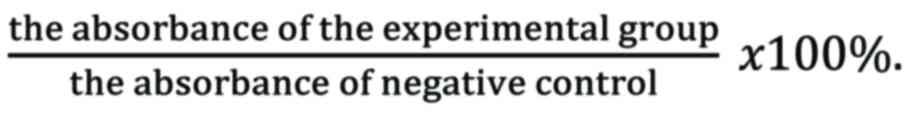Introduction
Adenomyosis is a benign gynecological disorder
defined by the ectopic presence of endometrial tissue in the
myometrium. It is clinically characterized by diffuse enlargement
of the uterus, secondary dysmenorrhea and irregular vaginal
bleeding (1). The prevalence of
adenomyosis worldwide ranges widely from 8.8-61.5%, and the gold
standard for diagnosis is the histopathologic examination of the
uterus after hysterectomy (2). In
recent years, patients with adenomyosis tend to be younger and the
incidence of the disease has increased annually (3). Thus, it is of great importance to
unveil the etiology and pathogenesis of adenomyosis. It is commonly
accepted that adenomyosis results from the increased invasive
properties of endometrial cells, including increased proliferation
and migration and decreased apoptosis. These changes facilitate the
migration of endometrial cells across the boundary between the
endometrium and the myometrium, along with an excessive
proliferation of ectopic endometrial cells in the myometrium
(4,5). Therefore, targeting the
proliferation, migration and apoptosis of endometrial cells might
be a promising therapeutic strategy in adenomyosis treatment
(6,7).
The proinflammatory cytokine interleukin (IL)-6 is
elevated in eutopic and ectopic endometrium in women with
adenomyosis compared with control endometrium, probably playing an
important role in the formation of ectopic endometrial implants in
adenomyosis (8-10).
IL-6 acts as a growth regulator of human endometrial stromal cells
through its receptor IL-6R (11).
Once bound to IL-6R, IL-6 can activate Janus kinase 2 (JAK2) and
trigger the phosphorylation and nuclear localization of signal
transducer and STAT3 (12-14).
The IL-6/JAK2/STAT3 pathway plays a key role in the growth and
development of human types of cancer, including endometrial
carcinoma (15,16). The IL-6/JAK2/STAT3 signaling
pathway is hyperactivated in human endometrial cells cocultured
with macrophages and promotes epithelial-mesenchymal transition in
adenomyosis (10). Thus,
IL-6/JAK2/STAT3 may promote the invasive behavior of endometrial
cells in adenomyosis, but the mechanism underlying the activation
of IL-6/JAK2/STAT3 in adenomyosis remains to be elucidated.
Exosomes are a subtype of extracellular vesicles
characterized by a diameter of 30-150 nm and the presence of marker
proteins such as CD63 and CD9. Exosomes can transfer molecular
cargoes, such as DNA, RNA, proteins and lipids, from the parental
cells to the recipient cells, playing an important role in
intercellular communication (17-19).
Endometrial cell-derived exosomes contain important cargoes that
are involved in the pathogenesis of endometriosis (20). Proinflammatory cytokines are
increased in endothelial cells treated with exosomes from
patient-derived endometriotic epithelial cells (21). Hence, these studies suggest that
exosomes from endometrial cells play a role in cell behavior and
immune modulation of the recipient cells. Thus, it was hypothesized
that endometrial cell exosomes might mediate the communication
between the endometrium and the myometrium through IL-6 signaling,
contributing to the development of adenomyosis.
The present study isolated primary adenomyotic
myometrial (AM) cells and eutopic endometrial cells from patients
with adenomyosis and examined the effects and the underlying
mechanism of endometrial cell-derived exosomes on AM cell
proliferation, apoptosis, cell cycle distribution and migration.
The findings suggested that endometrial cell exosomes promote the
invasive properties of AM cells by activating IL-6/JAK2/STAT3
signaling, providing new information about the etiology of
adenomyosis.
Materials and methods
Sample selection
A total of 10 women (mean age, 46 years old) with
adenomyosis were recruited from the First Affiliated Hospital of
Guangdong Pharmaceutical University (Guangdong, China) between
March 2020 and May 2021. None of the patients had received hormones
or similar drug therapy within 6 months prior to the study. Biopsy
specimens of endometrial and adenomyotic tissue were collected
during surgery. Adenomyosis was confirmed by pathological
examination. Endometrial tissue samples were collected from the
endometrium without visible infection. The study was approved by
the Research Ethics Committee of The First Affiliated Hospital of
Guangdong Pharmaceutical University [approval no. 2021-(31)]. All participants provided informed
consent before the study.
Isolation and characterization of
primary AM cells and eutopic endometrial cells
The adenomyotic and endometrial tissue specimens
were digested with collagenase type 1 (MilliporeSigma) to obtain
primary AM cells and endometrial cells. Cells were cultured in
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 1% penicillin/streptomycin and 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). The cells were passaged
every 5-7 days and only the cells in passages 3-6 were used for the
present study. In order to characterize the primary AM and
endometrial cells, immunocytochemistry was performed to detect the
expression of surface markers in the cells of passage 1. Briefly,
cells were blocked with normal goat serum (OriGene Technologies,
Inc.) at room temperature for 60 min and incubated with the primary
antibody against keratin (cat. no. ZM-0060; OriGene Technologies,
Inc.), vimentin (cat. no. ZM-0260; OriGene Technologies, Inc.), or
actin (cat. no. ZM-0003; OriGene Technologies, Inc.; all diluted
1:200) overnight at 4˚C. The cells were incubated with a secondary
antibody (cat. no. TA130040; OriGene Technologies, Inc.) for 30 min
at 37˚C and visualized with an Olympus PM-20 optical microscope
(Olympus Corporation).
Isolation and characterization of
exosomes from endometrial cells
The endometrial cells were cultured in serum-free
high-glucose DMEM for 24 h. The supernatant was collected for
exosome isolation using a MagCapture exosome extraction kit
(FUJIFILM Wako Pure Chemical Corporation) following the
manufacturer's instructions. Briefly, the extraction reagent was
mixed with the cell supernatant at 1:5. After centrifugation
(10,000 x g, 30 min, 4˚C) overnight at 4˚C the supernatant was
discarded. The exosome pellet was resuspended in phosphate-buffered
saline solution and identified by transmission electron microscopy
(TEM), nanoparticle tracking analysis and western blot
analysis.
