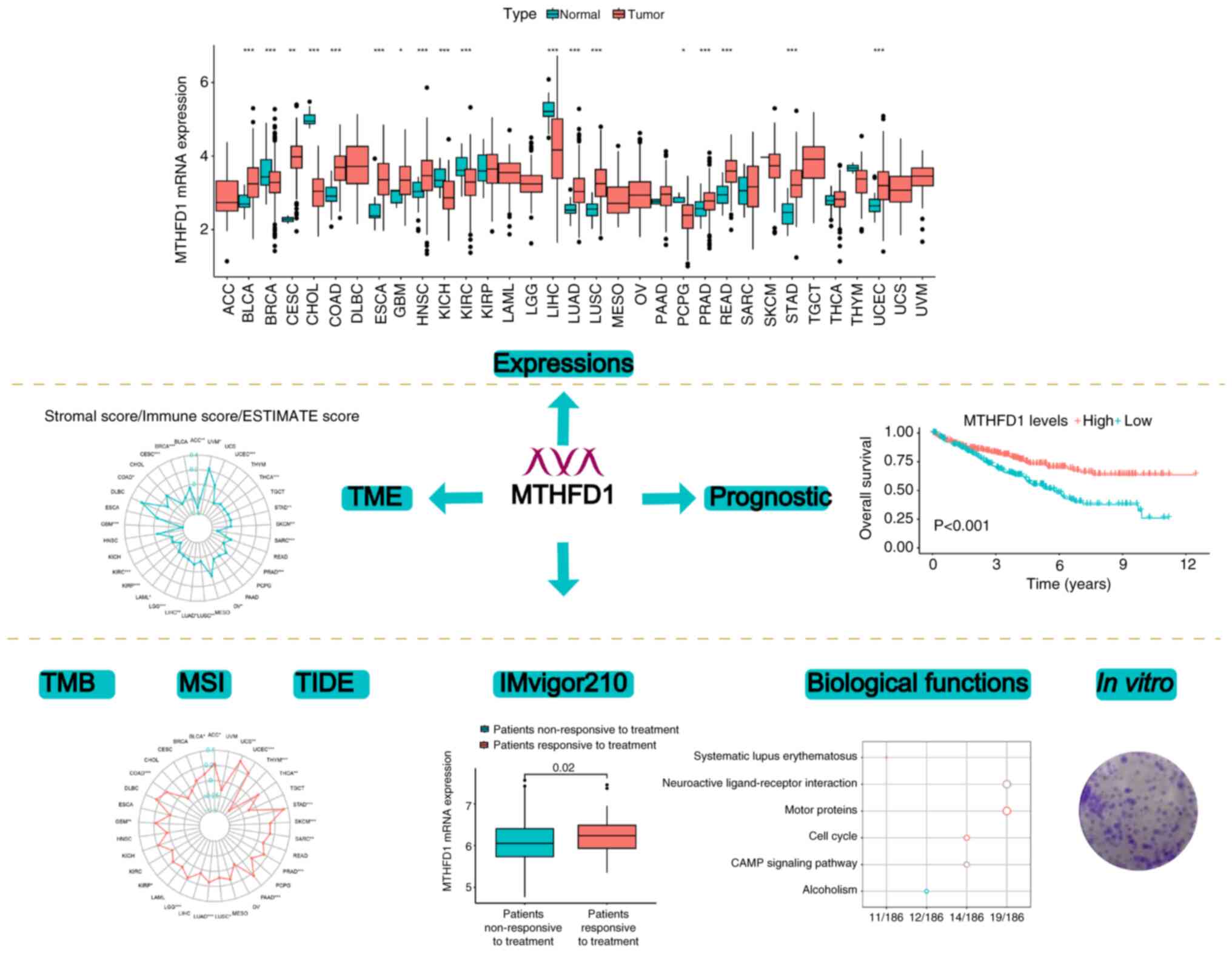
Introduction
By 2040, the global number of patients to be newly
diagnosed with cancer is estimated to be 28.4 million, representing
a 47% increase from 2020(1). This
substantial rise in incidence is a notable threat to human health
and survival, which is an economic burden on society. Despite
advances in personalized and precision cancer therapies, several
challenges remain. The lack of sensitive early diagnostic
biomarkers results in ~52.8% of patients being diagnosed already
with advanced stage cancer on first presentation (2). Additionally, a proportion of patients
will experience recurrence and metastasis even after comprehensive
treatment, leading to 5-year survival rates of <50% for several
types of cancer (2). For instance,
in head and neck squamous cell carcinoma (HNSC), ~50.6% of
recurrences occur within 6 months post-treatment, with 88.6%
occurring within 2 years (3).
Similarly, patients with colorectal cancer face a 5-year recurrence
rate of ~26.9%, with survival rates dropping significantly in
advanced stages (4). These issues
underscore the importance of identifying early diagnostic
biomarkers whilst also elucidating the molecular and cellular
mechanisms underlying tumor progression.
During cancer development, the reprogramming of
amino acid metabolism is an important process (5). Numerous studies have demonstrated the
role of amino acid metabolic reprogramming in the malignant
progression of cancer cells (5-7).
Upregulation of the cysteine/glutamate transporter 2 (ASCT2) has
been reported to promote glutamine uptake in colon cancer and lung
adenocarcinoma cells, thereby creating a cycle of abnormal
proliferation (8). In HNSC and
triple-negative basal-like breast cancer xenograft models, knocking
down ASCT2 was found to reduce the activity of mTOR complex 1 to
inhibit tumor growth (9-11).
Additionally, in colorectal cancer cells, activating mutations in
the KRAS gene, particularly the common G12D and G13D variants,
reprogram cellular metabolism by enhancing glutamine uptake
(12). This occurs through
upregulation of glutamine transporters and increased
glutaminolysis, providing intermediates for the tricarboxylic acid
cycle to support tumor growth (13). Additionally, the L-type amino acid
transporter 1 (LAT1), encoded by SLC7A5, is often overexpressed in
colorectal cancer cells (13).
LAT1 facilitates the uptake of essential amino acids such as
leucine, which activates the mTOR signaling pathway, thereby
promoting protein synthesis and cell proliferation (14). Blocking the cystine antiporter
solute carrier family 7 member 11/glutathione axis selectively can
also inhibits the proliferation of KRAS-mutant non-small cell lung
cancer (15,16). These aforementioned findings
suggest that targeting amino acid metabolism may be a potentially
viable therapeutic option, but currently there is a lack of
research in this area.
Methylenetetrahydrofolate dehydrogenase 1 (MTHFD1)
is an important regulatory factor in the progression of various
types of cancer, including gallbladder, pancreatic, metastatic
colorectal and non-small cell lung cancers (17-20).
Over the past decade, its association with amino acid metabolism
has been garnering attention in the field of cancer research. In
pancreatic cancer, MTHFD1 was found to be activated by
decrotonylation at the Lys354 and Lys553 sites, which enhances
resistance to ferroptosis and promotes cancer development (21). MTHFD1 mainly participate in
one-carbon metabolism, regulating the metabolism of several amino
acids, particularly serine, glycine, histidine, methionine,
tryptophan and lysine (22-25).
By modulating the metabolism of these amino acids, MTHFD1 can in
turn regulate nucleic acid synthesis, methylation reactions,
protein modification and cell proliferation (26). Since these four pathways are
important for the proliferation of tumor cells (27-30),
MTHFD1 may yet serve a key role in cancer progression through its
regulation of amino acid metabolism. Therefore, MTHFD1 may have
value as a therapeutic target against cancer. However, to the best
of our knowledge, the specific roles of MTHFD1 in various types of
cancer are yet to be elucidated, highlighting a gap in the latest
research field.
Therefore, the present study aimed to fill this gap
using the Cancer Cell Line Encyclopedia (CCLE) database, the
Clinical Proteomic Tumor Analysis Consortium (CPTAC), The Cancer
Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx)
project to investigate the expression patterns, prognostic value
and correlations of MTHFD1 with immune cell infiltration in several
types of cancer. The predictive performance of MTHFD1 expression
levels on immunotherapy outcomes was also investigated.
Additionally, in vitro cell experiments were used to
investigate the role of MTHFD1 in lung adenocarcinoma (LUAD) and
clear cell renal cell carcinoma (KIRC) and to further examine its
molecular mechanisms in cancer progression.
Materials and methods
Data retrieval
mRNA expression matrices, clinical information,
survival data and tumor mutational burden (TMB) for 33 types of
tumor were obtained from TCGA (https://www.cancer.gov/tcga) and the GTEx (https://gtexportal.org/home/) databases. The TCGA data
were accessed using the UCSC Xena platform (https://xenabrowser.net/) by searching for ‘TCGA
Pan-Cancer’ datasets. RNA-seq data (HTSeq-FPKM format) and
corresponding clinical information were downloaded. Samples lacking
survival information were excluded. The GTEx data were obtained
from the GTEx Portal (v8 release), selecting normal tissues
anatomically corresponding to the tumor tissues under study.
RNA-seq transcripts per million data were used. The ‘IMvigor210’
dataset, consisting of RNA-sequencing data and clinical information
from patients with metastatic urothelial carcinoma treated with
atezolizumab, was accessed from the website (http://research-pub.Gene.com/imvigor210corebiologies).
Additionally, MTHFD1 mRNA expression data in tumor cells was
obtained from the CCLE (https://sites.broadinstitute.org/ccle) database, using
the keyword ‘MTHFD1’. MTHFD1 protein expression levels in tumor and
normal tissues across 10 types of cancer were further validated
using the ‘CPTAC analysis’ module of the University of Alabama at
Birmingham Cancer Data Analysis Portal (http://ualcan.path.uab.edu/analysis-prot.html).
Fig. 1 presents a flowchart of the
present study.
Immunological analysis
The ‘estimation of stromal and immune cells in
malignant tumor tissues using expression data’ (ESTIMATE) R package
(version 1.0.13) (31) was used to
compute the tumor microenvironment (TME) status, including the
stromal score, immune score and ESTIMATE score, across 33 types of
cancer using RNA-seq data obtained from TCGA. Additionally, the
cell-type identification by estimating relative subsets of RNA
transcripts (CIBERSORT) algorithm (32) was used to calculate the abundance
of the different immune cell infiltrates. Microsatellite
instability (MSI) and tumor immune dysfunction and exclusion (TIDE)
scores were determined using the TIDE website (http://tide.dfci.harvard.edu/), with all analyses
performed using the default parameters. Radar plots depicting
associations with MTHFD1 expression were generated using the ‘fmsb’
package (version 0.7.6) (https://CRAN.R-project.org/package=fmsb).
Biological function analysis
The activity of MTHFD1 was assessed using the single
sample gene set enrichment analysis (ssGSEA) algorithm implemented
through the ‘GSVA’ (version 1.48.0) (33) and ‘GSEABase’ (version 1.62.0)
packages (https://bioconductor.org/packages/GSEABase). To
explore the biological pathways potentially regulated by MTHFD1,
differentially expressed genes (DEGs) between the high and low
MTHFD1 expression groups were identified using the ‘limma’ package
(version 3.56.1) (34), with an
absolute log2 fold-change >0.585 and a false discovery rate
<0.05 as the cutoff thresholds. Subsequently, Gene Ontology (GO)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment
analyses were performed using the ‘clusterProfiler’ package
(version 4.4.1) (35) to elucidate
the functional roles of these DEGs. In these analyses, terms and
pathways were considered significantly enriched if the adjusted
P-value was <0.05, indicating that the enrichment was
statistically significant after correcting for multiple
testing.
