Introduction
Chronic lymphocytic leukemia (CLL) and small
lymphocytic lymphoma (SLL) are different manifestations of a mature
B-cell neoplasm with a progressive accumulation of monoclonal B
lymphocytes. CLL accounts for ~25-35 and 10% of all cases of
leukemia in the United States and Asian countries, respectively
(1,2). The incidence rate of SLL is
remarkably low in Asia and compared with patients with CLL/SLL in
Western countries, Asian patients with CLL/SLL are younger and have
an adverse prognosis (3). Compared
with other types of leukemia or lymphoma, cutaneous infiltration by
malignant leukocytes is far less common in patients with CLL/SLL
(4). The current study reported a
rare case of SLL with cutaneous and musculotendinous involvement in
the left lower extremity as the initial symptom.
Case report
A 65-year-old Asian man with chronic hepatitis B
virus infection presented with left calf and ankle pain along with
swelling and erythema lasting for 2 months. Although the patient
was treated for presumed cellulitis with empiric antibiotics, no
clinical improvement was observed. The patient arrived at the
emergency room of the Tri-Service General Hospital (Taipei, Taiwan)
with symptoms of progressive painful swelling and difficulty
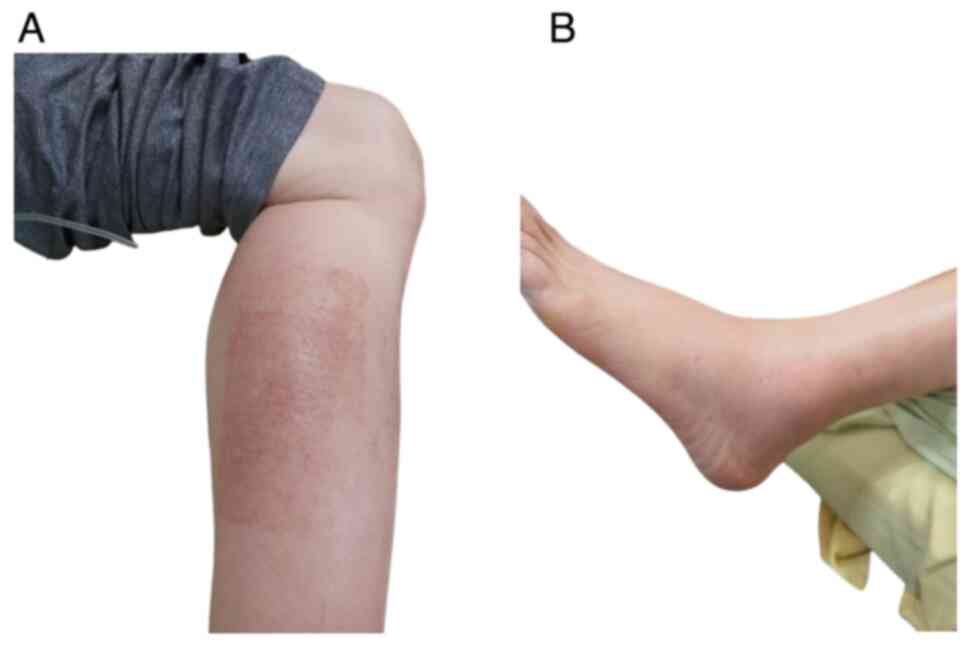
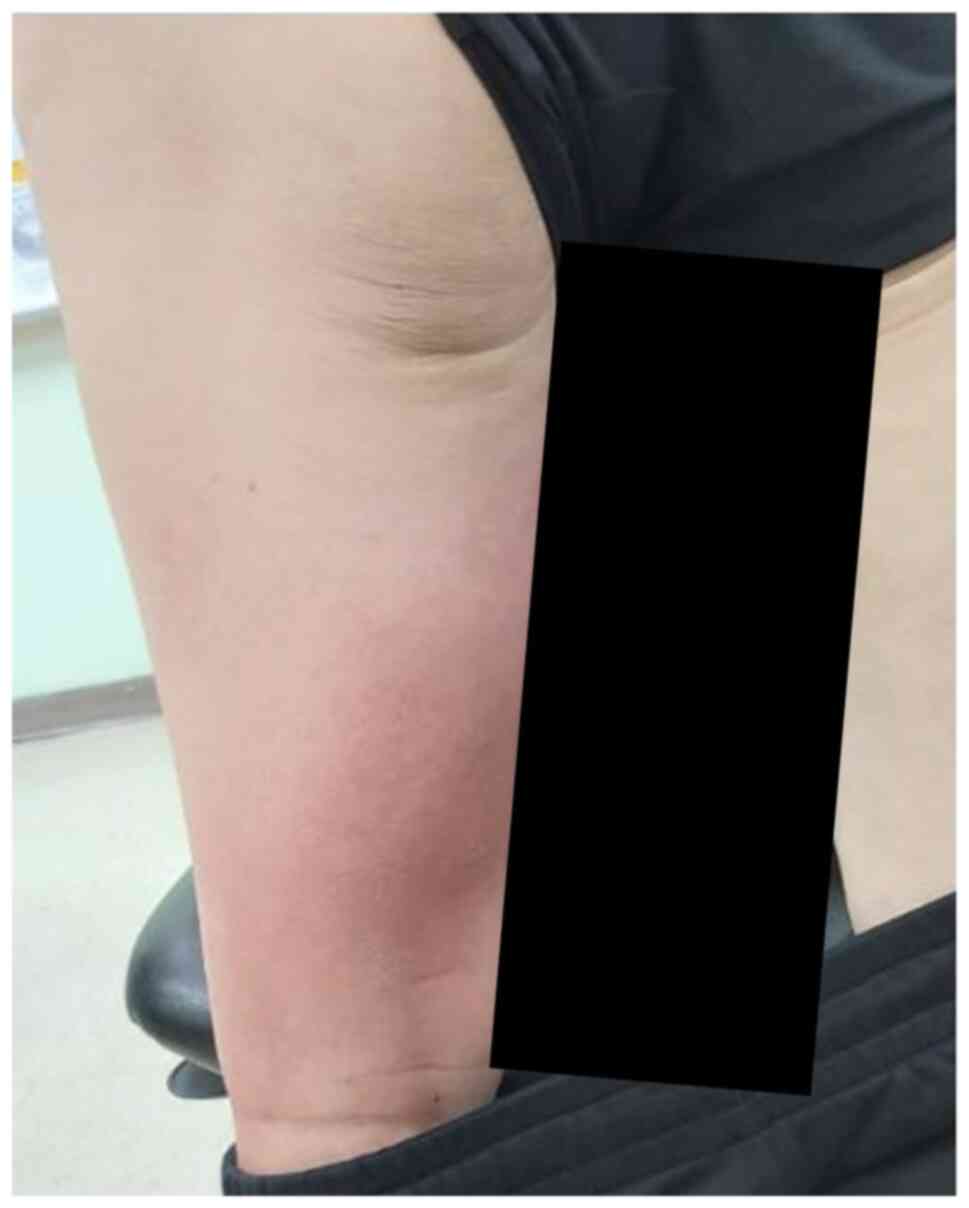
walking in October 2021. On examination, erythematous changes over
the left ankle and calf along with tenderness were observed
(Fig. 1). Homan's sign of the left
lower extremity was negative and the results of initial biochemical
tests were inconclusive, except for mild bicytopenia and elevation
of C-reactive protein levels. The hemoglobin level was 8.2 g/dl
(reference range: 13.5-17.5 g/dl in adult males), the mean
corpuscular volume was 80.4 fl (reference range: 80-100 fl in
adults) and the platelet count was 145x103/µl (reference
range: 150-350x103/µl in adults). The serum C-reactive
protein level was 0.83 mg/dl (reference range: >0.3 mg/dl in
adults). There was no leukocytosis or lymphocytosis. The total
white blood cell count was 6.78x103/µl (reference range:
4.5-11x103/µl in adults), the absolute neutrophil count
was 2.12x103/µl. (reference range:
1.8-7.7x103/µl in adults) and the absolute lymphocyte
count was 3.02x103/µl (reference range:
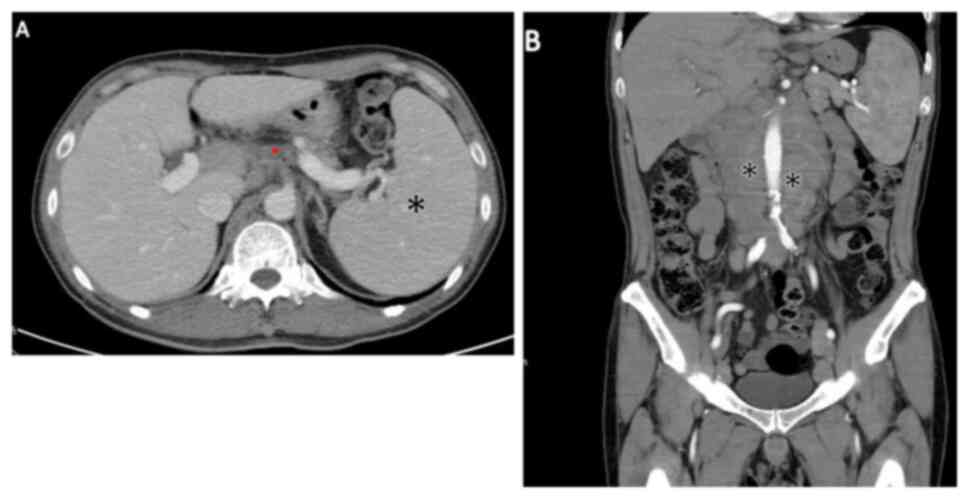
1.5-4x103/µl in adults). Abdominal computed tomography
(CT) revealed multiple paraaortic, iliac and inguinal
lymphadenopathy (Fig. 2).
