Introduction
Traumatic brain injury (TBI) is becoming a major
public health concern, due to a steadily rising annual incidence of
~50 million individuals (1-4).
TBI involves a heterogeneous set of functional, anatomical and
histological alterations that are induced by external physical
forces exerting excessive forces to the brain (5-9).
This in turn ultimately leads to neuronal or glial cell apoptotic
and necrotic damage, blood vessel rupture, thrombosis, disruption
of the blood-brain barrier, skull fractures and/or meningeal tears
(3,5-9).
This type of sudden primary injury in TBI can present as a number
of pathophysiological characteristics, including macroscopic focal
or diffuse lesions, hematomas, haemorrhages, cerebral contusions
and/or diffuse axonal injuries, which may not be treatable
(3,9,10).
In addition, delayed neuronal damage may be inflicted by secondary
insults involving several molecular, biochemical and
neuroinflammatory disturbances, which can last from several minutes
to even months after the first mechanical insult (9,11).
The profile of primary or secondary pathology is dependent on the
injury mechanism, existence of concurrent injuries and
comorbidities and treatment effectiveness (12,13).
These aforementioned events are generally accompanied with robust
local and/or systemic immune activation (14). Therefore, targeting certain
immunological pathways may prove beneficial for developing future
TBI treatment strategies (14).
The primary brain injury may involve damage to the
intracranial contents, volume-occupying effects, in addition to
neuronal, glial and/or cerebral vascular dysregulation (3,8). By
contrast, secondary brain injury depends on the activation status
of several interconnected pathophysiological pathways (10). The cell populations that will
either undergo apoptotic cell death or sustain the significant
functional impairments are determined by the degree of activation
of these complex pathways and processes (8,10).
A variety of metabolic and/or molecular cascades can
be activated in TBI, ultimately leading to elevated intracellular
concentrations of calcium and sodium, mitochondrial dysfunction,
free radical production, oxidative phosphorylation impairment,
apoptosis activation, cumulative release of neurotransmitters, and
in energy expenditure (11,15,16).
Pro-inflammatory, anti-inflammatory cytokines and
chemokines can be secreted by neurons, glial cells and systemic
immune cells, which can also serve a significant role in
intracellular pathological signalling (8,11).
Mechanical processes such as volume-occupying
traumatic lesions can cause cerebral oedema, ischaemia, recurrent
haemorrhage, impaired cerebral autoregulation, decreased cerebral
perfusion pressure, increased intracranial pressure and herniation
syndromes (17).
Systemic processes can result in a variety of
conditions, including reduced cerebral blood flow, electrolyte
disorders, hyperglycaemia, hypoglycaemia, hypoxia, anaemia, hypo-
or hypercapnia, acid-base disorders and seizures (2).
On a molecular level, mechanical energy transfer can
disrupt neurotransmitter circuits and homeostasis, even if there is
no anatomical damage on cellular or macroscopic levels (16).
Neuroinflammation is normally orchestrated through a
coordinated web of neuronal and microglial signalling (18-20).
It possesses a diverse array of both beneficial and neurotoxic
components (18-20).
There is also emerging evidence that an isolated incidence of brain
injury can lead to complex immunological alterations in the levels
of circulating leukocytes, complement proteins, pro- or
anti-inflammatory cytokines and coagulation factors (18-20).
Subsequently, pro-inflammatory cytokines activate M1
macrophages to repair damage, whereas anti-inflammatory cytokines
can stimulate M2 macrophages, which serves to regenerate
the neural tissue (11,12).
TBI severity is commonly classified as severe,
moderate or mild (Table SI). In
addition, certain biomarkers, CT imaging prognostic scales and
multidimensional computer prognostic models are also currently used
to determine the severity of TBI (13,15,21).
In particular, because of its simplicity, low cost, speed, ability
to reveal osseous structures and surgical lesions, head CT scan
remains to be the gold standard for the initial evaluation of an
injured patient with a TBI (15).
In terms of molecular biomarkers, ubiquitin C-terminal hydrolase L1
(UCH-L1) is considered to be a protein biomarker for neuronal cell
body injury, whereas glial fibrillary acidic protein (GFAP) is
generally considered as a biomarker for astro-glial injury
(10,22). UCH-L1 is a deubiquitinating
neuronal enzyme, whilst GFAP is a monomer of intermediate filaments
forming the astrocytic cytoskeleton (23-25).
The i-STAT TBI Plasma test, which measures both UCH-L1 and GFAP,
has been approved by the U.S. Food and Drug Administration for the
detection of possible candidates for CT scan, between patients with
mild TBI discrimination in adults (26). Apart from GFAP, astroglial
calcium-binding protein B (S100B) is also one of the most
extensively studied TBI biomarkers (27-33).
Scandinavian Guidelines for Initial Management of Minimal, Mild and
Moderate Head Injuries in Adults have already incorporated S100B,
to triage patients with mild TBI for brain imaging (34). Furthermore, two large clinical
trials (INTREPID used GFAP and UCH-L1 and Bio-ProTECT used GFAP,
S100B and UCH-L1) have incorporated these three biomarkers for
evaluating treatment efficacy (10).
IL-6 is a member of the IL-6 cytokine family
(35). Although it is generally
considered to be a pro-inflammatory cytokine, a recent study has
revealed that it can also possess anti-inflammatory properties
(36). A large group of different
immune, epithelial, neuronal or astroglial cells can release IL-6,
which in turn triggers a multitude of biological cascades (35,37,38).
Amongst the list of reported metabolic and neurotrophic functions,
an essential role in neuronal survival during TBI-induced
neuroinflammation has also been ascribed to this important family
of cytokines (36,39,40).
By contrast, IL-8 is a chemoattractant cytokine that is produced by
various cell types, including monocytes, neutrophils, fibroblasts,
endothelium, epithelial cells and cancer cells (41). Unlike other cytokines, IL-8 has a
distinct specificity for attracting neutrophils towards
inflammatory regions, in addition to recruiting epithelial cells
for angiogenesis and tissue healing (41-44).
It has been extensively studied in a variety of inflammatory
responses, including fever, sepsis and carcinogenesis (41,45).
However, its role in the pathophysiology of the neuroinflammation
following TBI remains unclear. IL-10 is considered to be an
anti-inflammatory cytokine that can inhibit the expression of
pro-inflammatory factors, such as IL-1β, TNF-α, IL-1α, IL-6 and
IL-8 (46-50).
In terms of its neurological role, IL-10 can appear to mediate the
recovery process following TBI-induced neuroinflammation (48). Additionally, IL-10 has been
reported to facilitate cytokine storm resolution, to prevent
prolonged secondary brain damage (51). Monocytes and lymphocytes can both
secrete IL-10, which stimulates the IL-10/Janus kinase 1/STAT3
pathway to suppress these damaging inflammatory processes (47,49,50).
A number of studies have previously examined the
potential changes in the serum levels of pro- or anti-inflammatory
cytokines during the acute phase following TBI in animal models and
humans (Table SII, Table SIII, Table SIV, Table SV and Table SVI). Although results remain
inconclusive, they generally suggest the upregulation of IL-6, IL-8
and IL-10 following TBI compared with that in healthy controls.
However, studies examining the association of cytokine levels,
measured at the time of injury and/or soon after, with injury
severity in human patients remain elusive. This is compounded by
the potential value of applying cytokine levels for disease
prognosis receiving next to no attention. To the best of our
knowledge, there is insufficient information on the association of
IL-6, IL-8 and IL-10 with the extant set of clinical, imaging,
laboratory indices of injury severity and clinical prognosis in
TBI. Instead, the vast majority of previous studies tended to focus
on the association of each interleukin with a limited set of
clinical parameters.
Therefore, present study attempted a more holistic
evaluation of the possible relationship among some of the
inflammatory serum biomarkers and TBI severity and prognosis.
Numerous variables, including epidemiological, clinical,
laboratory, imaging, specific neurobiomarkers, complications and
functional outcomes were recorded and analysed. The present study
had the following two primary objectives: i) To assess the value of
IL-6, IL-8 and IL-10 and cell injury markers (such as UCH-L1 and
GFAP) as potential complementary TBI severity classification
indices upon admission, in combination with standardised existing
clinical, imaging variables and scoring systems; and ii) To assess
the possible prognostic value of ILs, as assessed using validated
functional outcome scoring scales.
Patients and methods
Patient recruitment
In the present single-centre, prospective
observational study, adult and paediatric patients from a
Neurosurgical Department and two Intensive Care Units (ICU) of
University General Hospital of Heraklion, in eastern Crete, Greece
were recruited between 2019 and 2022. Adult (>16 years old)
patients who were consecutively admitted with mild, moderate or
severe TBI, as described in the introduction section, were eligible
for enrolment. A smaller pilot group of paediatric patients (aged
1-16 years) was also enrolled for comparison, to clarify whether
paediatric patients with TBI show similar neuroinflammatory
responses to adults. A group of healthy adult individuals served as
the control group for comparisons. Inclusion criteria: i) Patients
with mild, moderate or severe TBI, with any type of haemorrhagic
traumatic brain imaging findings; ii) Patients or patients' legal
representatives were able or willing to provide written informed
consent; and iii) Blood sampling feasible within the first 12 h
after TBI; iv) Blood sampling feasible before any surgical
intervention. Exclusion criteria: i) Previous history of
neurological disease, or CNS malignancy, ii) Concurrent acute
infectious, neoplastic, inflammatory or immunological disease; iii)
Previous TBI or CNS surgery; and iv) Patients with blast or
penetrative injuries.
Data acquisition
Demographic and clinical data, co-occurring injuries
and comorbidities, imaging findings, injury severity scoring
systems, surgical interventions and clinical outcomes were
recorded. Additionally, the occurrence of possible related
complications was recorded throughout the first 7 days and at 6
months following the injury.
Variables collected. Clinical
diagnostic variables
Signs and symptoms, types of injury, co-occurrence
of multiple traumatic injuries, Glasgow Coma Scale (GCS; Table SI) score, motor component of the
GCS (mGCS) score (Table SVII),
Karnofsky Performance Scale (KPS) score (Table SVIII), Modified Rankin Scale score
(Table SIX), Eastern Cooperative
Oncology Group/WHO (ECOG/WHO) score (Table SX), pupil size and reactivity and
vital signs (hypotension and hypoxia) were obtained upon admission.
The Injury Severity Score (ISS) was also calculated (Table SXI). GCS was used as the primary
clinical parameter for TBI severity definition, whilst the
systematic traumatic injuries of patients were assessed through
ISS.