Cell culture and transfection
The LUAD cell line A549 and the KIRC cell line 786-O
were purchased from the American Type Culture Collection. Cells
were cultured in RPMI-1640 medium (MilliporeSigma) supplemented
with 10% FBS (Thermo Fisher Scientific, Inc.) and 1% penicillin and
streptomycin. The cells were maintained at 37˚C with 5%
CO2 in a humidified incubator. During culture, the
medium was refreshed every 2-3 days or as needed to ensure optimal
growth conditions. Cells were passaged upon reaching ~80%
confluence. Following the manufacturer's protocol, cells were
transfected with a negative control (NC)-overexpression (OE) vector
[empty pLV-CMV-MCS-PGK-Puro; OBiO Technology (Shanghai) Corp.,
Ltd.] or an OE-MTHFD1 [lentiviral vector backbone:
pLV-CMV-MCS-PGK-Puro; OBiO Technology (Shanghai) Corp., Ltd.]. For
gene knockdown experiments, cells were transfected with NC-small
interfering (si)RNA or si-MTHFD1 [both OBiO Technology (Shanghai)
Corp., Ltd.]. Lipo8000™ (Beyotime Institute of
Biotechnology) was used for both plasmids (including lentiviral
vectors) and siRNAs. For lentiviral vector transfection during
packaging, 2 µg of plasmid DNA was used per well. For siRNA
transfection, 50 nM siRNA was used per well. Lentiviral infection
was performed by incubating cells with viral particles in the
presence of Polybrene (5-8 µg/ml). Transfected or infected cells
were incubated at 37˚C with 5% CO2 for 48 h. After
confirming transfection efficiency by western blot analysis, cells
were used for subsequent experiments, typically at 24-48 h
post-transfection, depending on the specific requirements of the
experiments. The specific sequences of the constructs are shown in
Table SI.
Western blotting
Proteins from cells were extracted using RIPA buffer
(Cell Signaling Technology, Inc.). Equal amounts of protein were
loaded, separated using SDS-PAGE and then transferred onto PVDF
membranes. The protein concentration was determined using the
bicinchoninic acid assay. The mass of protein loaded per lane was
20 µg. The SDS-PAGE gel used was a 10% polyacrylamide gel. The
membranes were blocked with 5% non-fat milk in TBST (containing
0.1% Tween-20) for 1.5 h at room temperature. Subsequently, the
membranes were incubated overnight at 4˚C with primary antibodies
against MTHFD1 (1:2,000; cat. no. ab226341; Abcam) or GAPDH
(1:10,000; cat. no. ab263962; Abcam) following the manufacturer's
protocol. After washing with TBST (containing 0.1% Tween-20), the
membranes were incubated with a secondary Goat Anti-Rabbit IgG
H&L (HRP) antibody (1:4,000; cat. no. ab6721; Abcam) for 2 h at
room temperature. Following additional washes with TBST(containing
0.1% Tween-20), protein bands were visualized using ECL
chemiluminescence (cat. no. 34580; Thermo Fisher Scientific, Inc.),
followed by imaging and semi-quantification of the protein
expression levels using Image Studio Lite software (version 5.0)
(LI-COR Biosciences).
Colony formation assay
Following the aforementioned treatments, cells were
trypsinized and seeded into 6-well plates at a density of 500
cells/well and incubated at 37˚C with 5% CO2 for 14
days, with the media being changed every 2-3 days to ensure optimal
growth conditions. After incubation, the cells were fixed with 4%
paraformaldehyde at room temperature for 15 min and stained with
crystal violet at room temperature for 30 min. After washing with
phosphate-buffered saline (PBS), colony formation was observed
under an inverted phase-contrast light microscope, and the number
of colonies was counted manually using visual inspection. Colonies
were defined as clusters of >50 cells.
Transwell migration assays
A Transwell migration assay was used to assess the
migration of cells. Cells in the logarithmic growth phase were
harvested and digested with trypsin. The cells were resuspended in
serum-free DMEM medium and adjusted to a density of
1x105 cells/ml. A total of 200 µl of the cell suspension
was added to the upper chamber of the Transwell insert (8 µm pore
size), whilst 500 µl supplemented DMEM was added to the lower
chamber. The DMEM was supplemented with 10% FBS and 1%
penicillin-streptomycin. The cells were incubated at 37˚C with 5%
CO2 for 48 h. After incubation, the Transwell inserts
were removed and the cells on the membrane were fixed with 4%
paraformaldehyde at room temperature for 15 min. Subsequently, the
cells were stained with 0.1% crystal violet solution at room
temperature for 15 min and then washed with PBS to remove residual
crystal violet. Cells in the upper chamber of the Transwell
membrane that had not migrated were removed using a cotton swab,
whereas migratory cells in the lower chamber were counted manually
using a light microscope.
Statistical analysis
In vitro experiments were repeated three
times. In the bioinformatics analysis, comparisons of the MTHFD1
expression levels between two groups was performed using the
Wilcoxon rank-sum test. For comparisons involving three or more
groups, the Kruskal-Wallis test was used, followed by the
Bonferroni post hoc test for significant results. For the in
vitro experiments, the mean ± standard deviation values of the
different groups were compared using one-way ANOVA. When
significant differences were found, the least significant
difference post hoc test was used for pairwise comparisons. Pearson
correlation analysis was used for correlation analysis. The
prognostic value of MTHFD1 expression levels in tumors was
investigated using univariate Cox regression analysis. Kaplan-Meier
(KM) survival curves for patients in each group were produced, and
comparisons were performed using log-rank tests. Additionally,
multivariate Cox regression analysis was performed to evaluate the
independent prognostic value of MTHFD1 expression levels, adjusting
for potential confounding factors such as age, sex and clinical
stage. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using R software (version 4.2.1; The R Foundation for Statistical
Computing) and GraphPad Prism (version 9.5.1; Dotmatics).
Results
MTHFD1 exhibits differential
expression in 33 types of tumors
An analysis of the MTHFD1 mRNA expression levels
across 33 types of tumor was performed. The results revealed
significant differences in the MTHFD1 expression levels between 18
tumors and their normal tissue counterparts. Specifically, MTHFD1
was upregulated in the tumor tissues of bladder urothelial
carcinoma (BLCA), cervical squamous cell carcinoma (CESC), colon
adenocarcinoma (COAD), esophageal carcinoma (ESCA), glioblastoma
multiforme (GBM), HNSC, LUAD, lung squamous cell carcinoma (LUSC),
prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ),
stomach adenocarcinoma (STAD) and uterine corpus endometrial
carcinoma (UCEC). However, the expression of MTHFD1 was
downregulated in breast invasive carcinoma (BRCA),
cholangiocarcinoma (CHOL), kidney chromophobe (KICH), KIRC, liver
hepatocellular carcinoma (LIHC) and pheochromocytoma and
paraganglioma (PCPG) (Fig. 2A).
Additionally, across all tumor tissues, LIHC exhibited the highest
expression level of MTHFD1, whereas PCPG had the lowest (Fig. 2B). Furthermore, using the ssGSEA
algorithm, it was observed that MTHFD1 activity (enrichment score)
was increased in 21 tumor tissues compared with normal tissues
(including BLCA and BRCA; Fig.
2C). No tumors showed a significant decrease. Additionally,
diffuse large B-cell lymphoma (DLBC) had the highest MTHFD1
activity and PRAD the lowest across all of the tumors investigated
(Fig. 2D). Variations in the
MTHFD1 expression levels were also observed across different
clinical stages in COAD, KIRC, LUSC, pancreatic adenocarcinoma
(PAAD) and thyroid carcinoma (THCA; Fig. 2E). Specifically, in COAD, the
MTHFD1 expression level of stage I was significantly increased
compared with that of stage IV. Additionally, in KIRC, the MTHFD1
expression level of stage I was significantly higher compared with
that of stages II, III and IV. However, in LUSC, PAAD and THCA, the
MTHFD1 expression levels of stage I were markedly lower compared
with those of stage II (Fig. 2E).
Using the cBioPortal database, the distribution of MTHFD1 mutations
across various tumors was next investigated, where skin cutaneous
melanoma (SKCM) was revealed to have the highest mutational
frequency (Fig. 2F). As shown in
Fig. 2G, the expression of MTHFD1
was found to be the lowest in KIRC cell lines and highest in small
cell lung cancer cell lines. Furthermore, data from the CPTAC
database indicated that the expression level differences in MTHFD1
between tumor and normal tissues in BRCA, COAD, KIRC, UCEC, LUAD,
GBM and LIHC were consistent with the data from TCGA (Fig. 2H). In summary, these findings
demonstrate the diverse expression patterns of MTHFD1 across
different tumors, suggesting differences in its role and the
potential for further research.
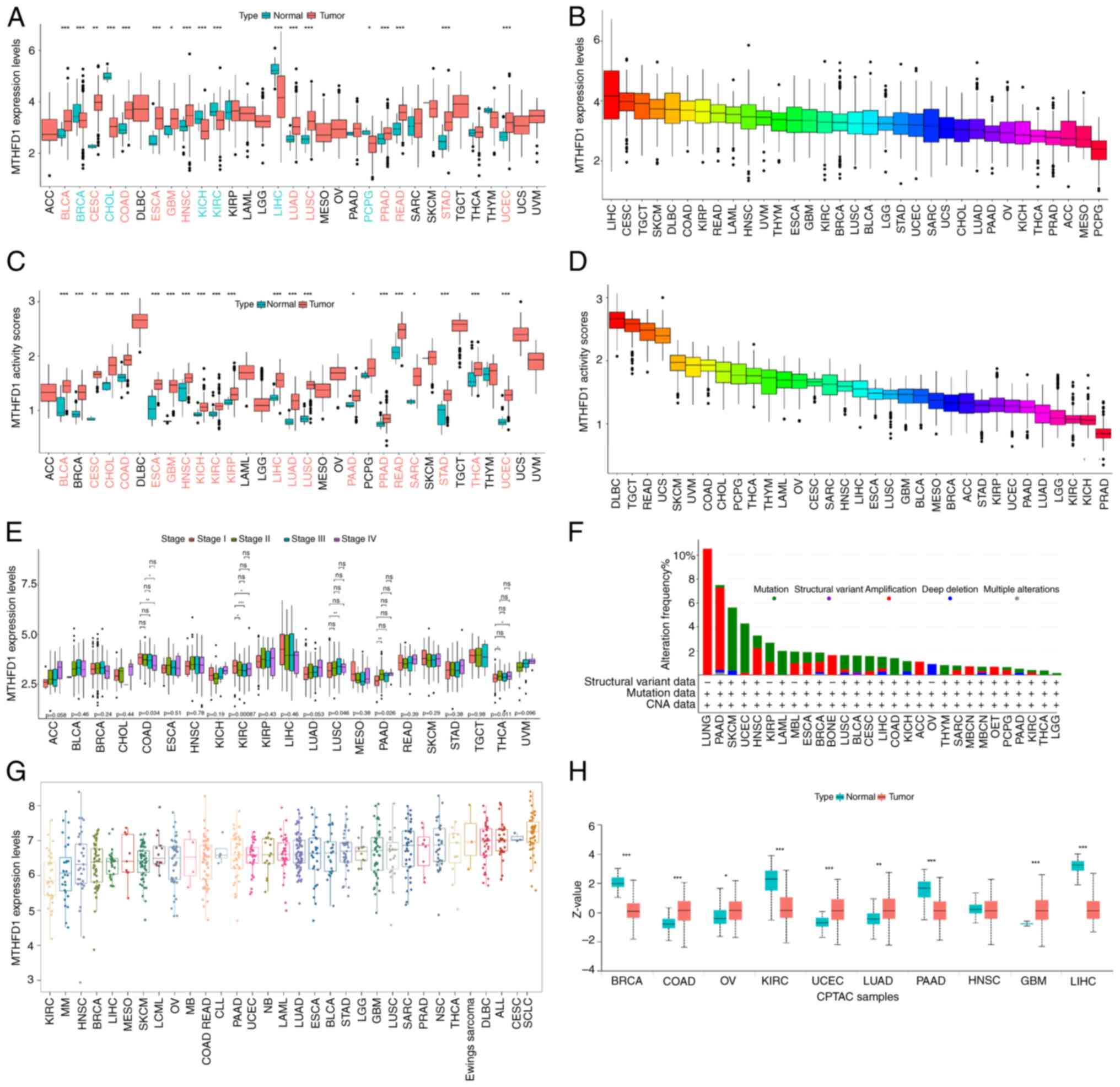 | Figure 2Analysis of MTHFD1 mRNA expression
levels, activity and alterations across 33 types of cancer. (A)
Boxplots showing MTHFD1 mRNA expression levels in tumor tissues
compared with normal tissues across different types of cancer. (B)
Comparison of MTHFD1 mRNA expression levels across different types
of cancer. (C) Boxplots indicating the MTHFD1 activity levels, as
determined by the ssGSEA algorithm, in tumor tissues compared with
normal tissues across different types of cancer. (D) Distribution
of MTHFD1 activity, as determined by the ssGSEA algorithm, across
different types of tumor. (E) MTHFD1 mRNA expression levels across
different clinical stages in different types of tumor. (F) Bar
graph showing the frequency of alterations in MTHFD1 within
different types of cancer. (G) MTHFD1 expression levels across
different cancer cell lines. (H) Comparison of MTHFD1 expression
levels between tumor and normal tissues in the CPTAC dataset.
