1. Introduction
Lung transplantation (LTx) is a key therapeutic
option for patients with end-stage pulmonary disease, particularly
when other medical and interventional treatments-such as long-term
oxygen therapy, pulmonary rehabilitation, pharmacological
management (e.g., bronchodilators, corticosteroids) or noninvasive
ventilation-fail to achieve adequate disease control (1). With advancements in anesthesia and
surgical techniques in the past decades, short- and long-term
survival have improved and the use of LTx has gradually expanded
(2-4).
Anesthetic management involves specific challenges,
particularly during the critical periods of LTx, such as the
induction of anesthesia, the start of positive pressure
ventilation, establishment and maintenance of single-lung
ventilation, clamping and unclamping of the pulmonary artery (PA)
and reperfusion of the transplanted lung (5). Tailoring anesthesia management to the
underlying lung disease of the patient and the surgical process,
with a particular emphasis on maintaining hemodynamic stability, is
critically important (5). Although
standard non-invasive monitoring-such as electrocardiography,
non-invasive blood pressure measurement, pulse oximetry and
capnography-is commonly used, hemodynamic monitoring during LTx
must be tailored to the specific needs of each patient. PA
catheters are a standard monitoring tool that provide valuable
hemodynamic data, including PA pressure, cardiac output and mixed
venous oxygen saturation, which are essential for guiding
anesthesia and fluid management (6). However, transesophageal
echocardiography (TEE) works by inserting a catheter with an
ultrasound probe into the esophagus, enabling close-proximity,
multi-plane scanning of cardiac structures. This allows for
high-resolution, real-time imaging of cardiac anatomy, blood flow
and functional status. In addition, during intraoperative
extracorporeal membrane oxygenation (ECMO) support, hemodynamic
measurements obtained via pulmonary artery catheters or other
standard monitors may be compromised due to altered flow patterns
and non-pulsatile circulation; however, TEE provides direct
visualization of vascular structures and cardiac function, offering
multidimensional parameters and thereby allowing for a more
comprehensive and accurate hemodynamic evaluation (6).
TEE was first proposed in 1976, and can produce both
two-dimensional (2D) and three-dimensional (3D) images with high
resolution (5,7). This enables clinicians to detect
critical intraoperative pathophysiological changes, such as right
ventricular dysfunction or pulmonary vascular anastomotic
abnormalities, at an early stage and to implement timely
interventions to prevent serious complications (5). The guidelines established by the
American Society of Anesthesiologists and the Society of
Cardiovascular Anesthesiologists in 2020 recommend the use of TEE
in the management of patients undergoing LTx (8). Notably, the latest guidelines from
the International Society for Heart and Lung Transplantation for
the first time classify the use of TEE in LTx as class I
recommendation, indicating that there is evidence and/or general
agreement that the procedure is beneficial, useful and effective in
clinical practice (9). Current
guidelines consistently recommend initiating TEE promptly, as
standard hemodynamic assessments do not adequately inform clinical
decision-making (7-9). As a
multidisciplinary diagnostic and monitoring modality, the
intraoperative application of TEE necessitates close collaboration
among anesthesiology, surgical and critical care teams for
integrated image interpretation and real-time decision support
(10). Furthermore, TEE has been
integrated into critical care ultrasonography protocols,
particularly in mechanically ventilated patients, where its bedside
evaluation of cardiac function has been widely validated (11). Perioperative use of TEE facilitates
optimized hemodynamic management, thereby improves patient outcomes
by enabling timely interventions, reducing perioperative
complications, and supporting better organ perfusion (12). Nevertheless, comprehensive and
updated evidence summarizing the role of TEE during LTx remains
limited.
The present review summarizes the latest advances in
the application of TEE in LTx, with a particular focus on its role
in diagnosing and managing intraoperative complications. Compared
with previous reviews, the present review provides updated insights
into the integration of TEE in the intraoperative management of
ECMO (13,14).
2. Monitoring of LTx with TEE
TEE provides comprehensive, real-time data that
enable precise management tailored to each phase of LTx (8). Table
I outlines the essential TEE monitoring contents recommended
throughout the four main stages of the procedure (8,13,14).
After anesthesia induction, initial hemodynamic fluctuations are
common (5). Therefore, once
hemodynamic stability is achieved during Period 1, a baseline TEE
examination should be performed prior to chest opening, providing a
reference for the assessment of subsequent changes (13). During Period 1, preparatory steps
such as positive pressure ventilation and single-lung ventilation
may also induce hemodynamic fluctuations, requiring close TEE
monitoring to assess changes and adjust the management plan
(13). Period 3, defined as the
release of the PA and atrial [or pulmonary vein (PV)] clamps and
subsequent reperfusion of the transplanted lung, occurs in rapid
succession. Therefore, for monitoring and management purposes, they
are considered as a single critical period (Table I) (13,14).
 | Table ITransesophageal echocardiography
monitoring recommended across the LTx procedure. |
Table I
Transesophageal echocardiography
monitoring recommended across the LTx procedure.
| | Critical periods of
LTx |
|---|
| TEE monitoring | Period
1a | Period
2b | Period
3c | Period
4d |
|---|
| Cardiac
structure | Y | Y | Y | Y |
| Cardiac systolic
function | Y | Y | Y | Y |
| Cardiac diastolic
function | Y | Y | Y | Y |
| Guide ECMO cannula
placement | Y | N | N | N |
| Troubleshooting for
ECMO | N | Y | Y | Y |
| PV flow | Y | N | Y | Y |
| PA flow | Y | N | Y | Y |
| Anastomotic
site | N | N | Y | Y |
| Air embolism | N | N | Y | N |
| Thrombosis | N | N | Y | Y |
| Pulmonary
edema | N | N | Y | Y |
| Atelectasis | N | N | N | Y |
| Pericardial
effusion | N | N | N | Y |
| Pleural
effusion | N | N | N | Y |
3. Intraoperative TEE during hemodynamic
instability
Due to the proximity of the probe to the heart and
major blood vessels, TEE offers an ultrasonic window that
facilitates detailed visualization of cardiac anatomy and
hemodynamic characteristics during LTx (15). In 2021, the International Consensus
Recommendations for Anesthetic and Intensive Care Management of LTx
approved the use of TEE for hemodynamic monitoring during the
preoperative, intraoperative, and postoperative phases of
perioperative management (16).
Hemodynamic instability due to right
cardiac dysfunction
Pulmonary impairment in patients undergoing LTx
typically leads to cardiac compensation that can result in
long-term structural changes, often manifesting as pulmonary
hypertension (PH), right ventricular (RV) dilation and abnormal
ventricular septum (such as thickening or morphological changes)
(17,18). Before transplantation (designated
as period 1 in Table I), the heart
and PA should be systematically assessed to establish baseline
values, which helps identify the cause of hemodynamic instability
(14). During transplantation
(periods 2 and 3, Table I), acute
hemodynamic changes caused by PA clamping/release, increased
pulmonary vascular resistance (PVR) due to reperfusion, or shifts
in RV load upon ECMO initiation/withdrawal can worsen pre-existing
right heart dysfunction (19,20).