TEM examination
TEM was used to examine the morphology of the
exosomes. The samples were prepared as previously described
(22). The sample was fixed with
2.5% glutaraldehyde at 4˚C for 20 min and washed with PBS 3 times
for 2 min each time. Then, 10 µl exosome resuspension was added to
the copper mesh and adsorbed at room temperature for 10 min. And 10
µl of 2% phosphotungstic acid (pH 6.8) was added to the copper mesh
to stain the sample at room temperature for 5 min. The exosomes
were observed under a JEM-1200EX microscope (JEOL, Japan) at a
magnification of x6,000.
Cell proliferation assay
AM cells were seeded in a 96-well plate at a density
of 1x105 cells per well and cultured overnight. After
starvation for 24 h, the cells were incubated with 1x104
endometrial cell-derived exosomes for 24 h. Untreated cells were
used as a negative control. Then, the cells were incubated with MTT
solution (Hangzhou Haotian Biotechnology, Co., Ltd.) for 4 h at
37˚C. The absorbance was measured at 490 nm using a microplate
reader. Cell viability was calculated as
Cell migration assay
Cell migration was investigated using a
wound-healing assay. AM cells were seeded in a 12-well plate and
grown overnight. A scratch was made using a sterile pipette tip.
The cells were incubated with exosomes in the presence or absence
of 20 µM tocilizumab (Roche Diagnostics) in a serum-free medium.
Untreated cells were used as a negative control. Images were
acquired at 0 and 24 h after incubation using a PM-20 optical
microscope (Olympus Corporation). The wound area was measured using
ImageJ software (version 1.53c; National Institutes of Health). The
percentage of wound closure was calculated as
Flow cytometry assay
Cell apoptosis and cell cycle were examined using
flow cytometry. For the apoptosis assay, AM cells were seeded in a
96-well plate at a density of 1x105 cells per well and
cultured overnight. After starvation for 24 h, the cells were
treated with 1x104 endometrial cell-derived exosomes.
Untreated cells were used as a negative control. The cells were
added with 5 µl Annexin V-FITC and stained with 5 µl propidium
iodide (PI), avoiding light at 4˚C for 15 min by using a FITC
Annexin V apoptosis detection kit (BD Biosciences) following the
manufacturer's instructions.
For the cell cycle analysis, AM cells were seeded in
a 24-well plate at a density of 5x104 cells per well and
cultured for 24 h. The cells were treated with exosomes in the
presence or absence of tocilizumab for 48 h. Untreated cells were
used as a negative control. The cells were harvested and fixed in
pure ethanol at 4˚C overnight. The cells were resuspended in DNA
staining solution and incubated in the dark for 30 min at room
temperature. Flow cytometry analysis was performed using an ALTRA
flow cytometer (Beckman Coulter). Data were analyzed using the
EXPO32 software (version 1.1.2; Applied Cytometry). The percentage
of apoptotic rate was calculated as the percentage of early
apoptotic cells + the percentage of late apoptotic cells.
Western blot analysis
AM cells were cultured in a six-well plate and
treated with exosomes in the presence or absence of 10 ng/ml
tocilizumab for 48 h at 37˚C in a serum-free medium. Untreated
cells were used as a negative control. Cells were lysed with 200 µl
RIPA lysis buffer (Thermo Fisher Scientific, Inc.) and protease
inhibitors were added to each plate and placed on ice for 5 min,
and 5x loading buffer was added and mixed well, then the samples
were denatured in a boiling water bath for 10 min. Total protein
was detected by using the BCA protein quantification kit (cat. no.
23250; Thermo Fisher Scientific, Inc. USA) and subjected to 8-12%
SDS-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes. The membranes were washed, blocked at
room temperature for 1 h with a 5% skim milk powder solution and
incubated with antibodies to detect the expression of IL-6 (cat.
no. ab214429; 1:1,000; Abcam), JAK2 (cat. no. ab32101; 1:1,000;
Abcam), phosphorylated (p-)JAK2 (cat. no. ab195055; 1:2,000;
Abcam), STAT3 (cat. no. ab68153; 1:2,000; Abcam), p-STAT3 (cat. no.
ab76315; 1:1,000; Abcam), CD63 (cat. no. ab134045; 1:1,000; Abcam),
CD9 (cat. no. ab236630; 1:1,000; Abcam) and GAPDH (cat. no. ab9485;
1:2,500; Abcam), followed by incubation with HRP-linked secondary
antibody (cat. no. HS201-01; Boster Biological Technology; 1:5,000)
for 1 h at room temperature. Then the protein bands were visualized
using an ECL kit (cat. no. 34075; Thermo Fisher Scientific, Inc.).
And the grey values of the protein bands were quantified with
Quantity One 4.1 software (Bio-Rad Laboratories, Inc.) and the
results were the average of three independent experiments.
Reverse transcription-quantitative
(RT-q) PCR
The cells (1x106) in each group were
treated with TRIzol® (Thermo Fisher Scientific, Inc.)
and chloroform was added at a ratio of 200 µl of chloroform to 1 ml
of TRIzol. The aqueous phase of the upper layer was extracted after
centrifugation (12,000 x g, 5 min, 4˚C). Isopropanol was added in a
ratio of 0.5 ml of isopropanol to 1 ml of TRIzol and then mixed and
the supernatant was discarded after centrifugation (12,000 x g, 10
min, 4˚C). Ethanol was added in a ratio of 1 ml of 75% ethanol to 1
ml of TRIzol and the supernatant was discarded after centrifugation
(7,500 x g, 5 min, 4˚C). The bottom layer was allowed to air-dry at
room temperature for 5-10 min and mixed with 20 µl of DEPCI. After
10 min, 20 µl DEPC water was added to dissolve the precipitate.
