Introduction
Oral cancer is a potentially fatal disease with a
5-year survival rate of 49.4%, and its incidence continues to
increase by 2-3% per year (1,2).
Furthermore, ~90% of all cases of oral cancer are histologically
diagnosed as oral squamous cell carcinoma (OSCC) (3). The Global Cancer Observatory
predicted a 40% increase in the incidence of OSCC by 2040, leading
to a subsequent 0.6% rise in mortality rates (2,4). At
present, surgical resection combined with radiotherapy or
chemotherapy represents the primary therapeutic option for OSCC
treatment (5,6). Neoadjuvant chemotherapy containing
docetaxel, cisplatin and 5-fluorouracil (5-FU) remains the
prominent adjuvant therapy for advanced oral cancers (7). However, drug resistance presents a
formidable challenge when treating patients with OSCC (8); therefore, the development of novel
drugs to improve the treatment of OSCC is urgently warranted.
Strategies for targeting molecular pathways in oral
cancer cells have been intensively studied (9,10).
The overexpression of EGFR, PI3K/Akt and mTOR has been identified
in oral cancer tissues, and this increase in expression levels is
associated with poor prognosis and survival rates (11,12);
therefore, targeting these factors is a potentially promising
strategy for oral cancer therapy (13,14).
Alternatively, approaches to inhibiting VEGF signaling have been
developed (15). These target
therapies have provided encouraging results, increasing
progression-free and overall survival in clinical trials (10,15);
however, the heterogeneity of molecular pathways remains a
challenge for oral cancer treatment.
Harmine is a β-carboline alkaloid with a diverse
range of biological functions, including anti-inflammatory,
anti-diabetic and anti-microbial activities, and it is isolated
from the seeds of Peganum harmala L. (16,17).
Harmine has been shown to be a potential herbal medicine effective
against certain types of cancers, including bladder cancer, thyroid
cancer and neuroblastoma (18,19),
as harmine extracts have been reported to possess anticancer
activity through inducing apoptosis, autophagy, targeting abnormal
cell proliferation by regulating the cell cycle, angiogenesis and
metastasis (20,21). Harmine hydrochloride (HH) is a
harmine derivative that has improved stability compared with
harmine and it is more easily absorbed by tissues (22). HH has been shown to exhibit
anti-proliferative activity by inducing apoptosis, depleting the
pools of cancer stem cells and decreasing the rate of cell invasion
by interfering with signaling pathways in gastric cancer,
glioblastoma cells and hepatoma cell lines (17,22,23).
However, to the best of our knowledge, the anti-proliferative
activity of HH on OSCC cells has yet to be reported. The aim of the
present study was to analyze the antiproliferation activity of HH
and investigate the possible signaling pathways involved in
HH-mediated cytotoxicity in oral cancer cells.
Materials and methods
Cell lines and cell culture
The human OSCC cell lines SCC-4 and SCC-25 were
purchased from the Food Industry Research and Development
Institute, and subsequently cultured following the instructions
provided by the supplier. Specifically, SCC-4 cells were cultured
in DMEM:F12 medium (cat. no. 11320033; Thermo Fisher Scientific,
Inc.) containing 10% Gibco® FBS (cat. no. 104037028;
Thermo Fisher Scientific, Inc.), 2 mM L-glutamine and 1%
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; cat.
no. 15140122; Thermo Fisher Scientific, Inc.). SCC-25 cells were
cultured in DMEM:F12 medium containing 10% FBS, 1.5 g/l sodium
bicarbonate (cat. no. 25080094), 2.5 mM L-glutamine (cat. no.
25030081), 15 mM HEPES (cat. no. 15630-080) and 0.5 mM sodium
pyruvate (cat. no. 11360070; all from Thermo Fisher Scientific,
Inc.), with the additional provision of 400 ng/ml hydrocortisone
(cat. no. H0888; Millipore Sigma). All the other supplements for
cell cultures, with the exception of hydrocortisone, were obtained
from Thermo Fisher Scientific, Inc. Cells were cultured in a
humidified atmosphere containing 5% CO2 at 37˚C and
tested to ensure that they were free of mycoplasma
contamination.
Reagents and antibodies
HH (cat. no. SMB00461) was purchased from Millipore
Sigma. The protein extraction buffer, M-PER (cat. no. 78501; Thermo
Fisher Scientific, Inc.), was used to lyse and extract proteins for
subsequent western blotting analyses. The protease inhibitor
cocktail (cat. no. 5971, 0.1%). was purchased from Cell Signaling
Technology Inc. A 5 mM concentration of pan-caspase inhibitor
Z-VAD-FMK (cat. no. V116; Millipore Sigma) was used to verify the
apoptotic pathway. A 5 mM concentration of Rhodamine 123 (cat. no.
R8004; Millipore Sigma) was used to detect the mitochondrial
membrane potential (MMP). A 20 mM concentration of PD98059 (cat.
no. 513000; Millipore Sigma), 20 mM of SP600125 (cat. no. 4420119;
Millipore Sigma) and 10 mM of SB203580 (cat. no. 559389; Millipore
Sigma) were used to inhibit signaling pathways in OSCC cells. The
primary antibodies for the detection of apoptosis, namely the
anti-caspase-3 (cat. no. 9662, 1:1,000), anti-caspase-8 (cat. no.
9746, 1:1,000), anti-caspase-9 (cat. no. 9502, 1:1,000), anti-poly
(ADP-ribose) polymerase (anti-PARP) (cat. no. 9542, 1:1,000) and
Bcl-xL (cat. no. 2764, 1:1,000) antibodies, were sourced from Cell
Signaling Technology, Inc. The primary antibodies for the
verification of cell cycle arrest, namely anti-CDK6 (cat. no.
13331, 1:1,000), anti-cyclin E2 (cat. no. 4132, 1:1,000), anti-p21
(cat. no. 2947, 1:1,000) and anti-p27 (cat. no. 2552, 1:1,000)
antibodies, were purchased from Cell Signaling Technology, Inc. The
primary antibodies for the signaling pathways, including p-ERK
(cat. no. 4377, 1:1,000), ERK (cat. no. 4695, 1:1,000), p-AMPK
(cat. no. 2531, 1:1,000), AMPK (cat. no. 2532, 1:1,000), p-MAPK9
(SAPK)/JNK (cat. no. 9251, 1:1,000), SAPK/JNK (cat. no. 9252,
1:1,000), p-MAPK (cat. no. 9215, 1:1,000) and MAPK (cat. no. 9212,
1:1,000) antibodies, were purchased from Cell Signaling
Technologies, Inc. The expression level of GAPDH (cat. no. 2118,
1:5,000; Cell Signaling Technology, Inc.) was used as a loading
control for western blotting analysis.
Cell viability analysis
The inhibition of growth activity of HH on the OSCC
cells was evaluated using the Cell Counting Kit 8 (CCK-8) assay
(cat. no. 96992; Millipore Sigma). Briefly, different
concentrations of HH were applied to 1x104 cells seeded
in 96-well plates, followed by incubation for 24, 48 and 72 h at
37˚C in a humidified incubator. The CCK-8 reagent was added, and
the absorbance was subsequently read at 490 nm after 2 h. The
optical density (OD) of DMSO vehicle-treated cells (used as the
control) was considered to be 100%. The OD values of HH-treated
cells were divided by the value of control group, and the resulting
ratios were considered to represent the survival rate of cells
following HH treatment.
Clonogenic assay
The clonogenic assay measures the ability of a
single cell to form a colony, defined as ≥50 cells. The clonogenic
assays were performed to determine the long-term cytotoxicity of HH
on OSCC cells. Briefly, 3x103 cells were seeded in a
6-well plate. HH was added to the 6-well plate the next day. After
7 days of incubation, HH-treated or control OSCC cells were fixed
with 10% formaldehyde for 30 min at 25˚C and visualized using 0.05%
crystal violet (Millipore Sigma) staining for 15 min at 25˚C.
ImageJ software (version 1.43, National Institutes of Health) was
used to scan and analyze the colonies. The colony number of cells
treated with the DMSO vehicle (control) was considered to be 100%.
The colony numbers of HH-treated cells were divided by those of the
control group, and the resulting ratios were considered to
represent the rate of HH inhibition of oral cancer cell colony
formation.
