Introduction
Reconstruction of soft tissue defects in the
trochanteric area can be complex and difficult. Defects resulting
from sarcoma resections in patients exposed to radiation therapy
further complicate the reconstructive procedure (1). Radiotherapy suppresses wound healing
by altering collagen production, rendering the microvasculature of
an irradiated area weaker, fragile and compromised (2). Therefore, careful handling and
meticulous care must be considered with irradiated vessels, as they
are more friable and prone to damage (3). Despite the beneficial effects of
radiotherapy in the management of soft tissue sarcoma, 10-25% of
sarcomas recur locally (4).
Treatment of the recurrence mandates additional tumor resection,
radiotherapy and reconstruction.
Reconstructive options for such large
three-dimensional defects include locoregional muscle,
myocutaneous, or fasciotcutaneous flaps and free flaps. Examples of
locoregional options are the gluteus maximus (GM) flap, posterior
gluteal thigh flap, tensor fascia lata (TFL) flap and anterolateral
thigh (ALT) flap. The vastus lateralis (VL) muscle flap is a good
choice to fill the dead space with a success rate equal to that of
the well-known ALT flap. It was first utilized in 1977 by Minami
et al (5) for
reconstruction of trochanteric pressure ulcers. In 1982, Bovet
et al (6) described the VL
myocutaneous flap, concluding that it has favorable results in
trochanteric reconstructions. The purpose of the present report is
to share our experience in harvesting the VL flap after prior
harvest of the neighboring ALT flap for reconstruction of a
recurrent trochanteric sarcoma. All procedures followed were in
accordance with the ethical standards of the responsible committee
on human experimentation (institutional and national) and with the
Helsinki Declaration of 1975, as revised in 2008. Informed consent
was obtained from the patient included in the study.
Case report
Patient
A 54-year-old man presented with a recurrent
myxofibrosarcoma involving the right greater trochanter region to
King Abdulaziz University Hospital in Jeddah, Saudi Arabia. The
patient had undergone multiple attempts to achieve a curative
resection and reconstruction, in addition to multiple sessions of