RV function and PA pressure show improvement and a trend towards
normalization after LTx (period 4, Table I) and post-transplant RV function
may serve as a prognostic indicator for patient outcomes (21). Although RV dysfunction is not
unique to LTx, it is commonly observed during the procedure;
therefore, continuous intraoperative monitoring of right heart
function is highly recommended (13). TEE is regarded as a valuable and
widely accepted technique to guide the intraoperative management of
PH and RV failure during LTx.
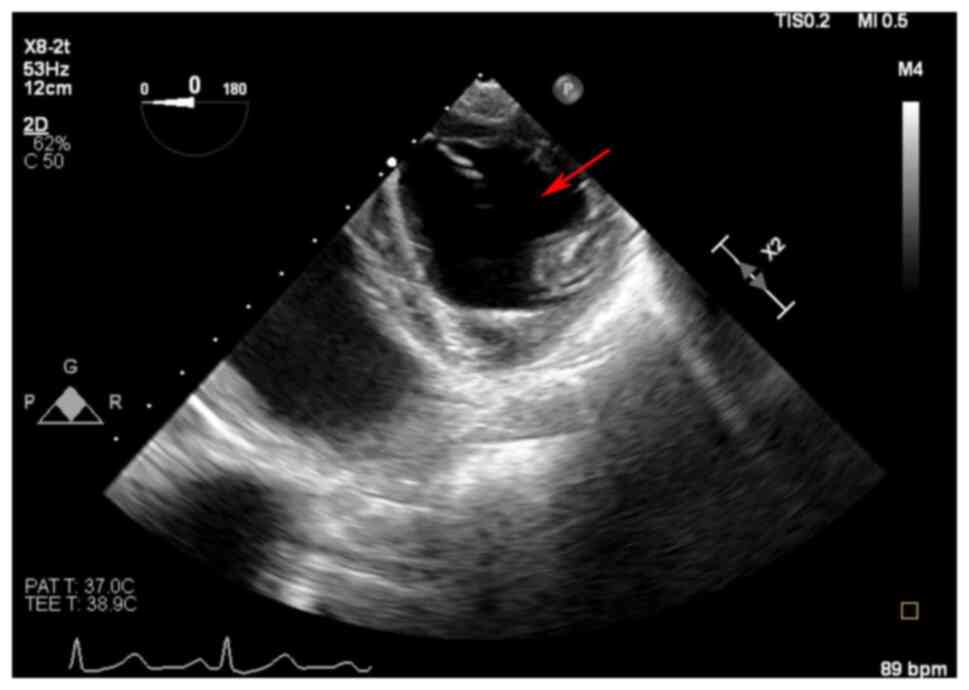
For the assessment of RV dysfunction, 2D TEE imaging
reveals RV dilation with a corresponding decrease in left
ventricular (LV) size (14). An
example case in Fig. 1 shows
intraoperative RV failure, with an enlarged right ventricle
compressing the left ventricle into a distinct D-shaped
configuration, indicative of RV pressure overload (22,23).
Normal RV diameters are defined as 2.5-4.1 cm at the basal level
and 1.9-3.5 cm at the mid-level, with a longitudinal dimension of
5.9-8.3 cm (24). RV dysfunction
is often associated with tricuspid valve (TV) abnormalities. Severe
tricuspid regurgitation is typically associated with tricuspid
annular dilation, indicated by a jet area >10 cm2 and
a venous constriction width >7 mm (14,25).
RV systolic dysfunction is identified by a tricuspid annular plane
systolic excursion (TAPSE) of <17 mm and a RV fractional area
change (RVFAC) of <35% (25).
Hemodynamic instability due to left
cardiac dysfunction
To date, reviews on LTx have primarily focused on
right heart function but have rarely mentioned left heart function;
however, LV function serves a critical role in maintaining the
overall hemodynamic stability during and after LTx (13,14).
LV preload is reduced by frequent RV dysfunction in patients with
chronic lung disease (26). If
prolonged, this reduction may lead to LV myocyte atrophy, which can
be related to reduced myocardial contractility and diastolic
dysfunction (27). LV diastolic
dysfunction increases left atrial pressure and pulmonary venous
congestion, thereby worsening cardiogenic pulmonary edema and
risking primary graft dysfunction (PGD) misdiagnosis (28). Therefore, monitoring of LV function
is crucial for identifying potential issues, optimizing fluid
management, assessing the indirect effects of RV function and
preventing postoperative complications.
By monitoring the blood flow velocity and
cross-sectional area of the LV outflow tract, TEE can calculate the
stroke volume and cardiac output per beat, thereby reflecting the
overall function of the left ventricle (29). Numerous conventional parameters
used to evaluate diastolic function, including early (E) and late
transmitral inflow velocities and deceleration time, are influenced
by loading conditions, limiting their ability to reliably assess
impaired relaxation (30). By
contrast, the E/mitral annular velocity (E/é) ratio is less
affected by elevated filling pressures and is closely associated
with the isovolumic relaxation time constant, making it a more
accurate reflection of delayed relaxation (30). Elevated E/é ratios are indicative
of more severe diastolic dysfunction and increased filling
pressures, with an E/é value of >8 considered highly sensitive
for diagnosing diastolic dysfunction (30). The present review provides a
summary of the latest research on TEE monitoring of left heart
function in LTx. Notably, there may be future possibilities to
combine artificial intelligence with TEE to automatically evaluate
LV function during hemodynamic monitoring.
Hemodynamic instability due to
vascular anastomosis-related complications
LTx is a complex procedure involving vascular
anastomosis between the two PAs, and between the PV and left atrium
(31). To date, five types of
vascular anastomosis-related complications have been described in
the literature (32). Type I is
described as anastomotic buckling (due to excessive donor vessel
length) and anastomotic deformation (due to insufficient donor
vessel length), or hilar malalignment, leading to anastomotic
stenosis and thrombosis. Type II is caused by transposition of the
donor vessel relative to the recipient vessel. Type III refers to
true anastomotic stenosis caused by suture overtightening or
misalignment. Type IV is caused by intraluminal obstruction,
secondary to thrombosis or occlusion. Type V refers to stenosis
with an additional luminal mass effect. The presence of anastomotic
complications in the PA may impede blood flow to the lung
allograft, thereby increasing RV afterload and leading to PH, RV
dysfunction, hypotension, hypoxemia, PGD, and eventually allograft
failure and death (33-36). Anastomotic
obstruction of the PV may lead to similar adverse outcomes. In
severe cases, obstruction of the PA and PV may require
extracorporeal life support (ECLS) (37). To prevent these complications,
early intraoperative diagnosis is crucial. Utilizing TEE to assess
vascular anastomoses during periods 3 and 4 can aid the timely
identification of PA or PV obstructions. This proactive approach
may help stabilize hemodynamics, reduce the risk of allograft
failure and improve patient survival outcomes (35,38).