*P<0.05, **P<0.01 and
***P<0.001. For certain cancer types (e.g., ACC, UCS,
UVM), the ‘normal’ MTHFD1 expression levels are not shown as these
data are not available in the TCGA database used for analysis.
MTHFD1, methylenetetrahydrofolate dehydrogenase 1; ACC, adrenal
carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast
invasive carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse large
B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous cell carcinoma; KIRC,
kidney renal clear cell carcinoma; KIRP, kidney renal papillary
cell carcinoma; LGG, low-grade glioma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic
adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD,
prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC,
sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach
adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid
carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UVM, uveal melanoma; CAN, copy number alteration; CTPA,
CPTAC dataset for protein expression in cancer; ssGSEA,
single-sample gene set enrichment analysis; Z-Score, standardized
value for the data points. |
MTHFD1 exhibits prognostic value in 33
types of tumors
Subsequently, the prognostic values of MTHFD1
expression levels in various tumors were investigated. Univariate
Cox regression analysis revealed that MTHFD1 was identified as a
prognostic factor for overall survival (OS) in adrenocortical
carcinoma (ACC), KICH, KIRC, LUAD, mesothelioma (MESO), PAAD, PCPG
and uveal melanoma (UVM; Fig. 3A).
KM survival analysis was performed to assess the association
between MTHFD1 expression and survival outcomes. Only cancer types
with statistically significant differences (P<0.05) in the KM
analysis were included for visualization, independent of the
univariate Cox regression results. Patients with low MTHFD1
expression levels had an increased OS compared with those with high
MTHFD1 expression levels in ACC, KICH, LUAD, PAAD, PCPG and UVM.
However, the opposite was demonstrated in CESC, KIRC, STAD and
thymoma (THYM; Fig. 3B-K).
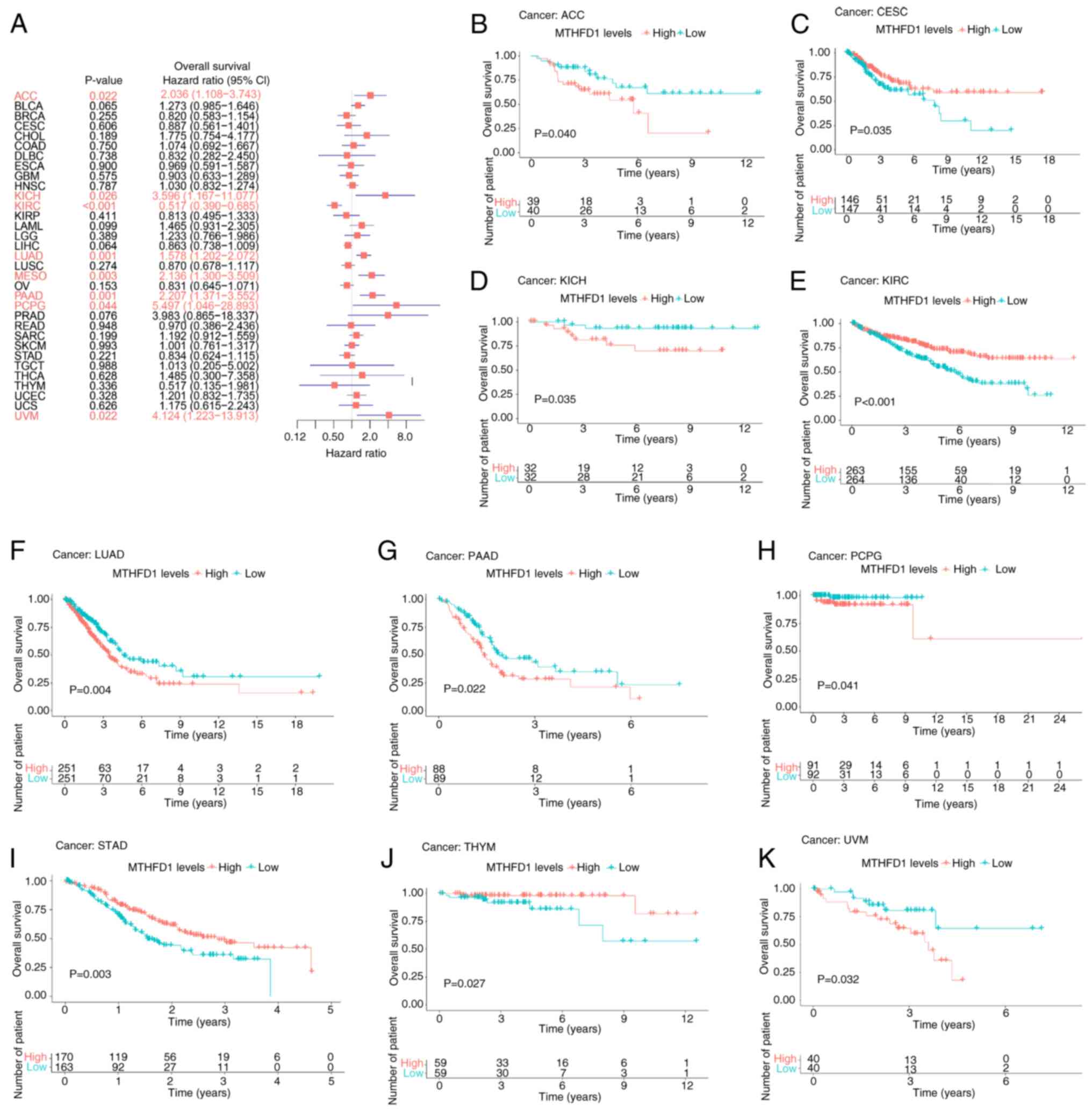 | Figure 3Prognostic value of MTHFD1 in 33
types of tumor based on OS. (A) Forest plot indicating the
predictive value of MTHFD1expression for OS across different types
of cancer. Kaplan-Meier survival curves demonstrating the
differences in OS between patients with high or low MTHFD1
expression levels in (B) ACC, (C) CESC, (D) KICH, (E) KIRC, (F)
LUAD, (G) PAAD, (H) PCPG, (I) STAD, (J) THYM and (K) UVM. MTHFD1,
methylenetetrahydrofolate dehydrogenase 1; OS, overall survival;
ACC, adrenal carcinoma; BLCA, bladder urothelial carcinoma; BRCA,
breast invasive carcinoma; CESC, cervical squamous cell carcinoma;
CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse
large B-cell lymphoma; ESCA, esophageal carcinoma; GBM,
glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney
renal papillary cell carcinoma; LGG, low-grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma;
PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; TGCT, testicular germ cell tumor; THCA,
thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UVM, uveal melanoma. |
Results of progression-free survival (PFS) analysis
indicated that MTHFD1 expression was associated with PFS in ACC,
KICH, KIRC, LUAD, MESO, PAAD, SARC, STAD, THCA and UVM (Fig. 4A). KM curves demonstrated that
patients with low MTHFD1 expression levels had a longer PFS
compared with those with high expression levels in ACC, KICH, LUAD,
SARC, THCA and UVM. However, the opposite was revealed in KIRC and
STAD (Fig. 4B-I).
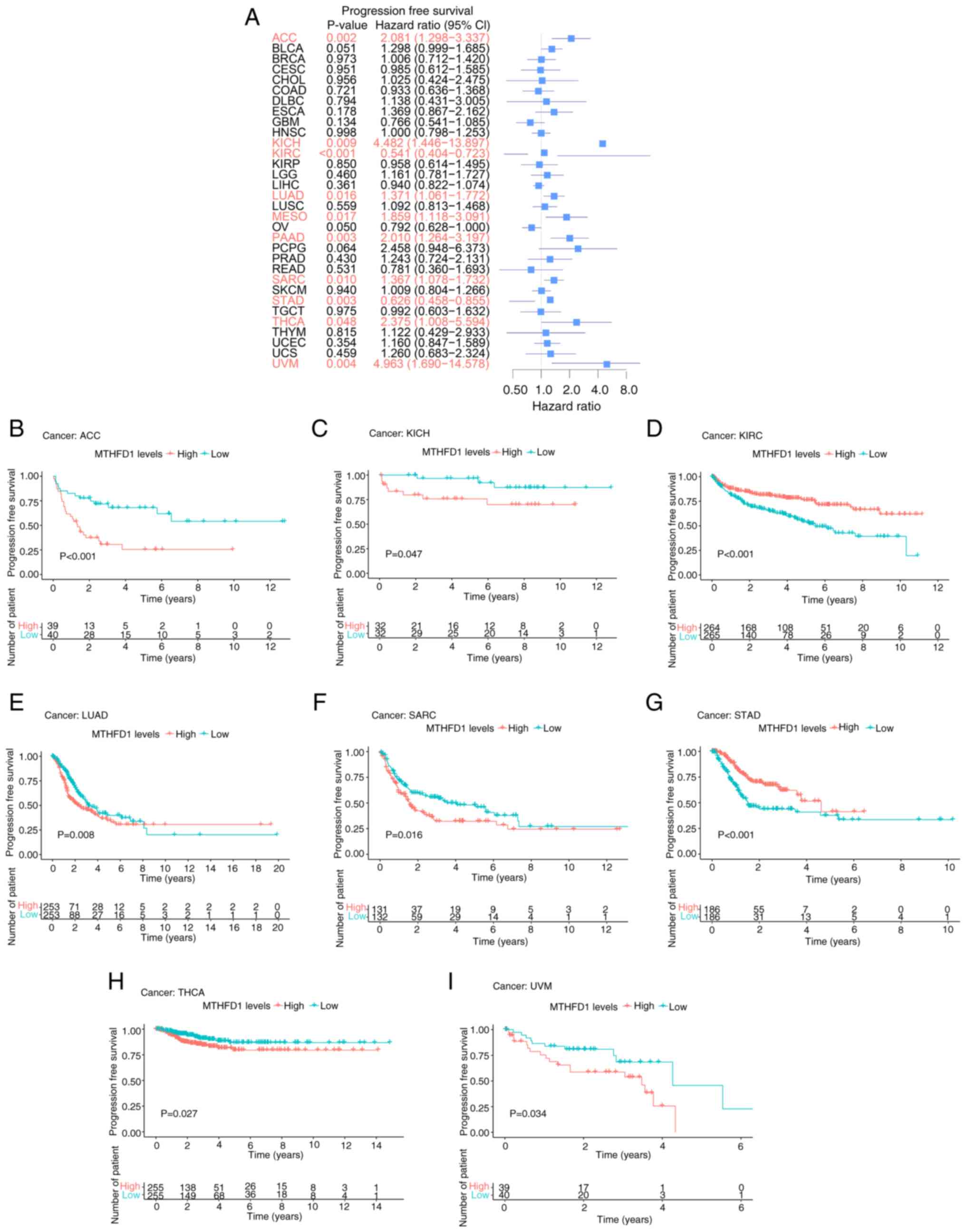 | Figure 4Prognostic value of MTHFD1 in 33
types of tumor based on PFS. (A) Forest plot indicating the
predictive value of MTHFD1 for PFS across different types of
cancer. Kaplan-Meier survival curves demonstrating the differences
in PFS between patients with high or low MTHFD1 expression levels
in (B) ACC, (C) KICH, (D) KIRC, (E) LUAD, (F) SARC, (G) STAD, (H)
THCA and (I) UVM. PFS, progression-free survival; MTHFD1,
methylenetetrahydrofolate dehydrogenase 1; ACC, adrenal carcinoma;
BLCA, bladder urothelial carcinoma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse large
B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous cell carcinoma; KIRC,
kidney renal clear cell carcinoma; KIRP, kidney renal papillary
cell carcinoma; LGG, low-grade glioma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic
adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD,
prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC,
sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach
adenocarcinoma; TGCT, testicular germ cell tumor; THCA, thyroid
carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UVM, uveal melanoma. |
Subsequent disease-specific survival (DSS) analysis
revealed that, excluding CESC and THYM, which were not
significantly associated with OS or DSS, the survival trends for
other cancer types were consistent with those observed for OS.
Furthermore, in COAD, patients with high MTHFD1 expression levels
had a longer DSS rate compared with those with low expression
levels (Fig. 5A-J).