Brain imaging diagnostic variables. The
different types of traumatic lesions found on brain CT scanning
(General Electric Revolution CT-GSI) upon admission, the presence
and types of skull fractures, the patency of basal cisterns
(Table SXII), the presence of
midline shift (Table SXIII) and
the volume of space-occupying haemorrhagic lesions (Table SXIV) were all recorded. The
scores for the following TBI imaging scales were calculated: i)
Rotterdam CT; ii) Marshall CT Classification; iii) Stockholm CT;
and iv) Helsinki CT (Table
SXV).
Outcome scales. The KPS and Glasgow Outcome
Scale (GOS) (Table SXVI) scores
on day 7 post-injury and Glasgow Outcome Scale-Extended (GOS-E;
Table SXVII) and mortality at 6
months post-injury were used as outcome variables.
Prognostic models
The Corticosteroid Randomisation After Head Injury
(CRASH) predictive model was also calculated (Table SXV).
Assays
Blood samples were obtained within 12 h from
patients with TBI and on day 7 after the TBI, before the serum was
stored at -80˚C until further quantification analysis. IL-6 (Cat.
no. 430504; BioLegend, Inc.), IL-8 (Cat. no. 431504; BioLegend,
Inc.) and IL-10 (Cat. no. 430604; BioLegend, Inc.), GFAP (cat. no.
E-EL-H6093; Elabscience Biotechnology, Inc.) and UCH-L1 (cat. no.
E-EL-H2377; Elabscience Biotechnology Inc.) were measured through
ELISA according to manufacturer's protocols. All specimens were
assayed in duplicate. The sensitivities of the assays were 4 pg/ml
for IL-6, 8 pg/ml for IL-8, 2 pg/ml for IL-10, 9.38 pg/ml for GFAP
and 46.88 pg/ml for UCH-L1. The detection range was 7.8-500 pg/ml
for IL-6, 15.6-1,000 pg/ml for IL-8, 3.9-250 pg/ml for IL-10,
15.63-1,000 pg/ml for GFAP and 78.13-5,000 pg/ml for UCH-L1.
Statistical analysis. Univariate
analyses
Descriptive statistics of serum biomarkers are
presented for the three study groups (adult or paediatric TBI
patients, and healthy adults). Categorical variables are described
as absolute values and frequencies. The Kolmogorov-Smirnov and
Shapiro-Wilk analyses were used to determine whether a normal
distribution model fitted the observations as appropriate. Based on
this test of normality, quantitative clinical variables are
expressed as the mean ± standard deviation (parametric analyses),
or as median and interquartile range (non-parametric analyses).
Non-parametric tests were used to handle serum biomolecule data,
given significant deviations from normality. Spearman's correlation
coefficient was used for correlations between two continuous
variables and χ2 test for categorical variables,
respectively. Non-parametric group differences were examined using
the Mann-Whitney U-test, or the Kruskal-Wallis independent samples
test with post hoc Dunn's pairwise tests, in the event that an
independent variable consisted of > two groups. Receiver
Operating Characteristic Curves (ROC) were used to examine the
response function of potential biomolecule predictors toward
specific outcome variables. The ‘optimal’ cut off point for the
best sensitivity-specificity combination of the selected
discriminators was calculated by the Youden index (J), and
confirmed by the Closest to (0,1) Criteria (52).
Multivariate analyses
Selected variables that displayed statistically
significant associations with specific outcomes were entered into a
multivariate logistic regression model to identify parameters that
were independently associated with an adverse outcome. Nagelkerke
R2 goodness of fit value was used to evaluate the best-fitting
model.
Statistical analyses were performed using the SPSS
software for Windows (version 25; IBM Corp.). A P-value <0.05
was considered as an indicator of statistically significant
differences.
Results
The subsequent analyses refer to the adult
population, unless differently specified.
Patient characteristics
In total, 109 adult cases were eligible for
inclusion into the present study (66.1% males and 33.9% females;
mean age, 62.37±22 years). Mechanisms of brain injury included
falls from <1 m (44%) or >1 m (16.8%), road vehicle accidents
(22.4%), pedestrian involved accidents (6.4%), bicycle or skating
accidents (4%), assaults (4%) or object percussion injuries (2.4%).
The control group consisted of 20 healthy adult volunteers (55%
males and 45% females; mean age, 39.0±9.6 years). A smaller
paediatric pilot group of 17 patients was also enrolled (age range,
1-16 years; mean age, 10.2±4.5 years). Demographic, imaging and
clinical characteristics of the patients are summarized in Table I.
 | Table IPatient demographic and clinical
characteristics. |
Table I
Patient demographic and clinical
characteristics.
| Variable | Adults (n=109) | Children
(n=17) | P-value |
|---|
| Sex, n (%) | | | 0.289 |
|
Males | 72 (66.1) | 13 (76.5) | |
|
Females | 37 (33.9) | 4 (23.5) | |
| Age, mean (SD) | 62.37(22) | 10.2 (4.5) | - |
| GCS score, median
(IQR) | 14 (6-15) | 14 (10-15) | 0.642 |
| ISS, median
(IQR) | 14.5 (9-25) | 9 (6-21) | 0.312 |
| LOS (days), median
(IQR) | 15 (8-30) | 7 (4-14) | 0.191 |
| WBC x103
(cells/µl), median (IQR) | 12 (8.6-15.6) | 13 (10-19.2) | 0.078 |
| CRP (mg/dl), median
(IQR) | 1.2 (0.3-2.6) | 0.19
(0.06-0.76) | 0.002 |
| Marshall CT
Classification score, n (%) | | | 0.918 |
|
I + II | 60(55) | 11 (64.7) | |
|
III +
IV | 4 (3.7) | 6 (35.3) | |
|
V + VI | 45 (41.3) | 0 (0) | |
| Rotterdam CT score,
median (IQR) | 3 (2-3) | 2 (2-3) | 0.013 |
| Stockholm CT score,
median (IQR) | 1.7 (1-2.5) | 1 (0.7-1.5) | 0.034 |
| Helsinki CT score,
median (IQR) | 2.5 (2-6) | 1 (0-2) | 0.039 |
The majority of adult patients exhibited clinical
presentations consistent with mild TBI (66.9%) upon admission,
whereas 16.5% patients suffered from moderate TBI and 16.6% from
severe TBI. The 6-month mortality rate in the adult TBI patient
sample was 23.5%. By contrast, the majority of children exhibited
clinical presentation consistent with mild TBI (66.7%) upon
admission, whereas 14.3% patients suffered from moderate TBI and
