Introduction
Multiple myeloma (MM) is a hematological malignancy
characterized by the clonal proliferation of plasma cells within
the bone marrow, leading to disruption of normal hematopoiesis and
immune regulation (1,2). This dysregulation results in a wide
range of systemic complications, including anemia,
immunosuppression, osteolytic lesions, hypercalcemia and renal
dysfunction (3). Although MM
primarily involves the bone marrow, extramedullary manifestations
occur in ~4% of cases, with cutaneous involvement being
exceptionally rare and affecting <1% of patients (4).
Cutaneous features of MM are diverse and often
non-specific, including conditions such as light chain amyloidosis,
cryoglobulinemia, xanthomatosis, polyneuropathy, organomegaly,
endocrinopathy, monoclonal gammopathy and skin changes syndrome,
and sclerodermoid changes (5,6).
Among these, follicular spicules of MM (FSMM) represent an uncommon
but highly specific dermatological finding. Clinically, FSMM
presents as fine, keratotic, spiny projections emerging from hair
follicles, predominantly distributed over the nasal and facial
regions (7). Histopathological
examination typically reveals eosinophilic keratinous material
within the follicles, frequently containing monoclonal
immunoglobulin deposits that correspond to circulating paraproteins
(8).
The precise pathogenesis of FSMM remains poorly
defined (9). Recent studies have
emphasized that abnormal deposition of monoclonal proteins within
the follicular epithelium may contribute to the development of
hyperkeratotic changes (10-12).
Furthermore, a recent case report in patients with advanced chronic
kidney disease (CKD) highlighted the diagnostic challenge in
distinguishing FSMM from other CKD-associated skin changes
(12). Differential diagnoses
include other hyperkeratotic conditions such as vitamin A
deficiency, CKD-related dermatoses, HIV/acquired immunodeficiency
syndrome and paraneoplastic syndromes; however, FSMM can be
reliably distinguished through histological and immunofluorescence
analysis demonstrating MM-specific monoclonal protein deposition
(13).
The present report describes a clinically and
histologically confirmed case of FSMM in a 59-year-old man with
newly diagnosed MM and end-stage renal disease undergoing
hemodialysis. The case details, including dermatological,
hematological, as well as histopathological findings, underscore
the diagnostic value of FSMM as an early cutaneous marker of MM.
The present case contributes to the limited literature on FSMM,
emphasizing the importance of dermatological vigilance and
suggesting that prompt recognition of this rare phenotype may
facilitate earlier diagnostic workup and improved patient
management.
Case report
Patient information and clinical
evaluation
The present report describes the case of a
59-year-old male patient diagnosed with MM and stage 5 CKD.
Clinical data, including medical history, symptoms and physical
examination findings, were collected at the time of hospital
admission in February 2024 at The First Affiliated Hospital of
Chongqing Medical and Pharmaceutical College (Chongqing, China).
The patient underwent a comprehensive assessment involving
hematological analysis, biochemical tests, karyotype analysis,
immunofixation electrophoresis (IFE) and bone marrow biopsy. The
serum vitamin A levels of the patient were within the normal range.
Additionally, the patient tested negative for HIV via both
serological and molecular methods.
Laboratory tests
Venous blood samples were collected in
EDTA-containing tubes. The samples were allowed to clot at room
temperature for 30 min and then centrifuged at 2,000 x g for 10 min
at 4˚C. The resulting supernatant (serum) was carefully extracted
and used for biochemical and immunoassay analyses. Complete blood
count was performed using an automated hematology analyzer (Beckman
Coulter, Inc.). Serum calcium, creatinine, globulin, total protein
and C-reactive protein (CRP) levels were measured using a
biochemical analyzer (Roche Cobas 8000; Roche Diagnostics). Serum
protein electrophoresis (SPE) and IFE were conducted using an
agarose gel electrophoresis system (Sebia). Thyroid function tests,
IL-6 and calcitonin levels were measured using
electrochemiluminescence immunoassay kits (cat. no. 07027838 for
IL-6 and cat. no. 05109342 for calcitonin; Roche Elecsys; Roche
Diagnostics), and assays were performed on a Cobas e801 analyzer in
accordance with the manufacturer's protocols. Serum ferritin and
folate levels were determined using chemiluminescence immunoassay
kits (cat. no. 7K61 for ferritin and cat. no. 8K41 for folate;
Abbott Architect) as per the manufacturer's instructions. Serum
albumin was measured using the same biochemical analyzer (Roche
Cobas 8000; Roche Diagnostics) following the manufacturer's
guidelines.
