Chronic subdural hematoma (CSDH) represents a
prevalent neurosurgical condition in the geriatric population, with
annual incidence rates ranging from 1.0 to 13.1 per 100,000
individuals (1), exhibiting a
predilection for patients over 65 years of age (2). Epidemiological studies document a
rising global incidence trend, attributed to expanding indications
for antithrombotic therapies and age-related traumatic mechanisms
(3). Burr hole drainage (BHD)
constitutes the standard neurosurgical intervention for
uncomplicated CSDH, while craniotomy with membranectomy is reserved
for cases with septated hematoma or recurrence after initial
drainage (4). CSDH is a benign
disease, but it frequently has an unfavorable prognosis due to its
high recurrence rate. In adults, glioma is the most common brain
malignancy, accounting for ~30% of all central nervous system
tumors (5,6). Gliomas represent the most prevalent
tumors of the central nervous system, constituting 80% of all
malignant primary brain neoplasms (7). Among these, glioblastoma multiforme
(GBM) is particularly notable, with a global incidence rate of 5 to
8 cases per 100,000 individuals (6). Despite significant advancements in
the development of novel anti-tumor therapeutics, GBM persists as
the most aggressive primary CNS malignancy, maintaining an
exceptionally dismal prognosis characterized by a median overall
survival duration of merely 12-15 months post-diagnosis (6,8).
Chronic subdural hematomas and cerebral neoplasms constitute major
intracranial pathologies in the geriatric population (6,9).
Subdural hematoma following brain tumor surgery is an infrequent
occurrence, where glioma-associated subdural hematoma has been
sporadically documented (10-12).
However, to the best of our knowledge, there is a paucity of
literature on gliomagenesis following subdural hematoma. Therefore,
in the present report, the case of a patient who developed a glioma
subsequent to a subdural hematoma is documented. The anatomic
colocalization and temporal proximity of these disease entities
raise the hypothesis of a potential etiopathogenic link between
chronic subdural hematomas and subsequent gliomagenesis.
A 62-year-old male with a history of lower extremity
deep vein thrombosis underwent long-term anticoagulation therapy
with oral rivaroxaban at a maintenance dosage of 10 mg once daily
over a 5-year period. However, the patient began experiencing
symptoms of headaches in April 2023, which led to the cessation of
rivaroxaban treatment. The patient presented to Beijing Tiantan
Hospital, Capital Medical University (Beijing, China), for
comprehensive diagnostic evaluation to assess disease progression
and therapeutic efficacy in April 2023. A CT scan of the head
revealed the presence of a chronic subdural hematoma on the left
side of the head. Conservative management with the oral medication
Lipitor (atorvastatin) 10 mg daily administered for one month was
implemented, but proved ineffective, as the patient's intermittent
headaches demonstrated progressive clinical deterioration. In June
2023, the patient exhibited gait instability and speech disfluency.
A re-examination of the head CT scan at the same hospital revealed
that the size of the left frontotemporal parietal subdural hematoma
had increased in size, with the maximum thickness demonstrating an
interval growth of 4-5 mm. Subsequently, a left parietal drilling
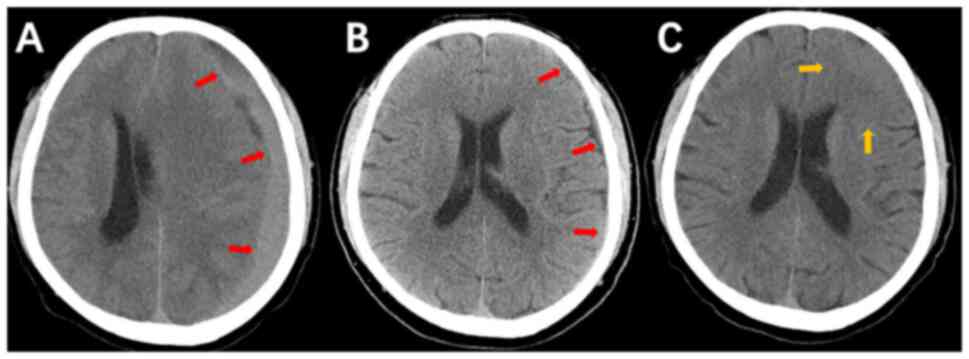
and drainage procedure was performed at the Beijing Tiantan
Hospital of Capital Medical University (Fig. 1A), postoperative imaging
demonstrated resolution of subcranial abnormal density shadows and
decreased midline deviation. A CT scan of the head conducted on day
4 following surgery revealed the presence of a left frontotemporal
parietal subdural hematoma, in addition to evidence of drilling and
drainage in the left parietal region. A cerebral herniation
phenomenon was also observed (Fig.