radiotherapy. The first excision was performed at another hospital
in January 2012.
The patient presented to King Abdulaziz University
Hospital with recurrence 10 months after that operation, in
November 2012, and was admitted for work-up and multidisciplinary
team (MDT) discussion. The work-up consisted of routine laboratory
work, computed tomography (CT) scans, magnetic resonance imaging
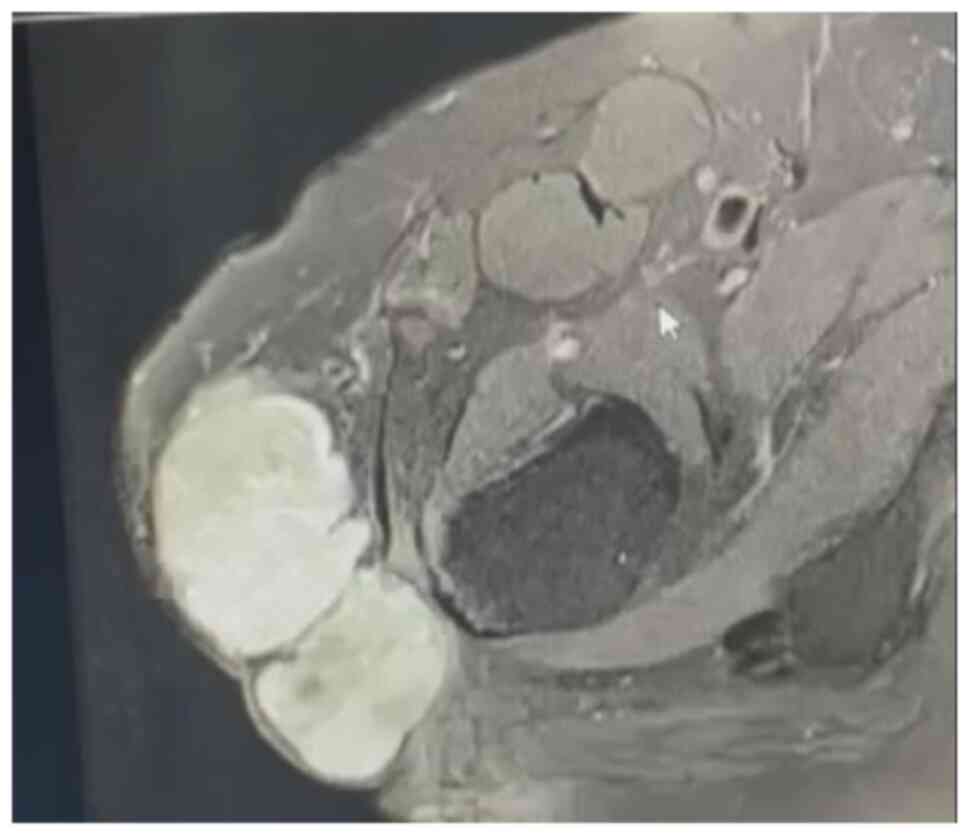
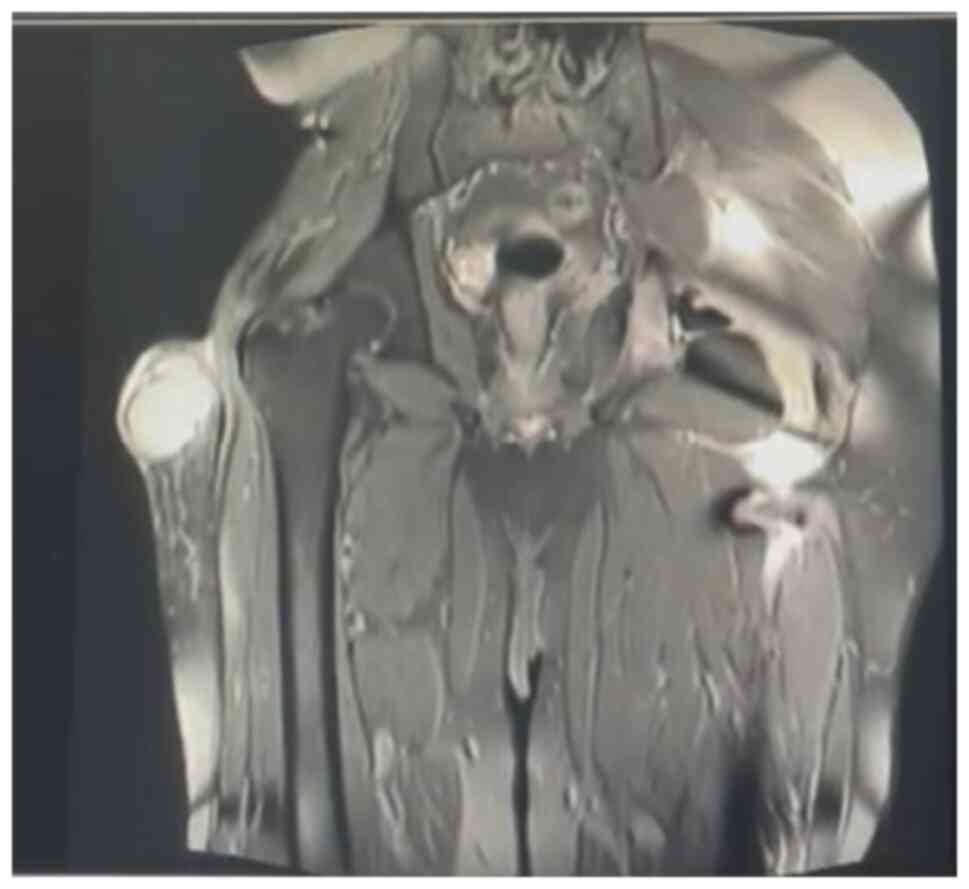
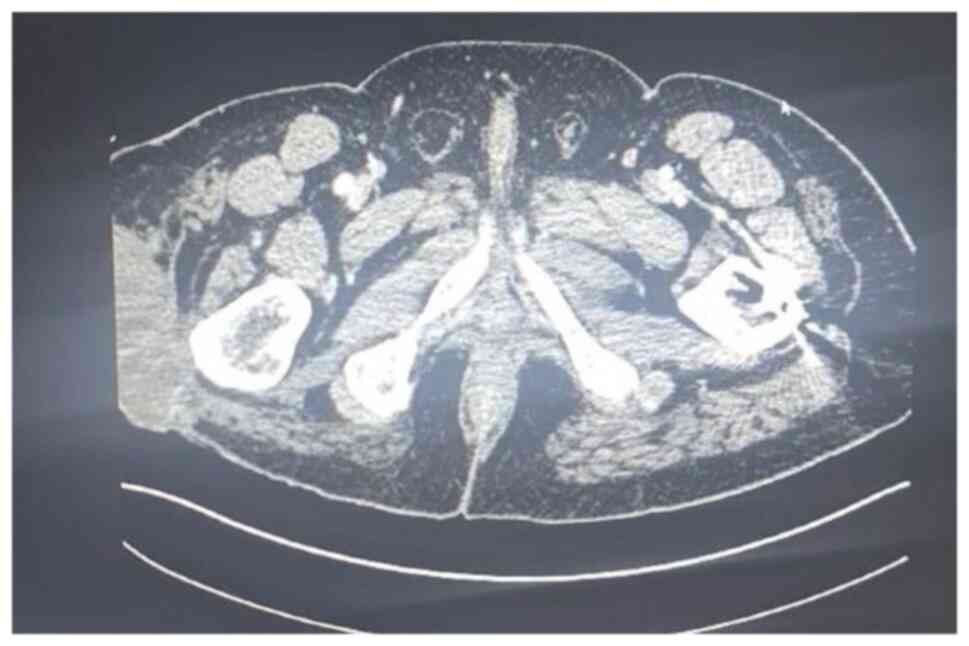
(MRI), bone scan and histopathological review of the tissue samples
from the first excision (Fig. 1,
Fig. 2, Fig. 3 and Fig. 4). The diagnosis was confirmed to be
malignant fibrosarcoma. MDT consensus was to start with
neo-adjuvant chemotherapy followed by surgical resection. After
receiving two cycles of chemotherapy a repeated MRI revealed poor
response to chemotherapy and increase in tumor size. Therefore,
surgical resection was performed in February 2013. Tumor negative
margin was achieved and primary closure of the wound was done
followed by adjuvant radiotherapy.
In September 2016, the patient presented with
cellulitis in the same area with a suspicious mass. An MRI revealed
a suspicious lesion and an incisional biopsy confirmed the second
recurrence. Metastatic work-up was negative for distant metastasis.
In October 2016, the patient underwent resection and reconstruction
with a pedicled ALT flap. Following the excision, the patient was
reviewed by medical and radiation oncology and it was determined
that there was no need for adjuvant therapy at this stage.
In April 2018, a follow-up MRI revealed a new lesion
in the same area. The third recurrence was confirmed with an
ultrasound-guided biopsy. A MDT meeting concluded that there would
be no benefit from chemotherapy as the tumor was chemo-resistant.
Therefore, the surgical team proceeded with the resection in July
2018. The excision included removal of the previous ALT flap and
final pathology revealed negative margins. This resection was
complicated by an injury to the sciatic nerve, which required
surgical repair and prolonged post-operative rehabilitation. The
wound was initially managed by negative pressure wound therapy
(NPWT) dressings followed by skin grafting.