The mid-esophageal ascending aortic short- and
long-axis views are key TEE views for the evaluation of PA
anastomoses after transplantation (35). For right PA anastomosis, optimizing
the probe angle and multiplane angle (0-60˚) allows for improved
assessment of hemodynamic parameters, including turbulence, peak
velocity and pressure gradients (35). Due to anatomical obstructions, left
PA anastomosis is challenging to visualize directly and often
requires indirect assessment through PV Doppler signals or
intraoperative contact echocardiography for additional imaging
support (35). A previous case
series and review of vasculopathy concluded that a diagnosis of
obstruction after vascular anastomosis requires a diameter of ≥75%
of the native PA and evidence of laminar, nonturbulent flow
(35). Clinically significant
symptoms of PA obstruction occur with a mean peak pulmonary
arterial velocity of >2.6 m/sec or a pulmonary arterial lumen
diameter of <0.8 cm postoperatively (39). If the pressure gradient at the
anastomotic site is >64 mmHg, it may indicate a serious
obstruction (35).
The assessment of PV anastomoses following LTx using
TEE requires optimization of specific imaging views to visualize
individual PVs. The left upper PV is optimally assessed using the
mid-esophageal 2-chamber view, with slight probe withdrawal to
visualize its position super posterior to the left atrial appendage
(35). The left lower PV, often
challenging to visualize due to its deeper location, requires the
mid-esophageal 4-chamber (ME4Ch) view, with probe
rotation and advancement to enhance imaging (35). Similarly, the right upper PV and
right lower PV are accessed via the ME4Ch view, with
appropriate probe rotation and positional adjustments (35). Color Doppler helps locate PVs and
detect turbulence or abnormal flow, while spectral Doppler
quantifies velocities and triphasic waveforms to diagnose
anastomotic issues such as stenosis or thrombosis (10). In vascular anastomosis-related
complications, TEE can monitor obstruction. Peak PV velocities
>100 cm/sec suggest possible obstruction; velocities >170
cm/sec with a clearly elevated baseline strongly indicate
obstruction. If velocities fall between 100 and 170 cm/sec,
additional signs such as turbulence or elevated baseline should be
assessed (34,40). However, hyperdynamic myocardial
function can be caused by drugs that have a positive inotropic
effect or reduce PVR, increase cardiac output and thus PV flow
velocity, which needs to be differentiated from true vascular
obstruction. If possible, all four PVs should be examined, as a
notable increase in flow velocity in one PV is indicative of severe
obstruction. A PV diameter >0.5 cm has been shown to reduce the
risk of thrombosis and unilateral pulmonary edema, whereas a PV
diameter <0.25 cm has been described as the threshold for
transplant failure (28,32).
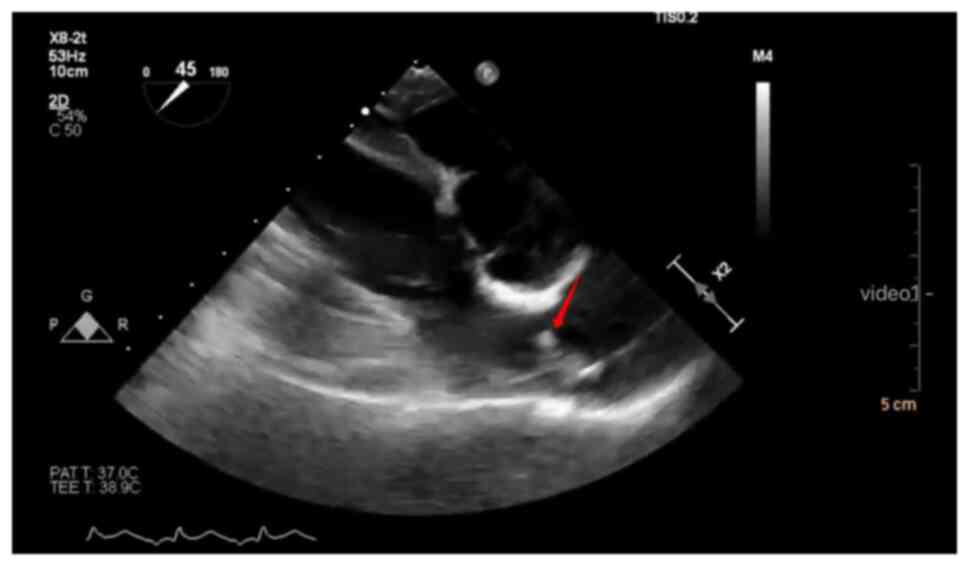
An echogenic mass originating from one of the PVs
and extending into the left atrium observed on 2D echocardiography
suggests the presence of a thrombus (33). When a thrombus appears in the
cardiovascular system, TEE shows visible bright floating objects
(Fig. 2). A clearer dynamic
visualization is provided in Video
S1. Even in the absence of a visible mass, Doppler imaging
should be utilized to assess for PV thrombosis or obstruction
(33). Normal systolic (S wave)
and diastolic (D wave) flow velocities typically range between
30-60 cm/sec (33). A spectral
Doppler velocity >100 cm/s, the presence of turbulence or a
shift in the usual systolic flow dominance (S wave > D wave) may
indicate marked PV obstruction, warranting further investigation
(33). The diagnostic accuracy of
TEE in identifying intracardiac thrombi is well-established. TEE
has a sensitivity of 100% and a specificity of 99% for detecting
thrombi within the left atrium (41).
At present, pulmonary vascular occlusion after LTx
is treated with surgical revision of the anastomotic site,
endovascular angioplasty, endovascular stenting of the anastomosis,
conservative thrombolysis and anticoagulant therapy (39,42,43).
Given that thrombosis is detrimental to allograft and patient
survival, clinicians should be familiar with the clinical and
echocardiographic patterns of thrombosis during the early
postoperative period after LTx (44). Based on the TEE findings, the size
and flow velocity of a thrombus at the anastomotic site can be used
to guide postoperative management (44). Systemic anticoagulation therapy is
recommended if clinically feasible. If anticoagulation is not
feasible, the thrombus is small or the blood flow velocity does not
indicate severe obstruction, conservative management is
recommended. In cases of acute thrombosis with large thrombi or
hemodynamic instability, thrombectomy and anastomotic repair should
be considered (45).
Other causes of hemodynamic
instability
In addition to the aforementioned causes, other
causes of hemodynamic instability during LTx can be diagnosed using
TEE. A hallmark of LTx is the restrictive fluid replacement
strategy commonly employed during the perioperative period to
minimize complications such as graft edema and RV overload
(46). While this approach is
crucial for protecting graft function, it can lead to low
intravascular volume and reduced preload, potentially resulting in
hypotension and impaired cardiac output. These hemodynamic
challenges require precise volume assessment and management. TEE is
an indispensable tool for the evaluation of the volume status of
the heart and large vessels in this context. For example, in the
transgastric short-axis view, under conditions of low volume,
particularly during LV contraction, the papillary muscles may draw
closer together, even making contact, which is a phenomenon
referred to as ‘kissing papillary muscles’ (Video S2) (47). While ‘kissing papillary muscles’
are observed in some cases as a result of reduced afterload, they
are more frequently associated with hypovolemia during LTx. This
highlights the importance of considering both preload and afterload
when assessing volume status. It is also critical to note that
volume assessments should be made during diastole, as systolic
measurements do not accurately reflect ventricular volume.