Cell cycle analysis
To determine whether HH treatment could affect the
cell cycle profile of OSCC cells, 1x105 cells were
seeded in 6-well plates. Following 24 h of synchronization in a
serum-free DMEM:F12 medium, cells were changed to 10% FBS DMEM:F12
medium and treated with HH for 12, 24, 36 and 48 h in a humidified
atmosphere containing 5% CO2 at 37˚C. A 100% methanol
solution (cat. no. 34860; Millipore Sigma) was used to fix cells
for 24 h at 4˚C. The DNA content was assessed by incubating the
cells with 0.05 mg/ml propidium iodide (PI) (cat. no. 537060;
Millipore Sigma) and 2 mg/ml RNase (cat. no. 11119915001; Roche
Diagnostics) solution at 25˚C in the dark for 30 min, followed
subsequently by flow cytometric analysis using the BD FACSCanto™ II
(BD Biosciences) and the BD FACSDiva™ Software (version 6.0, BD
Biosciences). The cell cycle profile was assessed using Modfit LT
(version 3.3, Verity Software House) for cell cycle analysis. In
brief, after staining cellular DNA with PI and analyzing the data
using flow cytometric software, the results were further processed
using ModFit analysis. This analysis produced fluorescence
intensity graphs based on the DNA content, which allowed the
identification of cell proportions in the G0/G1, S and G2/M phases
of the cell cycle, thereby determining how HH affects the cell
cycle in oral cancer.
Flow cytometric analysis for the
detection of apoptosis
The OSCC cells were treated with HH for 24 or 48 h
in a humidified atmosphere containing 5% CO2 at 37˚C.
Apoptotic cells were subsequently stained with annexin V-FITC and
PI using an apoptosis detection kit (cat. no. K101-100; BioVision,
Inc.). During early apoptosis, the inner cell membrane flips
outwards, exposing phosphatidylserine, which is able to bind to
annexin V-FITC. PI is a DNA intercalating dye that is able to enter
the nucleus of cells with damaged membranes (22). The fluorescence intensities of
annexin V-FITC and PI were detected by flow cytometry using the BD
FACSCanto™ II (BD Biosciences) and BD FACSDiva™ software (version
6.0, BD Biosciences). The apoptotic cell ratio was calculated as
the sum of early apoptosis (FITC fluorescence only) and late
apoptosis (where both FITC and PI fluorescence were detected).
Flow cytometric analysis for the
detection of MMP
A total of 1x105 cells OSCC cells were
treated with HH for 24 or 48 h in 6-well plates in a humidified
atmosphere containing 5% CO2 at 37˚C. After treatment,
the cells were detached using 0.25% Trypsin-EDTA (cat. no.
25200056, Thermo Fisher Scientific, Inc.), washed with PBS and
stained with 5 µM Rhodamine 123 (Millipore Sigma) at 37˚C for 30
min. After staining, the cells were washed with PBS and analyzed by
flow cytometry using the BD FACSCanto™ II (BD Biosciences) and BD
FACSDiva™ software (version 6.0, BD Biosciences).
Cell lysate preparation and western
blotting
The proteins of HH-treated SCC-4 and SCC-25 cells
were lysed and extracted using M-PER mammalian protein extraction
containing a 0.1% protease inhibitor cocktail (cat. no. 7012; Cell
Signaling Technology, Inc.). The protein concentration was
determined using the Bio-Rad Protein Assay reagent (cat. no.
5000001; Bio-Rad Laboratories, Inc.). SDS-PAGE, using a 12.5% gel,
was performed to separate 40 µg of each sample, followed by
transfer on to a PVDF membrane (cat. no. 1620177; Bio-Rad
Laboratories, Inc.). The membrane was blocked in 5% bovine serum
albumin (cat. no. A2153, MilliporeSigma) at 25˚C for 1 h. The
membrane was probed with the primary specific antibodies in 5% BSA
(cat. no. A2153, MilliporeSigma) as indicated in the reagent and
antibodies section at 4˚C for 16 h. Subsequently, the membrane was
incubated with horseradish peroxidase-conjugated secondary
antibodies (anti-mouse, cat. no. AB_10015289; anti-rabbit, cat. no.
AB_2337936; both purchased from Jackson ImmunoResearch
Laboratories, Inc.) at 25˚C for 2 h. Protein expression was
detected using an enhanced chemiluminescence horseradish peroxidase
substrate detection kit (cat. no. WBKLS0500; Millipore Sigma).
Finally, quantification of protein expression was performed by
analyzing the densities of the gray bands using a UVP BioSpectrum
800 Imaging System.
Migration and invasion assay
For the migration assay, 2x105 OSCC cells
in 200 µl serum-free DMEM:F12 medium were seeded in the upper
chamber of 8 µm cell culture inserts (cat. no. PI8P01250; Millipore
Sigma), and 10, 15 or 20 µM of HH for SCC-4 cells and 5, 10 or 15
µM of HH for SCC-25 cells were added. For cell migration
experiments, DMEM:F12 medium containing 20% FBS was added to the
lower chamber, and the cells were incubated for 24 or 48 h in a
humidified atmosphere containing 5% CO2 at 37˚C.
Following incubation, non-migrated cells in the upper chamber were
carefully removed using a cotton swab. The migrated cells were
fixed with 4% paraformaldehyde for 10 min at 25˚C, washed with PBS,
subsequently stained with 0.2% crystal violet for 30 min at 25˚C.
The excess dye was washed off using PBS samples were imaged using
the IX83 inverted light microscope (Olympus Corporation). The fixed
migrated cells were then counted using ImageJ software. For the
invasion assay, the protocol followed was identical with that of
the migration assay, except that 100 µl Matrigel™ (Corning, Inc.)
diluted in serum-free DMEM:F12 medium at a concentration of 1:5 was
added to the upper chamber and incubated at 37˚C for 2 h.