Total RNA was extracted according to manufacturer's instructions
and the absorbance was measured at 260 and 280 nm for quality
control and quantification. The RNA was reverse transcribed into
cDNA according to the manufacturer's instruction of the TaKaRa
Reverse Transcription Kit (Takara Bio, Inc.) and then diluted
10-fold, using a 10 µl system: 2 µl template cDNA, 0.4 µl each of
the upper and lower primers and 5 µl amplification probe II and
then make up the total amount to 10 µl with sterilized
double-distilled water and then placed into a fluorescent
quantitative PCR instrument for the reaction using a Super SYBR
Green kit (Transgene SA). Gene amplification was carried out
according to the manufacturer's instructions: 95˚C for 10 min; and
40 cycles of 95˚C for 10 s and 60˚C for 34 sec. All reactions were
run in triplicate and the gene expression was quantified using the
-2ΔΔCq method (23),
with GAPDH as the internal reference. The primer sequences were
GAPDH (internal reference) F 5'-GAAGGTGAAGGTCGGGAGTC-3' and R
5'-GAAGATGGTGATGGGATTTC-3'; IL-6 F 5'-ACTCACCTCTTCAGAACGAATTG-3'
and R 5'-CCATCTTTGGAAGGTTCAGGTTG-3'; JAK2 F
5'-GTTTGGAGCTTTGGAGTGGTT-3' and R 5'-AATCATACGCATAAATTCCGC-3';
STAT3 F 5'-CACCACCAAGCGAGGACT-3' and R
5'-CAGCCAGACCCAGAAGGA-3'.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed using SPSS 19.0 (IBM Corp.). Statistical analysis was
conducted using one-way ANOVA. The differences between groups were
analyzed using the Bonferroni method when homogeneity of variance
was observed and Tamhane's T2 test when heterogeneity of variance
was observed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of primary cells and
exosomes
AM and endometrial cells from patients were
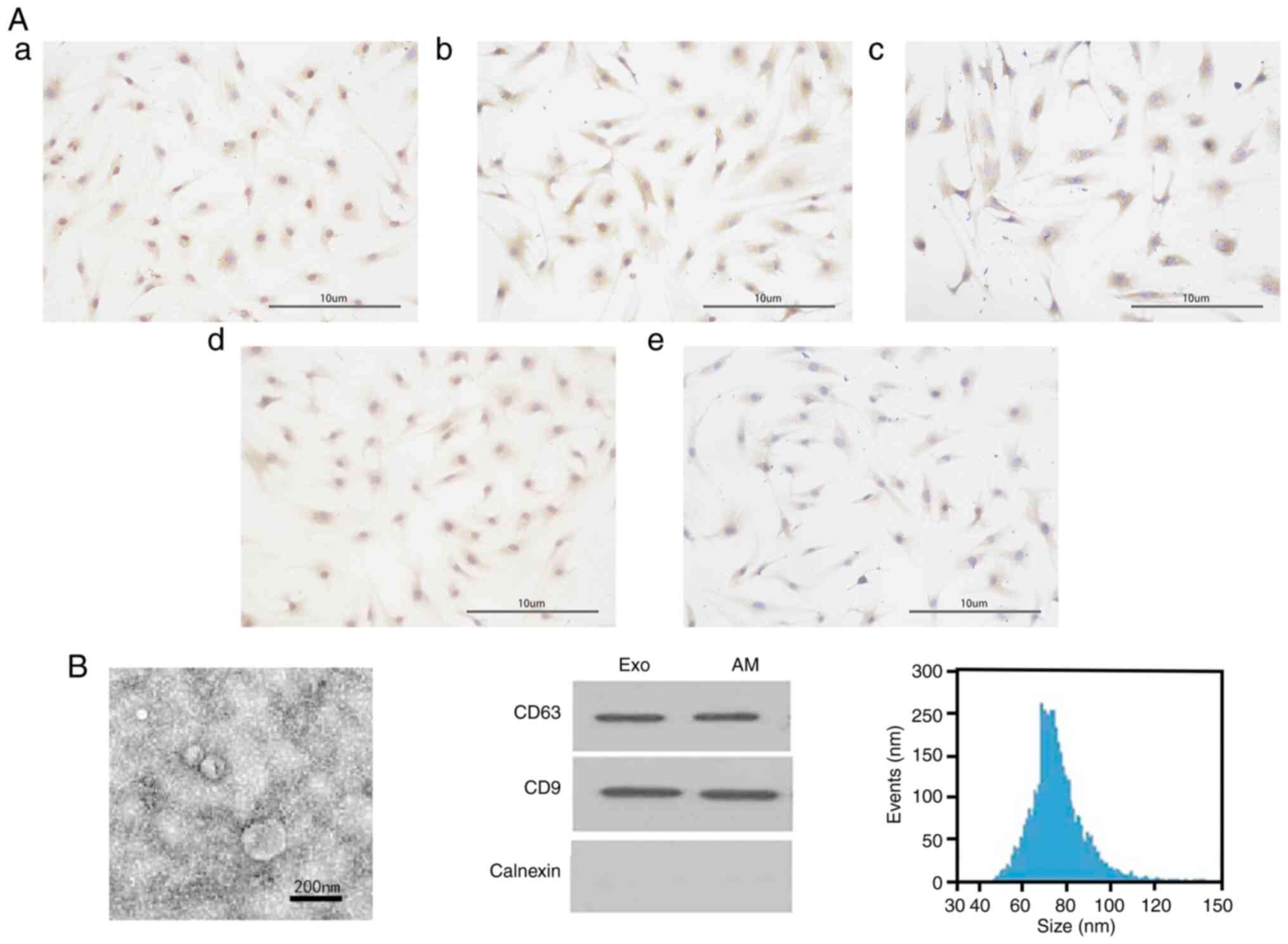
characterized by surface markers. Immunocytochemical staining
showed that AM cells expressed keratin, vimentin and actin
(Fig. 1Aa-c), whereas endometrial
cells expressed keratin and vimentin (Fig. 1Ad-e). Under TEM, endometrial
cell-derived exosomes appeared as spheres with clear and holonomic
membranes. Western blot analysis showed that the exosomes expressed
the typical exosomal markers CD63 and CD9 but not the endoplasmic
reticulum protein calnexin. The diameters of the exosomes ranged
from 45 to 120 nm, peaking at 73 nm (Fig. 1B). These results suggest that the
primary AM and endometrial cells and endometrial cell exosomes were
successfully obtained.