1B). At 4 days after surgery, a subdural hematoma remained. The
postoperative examination revealed no significant abnormality and
the incision had healed to the expected standard (grade A). The
sutures were removed at the conclusion of the healing process, and
the patient was discharged from the hospital for observation, and a
course of traditional Chinese medicine was initiated. The patient
commenced treatment with a Chinese medicine practitioner at the
same hospital from the day of discharge, who prescribed a course of
Chinese medicinal remedies to facilitate the reduction in size of
the subdural hematoma. The herbal remedies this patient was
administered included the following: 20 g Leonurus artemisia
Sweet, 9 g Semen persicae, 5 g Hirudo, 9 g Flos carthami and 20 g
Atractylodes macrocephala Koidz. The duration of this
prescription was 28 days, with a single dose administered in the
morning and evening. The traditional Chinese medicine therapeutic
drug combination utilized in the treatment of this patient is
protected by a patent (designated patent no. ZL 2022 1 1276741.3).
Following the completion of the initial prescription, the patient
continued pharmacological therapy with the following formulation
for 30 consecutive days via twice daily administration (morning and
evening doses): 30 g Leonurus artemisia Sweet, 9 g Semen
persicae, 5 g Hirudo, 9 g Flos carthami and 3 g Notoginseng
Radix Et Rhizoma. Following 58 days of traditional Chinese
medicine, a head CT scan at the same hospital revealed that the
subdural hematoma had diminished in size, suggesting it had
undergone slight absorption. Physical examination revealed no
neurological deficits, and the patient reported complete resolution
of cephalalgia symptoms.
In September 2023, the patient developed a
hypertensive crisis manifesting as severe blood pressure elevation
(systolic 208 mmHg/diastolic 116 mmHg). The CT scan revealed a
notable reduction in the chronic subdural hematoma in the left
frontotemporal parietal lobe, accompanied by a suspected slight
increase in the density of the left frontal gyrus (Fig. 1C). Further observation was
therefore conducted. In November 2023, the patient presented with
symptoms suggestive of the recurrence of the subdural hematoma,
including a 1-h headache. Consequently, the patient was promptly
transferred to the Emergency Department Beijing Tiantan Hospital
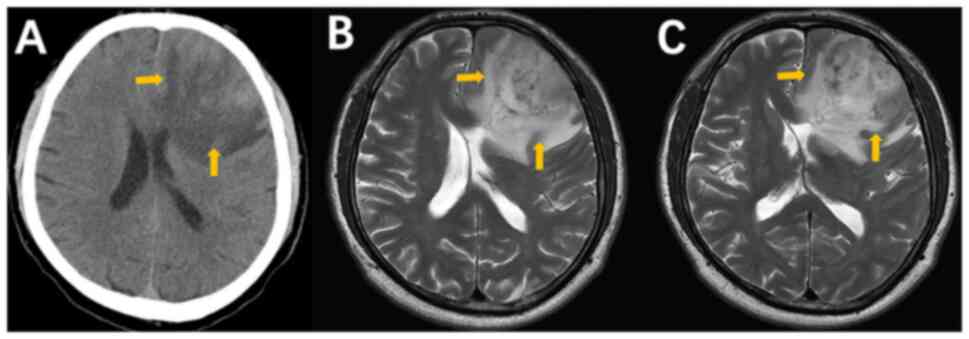
for a CT scan of the head. The scan revealed the presence of clumps
and mixed high-density shadows in the left frontal lobe,
accompanied by peripheral flaccid oedema, suspected hemorrhagic
lesions and cerebral herniation (Fig.
2A). The following day, the patient underwent a routine
examination and MRI of the head at the same hospital. The MRI
results revealed a high-grade glioma in the left frontal lobe
(Fig. 2B and C). Specifically, the patient exhibited
left frontal lobe occupancy for a brief period, accompanied by
compression and deformation of the ventricles, right deviation of
the midline and substantial enhancement of the enhancement scans,
manifesting as a wreath measuring ~50x55x48 mm. The necrotic areas
were characterized by a mixture of solid portions and uneven
signals. The presence of irregular thick ring-like enhancements
(Fig. 2B and C), indicative of a necrotic core
accompanied by a peripheral enhancement band, is a hallmark feature
of high-grade glioma (5). These
necrotic areas were intermingled with old hemorrhage, where the
absence of a discernible cystic wall structure following
enhancement was notable (Fig. 2B
and C). MRI revealed an abnormal
mass of signals in the left frontal lobe. The lesion exhibited
marked enhancement on the subsequent enhancement scan and was
surrounded by a substantial Fluid attenuated inversion recovery
(FLAIR) high-signal shadow. This abnormality involved the left
parietal lobe, basal ganglia and corpus callosum, accompanied by
local narrowing of the sulcus fissure, deformation of the bilateral
ventricles by compression and a rightward shift of the midline.
Necrotic areas were characterized by a mixture of solid portions
and uneven signals. The presence of irregular thick ring-like
enhancements, indicative of a necrotic core accompanied by a
peripheral enhancement band, is a hallmark feature of high-grade
glioma. These necrotic areas are often intermingled with old
hemorrhage and the absence of a discernible cystic wall structure
following enhancement is notable. Therefore, it was highly
suspected that the patient's disease was a high-grade glioma, due
to the severe headaches and the rapid growth of the tumor.