19% with severe TBI. The 6-month mortality rate was 5.6% for the
paediatric TBI patient sample.
Group differences
Group differences of the studied protein levels are
presented in Table II. Serum
levels of IL-6 and IL-10 on day 1 were found to be significantly
elevated in adult patients with TBI compared with those in healthy
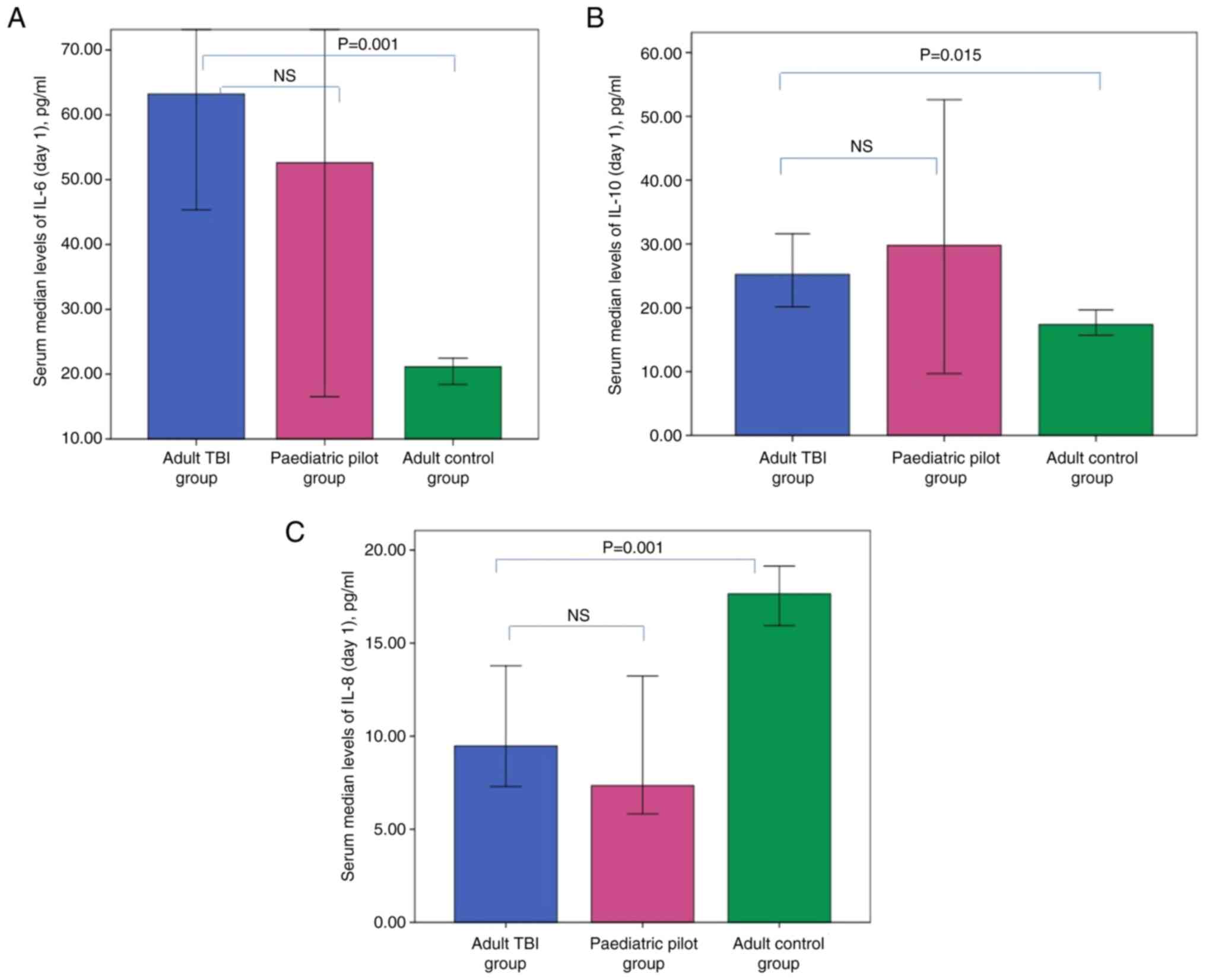
individuals (P=0.001 and 0.015 respectively) (Fig. 1). No significant differences were
recorded among the adult and paediatric patient groups regarding
the day 1 serum levels of IL-6, IL-8 and IL-10. On day 1, adult
patients with TBI displayed significantly lower IL-8 (P=0.004) and
significantly higher UCH-L1 levels (P=0.001) compared with those in
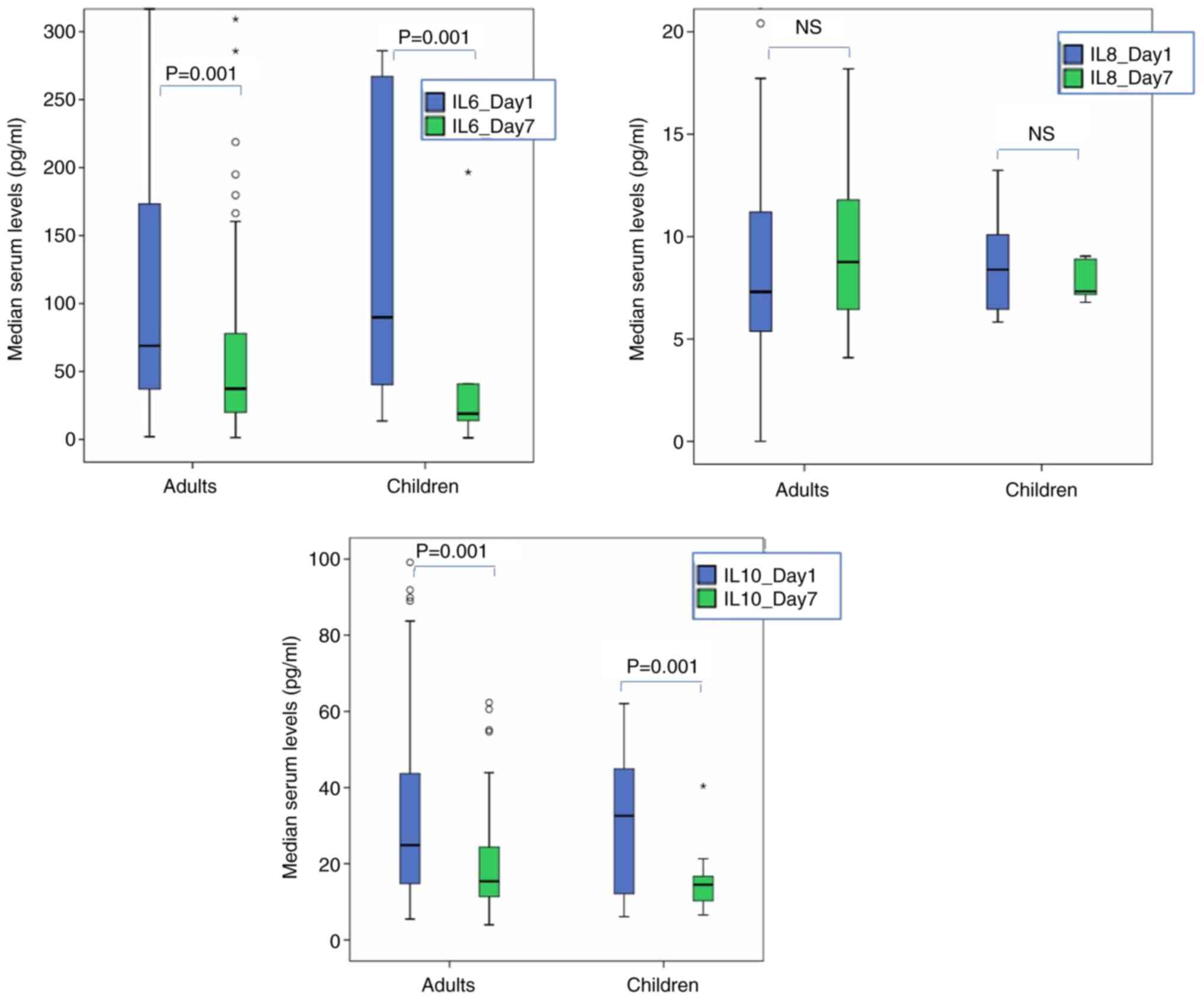
the control group. Consecutive measurements of inflammatory markers
revealed significant reduction for IL-6 and IL-10, or elevation for
UCH-L1 serum levels for adult TBI cases (P<0.021 for all)
(Fig. 2). It should be noted that
the IL data were not included in subsequent longitudinal analyses
in cases of missing values (which explains the differences in
medians among Table II and
Fig. 2) or if a given patient
underwent any surgical procedures during the first 7 days following
the TBI. These measures were taken to avoid confounding
measurements of IL on day 7.
 | Table IISerum levels of the biomolecules
measured in patients with TBI compared with healthy controls. |
Table II
Serum levels of the biomolecules
measured in patients with TBI compared with healthy controls.
| Biomolecules | Adult TBI
patients | Paediatric TBI
patients | Healthy adults |
|---|
| IL-6 Day1 | 63
(34-179)a | 52.6
(19.2-272) | 21.1 (18.2-23) |
| IL-6 Day7 | 37.2 (18.7-78) | 17.7 (13-40.7) | - |
| IL-8 Day1 | 9.5
(6.6-16.9)a | 7.35 (6-13.2) | 17.6 (15.6-19) |
| IL-8 Day7 | 10.6 (7-16.2) | 8.9 (7.3-15) | - |
| IL-10 Day1 | 25.2
(15-44.5)a | 29.8 (12.1-45) | 17.4 (15.8-20) |
| IL-10 Day7 | 15.3 (11-24.4) | 14.5
(10.3-16.7) | - |
| UCH-L1 Day1 | 207.2
(110-472)a | 190 (140-540) | 54 (46-105) |
| UCH-L1 Day7 | 323
(136-556.3) | 279
(210-393.4) | - |
| GFAP Day1 | 55.7 (27.3-94) | 46.2 (15.5-81) | 43.7 (24.5-71) |
| GFAP Day7 | 64 (35-88.5) | 29.4
(14.6-58.6) | - |
Associations of inflammatory indices
with TBI severity in adults
On day 1, adult patients who suffered from severe
TBI (as indicated by GCS<9) displayed significantly higher
levels of IL-6 (P=0.001), IL-10 (P=0.009), and GFAP (P=0.001)
compared with those in patients with mild or moderate TBI.
Similarly, patients who had lower mGCS on day 1(as indicated by
mGCS≤3) showed significantly higher levels of IL-6 (P=0.001), IL-10
(P=0.035) and GFAP (P=0.028). In addition, patients who suffered
from severe injuries throughout the body as indexed by scores of
ISS >24 displayed significantly elevated IL-6 (P=0.006), IL-10
(P=0.047) and GFAP (P=0.006) levels on day 1, compared with those
in the remaining patients (ISS≤24). Patients who scored <50
points according to KPS also had significantly higher levels of
IL-6 and IL-10 (P=0.004 for both) on day 1, compared with those who
scored >40. Similarly, patients who scored >3 according to
MRS classification displayed significantly elevated IL-6 (P=0.005),
IL-10 (P<0.001) and UCH-L1 (P=0.037) levels on day 1, compared
with those who scored <4. However, patients who scored >2
according to ECOG/WHO classification displayed significantly
elevated IL-6 (P=0.017) and IL-10 (P<0.001) levels, but
significantly lower UCH-L1 (P=0.022) levels on day 1, compared with
those who scored <3.
Inflammation biomarkers and imaging
findings upon admission (day 1) in adult TBI patients
Higher levels of IL-6 and IL-10 on day 1 were found
to be connected with increased risk according to the imaging
indices of TBI severity, such as basal cistern compression
(P<0.007), midline shift >5 mm (P<0.003) and larger total
lesion volume (P<0.006). The association between inflammatory
markers and the finding of traumatic intraventricular haemorrhage
or traumatic subarachnoid haemorrhage was found to be negligible.
Severe cases, found according to higher scores on the Stockholm and
Rotterdam CT scales, were correlated with elevated IL-6
(rs=0.4 and 0.274, respectively; P<0.009 for all),
IL-10 (rs=0.323 and 0.305, respectively; P<0.003 for
all) and GFAP (rs=0.203 and 0.213, respectively;
P<0.045 for all) on day 1. In addition, severe cases according
to higher scores on the Marshall CT Classification and Helsinki CT
scales were correlated with elevated IL-6 (rs=0.386 and
0.446, respectively; P<0.001 for all) and IL-10
(rs=0.272 and 0.335, respectively; P<0.009 for all)
on day 1. However, the association between UCH-L1 and none of the
severity CT scores used reached significance. The CRASH head injury
prognostic score (14-day mortality risk or 6-month mortality and
severe disability risk) was found to be positively correlated with
IL-6, GFAP and UCH-L1 (rs=0.372, 0.357 and 0.369
respectively; P<0.031 for all) on day 1.
Inflammation biomarker-independent
associations with functional outcomes and 6-month mortality
Adult patients who did not survive during the first
6 months following TBI (n=32) had significantly elevated IL-6,
IL-10 (on day 1) and UCH-L1 levels (on days 1 and 7; P<0.018)
compared with those in survivors. In particular, adult patients who
exhibited an unfavourable outcome on day 7 (n=22 as indicated by a
score <4 according to GOS) displayed significantly elevated IL-6
(P=0.003) and IL-10 (P=0.007) levels on day 1. Similarly, patients
who had an unfavourable outcome at 6 months (n=45 as indicated by a
score <5 according to GOS-E) exhibited significantly elevated
IL-6 (P=0.045), IL-10 (P=0.048) and UCHL-1 (P=0.042) on day 1.
Significantly elevated IL-6 and IL-10 levels on day 1 were also
recorded for patients with more severe functional state according
to KPS (as indicated by a score <50) of day 7 compared with
those who scored >40 (P<0.004 for all).