Statistical analysis
The results were analyzed for statistical
significance using GraphPad Prism software (version 7.0;
Dotmatics). One-way ANOVA followed by Bonferroni's post hoc
multiple comparison test was used to analyze data. All in
vitro experiments were performed in triplicate and data are
shown as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
HH exhibits growth inhibitory activity
in human OSCC cells
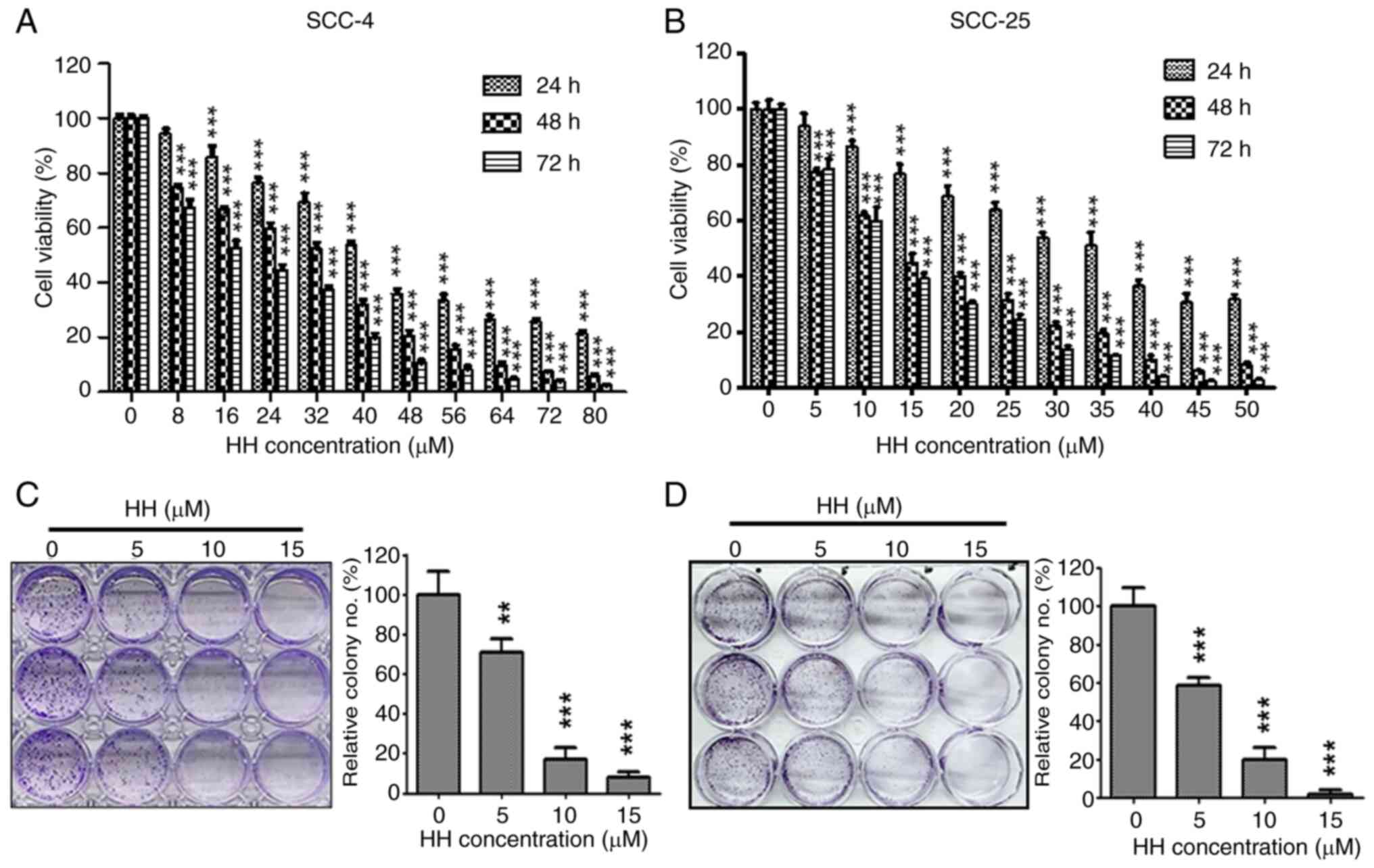
To assess whether HH exerts effective anti-growth
activity on human OSCC cells, SCC-4 and SCC-25 cells were treated
with HH, and the resulting cell survival rates were analyzed using
CCK-8 and clonogenic assays. HH treatment was found to exert a
cytotoxic effect on both SCC-4 and SCC-25 cells in a concentration-
and time-dependent manner (Fig. 1A
and B). The IC50 values
were determined to be 41.73, 32.92 and 18.7 µM in SCC-4 cells, and
35.41, 13.52 and 12.38 µM in SCC-25 cells, after 24, 48 and 72 h of
treatment with HH, respectively. A clonogenic assay was further
used to determine the long-term cytotoxicity of HH on OSCC cells.
The number of colonies was reduced in a concentration-dependent
manner following HH treatment. At a low concentration (5 µM), HH
treatment of SCC-4 and SCC-25 cells resulted in a statistically
significant reduction in colony formation ability. As the
concentration increased to 10 µM, the number of colonies of SCC-4
and SCC-25 cells decreased to <50% of the control group. At the
highest concentration (15 µM), colony formation was reduced to ~10%
of the control group (Fig. 1C and
D). Taken together, these data
suggested that HH treatment may exert an inhibitory effect on human
OSCC cell proliferation.
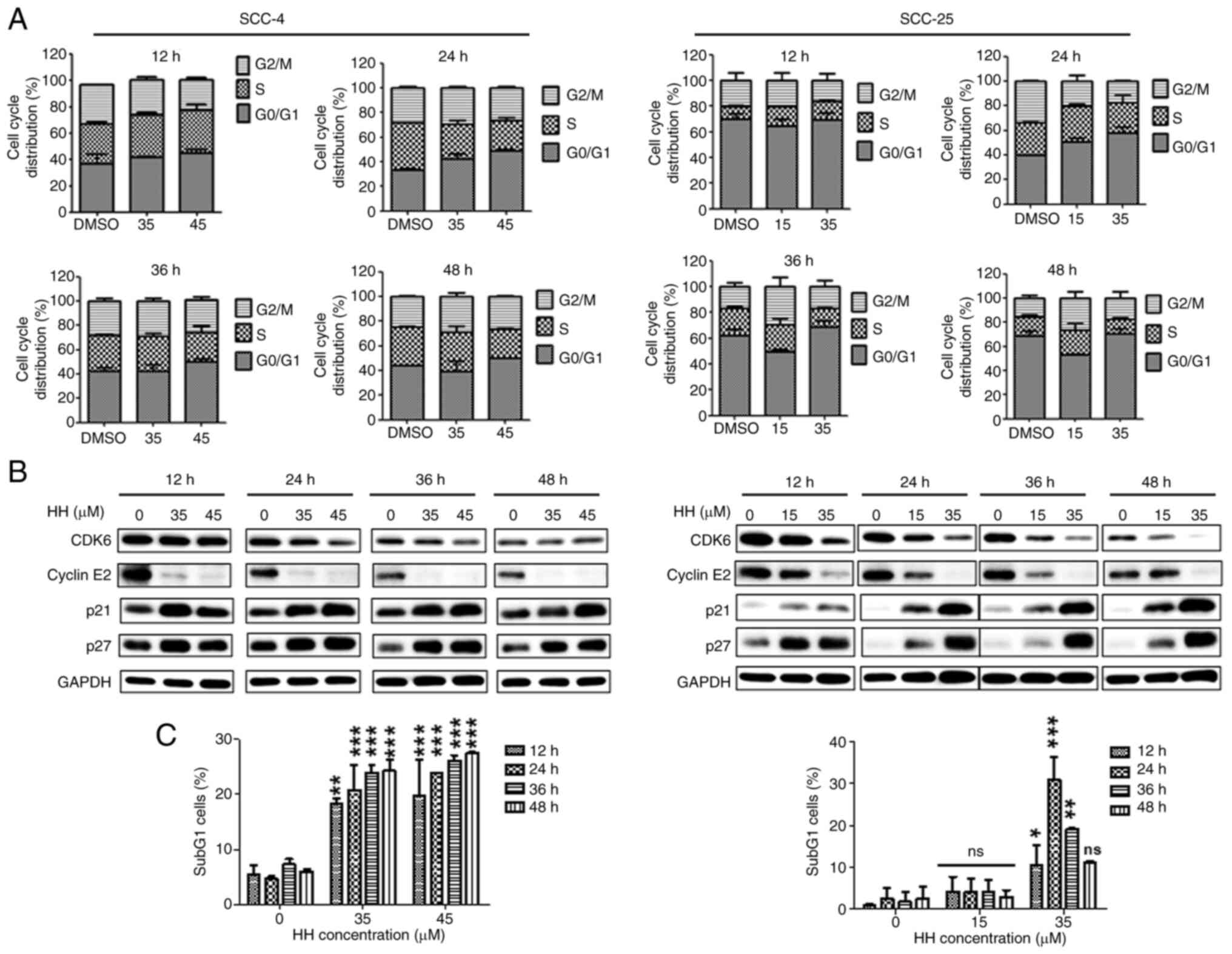
HH causes cell cycle arrest at the
G0/G1 phase
To analyze the underlying mechanisms via which HH
inhibits the growth of OSCC cells, flow cytometric analysis was
performed to determine the cell cycle distribution profiles
following HH treatment. After HH treatment of SCC-4 and SCC-25
cells for 12, 24, 36 and 48 h, the cell cycle was found to have
been arrested at the G0/G1 phase in a concentration-dependent
manner (Figs. 2A and S1). Furthermore, the expression levels
of cell cycle marker proteins were determined using western
blotting. CDK6 performs its role in conjunction with CDK4 to
facilitate cell cycle progression from the G1 phase to the S phase
(24,25). Cyclin E2 performs a supportive role
in a rate-limiting step for G1 progression (26). Furthermore, the proteins p21 and
p27 are known to bind to CDKs and to inhibit cell cycle progression
(27,28). The protein expression levels of
CDK6 and cyclin E2 were decreased upon treatment with HH (Fig. 2B). By contrast, the protein
expression levels of p21 and p27 increased. Taken together, the
results of the flow cytometry and western blotting experiments
suggested that HH treatment induced arrest of the SCC-4 and SCC-25
cells at the G0/G1 phase. Additionally, HH treatment increased the
ratio of sub-G1 phase cells (Fig.