Combining this understanding with other TEE parameters-such as left
ventricular end-diastolic area, stroke volume variation,
velocity-time integral and collapsibility of the inferior vena
cava-allows for precise fluid administration to optimize
hemodynamics while avoiding volume overload, a key consideration in
the restrictive fluid management strategy of LTx (48).
If the size of the chest cavity does not match that
of the donor lung, hemodynamic instability may occur because of
chest wall compression during chest closure (49). In this case, TEE findings reveal
donor lung compression with reduced LV filling, elevated PA
pressures and septal shift toward the left, indicative of increased
RV strain. These findings strongly suggest a donor/recipient size
mismatch; therefore, delayed chest closure may be considered an
appropriate intervention to alleviate lung compression and restore
hemodynamic stability (50).
Use of drugs for hemodynamic
management under TEE guidance
Hypervolemia is associated with pulmonary edema in
patients undergoing thoracic surgery; therefore, it should be
prevented in patients undergoing LTx (51). Drugs can be used to maintain
hemodynamic stability and prevent pulmonary edema (46); however, if drugs fail to maintain
organ tissue perfusion, fluid resuscitation should be considered.
Inotropic support should be administered based on baseline cardiac
function, particularly RV function and the degree of vasodilation
in patients with elevated PA pressure (52). The management of hemodynamic
instability in patients undergoing LTx involves the use of drugs to
support positive muscle strength and the restoration of systemic
vascular resistance (SVR) and PVR (52). Certain patients may experience
notable blood loss during surgical procedures, necessitating volume
resuscitation to restore hemodynamic stability (53). TEE monitoring serves a critical
role in guiding therapeutic decisions, including the selection of
appropriate drugs or blood products. For instance, the appearance
of ‘kissing papillary muscles’ on TEE, which are indicative of
severe hypovolemia, may indicate the need for volume resuscitation,
while a reduced TAPSE or RVFAC may suggest compromised RV function
and the need for inotropic support (14,25).
During surgery, pulmonary arterial hypertension is
primarily managed with inhaled nitric oxide (NO), inhaled
prostaglandin E1 analogs, and intravenous milrinone or
prostaglandin E1 analogs (54). NO is essential for reducing PVR in
ischemia-reperfusion injury (55).
Prior to the adoption of inhaled NO, prostaglandin E1
analogs were the mainstay for controlling RV load in patients with
PH; however, their long half-life often causes hypotension,
especially in patients with RV dysfunction reliant on adequate
coronary perfusion pressure (39,56).
Milrinone, a phosphodiesterase-3 inhibitor, has exhibited efficacy
in reducing lung injury in acute lung injury models; however, its
use in LTx remains limited (57).
Vasopressin can increase SVR without elevating PVR, optimizing RV
perfusion without adding to the afterload (58). Additionally, dopamine, adrenaline,
norepinephrine and dobutamine are commonly employed as positive
inotropes during LTx.
TEE also facilitates real-time monitoring of the
response to these therapies. For example, improvements in TAPSE and
RVFAC can indicate effective inotropic support, while reductions in
tricuspid regurgitation velocity reflect successful pulmonary
vasodilation. Morphological changes, including resolving RV
dilation or septal flattening, may further confirm the efficacy of
interventions. By enabling continuous assessment of RV function and
load, TEE ensures timely adjustments to pharmacological therapies,
helping to optimize fluid management, maintain hemodynamic
stability and prevent complications such as pulmonary edema or
right heart failure.
TEE effectively utilizes real-time imaging and
quantitative parameters to assess the potential causes of
hemodynamic instability, including cardiac dysfunction,
complications related to vascular anastomosis and abnormalities in
volume status. TEE also provides valuable guidance for clinical
interventions, including determining the need for pharmacological
support, optimizing drug selection and evaluating whether to
initiate ECLS.
4. TEE during guided ECMO in LTx
The use of ECLS in LTx has steadily increased over
the past decade, since the first successful LTx involving
cardiopulmonary bypass (CPB) (59,60).
In cases of limited donor availability and poor overall recipient
condition, ECLS is used during LTx to ensure hemodynamic stability
(61). The main advantage of ECLS
is that it prevents volume overload and reperfusion injury in the
first transplanted lung during bilateral LTx (62). The types of ECLS include CPB,
venovenous ECMO (VV ECMO) and venoarterial ECMO (VA ECMO) (52).
ECLS is an important tool used to enhance surgical
safety. The use of VV ECMO or VA ECMO as alternatives to CPB has
become more popular (59). In
2022, the American Association of Thoracic Surgeons published an
expert consensus document on the use of MCLS in LTx (59). This document provides 36
recommendations to guide professionals involved in the care of
patients with end-stage lung disease who are considering
transplantation. Among the recommendations, TEE is emphasized as a
critical tool for evaluating ECMO dysfunction and related
complications. These complications, including malpositioned
cannulas, thrombosis, pericardial tamponade or inadequate
ventricular unloading, require prompt and accurate assessment. The
importance of TEE in this context is underscored by a strong
consensus and a 92% agreement score among experts (59). Several studies have reported the
use of TEE to guide ECMO; however, a comprehensive summary is
lacking (63-65). Therefore,
the present review summarizes the findings of the relevant
studies.
ECMO involves cannulation of blood vessels to create
a bypass circuit consisting of a pump and a membrane oxygenator
(63). Bicaval VV ECMO is a
well-recognized and validated therapy that requires the use of
single or double peripheral venous access for the insertion of two
different-sized cannulas to achieve adequate blood oxygenation
(65). Traditionally, VV ECMO was
performed using two cannulas [a femoral venous drainage cannula and
an internal jugular (IJ), subclavian or second femoral venous
cannula] to return blood to or near the right atrium (RA); however,
the recently developed dual-lumen cannula can be used independently
in the IJ vein (63). To ensure
correct cannula positioning during surgery, TEE is an invaluable
tool for both initial placement and continuous monitoring. Proper
positioning requires the drainage port to reside in the superior
vena cava (SVC) and the return port in the RA, with flow directed
toward the TV (63). Using TEE,
the mid-esophageal 4-chamber view can confirm the flow direction
toward the TV, while the mid-esophageal SVC long-axis view verifies
the location of the drainage port in the SVC (63). Additionally, the transgastric RV
inflow-outflow view can confirm that the return port is in the RA
without entering the right ventricle or contacting the atrial wall
(63). In cases of malpositioning,
TEE may reveal abnormal flow patterns, turbulence or mechanical
compression of cardiac structures. These findings highlight the
importance of continuous monitoring and adjustment to ensure
optimal cannula function and prevent complications.