The neurosurgeons from Beijing Tiantan Hospital of
Capital Medical University then performed a left frontal hiatus
resection in November 2023 to remove the intracranial occupancy.
The resected tumor was then subjected to an immunohistochemistry
test for protein markers, yielding the following results: Glial
fibrillary acidic protein (GFAP) (+), Olig-2 (+), isocitrate
dehydrogenase (IDH)1 R132H (-), IDH2 R172K (-),
α-thalassemia/mental retardation X-linked (ATRX; +), p53(even +)
and Ki-67 (30%). The diagnosis was left frontal glioblastoma, not
otherwise specified, central nervous system and World Health
Organization (WHO) grade 4(5).
This diagnosis was based on a number of key molecular markers,
including IDH wild-type status, high proliferative index, p53
positivity and ATRX positivity. IDH mutations (such as IDH1 R132H
or IDH2 R172) are key molecular markers for differentiating the
subtypes of glioblastoma (5,13-15).
IDH-wild-type glioblastomas account for ~90% of all glioblastomas
and have a poor prognosis (13).
Ki-67 is a marker of cell proliferative activity. In high-grade
gliomas (WHO grade 4), Ki-67 positivity is frequently >10% and
increases with increasing malignancy (16). A positivity rate of 30% strongly
indicated the high proliferative activity of this tumor. p53, a
tumor suppressor protein encoded by TP53, exhibits low basal
expression and rapid degradation in normal cells, rendering it
undetectable by standard immunohistochemistry (IHC) (17,18).
In tumors, p53 positivity reflects aberrant stabilization of mutant
or dysregulated protein. p53 expression is indicative of a TP53
gene missense mutation and is commonly observed during the
progressive stages of glioblastoma (19). p53 positivity is associated with
tumor invasiveness, resistance to therapy and a poor prognosis,
where the rate of apparent p53 positivity is significantly higher
in high-grade gliomas (50-70%) compared with that in its low-grade
(approximately 10-30%) counterparts (13,20,21).
The aforementioned findings aligned with the WHO grade 4 criteria
for glioblastoma (5,22), thereby excluding low-grade gliomas.
Mutation in the ATRX gene is typically associated with IDH mutant
gliomas (such as in secondary glioblastoma). Conversely, ATRX(+) is
an indication of an IDH wild-type (22,23),
thereby supporting this diagnosis. Positivity for GFAP is a marker
of astrocyte differentiation, whilst Olig-2 positivity not only
suggested that the tumor may be characterized by mixed glial cell
differentiation, but also helped to exclude metastatic tumors or
neuronal malignancies (such as neuroblastoma).
In total, 7 days after brain surgery, the patient
demonstrated a positive recovery trajectory and met the discharge
criteria set by the hospital. In addition, the patient's condition
was stable during the post-operative recovery, with no
complications, such that the patient was largely able to care for
themself. Therefore, the hospital considered arranging for
discharge, in line with the wishes of the family of the patient.
Subsequently, the patient was transferred to the Beijing Haidian
Hospital (Beijing, China), a more convenient local hospital, for
the administration of temozolomide chemotherapy (75 mg/m² daily
during radiotherapy, followed by 150-200 mg/m² for 5 days/28-day
cycle), combined with standard radiotherapy delivered as 2 Gy per
fraction, five fractions weekly over six weeks (total dose 60 Gy in
30 fractions), aiming to reducing the risk of tumor recurrence.
At the subsequent follow-up visit 1 month after