Endometrial cell exosomes inhibit
apoptosis of AM cells
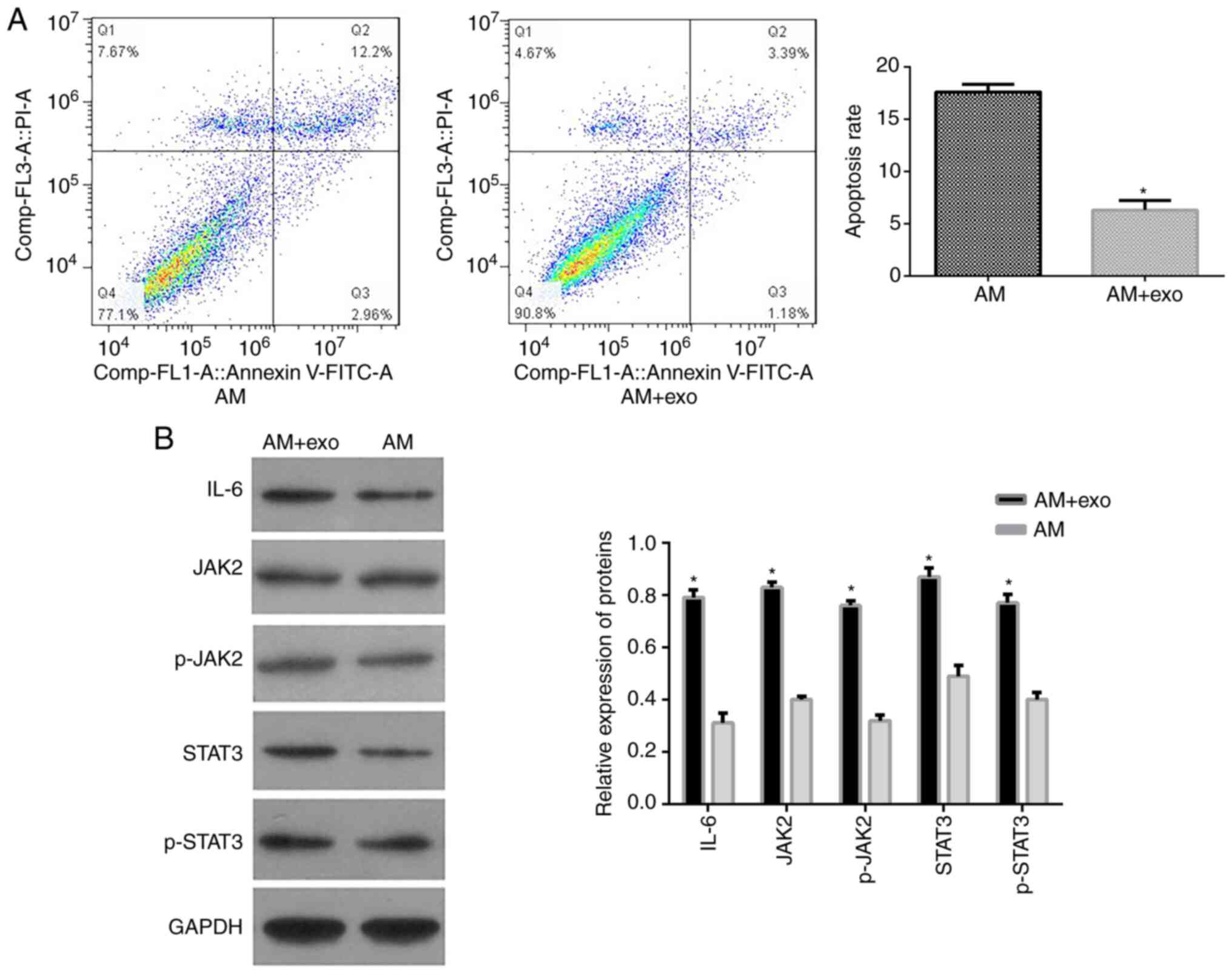
AM cells were treated with endometrial cell exosomes
to examine cell apoptosis. As shown in Fig. 2A, exosome treatment significantly
reduced the apoptotic rate of AM cells compared with the control
(5.28±1.87% vs. 18.64±3.97%, P<0.05), suggesting that
endometrial cell exosomes inhibit apoptosis of adenomyotic
cells.
Endometrial cell exosomes enhance IL-6
expression and JAK2/STAT3 activation of AM cells
IL-6 upregulation is closely involved in the
pathogenesis of adenomyosis (9).
Thus, IL-6 expression and JAK2 and STAT3 phosphorylation were
detected in AM cells exposed to exosomes. Western blotting revealed
that endometrial cell exosomes significantly enhanced IL-6, JAK2,
p-JAK2, STAT3 and p-STAT3 protein expression (Fig. 2B), suggesting that the activation
of IL6/JAK2/STAT3 signaling contributes to the effect of
endometrial cell exosomes on AM cells.