At two years later, a fourth recurrence was
identified on follow-up assessment. An MRI and CT scan performed in
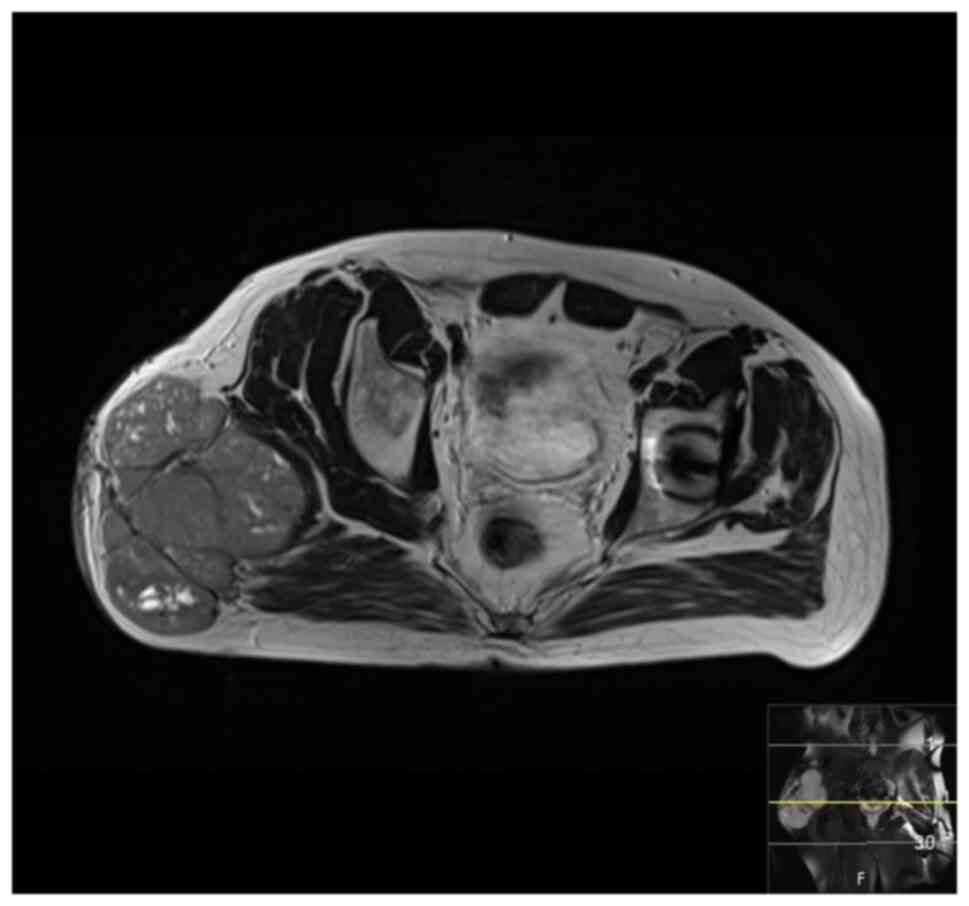
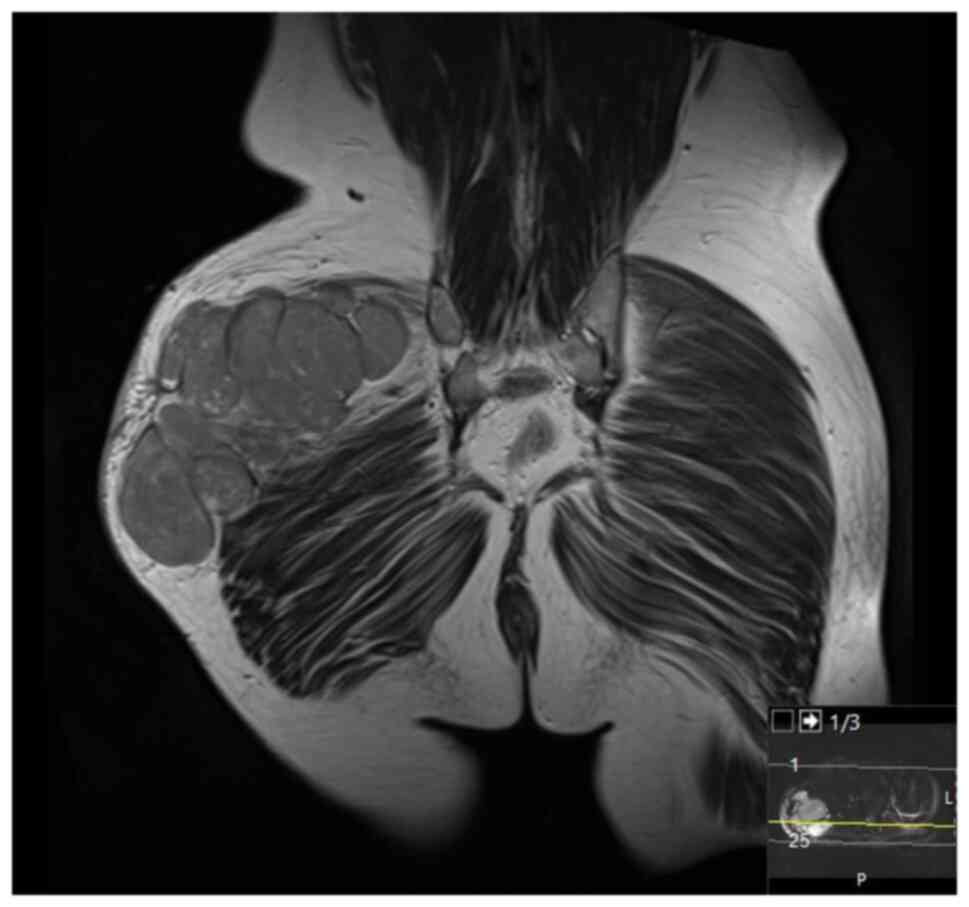
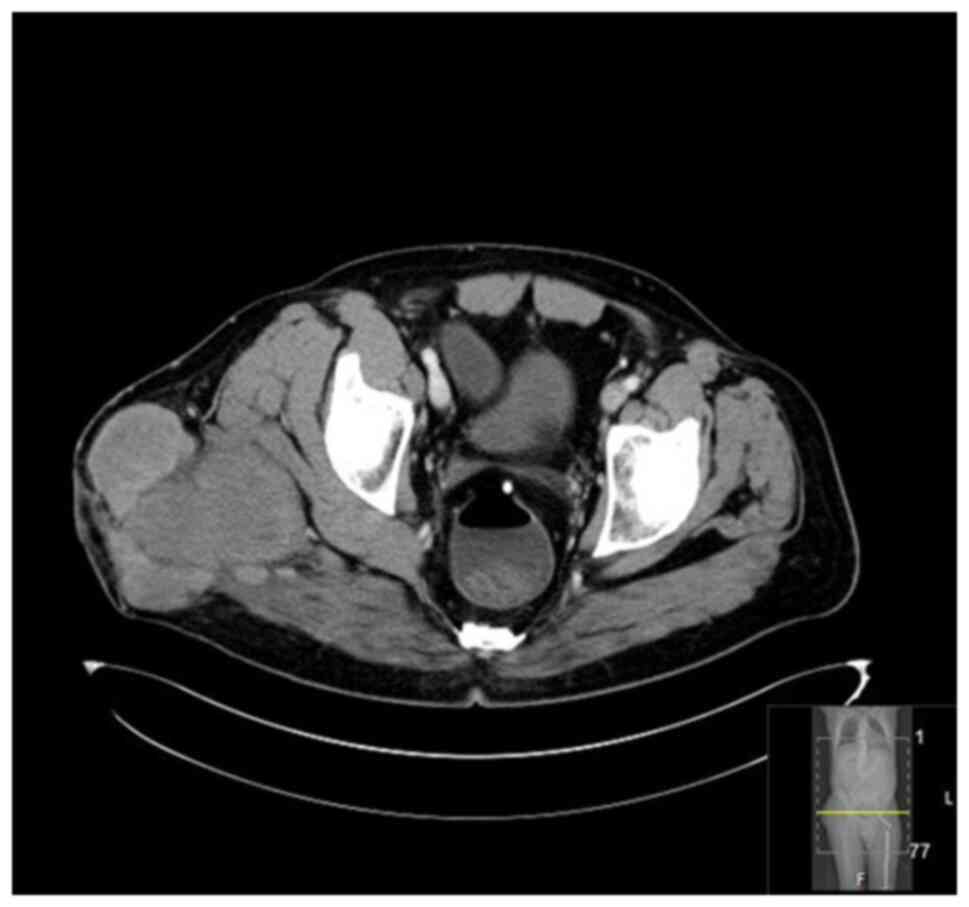