surgery, it was ascertained through a video call that the patient
was capable of performing simple domestic tasks, such as sweeping
and mopping the floor, while undergoing radiotherapy and
chemotherapy. Thereafter, the patient was monitored on a monthly
basis by Beijing Tiantan Hospital of Capital Medical University
through telephone calls to ascertain their general condition. The
patient exhibited consistent cooperation throughout the follow-up
period, which continued until June 2024, when the patient condition
was deemed stable. Thereafter, follow-up visits were conducted
every two months. In September 2024, the patient began to
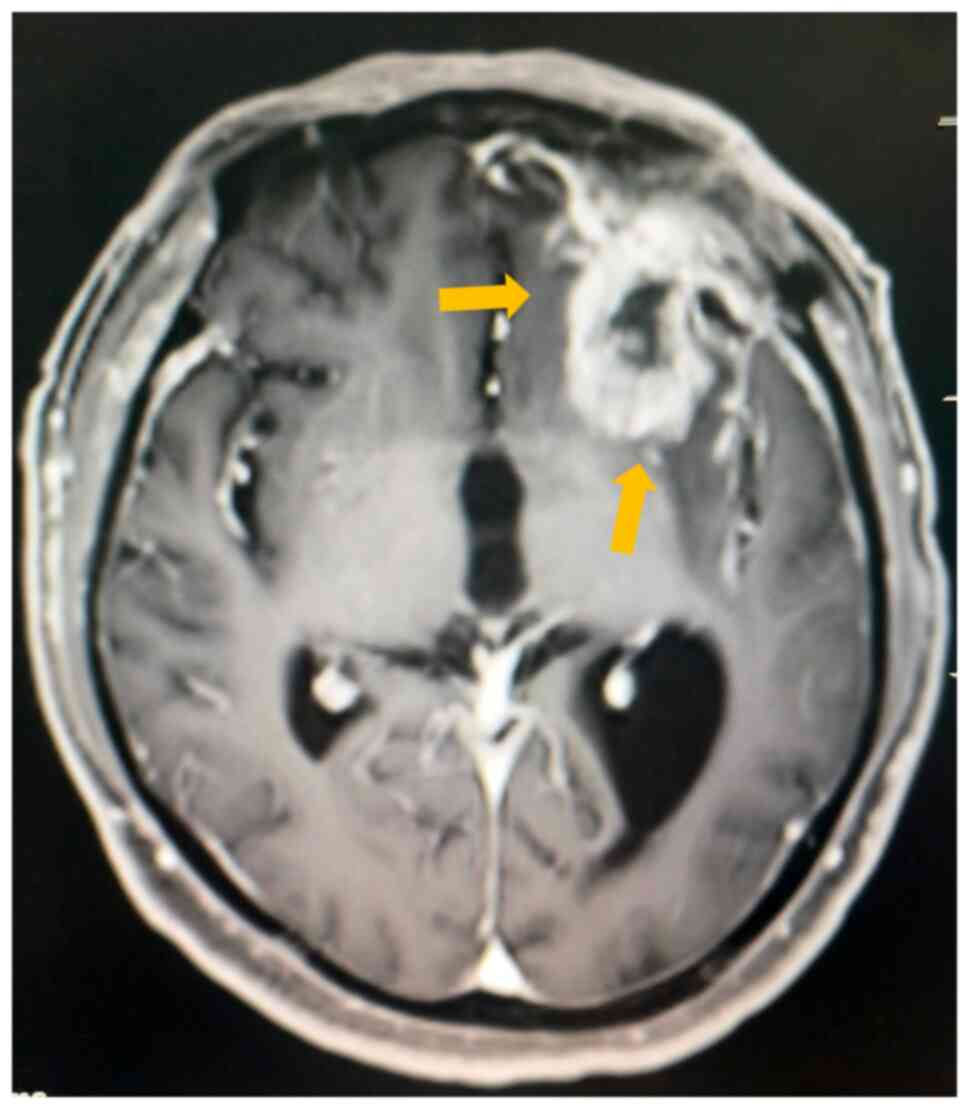
experience speech disorders. An MRI review was conducted at Beijing
Tiantan Hospital of Capital Medical University in September 2024,
which indicated the suspicion of tumor recurrence (Fig. 3). Postoperative MRI revealed a
well-demarcated surgical cavity in the left frontal lobe with
peripheral irregular annular enhancement involving the frontobasal
ganglia region. Adjacent dural linear enhancement was noted,
radiologically consistent with recurrent neoplasm. However,
neurosurgeons deemed the second operation to carry a high risk,
leading to the decision to continue with radiotherapy treatment.
The most recent follow-up in January 2025 revealed that the patient
had undergone treatment for cholecystitis at Peking University
International Hospital (Beijing, China) in December 2024.
At present, although the patient remains at high
risk of glioma recurrence, treatment is still being administered
for pre-squared glioma recurrence.
Gliomas account for the majority (80%) of all
primary malignant brain tumors. In particular, Glioblastoma
multiforme is a type of glioma with the highest degree of
malignancy and with a poor prognosis (6-8).
Despite the use of advanced antitumor therapies, including
anti-angiogenic agents (bevacizumab targeting VEGF-A), immune
checkpoint blockade (anti-PD-1 monoclonal antibodies),
tumor-treating fields and molecularly targeted therapies
(EGFRvIII-specific CAR-T cells) (24-27),
the median survival time of patients with glioblastoma is 14-15
months (28). By contrast, CSDH is
one of the most prevalent neurosurgical diseases necessitating
surgical treatment in the elderly, with an annual incidence
exceeding 100 per 100,000 individuals aged over 80 years (1,2,29),
where a range of complications, including headache, emesis and
epilepsy, can occur (8). To the
best of our knowledge, there have been no reports of gliomagenesis
following subdural hematoma (30).
In the present case, a patient who developed a glioma following the
development of subdural hematoma was documented.
The patient with a five-year history of rivaroxaban
therapy for venous thrombosis (known to increase hemorrhage risk
(31) demonstrated initial CT
findings of mixed-density lesions suggestive of acute hemorrhage.
These features were more likely be associated with nascent membrane
formation and increased exudation and hemorrhage caused by
long-term use of rivaroxaban (32-35).
The patient subsequently developed an ipsilateral glioma following
resolution of the subdural hematoma. There are several potential
explanations for this causal relationship. CSDH is defined as the
accumulation of hematoma fluid that is comprised of blood, body
fluids and blood products, situated between the dural and arachnoid
membranes (36). The accumulation
of inflammatory cells has been reported to serve a role in the
formation of the hematoma membrane and fluid (32,37).