Therefore, an excisional biopsy of the left inguinal region was
performed in November 2021 and the histopathological report
revealed B-cell lymphoma characterized by lymphoid structure
effacement with monotonous small-sized hyperchromatic malignant
cells. Protocols are provided in Data
S1 and the protocols of IHC for the same markers are the same
over different tissue (lymph node, bone marrow and skin).
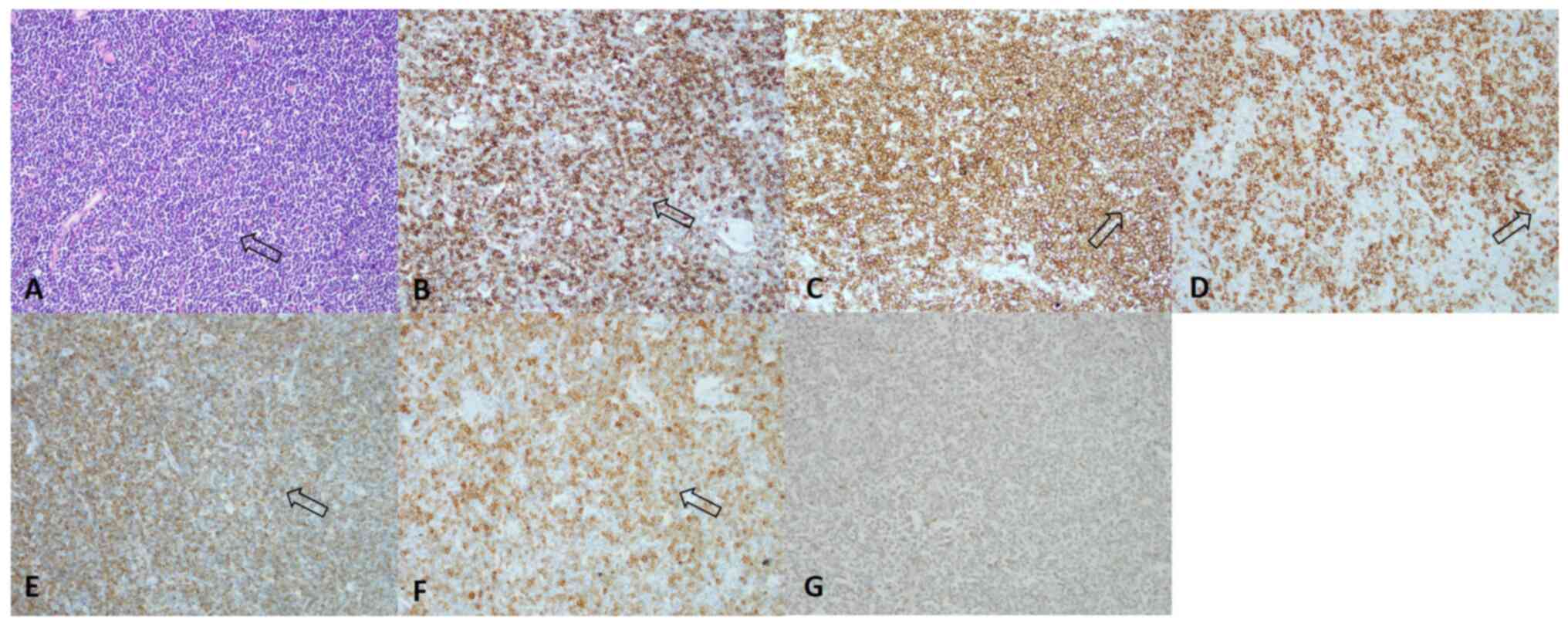
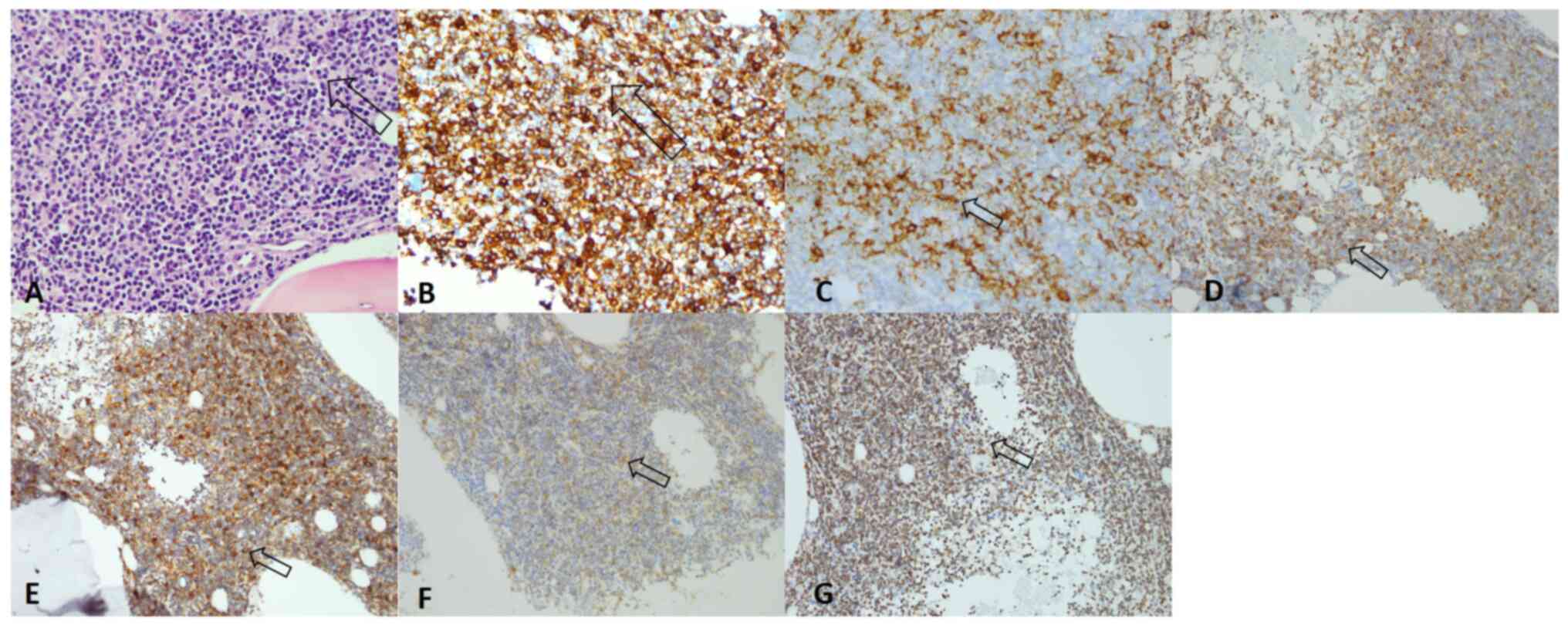
Immunohistochemical (IHC) staining results (positive for CD5, CD20,
CD23, Bcl-2, CD79a and negative for cyclin D1 for malignant cells)
indicated CLL/SLL (Figs. 3 and
S1). The degree of small round
lymphocyte infiltration in the lymph node was ~90%. In addition,
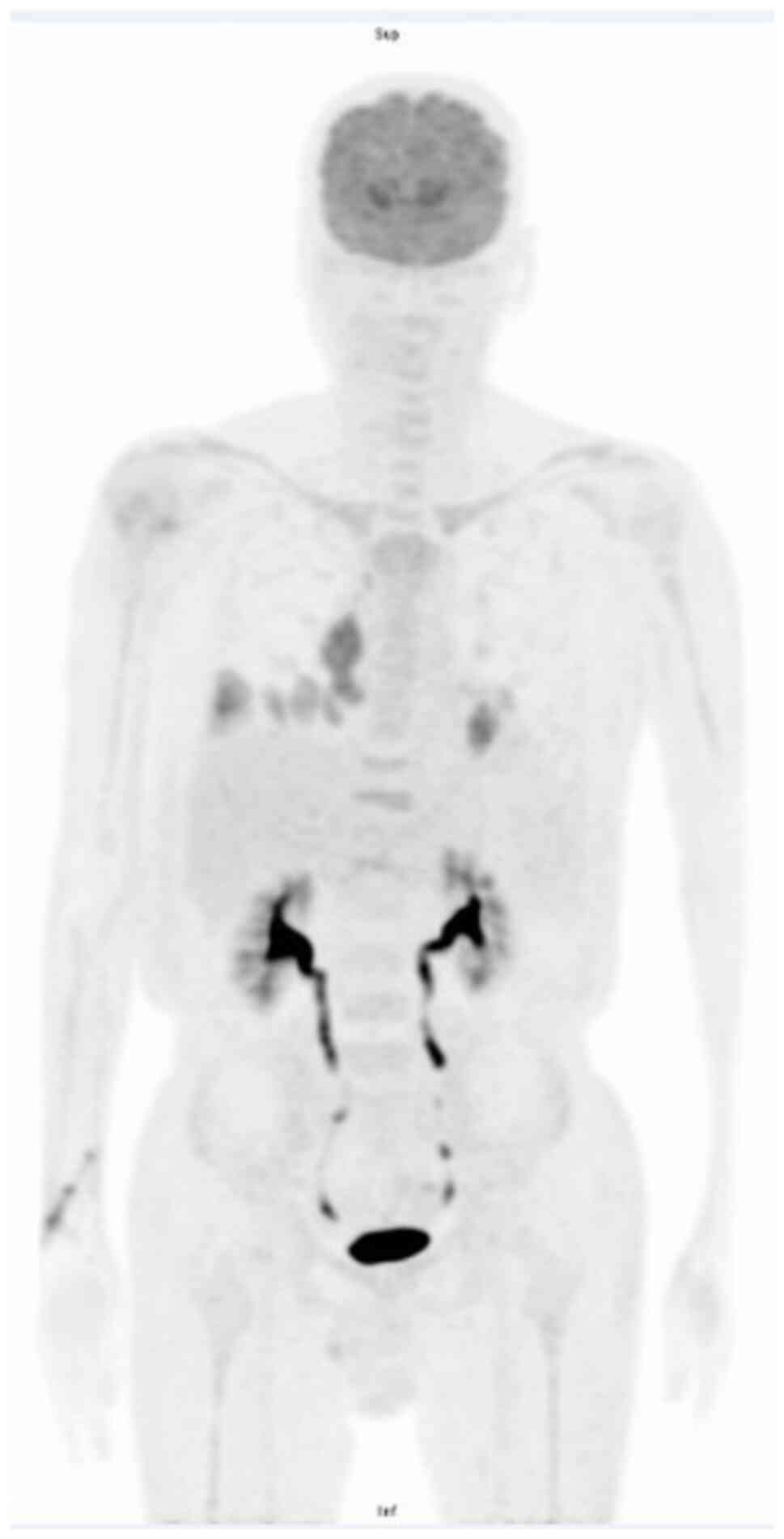
after fasting for 6 h, the patient underwent 18-fluorodeoxyglucose
positron emission tomography/computed tomography
(18F-FDG PET/CT) in November 2021 (protocol provided in
Data S2, which revealed multiple
low-grade fluorodeoxyglucose uptake nodules over several lymphatic
sites, including gastrohepatic and hepatoduodenal ligaments,
para-aortocaval spaces and bilateral iliac chains, except for the
skin and musculoskeletal system (Fig.
