yeh CC, Wu JY, Lee GL, Wen HT, Lin P and
Kuo CC: Vanadium derivative exposure promotes functional
alterations of vsmcs and consequent atherosclerosis via
ROS/p38/NF-κB-Mediated IL-6 production. Int J Mol Sci.
20(6115)2019.PubMed/NCBI View Article : Google Scholar
|