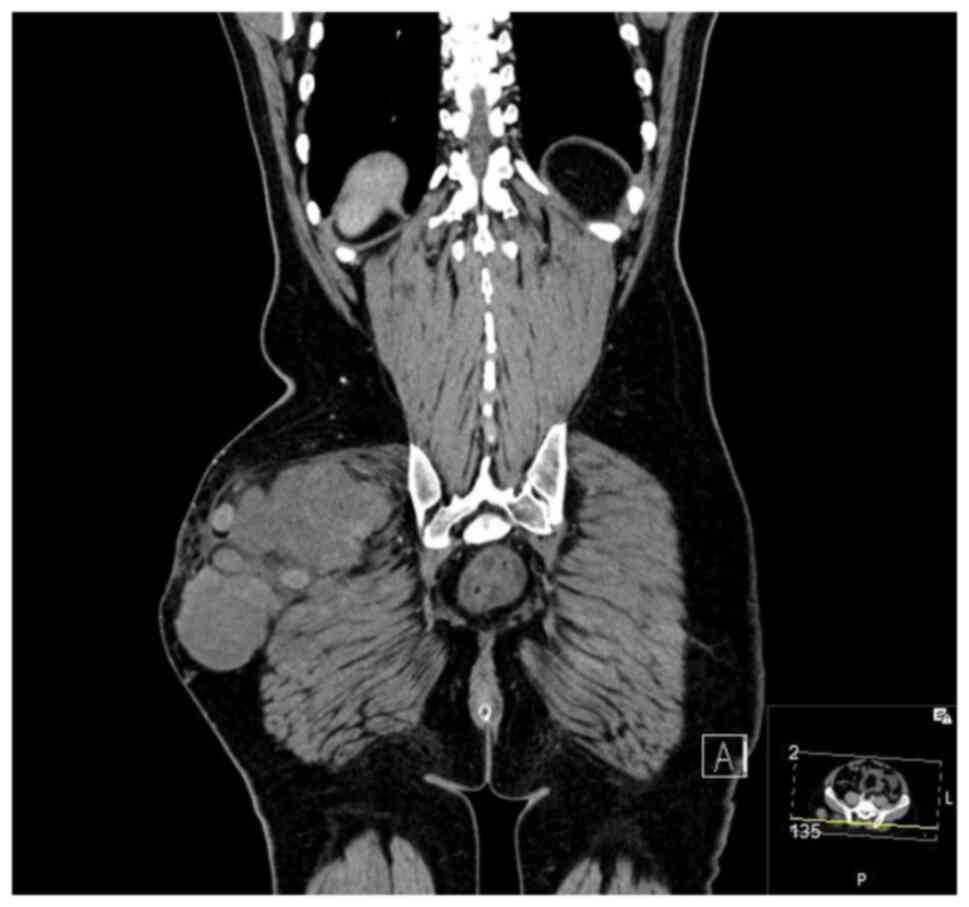
September 2020 showed a mass measuring 8.5x4x5 cm over the greater
trochanter extending to the fascia, with no abnormal signal in the
muscle or bone and no regional lymphadenopathy (Fig. 5, Fig.