IL-6 inhibition abolishes endometrial
cell exosome-induced proliferation, cell cycle progression and
migration of AM cells
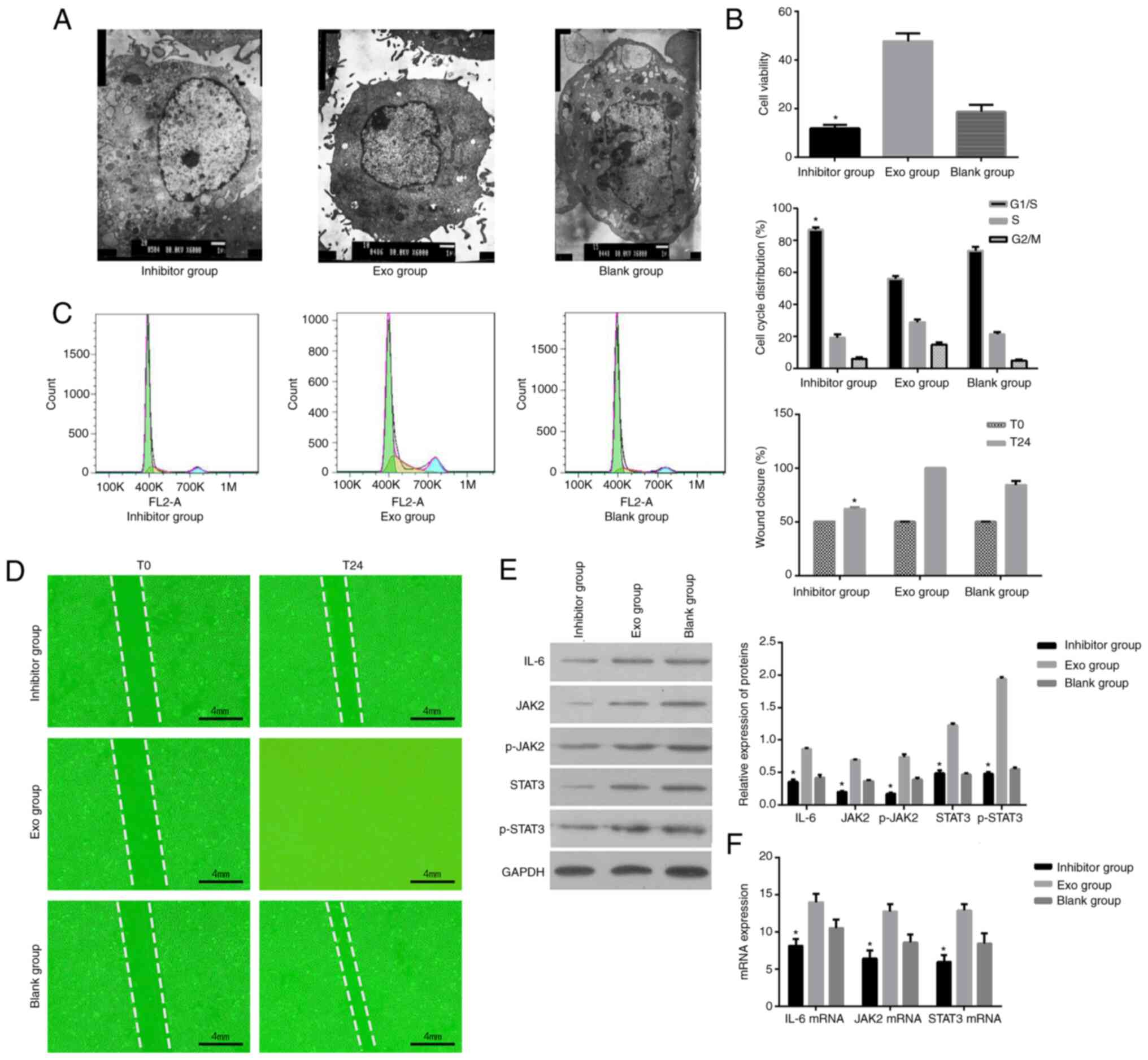
In order to investigate whether IL-6 mediates the
effects of endometrial cell exosomes on AM cells, AM cells were
treated with exosomes in the presence or absence of IL-6 inhibitor
tocilizumab. As shown in Fig. 3A,
AM cells exposed to exosomes and tocilizumab exhibited apoptotic
features, including decreases in size, cytoplasmic and nuclear
condensation, massive accumulation of vacuoles in mitochondria,
massive aggregation and chromatin fragmentation. These phenomena
were not observed in untreated cells or cells exposed to exosomes
alone. Furthermore, the MTT assay showed that the cell viability of
exosome-treated cells was markedly increased in the absence of
tocilizumab compared with untreated cells (P<0.05) but
substantially decreased in the presence of tocilizumab (P<0.05;
Fig. 3B). In addition, the
percentage of exosome-treated cells in S-phase was significantly
decreased in the presence of tocilizumab compared with untreated
cells (17.67±1.37% vs. 28.17±2.76%, P<0.05). The presence of
tocilizumab blocked the cells in the G1/S phase (78.57±3.92% vs.
51.59±4.85%, P<0.05; Fig. 3C).
Moreover, the wound-healing assay showed that endometrial cell
exosomes promoted the migration of AM cells compared with untreated
cells and it was abolished by tocilizumab (Fig. 3D). Western blot analysis
demonstrated that tocilizumab reversed the activation of
IL-6/JAK2/STAT3 signaling in AM cells induced by endometrial cell
exosomes (Fig. 3E). As shown in
Fig. 3F, IL-6, JAK2 and STAT3 mRNA
expression was higher in the exosome group than in the blank group.
The expression of the IL-6, JAK2 and STAT3 mRNA was significantly
reduced by the addition of the IL-6 inhibitor and the difference
was statistically significant compared with the exosome group
(P<0.05). Taken together, these data suggested that endometrial
cell exosomes promoted cell proliferation, cell cycle progression
and migration of AM cells and that IL-6 signaling was involved.
Discussion
The etiology of adenomyosis remains elusive and the
IL-6/JAK2/STAT3 pathway is involved in the pathogenesis of
adenomyosis (10). The present
study investigated whether endometrial cell exosomes contributed to
the invasive behavior of AM cells through the IL-6/JAK2/STAT3
pathway. It was demonstrated that incubation with endometrial cell
exosomes significantly promoted cell proliferation, migration and
cell cycle progression of AM cells while suppressing AM cell
apoptosis, along with enhancement in IL-6 production and JAK2/STAT3
phosphorylation. The IL-6 inhibitor tocilizumab effectively
reversed the effects of endometrial cell exosomes on AM cell
proliferation and migration and blocked the cells in the
G1/S phase, accompanied by a substantial attenuation of
IL-6/JAK2/STAT3 signaling. Thus, endometrial cell exosomes may
contribute to the development of adenomyosis by promoting cell
proliferation, migration and cell cycle progression of AM cells
through the IL-6/JAK2/STAT3 pathway.