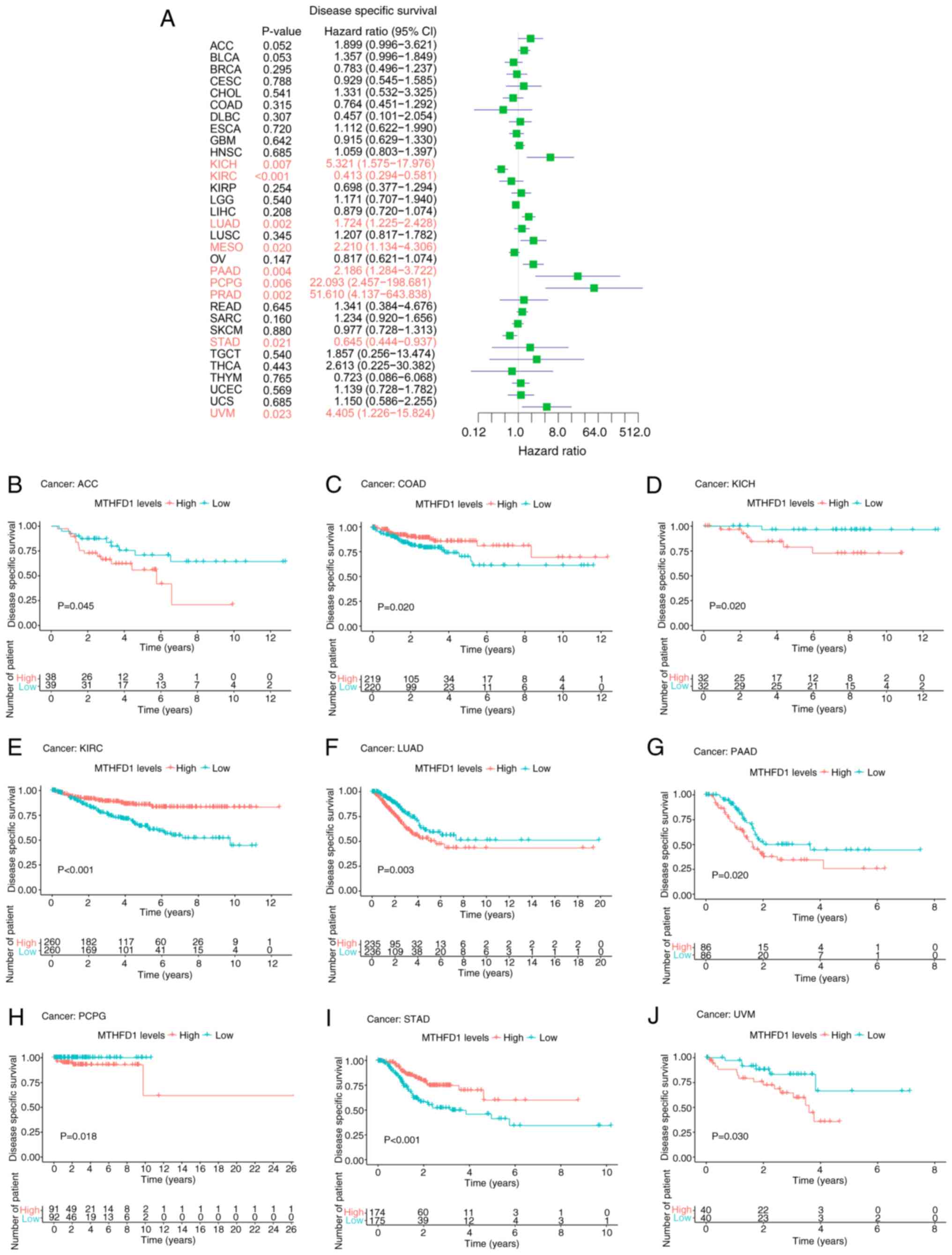 | Figure 5Prognostic value of MTHFD1 in 33
types of tumor based on DSS. (A) Forest plot showing the predictive
value of MTHFD1 for DSS across different types of cancer.
Kaplan-Meier survival curves demonstrating the differences in DSS
between patients with high or low MTHFD1 expression levels in (B)
ACC, (C) COAD, (D) KICH, (E) KIRC, (F) LUAD, (G) PAAD, (H) PCPG,
(I) STAD and (J) UVM. MTHFD1, methylenetetrahydrofolate
dehydrogenase 1; DSS, disease-specific survival; ACC, adrenal
carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast
invasive carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse large
B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous cell carcinoma; KIRC,
kidney renal clear cell carcinoma; KIRP, kidney renal papillary
cell carcinoma; LGG, low-grade glioma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic
adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD,
prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC,
sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach
adenocarcinoma; TGCT, testicular germ cell tumor; THCA, thyroid
carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UVM, uveal melanoma. |
Taken together, although the expression levels of
MTHFD1 may have prognostic value in various types of tumors,
consistent results between the Kaplan-Meier and Cox regression
analyses are only demonstrated for a subset of tumors. For example,
for OS, consistent results were indicated for ACC, KICH, KIRC,
LUAD, PAAD, PCPG and UVM. In addition, for PFS, consistent results
were revealed for ACC, KICH, KIRC, LUAD, SARC, STAD, THCA and UVM.
Additionally, for DSS, consistent findings were demonstrated for
KICH, KIRC, LUAD, PAAD, PCPG, STAD and UVM. These consistent
results between the Kaplan-Meier and Cox regression analyses
suggested that the expression level of MTHFD1 may be reliable as a
prognostic marker in these types of tumors, indicating its
potential utility in clinical decision-making.
MTHFD1 exhibits potential inhibition
of immune cell infiltration in 33 types of tumors
Given the differential expression of MTHFD1 across
the various tumors and its potential prognostic value, coupled with
the important role of the TME in tumor progression (36), the association between MTHFD1
expression levels and immune cell infiltration was further analyzed
using multiple algorithms. The ESTIMATE algorithm indicated a
negative correlation between MTHFD1 expression levels and stromal
score in 21/33 types of tumor, excluding UVM, USC, PCPG, PAAD,
MESO, LIHC, LAML, KICH, esophageal adenocarcinoma (ESAC), DLBC,
CHOL, and BLCA (Fig. 6A).
Additionally, there was a negative correlation between MTHFD1
expression levels and immune cell infiltration in 16/33 types of
tumor (such as ACC, UCEC, THCA and SKCM) and a positive correlation
only in UVM (Fig. 6B). The
ESTIMATE score was positively correlated with UVM, whereas it was
negatively correlated in 19/33 types of tumor (such as ACC and
UCEC; Fig. 6C). This suggested
that an increased MTHFD1 expression level may inhibit immune cell
activity in the majority of tumors. Further analysis using the
CIBERSORT algorithm revealed a negative correlation between MTHFD1
expression levels and the infiltration abundance of the majority of
immune cell types. For example, in COAD, MTHFD1 expression was
positively correlated with the infiltration abundance of resting
mast cells and M1 macrophages, while in UCEC, it was positively
correlated with CD8+ T cells. By contrast, in KIRC and
LGG, MTHFD1 expression levels showed a negative correlation with
CD8+ T cell infiltration. Additionally, MTHFD1 was
negatively correlated with pro-tumor immune cells, such as
regulatory T cells (Tregs), but positively correlated with
antitumor immune cells, including M1 macrophages (Fig. 6D). In summary, the findings from
multiple algorithms indicated that high MTHFD1 expression levels
may suggest an immunosuppressive state, whilst low expression
levels may indicate immune activation. This highlighted a possible
potential for MTHFD1 in tumor immunology research.
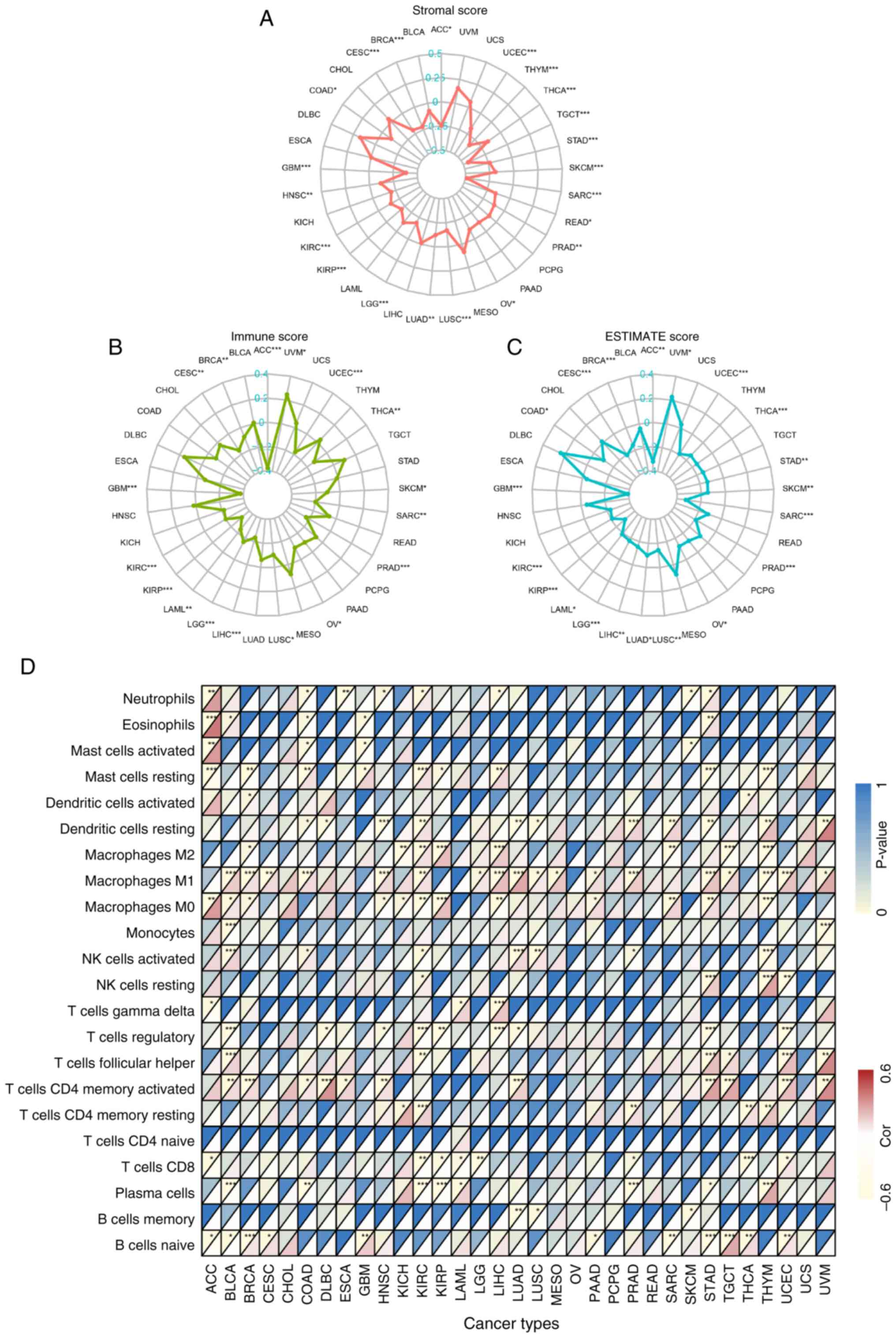 | Figure 6Correlation between the MTHFD1
expression level and the tumor microenvironment in 33 types of
tumor. Radar plots demonstrating the correlation between MTHFD1
expression levels and (A) stromal, (B) immune and (C) ESTIMATE
scores in different types of cancer. (D) Heatmap indicating the
correlation between MTHFD1 expression levels and the abundance of
22 immune cell infiltrates in different types of cancer. In each
grid, the lower triangle represents the correlation coefficient,
where red indicates positive correlation and yellow indicates
negative correlation. The upper triangle represents the
corresponding P-value, with deeper blue indicating a smaller
P-value (greater statistical significance). *P<0.05,
**P<0.01 and ***P<0.001. MTHFD1,
methylenetetrahydrofolate dehydrogenase 1; ACC, adrenal carcinoma;
BLCA, bladder urothelial carcinoma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse large
B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous cell carcinoma; KIRC,
kidney renal clear cell carcinoma; KIRP, kidney renal papillary
cell carcinoma; LGG, low-grade glioma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic
adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD,
prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC,
sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach
adenocarcinoma; TGCT, testicular germ cell tumor; THCA, thyroid
carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UVM, uveal melanoma. |
MTHFD1 as a potential biomarker for
predicting immunotherapy efficacy in 33 types of tumors
Immunotherapy, as a relatively non-invasive
treatment modality, has potential in tumor management. However, its
clinical application is notably hindered due to a lack of efficacy
in 60-80% of patients with solid tumors (37,38).