4). Histology of a bone marrow biopsy (November 2021) indicated
a homogenous population of small lymphocytes infiltrating the
myeloid tissue. The IHC staining results (malignant cells positive
for CD5, CD20, CD23, CD43, CD79a and Bcl-2) were in favor of a
prognosis of CLL/SLL (Fig. 5). The
degree of small round lymphocyte infiltration in the bone marrow
was ~70%. According to the International Workshop on CLL guidelines
(5), the definition of SLL
requires the presence of lymphadenopathy and the absence of
cytopenias caused by a clonal marrow infiltrate. In addition, the
number of B lymphocytes in the peripheral blood should be
#x003C;5x109/l. In SLL, the diagnosis should be
confirmed by histopathological evaluation of a lymph node biopsy or
biopsy of other tissues. A diagnosis of SLL in Lugano stage IV,
with multiple lymphadenopathies and bone marrow infiltration, was
established and fluorescence in situ hybridization analysis
of the short arm of chromosome 17(6) revealed no deletions (the FISH panels
including the probes for 11q22.3 (ATM), 17p13.1 (Tp53), 12p11.1-q11
(CEP 12), 13q34, 13q14.3 (LSI D13S319) were all from Vysis, Inc.
Based on the MABLE study (7) and
the reimbursement of the National Health Insurance (NHI), the
patient received one treatment course of chemoimmunotherapy with
rituximab and bendamustine (RB) in November 2021. The RB regimen
was as follows: Rituximab 375 mg/m2 intravenously (IV)
on day 1, plus RB 90 mg/m2 IV on days 1 and 2. Although
the patient's cutaneous symptoms on the left calf and ankle
improved in the following two weeks, new erythematous skin changes
developed over the dorsal side of the left internal thigh two
months later from initial encounter (Fig. 6).
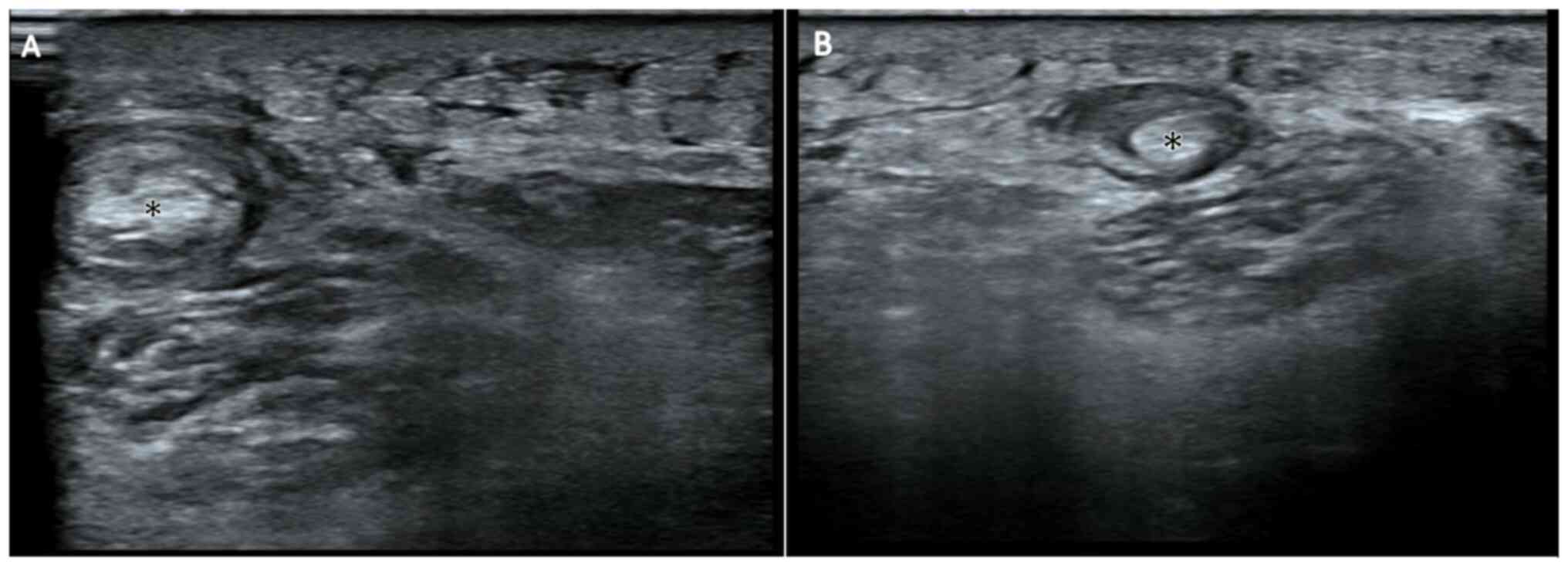
Ultrasonography of the left posterior thigh in
December 2021 demonstrated target-like lesions over semimembranosus
muscles and tendons with a hypoechoic rim and hyperechoic core
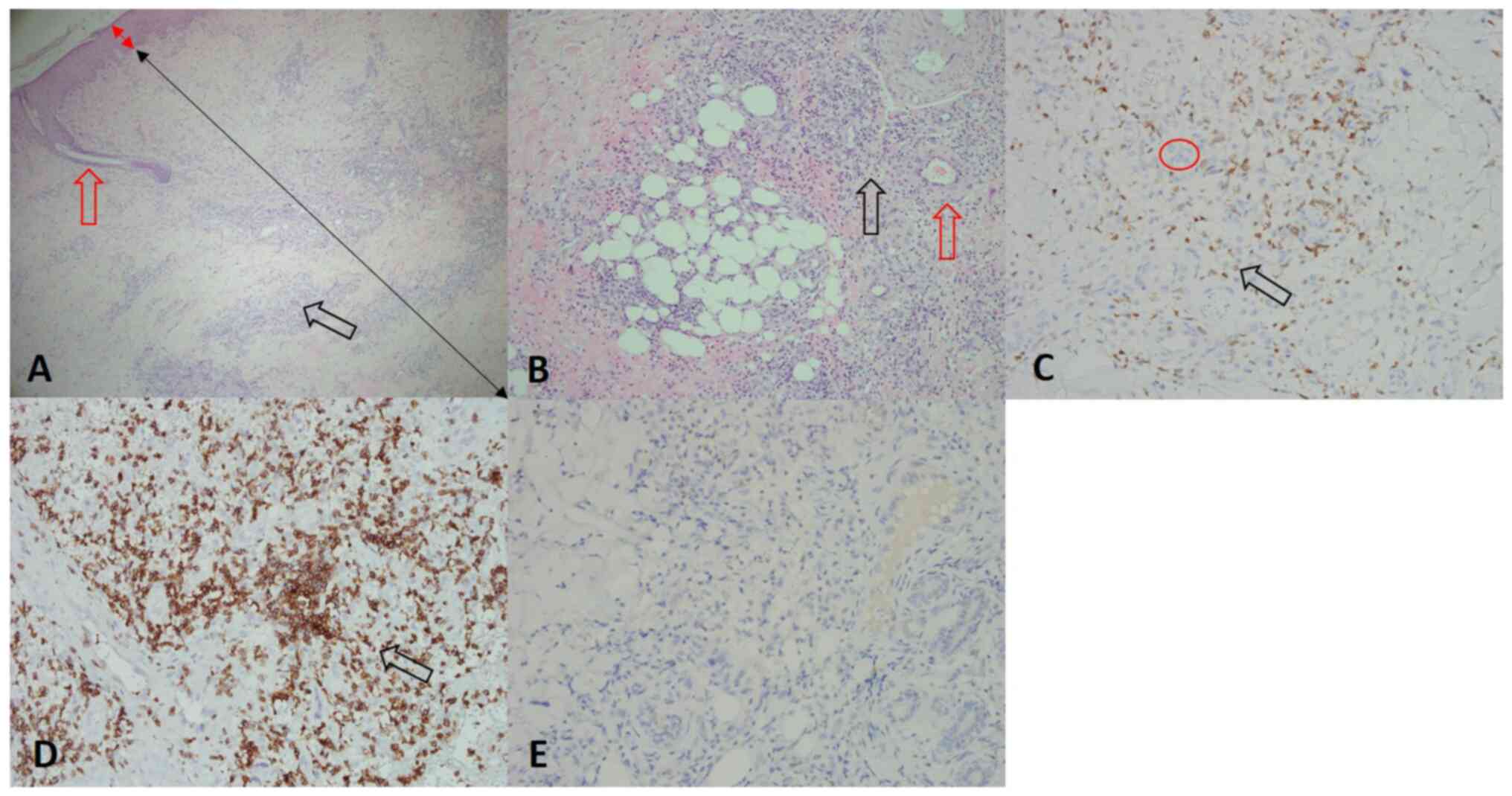
(Fig. 7). Examination of a skin
biopsy of the lesion site in December 2021 revealed atypical small
lymphocytes with perivascular infiltration of the skin dermis. The
severity of atypical small lymphocyte infiltration in the dermal
layer was ~50%. In addition, the results of IHC staining were
positivity of reactive T cells for CD3 (Fig. 8C), as well as positivity of
malignant cells (the abnormal lymphocytes that exhibit staining are
the malignant cells) for CD5 (Fig.
8D) but negativity for CD3 (Fig.
8C) and CD20 (Fig. 8E).