Exosomes produced by pathological tissues have
detrimental effects on the surrounding tissues and contribute to
the development and progression of several diseases (24-27).
Exosomes from pathological lesions can also exert toxic effects on
nearby cells (24,25,28).
Endometrial cell-derived exosomes and their cargoes contribute to
the pathophysiology of endometriosis through multiple signaling
pathways involved in cell proliferation, migration, apoptosis,
inflammation and angiogenesis (20,29).
Exosomes are also involved in developing endometrial lesions and
types of cancer (30). However,
few studies have assessed the role of endometrial cell exosomes in
adenomyosis. Adenomyosis is closely related to endometriosis and
occasionally coexists with endometriosis (31). Adenomyosis and endometriosis have
been considered different phenotypes of a single disease (32). Thus, endometrial cell exosomes may
also contribute to the etiology of adenomyosis. As expected, the
results of the present study showed that endometrial cell exosomes
significantly inhibited apoptosis of AM cells compared with control
cells. Inhibition of apoptosis plays an important role in the
pathogenesis of endometriosis and adenomyosis. Therapeutic agents
that accelerate apoptosis have shown efficacy against adenomyosis
(33,34). Therefore, endometrial cell exosomes
may exacerbate adenomyosis by suppressing the apoptosis of AM
cells.
IL-6 plays a central role in the growth of
endometrial cells. Dysregulation of IL-6 signaling may cause
disorders of the endometrium, such as endometriosis and endometrial
cancer (35,36). In women with adenomyosis, IL-6
upregulation has been frequently seen in endometrial stromal cells,
macrophages, endometrial biopsies, adenomyosis-derived mesenchymal
stem cells and the peritoneal fluid (37-41).
Incubation with exosomes from different sources can induce IL-6
production by endometrial cells (42,43).
It was therefore hypothesized that IL-6 was involved in the effect
of endometrial cell exosomes on the growth of AM cells. The results
showed that AM cells incubated with endometrial cell exosomes had
markedly enhanced IL-6 expression compared with untreated cells,
consistent with the original hypothesis.
Although adenomyosis is a benign condition, AM cells
share some characteristics with cancer cells. Women with
adenomyosis are at a higher risk of endometrial cancer and
adenomyosis is considered a precursor of endometrial cancer
(44,45). Since the IL-6/JAK/STAT3 pathway is
hyperactivated in endometrial cancer (16,46,47),
AM cells were treated with exosomes in the presence or absence of
an IL-6 inhibitor to investigate whether IL-6 is required for the
effects of endometrial cell exosomes on AM cell behavior. The
results revealed that IL-6 inhibition completely reversed
endometrial cell exosome-induced proliferation, cell cycle
progression and migration of AM cells, suggesting that endometrial
cell exosomes promote the invasive properties of AM cells through
the activation of IL-6 signaling.
Age is probably a major contributor in the
development of adenomyosis since most patients are diagnosed in
their 40s and 50s when symptoms appear (48,49),
but the true incidence is unknown (48) and it is possible that younger women
can have asymptomatic adenomyosis. Of note, the reported prevalence
varies widely from 1-70% (48-50).
Whether symptomatic adenomyosis results from slowly progressing
lesions that take years to develop symptoms or whether aging plays
a role in the development of adenomyosis remains unknown and the
present study was not designed to answer that question.
Nevertheless, inflammation and aging are two sides of the same
medal (51-54).
Since the present study reported a strong role of IL-6, a
proinflammatory cytokine (12-14),
in the development of adenomyosis and since aging is associated
with a low-grade basal inflammatory state (51-54),
it is possible that aging contributes to the development of
adenomyosis. That hypothesis will have to be tested in future
studies.
The present study has some limitations that need to
be addressed in the future. First, the exosomal cargo that promotes
IL-6 production by AM cells remains unknown. Second, the
application of a JAK2/STAT3 inhibitor is required to verify whether
JAK2/STAT3 is essential to mediate the effects of endometrial cell
exosomes on AM cell growth. Third, the tissue samples were not
subjected to immunohistochemistry and had to be used to isolate
AMs. Indeed, the sample was limited to ~1 cm3 to not
interfere with the postoperative pathological examination and the
study of adenomyosis tissues will be further supplemented and
improved in future animal experiments. Fourth, a key limitation is
the lack of the immune system context. Indeed, tocilizumab has
direct effects on IL-6 but also has effects on immune cells
(55). The immune context will
have to be examined in vivo.
In conclusion, endometrial cell-derived exosomes
promoted cell proliferation, migration and cell cycle progression
of AM cells through IL-6/JAK2/STAT3 activation, suggesting that
endometrial cells exosomes mediated the crosstalk between the
endometrium and the myometrium via IL-6 signaling and thus
facilitated the development of adenomyosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Basic and
Applied Basic Research Fund of Guangdong Province (grant no.
2019A1515111175), the Characteristic Innovation Project of
Guangdong Universities (Natural Science; grant no. 2021KTSCX054),
the Key Discipline Construction Project of TCM of Guangdong
Pharmaceutical University (grant no. ZY2021M05) and the Project of
Guangdong Provincial Administration of Traditional Chinese Medicine
(grant no. 20241169).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XJ and XC performed the experiments, participated in
collecting data and drafted the manuscript. XJ and XC performed the
statistical analysis and participated in its design. XJ
participated in acquiring, analyzing, or interpreting data and
drafted the manuscript. Both authors read and approved the final
manuscript. XJ and XC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of The First Affiliated Hospital of Guangdong
Pharmaceutical University [approval no. 2021-(31)]. All participants provided informed
consent before the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nirgianakis K, Kalaitzopoulos DR, Schwartz
ASK, Spaanderman M, Kramer BW, Mueller MD and Mueller M: Fertility,
pregnancy and neonatal outcomes of patients with adenomyosis: A
systematic review and meta-analysis. Reprod Biomed Online.