Karyotype analysis
Peripheral blood samples were collected in
heparinized tubes and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1% phytohemagglutinin-M
(cat. no. L8754; Sigma-Aldrich; Merck KGaA;) for 72 h at 37˚C in a
5% CO2 incubator. After incubation, colchicine (cat. no.
C9754; Sigma-Aldrich; Merck KGaA) was added at a final
concentration of 0.1 µg/ml and the cells were incubated for an
additional 2 h at 37˚C. Following colchicine treatment, lymphocytes
were harvested by centrifugation (1,000 x g for 10 min) and
hypotonic treatment using 0.075 M KCl for 20 min at 37˚C. Cells
were then fixed with freshly prepared in methanol:acetic acid (3:1)
fixative at room temperature for 30 min (three changes). Metaphase
chromosome spreads were prepared and subjected to G-banding using
standard trypsin-Giemsa staining. Chromosomal abnormalities were
analyzed under a light microscope (Olympus BX53; Olympus
Corporation) and described in accordance with the International
System for Human Cytogenomic Nomenclature (ISCN 2020) (14).
SPE and IFE
SPE was performed using a semi-automated agarose gel
electrophoresis system (Hydrasys 2 Scan; Sebia) according to the
manufacturer's protocol, to detect the presence of monoclonal
M-spike bands in the γ region. IFE was conducted using antisera
specific for IgG, IgA, IgM, and κ and λ light chains (Hydragel IF
kit; cat. no. 4460; Sebia) following the manufacturer's
instructions. After electrophoresis and fixation, the gels were
stained and imaged using a gel documentation and analysis system
(Bio-Rad Gel Doc XR+; Bio-Rad Laboratories, Inc.).
Bone marrow biopsy and
histopathological examination
A bone marrow biopsy was performed under local
anesthesia using 2% lidocaine hydrochloride (5 ml; Hubei Tianyao
Pharmaceutical Co., Ltd.). The procedure was conducted using an
11-gauge Jamshidi needle (Cardinal Health Canada, Inc.). The biopsy
specimen was immediately fixed in 10% neutral buffered formalin
(Wuhan Servicebio Technology Co., Ltd.) at room temperature
(20-25˚C) for 24 h, followed by decalcification in 10% EDTA
solution (Wuhan Servicebio Technology Co., Ltd.) for 48 h. Samples
were dehydrated, embedded in paraffin and sectioned into 4-µm
slices using a microtome. The sections were rehydrated in
descending alcohol series (100, 95, 85 and 75% ethanol) and rinsed
with PBS (pH 7.4). Hematoxylin and eosin (H&E) staining was
performed using Mayer's hematoxylin (Wuhan Servicebio Technology
Co., Ltd.) for 5 min at room temperature, followed by eosin (1%)
staining for 2 min at room temperature (20-25˚C). Slides were
washed and mounted for microscopic observation under a light
microscope (BX53, Olympus Corporation).
For immunohistochemical (IHC) analysis,
paraffin-embedded sections underwent antigen retrieval in citrate
buffer (pH 6.0; Wuhan Servicebio Technology Co., Ltd.) at 95˚C for
20 min using a water bath. After cooling and washing with PBS,
endogenous peroxidase activity was blocked using 3% hydrogen
peroxide for 10 min at room temperature. Non-specific binding was
blocked with 5% normal goat serum (Wuhan Servicebio Technology Co.,
Ltd.) for 20 min at room temperature. No permeabilization step was
applied, as all antibodies targeted surface or membrane-associated
antigens. Primary antibodies were incubated overnight at 4˚C,
including: CD138 (cat. no. ab34164; dilution 1:100; Abcam), CD56
(cat. no. ab75813; dilution 1:200; Abcam), κ light chain (cat. no.