According to the patient's medical records and IHC results, this
finding was consistent with lymphoma cutis, with suspected
semimembranosus involvement. The patient had received two cycles of
the RB regimen (November and December 2021) and the regimen was
switched to R-CHOP (rituximab, cyclophosphamide, doxorubicin,
vincristine and prednisolone) in January 2022 under reimbursement
in Taiwan because of the inadequate treatment response and the
unaffordability of the Bruton's tyrosine kinase inhibitor (BTKi)
combination (the BTKi used in CLL/SLL includes ibrutinib,
acalabrutinib and zanubrutinib) at that time. The dosages of R-CHOP
included intravenous infusion of rituximab (375 mg/m2)
on day 1, cyclophosphamide (750 mg/m2) on day 1,
doxorubicin (50 mg/m2) on day 1, vincristine (1.4
mg/m2) on day 1 and oral prednisone (40
mg/m2) on days 1 to 5, for every three weeks per cycle
(R-CHOP is typically given in about six cycles and each cycle is
spaced three weeks apart). The patient's symptoms gradually
improved after three consecutive treatment courses. The follow-up
18F-FDG PET/CT 4.5 months after the initial encounter
for the evaluation of treatment response revealed a stable disease
status compared with the initial scan, without any evidence of
substantially abnormal FDG uptake (Fig. 9). Considering the possible
significant adverse events of chemotherapy, including
myelosuppression, nausea and fatigue, after 6 cycles of R-CHOP,
ibrutinib was administered at the patient's own expense and
rituximab infusion until the supply of rituximab for reimbursement
was exhausted in January 2024(8).
Ibrutinib was also discontinued at that month due to its cost. The
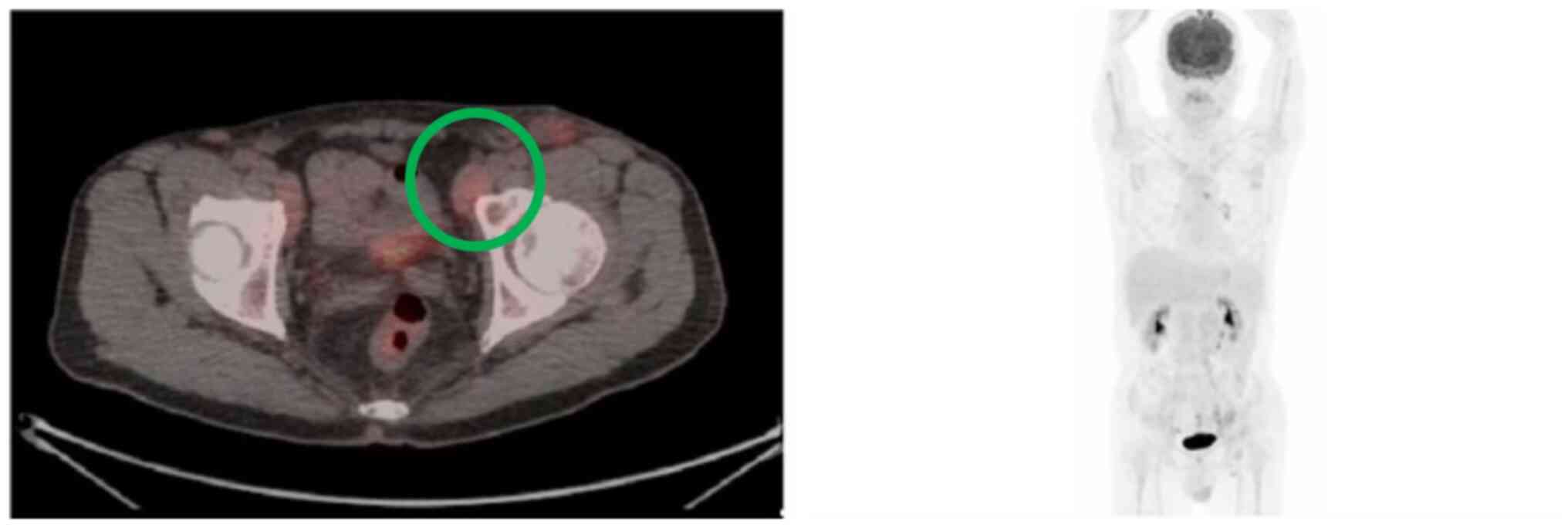
patient underwent a whole-body PET scan in January 2024 (Fig. 10A). Compared to the previous scan
from August 2023 [little interval change with moderate FDG-avidity
over several lymph nodes involving the para-vascular [maximal
standardized uptake value (SUVmax)=4.7], right lower paratracheal
(SUVmax=4.4), left-sided paraaortic (SUVmax=6.3) and RLL nodule
(SUVmax=5.0), suspicious residual malignancy in stable status],
which revealed residual malignancy in a stable state [Deauville
(9) score, 4], the current imaging
results may indicate lymphoma, likely accompanied by inflammation,
affecting the mediastinum, right lower lobe of the lung and skin on
the left buttock, with the patient remaining in a relatively stable
condition during treatment. In September 2024, the patient had
another whole-body PET scan (Fig.