TEE provides guidance for all three key steps of
cannulation: Wire placement, assessment of the precise depth of
cannulation in the inferior vena cava (IVC) and orientation of the
reperfusion port to the TV (66).
Proper insertion and accurate positioning of the cannula are
important for the safety and optimal performance of the dual-lumen
cannula. Malpositioned cannulas can result in hemodynamic
instability. For example, a misaligned return port may direct flow
against the atrial wall, causing turbulence or inadequate flow
(63). Similarly, an improperly
placed drainage port may fail to fully capture venous return. TEE
findings such as turbulence, low flow velocity or changes in
chamber size (such as RV dilation) can indicate malpositioning
(63). Structural injuries, such
as atrial wall damage or vessel perforation, may be identified on
TEE by the presence of hematomas, pericardial effusion or abnormal
contact between the cannula and cardiac structures (63). Early detection of such issues
allows for immediate correction of cannula position and, if
necessary, surgical intervention to address injuries (67-69).
Thrombosis is the main complication associated with
low ECMO flow (70). TEE is used
to guide the diagnosis and treatment of thrombosis during ECMO
(71-73).
Obstruction of the cannula in ECMO is detected based on reduced
blood flow velocity, hemodynamic instability and poor blood
oxygenation. In addition, if refractory hypoxemia occurs during
surgery after other causes (e.g., inadequate ECMO support due to
insufficient flow, sweep gas failure or oxygenator dysfunction)
have been excluded, TEE should be used for the early detection of a
malpositioned cannula (63). In
most cases, malpositioning of the cannula is predicted by
deteriorating oxygenation during ECMO (63). TEE can be used to detect other
complications related to cannulation such as hemothorax,
pneumothorax and mediastinal or pericardial bleeding (63).
The risk of catheter malpositioning and tamponade
may be higher in children than in adults because children have
smaller, thinner blood vessels and smaller heart chambers (65,74).
In addition, a particular concern in the pediatric population is
the formation of a conduit loop, despite TEE images revealing the
entry of the guidewire tip into the RV (63). After 2D TEE is used to guide the
guidewire position, 3D TEE should be used to exclude the
possibility of the formation of a guidewire loop during
advancement. The oxygenation efficiency of the ECMO circuit depends
on the flow rate of the pump relative to the cardiac output of
patients. Accordingly, oxygenation in patients should be increased
with increasing ECMO flow rate. If this does not occur, blood
should be suspected to recirculate between the inflow and outflow
cannulas, indicating the formation of a conduit loop (64). A case report of cardiac perforation
during placement of a dual-lumen catheter in neonates highlighted
that slight movement of the catheter in this population may lead to
tip misalignment, and that using wire navigation to connect the RA
to the IVC may be particularly difficult and dangerous (74). Direct contact between the tip of
the wire or dilator and the IVC wall should be avoided. Neonatal
experts recommend that TEE should be performed after catheter
placement and before initiating ECMO to predict complications
related to ECMO and to prevent cardiac arrest during immediate
drainage (74).
A recent study has shown that TEE-guided ECMO is
increasingly used in LTx due to its low radiation dose and bedside
convenience (75). The present
review summarizes specific monitoring of TEE in patients undergoing
ECMO during LTx. However, studies on ECMO weaning during LTx and
data validating the effectiveness of TEE in guiding ECMO are
lacking.
5. Use of TEE in other aspects of LTx
Before LTx, the interatrial septum should be checked
for the presence of a patent foramen ovale or an atrial septal
defect (34). In the presence of a
large atrial septal defect, RV pressure after LTx may lead to
severe left-to-right shunting, resulting in high blood flow and
transplant failure (76).
TEE can be used to detect the formation of a
bronchovenous fistula (BVF) during LTx, especially in patients with
severe pleural adhesions (77).
BVF is a rare complication that can cause arterial gas embolism in
important organs (including the heart and brain), leading to high
mortality rates. Taguchi et al (77) reported that patients often receive
high airway pressure ventilation during LTx and the surgical
procedure can easily damage the bronchi and pulmonary vessels,
leading to BVF. TEE can detect bubbles in the left side of the
heart, facilitating early diagnosis and prompt treatment of BVF.
The treatment goal is to maintain hemodynamic stability and
minimize air entry into the bloodstream (reducing airway pressure
and single-lung ventilation). Timely administration of pure oxygen
can effectively treat hypoxemia and reduce air bubbles by
eliminating nitrogen. TEE is an effective tool for the early
diagnosis of BVF during LTx, which can improve the prognosis
(77).
As mentioned in the 2020 Guidelines for the Use of
TEE to Assist with Surgical Decision-Making in the Operating Room,
TEE can help diagnose gas embolism in the left ventricle during
reperfusion when the left atrial clamp is removed and the donor
lung is implanted (8).
For certain specialized intraoperative support
techniques, including the novel hybrid VA ECMO/CPB circuit, TEE
monitoring can be utilized to adjust the ECMO flow rate and
determine whether mode conversion is necessary (78,79).
After chest closure, TEE should be performed to
determine the presence of atelectasis and pulmonary edema, or
pulmonary tamponade occurring during severe emphysema or excessive
lung inflation, providing a reference value for subsequent patient
ventilation and volume management (80).
6. Conclusion and future perspectives
LTx is a complex procedure for both surgeons and
anesthesiologists. Successful anesthesia during LTx can maintain
hemodynamic stability and improve the prognosis. Therefore,
anesthesiologists should possess expertise in TEE to manage
intraoperative anesthesia and care. In addition, communication
between anesthesiologists and LTx teams during surgery is crucial
for achieving an optimal prognosis.
Current research primarily focuses on intraoperative
monitoring, lacking comprehensive integration and analysis of
continuous perioperative TEE data, thereby limiting a thorough
evaluation of its clinical value and the assessment of
multidisciplinary collaboration on patient outcomes (13,77).
Future research should establish a robust multidisciplinary
integration framework, spanning preoperative assessment,
intraoperative monitoring, intensive care unit applications and
postoperative follow-up. Such an integrative approach would not
only provide cohesive support for perioperative decision-making but
also identify dynamic clinical changes often overlooked by
intermittent monitoring, ultimately optimizing patient management
strategies and improving clinical outcomes.
Moreover, the application of TEE in pediatric LTx
remains underexplored. Current practice in this population
primarily relies on experience extrapolated from adult cases, with
limited evidence from large-scale studies or pediatric-specific
guidelines. Future investigations should focus on assessing the
role of perioperative TEE in improving graft survival and reducing
complications in children. Additionally, efforts are warranted to
develop TEE probes and imaging modalities tailored to the unique
anatomical features of pediatric patients.
Supplementary Material
Supplementary Data.
Visible bright floating objects. The
video obtained by the authors from a 14-year-old male patient
undergoing lung transplantation for obliterative
bronchiolitis.