A multivariate predictive logistic regression
analysis was conducted to distinguish adult patients with TBI at
risk of an unfavourable outcome based on GOS-E. The following
variables were included into the model: IL-6, IL-10 and UCH-L1
levels on days 1 and 7; age; serum glucose levels; GCS; MRS; and
KPS upon admission. The final model was associated with acceptable
fit to the data (χ2=11.28, P=0.004, Nagelkerke
R2=0.458) with the following significant predictors:
IL-6 on day 1 [Exp (B)=0.987; P=0.025] and UCH-L1 of day 1 [Exp
(B)=0.993; P=0.032], along with age (P=0.014) and GCS (P=0.045;
Table III).
 | Table IIIMultivariate logistic regression of
potential predictors of an unfavourable outcome based on GOS-E. |
Table III
Multivariate logistic regression of
potential predictors of an unfavourable outcome based on GOS-E.
| Variable | Odds ratio | 95% CI | P-value |
|---|
| IL-6 Day 1 | 0.987 | 0.975-0.998 | 0.025a |
| IL-10 Day 1 | 1.009 | 0.925-1.100 | 0.843 |
| UCH-L1 Day 1 | 0.993 | 0.987-0.999 | 0.032a |
| Age | 0.907 | 0.840-0.980 | 0.014a |
| Glucose | 1.029 | 0.997-1.062 | 0.077 |
| GCS | 1.768 | 1.013-3.084 | 0.045a |
| MRS | 0.542 | 0.263-1.119 | 0.098 |
| KPS | 0.978 | 0.911-1.044 | 0.468 |
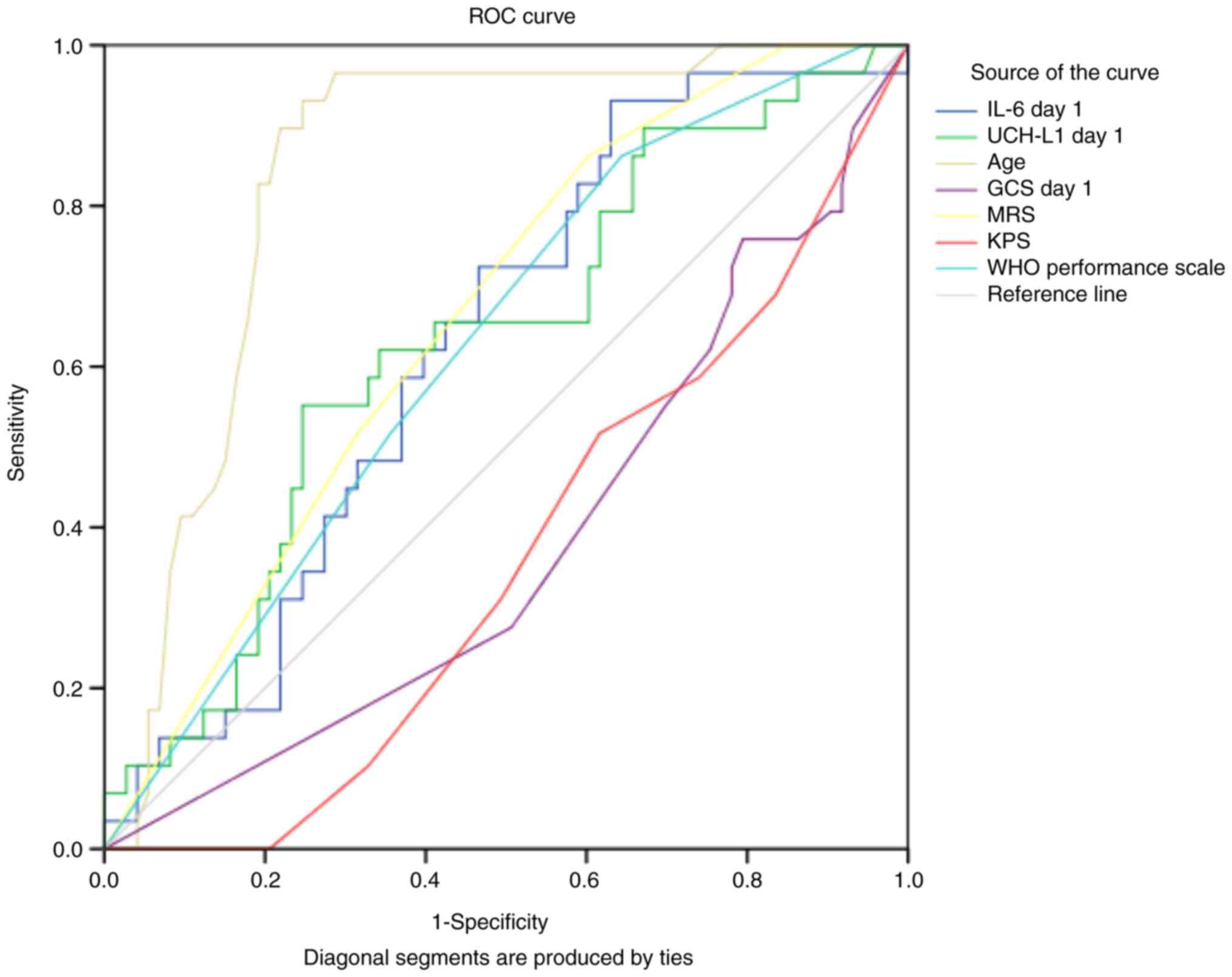
ROC analysis in adults revealed that without
considering other potential predictors, age, IL-6 and UCH-L1 on day
1 were marginally acceptable, independent predictors of 6-month
severe disability based on GOS-E (Fig.
3; Table IV). The optimal
cut-off values according to the Youden index (J) were 55.51 pg/ml
(sensitivity 73% and specificity 55%) for IL-6 and 204 pg/ml for
UCH-L1 (sensitivity 65% and specificity 59%).
 | Table IVData for ROC curve showing the
independent discriminators of an unfavourable outcome for patients
with TBI based on GOS-E. |
Table IV
Data for ROC curve showing the
independent discriminators of an unfavourable outcome for patients
with TBI based on GOS-E.
| | Asymptotic 95%
confidence interval |
|---|
| Prognostic
discriminators | Area | Std. error | Asymptotic
sig. | Lower bound | Upper bound |
|---|
| IL-6 day 1
(pg/ml) | 0.632 | 0.057 | 0.045 | 0.515 | 0.740 |
| UCH-L1 day 1
(pg/ml) | 0.640 | 0.062 | 0.033 | 0.519 | 0.761 |
| Age (years) | 0.758 | 0.048 | 0.001 | 0.716 | 0.904 |
| GCS day 1 | 0.366 | 0.063 | 0.041 | 0.243 | 0.489 |
| MRS | 0.673 | 0.058 | 0.008 | 0.560 | 0.787 |
| KPS | 0.337 | 0.058 | 0.013 | 0.223 | 0.451 |
| ECOG/WHO | 0.644 | 0.058 | 0.028 | 0.526 | 0.762 |
Other associations in adult patients
with TBI
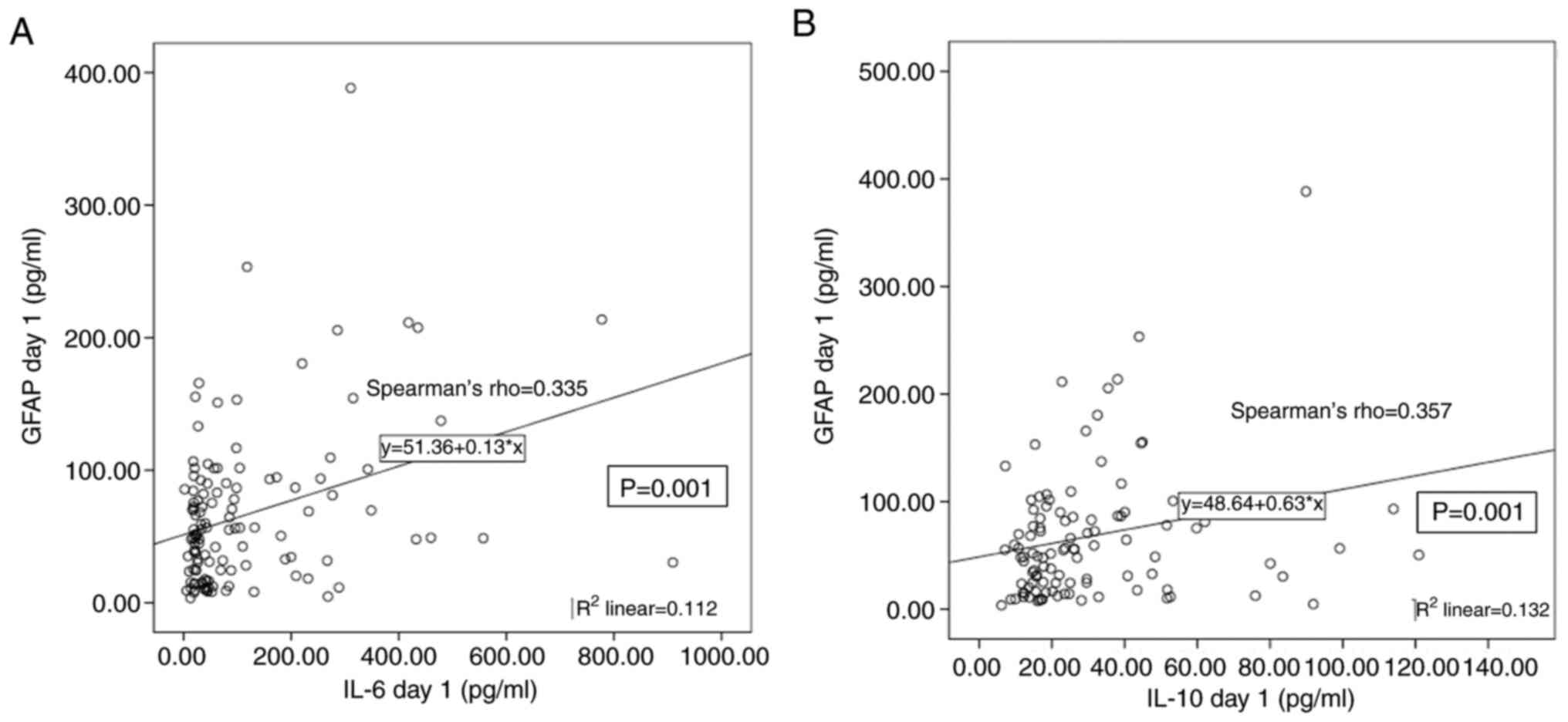
GFAP levels on day 1 were positively correlated with
IL-6 and IL-10 (rs=0.335 and 0.357, respectively;
P=0.001 for both; Figs. 4 and
5), whilst a significant negative
correlation was found between UCH-L1 and IL-8 levels on day 1
(rs=-0.302; P=0.001). Additionally, elevated IL-6 and
IL-10 on days 1 and 7 were correlated with increased risk for ICU
admission (rs=0.329 and 0.510 respectively; P<0.005
for all). Among the common laboratory markers, IL-6 and IL-10
levels on day 1 were positively correlated with white blood cell
and neutrophil counts (rs=0.271 and 0.282 respectively;
P<0.05 for both), glucose (rs=0.265 and 0.313
respectively; P<0.025 for both), troponin (rs=0.285;
P=0.001 only for IL-6) and creatine phosphokinase (rs
<0.218; P<0.041 for both). GFAP levels on day 1 were
positively correlated with glucose (rs=0.199; P=0.044)
and troponin (rs=0.3; P=0.002). No significant
correlations among these common laboratory markers and IL-8 or
UCH-L1 could be found.