6 and Fig. 7). Metastatic
work-up remained negative. Following the MDT recommendation, the
patient received 25 sessions of neo adjuvant radiotherapy. The
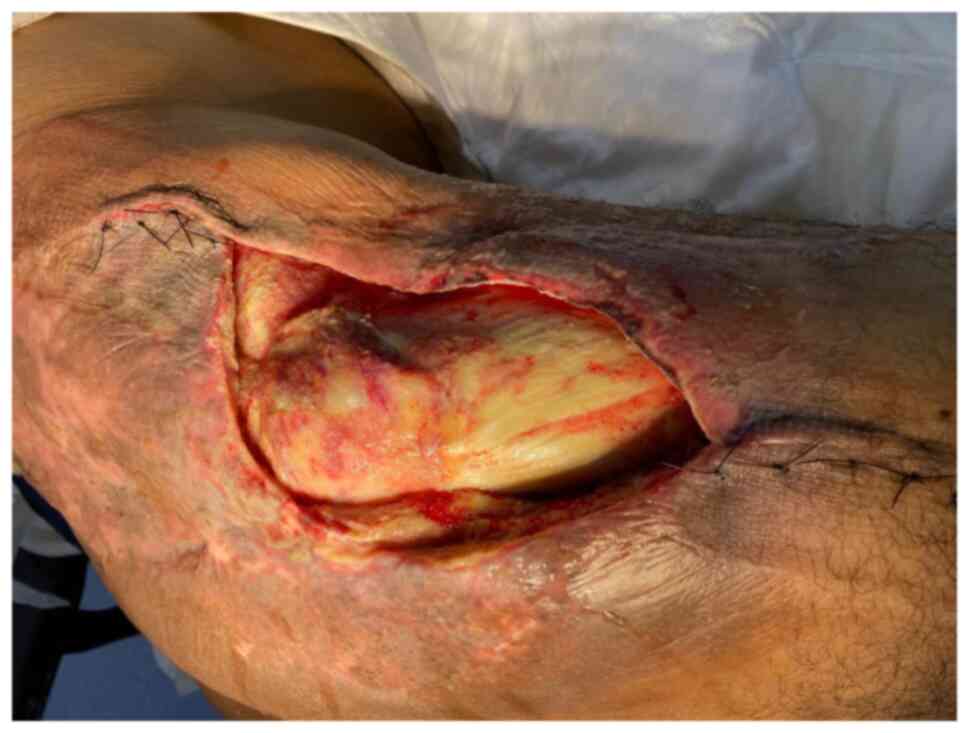
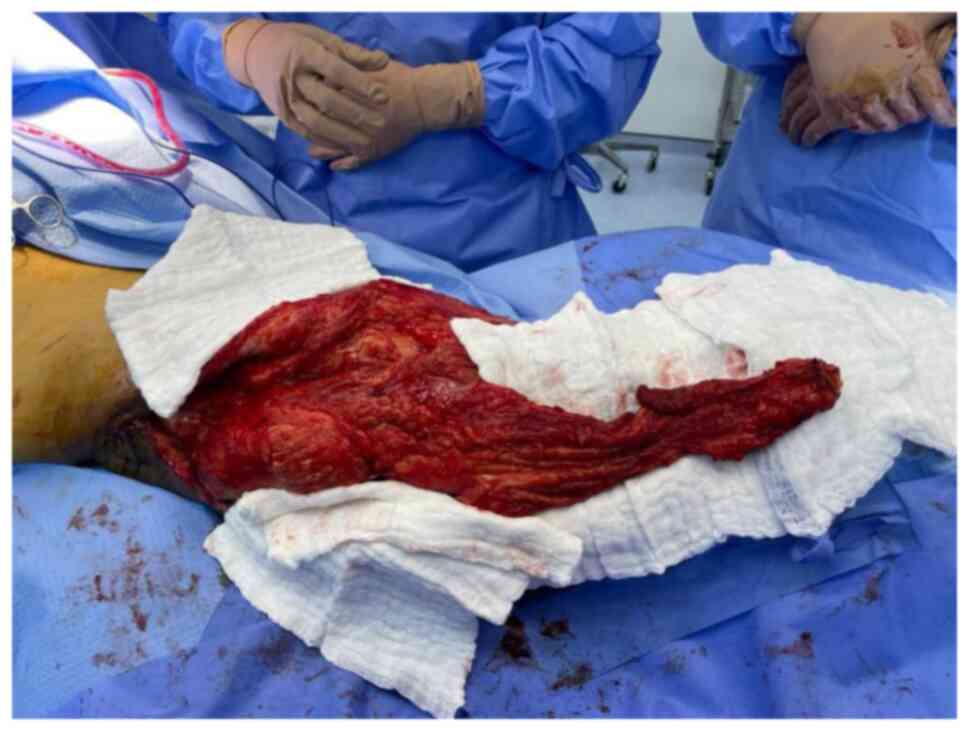
fifth excision was performed in April 2021. This resulted in a
large trochanteric defect of 15x10 cm surrounded by poor quality
irradiated skin (Fig. 8). The
final pathology confirmed complete resection of a high-grade
myxofibrosarcoma with negative margins. The wound was initially
managed by NPWT and reconstruction with the VL muscle flap was
planned for coverage of the defect.
Flap harvest
Following a complete resection with clear margins,
the VL muscle was partially exposed within the floor of the defect.
The incision was extended distally to assess the quality and
perfusion of the muscle. Multiple patent muscular branches were
identified in the medial and deep parts of the muscle. The VL
muscle flap was carefully divided from the tendonous insertion
distally, 10 cm proximal to the patella. The quadriceps tendon was
preserved to reduce the risk of patellar instability. The minor
pedicle originating from the lateral superior genicular artery was
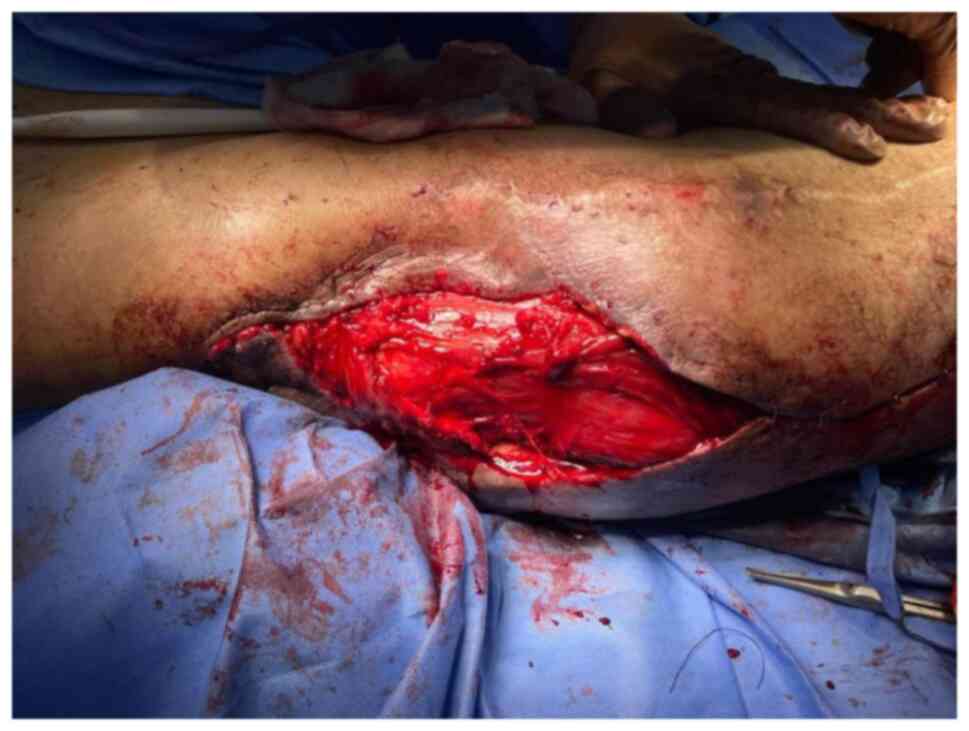
ligated distally. The flap was elevated distal to proximal
(Fig. 9). The vascularity to the
VL was well maintained, despite the need to sacrifice a few
branches entering the distal half of the muscle. The VL flap was
turned over into the defect, while preserving the proximal vascular
supply. The flap rested easily with no tension over the defect and
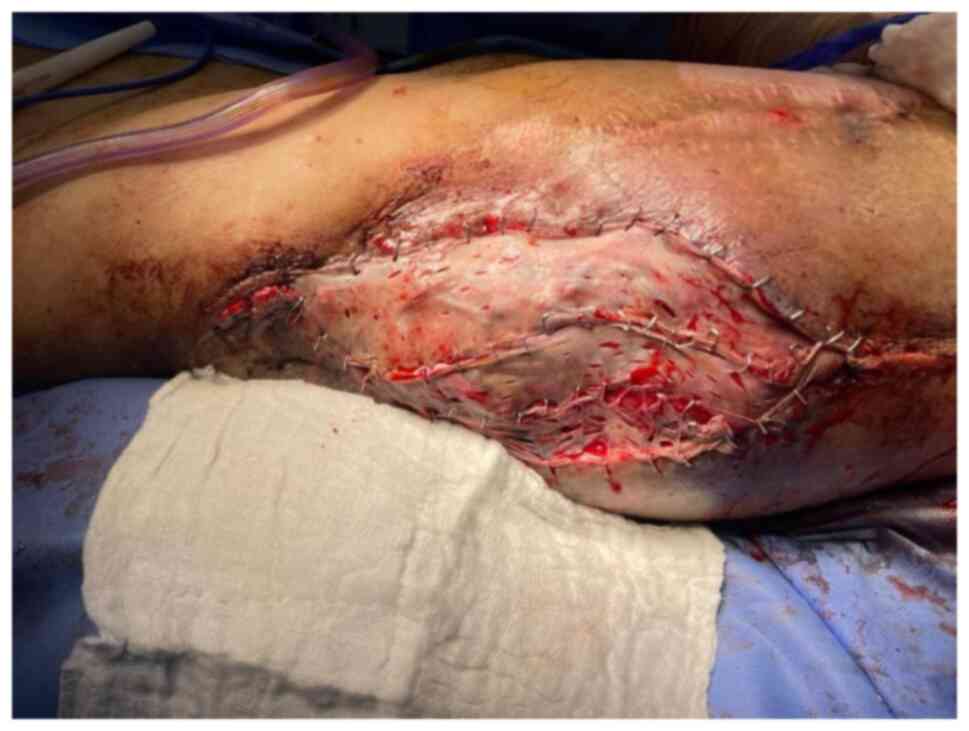
covered the exposed trochanteric bone (Fig. 10). The donor site was closed
primarily. A meshed partial thickness skin graft was placed over
the flap (Fig. 11) and NPWT
dressing was applied.