Vascular endothelial growth factor (VEGF) is of interest due to its
elevated mRNA expression levels in CSDH hematoma fluid, membranes
and neutrophils according to a previous study (38). VEGF also serves a key role in
promoting angiogenesis. Hypoxia-inducible factor (HIF-α) represents
a product of the cellular response to a hypoxic environment
(39). The role of HIF-α and VEGF
in tumor invasion and metastasis has been demonstrated, where there
is a significant and positive association between VEGF expression
and tumor invasive capacity (40).
The formation of CSDH hematoma membranes and effusions is
accompanied by elevated levels of proinflammatory factors such as
TNF-α, IL-6, VEGF and MMP-9 (41-43),
and the aggregation of inflammatory cells (32,41).
Despite the absence of a direct experimental model that can
conclusively demonstrate a causal relationship between CSDH-induced
inflammation and glioma response, the existing literature provides
support for the notion that these inflammatory factors serve a role
in the glioma microenvironment. This role is considered to involve
the regulation of tumor cell behavior through the activation of
different signaling pathways such as IL-6/JAK/STAT3 Pathway,
TNF-α/NF-κB Pathway and TGF-β/Smad Pathway (32,44-46).
Further studies are required to elucidate the potential role of
aforementioned cytokines. Abnormal activation of astrocytes can be
another cause of this causal relationship, since astrocyte-specific
alterations can increase the risk of glioma (47,48).
The effects of local inflammation caused by CSDH can induce a
reactive phenotype in astrocytes, including a phenotype that
promotes glioblastoma, due to the release of IL-1β and TNF-α
(49,50). The presence of extravasated blood
leads to platelet activation, which results in the release of
platelet-derived growth factor (PDGF) (51). PDGF serves a multifaceted role in
tumor biology (52), as it can
regulate angiogenesis and development, whilst inducing the
proliferation and activation of astrocytes (47,51).
Conversely, pathological alterations resulting from CSDH, including
astrocyte swelling and a persistent increase in S-100β expression,
have been observed in an acute subdural hematoma rat model
(53,54). Additionally, S-100β and MMPs can
indirectly activate microglia and astrocytes. Previous experimental
studies have demonstrated that the S-100β protein can stimulate the
overexpression of primary cortical astrocytes in rats by activating
the NF-κB pathway (55-57)
Activation of NF-κB has been demonstrated to inhibit p53-mediated
apoptosis, primarily through the activation of NF-κB signaling by
binding to RAGE (Receptor for Advanced Glycation End Products)
(54,58,59).
This process is associated with the inhibition of cell
proliferation and the induction of apoptosis. Consequently, S-100β
may be involved in the proliferation of reactive astrocytes in a
synergistic manner. In the aftermath of parenchymal or structural
brain injury, such as traumatic brain injury (60), astrocytes undergo reactive
proliferation and migrate to the site of injury, thereby increasing
the risk of malignancy (61-65).
Neutrophils are another candidate for this potential causal
relationship. Neutrophils have been identified to be key players in
the development and recurrence of CSDH, as evidenced by numerous
studies (32,66-68).
It is therefore conceivable that the increased reactivity of
neutrophils during CSDH may be involved in the pathogenesis and
recurrence of gliomagenesis (69-71).
Neutrophils exert their inflammatory functions through a number of
mechanisms, including phagocytosis, degranulation and the release
of neutrophil extracellular traps (NETs) (72,73).
The oncogenic role of neutrophils and their NETs is manifested
primarily through the induction of DNA damage, angiogenesis and
immunosuppression, as evidenced by previous reports (74,75).
Further evidence from the present patient for testing this
hypothesis could not be obtained. This remains an important
hypothesis for future studies due to the association between the
aforementioned factors and gliomagenesis. Furthermore, the
immunohistochemistry results obtained from the tumor resection in
the present case indicated the presence of astrocyte activation,
along with potential activation of oligodendrocytes. Additionally,
30% of the tumor cells exhibited proliferative activity within the
patient's glioma tissues, suggesting that the tumor was
characterized by rapid growth and marked invasiveness. Since this
phenomenon was only identified subsequent to the removal of the
patient's glioma, it was not feasible to further refine the
immunohistochemical results to confirm the relationship between
CSDH and glioma.
To conclude, the pathological alterations resulting
from CSDH may be linked to the emergence of gliomas. Although
gliomagenesis following CSDH is a rare occurrence, it is
nevertheless a phenomenon that should not be overlooked. Therefore,
it is recommended that further mechanistic studies be conducted on
this topic.
Not applicable.
Funding: The present report was supported by the National
Natural Science Foundation of China (grant no. 82074350).
The data generated in the present study may be
requested from the corresponding author.