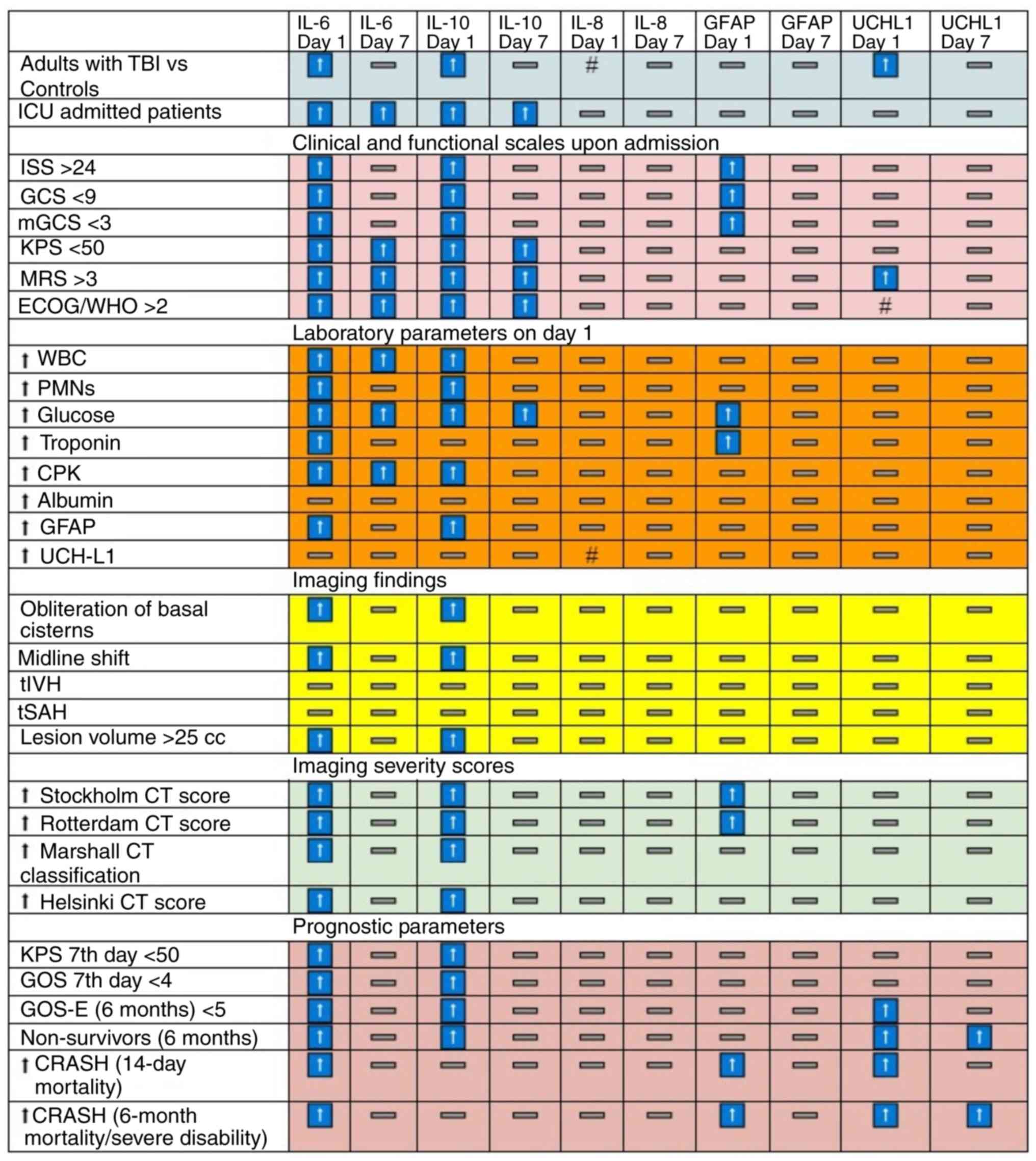 | Figure 4Significant associations of
biomarkers with the clinical, diagnostic and prognostic parameters
for the adult population of the study. Arrows indicate significant
positive (↑) or negative (#) associations of interleukins. CRASH,
corticosteroid randomisation after significant head injury; CPK,
creatine phosphokinase; ECOG/WHO score, Eastern Cooperative
Oncology Group score/WHO score; GCS, Glasgow Coma Scale; GFAP,
glial fibrillary acidic protein; GOS, Glasgow Outcome Score; GOS-E,
Glasgow Outcome Scale-Extended; ICU, Intensive Care Unit; ISS,
Injury Severity Score; KPS, Karnofsky Performance Scale; LOS,
length of stay; mGCS, Motor Component of Glasgow Coma Scale; MRS,
Modified Rankin Scale; PMNs, neutrophils, tIVH, traumatic
intraventricular haemorrhage; tSAH, traumatic subarachnoid
haemorrhage; UCH-L1, ubiquitin C-terminal hydrolase L1; WBC, white
blood cells. |
In terms of paediatric patients with TBI, a
significant positive correlation between GFAP and IL-10 levels
(rs=0.555; P=0.029) on day 1 was found, whilst a
significant negative correlation between UCH-L1 and IL-8
(rs=-0.554; P=0.04) was recorded.
Discussion
In the present study, it was found that the levels
of IL-6, IL-10 and UCH-L1 are significantly elevated on admission
in adult patients with TBI compared with healthy individuals,
whilst the levels of IL-6 and IL-10 tended to decrease over the
first week post-injury. In addition, relatively higher serum levels
of IL-6 and UCH-L1 were found to be predictive of increased
mortality and poorer functional outcome at 6 months post-injury,
even after controlling for other demographic parameters (such as
age), common laboratory parameters (such as glucose) and clinical
indices of injury severity (such as KPS and MRS scores). By
contrast, the vast majority of previous studies focused on
associations of separate IL with a limited set of parameters.
UCH-L1 has been previously proposed to be an
important index of TBI classification and prognostication (23,53),
whilst other studies also support their significant associations
with outcome (54). However, they
did not highlight their potential predictive power in TBI
prognostic models (54). IL-6 and
IL-10 are important inflammatory cytokines with primary protective
roles against pathogenic, traumatic or stressful insults that are
regularly present in relatively low levels, even in healthy
individuals (36,37,39,49).
Results from the present study support the notion that
post-traumatic inflammatory and anti-inflammatory mechanisms start
concurrently during the early stages of TBI to maintain the ideal
inflammatory equilibrium (55).
Previously reported findings are in accordance with
multiple human studies, which showed the statistically significant
upregulation of IL-6 concentrations in patients with TBI compared
with those in controls (56-73).
However, it should be noted that two large-scale studies
(Ntotal=245) failed to detect significant differences
(71). In particular, three
studies have reported a significant negative correlation between
IL-6 and admission GCS (72,74,75),
but other studies failed to find such a relationship (67,76,77).
In addition, four studies have discovered a statistically
significant positive correlation between IL-6 and severe imaging
findings (such as size of lesions and traumatic subarachnoid
haemorrhage) (78-81).
However, other previous studies have reported that IL-6
concentration is significantly associated with the concentration of
various TBI-specific neurological biomarkers (such as nerve growth
factor, S100 calcium-binding protein B and neuron-specific enolase)
(58,80). Furthermore, several studies
consistently highlighted the predictive value of IL-6 for mortality
or functional outcome (47,67,76,77,81-93).
Clinical prognosis in TBI may depend on both local and systemic
components, since nerve tissue damage stimulates neuroinflammation
through both local and systemic inflammatory cells (12). In the present report, a significant
association of IL-6 with several indices of diagnostic or
prognostic classification of ΤΒΙ was documented, highlighting the
potential value of IL-6 as a complementary index of both localised
neural tissue damage and multisystem pathology. This is
complementary to other TBI-specific neurobiomarkers, such as GFAP
and UCH-L1.
The present analysis demonstrated consistent
associations among IL-10 and multiple variables associated with the
diagnostic and prognostic classification of ΤΒΙ. Increased values
of IL-6 and IL-10 have both been connected with an increased risk
for ICU admission, lower scores in GCS, lower scores in mGCS and
lower scores in KPS, higher scores in ECOG/WHO and ISS upon
admission, higher blood levels of multiple common laboratory
markers (such as white blood cells, polymorphonuclear leukocytes,
glucose, creatine phosphokinase), higher blood levels of GFAP and
with positive imaging findings (midline shift >5 mm,
obliteration of basal cisterns, larger volume of lesions, higher
values in Stockholm, Rotterdam, Marshall and Helsinki CT scales).
The role of IL-10 as an index of injury severity and prognosis of
TBI has previously been highlighted in the literature (47,49,57,94-96),
although a negative finding has also been found (97). Therefore, existing evidence appears
to be stronger regarding the association of elevated IL-10 with
mortality or with unfavourable functional outcomes as indexed by
GOS or GOS-E (47,57,89,94,98-101).
The relationship of IL-10 with admission GCS, complications or
other biomarker levels has not been sufficiently examined to
date.
Serum levels of IL-8 were found to be significantly
lower in patients with TBI compared with those in healthy adult
controls with relatively stable levels throughout the week 1
post-injury. Due to the neutrophil chemoattractant properties that
have been attributed to IL-8(102), this finding may not be
surprising. Systemic serum IL-8 levels could be suppressed during
TBI, due to its valuable role locally as a neuroinflammation danger
signal. In addition, it has been reported that IL-8 is involved in
angiogenesis, which is hypothesised to promote neurodegeneration
(42,103). However, early and sustained
downregulation of IL-8 may confer a protective response against
neurodegeneration. This possibility warrants further investigation
(104). Therefore, the present
results do not support the diagnostic (67,74,103,105-108)
or prognostic significance of IL-8 in TBI (67,87,89,93,108-111).
A potential novelty of the present study is that a
pilot group of paediatric patients was also included, given the
scarcity of paediatric TBI data. However, the present study has
certain limitations. A sample of consecutive patients with TBI was
enrolled, whose age varied widely. By contrast, the control group
consisted of significantly younger healthy volunteers. Moreover,
the largest part of the present study was conducted during the
COVID-19 pandemic, which could have influenced the demographic and
TBI mechanism and severity characteristics of the patients. Further
studies will be needed to clarify the preliminary results of the
present study with regards to inflammatory biomarkers. Another
limitation concerns the pilot paediatric population, which
consisted of only a small number of children, offering little
information in drawing remarkable conclusions. This was compounded
by the lack of control paediatric individuals in the present
study.