‘Kissing papillary muscles’. The video
obtained by the authors from a 59-year-old female patient
undergoing lung transplantation for interstitial lung disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JX and ZX conceived and designed the review,
conducted the literature search and analysis, drafted the main
sections and revised the manuscript. GL, YL and YY assisted with
the literature search, data integration and discussion and
contributed to manuscript revision. JZ and QG prepared the figures,
organized the literature and contributed to content editing. XT and
RW ensured the scientific accuracy and clinical relevance of the
review and participated in the final revision. MY supervised the
study, guided manuscript preparation and conducted the final
review. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martin AK, Renew JR, Jayaraman AL, Murray
AW, Fritz AV and Ramakrishna H: Analysis of outcomes in lung
transplantation. J Cardiothorac Vasc Anesth. 33:1455–1466.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Young KA and Dilling DF: The future of
lung transplantation. Chest. 155:465–473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chambers DC, Perch M, Zuckermann A,
Cherikh WS, Harhay MO, Hayes D Jr, Hsich E, Khush KK, Potena L,
Sadavarte A, et al: The international thoracic organ transplant
registry of the international society for heart and lung
transplantation: Thirty-eighth adult lung transplantation
report-2021; focus on recipient characteristics. J Heart Lung
Transplant. 40:1060–1072. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bottiger B, Klapper J, Fessler J, Shaz BH
and Levy JH: Examining bleeding risk, transfusion-related
complications, and strategies to reduce transfusions in lung
transplantation. Anesthesiology. 140:808–816. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim HJ, Shin SW, Park S and Kim HY: A
review of anesthesia for lung transplantation. J Chest Surg.
55:293–300. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Su Y, Liu K, Zheng JL, Li X, Zhu DM, Zhang
Y, Zhang YJ, Wang CS, Shi TT, Luo Z and Tu GW: Hemodynamic
monitoring in patients with venoarterial extracorporeal membrane
oxygenation. Ann Transl Med. 8(792)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsumoto M, Oka Y, Lin YT, Strom J,
Sonnenblick EH and Frater RW: Transesophageal echocardiography; for
assessing ventricular performance. N Y State J Med. 79:19–21.
1979.PubMed/NCBI
|
|
8
|
Nicoara A, Skubas N, Ad N, Finley A, Hahn
RT, Mahmood F, Mankad S, Nyman CB, Pagani F, Porter TR, et al:
Guidelines for the use of transesophageal echocardiography to
assist with surgical decision-making in the operating room: A
surgery-based approach: From the American society of
echocardiography in collaboration with the society of
cardiovascular anesthesiologists and the society of thoracic
surgeons. J Am Soc Echocardiogr. 33:692–734. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martin AK, Mercier O, Fritz AV, Gelzinis
TA, Hoetzenecker K, Lindstedt S, Marczin N, Wilkey BJ, Schecter M,
Lyster H, et al: ISHLT consensus statement on the perioperative use
of ECLS in lung transplantation: Part II: Intraoperative
considerations. J Heart Lung Transplant: October 23, 2024 (Epub
ahead of print).
|
|
10
|
American Society of Anesthesiologists and
Society of Cardiovascular Anesthesiologists Task Force on
Transesophageal Echocardiography. Practice guidelines for
perioperative transesophageal echocardiography. An updated report
by the American society of anesthesiologists and the society of
cardiovascular anesthesiologists task force on transesophageal
echocardiography. Anesthesiology. 112:1084–1096. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Teran F, Burns KM, Narasimhan M, Goffi A,
Mohabir P, Horowitz JM, Yuriditsky E, Nagdev A, Panebianco N, Chin
EJ, et al: Critical care transesophageal echocardiography in
patients during the COVID-19 pandemic. J Am Soc Echocardiogr.
33:1040–1047. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vieillard-Baron A, Millington SJ,
Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, Pinsky MR, Pulido J,
Mayo P and Fletcher N: A decade of progress in critical care
echocardiography: A narrative review. Intensive Care Med.
45:770–788. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Serra E, Feltracco P, Barbieri S, Forti A
and Ori C: Transesophageal echocardiography during lung
transplantation. Transplant Proc. 39:1981–1982. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan Z, Roscoe A and Rubino A:
Transesophageal echocardiography in heart and lung transplantation.
J Cardiothorac Vasc Anesth. 33:1548–1558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hahn RT, Abraham T, Adams MS, Bruce CJ,
Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W and
Picard MH: Guidelines for performing a comprehensive
transesophageal echocardiographic examination: Recommendations from
the American society of echocardiography and the society of
cardiovascular anesthesiologists. J Am Soc Echocardiogr.
26:921–964. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Marczin N, de Waal EEC, Hopkins PMA,
Mulligan MS, Simon A, Shaw AD, Van Raemdonck D, Neyrinck A, Gries
CJ, Algotsson L, et al: International consensus recommendations for
anesthetic and intensive care management of lung transplantation.
An EACTAIC, SCA, ISHLT, ESOT, ESTS, and AST approved document. J
Heart Lung Transplant. 40:1327–1348. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mocumbi A, Humbert M, Saxena A, Jing ZC,
Sliwa K, Thienemann F, Archer SL and Stewart S: Pulmonary
hypertension. Nat Rev Dis Primers. 10(1)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson J, Yang Y, Bian Z, Schena G, Li Y,
Zhang X, Eaton DM, Gross P, Angheloiu A, Shaik A, et al: Systemic
hypoxemia induces cardiomyocyte hypertrophy and right ventricular
specific induction of proliferation. Circ Res. 132:723–740.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Starke H, von Dossow V and Karsten J:
Intraoperative circulatory support in lung transplantation: Current
trend and its evidence. Life (Basel). 12(1005)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hoeper MM and Granton J: Intensive care
unit management of patients with severe pulmonary hypertension and
right heart failure. Am J Respir Crit Care Med. 184:1114–1124.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kusunose K, Tsutsui RS, Bhatt K, Budev MM,
Popović ZB, Griffin BP and Bolen MA: Prognostic value of RV
function before and after lung transplantation. JACC Cardiovasc
Imaging. 7:1084–1094. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sullivan B, Puskas F and
Fernandez-Bustamante A: Transesophageal echocardiography in
noncardiac thoracic surgery. Anesthesiol Clin. 30:657–669.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Louie EK, Rich S, Levitsky S and Brundage
BH: Doppler echocardiographic demonstration of the differential
effects of right ventricular pressure and volume overload on left
ventricular geometry and filling. J Am Coll Cardiol. 19:84–90.
1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
American society of echocardiography and the European association
of cardiovascular imaging. J Am Soc Echocardiogr. 28:1–39.e14.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lancellotti P, Tribouilloy C, Hagendorff
A, Popescu BA, Edvardsen T, Pierard LA, Badano L and Zamorano JL:
Scientific Document Committee of the European Association of
Cardiovascular Imaging. Recommendations for the echocardiographic
assessment of native valvular regurgitation: An executive summary
from the European association of cardiovascular imaging. Eur Heart
J Cardiovasc Imaging. 14:611–644. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vizza CD, Lynch JP, Ochoa LL, Richardson G
and Trulock EP: Right and left ventricular dysfunction in patients
with severe pulmonary disease. Chest. 113:576–583. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tudorache I, Sommer W, Kühn C, Wiesner O,
Hadem J, Fühner T, Ius F, Avsar M, Schwerk N, Böthig D, et al: Lung
transplantation for severe pulmonary hypertension-awake
extracorporeal membrane oxygenation for postoperative left
ventricular remodelling. Transplantation. 99:451–458.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yoon J and Salamanca-Padilla Y: Effect of
left ventricular diastolic dysfunction on development of primary
graft dysfunction after lung transplant. Curr Opin Anaesthesiol.