Therefore, there is value in identifying effective predictive
biomarkers for immunotherapy response. In 2020, TMB received Food
and Drug Administration (FDA) approval for use as a biomarker to
guide the selection of treatments for patients with solid tumors
exhibiting high TMB (39).
Therefore, the association between MTHFD1 and TMB was next assessed
in the present study. The results revealed a correlation between
MTHFD1 expression levels and TMB in 17 types of tumor, including
ACC, uterine carcinosarcoma, UCEC and THYM (Fig. 7A). Specifically, a positive
correlation was observed in ACC, uterine carcinosarcoma, UCEC and
other tumors, while a negative correlation was found in THYM.
However, the TIDE algorithm showed no correlation in only four
types of tumor, namely MESO, LIHC, DLBC and CHOL (Fig. 7B). The TIDE algorithm was used to
evaluate the tumor immune dysfunction and exclusion, and all of
these tumors showed a negative correlation between MTHFD1
expression levels and TIDE scores. In 2017, the FDA first approved
the use of the programmed cell death protein 1 (PDCD1) inhibitor
pembrolizumab (also known as Keytruda) for treating patients with
solid tumors with high MSI or mismatch repair deficiency (40). Therefore, the association between
MTHFD1 and MSI was also assessed in the present study. This
revealed an association in nine types of tumors (Fig. 7C). Positive correlations were
observed in UVM, UCEC, STAD, SARC and COAD, while negative
correlations were found in THCA, HNSC, DLBC and BRCA. Furthermore,
analysis of the IMvigor210 dataset revealed an increased expression
of MTHFD1 in patients that responded to immune checkpoint
inhibitors (ICBs) compared with those that did not respond
(Fig. 7D). Considering the
therapeutic potential of ICB, the correlations between MTHFD1
expression and the different ICB-related gene expression levels
were further investigated using data from TCGA. There was a
negative correlation between MTHFD1 expression levels and various
genes, including PDCD1 in KIRC, KIRP, LIHC, THCA and THYM, CD274 in
MESO, CTLA-4 in CESC, GBM, LGG, LIHC and PRAD, and TIGIT in KIRC,
LGG and LIHC. By contrast, a positive correlation between MTHFD1
expression levels and PDCD1 was observed in BLCA, HNSC, LUAD and
UVM, as well as with CD274 in KIRC, CTLA-4 in BLCA, and TIGIT in
BLCA and UVM (Fig. 7E). In
summary, these results highlighted the potential of MTHFD1 as a
predictive biomarker for the response to immunotherapy in a number
of tumor types.
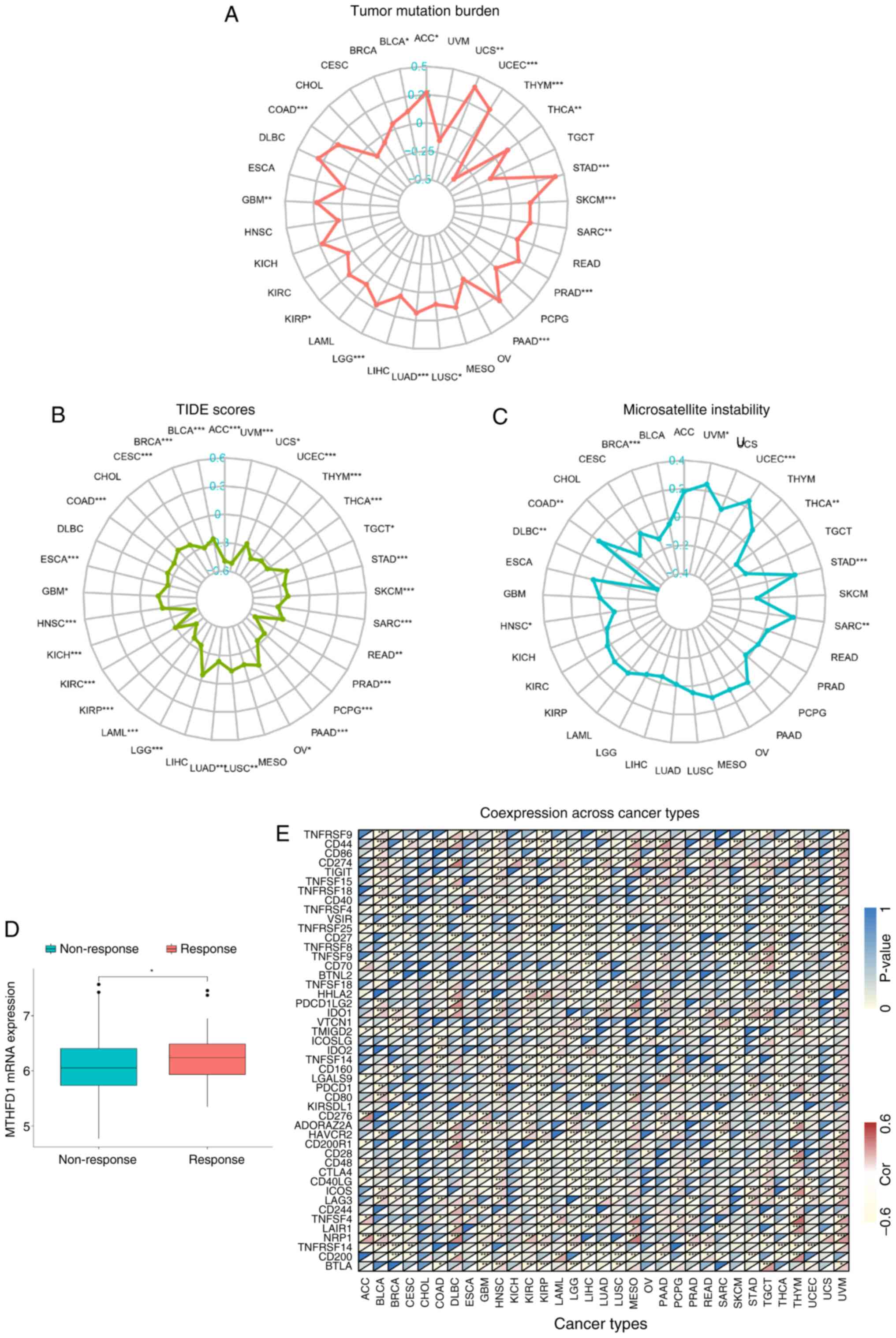 | Figure 7Predictive potential of MTHFD1 in
immunotherapy across 33 types of tumor. Radar plots demonstrating
the correlation between MTHFD1 and (A) tumor mutational burden, (B)
TIDE scores and (C) microsatellite instability in different types
of cancer. (D) Boxplot of the IMvigor210 dataset showing the
differences in MTHFD1 expression levels between patients who
responded to atezolizumab (PD-L1 inhibitor) treatment and those who
did not. (E) Heatmap indicating the correlation between the
expression of MTHFD1 and 48 immune checkpoint blockade-related
genes in different types of cancer. In each grid, the lower
triangle represents the correlation coefficient, where red
indicates positive correlation and yellow indicates negative
correlation. The upper triangle represents the corresponding
P-value, with deeper blue indicating a smaller P-value (greater
statistical significance). *P<0.05,
**P<0.01 and ***P<0.001. MTHFD1,
methylenetetrahydrofolate dehydrogenase 1; TIDE, tumor immune
dysfunction, and exclusion; ACC, adrenal carcinoma; BLCA, bladder
urothelial carcinoma; BRCA, breast invasive carcinoma; CESC,
cervical squamous cell carcinoma; CHOL, cholangiocarcinoma; COAD,
colon adenocarcinoma; DLBC, diffuse large B-cell lymphoma; ESCA,
esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and
neck squamous cell carcinoma; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG,
low-grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian
serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma;
READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell
tumor; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus
endometrial carcinoma; UVM, uveal melanoma. |
MTHFD1 exhibits distinct roles in LUAD
and KIRC
As the expression of MTHFD1 was upregulated in LUAD
tumors, where high MTHFD1 expression levels were associated with
reduced OS, PFS and DSS in patients with LUAD, with opposite trends
revealed for KIRC tumors, the specific effects of MTHFD1 in LUAD
and KIRC tumor cells was investigated further. The median
expression level of MTHFD1 was calculated by ranking all of the
expression level values for each type of tumor and selecting the
50th percentile. For LUAD, the median was determined to be
3.033679, whereas for KIRC it was 3.289875. These median values are
indicated on the Kaplan-Meier curves in Fig. 3E and F, which were used as the cut-off
thresholds to stratify patients into groups of high or low
expression levels. Using these thresholds in the subsequent
differential expression analysis (with an absolute log-fold change
cutoff of 0.585 and a false discovery rate of 0.05, 818 and 264
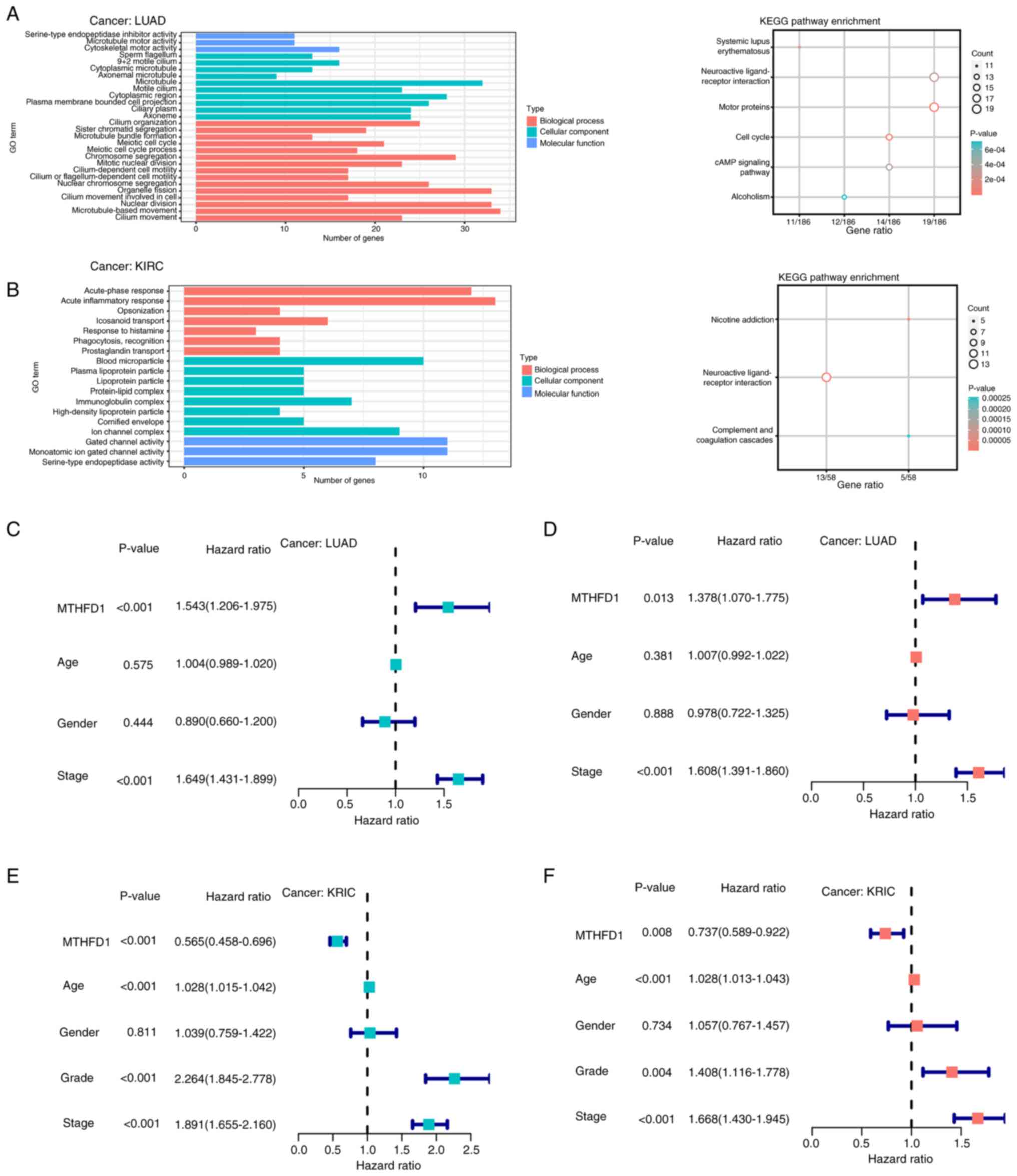
DEGs were identified in LUAD and KIRC, respectively. Fig. 8 presents the results of GO and KEGG
analyses of DEGs in LUAD and KIRC. In LUAD (Fig. 8A), the top enriched biological
processes included ‘organelle fission’, ‘nuclear division’ and
‘microtubule-based movement’. Cellular components enriched in LUAD
were ‘microtubule’ and ‘cytoplasmic region’, while molecular
functions included ‘cytoskeletal motor activity’. KEGG pathway
analysis in LUAD revealed significant enrichment in ‘cAMP signaling
pathway’, ‘neuroactive ligand-receptor interaction’ and ‘motor
proteins’. In KIRC (Fig. 8B), the
top biological processes enriched were ‘acute-phase response’,
‘acute inflammatory response’ and ‘icosanoid transport’. The
cellular components enriched in KIRC included ‘ion channel complex’
and ‘blood microparticle’, while molecular functions included
‘gated channel activtity’ and ‘serine-type endopeptidase activity’.