WW was responsible for study design, case data
collection, clinical analysis and writing of the first draft of the
paper. YPF took the lead in constructing the study framework,
analyzed data, and was responsible for multiple revisions of the
manuscript and final academic control. YPF and WW confirm the
authenticity of all the raw data. TY participated in the data
interpretation. LL created diagnostic imaging charts, participated
in case discussions and provided key clinical insights. XYL created
diagnostic imaging charts and analyzed the results of pathology
reports. All authors read and approved the final version of the
manuscript.
The present study was approved by the Ethics
Committee of Beijing Tiantan Hospital, Capital Medical University
(Beijing, China) (approval no: HX-A-2023054) .
Written informed consent was obtained from the
patient, who agreed to the publication of data and images.
The authors declare that they have no competing
interests.
|
1
|
Almenawer SA, Farrokhyar F, Hong C,
Alhazzani W, Manoranjan B, Yarascavitch B, Arjmand P, Baronia B,
Reddy K, Murty N and Singh S: Chronic subdural hematoma management:
A systematic review and meta-analysis of 34,829 patients. Ann Surg.
259:449–457. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bartek J Jr, Sjåvik K, Dhawan S, Sagberg
LM, Kristiansson H, Ståhl F, Förander P, Chen CC and Jakola AS:
Clinical course in chronic subdural hematoma patients aged 18-49
compared to patients 50 years and above: A multicenter study and
meta-analysis. Front Neurol. 10(311)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ducruet AF, Grobelny BT, Zacharia BE,
Hickman ZL, DeRosa PL, Andersen KN, Sussman E, Carpenter A and
Connolly ES Jr: The surgical management of chronic subdural
hematoma. Neurosurg Rev. 35:155–169. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Henry J, Amoo M, Kissner M, Deane T,
Zilani G, Crockett MT and Javadpour M: Management of chronic
subdural hematoma: A systematic review and component network
meta-analysis of 455 studies with 103 645 cases. Neurosurgery.
91:842–855. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2016-2020. Neuro Oncol. 25 (12
Suppl 2):iv1–iv99. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weller M, Wen PY, Chang SM, Dirven L, Lim
M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers.
10(33)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang W and Huang J: Chronic subdural
hematoma: Epidemiology and natural history. Neurosurg Clin N Am.
28:205–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kotwica Z and Zawirski M: Subdural
hematoma caused by cerebral tumors. Zentralbl Neurochir.
47:259–262. 1986.PubMed/NCBI
|
|
11
|
Coombs JB, Coombs BL and Chin EJ: Acute
spontaneous subdural hematoma in a middle-aged adult: Case report
and review of the literature. J Emerg Med. 47:e63–e68.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pavlov V, Bernard G and Chibbaro S:
Chronic subdural haematoma management: An iatrogenic complication.
Case report and literature review. BMJ Case Rep.
2012(bcr1220115397)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Picca A, Berzero G, Di Stefano AL and
Sanson M: The clinical use of IDH1 and IDH2 mutations in gliomas.
Expert Rev Mol Diagn. 18:1041–1051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ohgaki H and Kleihues P: The definition of
primary and secondary glioblastoma. Clin Cancer Res. 19:764–772.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Colman H, Zhang L, Sulman EP, McDonald JM,
Shooshtari NL, Rivera A, Popoff S, Nutt CL, Louis DN, Cairncross
JG, et al: A multigene predictor of outcome in glioblastoma. Neuro
Oncol. 12:49–57. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 World Health Organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2(a001008)2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Watanabe K, Tachibana O, Sata K, Yonekawa
Y, Kleihues P and Ohgaki H: Overexpression of the EGF receptor and
p53 mutations are mutually exclusive in the evolution of primary
and secondary glioblastomas. Brain Pathol. 6:217–223.
1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cancer Genome Atlas Research Network. Brat
DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay
E, Miller CR, Vitucci M, et al: Comprehensive, integrative genomic
analysis of diffuse lower-grade gliomas. N Engl J Med.
372:2481–2498. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wiestler B, Capper D, Holland-Letz T,
Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M and
Wick W: ATRX loss refines the classification of anaplastic gliomas
and identifies a subgroup of IDH mutant astrocytic tumors with
better prognosis. Acta Neuropathol. 126:443–451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dummer R, Lebbé C, Atkinson V, Mandalà M,
Nathan PD, Arance A, Richtig E, Yamazaki N, Robert C, Schadendorf
D, et al: Combined PD-1, BRAF and MEK inhibition in advanced
BRAF-mutant melanoma: Safety run-in and biomarker cohorts of
COMBI-i. Nat Med. 26:1557–1563. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miao H, Choi BD, Suryadevara CM,
Sanchez-Perez L, Yang S, De Leon G, Sayour EJ, McLendon R, Herndon
JE II, Healy P, et al: EGFRvIII-specific chimeric antigen receptor
T cells migrate to and kill tumor deposits infiltrating the brain
parenchyma in an invasive xenograft model of glioblastoma. PLoS
One. 9(e94281)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Azimi P, Yazdanian T and Ahmadiani A: mRNA
markers for survival prediction in glioblastoma multiforme
patients: A systematic review with bioinformatic analyses. BMC
Cancer. 24(612)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rauhala M, Helén P, Huhtala H, Heikkilä P,
Iverson GL, Niskakangas T, Öhman J and Luoto TM: Chronic subdural
hematoma-incidence, complications, and financial impact. Acta
Neurochir (Wien). 162:2033–2043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Iihara K, Saito N, Suzuki M, Date I, Fujii
Y, Houkin K, Inoue T, Iwama T, Kawamata T, Kim P, et al: The Japan
neurosurgical database: Statistics update 2018 and 2019. Neurol Med
Chir (Tokyo). 61:675–710. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen X, Huang W, Sun A, Wang L, Mo F and
Guo W: Bleeding risks with novel oral anticoagulants especially
rivaroxaban versus aspirin: A meta-analysis. Thromb J.