ZM-0317; dilution 1:150; OriGene Technologies, Inc.) and λ light
chain (cat. no. ZM-0318; dilution 1:150; Origene Technologies,
Inc.). After cooling, sections were washed with phosphate-buffered
saline (PBS, pH 7.4). After washing, HRP-conjugated secondary
antibodies (goat anti-rabbit/mouse IgG; cat. no. PV-6000; dilution
1:200; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) were
applied for 30 min at room temperature. Visualization was achieved
using 3,3'-diaminobenzidine (cat. no. ZLI-9018; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) as the chromogenic substrate,
followed by counterstaining with hematoxylin. Slides were observed
under a light microscope (Olympus BX53; Olympus Corporation).
Appropriate positive and negative controls were included for each
staining run. IHC procedures were performed using the automated
BenchMark ULTRA staining system (Roche Diagnostics GmbH) according
to manufacturer protocols where applicable.
Findings. Basic information
The patient was a 59-year-old man with a medical
history significant for stage 5 CKD, who was receiving maintenance
hemodialysis. The patient presented in February 2024 with
complaints of generalized weakness persisting for 2 days. Over the
previous month, the patient had been hospitalized in the Department
of Gastroenterology of the First Affiliated Hospital of Chongqing
Medical and Pharmaceutical College for symptoms including cough,
sputum production and melena. Laboratory findings during that
hospitalization revealed impaired renal function and anemia,
ultimately leading to the diagnosis of stage 5 CKD. The patient had
been undergoing regular hemodialysis three times per week, with
hemoglobin levels consistently maintained at ~60 g/l (normal range,
120-160 g/l), indicating persistent moderate-to-severe anemia.
Clinical observations included notable anemia,
hyperphosphatemia, hypocalcemia and general malaise. Additionally,
the patient experienced an intermittent cough with hemoptysis,
melena of unspecified quantity and progressive abdominal
distension. Despite these symptoms, urination remained normal, and
the patient had a significant unintentional weight loss of ~9 kg.
Prior to the onset of these symptoms, the patient had been
generally healthy, and had no history of hypertension, coronary
artery disease or diabetes mellitus.
At the time of admission in February 2024, the
patient reported a prior diagnosis of hypothyroidism and had been
taking levothyroxine sodium at a dose of 50 µg per day for the past
2 months. They had no known history of dysentery, malaria, viral
hepatitis, tuberculosis or other infectious diseases, nor any known
exposure to hepatitis or tuberculosis. The patient denied any