S1). Furthermore, after treating SCC-4 cells with HH for 12,
24, 36 and 48 h, the number of subG1 cells significantly increased
in a concentration- and time-dependent manner. This effect was
particularly significant at the highest concentration of 45 µM. By
contrast, when SCC-25 cells were treated with 15 µM HH, the subG1
ratio showed a slight increase at 12, 24, 36 and 48 h; however,
this increase was not statistically significant. Treatment with 35
µM HH for 12 and 24 h resulted in a significant increase in the
subG1 cell ratio compared with the controls. However, as the
treatment duration increased, the subG1 cell ratio in SCC-25 cells
decreased, and by 48 h, the difference was no longer statistically
significant (Fig. 2C).
Collectively, these results suggested that HH treatment induces
G0/G1 cell cycle arrest and may subsequently trigger OSCC
apoptosis.
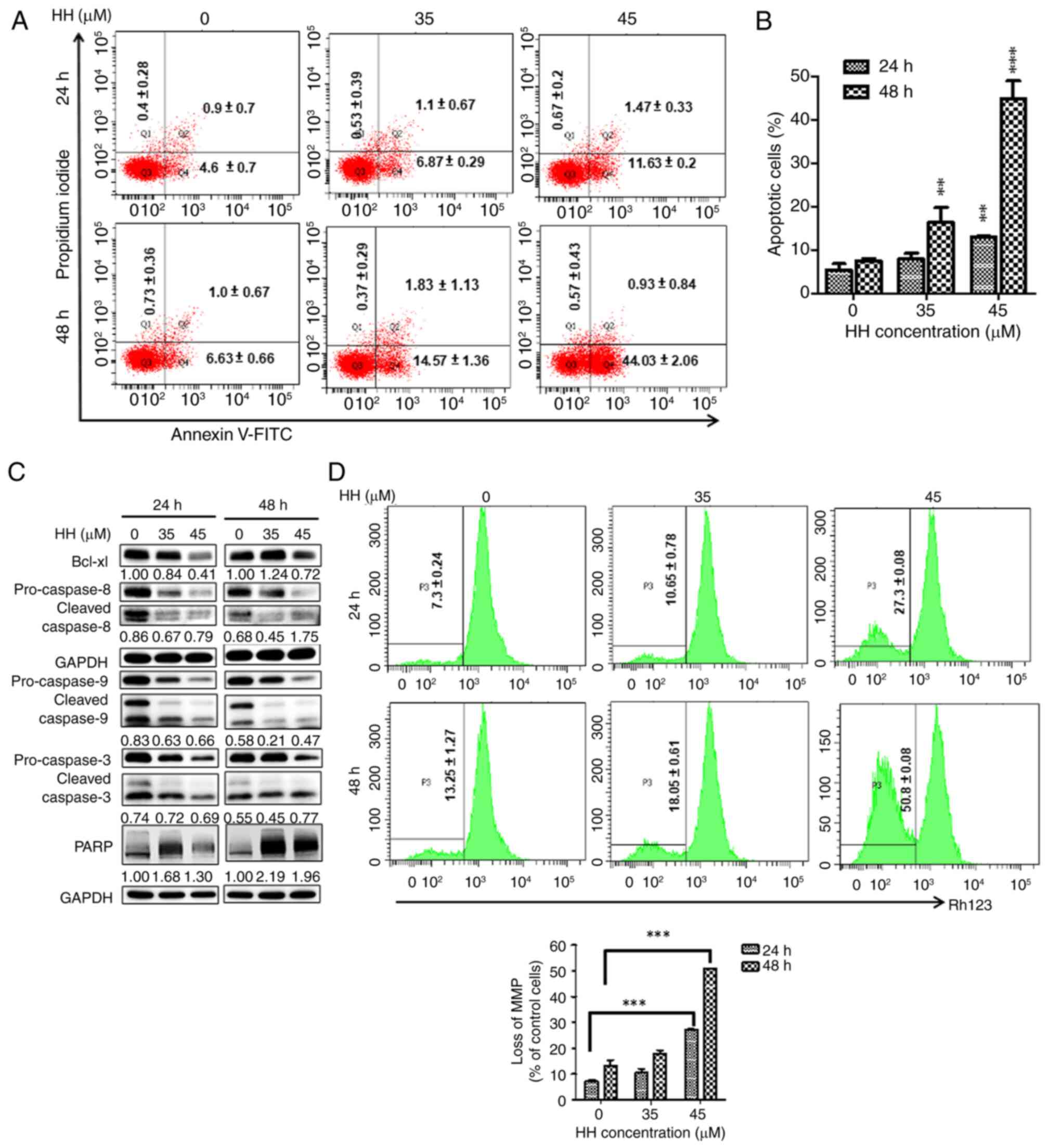
HH induces apoptosis in OSCC
cells
As aforementioned, HH treatment resulted in an
increase in the population of sub-G1 cells in OSCC cells. The
extent of apoptosis was further assessed in HH-treated OSCC cells
using flow cytometric analysis with annexin V/PI double staining.
Following HH treatment, annexin-V staining was significantly
increased at all HH concentrations tested in the SCC-4 cells
compared with the controls, suggesting the occurrence of apoptotic
cell death (Fig. 3A and B). The expression levels of
apoptosis-associated proteins were further determined using western
blotting. The level of cleaved PARP was increased following HH
treatment at 24 and 48 h (Fig.
3C). Moreover, increased cleaved/proform ratios of caspases-3,
-8 and -9 were observed at 48 h upon treatment of the SCC-4 cells
with a higher concentration of HH. By contrast, the expression
level of the anti-apoptotic factor, Bcl-xL, was found to decrease
(Fig. 3C). Taken together, these
data suggested that intrinsic and extrinsic caspases may be
involved in HH-induced apoptosis. Subsequently, changes in the MMP
following HH treatment in SCC-4 cells were examined to confirm the
involvement of the intrinsic caspase pathway. An observed increase
in rhodamine 123 staining was indicative of a loss of MMP, which
showed a significant increase with increased HH treatment
concentration and duration. At 24 h, the cells treated with 45 µM
demonstrated an ~4X increase in rhodamine 123 compared with the
control group, and this was significantly increased further at the
48 h, suggesting the involvement of the intrinsic caspase pathway
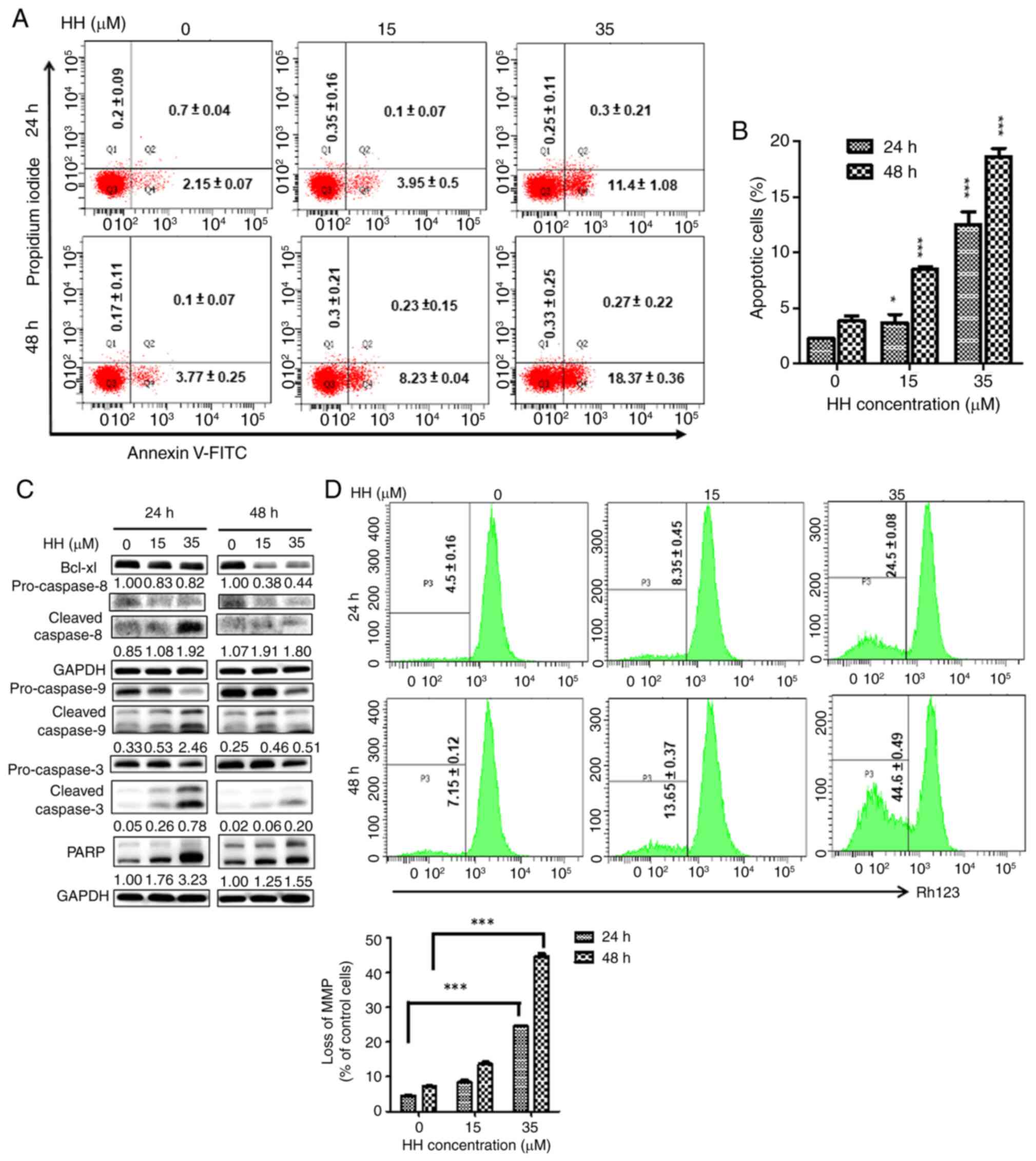
in HH-triggered apoptosis in SCC-4 cells (Fig. 3D). Similarly, HH treatment
increased the level of apoptosis in SCC-25 cells. Following HH
treatment, annexin-V staining was significantly increased at all HH
concentrations in SCC-25 cells. When cells were treated with a high
concentration of 35 µM HH, apoptosis was ~4X higher compared with
that of the control group, regardless of the treatment duration,
suggesting the occurrence of apoptotic cell death in HH-treated
SCC-25 cells (Fig. 4A and B). Subsequent western blotting analysis
further confirmed increases in the protein expression levels of
caspases-3, -8, -9 and cleaved PARP, with a concomitant decrease in
the protein expression level of the anti-apoptotic factor, Bcl-xL,
in HH-treated SCC-25 cells (Fig.