19(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Edlmann E, Giorgi-Coll S, Whitfield PC,
Carpenter K and Hutchinson PJ: Pathophysiology of chronic subdural
haematoma: Inflammation, angiogenesis and implications for
pharmacotherapy. J Neuroinflammation. 14(108)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiang R, Wang NP, Tanaka KA, Levy JH,
Guyton RA, Zhao ZQ and Vinten-Johansen J: Factor Xa induces tissue
factor expression in endothelial cells by P44/42 MAPK and
NF-κB-dependent pathways. J Surg Res. 169:319–327. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Entezami P, Boulos A, Paul A,
Nourollahzadeh E and Dalfino J: Contrast enhancement of chronic
subdural hematomas after embolization of the middle meningeal
artery. Interv Neuroradiol. 25:596–600. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nordström CH: Letter to the editor: Rapid
selective brain cooling with PolarCap(®)-A commercial delusion. J
Neurotrauma. 40:1257–1258. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lepić M and Sato H: Editorial: Chronic
subdural hematoma (CSDH)-a well-known unknown. Front Neurol.
15(1461117)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jensen T, Olsen MH, Christoffersen C,
Binderup T and Fugleholm K: The cellular composition of chronic
subdural hematoma. Acta Neurochir (Wien). 166(208)2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Isaji T, Osuka K, Ohmichi Y, Ohmichi M,
Naito M, Nakano T, Iwami K and Miyachi S: Expression of
angiopoietins and angiogenic signaling pathway molecules in chronic
subdural hematomas. J Neurotrauma. 37:2493–2498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Patel SA, Nilsson MB, Le X, Cascone T,
Jain RK and Heymach JV: Molecular mechanisms and future
implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res.
29:30–39. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shen J, Zhang Y and Wu X: Rapamycin
promotes hematoma resorption and enhances endothelial cell function
by suppressing the mTOR/STAT3 signaling in chronic subdural
hematoma. Exp Cell Res. 433(113829)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Suzuki M, Endo S, Inada K, Kudo A,
Kitakami A, Kuroda K and Ogawa A: Inflammatory cytokines locally
elevated in chronic subdural haematoma. Acta Neurochir (Wien).
140:51–55. 1998.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Su GJ, Gao J, Wu CW, Zou JF, Zhang D, Zhu
DL, Liu J, Zhang JH and Huang XJ: Serum levels of MMP-8 and MMP-9
as markers in chronic subdural hematoma. J Clin Med.
11(902)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Galvão RP and Zong H: Inflammation and
gliomagenesis: Bi-Directional communication at early and late
stages of tumor progression. Curr Pathobiol Rep. 1:19–28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bhat K, Balasubramaniyan V, Vaillant B,
Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L,
James JD, Goodman LD, et al: Mesenchymal differentiation mediated
by NF-κB promotes radiation resistance in glioblastoma. Cancer
Cell. 24:331–346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Katz AM, Amankulor NM, Pitter K, Helmy K,
Squatrito M and Holland EC: Astrocyte-specific expression patterns
associated with the PDGF-induced glioma microenvironment. PLoS One.
7(e32453)2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mega A, Nilsen MH, Leiss LW, Tobin NP,
Miletic H, Sleire L, Strell C, Nelander S, Krona C, Hägerstrand D,
et al: Astrocytes enhance glioblastoma growth. Glia. 68:316–327.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pripp AH and Stanišić M: The correlation
between pro- and anti-inflammatory cytokines in chronic subdural
hematoma patients assessed with factor analysis. PLoS One.
9(e90149)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hyvärinen T, Hagman S, Ristola M, Sukki L,
Veijula K, Kreutzer J, Kallio P and Narkilahti S: Co-stimulation
with IL-1β and TNF-α induces an inflammatory reactive astrocyte
phenotype with neurosupportive characteristics in a human
pluripotent stem cell model system. Sci Rep.
9(16944)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Raica M and Cimpean AM: Platelet-Derived
growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for
antitumor and antiangiogenic therapy. Pharmaceuticals (Basel).