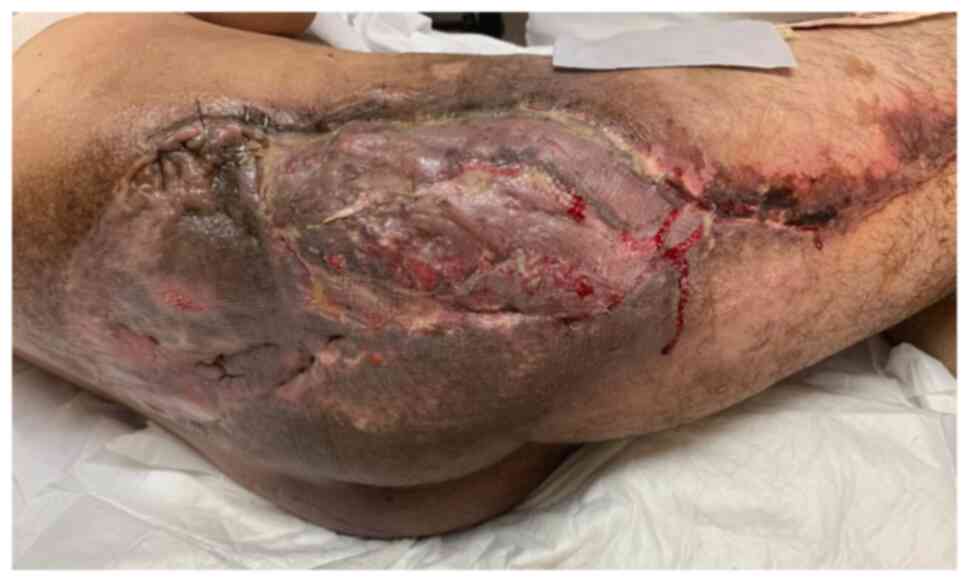
Successful flap survival was achieved with favorable
skin graft take (Fig. 12). The
wound progressed towards complete healing. The patient recovered
and was discharged from the hospital in June 2021. The patient
required physiotherapy and eventually returned to their baseline
health status. Follow-up MRI scan revealing no signs of tumor
recurrence (July 2021). At the last reported follow-up, three years
post-procedure, the patient remained disease-free (Fig. 13).
Anatomy
The VL muscle flap is classified as type I Mathes
and Nahai based on its vascular supply (7). It can be utilized as a muscle or
musculocutaneous flap and it has a skin paddle similar to that of
the ALT flap. The main pedicle of the VL arises from the lateral
circumflex femoral artery (LCFA), a large branch of the deep
femoral artery. It trifurcates to the ascending, transverse and
descending branches. The main pedicle of the VL is the descending
branch of the lateral circumflex femoral artery (d-LCFA) and vein.
The ALT flap similarly depends on the d-LCFA as its main vascular
supply, mainly through its perforators (8). Anatomical variations are not rare in
this sequence; in up to 44% of anatomical cases, there is an
oblique branch arising commonly from the d-LCFA, which serves as
the dominant perforator of the ALT flap when present (9). The d-LCFA runs in the intermuscular
septum between the rectus femoris and the VL for a variable
distance before entering the substance of the VL (10). The vessel diameter is >2 mm,
with a pedicle length ranging between 8-16 cm (10). Proximally, the d-LCFA gives off a
large branch to the rectus femoris muscle, known as the rectus
femoris branch (8,11). The VL muscle is innervated through
segmental muscular branches of the femoral nerve (12).