4C). Loss of MMP was further detected through the increase of
rhodamine 123 staining in SCC-25 cells, which intensified with
increased HH concentration and duration. At 24 h, 35 µM HH
treatment increased rhodamine 123 staining ~4X compared with the
control, which increased significantly at 48 h, suggesting
intrinsic caspase pathway involvement in HH-induced apoptosis in
SCC-25 cells (Fig. 4D). Taken
together, these results demonstrated that HH treatment induced
apoptosis in human SCC-4 and SCC-25 OSCC cells.
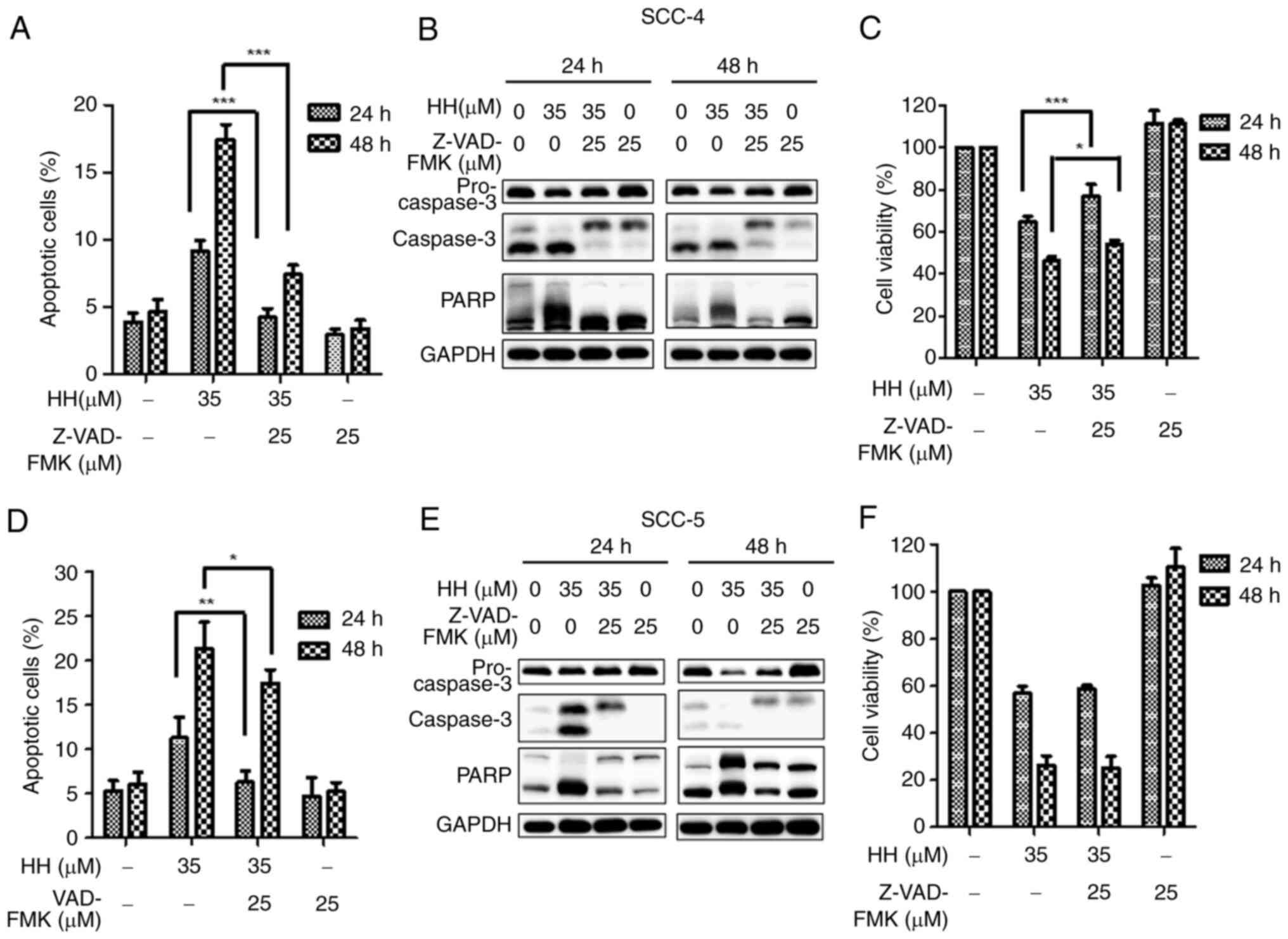
Caspases are involved in HH-induced
apoptotic cell death in OSCC cells
Experiments were subsequently devised to further
determine the involvement of caspases in HH-induced apoptosis in
OSCC cells. A pan-caspase inhibitor, Z-VAD-FMK, was applied to OSCC
cells prior to HH treatment. When SCC-4 cells were pretreated with
Z-VAD-FMK before HH treatment, HH-mediated apoptosis was
significantly attenuated in SCC-4 cells compared with cells that
were not pretreated. (Figs. 5A and
S2A). This result was further
confirmed through the identification of decreased levels of
apoptosis-associated proteins via western blotting, including the
cleaved forms of caspase-3 and PARP (Fig. 5B). Furthermore, comparing HH
treatment alone with Z-VAD-FMK pretreatment followed by HH
treatment of SCC-4 cells, it was demonstrated that pretreatment
with Z-VAD-FMK before HH treatment increased cell viability
compared with HH treatment alone (Fig.
5C). Taken together, these findings suggested that HH induced
caspase-dependent apoptotic cell death in SCC-4 cells. Caspase
activation is also an important factor in HH-mediated apoptosis in
SCC-25 cells. Although decreases in both the numbers of apoptotic
cells (Figs. 5D and S2B) and the protein expression levels of
cleaved caspase-3 and PARP in Z-VAD-FMK-pretreated SCC-25 cells
compared with the HH-treated SCC-25 cells (Fig. 5E) were observed, by contrast with
the results obtained in SCC-4 cells, an increase in cell viability
was not observed in Z-VAD-FMK pretreated SCC-25 cells compared with
the HH-treated SCC-25 cells (Fig.