3:572–599. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chaudhary PK and Kim S and Kim S: An
insight into recent advances on platelet function in health and
disease. Int J Mol Sci. 23(6022)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xu X, Wang D, Han Z, Wang B, Gao W, Fan Y,
Li F, Zhou Z, Gao C, Xiong J, et al: A novel rat model of chronic
subdural hematoma: Induction of inflammation and angiogenesis in
the subdural space mimicking human-like features of progressively
expanding hematoma. Brain Res Bull. 172:108–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wajima D, Nakagawa I, Nakase H and
Yonezawa T: Neuroprotective effect of suppression of astrocytic
activation by arundic acid on brain injuries in rats with acute
subdural hematomas. Brain Res. 1519:127–135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Asano T, Mori T, Shimoda T, Shinagawa R,
Satoh S, Yada N, Katsumata S, Matsuda S, Kagamiishi Y and Tateishi
N: Arundic acid (ONO-2506) ameliorates delayed ischemic brain
damage by preventing astrocytic overproduction of S100B. Curr Drug
Targets CNS Neurol Disord. 4:127–142. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Patabendige A, Singh A, Jenkins S, Sen J
and Chen R: Astrocyte activation in neurovascular damage and repair
following ischaemic stroke. Int J Mol Sci. 22(4280)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bianchi R, Giambanco I and Donato R:
S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1
Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha.
Neurobiol Aging. 31:665–677. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kumar M, Meode M, Blough M, Cairncross G
and Bose P: PDGF gene expression and p53 alterations contribute to
the biology of diffuse astrocytic gliomas. NPJ Genom Med.
8(6)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shaw SS, Schmidt AM, Banes AK, Wang X,
Stern DM and Marrero MB: S100B-RAGE-mediated augmentation of
angiotensin II-induced activation of JAK2 in vascular smooth muscle
cells is dependent on PLD2. Diabetes. 52:2381–2388. 2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tyagi V, Theobald J, Barger J, Bustoros M,
Bayin NS, Modrek AS, Kader M, Anderer EG, Donahue B, Fatterpekar G
and Placantonakis DG: Traumatic brain injury and subsequent
glioblastoma development: Review of the literature and case
reports. Surg Neurol Int. 7(78)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sirko S, Schichor C, Della Vecchia P,
Metzger F, Sonsalla G, Simon T, Bürkle M, Kalpazidou S, Ninkovic J,
Masserdotti G, et al: Injury-specific factors in the cerebrospinal
fluid regulate astrocyte plasticity in the human brain. Nat Med.
29:3149–3161. 2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Michinaga S and Koyama Y:
Pathophysiological responses and roles of astrocytes in traumatic
brain injury. Int J Mol Sci. 22(6418)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Munch TN, Gørtz S, Wohlfahrt J and Melbye
M: The long-term risk of malignant astrocytic tumors after
structural brain injury-a nationwide cohort study. Neuro Oncol.
17:718–724. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Laird MD, Vender JR and Dhandapani KM:
Opposing roles for reactive astrocytes following traumatic brain
injury. Neurosignals. 16:154–164. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Holl DC, Volovici V, Dirven C, Peul WC,
van Kooten F, Jellema K, van der Gaag NA, Miah IP, Kho KH, den
Hertog HM, et al: Pathophysiology and nonsurgical treatment of
chronic subdural hematoma: From past to present to future. World
Neurosurg. 116:402–411.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kim KH and Lee Y: Medical management of
chronic subdural hematoma. Korean J Neurotrauma. 19:288–297.
2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Georgountzos G, Gkalonakis I,
Anastasopoulos L, Stranjalis G and Kalamatianos T: Biofluid
biomarkers in the prognosis of chronic subdural hematoma: A
systematic scoping review. Diagnostics (Basel).
13(2449)2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nat Cell Biol. 2:737–744.
2000.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Su GJ, Zhang D, Wu JN, Deng YH, Wu CW,
Zhang XJ and Huang XJ: Immunoexpression of MMP-8 and MMP-9 in
chronic subdural hematoma. Front Neurol. 13(988854)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Saleem M, Kweon MH, Johnson JJ, Adhami VM,
Elcheva I, Khan N, Hafeez BB, Bhat KM, Sarfaraz S, Reagan-Shaw S,
et al: S100A4 accelerates tumorigenesis and invasion of human
prostate cancer through the transcriptional regulation of matrix
metalloproteinase 9. Proc Natl Acad Sci USA. 103:14825–14830.
2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zha C, Meng X, Li L, Mi S, Qian D, Li Z,
Wu P, Hu S, Zhao S, Cai J and Liu Y: Neutrophil extracellular traps
mediate the crosstalk between glioma progression and the tumor
microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med.
17:154–168. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wang M, Lv X, Wang Y, Li Y, Li H, Shen Z
and Zhao L: Biomarkers of peripheral blood neutrophil extracellular
traps in the diagnosis and progression of malignant tumors. Cancer
Med. 13(e6935)2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang S, Guo M, Liu Q, Liu J and Cui Y:
Neutrophil extracellular traps induce a hypercoagulable state in
glioma. Immun Inflamm Dis. 9:1383–1393. 2021.PubMed/NCBI View Article : Google Scholar
|