33:10–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dark PM and Singer M: The validity of
trans-esophageal Doppler ultrasonography as a measure of cardiac
output in critically ill adults. Intensive Care Med. 30:2060–2066.
2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Porteous MK, Ky B, Kirkpatrick JN,
Shinohara R, Diamond JM, Shah RJ, Lee JC, Christie JD and Kawut SM:
Diastolic dysfunction increases the risk of primary graft
dysfunction after lung transplant. Am J Respir Crit Care Med.
193:1392–1400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kumar N, Essandoh M, Bhatt A, Whitson BA,
Sawyer TR, Flores A, Awad H, Dimitrova G, Gorelik L, Bhandary S, et
al: Pulmonary cuff dysfunction after lung transplant surgery: A
systematic review of the evidence and analysis of its clinical
implications. J Heart Lung Transplant. 38:530–544. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Batra K, Chamarthy MR, Reddick M, Roda MS,
Wait M and Kalva SP: Diagnosis and interventions of vascular
complications in lung transplant. Cardiovasc Diagn Ther. 8:378–386.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Evans A, Dwarakanath S, Hogue C, Brady M,
Poppers J, Miller S and Weiner MM: Intraoperative echocardiography
for patients undergoing lung transplantation. Anesth Analg.
118:725–730. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Siddique A, Bose AK, Özalp F, Butt TA,
Muse H, Morley KE, Dark JH, Parry G and Clark SC: Vascular
anastomotic complications in lung transplantation: A single
institution's experience. Interact Cardiovasc Thorac Surg.
17:625–631. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abrams BA, Melnyk V, Allen WL, Subramaniam
K, Scott CD, Mitchell JD, Seres T and Martin AK: TEE for lung
transplantation: A case series and discussion of vascular
complications. J Cardiothorac Vasc Anesth. 34:733–740.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
González-Fernández C, González-Castro A,
Rodríguez-Borregán JC, López-Sánchez M, Suberviola B, Francisco
Nistal J and Martín-Durán R: Pulmonary venous obstruction after
lung transplantation. Diagnostic advantages of transesophageal
echocardiography. Clin Transplant. 23:975–980. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Iyer MH, Bhatt A, Kumar N, Hussain N and
Essandoh MK: Transesophageal echocardiography for lung
transplantation: A new standard of care? J Cardiothorac Vasc
Anesth. 34:741–743. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang YC, Cheng YJ, Lin YH, Wang MJ and
Tsai SK: Graft failure caused by pulmonary venous obstruction
diagnosed by intraoperative transesophageal echocardiography during
lung transplantation. Anesth Analg. 91:558–560. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kumar N, Hussain N, Kumar J, Essandoh MK,
Bhatt AM, Awad H, Perez WJ, Whitson BA, Ganapathi AM, Mokadam NA,
et al: Evaluating the impact of pulmonary artery obstruction after
lung transplant surgery: A systematic review and meta-analysis.
Transplantation. 105:711–722. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wakefield BJ and Alfirevic A: Pulmonary
venous flow after lung transplantation: Turbulence and high
velocities. J Cardiothorac Vasc Anesth. 34:1985–1989.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hemamalini P, Paramasivan S, Dutta P and
Attawar S: The role of transesophageal echocardiography in
evaluation and management of hypoxia following lung
transplantation. Ann Card Anaesth. 25:356–358. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schulman LL, Anandarangam T, Leibowitz DW,
Ditullio MR, McGregor CC, Galantowicz ME and Homma S: Four-year
prospective study of pulmonary venous thrombosis after lung
transplantation. J Am Soc Echocardiogr. 14:806–812. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fadel BM, Abdulbaki K, Nambiar V, Al Amri
M, Shahid M, Khouqeer F and Canver C: Dual thrombosis of the
pulmonary arterial and venous anastomotic sites after single lung
transplantation: Role of transesophageal echocardiography in
diagnosis and management. J Am Soc Echocardiogr. 20:38.e9–e12.
2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nahar T, Savoia MT, Liguori C, DiTullio
MR, Schulman LL and Homma S: Spontaneous resolution of pulmonary
venous thrombosis after lung transplantation. J Am Soc
Echocardiogr. 11:209–212. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chaaya G and Vishnubhotla P: Pulmonary
vein thrombosis: A recent systematic review. Cureus.
9(e993)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schultze BS: Fluid management in lung
transplant patients. Nurs Clin North Am. 52:301–308.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reuter DA, Felbinger TW, Schmidt C,
Moerstedt K, Kilger E, Lamm P and Goetz AE: Trendelenburg
positioning after cardiac surgery: Effects on intrathoracic blood
volume index and cardiac performance. Eur J Anaesthesiol. 20:17–20.
2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nagueh SF, Appleton CP, Gillebert TC,
Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka
PA and Evangelista A: Recommendations for the evaluation of left
ventricular diastolic function by echocardiography. J Am Soc
Echocardiogr. 22:107–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Denault A, Ferraro P, Couture P,
Boudreault D, Babin D, Poirier C and Buithieu J: Transesophageal
echocardiography monitoring in the intensive care department: The
management of hemodynamic instability secondary to thoracic
tamponade after single lung transplantation. J Am Soc Echocardiogr.
16:688–692. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen F, Matsukawa S, Ishii H, Ikeda T,
Shoji T, Fujinaga T, Bando T and Date H: Delayed chest closure
assessed by transesophageal echocardiogram in single-lobe lung
transplantation. Ann Thorac Surg. 92:2254–2257. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Assaad S, Popescu W and Perrino A: Fluid
management in thoracic surgery. Curr Opin Anaesthesiol. 26:31–39.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Murray AW, Boisen ML, Fritz A, Renew JR
and Martin AK: Anesthetic considerations in lung transplantation:
Past, present and future. J Thorac Dis. 13:6550–6563.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Vajter J, Vachtenheim J Jr, Prikrylova Z,
Berousek J, Vymazal T, Lischke R, Martin AK and Durila M: Effect of
targeted coagulopathy management and 5% albumin as volume
replacement therapy during lung transplantation on allograft
function: A secondary analysis of a randomized clinical trial. BMC
Pulm Med. 23(80)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tomasi R, Betz D, Schlager S, Kammerer T,
Hoechter DJ, Weig T, Slinger P, Klotz LV, Zwißler B, Marczin N and
von Dossow V: Intraoperative anesthetic management of lung
transplantation: Center-specific practices and geographic and
centers size differences. J Cardiothorac Vasc Anesth. 32:62–69.