KEGG analysis in KIRC revealed significant pathways, such as
‘nicotine addiction’, ‘neuroactive ligand-receptor interaction’ and
‘complement and coagulation cascades’. These results highlight the
involvement of immune-related pathways and metabolic processes in
tumor progression across both cancer types. Furthermore, subsequent
univariate and multivariant Cox analysis (the latter adjusted for
age, sex and stage) revealed that MTHFD1 expression level was an
independent risk factor for prognosis in LUAD (Fig. 8C and D) and an independent protective factor
for prognosis in KIRC (Fig. 8E and
F). These findings suggested that
MTHFD1 may have differential roles in different types of
tumors.
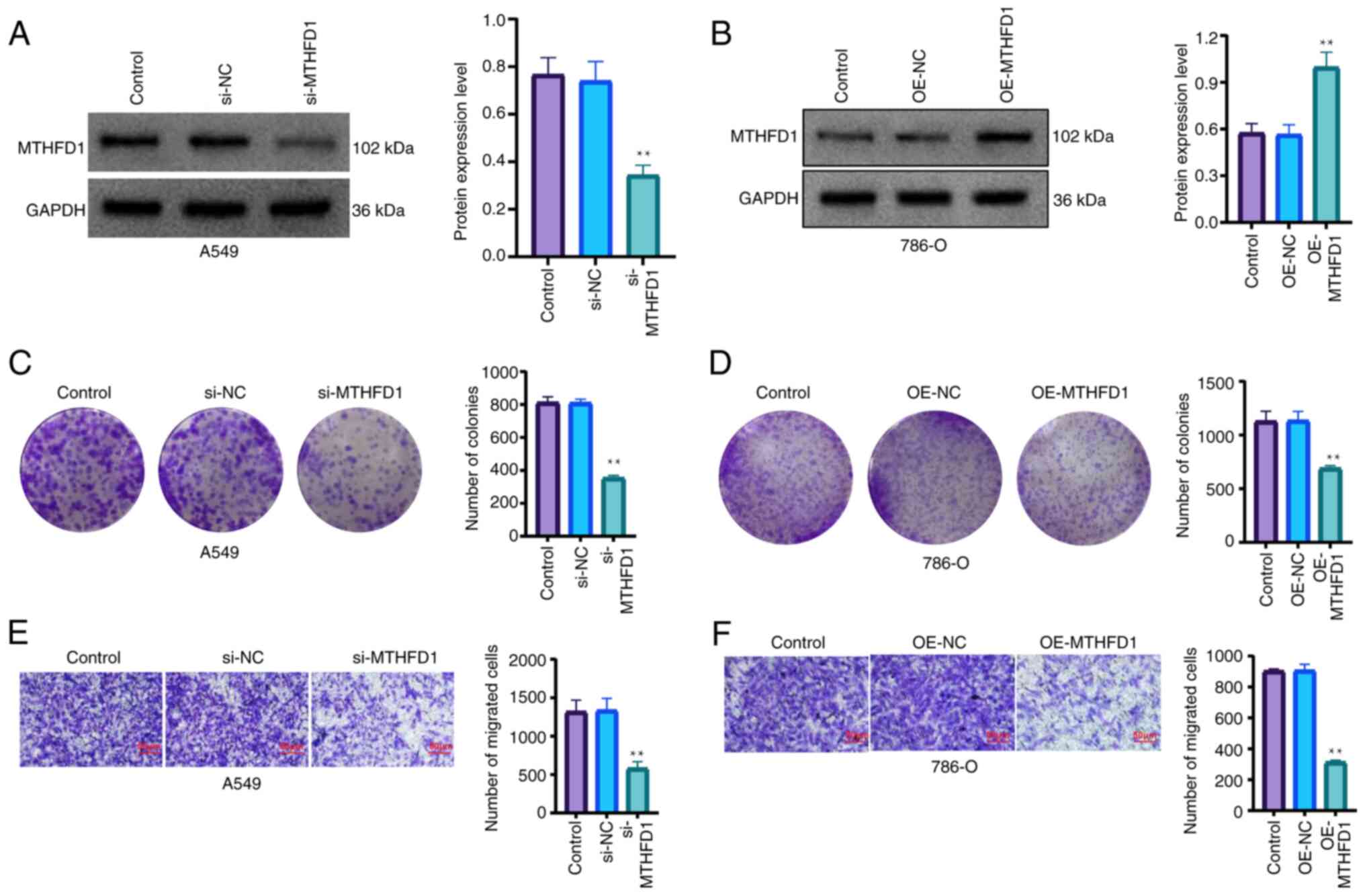
Furthermore, in vitro experiments
demonstrated that knocking down the expression of MTHFD1 in a LUAD
cell line significantly inhibited the proliferation and migration
of cells (Fig. 9A-C). In addition,
the overexpression of MTHFD1 in a KIRC cell line significantly
reduced the proliferation and migration of cells (Fig. 9D-F). Taken together, these results
indicated that MTHFD1 may have an oncogenic role in LUAD, as well
as a tumor suppressive role in KIRC.
Discussion
The present study investigated the expression levels
of the amino acid metabolism-related gene MTHFD1 across 33 types of
cancer. The impact of MTHFD1 on the prognosis and immune status,
particularly in predicting immunotherapy efficacy, was also
assessed. The results of the present study indicated that MTHFD1
had a role in various types of cancer, including LUAD and KIRC.
Data from TCGA indicated that MTHFD1 had differential expression
levels in 18 types of cancer compared with those in their normal
tissue counterparts. Among these, 13 types of cancer indicated
upregulated MTHFD1 expression levels in tumor tissues, including
BLCA, CESC, COAD, ESCA, GBM, HNSC, LUAD, LUSC, PRAD, READ, STAD and
UCEC. By contrast, decreased MTHFD1 expression levels were
indicated in BRCA, KICH, KIRC, LIHC and PCPG. Additionally, data
from the CPTAC also indicated these trends in the expression of
MTHFD1 in BRCA, COAD, KIRC, UCEC, LUAD, GBM and LIHC. This
suggested that MTHFD1 may have mechanistic differences in various
tumors.
Further analysis of prognostic data revealed that
high MTHFD1 expression levels were associated with a reduced OS in
ACC, KICH, LUAD, PAAD, PCPG and UVM. However, an opposite trend was
demonstrated in KIRC. PFS analysis indicated that patients with low
MTHFD1 expression levels had a longer PFS in ACC, KICH, LUAD, SARC,
THCA and UVM compared with those with high MTHFD1 expression
levels. However, the opposite was demonstrated in KIRC and STAD.
DSS trends mostly mirrored those of OS, except in ACC, where the
survival curve showed significance, but the association was not
statistically significant in the univariate Cox regression analysis
and was therefore excluded. By contrast, the association was
statistically significant in STAD.
Overall, the data obtained from a number of
databases suggested that the expression of MTHFD1 was downregulated
in KIRC compared with that in normal tissues, with high MTHFD1
expression levels associated with a favorable prognosis, which may
indicate a tumor suppressive role. However, in LUAD, MTHFD1 may
serve as an oncogene, since its expression levels and prognostic
trends were the opposite of those for KIRC. These findings align
with previous studies that indicate the variable roles of the same
gene/protein across different types of cancer. A previous study by
Li et al (41) revealed
that the microtubule associated monooxygenase, calponin and LIM
domain-containing gene is an oncogene in KIRC, but a tumor
suppressor in PAAD and LUAD. Another study by Cao et al
(42) reported that the lysine
N-methyltransferase 2C gene is a protective factor in KIRC and
ovarian serous cystadenocarcinoma, but a risk factor in LUSC and
UVM. There heterogeneity of tumors and the microenvironmental
differences may contribute to the diverse roles that a protein has
in different types of cancer (43). Furthermore, results of the ssGSEA
algorithm indicated that MTHFD1 activity was increased in 21 tumor
tissues compared with that in the normal tissue counterparts,
suggesting that MTHFD1 may play a dual role in cancer, by promoting
tumor growth and progression by modulating amino acid metabolism in
some cancers, while potentially inhibiting tumor development in
others.
To further investigate the differential roles of
MTHFD1 in various types of tumors and its influence on immune
cells, an in-depth analysis of its association with immune cell
infiltration was next performed. The results, as indicated using
the ESTIMATE algorithm, revealed a significant correlation between
MTHFD1 expression levels and immune cell infiltration across 17
types of tumors, albeit with notable differences among different
types of cancer. In UVM, MTHFD1 expression levels were positively
correlated with immune scores, suggesting an immunoactive
environment characterized by enhanced immune cell infiltration,
particularly CD4 T cells and dendritic cells, which may promote
antitumor immunity. By contrast, in ACC, MTHFD1 expression levels
were negatively correlated with immune scores. COAD, in which the
MTHFD1 expression levels were increased compared with those in
normal tissues, is positively correlated with pro-tumor immune
cells, such as resting mast cells. These cells secrete IL-10, TGF-β
and VEGF, which suppress immune responses and promote angiogenesis
(44-48).
By contrast, in other types of cancer, such as LIHC, in which the
MTHFD1 expression levels were decreased compared with those in
normal tissues, MTHFD1 was negatively correlated with pro-tumor
immune cells, such as Tregs, but was positively correlated with
antitumor immune cells, including M1 macrophages. M1 macrophages
secrete TNF-α and IL-12, which eliminate tumor cells and activate
other immune cells, including CD8+ T cells and natural
killer cells (49). By contrast,
Tregs can weaken the antitumor effect of the immune system by
inhibiting the activity of cytotoxic T cells and natural killer
cells (50). In KIRC, MTHFD1
expression was correlated with 13 types of immune cell types, with
6 types negatively correlated (e.g., plasma cells) and 7 types
positively correlated (e.g., neutrophils), suggesting robust immune
cell infiltration and a potential tumor-suppressing role. However,
in LUAD, MTHFD1 expression was correlated with only six types of
immune cells, with three types positively correlated (e.g., M1
macrophages) and three types negatively correlated (e.g., memory B
cells). These results indicate that MTHFD1 may influence tumor
progression by modulating immune cell activity. In certain types of
cancer, such as KIRC, LUAD and UVM, MTHFD1 may enhance antitumor
immune cell function, promoting an ‘activated’ immune
microenvironment. By contrast, in other types of cancer, it may
promote pro-tumor immune cell functions, resulting in an
immunosuppressive state. However, the complexity of cancer should
be acknowledged, as genes may impact tumor progression through
various mechanisms beyond immune cells, such as the regulation of
the cell cycle and proliferation by transcription factors such as
FOXM1, epigenetic modifications leading to gene silencing,
remodeling of the extracellular matrix in the tumor
microenvironment, and metabolic reprogramming to support rapid
tumor growth and invasion (51-53).