history of trauma, blood transfusions, drug allergies or food
allergies.
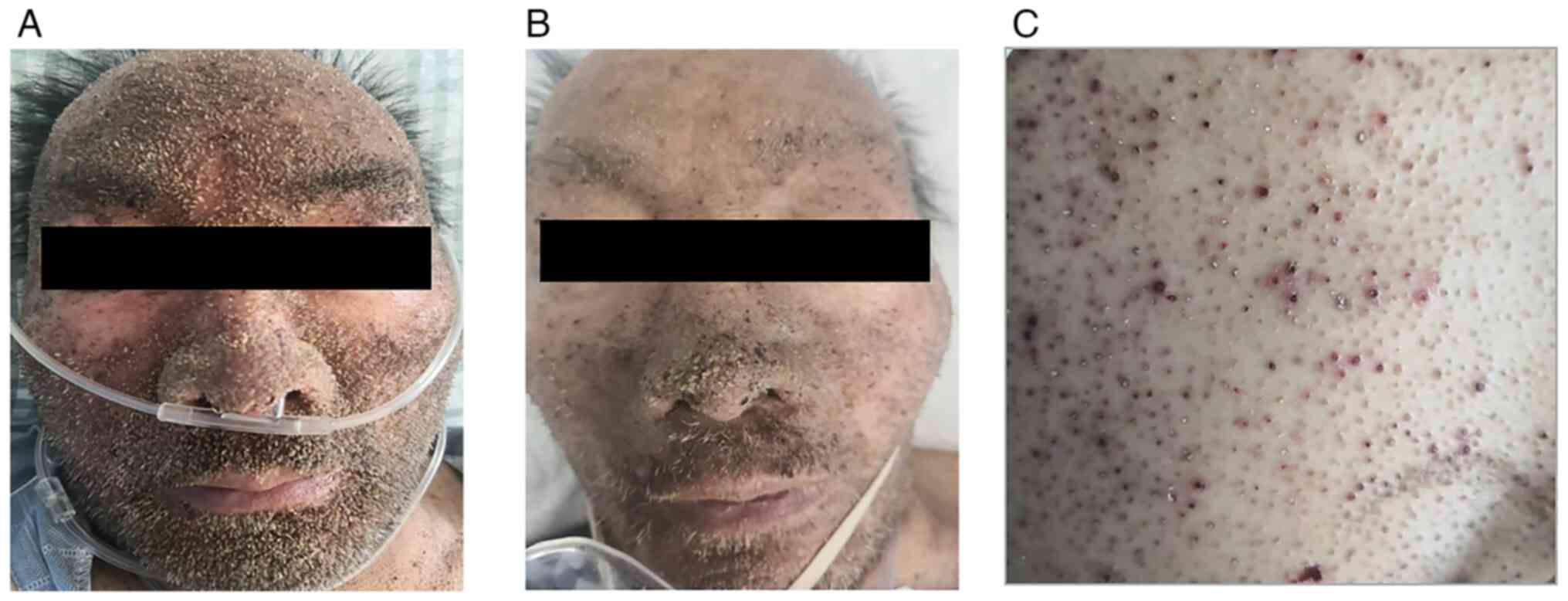
Physical examination. On presentation, the
patient was alert and oriented, with a dusky complexion.
Dermatological examination revealed numerous dense, pinpoint, brown
keratotic papules on the facial skin, consistent with the cutaneous
manifestations of MM.
The vital signs of the patient were as follows: Body
temperature, 36.4˚C (normal, 36.1-37.2˚C); pulse rate, 106 beats
per min (normal, 60-100 bpm); respiratory rate, 20 breaths per min
(normal, 12-20 breaths per min); and blood pressure, 115/78 mmHg
(normal, 90/60-120/80 mmHg). Respiratory system findings included
the following: Auscultation revealed slightly coarse breath sounds
bilaterally, with no evidence of dry or wet rales. Cardiovascular
system findings included the following: The heart rate was regular,
and no murmurs were detected in any of the cardiac valve regions.
In addition, the abdomen was flat, with periumbilical pigmentation;
it was soft, non-tender and exhibited no rebound tenderness or
muscle rigidity. Murphy's sign was negative and no mobile turbidity
was detected. In addition, bowel sounds were normal. In the lower
extremities, no edema was observed in either lower limb. Additional
findings included a 3-cm surgical scar on the left wrist. A
palpable tremor (thrill) was identified at the site of a previously
constructed arteriovenous fistula (AVF) for hemodialysis, located
on the left forearm. A continuous vascular murmur (bruit) was also
audible upon auscultation, consistent with a functioning AVF.
Inspection results. The physical examination
indicators of the patient are shown in Table I. Regarding the hematological
analysis, the patient exhibited anemia with a reduced red blood
cell count (1.54x1012/l), hemoglobin level (46 g/l) and
blood platelet count (75x109/l), which were all below
the reference ranges. The white blood cell count was within normal
limits. CRP levels were elevated at 13.09 mg/l (reference value,
≤10 mg/l) suggesting an inflammatory state. Notably high levels of
calcitonin (3.77 ng/ml; reference value, <0.15 ng/ml) and IL-6
(6,124.5 pg/ml; reference value, <6.6 pg/ml) were detected,
indicating an immune and inflammatory response associated with the
disease. The liver and kidney function test results were as
follows: Serum calcium was elevated at 2.96 mmol/l (reference
value, 2.25-2.75 mmol/l) and creatinine was markedly raised (566.53
µmol/l; reference value, 53-106 µmol/l), indicating renal
impairment. Protein markers, such as globulin (64.73 g/l; reference
value, 20-40 g/l) and total protein (102.86 g/l; reference value,
63-82 g/l), were also elevated.