10B). When compared to the FDG PET/CT from January 2024, the
latest imaging findings again suggested lymphoma, possibly mixed
with inflammation, involving the same areas post-treatment in a
relatively stable disease condition (Deauville score, 3-4). The
patient remains in a relatively stable cutaneous condition with
regular medical follow-up at three-month intervals without any
disease-specific therapy at the outpatient department.
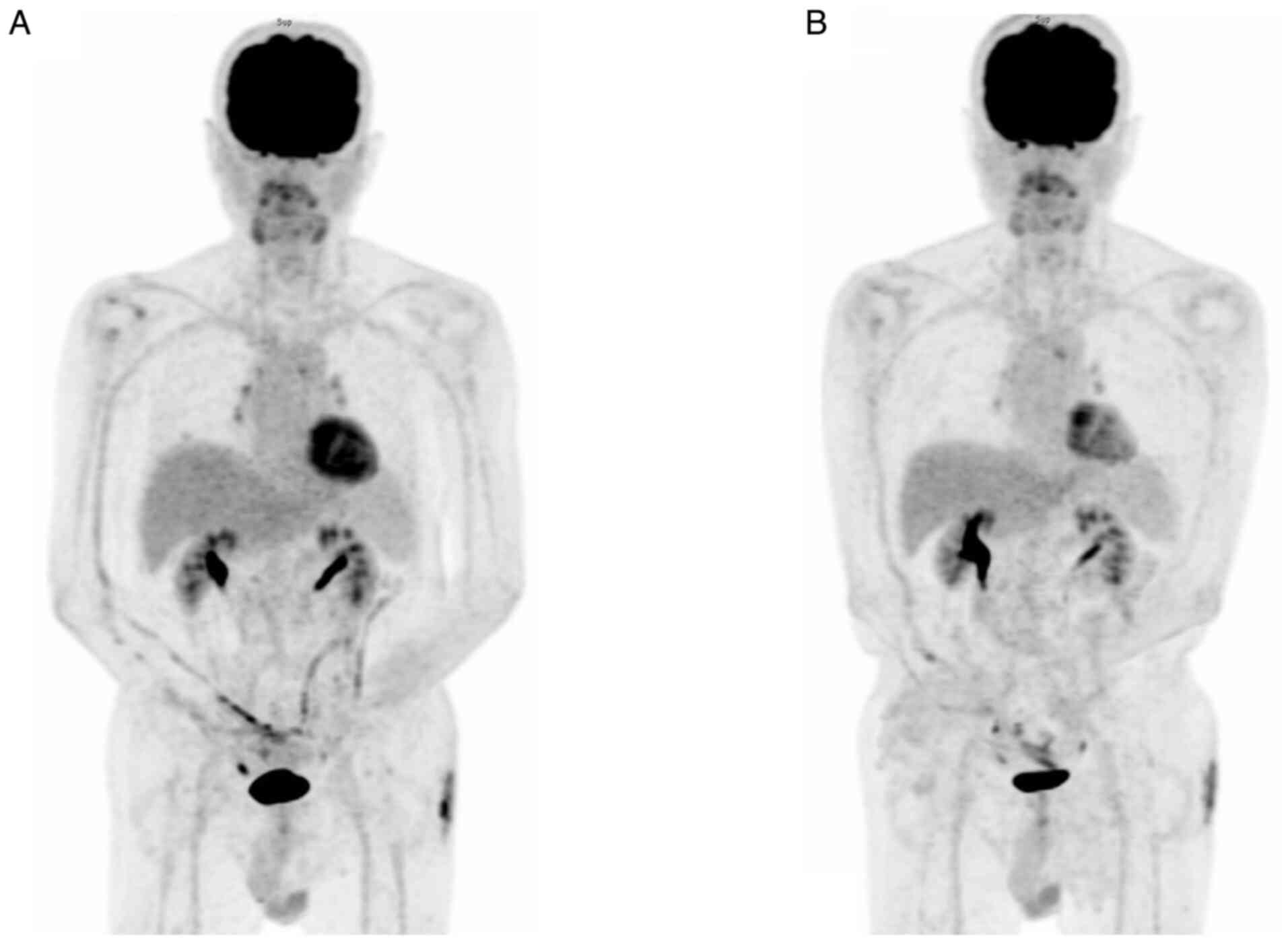 | Figure 10Follow-up PET scans. (A) PET scan in
January 2024 (about two years and three months after the first
encounter). Relatively stable appearance of the previously noted
FDG-avid lesions over the mediastinum (e.g. AP window LN, SUVmax:
4.1), RLL lung (SUVmax: 2.5) and cutaneous region of the left
buttock (SUVmax: 7.2), suggesting lymphoma (probably mixed with
inflammation) under treatment in a relatively stable disease
condition (SD; Deauville score, 4). Furthermore, normal
physiological FDG accumulation in the cerebral cortex, adenoids of
tonsils, vocal cords, heart, aorta, T-L spines, renal pelves,
ureters and bladder was seen. (B) PET scan in September 2024 (about
two years and eleven months later after first encounter). The study
showed a relatively stable appearance of the previously noted
FDG-avid lesions over the mediastinum (e.g. AP LN, SUVmax: 3.8;
previously 4.1), RLL lung (SUVmax: 1.6; previously 2.5) and
cutaneous region of the left buttock (SUVmax: 4.8; previously 7.2),
suggesting lymphoma (probably mixed with inflammation)
post-treatment in a relatively stable disease condition (SD;
Deauville score, 3-4). Furthermore, normal physiological FDG
accumulation in the cerebral cortex, adenoids of tonsils, vocal
cords, heart, aorta, T-L spines, renal pelves, ureters and bladder
was seen. PET, positron emission tomography; FDG,
18-fluorodeoxyglucose; RLL, right lower lobe; SD, stable disease;
AP window, aortopulmonary window; LN, lymph node; SUVmax, maximal
standardized uptake value. |
Discussion
Review of the literature indicated that
dermatomuscular manifestations of CLL/SLL are uncommon and may be
accompanied by various types of skin reactions, including papule,
nodule, patch, plaque or ulcerative lesion (10). The most common sites of skin
involvement are the head and neck rather than the trunk or
extremities (11). Patients with
these conditions are considered to be at an increased risk of
secondary cutaneous malignancies, such as squamous cell carcinoma
(12). The majority of cases with
skeletal muscle involvement are mostly diagnosed with diffuse large
B-cell lymphoma (13). The patient
of the current study presented with recurrent painful swelling and
erythema over the left extremity, which raised the suspicion of
malignancy and was confirmed by the histopathological report. This
clinical manifestation of leukemia/lymphoma originating from a
B-cell lineage, which mimicked recurrent cellulitis, increased the
difficulty of early diagnosis and delayed the standard therapy.