42:185–206. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Upson K and Missmer SA: Epidemiology of
Adenomyosis. Semin Reprod Med. 38:89–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lacheta J: Uterine adenomyosis:
Pathogenesis, diagnostics, symptomatology and treatment. Ceska
Gynekol. 84:240–246. 2019.PubMed/NCBI
|
|
4
|
Che X, Wang J, He J, Yu Q, Sun W, Chen S,
Zou G, Li T, Guo X and Zhang X: A new trick for an old dog: The
application of mifepristone in the treatment of adenomyosis. J Cell
Mol Med. 24:1724–1737. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gunther R and Walker C: Adenomyosis.
StatPearls, Treasure Island, FL, 2022.
|
|
6
|
Li J, Yanyan M, Mu L, Chen X and Zheng W:
The expression of Bcl-2 in adenomyosis and its effect on
proliferation, migration, and apoptosis of endometrial stromal
cells. Pathol Res Pract. 215(152477)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu W, Song Y, Li K, Zhang B and Zhu X:
Quercetin inhibits adenomyosis by attenuating cell proliferation,
migration and invasion of ectopic endometrial stromal cells. Drug
Des Devel Ther. 14:3815–3826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harmsen MJ, Wong CFC, Mijatovic V,
Griffioen AW, Groenman F, Hehenkamp WJK and Huirne JAF: Role of
angiogenesis in adenomyosis-associated abnormal uterine bleeding
and subfertility: A systematic review. Hum Reprod Update.
25:647–671. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bourdon M, Santulli P, Jeljeli M,
Vannuccini S, Marcellin L, Doridot L, Petraglia F, Batteux F and
Chapron C: Immunological changes associated with adenomyosis: A
systematic review. Hum Reprod Update. 27:108–129. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
An M, Li D, Yuan M, Li Q, Zhang L and Wang
G: Interaction of macrophages and endometrial cells induces
epithelial-mesenchymal transition-like processes in adenomyosis.
Biol Reprod. 96:46–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rier SE, Zarmakoupis PN, Hu X and Becker
JL: Dysregulation of interleukin-6 responses in ectopic endometrial
stromal cells: Correlation with decreased soluble receptor levels
in peritoneal fluid of women with endometriosis. J Clin Endocrinol
Metab. 80:1431–1437. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Candido S, Tomasello BMR, Lavoro A,
Falzone L and Gattuso Gand Libra M: Novel insights into epigenetic
regulation of IL6 pathway: In silico perspective on inflammation
and cancer relationship. Int J Mol Sci. 22(10172)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baran P, Hansen S, Waetzig GH, Akbarzadeh
M, Lamertz L, Huber HJ, Ahmadian MR, Moll JM and Scheller J: The
balance of interleukin (IL)-6, IL-6.soluble IL-6 receptor (sIL-6R),
and IL-6.sIL-6R.sgp130 complexes allows simultaneous classic and
trans-signaling. J Biol Chem. 293:6762–6775. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mihara M, Hashizume M, Yoshida H, Suzuki M
and Shiina M: IL-6/IL-6 receptor system and its role in
physiological and pathological conditions. Clin Sci (Lond).
122:143–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Che Q, Xiao X, Liu M, Lu Y, Dong X and Liu
S: IL-6 promotes endometrial cancer cells invasion and migration
through signal transducers and activators of transcription 3
signaling pathway. Pathol Res Pract. 215(152392)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells.
8(727)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li P, Kaslan M, Lee SH, Yao J and Gao Z:
Progress in exosome isolation techniques. Theranostics. 7:789–804.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Andreu Z and Yanez-Mo M: Tetraspanins in
extracellular vesicle formation and function. Front Immunol.
5(442)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Freger S, Leonardi M and Foster WG:
Exosomes and their cargo are important regulators of cell function
in endometriosis. Reprod Biomed Online. 43:370–378. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Khalaj K, Miller JE, Lingegowda H,
Fazleabas AT, Young SL, Lessey BA, Koti M and Tayade C:
Extracellular vesicles from endometriosis patients are
characterized by a unique miRNA-lncRNA signature. JCI Insight.
4(e128846)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jung MK and Mun JY: Sample preparation and
imaging of exosomes by transmission electron microscopy. J Vis Exp.
56482:2018.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Othman N, Jamal R and Abu N:
Cancer-Derived exosomes as effectors of key inflammation-related
players. Front Immunol. 10(2103)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li KL, Huang HY, Ren H and Yang XL: Role
of exosomes in the pathogenesis of inflammation in Parkinson's
disease. Neural Regen Res. 17:1898–1906. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Isola AL and Chen S: Exosomes: The
messengers of health and disease. Curr Neuropharmacol. 15:157–165.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan Y, Chen Z and Zhang M: Role of
exosomes in the pathogenesis, diagnosis, and treatment of central
nervous system diseases. J Transl Med. 20(291)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Genc S, Pennisi M, Yeni Y, Yildirim S,
Gattuso G, Altinoz MA, Taghizadehghalehjoughi A, Bolat I, Tsatsakis
A, Hacımüftüoğlu A and Falzone L: Potential neurotoxic effects of
glioblastoma-derived exosomes in primary cultures of cerebellar
neurons via oxidant stress and glutathione depletion. Antioxidants
(Basel). 11(1225)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang M, Wang X, Xia X, Fang X, Zhang T
and Huang F: Endometrial epithelial cells-derived exosomes deliver
microRNA-30c to block the BCL9/Wnt/CD44 signaling and inhibit cell
invasion and migration in ovarian endometriosis. Cell Death Discov.
8(151)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Soroczynska K, Zareba L, Dlugolecka M and
Czystowska-Kuzmicz M: Immunosuppressive extracellular vesicles as a
linking factor in the development of tumor and endometriotic
lesions in the gynecologic tract. Cells. 11(1483)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bordonne C, Puntonet J, Maitrot-Mantelet
L, Bourdon M, Marcellin L, Dion E, Plu-Bureau G, Santulli P and
Chapron C: Imaging for evaluation of endometriosis and adenomyosis.