5F). One possibility to account for this result is that SCC-25
cells may be sensitive to the combined treatment of Z-VAD-FMK and
HH. In addition, alternative pathways to apoptosis may contribute
to HH-induced cell death in SCC-25 cells.
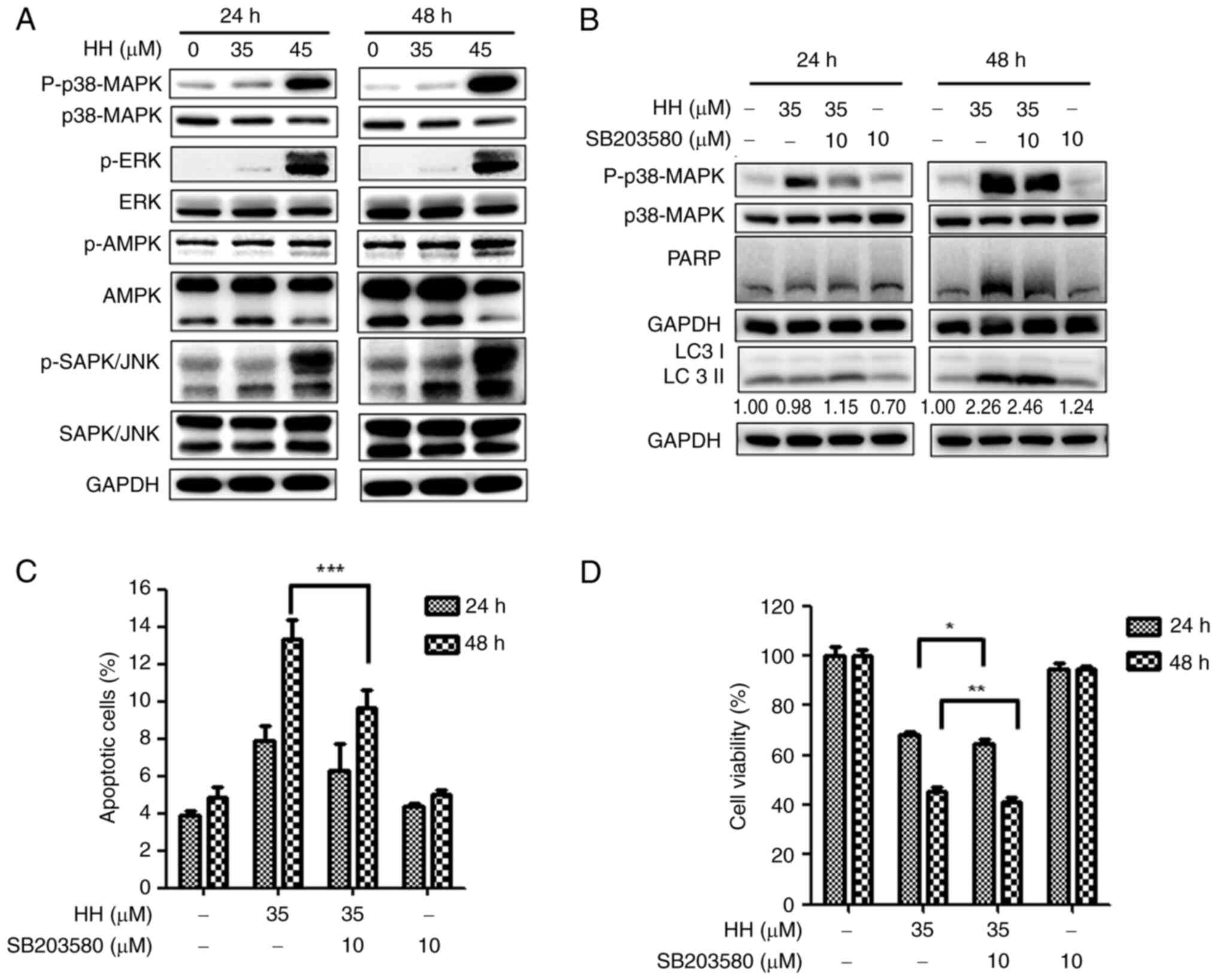
HH induces apoptosis in SCC-4 cells
through the MAPK pathway
Given that HH has previously been shown to modulate
the MAPK, ERK and JNK signaling pathways in breast cancer and
hepatocellular carcinoma cells (29,30),
western blotting analysis was performed to investigate which HH
target triggered SCC-4 apoptotic cell death. HH treatment was found
to activate each of the MAPK, ERK and JNK pathways, as evidenced by
the increased expression levels of the respective phosphorylated
proteins (Fig. 6A). Furthermore,
suppression of ERK and JNK signaling through treatment with the
inhibitors PD98059 and SP600125 respectively did not lead to any
decrease in the protein expression level of cleaved PARP (Fig. S3); only applying the MAPK pathway
inhibitor, SB203580, led to a decrease in the protein expression of
cleaved PARP and the apoptotic cell ratio following HH treatment
(Fig. 6B and C), suggesting that the MAPK pathway is
involved in HH-triggered apoptosis in SCC-4 cells. Taken together,
these results suggested that HH triggers SCC-4 cell apoptosis
through activating the MAPK pathway.
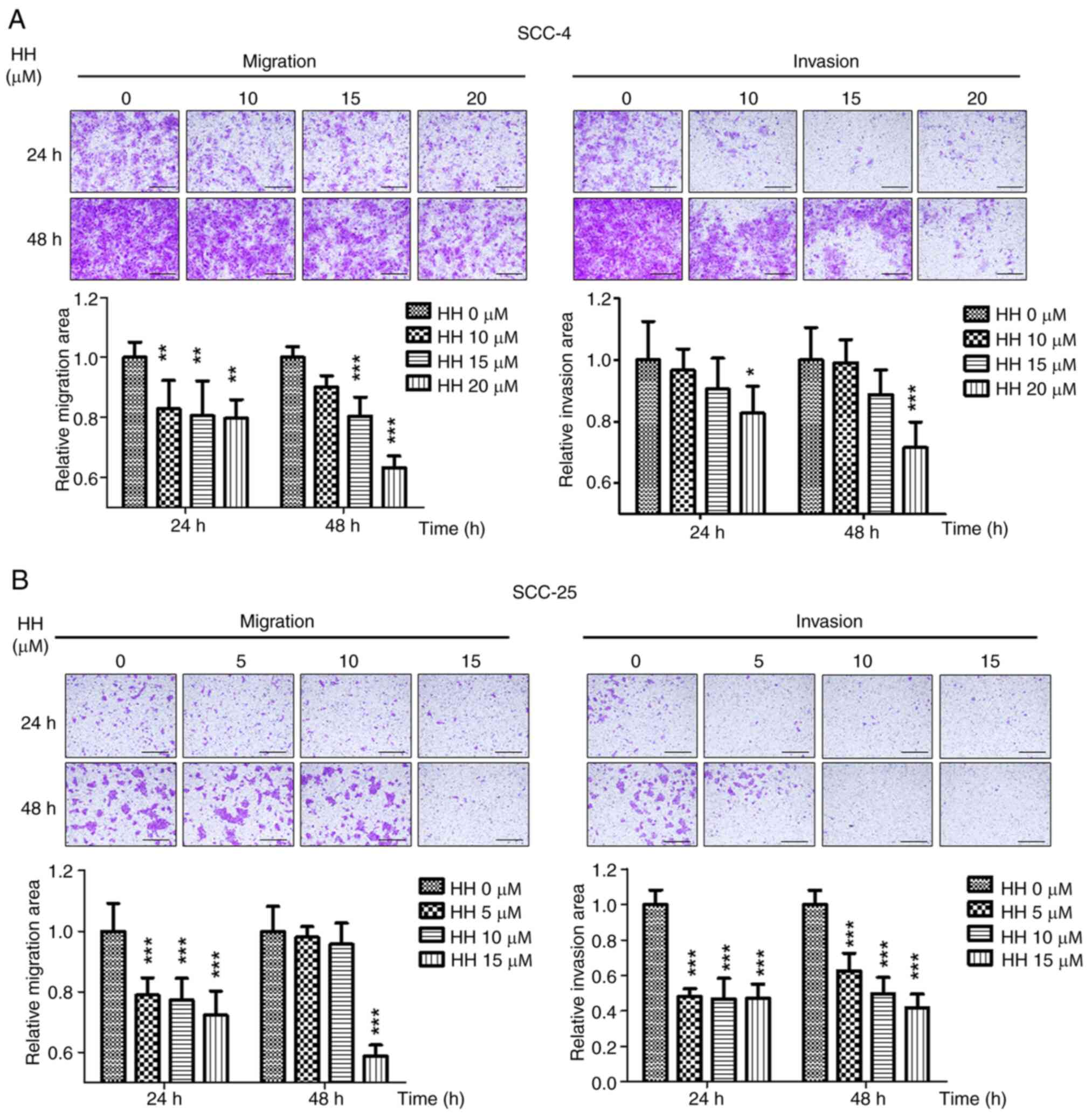
HH inhibits migration and invasion in
OSCC cells
Local invasion is one of the factors that
contributes to the poor prognosis of OSCC (31). To evaluate whether HH could inhibit
the invasion of OSCC cells, the migratory and invasive capabilities
of SCC-4 and SCC-25 cells were assessed following HH treatment.