IHC staining of the left inguinal lymph node biopsy
revealed positivity of malignant cells for CD5, CD20, CD23, Bcl-2
and CD79a and negativity for cyclin D1. IHC analysis of the bone
marrow aspirate indicated positive staining of malignant cells for
CD5, CD20, CD23, CD43, CD79a and Bcl-2. It was possible to
differentiate the origin of B cells of CLL/SLL from the other
possible B-cell lineage lymphoma (14). Histological analysis of the skin
biopsy by H&E staining indicated infiltration of abnormal
hyperchromatic cells. After IHC processing, positive CD5 staining
of malignant cells, along with negative CD3 and CD20 staining of
malignant cells, were noted. Based on the previous therapeutic
course, which included rituximab infusion, the likely diagnosis was
SLL with infiltration of the dermal layer of the left thigh.
Cyclin D1 serves as a valuable marker for
differentiating mantle cell lymphoma (MCL) from other small B-cell
lymphomas, as MCL typically expresses high levels of cyclin D1.
Most tumors also exhibit CD19, CD20 and moderately high levels of
surface immunoglobulin (Ig) (usually IgM and IgD, with either κ or
λ light chains). MCL is generally CD5-positive and CD23-negative,
which aids in distinguishing it from CLL and SLL. The diagnosis of
MCL is usually confirmed through a biopsy of lymph nodes, tissue
and bone marrow, revealing the characteristic morphology of
monomorphic small- to medium-sized lymphoid cells with irregular
nuclear contours. The hallmark chromosomal translocation
t(11;14)(q13;q32) is present in most cases of MCL and is
responsible for the abnormal expression of cyclin D1, which is not
typically found in normal lymphocytes. Immunohistochemistry can
detect cyclin D1 in ~98% of MCL cases (15).
The left inguinal lymph node biopsy specimen
exhibited IHC staining for cyclin D1 and accordingly, mantle cell
lymphoma was less considered. In addition, the IHC staining of
lymph node and bone marrow specimens both demonstrated small,
atypical lymphocytes with positive staining for CD5 and CD23, which
excluded mantle-cell lymphoma, marginal zone lymphoma and large
B-cell lymphoma. Besides, lymphoplasmacytic lymphoma (LPL) and SLL
are both characteristic of abnormal small lymphocytes on H&E
staining. The typical IHC staining result of malignant cells of LPL
may be negative for CD5 but positive for CD138 on the IHC stain.
The clinical presentation of patients with LPL is varied and can
present with symptoms related to tumor infiltration
(lymphadenopathy, organomegaly, cytopenias) or monoclonal protein
production (hyperviscosity, neuropathy). The pathology of LPL is
typically composed of small B cells, plasmacytoid lymphocytes and
plasma cells involving the bone marrow. The above findings were
inconsistent with the pathological IHC staining for the present
case. As there was no peripheral blood lymphocytosis, the diagnosis
was SLL rather than CLL. The diagnosis of CLL requires the presence
of at least 5x109/l B lymphocytes in the peripheral
blood. Confirmation of the clonality of these B lymphocytes is
achieved by demonstrating Ig light chain restriction through flow
cytometry. However, numerous cases of SLL do not exhibit B
lymphocytosis and flow cytometry is not typically part of the
diagnostic criteria for this disease. In addition, our hospital
does not routinely provide access to flow cytometry or Ig heavy
chain variable assessments. For the patient of the present study,
the musculotendinous involvement of lymphoma over the left
posterior thigh was suspected according to the clinical images and
the patient refused muscle biopsy due to the invasiveness of this
procedure.
Currently, the Lugano classification is used for
lymphoma staging with the aid of a PET/CT scan, which was derived
from the Ann Arbor staging system (16). The patient was staged using the
Lugano classification instead of the Ann Arbor staging system
because PET-CT is effective for assessing response in FDG-avid
histology using a 5-point scale, while CT is preferred for cases
with low or variable FDG avidity, particularly when aggressive
transformation is suspected (16).
SLL with Lugano stage IV was finally confirmed because the lymphoma
involved multiple lymph nodes and bone marrow, without peripheral
blood lymphocytosis.
According to the National Comprehensive Cancer
Network guidelines for CLL/SLL, the preferred therapeutic regimens
for CLL/SLL without del(17p)/TP53 mutation include BTKi
with/without anti-CD20 monoclonal antibodies, venetoclax with
obinutuzumab or (chemo)immunotherapy (17). BTKi and venetoclax are not covered
under the reimbursement policies of the NHI in Taiwan based on the
patient's diagnosis and clinical condition. After discussion with
the patient and his family about therapeutic effects, possible
adverse events and the cost-effectiveness, they opted for ibrutinib
at their personal expense and rituximab infusion until the
reimbursed supply of rituximab was exhausted. The initial choice of
the RB regimen for the patient was based on the MABLE study
(7) and the reimbursement policies
of the NHI. In addition, SLL is typically classified as an indolent
lymphoma, which is why the treatment strategy did not initially
include the R-CHOP regimen, as this is primarily indicated for more
aggressive non-Hodgkin's lymphomas, such as diffuse large B-cell
lymphoma or advanced follicular lymphoma. The patient received two
cycles of the RB regimen in late November and late December 2021.
However, due to an inadequate treatment response, extranodal
involvement and the high cost of BTKi, the regimen was switched to
R-CHOP in late January 2022 under NHI reimbursement.
Concerning the skin biopsy results of the left
thigh, the IHC staining results were positive for CD5 but negative
for CD23 and CD20. In terms of the phenotypic changes of IHC
staining of CD20-positive B-cell lymphoma, Maeshima et al
(18) observed that after
rituximab-containing chemotherapies, CD20 protein-negative or
-decreased phenotypic changes were confirmed in 5 cases among 36
patients when the disease progressed. Until a CD20-negative
phenotypic change occurred, 4 to 14 cycles of rituximab were
administered and the duration of relapse or progression from their
first treatment with rituximab ranged from 2 to 81 months. There is
no exact duration from initiation of rituximab-containing therapy
to CD20-negative transformation due to the rarity of this
population. Of note, due to IHC staining results of CD20 changing
after rituximab therapy (one of the chimeric monoclonal antibodies
targeted against CD20), which is one of the major mechanisms of
treatment resistance in non-Hodgkin B cell lymphomas (19), clinicians are required to follow up
the treatment responses closely and switch the regimen in a timely
manner. A limitation of the present study is that IHC co-staining
could not be performed at our laboratory due to the lack of
applicable antibodies, which was an obstacle for differential
diagnosis.