In conclusion, patients with TBI may have multiple
injuries and/or complications. The prognosis of a patient with TBI
is dependent on the presence of both local and systemic responses,
in addition to that of damage to other tissues. Therefore, an
adequate TBI prognostic model should take into consideration all
aspects of systematic inflammation and local neuroinflammation,
which is generated by both localised neural tissue damage and
systemic immune responses. At present, a significant number of
CNS-specific or non-specific inflammation biomarkers are being
studied for clinical use. However, to the best of our knowledge, no
reliable biomarker or group of biomarkers with adequate sensitivity
or specificity for TBI severity classification or prognostication
have been found to date. In the present study, three systemic
biomarkers associated with inflammatory processes, IL-6, IL-8 and
IL-10, in addition to two specific biomarkers associated with nerve
tissue injury, UCH-L1 and GFAP, were examined. The present study
indicated that IL-6 and IL-10, but not IL-8, may serve to be
independent TBI severity discriminators in neurocritical patients
with TBI, based on a holistic comparison with already authorised
TBI neurological biomarkers (UCH-L1 and GFAP), clinical and imaging
tools. Furthermore, IL-6 and UCH-L1 seemed to act as viable
prognostic TBI biomarkers. Therefore, the incorporation of
inflammation biomarkers IL-6 and IL-10, alongside TBI-specific
neurological biomarkers (such as UCH-L1) into diagnostic and
prognostic models may optimise the guidance of and enhance current
existing clinical decisions and practices, to facilitate outcome
prognostication.
Supplementary Material
GCS.
Summary of studies on IL-6 in animals
with TBI.
Summary of studies on IL-6 in human
TBI populations.
Studies on IL-8 in human populations
suffering from TBI.
Studies on IL-10 in animals with
provoked TBI.
Research on IL-10 studied in humans
suffering from TBI.
mGCS.
Karnofsky Performance Scale.
MRS.
ECOG/WHO score.
ISS.
Compression of basal cisterns on the
head CT.
Midline shift on head CT scan.
Volume of hemorrhagic lesions.
Imaging CT tools.
GOS.
GOS-E.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by ELKE (grant no.
KA:10344; Research Committee, University of Crete, School of
Medicine, Heraklion, Greece).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT, AV, PS and MV conceptualized the study. CT, MM,
EP, SL, NM, KN and AT were in charge of data curation,
investigation and formal analysis. The methodology of the study was
designed by CT, MM, SL, NM, KN, AT, SI, AV, PS and MV. CT, SL, NM,
KN, AT, SI, AV, PS and MV performed data validation. Data
visualization was carried out by CT and MM. CT, SL, NM, KN, AT, SI,
AV, PS and MV confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Institutional (University of Crete) ethics committee
approval was obtained for the present study [approval nos.
198/14.11.2019 (first) and 132/07.09.2022 (revised); Heraklion,
Crete, Greece]. Approval was acquired for both participation and
publication of the study's findings. All data were
de-identified.
Patient consent for publication
Written informed consent was obtained from the
patients or patients’ representatives (surrogate decision makers)
before inclusion into the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mollayeva T, Mollayeva S and Colantonio A:
Traumatic brain injury: Sex, gender and intersecting
vulnerabilities. Nat Rev Neurol. 14:711–722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
NAEMT in Cooporetion with American College
of Surgeons, Committee Trauma. Chapter 8 Head Trauma. In: PHTLS,
Prehospital Trauma Life Support. Ninth. Burlington, MA: Jones &
Bartlett Learning; 2023.
|
|
3
|
Khellaf A, Khan DZ and Helmy A: Recent
advances in traumatic brain injury. J Neurol. 266:2878–2889.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vella MA, Crandall ML and Patel MB: Acute
management of traumatic brain injury. Surg Clin North Am.
97:1015–1030. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Williamson C and Rajajee V: Traumatic
brain injury: Epidemiology, classification, and pathophysiology.
In: UpToDate. UpToDate, Post TW. UpToDate, Waltham, MA; 2023.
|
|
6
|
Menon DK, Schwab K, Wright DW and Maas AI:
Demographics and Clinical Assessment Working Group of the
International and Interagency Initiative toward Common Data
Elements for Research on Traumatic Brain Injury and Psychological
Health. Position statement: Definition of traumatic brain injury.
Arch Phys Med Rehabil. 91:1637–1640. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Capizzi A, Woo J and Verduzco-Gutierrez M:
Traumatic brain injury: An overview of epidemiology,
pathophysiology, and medical management. Med Clin North Am.
104:213–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dixon KJ: Pathophysiology of traumatic
brain injury. Phys Med Rehabil Clin N Am. 28:215–225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Galgano M, Toshkezi G, Qiu X, Russell T,
Chin L and Zhao LR: Traumatic brain injury: Current treatment
strategies and future endeavors. Cell Transplant. 26:1118–1130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein
R, Tyndall JA and Manley GT: An update on diagnostic and prognostic
biomarkers for traumatic brain injury. Expert Rev Mol Diagn.
18:165–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pearn ML, Niesman IR, Egawa J, Sawada A,
Almenar-Queralt A, Shah SB, Duckworth JL and Head BP:
Pathophysiology associated with traumatic brain injury: Current
treatments and potential novel therapeutics. Cell Mol Neurobiol.
37:571–585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sulhan S, Lyon KA, Shapiro LA and Huang
JH: Neuroinflammation and blood-brain barrier disruption following
traumatic brain injury: Pathophysiology and potential therapeutic
targets. J Neurosci Res. 98:19–28. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
MRC CRASH Trial Collaborators. Perel P,
Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, Roberts I,
Shakur H, Steyerberg E and Yutthakasemsunt S: Predicting outcome
after traumatic brain injury: Practical prognostic models based on
large cohort of international patients. BMJ. 336:425–429.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kempuraj D, Selvakumar GP, Ahmed ME,
Raikwar SP, Thangavel R, Khan A, Zaheer SA, Iyer SS, Burton C,
James D and Zaheer A: COVID-19, mast cells, cytokine storm,
psychological stress, and neuroinflammation. Neuroscientist.
26:402–414. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Najem D, Rennie K, Ribecco-Lutkiewicz M,
Ly D, Haukenfrers J, Liu Q, Nzau M, Fraser DD and Bani-Yaghoub M:
Traumatic brain injury: Classification, models, and markers.
Biochem Cell Biol Biochim Biol Cell. 96:391–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McGinn MJ and Povlishock JT:
Pathophysiology of traumatic brain injury. Neurosurg Clin N Am.
27:397–407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mark S: Greenberg. Handbook of
Neurosurgery [Internet]. [cited 2022 May 30]. Available from:
https://medone.thieme.com/ebooks/cs_9872229?context=coverpage&fromSearch=false#/ebook_cs_9872229_d1e266595.
|
|
18
|
Karve IP, Taylor JM and Crack PJ: The
contribution of astrocytes and microglia to traumatic brain injury.
Br J Pharmacol. 173:692–702. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Johnston RB: An overview of the innate
immune system. In: UpToDate Post TW. UpToDate, Waltham, MA;
2023.
|
|
20
|
Jassam YN, Izzy S, Whalen M, McGavern DB
and El Khoury J: Neuroimmunology of Traumatic Brain Injury: Time
for a Paradigm Shift. Neuron. 95:1246–1265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prognostic calculator|TBI-IMPACT.org [Internet]. [cited 2022 Dec 4].
Available from: http://www.tbi-impact.org/?p=impact/calc.
|
|
22
|
Mozaffari K, Dejam D, Duong C, Ding K,
French A, Ng E, Preet K, Franks A, Kwan I, Phillips HW, et al:
Systematic review of serum biomarkers in traumatic brain injury.
Cureus. 13(e17056)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nishimura K, Cordeiro JG, Ahmed AI,
Yokobori S and Gajavelli S: Advances in traumatic brain injury
biomarkers. Cureus. 14(e23804)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang KKW, Kobeissy FH, Shakkour Z and
Tyndall JA: Thorough overview of ubiquitin C-terminal hydrolase-L1
and glial fibrillary acidic protein as tandem biomarkers recently
cleared by US Food and Drug Administration for the evaluation of
intracranial injuries among patients with traumatic brain injury.
Acute Med Surg. 8(e622)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hier DB, Obafemi-Ajayi T, Thimgan MS,
Olbricht GR, Azizi S, Allen B, Hadi BA and Wunsch DC II: Blood
biomarkers for mild traumatic brain injury: A selective review of
unresolved issues. Biomark Res. 9(70)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Middleton J: UCH-L1 and GFAP Testing
(i-STAT TBI Plasma) for the detection of intracranial injury
following mild traumatic brain injury. Am Fam Physician.
105:313–314. 2022.PubMed/NCBI
|
|
27
|
Korfias S, Stranjalis G, Papadimitriou A,
Psachoulia C, Daskalakis G, Antsaklis A and Sakas DE: Serum S-100B
protein as a biochemical marker of brain injury: A review of
current concepts. Curr Med Chem. 13:3719–3731. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amoo M, Henry J, O'Halloran PJ, Brennan P,
Husien MB, Campbell M, Caird J, Javadpour M and Curley GF: S100B,
GFAP, UCH-L1 and NSE as predictors of abnormalities on CT imaging
following mild traumatic brain injury: A systematic review and
meta-analysis of diagnostic test accuracy. Neurosurg Rev.
45:1171–1193. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Korfias S, Stranjalis G, Boviatsis E,
Psachoulia C, Jullien G, Gregson B, Mendelow AD and Sakas DE: Serum
S-100B protein monitoring in patients with severe traumatic brain
injury. Intensive Care Med. 33:255–260. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Janigro D, Mondello S, Posti JP and Unden
J: GFAP and S100B: What you always wanted to know and never dared
to ask. Front Neurol. 13(835597)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Frankel M, Fan L, Yeatts SD, Jeromin A,
Vos PE, Wagner AK, Wolf BJ, Pauls Q, Lunney M, Merck LH, et al:
Association of very early serum levels of S100B, glial fibrillary
acidic protein, Ubiquitin C-Terminal Hydrolase-L1, and spectrin
breakdown product with outcome in ProTECT III. J Neurotrauma.
36:2863–2871. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thelin EP, Zeiler FA, Ercole A, Mondello
S, Büki A, Bellander BM, Helmy A, Menon DK and Nelson DW: Serial
sampling of serum protein biomarkers for monitoring human traumatic
brain injury dynamics: A systematic review. Front Neurol.