When compared with the control group, HH treatment significantly
reduced SCC-4 cell migration in a dose-dependent manner at 24 and
48 h. However, when compared with the control group, only 20 µM HH
significantly inhibited invasion, while 10 and 15 µM showed no
statistically significant effect at 24 or 48 h after HH treatment
(Fig. 7A). A similar trend was
observed in the SCC-25 cells; compared with the control group, 5,
10 or 15 µM HH treatment significantly reduced migration by ~20% at
24 h, whereas only 15 µM HH treatment demonstrated a significant
effect at 48 h. Compared with the control group, invasion decreased
significantly by ~50% in cells treated with 5, 10 or 15 µM HH at
both 24 and 48 h. (Fig. 7B).
Therefore, the present study demonstrated that HH exhibited
cytotoxicity on oral cancer cells and that treatment with HH
triggered cell cycle arrest at the G0/G1 phase. It was also
demonstrated that activation of the MAPK signaling pathway was
involved in HH-induced caspase-dependent apoptosis in SCC-4
cells.
Discussion
Despite improvements in the treatment of oral
cancer, including surgical resection combined with radiotherapy or
chemotherapy, the morbidity and mortality rates of oral cancer
continue to increase worldwide. The annual incidence of this
condition has been documented as >377,000 cases and 0.6%
increase in mortality globally (1,2).
Developing drugs that are derived from natural herbs which may
provide reduced side effects is an attractive approach for cancer
therapy (32). The present study
demonstrated that HH, a derivative of the β-carboline alkaloid
harmine, exhibited effective anti-proliferative activity in two
OSCC cell lines via G0/G1 cell cycle arrest. Furthermore, the MAPK
pathway was demonstrated to be involved in HH-mediated apoptosis in
oral cancer cells.
Harmine possesses a diverse range of biological
activities; however, its low bioavailability and side effects, such
as severe weight loss and acute or delayed locomotor changes in
rats, restrict its applicability (16). Therefore, modifications have been
made to the β-carboline nucleus of harmine to synthesize
derivatives that are lacking these limitations (17). Among these derivatives, HH was
synthesized by combining harmine with hydrochloride to enhance its
water solubility and bioavailability, and its anti-proliferative
activity has been reported in gastric, hepatocarcinoma and colon
cancer cells (23,29,33).
In cancer cells, HH has been shown to cause cell cycle arrest in
the G2/M and the G0/G1 phases. The present study demonstrated that
HH caused G0/G1 arrest in both SCC-4 and SCC-25 oral cancer cells,
suggesting that HH may interfere with cell cycle progression at
different stages.
Apoptosis induces HH-mediated cell death in cancer
cells. Intrinsic or extrinsic pathways activate apoptosis, leading
to the activation of caspases (34). Loss of MMP leads to matrix
condensation, which subsequently facilitates the release of
cytochrome c, thereby activating caspases and cell apoptosis
(35). Bcl-xL, an anti-apoptotic
protein, inhibits apoptosis through impeding the transfer of
cytochrome c into the cytosol (36). When Bcl-xL is overexpressed, it has
a role in both drug resistance and relapse in ovarian cancer and
multiple melanoma (37),
suggesting that Bcl-xL may be a good target for cancer therapy. In
the present study, HH treatment led to decreased protein expression
levels of Bcl-xL in SCC-4 and SCC-25 cells. Moreover, flow
cytometric analysis results demonstrated a decrease in MMP and an
increase of the cleaved forms of caspases-3, -8 and -9. Similarly,
HH has been shown to antagonize the effects of Bcl-xL in
hepatocarcinoma and colon cancer cells (22,29,32),
suggesting that HH may be a potential anticancer drug for the
treatment of oral cancer.
Depending on the cell type and the underlying
conditions, such as cell culture passage number, the p38-MAPK
signaling pathways respond to stress and are involved in cell
survival or apoptosis (38). In
the present study, suppression of the MAPK pathways may reduce
HH-mediated apoptosis in SCC-4 cells, suggesting that HH-mediated
apoptosis occurs through the MAPK pathway in SCC-4 cells.
Similarly, HH activates the MAPK pathway to trigger apoptosis in
breast and hepatocarcinoma cancer cells (30,32).
The MAPK signaling pathway is activated in >50% of the recorded
cases of oral cancer (39).
Therefore, enhancing MAPK overexpression may be a viable option for
treating these types of cancers. However, in SCC-25 cells, the
present study did not demonstrate involvement of the MAPK pathway
in HH-mediated apoptotic cell death, suggesting that, for this cell
line, other signaling pathways may be involved. Further whole
genome sequencing may help to identify mechanisms that are
associated with HH-mediated cell death. The present study focused
solely on the cytotoxic effects of HH on OSCC cells, therefore,
performing in vivo animal experiments in the future should
provide deeper insights into the cytotoxic functions of HH against
OSCC. Additionally, synergistic cytotoxic effects between HH and
the currently used clinical drugs for OSCC, namely cisplatin and
5-FU, were not observed (data not shown).
In conclusion, the present study demonstrated that
HH exerted anti-proliferative activity in oral cancer cells through
inducing G0/G1 cell cycle arrest. Furthermore, the MAPK pathway was
involved in HH-induced apoptosis in the cell lines tested. These
results suggested that HH may have the potential for treating oral
cancer in the future.
Supplementary Material
HH induces G0/G1 cell cycle arrest in
oral cancer cells. (A) SCC-4 (left panel) and (B) SCC-25 (right
panel) cells were treated with HH for 12, 24, 36 or 48 h and the
cell cycle distribution was analyzed by flow cytometry. HH, harmine
hydrochloride.
HH-induced apoptosis in oral cancer
cells was caspase-dependent. The lower right quadrant represents
early apop-totic cells, indicated as
(FITC+/PI-); the upper right quadrant
represents late apoptotic cells, indicated as
(FITC+/PI+); the upper left quadrant
represents necrotic cells, indicated as
(FITC-/PI+); and the lower left quadrant
represents live cells, indicated as
(FITC-/PI-). The total number of apoptotic
cells is the sum of the cells in the lower right and upper right
quadrants. Cells were pretreated with Z-VAD-FMK before HH
treatment. The apoptotic cells were analyzed by flow cytometry in
(A) SCC-4 cells and (B) SCC-25 cells. HH, harmine
hydrochloride.
Signaling pathway inhibitors were
applied to HH-treated SCC-4 cells and protein expression levels
were analyzed by western blotting. (A) SCC-4 cells were treated
with (A) PD98059 or (B) SP600125 prior to HH treatment. PARP
protein expression was analyzed by western blotting. HH, harmine
hydrochloride; p, phosphorylated; PARP, poly (ADP-ribose)
polymerase. fect size; CI, confidence interval.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Ditmanson
Medical Foundation Chiayi Christian Hospital (grant no.
R110-047).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CYF and WTL conceived and designed the experiments.