In summary, due to the diverse etiologies of
dermatomuscular manifestations, it is crucial to actively monitor
treatment responses. The potential for malignancy should always be
included in the differential diagnoses. In addition, in newly
diagnosed cases of SLL, thorough investigation of skin changes or
symptoms is essential for accurate clinical staging and selection
of appropriate treatment regimens.
Supplementary Material
Hematoxylin and eosin stains of the
left inguinal lymph node biopsy. Generally, the lymph node is
penetrated by numerous afferent lymph vessels, which extend to the
deeper areas of the lymph node by way of the trabecular extensions
of the cortex. The paracortical region, the periphery of a lymph
node, is the lymphoid follicle. However, in these slices there is
infiltration of homogenous hyperchromatic cells in almost the
entire field of view. The lymph vessels and trabecular structures
could merely be seen in these slices. (A) Magnification, x40; (B)
magnification, x100; (C) magnification, x200.
Protocols for immunohistochemical
(IHC) and hematoxylin and eosin (H&E) staining.
Protocol for
18F-fludeoxyglucose-positron emission tomography/computed
tomography (18F-FDG-PET/CT).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YLC drafted the manuscript. SWL conceptualized and
designed the study. YYC processed the H&E and IHC staining of
the tissue specimens and provided the interpretation for
pathological results. YLC, SWL and YYC collected clinical
information and performed the analysis. TCH, YYC and SWL
interpreted the data and critically reviewed and revised the
manuscript. YLC and SWL confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Tri-Service General Hospital (Taipei, Taiwan;
approval no. A202215163).
Patient consent for publication
Informed consent was obtained from the patient for
the publication of this case report, including the personal medical
history and images without disclosing the patient's identity.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu SJ, Huang SY, Lin CT, Lin YJ, Chang CJ
and Tien HF: The incidence of chronic lymphocytic leukemia in
Taiwan, 1986-2005: A distinct increasing trend with birth-cohort
effect. Blood. 116:4430–4435. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ko BS, Chen LJ, Huang HH, Chen HM and
Hsiao FY: Epidemiology, treatment patterns and survival of chronic
lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) in
Taiwan, 2006-2015. Int J Clin Pract. 75(e14258)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Raufi A, Alsharedi M, Khelfa Y, Griswold
DC and Lebowicz Y: Leukemia cutis in a patient with chronic
lymphocytic leukemia presenting as bilateral helical nodules. SAGE
Open Med Case Rep. 4(2050313X16683624)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating M,
Montserrat E, Chiorazzi N, et al: iwCLL guidelines for diagnosis,
indications for treatment, response assessment, and supportive
management of CLL. Blood. 131:2745–2760. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Van Dyke DL, Werner L, Rassenti LZ,
Neuberg D, Ghia E, Heerema NA, Dal Cin P, Dell Aquila M,
Sreekantaiah C, Greaves AW, et al: The Dohner fluorescence in situ
hybridization prognostic classification of chronic lymphocytic
leukaemia (CLL): The CLL Research Consortium experience. Br J
Haematol. 173:105–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Michallet AS, Aktan M, Hiddemann W, Ilhan
O, Johansson P, Laribi K, Meddeb B, Moreno C, Raposo J, Schuh A, et
al: Rituximab plus bendamustine or chlorambucil for chronic
lymphocytic leukemia: Primary analysis of the randomized,
open-label MABLE study. Haematologica. 103:698–706. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Preetesh J, Michael JK, William GW,
Mariela S, Philip AT, Alessandra F, Zeev E, Hagop K, Susan O and
Jan AB: Long-term follow-up of treatment with ibrutinib and
rituximab in patients with high-risk chronic lymphocytic leukemia.
Clin Cancer Res. 23:2154–2158. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meignan M, Gallamini A and Haioun C:
Report on the first international workshop on interim-pet-scan in
lymphoma. Leuk Lymphoma. 50:1257–1260. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wagner G, Fenchel K, Back W, Schulz A and
Sachse MM: Leukemia cutis-epidemiology, clinical presentation, and
differential diagnoses. J Dtsch Dermatol Ge. 10:27–36.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lazarian G, Munger M, Quinquenel A,
Dilhuydy MS, Veronese L, Luque Paz D, Guièze R, Ledoux-Pilon A,
Paillassa J, Merabet F, et al: Clinical and biological
characteristics of leukemia cutis in chronic lymphocytic leukemia:
A study of the French innovative leukemia organization (FILO). Am J
Hematol. 96:E353–E356. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Velez NF, Karia PS, Vartanov AR, Davids
MS, Brown JR and Schmults CD: Association of advanced leukemic
stage and skin cancer tumor stage with poor skin cancer outcomes in
patients with chronic lymphocytic leukemia. JAMA Dermatol.
150:280–287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao S, Shu H and Yang H: Imaging features
of skeletal muscle lymphoma: A case report and literature review.
BMC Med Imaging. 21(136)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mollejo M and Piris MA: The complex
pathology and differential diagnosis of splenic and nodal marginal
zone lymphoma. Ann Lymphoma. 4:1–14. 2020.
|
|
15
|
Julie M Vose: Mantle cell lymphoma: 2017
update on diagnosis, risk-stratification, and clinical management.
Am J Hematol. 92:806–813. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance,
Australasian Leukaemia, Lymphoma Group, Eastern Cooperative
Oncology Group et al. Recommendations for initial
evaluation, staging, and response assessment of hodgkin and
non-hodgkin lymphoma: The lugano classification. J Clin Oncol.
32:3059–3068. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Comprehensive Cancer Network:
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (version
1.2024). https://www.nccn.org/professionals/physician_gls/pdf/cll_blocks.pdf.
|
|
18
|
Maeshima AM, Taniguchi H, Fujino T, Saito
Y, Ito Y, Hatta S, Yuda S, Makita S, Fukuhara S, Munakata W, et al:
Immunohistochemical CD20-negative change in B-cell non-Hodgkin
lymphomas after rituximab-containing therapy. Ann Hematol.
99:2141–2148. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hiraga J, Tomita A, Sugimoto T, Shimada K,
Ito M, Nakamura S, Kiyoi H, Kinoshita T and Naoe T: Down-regulation
of CD20 expression in B-cell lymphoma cells after treatment with
rituximab-containing combination chemotherapies: Its prevalence and
clinical significance. Blood. 113:4885–4893. 2009.PubMed/NCBI View Article : Google Scholar
|