2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kemming GI, Merkel MJ, Schallerer A,
Habler OP, Kleen MS, Haller M, Briegel J, Vogelmeier C, Fürst H,
Reichart B and Zwissler B: Inhaled nitric oxide (NO) for the
treatment of early allograft failure after lung transplantation.
Munich lung transplant group. Intensive Care Med. 24:1173–1180.
1998.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Aoe M, Trachiotis GD, Okabayashi K,
Manchester JK, Lowry OH, Cooper JD and Patterson GA: Administration
of prostaglandin E1 after lung transplantation improves early graft
function. Ann Thorac Surg. 58:655–661. 1994.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bueltmann M, Kong X, Mertens M, Yin N, Yin
J, Liu Z, Koster A, Kuppe H and Kuebler WM: Inhaled milrinone
attenuates experimental acute lung injury. Intensive Care Med.
35:171–178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Pelletier JS, Dicken B, Bigam D and Cheung
PY: Cardiac effects of vasopressin. J Cardiovasc Pharmacol.
64:100–107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Expert Consensus Panel. Hartwig M, Van
Berkel V, Bharat A, Cypel M, Date H, Erasmus M, Hoetzenecker K,
Klepetko W, Kon Z, et al: The American association for thoracic
surgery (AATS) 2022 expert consensus document: The use of
mechanical circulatory support in lung transplantation. J Thorac
Cardiovasc Surg. 165:301–326. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kaiser LR, Pasque MK, Trulock EP, Low DE,
Dresler CM and Cooper JD: Bilateral sequential lung
transplantation: The procedure of choice for double-lung
replacement. Ann Thorac Surg. 52:438–446. 1991.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gulack BC, Hirji SA and Hartwig MG: Bridge
to lung transplantation and rescue post-transplant: The expanding
role of extracorporeal membrane oxygenation. J Thorac Dis.
6:1070–1079. 2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Erkılınç A and Vayvada M: The use of
intraoperative extracorporeal membrane oxygenation in lung
transplantation: Initial institutional experience. Braz J
Cardiovasc Surg. 38:88–95. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Griffee MJ, Tonna JE, McKellar SH and
Zimmerman JM: Echocardiographic guidance and troubleshooting for
venovenous extracorporeal membrane oxygenation using the dual-lumen
bicaval cannula. J Cardiothorac Vasc Anesth. 32:370–378.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bautista-Rodriguez C, Sanchez-de-Toledo J
and Da Cruz EM: The role of echocardiography in neonates and
pediatric patients on extracorporeal membrane oxygenation. Front
Pediatr. 6(297)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Berdajs D: Bicaval dual-lumen cannula for
venovenous extracorporeal membrane oxygenation: Avalon© cannula in
childhood disease. Perfusion. 30:182–186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hemamalini P, Dutta P and Attawar S:
Transesophageal echocardiography compared to fluoroscopy for avalon
bicaval dual-lumen cannula positioning for venovenous ECMO. Ann
Card Anaesth. 23:283–287. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hirose H, Yamane K, Marhefka G and
Cavarocchi N: Right ventricular rupture and tamponade caused by
malposition of the avalon cannula for venovenous extracorporeal
membrane oxygenation. J Cardiothorac Surg. 7(36)2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yastrebov K, Manganas C, Kapalli T and
Peeceeyen S: Right ventricular loop indicating malposition of
J-wire introducer for double lumen bicaval venovenous
extracorporeal membrane oxygenation (VV ECMO) cannula. Heart Lung
Circ. 23:e4–e7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Tanaka D, Pitcher HT, Cavarocchi N and
Hirose H: Migrated avalon veno-venous extracorporeal membrane
oxygenation cannula: How to adjust without interruption of flow. J
Card Surg. 30:865–868. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zamper RPC, Bainbridge D, Nagpal D and
Fujii S: Management of a bi-caval dual lumen cannula clot
obstruction after TEE guided diagnosis: A case-report. Braz J
Anesthesiol. 70:55–58. 2020.PubMed/NCBI View Article : Google Scholar : (In
Portuguese).
|
|
71
|
Ruisanchez C, Sarralde JA,
Gonzalez-Fernandez C and Dominguez MJ: Sudden dysfunction of
veno-venous extracorporeal membrane oxygenation caused by
intermittent cannula obstruction: The key role of echocardiography.
Intensive Care Med. 43:1055–1056. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jingquan L, Fan Z, Ziqiang S, Jifu L,
Zongbin L, Xianghong Y, Renhua S and Jun H: Echocardiographic image
of extracorporeal membrane oxygenation cannula-associated inferior
vena cava thrombosis and filter implantation. Chest. 163:e275–e279.
2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Pavlov M, Babić Z and Bulj N: Unusual
pattern of inferior vena cava thrombosis after veno-arterial
extracorporeal membrane oxygenation: A report of two cases. Croat
Med J. 61:555–560. 2020.PubMed/NCBI
|
|
74
|
Czerwonko ME, Fraga MV, Goldberg DJ,
Hedrick HL and Laje P: Cardiovascular perforation during placement
of an avalon elite® bicaval dual lumen ECMO cannula in a
newborn. J Card Surg. 30:370–372. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wilkey BJ, Elliott T, Jones TE,
Vasilopoulos T, Gelzinis T, Bellomy M, Brodt JL, Knight J, Geube
MA, Fox JM, et al: Survey of extracorporeal life support
application in lung transplantation. Clin Transplant.
39(e70094)2025.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gorcsan J III, Edwards TD, Ziady GM, Katz
WE and Griffith BP: Transesophageal echocardiography to evaluate
patients with severe pulmonary hypertension for lung
transplantation. Ann Thorac Surg. 59:717–722. 1995.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Taguchi A, Kai S, Kimura K, Yutaka Y, Date
H and Fukuda K: Intraoperative diagnosis of bronchovenous fistula
during lung transplantation using transesophageal echocardiography.
J Cardiothorac Vasc Anesth. 36:2572–2574. 2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Martin AK, Fritz AV, Pham SM, Landolfo KP,
Sareyyupoglu B, Brown TE, Logvinov I, Li Z, Narula T, Makey IA and
Thomas M: Initial experience and outcomes with a hybrid
extracorporeal membrane oxygenation and cardiopulmonary bypass
circuit for lung transplantation. JTCVS Open. 16:1029–1037.
2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Martin AK, Harrison BA, Fritz AV, Landolfo
KP, Makey IA, Sareyyupoglu B, Brown TE, Johnson JL Jr, Pham SM and
Thomas M: Intraoperative management of a hybrid extracorporeal
membrane oxygenation circuit for lung transplantation. J Card Surg.
35:3560–3563. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Higny J, Forêt F and Laterre PF:
Three-dimensional critical care transesophageal echocardiography: A
bedside tool in the diagnosis and management of shock. Clin Case
Rep. 9(e05164)2021.PubMed/NCBI View Article : Google Scholar
|