Discussion
Myxofibrosarcomas are among the most common soft
tissue sarcomas (STS) presenting in extremities. It has a
predilection towards the lower extremity with usual presentation in
males between 60-80 years of age. Management of such tumors always
involves a multidisciplinary team and treatment options include
surgical resection, radiation and chemotherapy (13). Localized lesions are best managed
by wide surgical resection with a goal of free margins.
Radiotherapy and chemotherapy have limited roles and their benefit
has been debated. Some have even labeled such tumors as
radioresistant; however, this has been argued as the indication for
radiotherapy is more advanced cases and not due to the modality
itself. Chemotherapy mainly plays a role in metastatic disease and
has poor outcomes. They demonstrate high recurrence rates compared
to other STS, ranging from 20-60% (13). The best form of surveillance is by
clinical examination and MRI. To this day local control, which was
the main oncologic treatment goal of the present case, is mainly
achieved by adequate surgical resection in both margin width and
anatomic barrier with adjuvant radiotherapy (13).
Reconstruction of soft tissue defects over the
greater trochanter is challenging, as this is a known pressure
point and an area of bony projection. The present case was further
complicated by multiple resections, radiotherapy, previous
dissection of the main vascular pedicle in the region and
limitations in recipient vessels for free tissue transfer. All
these factors adversely affected the local microvascular network
and limited the locoregional reconstructive options. Upon
completion of resection with clear margins, VL was adjacent to the
defect and appeared to be the only available regional option to
attempt reconstruction. The VL is an optimal muscle flap with a
reliable vascular pedicle and a wide arc of rotation with minimal
donor site morbidity (14). The
present case was a case of VL harvest following previous pedicled
ALT flap reconstruction in the same area, both of which share a
common vascular pedicle.
Harvesting the VL flap for reconstruction of this
trochanteric defect allows for immediate reconstruction with a
locoregional option. This minimizes recovery time and number of
operations needed to resume function. Flap reconstruction of
resected soft tissue sarcoma allows for complete recovery with
superior functional outcomes compared to amputation (15). Moreover, immediate reconstruction
has resulted in decreased wound complications compared to delayed
or interval reconstruction (15).
Theoretically, harvesting the VL for reconstruction
is challenging due to prior utilization of the ALT flap and
dissection of the main shared pedicle, especially in an irradiated
bed. These alterations affect the muscle volume and the
vasculature, rendering it unpredictable and threatening the success
of the reconstruction plan. Intraoperatively, the VL segment was
well perfused and deemed viable to be utilized as a pedicled flap.
The minor perforators were pulsating and adequately perfusing the
flap. A restrictive approach to elevate the minimal required length
of the muscle was considered to preserve deep proximal vascular
branches as much as possible. Following the inset of the flap over
the defect, adequate muscle perfusion was examined by color and
healthy bleeding, with no signs of venous congestion. If the VL
muscle did not appear well vascularized on exploration, the backup
options were either to perform a delayed extended groin flap or to
create an arteriovenous (AV) loop in preparation for free tissue
transfer.
The VL flap is conventionally classified as Mathes
and Nahai type I. However, Toia et al (12) have delineated three distinct
partitions within the VL muscle, each with its unique blood supply:
The superficial partition is supplied by the d-LCFA, the
intermediate partition by the transverse branch of the same artery
and the deep partition by perforating branches of the deep femoral
artery and the deep branch of the superior lateral genicular artery
(12). In the present case,
multiple perforating branches were observed from both the middle
and deep partitions that were adequate and sufficient to supply the
flap.
Reconstructive procedures, especially in such
situations, require careful planning. Larger defects also limit the
choices of the surgeons; either to utilize regional flaps or free
flaps. In one case dealing with a large trochanteric and gluteal
defect (25x15 cm) as a result of sarcoma resection, the ALT was
utilized as a pedicled local flap and a recipient as a flow through
donor vessel, via the d-LCFA, for a free flap from the
contralateral ALT (1). In
comparison, the present defect was much smaller and the neighboring
ALT along with its pedicle had already been harvested and
subsequently excised due to tumor recurrence; therefore, such an
option was not available. However, combining flaps is a useful
technique for providing coverage of large defects.
Latissimus dorsi (LD) myocutaneous flap is a viable
alternative in the management of recurrent soft tissue sarcoma
(1,2). The LD flap offers coverage for large
defects and aids in restoring function to the affected limb. It
serves as a workhorse free flap option, offering adequate volume to
eliminate dead space and provides healthy well-vascularized tissue
from areas unaffected by radiation. However, due to the complexity
of the present case, a staged procedure would have been necessary,
involving the formation of an AV-loop followed by free tissue
transfer. Therefore, the drawbacks of pursuing such an option
include the necessity for a staged procedure, the need for
microsurgical expertise, a remote donor site, repositioning during
surgery and potential morbidity associated with LD harvest
(16,17). Whereas a number of these drawbacks
are not encountered with a locoregional option such as the VL flap,
it has also been reported to have minimal donor site morbidity
(14).
The superior posterior femoral fasciocutaneous flap
has been described in the reconstruction of greater trochanter
defects post resection of a recurrent malignant fibrous
histiocytoma involving soft tissue of the hip (18). In that case, the tumor extended
into the lateral GM and the TFL muscle. To achieve a free margin
part of the VL and Sartorius were resected, resulting in a large
defect. Similar to the present case, this defect underwent
excessive dissection, alongside complete resection of the TFL.
The GM flap has been reported in reconstruction of
trochanteric pressure sores as an advancement flap, with the
advantage of providing adequate muscle bulk and neighboring the
defect, allowing for a smaller doner site that can be closed
primarily with minor tension when an oblique design is used
(19). However, GM is unfavorable
in mobile patients. Due to the major functional deficit at the
donor site, this flap is usually preserved for paraplegic subjects
(19). In the present case, the
patient was mobile; therefore, the GM flap was not a preferred
option.
The most common defects in the trochanteric region
are due to pressure sores. As in the case of the defect described
in the present report they are large and often extensive.