Furthermore, GO and KEGG enrichment analyses suggested that in
LUAD, MTHFD1 may promote tumor progression by enhancing biological
processes such as ‘nuclear division’, ‘organelle fission’ and
‘microtubule-based movement’. Cellular components such as the
‘microtubule’ and ‘cytoplasmic region’ were enriched, while
molecular functions related to ‘cytoskeletal motor activity’ were
identified. KEGG pathway analysis revealed significant involvement
of pathways such as the ‘cAMP signaling pathway’, which could
support cell survival, and ‘neuroactive ligand-receptor
interaction’, potentially contributing to tumorigenesis. These
results highlight the role of MTHFD1 in driving key cellular
functions and metabolic pathways that promote LUAD progression.
MTHFD1 has been previously documented to enhance tumor cell
migration and invasion in colorectal cancer by modulating
cytoskeletal and adhesion molecules, such as by regulating actin
filament rearrangement and integrin expression, thereby increasing
the risk of metastasis (54). In
addition, its role in the cAMP signaling pathway may also
facilitate the proliferation of cancer cells, particularly in
bladder cancer, where it is involved in the induction of
7-dehydrocholesterol reductase, stimulating cholesterol synthesis
and activating cAMP signaling to promote metastasis (55). These mechanisms highlight the
potential of MTHFD1 as an oncogenic factor and a therapeutic target
in LUAD. By contrast, in KIRC, MTHFD1 may exert tumor suppressing
effects by regulating acute inflammatory responses, complement and
coagulation cascades and the functions of cell membrane channels.
These mechanisms may enhance antitumor immunity and facilitate the
clearance of cancer cells, as supported by studies on inflammation,
immune regulation, and coagulation pathways (56-59).
The multifaceted role of MTHFD1 in KIRC as a tumor suppressor
highlighted it as a potential therapeutic target. Potential
strategies to target MTHFD1 in KIRC include using gene silencing
techniques such as RNA interference or CRISPR-Cas9 to reduce MTHFD1
expression, developing small molecule inhibitors to block its
enzymatic activity, or employing gene therapy approaches to either
restore its function or inhibit its overexpression in tumor cells.
Preliminary in vitro experiments further indicated the
divergent roles of MTHFD1 in LUAD and KIRC, which highlighted its
potential in different types of cancer.
Given the role of immunotherapy in the treatment of
cancer (60), in the present
study, the efficacy of MTHFD1 in immunotherapy across 33 different
tumor types was analyzed using the TIDE algorithm. However, it
should be noted that MTHFD1 has not yet been FDA-approved as an
immunotherapy target for all these cancer types, and its efficacy
in immunotherapy remains under investigation. The results indicated
that a high expression of MTHFD1 was significantly associated with
poorer responses to immunotherapy, as indicated by negative
correlations with TIDE scores, suggesting that higher MTHFD1
expression is linked to immune escape and resistance to treatment
in most cancers studied. However, no significant correlation was
observed in LIHC, DLBC, MESO and CHOL. Further analysis from the
IMvigor database also suggested a role of MTHFD1 in immunotherapy.
TIDE scores and IMvigor data are used to reflect the efficacy
response of PDCD1/PD-L1 and cytotoxic T-lymphocyte-associated
protein 4 (CTLA-4) inhibitors (61,62)
A previous study identified MTHFD1 as a potential therapeutic
target in prostate cancer, associating its expression with poor
survival outcomes and suggesting that targeting MTHFD1 could
enhance immunotherapy efficacy (63). MTHFD1 is a key enzyme in the
one-carbon metabolism pathway and is responsible for converting
folate metabolites into active one-carbon units used for DNA
synthesis, nucleic acid synthesis and methionine regeneration.
These processes are important for sustaining rapid tumor cell
proliferation and immune cell activation (64-66).
By promoting these reactions involved in metabolite synthesis,
MTHFD1 provides sufficient metabolic support for T cells, enhancing
their activity within the TME, including in colorectal cancer,
bladder cancer and tongue squamous cell carcinoma (67). Adequate nucleic acid supply aids in
T cell proliferation, boosting their cytotoxic effects on tumor
cells and increasing the sensitivity of tumor cells to PDCD1/PD-L1
and CTLA-4 inhibitors, without promoting tumor cell proliferation
directly (68). Secondly, the role
of MTHFD1 in amino acid metabolism notably impacts the TME
(36,69). It participates in serine and
glycine synthesis, which are key for cell proliferation and
metabolic activities (22). By
promoting these metabolic pathways, MTHFD1 may reduce the capacity
of tumor cell immune evasion and in certain circumstances, such as
in colorectal and bladder cancer, inhibit the production of
immunosuppressive metabolites, such as adenosine (70-72).
Adenosine is a immunosuppressive molecule, where a reduction in its
levels can enhance the efficacy of ICBs (72). Additionally, MTHFD1 may influence
the balance of immune cell subsets, particularly by reducing the
proportion of Tregs, which are known immunosuppressive cells that
weaken immune responses by inhibiting the function of effector T
cells. MTHFD1 may regulate Treg activity through its metabolic
products, such as methylenetetrahydrofolate, formylmethionine,
serine and glycine (71). These
metabolites can reduce immune suppression and enhance antitumor
immune effects (73).
Additionally, similar to other genes, such as CTLA4 and DRD1
(74,75), methylation reactions involving
MTHFD1 not only regulate gene expression levels in tumor cells but
also affect PD-L1 and CTLA-4 expression levels in tumor and immune
cells, further improving the effectiveness of immunotherapy. The
results of the present study also demonstrated that MTHFD1 had
significant positive correlations with key ICB genes, such as PDCD1
in LUAD, CTLA-4 in BLCA and TIGIT in UVM. This suggested that
MTHFD1 may serve as a potential biomarker for evaluating the
response to immunotherapy. However, negative correlations with
certain immunosuppressive checkpoints, such as PDCD1 in KIRC, CD274
in MESO, CTLA-4 in CESC and TIGIT in KIRC, implied that MTHFD1 may
enhance antitumor immune responses by downregulating these
inhibitory pathways. Therefore, MTHFD1 is potentially valuable in
immunotherapy due to its possible modulation of metabolic pathways,
influence on immune cell functions and production of a favorable
TME. Future studies should focus on clinically validating these
findings and further investigating the specific biological
mechanisms of MTHFD1 in immunotherapy to develop novel therapeutic
strategies and enhance the efficacy of immunotherapy.
To the best of our knowledge, the present study was
the first to provide a comprehensive analysis of the amino acid
metabolism-related gene MTHFD1. The results indicted its varied
expression patterns across multiple types of cancer and highlighted
its associations with patient survival, tumor progression and the
immune microenvironment. The findings of the present study
highlighted the role of MTHFD1 in different tumor types and
indicated its potential application in precision medicine for
diagnosis and treatment. Furthermore, the present study indicated
that high MTHFD1 expression levels were associated with the
response of certain types of cancer to ICBs, such as drugs
targeting PDCD1/PD-L1 and CTLA-4. MTHFD1 demonstrated distinct
correlations with various immune checkpoint pathways, which
indicated its value as a potential biomarker for immunotherapy.
This evidence supports the translation of research on MTHFD1 from
the laboratory into a clinically significant diagnostic and
therapeutic target, paving the way for personalized treatment
strategies, and improving the effectiveness and precision of
immunotherapy.
Although the present study indicated the role of
MTHFD1 in a pan-cancer panel, it had a number of limitations. The
conclusions drawn used data from public databases and lacked
primary data obtained from patients. In addition, tumor tissues are
heterogeneous, where bulk data from databases, such as TCGA, may
not reflect the genetic changes within tumor cells. The present
study also lacked single-cell sequencing data to further
investigate the molecular mechanisms involved. In addition, the
present study lacked immune cell infiltration experiments using
animal and clinical specimens. The in vitro experiments
performed in the present study were limited to two types of cancer,
which therefore lacked a comprehensive analysis and mechanistic
investigation into other types of tumors. The present study also
lacked an investigation into the specific mechanisms underlying the
efficacy of immunotherapy.
In conclusion, the results of the present study
indicated the potential oncogenic role of MTHFD1 in LUAD and its
tumor suppressive role in KIRC in vitro. Furthermore, it was
demonstrated that high MTHFD1 expression levels were associated
with decreased immune cell infiltration in various types of cancer
and may serve as a predictive marker for the response to
immunotherapy. In future studies, MTHFD1 should be further
investigated to enhance the experimental and clinical data, which
may provide novel insights into its role in the prognosis and
treatment of tumors, particularly in the context of
immunotherapy.
Supplementary Material
Construct sequences used in the
present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SG and ChuP conceived the study. JY designed the
study. ChaP conducted the cell experiments and collected the data.
SG and ChuP wrote the manuscript. FP analyzed the data. SG and ChuP
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zeng H, Chen W, Zheng R, Zhang S, Ji JS,
Zou X, Xia C, Sun K, Yang Z, Li H, et al: Changing cancer survival
in China during 2003-15: A pooled analysis of 17 population-based
cancer registries. Lancet Glob Heal. 6:e555–e567. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang JH, Wu CC, Yuan KSP, Wu ATH and Wu
SY: Locoregionally recurrent head and neck squamous cell carcinoma:
Incidence, survival, prognostic factors, and treatment outcomes.
Oncotarget. 8:55600–55612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nors J, Iversen LH, Erichsen R, Gotschalck
KA and Andersen CL: Incidence of recurrence and time to recurrence
in stage I to III colorectal cancer: A nationwide Danish cohort
study. JAMA Oncol. 10:54–62. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu X, Ren B, Ren J, Gu M, You L and Zhao
Y: The significant role of amino acid metabolic reprogramming in
cancer. Cell Commun Signal. 22(380)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang J, Chen M, Yang Y, Liu Z, Guo W,
Xiang P, Zeng Z, Wang D and Xiong W: Amino acid metabolic
reprogramming in the tumor microenvironment and its implication for
cancer therapy. J Cell Physiol. 239(e31349)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Zhao Y, Song H, Li Y, Liu Z, Ye Z,
Zhao J, Wu Y, Tang J and Yao M: Metabolic reprogramming in tumor
immune microenvironment: Impact on immune cell function and
therapeutic implications. Cancer Lett. 597(217076)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Y, Zhao T, Li Z, Wang L, Yuan S and
Sun L: The role of ASCT2 in cancer: A review. Eur J Pharmacol.
837:81–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Z, Liu R, Shuai Y, Huang Y, Jin R,
Wang X and Luo J: ASCT2 (SLC1A5)-dependent glutamine uptake is
involved in the progression of head and neck squamous cell
carcinoma. Br J Cancer. 122:82–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cormerais Y, Vučetić M, Parks SK and
Pouyssegur J: Amino acid transporters are a vital focal point in
the control of mTORC1 signaling and cancer. Int J Mol Sci.
22(23)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Geldermalsen M, Wang Q, Nagarajah R,
Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N,
et al: ASCT2/SLC1A5 controls glutamine uptake and tumour growth in
triple-negative basal-like breast cancer. Oncogene. 35:3201–3208.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Najumudeen AK, Ceteci F, Fey SK, Hamm G,
Steven RT, Hall H, Nikula CJ, Dexter A, Murta T, Race AM, et al:
The amino acid transporter SLC7A5 is required for efficient growth
of KRAS-mutant colorectal cancer. Nat Genet. 53:16–26.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ohgaki R, Hirase Y, Xu M, Okanishi H and
Kanai Y: LAT1 expression in colorectal cancer cells is unresponsive
to HIF-1/2α accumulation under experimental hypoxia. Sci Rep.
14(19635)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hayase S, Kumamoto K, Saito K, Kofunato Y,
Sato Y, Okayama H, Miyamoto K, Ohki S and Takenoshita S: L-type
amino acid transporter 1 expression is upregulated and associated
with cellular proliferation in colorectal cancer. Oncol Lett.
14:7410–7416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang Y, Xu Y, Lu W, Ghergurovich JM, Guo
L, Blair IA, Rabinowitz JD and Yang X: Upregulation of antioxidant
capacity and nucleotide precursor availability suffices for
oncogenic transformation. Cell Metab. 33:94–109.e8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu K, Li K, Lv J, Feng J, Chen J, Wu H,
Cheng F, Jiang W, Wang J, Pei H, et al: Suppression of the
SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant
lung adenocarcinoma. J Clin Invest. 130:1752–1766. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang C, Chen J, Yu Z, Luo J, Li X, Zhou B
and Jiang N: Mining of RNA methylation-related genes and
elucidation of their molecular biology in gallbladder carcinoma.