8(300)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Korfias S, Papadimitriou A, Stranjalis G,
Bakoula C, Daskalakis G, Antsaklis A and Sakas DE: Serum
biochemical markers of brain injury. Mini Rev Med Chem. 9:227–234.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Minkkinen M, Iverson GL, Kotilainen AK,
Pauniaho SL, Mattila VM, Lehtimäki T, Berghem K, Posti JP and Luoto
TM: Prospective validation of the scandinavian guidelines for
initial management of minimal, mild, and moderate head injuries in
adults. J Neurotrauma. 36:2904–2912. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang S, Narazaki M, Metwally H and
Kishimoto T: Historical overview of the interleukin-6 family
cytokine. J Exp Med. 217(e20190347)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rose-John S: Interleukin-6 signalling in
health and disease. F1000Res 9: F1000 Faculty Rev-1013, 2020.
|
|
37
|
Rose-John S: Interleukin-6 family
cytokines. Cold Spring Harb Perspect Biol.
10(a028415)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sanchis P, Fernández-Gayol O, Vizueta J,
Comes G, Canal C, Escrig A, Molinero A, Giralt M and Hidalgo J:
Microglial cell-derived interleukin-6 influences behavior and
inflammatory response in the brain following traumatic brain
injury. Glia. 68:999–1016. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ooi SZY, Spencer RJ, Hodgson M, Mehta S,
Phillips NL, Preest G, Manivannan S, Wise MP, Galea J and Zaben M:
Interleukin-6 as a prognostic biomarker of clinical outcomes after
traumatic brain injury: A systematic review. Neurosurg Rev.
45:3035–3054. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li Z, Xiao J, Xu X, Li W, Zhong R, Qi L,
Chen J, Cui G, Wang S, Zheng Y, et al: M-CSF, IL-6, and TGF-β
promote generation of a new subset of tissue repair macrophage for
traumatic brain injury recovery. Sci Adv.
7(eabb6260)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matsushima K and Oppenheim JJ: Interleukin
8 and MCAF: Novel inflammatory cytokines inducible by IL 1 and TNF.
Cytokine. 1:2–13. 1989.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dobreva I, Waeber G, James RW and Widmann
C: Interleukin-8 secretion by fibroblasts induced by low density
lipoproteins is p38 MAPK-dependent and leads to cell spreading and
wound closure. J Biol Chem. 281:199–205. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lieberman MM, Sachanandani DM and Pinney
CA: Comparative study of neutrophil activation by chemiluminescence
and flow cytometry. Clin Diagn Lab Immunol. 3:654–662.
1996.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bickel M: The role of interleukin-8 in
inflammation and mechanisms of regulation. J Periodontol. 64 (5
Suppl):S456–S460. 1993.PubMed/NCBI
|
|
45
|
Hack CE, Hart M, van Schijndel RJ,
Eerenberg AJ, Nuijens JH, Thijs LG and Aarden LA: Interleukin-8 in
sepsis: Relation to shock and inflammatory mediators. Infect Immun.
60:2835–2842. 1992.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Woodcock T and Morganti-Kossmann MC: The
role of markers of inflammation in traumatic brain injury. Front
Neurol. 4(18)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lewis CT, Savarraj JPJ, McGuire MF,
Hergenroeder GW, Alex Choi H and Kitagawa RS: Elevated inflammation
and decreased platelet activity is associated with poor outcomes
after traumatic brain injury. J Clin Neurosci. 70:37–41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Maiti P, Peruzzaro S, Kolli N, Andrews M,
Al-Gharaibeh A, Rossignol J and Dunbar GL: Transplantation of
mesenchymal stem cells overexpressing interleukin-10 induces
autophagy response and promotes neuroprotection in a rat model of
TBI. J Cell Mol Med. 23:5211–5224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Csuka E, Morganti-Kossmann MC, Lenzlinger
PM, Joller H, Trentz O and Kossmann T: IL-10 levels in
cerebrospinal fluid and serum of patients with severe traumatic
brain injury: Relationship to IL-6, TNF-alpha, TGF-beta1 and
blood-brain barrier function. J Neuroimmunol. 101:211–221.
1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wen L, Wang YD, Shen DF, Zheng PD, Tu MD,
You WD, Zhu YR, Wang H, Feng JF and Yang XF: Exosomes derived from
bone marrow mesenchymal stem cells inhibit neuroinflammation after
traumatic brain injury. Neural Regen Res. 17:2717–2724.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Garcia JM, Stillings SA, Leclerc JL,
Phillips H, Edwards NJ, Robicsek SA, Hoh BL, Blackburn S and Doré
S: Role of Interleukin-10 in Acute Brain Injuries. Front Neurol.
8(244)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Unal I: Defining an optimal cut-point
value in ROC analysis: An alternative approach. Comput Math Methods
Med. 2017(3762651)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Korley FK, Jain S, Sun X, Puccio AM, Yue
JK, Gardner RC, Wang KKW, Okonkwo DO, Yuh EL, Mukherjee P, et al:
Prognostic value of day-of-injury plasma GFAP and UCH-L1
concentrations for predicting functional recovery after traumatic
brain injury in patients from the US TRACK-TBI cohort: An
observational cohort study. Lancet Neurol. 21:803–813.
2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Takala RSK, Posti JP, Runtti H, Newcombe
VF, Outtrim J, Katila AJ, Frantzén J, Ala-Seppälä H, Kyllönen A,
Maanpää HR, et al: Glial fibrillary acidic protein and ubiquitin
C-Terminal Hydrolase-L1 as outcome predictors in traumatic brain
injury. World Neurosurg. 87:8–20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
McKee CA and Lukens JR: Emerging roles for
the immune system in traumatic brain injury. Front Immunol.
7(556)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rodney T, Taylor P, Dunbar K, Perrin N,
Lai C, Roy M and Gill J: High IL-6 in military personnel relates to
multiple traumatic brain injuries and post-traumatic stress
disorder. Behav Brain Res. 392(112715)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bell MJ, Kochanek PM, Doughty LA, Carcillo
JA, Adelson PD, Clark RS, Whalen MJ and DeKosky ST: Comparison of
the interleukin-6 and interleukin-10 response in children after
severe traumatic brain injury or septic shock. Acta Neurochir
Suppl. 70:96–97. 1997.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kossmann T, Hans V, Imhof HG, Trentz O and
Morganti-Kossmann MC: Interleukin-6 released in human cerebrospinal
fluid following traumatic brain injury may trigger nerve growth
factor production in astrocytes. Brain Res. 713:143–152.
1996.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ondruschka B, Schuch S, Pohlers D, Franke
H and Dreßler J: Acute phase response after fatal traumatic brain
injury. Int J Legal Med. 132:531–539. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Thompson HJ, Martha SR, Wang J and Becker
KJ: Impact of age on plasma inflammatory biomarkers in the 6 months
following mild traumatic brain injury. J Head Trauma Rehabil.
35:324–331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bell MJ, Kochanek PM, Doughty LA, Carcillo
JA, Adelson PD, Clark RS, Whalen MJ and DeKosky ST: Interleukin-6
and interleukin-10 in cerebrospinal fluid after severe traumatic
brain injury in children. J Neurotrauma. 14:451–457.
1997.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liao Y, Liu P, Guo F, Zhang ZY and Zhang
Z: Oxidative burst of circulating neutrophils following traumatic
brain injury in human. PLoS One. 8(e68963)2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hergenroeder GW, Moore AN, McCoy JP,
Samsel L, Ward NH III, Clifton GL and Dash PK: Serum IL-6: A
candidate biomarker for intracranial pressure elevation following
isolated traumatic brain injury. J Neuroinflammation.
7(19)2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Terrell TR, Abramson R, Barth JT, Bennett
E, Cantu RC, Sloane R, Laskowitz DT, Erlanger DM, McKeag D, Nichols
G, et al: Genetic polymorphisms associated with the risk of
concussion in 1056 college athletes: A multicentre prospective
cohort study. Br J Sports Med. 52:192–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pierce ME, Hayes J, Huber BR, Jeromin A,
Fortier CB, Fonda JR, Lasseter H, Chaby L, McGlinchey R and Milberg
W: Plasma biomarkers associated with deployment trauma and its
consequences in post-9/11 era veterans: Initial findings from the
TRACTS longitudinal cohort. Transl Psychiatry.
12(80)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gill J, Motamedi V, Osier N, Dell K,
Arcurio L, Carr W, Walker P, Ahlers S, Lopresti M and Yarnell A:
Moderate blast exposure results in increased IL-6 and TNFα in
peripheral blood. Brain Behav Immun. 65:90–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hayakata T, Shiozaki T, Tasaki O, Ikegawa
H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka
H, et al: Changes in CSF S100B and cytokine concentrations in
early-phase severe traumatic brain injury. Shock Augusta Ga.
22:102–107. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Meier TB, Huber DL, Bohorquez-Montoya L,
Nitta ME, Savitz J, Teague TK, Bazarian JJ, Hayes RL, Nelson LD and
McCrea MA: A prospective study of acute blood-based biomarkers for
sport-related concussion. Ann Neurol. 87:907–920. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lustenberger T, Kern M, Relja B, Wutzler
S, Störmann P and Marzi I: The effect of brain injury on the
inflammatory response following severe trauma. Immunobiology.
221:427–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Meier TB, Guedes VA, Smith EG, Sass D,
Mithani S, Vorn R, Savitz J, Teague TK, McCrea MA and Gill JM:
Extracellular vesicle-associated cytokines in sport-related
concussion. Brain Behav Immun. 100:83–87. 2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Edwards KA, Gill JM, Pattinson CL, Lai C,
Brière M, Rogers NJ, Milhorn D, Elliot J and Carr W: Interleukin-6
is associated with acute concussion in military combat personnel.
BMC Neurol. 20(209)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yang DB, Yu WH, Dong XQ, Zhang ZY, Du Q,
Zhu Q, Che ZH, Wang H, Shen YF and Jiang L: Serum macrophage
migration inhibitory factor concentrations correlate with prognosis
of traumatic brain injury. Clin Chim Acta Int J Clin Chem.
469:99–104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
McKeating EG, Andrews PJ, Signorini DF and
Mascia L: Transcranial cytokine gradients in patients requiring
intensive care after acute brain injury. Br J Anaesth. 78:520–523.
1997.PubMed/NCBI View Article : Google Scholar
|
|
74
|
He LM, Qiu BH, Qi ST, Fang LX and Liu XJ:
Dynamic changes of serum interleukin-6 and interleukin-8 in
patients with acute traumatic brain injury and the clinical
significance. Nan Fang Yi Ke Da Xue Xue Bao. 29:999–1001.