YZL and HYH performed the experiments. PWZ and YNS analyzed the
data. CYF and WTL analyzed the data and wrote the manuscript. YZL
and CYF confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dong L, Xue L, Cheng W, Tang J, Ran J and
Li Y: Comprehensive survival analysis of oral squamous cell
carcinoma patients undergoing initial radical surgery. BMC Oral
Health. 24(919)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pekarek L, Garrido-Gil MJ, Sánchez-Cendra
A, Cassinello J, Pekarek T, Fraile-Martinez O, García-Montero C,
Lopez-Gonzalez L, Rios-Parra A, Álvarez-Mon M, et al: Emerging
histological and serological biomarkers in oral squamous cell
carcinoma: Applications in diagnosis, prognosis evaluation and
personalized therapeutics (Review). Oncol Rep.
50(213)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S,
Nice EC, Tang J and Huang C: Oral squamous cell carcinomas: State
of the field and emerging directions. Int J Oral Sci.
15(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng H, Sambrook PJ and Logan RM: The
treatment of oral cancer: An overview for dental professionals.
Aust Dent J. 56:244–252. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Eskander A, Dziegielewski PT, Patel MR,
Jethwa AR, Pai PS, Silver NL, Sajisevi M, Sanabria A and Doweck I:
Oral cavity cancer surgical and nodal management: A review from the
american head and neck society. JAMA Otolaryngol Head Neck Surg.
150:172–178. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fu JY, Yue XH, Dong MJ, Li J and Zhang CP:
Assessment of neoadjuvant chemotherapy with docetaxel, cisplatin,
and fluorouracil in patients with oral cavity cancer. Cancer Med.
12:2417–2426. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Atashi F, Vahed N, Emamverdizadeh P,
Fattahi S and Paya L: Drug resistance against 5-fluorouracil and
cisplatin in the treatment of head and neck squamous cell
carcinoma: A systematic review. J Dent Res Dent Clin Dent
Prospects. 15:219–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jiang Q, Xiao J, Hsieh YC, Kumar NL, Han
L, Zou Y and Li H: The role of the PI3K/Akt/mTOR axis in head and
neck squamous cell carcinoma. Biomedicines. 12(1610)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu L, Chen J, Cai X, Yao Z and Huang J:
Progress in targeted therapeutic drugs for oral squamous cell
carcinoma. Surg Oncol. 31:90–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu MJ, Johnson DE and Grandis JR:
EGFR-targeted therapies in the post-genomic era. Cancer Metastasis
Rev. 36:463–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ferreira DM, Neves TJ, Lima LGCA, Alves FA
and Begnami MD: Prognostic implications of the phosphatidylinositol
3-kinase/Akt signaling pathway in oral squamous cell carcinoma:
Overexpression of p-mTOR indicates an adverse prognosis. Applied
Cancer Research. 37(41)2017.
|
|
13
|
de Kort WWB, Spelier S, Devriese LA, van
Es RJJ and Willems SM: Predictive value of
EGFR-PI3K-AKT-mTOR-pathway inhibitor biomarkers for head and neck
squamous cell carcinoma: A systematic review. Mol Diagn Ther.
25:123–136. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Satgunaseelan L, Porazinski S, Strbenac D,
Istadi A, Willet C, Chew T, Sadsad R, Palme CE, Lee JH, Boyer M, et
al: Oral squamous cell carcinoma in young patients show higher
rates of EGFR amplification: Implications for novel personalized
therapy. Front Oncol. 11(750852)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Argiris A, Kotsakis AP, Hoang T, Worden
FP, Savvides P, Gibson MK, Gyanchandani R, Blumenschein GR Jr, Chen
HX, Grandis JR, et al: Cetuximab and bevacizumab: Preclinical data
and phase II trial in recurrent or metastatic squamous cell
carcinoma of the head and neck. Ann Oncol. 24:220–225.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen Q, Chao R, Chen H, Hou X, Yan H, Zhou
S, Peng W and Xu A: Antitumor and neurotoxic effects of novel
harmine derivatives and structure-activity relationship analysis.
Int J Cancer. 114:675–682. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang L, Li D and Yu S: Pharmacological
effects of harmine and its derivatives: A review. Arch Pharm Res.
43:1259–1275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jalali A, Dabaghian F and Zarshenas MM:
Alkaloids of peganum harmala: Anticancer biomarkers with promising
outcomes. Curr Pharm Des. 27:185–196. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu Y, Yu X, Yang L, Xue G, Wei Q, Han Z
and Chen H: Research progress on the antitumor effects of harmine.
Front Oncol. 14(1382142)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tarpley M, Oladapo HO, Strepay D, Caligan
TB, Chdid L, Shehata H, Roques JR, Thomas R, Laudeman CP, Onyenwoke
RU, et al: Identification of harmine and β-carboline analogs from a
high-throughput screen of an approved drug collection; profiling as
differential inhibitors of DYRK1A and monoamine oxidase A and for
in vitro and in vivo anti-cancer studies. Eur J Pharm Sci.
162(105821)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nafie E, Lolarga J, Lam B, Guo J,
Abdollahzadeh E, Rodriguez S, Glackin C and Liu J: Harmine inhibits
breast cancer cell migration and invasion by inducing the
degradation of Twist1. PLoS One. 16(e0247652)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang P, Huang CR, Wang W, Zhang XK, Chen
JJ, Wang JJ, Lin C and Jiang JW: Harmine hydrochloride triggers G2
phase arrest and apoptosis in MGC-803 cells and SMMC-7721 cells by
upregulating p21, activating caspase-8/Bid, and downregulating
ERK/Bad pathway. Phytother Res. 30:31–40. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Tan B, Li Y, Zhao Q, Fan L and Zhang M:
The impact of Harmine hydrochloride on growth, apoptosis and
migration, invasion of gastric cancer cells. Pathol Res Pract.
216(152995)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiong Y, Connolly T, Futcher B and Beach
D: Human D-type cyclin. Cell. 65:691–699. 1991.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goel S, Bergholz JS and Zhao JJ: Targeting
CDK4 and CDK6 in cancer. Nat Rev Cancer. 22:356–372.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gudas JM, Payton M, Thukral S, Chen E,
Bass M, Robinson MO and Coats S: Cyclin E2, a novel G1 cyclin that
binds Cdk2 and is aberrantly expressed in human cancers. Mol Cell
Biol. 19:612–622. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sherr CJ and Roberts JM: Inhibitors of
mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149–1163.
1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10(e19)2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim GD: Harmine hydrochloride triggers
G2/M cell cycle arrest and apoptosis in HCT116 cells through ERK
and PI3K/AKT/mTOR signaling pathways. Prev Nutr Food Sci.
26:445–452. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ock CW and Kim GD: Harmine hydrochloride
mediates the induction of G2/M Cell cycle arrest in breast cancer
cells by regulating the MAPKs and AKT/FOXO3a signaling pathways.
Molecules. 26(6714)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ling Z, Cheng B and Tao X:
Epithelial-to-mesenchymal transition in oral squamous cell
carcinoma: Challenges and opportunities. Int J Cancer.
148:1548–1561. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin SY, Wei WC, Jian FY and Yang NS:
Therapeutic applications of herbal medicines for cancer patients.
Evid Based Complement Alternat Med. 2013(302426)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim GD: Harmine hydrochloride induces G2/M
cell cycle arrest and apoptosis in SK-Hep1 hepatocellular carcinoma
cells by regulating mitogen-activated protein kinases and the
PI3K/AKT pathway. Prev Nutr Food Sci. 28:436–443. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lossi L: The concept of intrinsic versus
extrinsic apoptosis. Biochem J. 479:357–384. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome C release during apoptosis. Cell Death
Differ. 10:709–717. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kharbanda S, Pandey P, Schofield L,
Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, et
al: Role for Bcl-xL as an inhibitor of cytosolic cytochrome C
accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci U
S A. 94:6939–6942. 1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li M, Wang D, He J, Chen L and Li H:
Bcl-X(L): A multifunctional anti-apoptotic protein. Pharmacol Res.
151(104547)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cheng Y, Chen J, Shi Y, Fang X and Tang Z:
MAPK signaling pathway in oral squamous cell carcinoma: Biological
function and targeted therapy. Cancers (Basel).
14(4625)2022.PubMed/NCBI View Article : Google Scholar
|