Front Oncol. 11(621806)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen K, Wu S, Ye S, Huang H, Zhou Y, Zhou
H, Wu S, Mao Y, Shangguan F, Lan L and Chen B: Dimethyl fumarate
induces metabolic crisie to suppress pancreatic carcinoma. Front
Pharmacol. 12(617714)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tarragó-Celada J, Foguet C,
Tarrado-Castellarnau M, Marin S, Hernández-Alias X, Perarnau J,
Morrish F, Hockenbery D, Gomis RR, Ruppin E, et al: Cysteine and
folate metabolism are targetable vulnerabilities of metastatic
colorectal cancer. Cancers (Basel). 13(425)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ding K, Jiang J, Chen L and Xu X:
Methylenetetrahydrofolate dehydrogenase 1 silencing expedites the
apoptosis of non-small cell lung cancer cells via modulating DNA
methylation. Med Sci Monit. 24:7499–7507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zheng Y, Zhu L, Qin ZY, Guo Y, Wang S, Xue
M, Shen KY, Hu BY, Wang XF, Wang CQ, et al: Modulation of cellular
metabolism by protein crotonylation regulates pancreatic cancer
progression. Cell Rep. 42(112666)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pan S, Fan M, Liu Z, Li X and Wang H:
Serine, glycine and one-carbon metabolism in cancer (review). Int J
Oncol. 58:158–170. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fan J, Ye J, Kamphorst JJ, Shlomi T,
Thompson CB and Rabinowitz JD: Quantitative flux analysis reveals
folate-dependent NADPH production. Nature. 510:298–302.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu SC and Mato JM: S-adenosylmethionine in
liver health, injury, and cancer. Physiol Rev. 92:1515–1542.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
MacFarlane AJ, Perry CA, Girnary HH, Gao
D, Allen RH, Stabler SP, Shane B and Stover PJ: Mthfd1 is an
essential gene in mice and alters biomarkers of impaired one-carbon
metabolism. J Biol Chem. 284:1533–1539. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, et al: Sustained proliferation in
cancer: Mechanisms and novel therapeutic targets. Semin Cancer
Biol. 35 (Suppl 1):S25–S54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mullen NJ and Singh PK: Nucleotide
metabolism: A pan-cancer metabolic dependency. Nat Rev Cancer.
23:275–294. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dong Y, Zhao H, Li H, Li X and Yang S: DNA
methylation as an early diagnostic marker of cancer (review).
Biomed Rep. 2:326–330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dutta H and Jain N: Post-translational
modifications and their implications in cancer. Front Oncol.
13(1240115)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Becht E, Giraldo NA, Lacroix L, Buttard B,
Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman
C, Fridman WH and de Reyniès A: Estimating the population abundance
of tissue-infiltrating immune and stromal cell populations using
gene expression. Genome Biol. 17(218)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Newman AM, Liu CL, Green MR, Gentles AJ,
Feng W, Xu Y, Hoang CD, Diehn M and Alizadeh AA: Robust enumeration
of cell subsets from tissue expression profiles. Nat Methods.
12:453–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hänzelmann S, Castelo R and Guinney J:
GSVA: Gene set variation analysis for microarray and RNA-seq data.
BMC Bioinformatics. 14(7)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43(e47)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z,
Feng T, Zhou L, Tang W, Zhan L, et al: clusterProfiler 4.0: A
universal enrichment tool for interpreting omics data. Innovation
(Camb). 2(100141)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang W, Gu W, Tang H, Mai Z, Xiao H, Zhao
J and Han J: The emerging role of MTHFD family genes in regulating
the tumor immunity of oral squamous cell carcinoma. J Oncol.
2022(4867730)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao J, Ding X, Ji J, Zhang L and Luo C:
Efficacy and safety of immune checkpoint inhibitors rechallenge in
advanced solid tumors: A systematic review and meta-analysis. Front
Oncol. 14(1475502)2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ma W, Xue R, Zhu Z, Farrukh H, Song W, Li
T, Zheng L and Pan CX: Increasing cure rates of solid tumors by
immune checkpoint inhibitors. Exp Hematol Oncol.
12(10)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Anagnostou V, Bardelli A, Chan TA and
Turajlic S: The status of tumor mutational burden and
immunotherapy. Nat Cancer. 3:652–656. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hong YD, Enewold L, Halpern MT, Zeruto C
and Mariotto AB: Use of pembrolizumab among older adults with
cancer in the United States, before and after FDA approval of its
tumor-agnostic indication. Pharmacoepidemiol Drug Saf.
33(e5745)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li C, Xiao Y, Kong J, Lai C, Chen Z, Li Z
and Xie W: Elucidating the role of MICAL1 in pan-cancer using
integrated bioinformatics and experimental approaches. Cell Adh
Migr. 18:1–17. 2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cao W, Xie Y, Cai L, Wang M, Chen Z, Wang
Z, Xv J, Wang Y, Li R, Liu X and Wang W: Pan-cancer analysis on the
role of KMT2C expression in tumor progression and immunotherapy.
Oncol Lett. 28(444)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang A, Miao K, Sun H and Deng CX: Tumor
heterogeneity reshapes the tumor microenvironment to influence drug
resistance. Int J Biol Sci. 18:3019–3033. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tzorakoleftheraki SE and Koletsa T: The
complex role of mast cells in head and neck squamous cell
carcinoma: A systematic review. Medicina (Kaunas).
60(1173)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Derakhshani A, Vahidian F, Alihasanzadeh
M, Mokhtarzadeh A, Lotfi Nezhad P and Baradaran B: Mast cells: A
double-edged sword in cancer. Immunol Lett. 209:28–35.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mocellin S, Marincola FM and Young HA:
Interleukin-10 and the immune response against cancer: A
counterpoint. J Leukoc Biol. 78:1043–1051. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ribatti D, Ennas MG, Vacca A, Ferreli F,
Nico B, Orru S and Sirigu P: Tumor vascularity and
tryptase-positive mast cells correlate with a poor prognosis in
melanoma. Eur J Clin Invest. 33:420–425. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Watabe T, Takahashi K, Pietras K and
Yoshimatsu Y: Roles of TGF-β signals in tumor microenvironment via
regulation of the formation and plasticity of vascular system.
Semin Cancer Biol. 92:130–138. 2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chiba Y, Mizoguchi I, Furusawa J, Hasegawa
H, Ohashi M, Xu M, Owaki T and Yoshimoto T: Interleukin-27 exerts
its antitumor effects by promoting differentiation of hematopoietic
stem cells to M1 macrophages. Cancer Res. 78:182–194.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Togashi Y, Shitara K and Nishikawa H:
Regulatory T cells in cancer immunosuppression-implications for
anticancer therapy. Nat Rev Clin Oncol. 16:356–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong
W, Yi M and Xiang B: Regulatory mechanisms of PD-1/PD-L1 in
cancers. Mol Cancer. 23(108)2024.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sabit H, Arneth B, Abdel-Ghany S, Madyan
EF, Ghaleb AH, Selvaraj P, Shin DM, Bommireddy R and Elhashash A:
Beyond cancer cells: How the tumor microenvironment drives cancer
progression. Cells. 13(1666)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeutic targets. J Hematol Oncol. 15(61)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jechorek D, Haeusler-Pliske I, Meyer F and
Roessner A: Diagnostic value of syndecan-4 protein expression in
colorectal cancer. Pathol Res Pract. 222(153431)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zeng Y, Luo Y, Zhao K, Liu S, Wu K, Wu Y,
Du K, Pan W, Dai Y, Liu Y, et al: m6A-mediated induction of
7-dehydrocholesterol reductase stimulates cholesterol synthesis and
cAMP signaling to promote bladder cancer metastasis. Cancer Res.
84:3402–3418. 2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Palumbo JS and Degen JL: Mechanisms
linking tumor cell-associated procoagulant function to tumor
metastasis. Thromb Res. 120 (Suppl 2):S22–S28. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ricklin D, Hajishengallis G, Yang K and
Lambris JD: Complement: A key system for immune surveillance and
homeostasis. Nat Immunol. 11:785–797. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Monteith GR, McAndrew D, Faddy HM and
Roberts-Thomson SJ: Calcium and cancer: Targeting Ca2+ transport.
Nat Rev Cancer. 7:519–530. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ahrberg Y, Dallmann J, Freitag J, Hassan
A, Jung C, Kiefer J, Muralidharan AM, Peter M and Beck JD: CIMT
2024: Report on the 21st annual meeting of the association for
cancer immunotherapy. Hum Vaccin Immunother.
20(2381925)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kang K, Xie F, Wu Y, Han C, Bai Y, Long J,
Lian X and Zhang F: Genomic instability in lower-grade glioma:
Prediction of prognosis based on lncRNA and immune infiltration.
Mol Ther Oncolytics. 22:431–443. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Han H, Su H, Lv Z, Zhu C and Huang J:
Identifying MTHFD1 and LGALS4 as potential therapeutic targets in
prostate cancer through multi-omics mendelian randomization
analysis. Biomedicines. 13(185)2025.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hui Y, Leng J, Jin D, Wang G, Liu K, Bu Y
and Wang Q: BRG1 promotes liver cancer cell proliferation and
metastasis by enhancing mitochondrial function and ATP5A1 synthesis
through TOMM40. Cancer Biol Ther. 25(2375440)2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Tadic S and Martínez A: Nucleic acid
cancer vaccines targeting tumor related angiogenesis. Could mRNA
vaccines constitute a game changer? Front Immunol.
15(1433185)2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kubota Y, Han Q, Aoki Y, Masaki N, Obara
K, Hamada K, Hozumi C, Wong ACW, Bouvet M, Tsunoda T and Hoffman
RM: Synergy of combining methionine restriction and chemotherapy:
The disruptive next generation of cancer treatment. Cancer Diagn
Progn. 3:272–281. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Qiu Y, Xie E, Xu H, Cheng H and Li G:
One-carbon metabolism shapes T cell immunity in cancer. Trends
Endocrinol Metab. 35:967–980. 2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Pereira JA, Lanzar Z, Clark JT, Hart AP,
Douglas BB, Shallberg L, O'Dea K, Christian DA and Hunter CA: PD-1
and CTLA-4 exert additive control of effector regulatory T cells at
homeostasis. Front Immunol. 14(997376)2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Xiao B, Li G, Gulizeba H, Liu H, Sima X,
Zhou T and Huang Y: Choline metabolism reprogramming mediates an
immunosuppressive microenvironment in non-small cell lung cancer
(NSCLC) by promoting tumor-associated macrophage functional
polarization and endothelial cell proliferation. J Transl Med.
22(442)2024.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li Z, Ke H, Cai J, Ye S, Huang J, Zhang C,
Yuan M, Lan P and Wu X: MTHFD1 regulates autophagy to promote
growth and metastasis in colorectal cancer via the PI3K-AKT-mTOR
signaling pathway. Cancer Med. 13(e70267)2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang X and Wang Z: Targeting SHMTs and
MTHFDs in cancer: Attractive opportunity for anti-tumor strategy.
Front Pharmacol. 15(1335785)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Groth M, Moissiard G, Wirtz M, Wang H,
Garcia-Salinas C, Ramos-Parra PA, Bischof S, Feng S, Cokus SJ, John
A, et al: MTHFD1 controls DNA methylation in Arabidopsis. Nat
Commun. 7(11640)2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Manjili MH and Butler SE: Role of tregs in
cancer dormancy or recurrence. Immunol Invest. 45:759–766.
2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hoffmann F, Franzen A, de Vos L, Wuest L,
Kulcsár Z, Fietz S, Maas AP, Hollick S, Diop MY, Gabrielpillai J,
et al: CTLA4 DNA methylation is associated with CTLA-4 expression
and predicts response to immunotherapy in head and neck squamous
cell carcinoma. Clin Epigenetics. 15(112)2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Grant CE, Flis AL, Toulabi L, Zingone A,
Rossi E, Aploks K, Sheppard H and Ryan BM: DRD1 suppresses cell
proliferation and reduces EGFR activation and PD-L1 expression in
NSCLC. Mol Oncol. 18:1631–1648. 2024.PubMed/NCBI View Article : Google Scholar
|