2009.PubMed/NCBI(In Chinese).
|
|
75
|
Chiaretti A, Genovese O, Aloe L, Antonelli
A, Piastra M, Polidori G and Di Rocco C: Interleukin 1beta and
interleukin 6 relationship with paediatric head trauma severity and
outcome. Childs Nerv Syst. 21:185–194. 2005.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Park SH and Hwang SK: Prognostic value of
serum levels of S100 calcium-binding protein B, neuron-specific
enolase, and interleukin-6 in pediatric patients with traumatic
brain injury. World Neurosurg. 118:e534–e542. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Chiaretti A, Antonelli A, Riccardi R,
Genovese O, Pezzotti P, Di Rocco C, Tortorolo L and Piedimonte G:
Nerve growth factor expression correlates with severity and outcome
of traumatic brain injury in children. Eur J Paediatr Neurol.
12:195–204. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Vajtr D, Benada O, Kukacka J, Průša R,
Houstava L, Ťoupalík P and Kizek R: Correlation of ultrastructural
changes of endothelial cells and astrocytes occurring during blood
brain barrier damage after traumatic brain injury with biochemical
markers of BBB leakage and inflammatory response. Physiol Res.
58:263–268. 2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mellergård P, Åneman O, Sjögren F, Säberg
C and Hillman J: Differences in cerebral extracellular response of
interleukin-1β, interleukin-6, and interleukin-10 after
subarachnoid hemorrhage or severe head trauma in humans.
Neurosurgery. 68:12–19. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Pleines UE, Morganti-Kossmann MC, Rancan
M, Joller H, Trentz O and Kossmann T: S-100 beta reflects the
extent of injury and outcome, whereas neuronal specific enolase is
a better indicator of neuroinflammation in patients with severe
traumatic brain injury. J Neurotrauma. 18:491–498. 2001.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Singhal A, Baker AJ, Hare GMT, Reinders
FX, Schlichter LC and Moulton RJ: Association between cerebrospinal
fluid interleukin-6 concentrations and outcome after severe human
traumatic brain injury. J Neurotrauma. 19:929–937. 2002.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Aisiku IP, Yamal JM, Doshi P, Benoit JS,
Gopinath S, Goodman JC and Robertson CS: Plasma cytokines IL-6,
IL-8, and IL-10 are associated with the development of acute
respiratory distress syndrome in patients with severe traumatic
brain injury. Crit Care Lond Engl. 20(288)2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Davidson J, Cusimano MD and Bendena WG:
Post-Traumatic brain injury: Genetic susceptibility to outcome.
Neuroscientist. 21:424–441. 2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Woiciechowsky C, Schöning B, Cobanov J,
Lanksch WR, Volk HD and Döcke WD: Early IL-6 plasma concentrations
correlate with severity of brain injury and pneumonia in
brain-injured patients. J Trauma. 52:339–345. 2002.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Peltz CB, Kenney K, Gill J, Diaz-Arrastia
R, Gardner RC and Yaffe K: Blood biomarkers of traumatic brain
injury and cognitive impairment in older veterans. Neurology.
95:e1126–e1133. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Winter CD, Pringle AK, Clough GF and
Church MK: Raised parenchymal interleukin-6 levels correlate with
improved outcome after traumatic brain injury. Brain J Neurol.
127:315–320. 2004.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Nwachuku EL, Puccio AM, Adeboye A, Chang
YF, Kim J and Okonkwo DO: Time course of cerebrospinal fluid
inflammatory biomarkers and relationship to 6-month neurologic
outcome in adult severe traumatic brain injury. Clin Neurol
Neurosurg. 149:1–5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Feng MJ, Ning WB, Wang W, Lv ZH, Liu XB,
Zhu Y, Gao W, Jin HZ and Gao SS: Serum S100A12 as a prognostic
biomarker of severe traumatic brain injury. Clin Chim Acta Int J
Clin Chem. 480:84–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ferreira LCB, Regner A, Miotto KDL, de
Moura S, Ikuta N, Vargas AE, Chies JA and Simon D: Increased levels
of interleukin-6, -8 and -10 are associated with fatal outcome
following severe traumatic brain injury. Brain Inj. 28:1311–1316.
2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kumar RG, Diamond ML, Boles JA, Berger RP,
Tisherman SA, Kochanek PM and Wagner AK: Acute CSF interleukin-6
trajectories after TBI: Associations with neuroinflammation,
polytrauma, and outcome. Brain Behav Immun. 45:253–262.
2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Raheja A, Sinha S, Samson N, Bhoi S,
Subramanian A, Sharma P and Sharma BS: Serum biomarkers as
predictors of long-term outcome in severe traumatic brain injury:
Analysis from a randomized placebo-controlled phase II clinical
trial. J Neurosurg. 125:631–641. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zwirner J, Bohnert S, Franke H, Garland J,
Hammer N, Möbius D, Tse R and Ondruschka B: Assessing protein
biomarkers to detect lethal acute traumatic brain injuries in
cerebrospinal fluid. Biomolecules. 11(1577)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Crichton A, Ignjatovic V, Babl FE, Oakley
E, Greenham M, Hearps S, Delzoppo C, Beauchamp MH, Guerguerian AM,
Boutis K, et al: Interleukin-8 Predicts Fatigue at 12 months
post-injury in children with traumatic brain injury. J Neurotrauma.
38:1151–1163. 2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lagerstedt L, Azurmendi L, Tenovuo O,
Katila AJ, Takala RSK, Blennow K, Newcombe VFJ, Maanpää HR, Tallus
J, Hossain I, et al: Interleukin 10 and heart fatty acid-binding
protein as early outcome predictors in patients with traumatic
brain injury. Front Neurol. 11(376)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lagerstedt L, Egea-Guerrero JJ, Bustamante
A, Rodríguez-Rodríguez A, El Rahal A, Quintana-Diaz M,
García-Armengol R, Prica CM, Andereggen E, Rinaldi L, et al:
Combining H-FABP and GFAP increases the capacity to differentiate
between CT-positive and CT-negative patients with mild traumatic
brain injury. PLoS One. 13(e0200394)2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Posti JP, Takala RSK, Lagerstedt L,
Dickens AM, Hossain I, Mohammadian M, Ala-Seppälä H, Frantzén J,
van Gils M, Hutchinson PJ, et al: Correlation of blood biomarkers
and biomarker panels with traumatic findings on computed tomography
after traumatic brain injury. J Neurotrauma. 36:2178–2189.
2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Koivikko P, Posti JP, Mohammadian M,
Lagerstedt L, Azurmendi L, Hossain I, Katila AJ, Menon D, Newcombe
VFJ, Hutchinson PJ, et al: Potential of heart fatty-acid binding
protein, neurofilament light, interleukin-10 and S100
calcium-binding protein B in the acute diagnostics and severity
assessment of traumatic brain injury. Emerg Med J EMJ. 39:206–212.
2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Posti JP, Takala RSK, Raj R, Luoto TM,
Azurmendi L, Lagerstedt L, Mohammadian M, Hossain I, Gill J,
Frantzén J, et al: Admission levels of interleukin 10 and amyloid β
1-40 improve the outcome prediction performance of the Helsinki
computed tomography score in traumatic brain injury. Front Neurol.
11(549527)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Vedantam A, Brennan J, Levin HS, McCarthy
JJ, Dash PK, Redell JB, Yamal JM and Robertson CS: Early versus
late profiles of inflammatory cytokines after mild traumatic brain
injury and their association with neuropsychological outcomes. J
Neurotrauma. 38:53–62. 2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Schneider Soares FM, Menezes de Souza N,
Libório Schwarzbold M, Paim Diaz A, Costa Nunes J, Hohl A, Nunes
Abreu da Silva P, Vieira J, Lisboa de Souza R, Moré Bertotti M, et
al: Interleukin-10 is an independent biomarker of severe traumatic
brain injury prognosis. Neuroimmunomodulation. 19:377–385.
2012.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kirchhoff C, Buhmann S, Bogner V,
Stegmaier J, Leidel BA, Braunstein V, Mutschler W and Biberthaler
P: Cerebrospinal IL-10 concentration is elevated in non-survivors
as compared to survivors after severe traumatic brain injury. Eur J
Med Res. 13:464–468. 2008.PubMed/NCBI
|
|
102
|
Matsushima K, Yang D and Oppenheim JJ:
Interleukin-8: An evolving chemokine. Cytokine.
153(155828)2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Whalen MJ, Carlos TM, Kochanek PM,
Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Marion DW and Adelson
PD: Interleukin-8 is increased in cerebrospinal fluid of children
with severe head injury. Crit Care Med. 28:929–934. 2000.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hesse R, Wahler A, Gummert P, Kirschmer S,
Otto M, Tumani H, Lewerenz J, Schnack C and von Arnim CA: Decreased
IL-8 levels in CSF and serum of AD patients and negative
correlation of MMSE and IL-1β. BMC Neurol. 16(185)2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Rowland B, Savarraj JPJ, Karri J, Zhang X,
Cardenas J, Choi HA, Holcomb JB and Wade CE: Acute inflammation in
traumatic brain injury and polytrauma patients using network
analysis. Shock. 53:24–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Polat Ö, Uçkun ÖM, Tuncer C and Belen AD:
Is IL-8 level an indicator of clinical and radiological status of
traumatic brain injury? Ulus Travma Acil Cerrahi Derg. 25:193–197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Chaban V, Clarke GJB, Skandsen T, Islam R,
Einarsen CE, Vik A, Damås JK, Mollnes TE, Håberg AK and Pischke SE:
Systemic inflammation persists the first year after mild traumatic
brain injury: Results from the prospective Trondheim mild traumatic
brain injury study. J Neurotrauma. 37:2120–2130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rhodes J, Sharkey J and Andrews P: Serum
IL-8 and MCP-1 concentration do not identify patients with
enlarging contusions after traumatic brain injury. J Trauma.
66:1591–1598. 2009.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Mussack T, Biberthaler P, Kanz KG,
Wiedemann E, Gippner-Steppert C, Mutschler W and Jochum M: Serum
S-100B and interleukin-8 as predictive markers for comparative
neurologic outcome analysis of patients after cardiac arrest and
severe traumatic brain injury. Crit Care Med. 30:2669–2674.
2002.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Gopcevic A, Mazul-Sunko B, Marout J,
Sekulic A, Antoljak N, Siranovic M, Ivanec Z, Margaritoni M,
Bekavac-Beslin M and Zarkovic N: Plasma interleukin-8 as a
potential predictor of mortality in adult patients with severe
traumatic brain injury. Tohoku J Exp Med. 211:387–393.
2007.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Yang B, Sun X, Shi Q, Dan W, Zhan Y, Zheng
D, Xia Y, Xie Y and Jiang L: Prediction of early prognosis after
traumatic brain injury by multifactor model. CNS Neurosci Ther.
28:2044–2052. 2022.PubMed/NCBI View Article : Google Scholar
|