Classically the TFL flap has been utilized in reconstruction of
trochanteric pressure ulcers. However, larger and more complex
defects carry a high risk of complications, donor site morbidity
and flap failure. The VL is an alternative option with good
outcomes.
This case study is subject to inherent limitations,
notably its nature as a single-case study, which may not fully
capture the diversity of outcomes in similar clinical scenarios.
Additionally, the retrospective review of data encountered
challenges at the time of reporting due to missing information.
Despite these obstacles, the application of the VL flap in such a
complex case highlights its significant potential. Demonstrating
both robustness and adaptability, the VL flap proves to be a
reliable option, emphasizing its utility in the reconstruction of
trochanteric defects.
The VL is recognized for its versatility as a muscle
flap across a range of applications. Its reliability is evident
even in challenging situations in which the area has been subjected
to multiple surgical interventions, radiation and a prior ALT flap
harvest. With its rich vascularity and multifaceted utility, the VL
is an indispensable tool in the armamentarium of every
reconstructive surgeon.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
ZF was the main surgeon performing the
reconstructive procedure in this report, as well as the senior
author responsible for writing the paper and reviewing it for
oversights; ZF also provided a comprehensive revision of the
manuscript. AB contributed to the literature review, manuscript
development, format, review, drafting, data collection and design
of the manuscript. MA was a co-author who provided a comprehensive
revision of the manuscript structure, format and content. MA
participated in the design of the study, data collection and
interpretation and discussion. HA was a senior co-author who
provided the details of the case management course and helped in
manuscript development. ZF and MA confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1975, as revised in 2008. Informed written consent
was obtained from the patient included in the present study.
Patient consent for publication
Informed written consent for publication was
obtained from the patient included in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haque SA, Georgiou A and Woollard A:
Pedicled ipsilateral anterolateral thigh (ALT) with flow-through to
a secondary contralateral-free ALT flap for coverage of large
thigh, trochanteric and gluteal area defects: A case report.
Microsurgery. 41:276–279. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hohenberger P and Schwarzbach MH:
Management of locally recurrent soft tissue sarcoma after prior
surgery and radiation therapy. Recent Results Cancer Res.
179:271–283. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mulholland S, Boyd JB, McCabe S, Gullane
P, Rotstein L, Brown D and Yoo J: Recipient vessels in head and
neck microsurgery: Radiation effect and vessel access. Plast
Reconstr Surg. 92:628–632. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ballo MT, Zagars GK, Pollock RE, Benjamin
RS, Feig BW, Cormier JN, Hunt KK, Patel SR, Trent JC, Beddar S and
Pisters PW: Retroperitoneal soft tissue sarcoma: An analysis of
radiation and surgical treatment. Int J Radiat Oncol Biol Phys.
67:158–163. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Minami RT, Hentz VR and Vistnes LM: Use of
vastus lateralis muscle flap for repair of trochanteric pressure
sores. PIast Reconstr Surg. 60:364–368. 1977.PubMed/NCBI
|
|
6
|
Bovet JL, Nassif TM, Guimberteau JC and
Baudet J: The vastus lateralis musculocutaneous flap in the repair
of trochanteric pressure sores: Technique and indications. PIast
Reconstr Surg. 69:830–834. 1982.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mathes SJ and Nahai F: Classification of
the vascular anatomy of muscles: Experimental and clinical
correlation. Plast Reconstr Surg. 67:177–187. 1981.PubMed/NCBI
|
|
8
|
Wong CH and Wei FC: Anterolateral thigh
flap. Head Neck. 32:529–540. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wong CH, Ong YS and Wei FC: Revisiting
vascular supply of the rectus femoris and its relevance in the
harvest of the anterolateral thigh flap. Ann Plast Surg.
71:586–590. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei FC, Jain V, Celik N, Chen HC, Chuang
DC and Lin CH: Have we found an ideal soft-tissue flap? An
experience with 672 anterolateral thigh flaps. Plast Reconstr Surg.
109:2219–2226; discussion 2227-30. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mathes SJ and Nahai F: Reconstructive
surgery: Principles, Anatomy and Technique 2-Volume Set. Vol 2. 1st
edition. Churchill Livingstone, London. pp1233-1246, 1997.
|
|
12
|
Toia F, D'Arpa S, Brenner E, Melloni C,
Moschella F and Cordova A: Segmental anatomy of the vastus
lateralis: Guidelines for muscle-sparing flap harvest. Plast
Reconstr Surg. 135:185e–98e. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vanni S, De Vita A, Gurrieri L, Fausti V,
Miserocchi G, Spadazzi C, Liverani C, Cocchi C, Calabrese C,
Bongiovanni A, et al: Myxofibrosarcoma landscape: Diagnostic
pitfalls, clinical management and future perspectives. Ther Adv Med
Oncol. 14(17588359221093973)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rodaix C, Auregan JC, Lhuaire M, Feydy A,
Soubeyrand M and Biau D: The proximal vastus lateralis flap: An
anatomical and radiological study. Morphologie. 106:75–79.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stranix JT, Lee ZH, Lam G, Mirrer J, Rapp
T and Saadeh PB: Limb-sparing sarcoma reconstruction with
functional composite thigh flaps. Microsurgery. 38:466–472.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim J, Lee H, Pyon JK, Mun GH, Bang SI,
Jeon BJ and Lee KT: Association of unilateral latissimus dorsi
muscle harvest for breast reconstruction with postoperative spinal
posture. Plast Reconstr Surg. 150:644e–654e. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oberhofer HM, Samant SS, Swan CC, Wolfe
EM, Satteson ES, Leyngold MM and Chim H: Objective comparison of
donor-site morbidity following full and thoracodorsal
nerve-preserving split latissimus dorsi flaps. Plast Reconstr Surg.
149:966e–971e. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang R, Sun J, Wei X, Zhang H, Liu Y, Shi
M and Shi Y: Reconstruction of defects with the posterior femoral
fasciocutaneous flap after resection of malignant tumours of the
femoral greater trochanter, sacrococcygeal region and knee. J Plast
Reconstr Aesthet Surg. 62:221–229. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nisanci M, Sahin I, Eski M and Alhan D: A
new flap alternative for trochanteric pressure sore coverage:
Distal gluteus maximus musculocutaneous advancement flap. Ann Plast
Surg. 74:214–219. 2015.PubMed/NCBI View Article : Google Scholar
|