Minerva Obstet Gynecol. 73:290–303. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maruyama S, Imanaka S, Nagayasu M, Kimura
M and Kobayashi H: Relationship between adenomyosis and
endometriosis; Different phenotypes of a single disease? Eur J
Obstet Gynecol Reprod Biol. 253:191–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bilgic E, Guzel E, Kose S, Aydin MC,
Karaismailoglu E, Akar I, Usubutun A and Korkusuz P:
Endocannabinoids modulate apoptosis in endometriosis and
adenomyosis. Acta Histochem. 119:523–532. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang JH, Duan H, Wang S, Wang YY and Lv
CX: Upregulated microRNA let-7a accelerates apoptosis and inhibits
proliferation in uterine junctional zone smooth muscle cells in
adenomyosis under conditions of a normal activated hippo-YAP1 axis.
Reprod Biol Endocrinol. 19(81)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zarmakoupis PN, Rier SE, Maroulis GB and
Becker JL: Inhibition of human endometrial stromal cell
proliferation by interleukin 6. Hum Reprod. 10:2395–2399.
1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yoshioka H, Harada T, Iwabe T, Nagano Y,
Taniguchi F, Tanikawa M and Terakawa N: Menstrual cycle-specific
inhibition of the proliferation of endometrial stromal cells by
interleukin 6 and its soluble receptor. Am J Obstet Gynecol.
180:1088–1094. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS
and Ho HN: Decreased suppression of interleukin-6 after treatment
with medroxyprogesterone acetate and danazol in endometrial stromal
cells of women with adenomyosis. Fertil Steril. 86:1459–1465.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang JH, Wu MY, Chang DY, Chang CH, Yang
YS and Ho HN: Increased interleukin-6 messenger RNA expression in
macrophage-cocultured endometrial stromal cells in adenomyosis. Am
J Reprod Immunol. 55:181–187. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhihong N, Yun F, Pinggui Z, Sulian Z and
Zhang A: Cytokine profiling in the eutopic endometrium of
adenomyosis during the implantation window after ovarian
stimulation. Reprod Sci. 23:124–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK,
Lu KH, Chang CM and Chiou SH: Suppression of migratory/invasive
ability and induction of apoptosis in adenomyosis-derived
mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil
Steril. 94:1972–1979, 1979 e1-4. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ozcelik K, Capar M, Gazi Ucar M, Cakiotar
T, Ozcelik F and Tuyan Ilhan T: Are cytokine levels in serum,
endometrial tissue, and peritoneal fluid a promising predictor to
diagnosis of endometriosis-adenomyosis? Clin Exp Obstet Gynecol.
43:569–572. 2016.PubMed/NCBI
|
|
42
|
Paktinat S, Hashemi SM, Ghaffari Novin M,
Mohammadi-Yeganeh S, Salehpour S, Karamian A and Nazarian H:
Seminal exosomes induce interleukin-6 and interleukin-8 secretion
by human endometrial stromal cells. Eur J Obstet Gynecol Reprod
Biol. 235:71–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shi S, Tan Q, Liang J, Cao D, Wang S,
Liang J, Chen K and Wang Z: Placental trophoblast cell-derived
exosomal microRNA-1290 promotes the interaction between endometrium
and embryo by targeting LHX6. Mol Ther Nucleic Acids. 26:760–772.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zouzoulas OD, Tsolakidis D, Efstratiou I,
Pervana S, Pazarli E and Grimbizis G: Correlation between
adenomyosis and endometrial cancer: 6-year experience of a single
center. Facts Views Vis Obgyn. 10:147–152. 2018.PubMed/NCBI
|
|
45
|
Yeh CC, Su FH, Tzeng CR, Muo CH and Wang
WC: Women with adenomyosis are at higher risks of endometrial and
thyroid cancers: A population-based historical cohort study. PLoS
One. 13(e0194011)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Subramaniam KS, Omar IS, Kwong SC, Mohamed
Z, Woo YL, Mat Adenan NA and Chung I: Cancer-associated fibroblasts
promote endometrial cancer growth via activation of
interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 6:200–213.
2016.PubMed/NCBI
|
|
47
|
van der Zee M, Sacchetti A, Cansoy M,
Joosten R, Teeuwssen M, Heijmans-Antonissen C, Ewing-Graham PC,
Burger CW, Blok LJ and Fodde R: IL6/JAK1/STAT3 signaling blockade
in endometrial cancer affects the ALDHhi/CD126+ stem-like component
and reduces tumor Burden. Cancer Res. 75:3608–3622. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Struble J, Reid S and Bedaiwy MA:
Adenomyosis: A clinical review of a challenging gynecologic
condition. J Minim Invasive Gynecol. 23:164–185. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Senturk LM and Imamoglu M: Adenomyosis:
What is new? Womens Health (Lond). 11:717–724. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abbott JA: Adenomyosis and abnormal
uterine bleeding (AUB-A)-Pathogenesis, diagnosis, and management.
Best Pract Res Clin Obstet Gynaecol. 40:68–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Falzone L, Candido S, Docea AO and Calina
D: Editorial: Inflammation and aging in chronic and degenerative
diseases: Current and future therapeutic strategies. Front
Pharmacol. 13(1122786)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Machlin JH, Barishansky SJ, Kelsh J,
Larmore MJ, Johnson BW, Pritchard MT, Pavone ME and Duncan FE:
Fibroinflammatory signatures increase with age in the human ovary
and follicular fluid. Int J Mol Sci. 22(4902)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Protopapas A, Grimbizis G, Athanasiou S
and Loutradis D: Adenomyosis: Disease, uterine aging process
leading to symptoms, or both? Facts Views Vis Obgyn. 12:91–104.
2020.PubMed/NCBI
|
|
54
|
Sanchez-Prieto M, Sanchez-Borrego R,
Lubian-Lopez DM and Perez-Lopez FR: Etiopathogenesis of ovarian
cancer. An inflamm-aging entity? Gynecol Oncol Rep.
42(101018)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu Y, Zhang H, Zhang TX, Yuan M, Du C,
Zeng P, Huang Z, Jia D, Yang G, Shi FD and Zhang C: Effects of
tocilizumab therapy on circulating B cells and T helper cells in
patients with neuromyelitis optica spectrum disorder. Front
Immunol. 12(703931)2021.PubMed/NCBI View Article : Google Scholar
|