 | Table IPhysical examination indicators of
the patient. |
Table I
Physical examination indicators of
the patient.
| Variable | Inspection
results | Reference
value |
|---|
| Hematological
analysis | | |
|
White blood
cell count, x109/l | 4.63 | 3.50-9.50 |
|
Red blood
cell count, x1012/l | 1.54 | 4.0-5.50 |
|
Hemoglobin,
g/l | 46.00 | 120-160 |
|
Blood
platelet count, x109/l | 75.00 | 100-300 |
| C-reactive protein,
mg/l | 13.09 | ≤10.0 |
| Calcitonin,
ng/ml | 3.77 | <0.15 |
| IL-6, pg/ml | 6,124.50 | <6.60 |
| Liver and kidney
function analysis | | |
|
Serum
calcium, mmol/l | 2.96 | 2.25-2.75 |
|
Creatinine,
µmol/l | 566.53 | 53-106 |
|
Globulin,
g/l | 64.73 | 20-40 |
|
Total
protein, g/l | 102.86 | 63-82 |
| Serum protein
electrophoresis results | | |
|
Albumin,
g/l | 46.00 | 35-55 |
|
α1 globulin,
g/l | 3.10 | 0.01-0.03 |
|
M protein,
% | 36.70 | - |
|
Globulin,
g/l | 49.53 | 20-40 |
|
Serum κ
light chain, g/l | 3.87 | 6.29-13.50 |
|
Serum λ
light chain, g/l | 52.00 | 3.13-7.23 |
|
Immunoglobulin
A, g/l | 12.2 | 0.80-4.53 |
|
Immunoglobulin
G, g/l | 4.87 | 7.51-15.60 |
|
Immunoglobulin
M, g/l | 0.14 | 0.46-3.04 |
| Hepatitis B virus
surface antibody, mIU/ml | 909.71 | 0-10 |
| Hepatitis B virus
core antibody, IU/ml | 0.62 | <0.35 |
| Hepatitis B Virus e
antibody, IU/ml | <0.100 | <0.15 |
| Hepatitis B virus e
antigen, IU/ml | <0.050 | <0.10 |
| Thyroid function
tests | | |
|
Triiodothyronine,
nmol/l | 0.93 | 1.6-3.0 |
|
Thyrotropin,
mIU/l | 0.59 | 0.4-5.0 |
| Anemia tests | | |
|
Ferritin,
ng/ml | >3,000 | 25-350 |
|
Folate,
ng/ml | 3.03 | 5.21-20 |
SPE results indicated that the M protein percentage
was 36.7%, characteristic of monoclonal gammopathy. Other
immunoglobulin abnormalities included elevated IgA (12.2 g/l;
reference value, 0.71-3.35 g/l) and an increased λ light chain
(52.0 g/l; reference value, 3.13-7.23 g/l), further indicating a
monoclonal protein spike. Furthermore, the patient had a high level
of hepatitis B virus surface antibody (909.71 mIU/ml; reference
value, 0-10 mIU/ml), with normal levels for other hepatitis
markers. Thyroid function tests demonstrated a reduced
triiodothyronine level (0.93 nmol/l; below the normal range),
whereas thyrotropin (0.59 mIU/l) was within the reference range.
The results of the anemia test revealed that ferritin levels were
high (>3,000 ng/ml; reference value, 25-350 ng/ml), suggesting
chronic disease-related anemia, whereas folate levels were low
(3.03 ng/ml; reference value, 5.21-20 ng/ml), which could
contribute to anemia. These results indicated a comprehensive
systemic involvement in the patient, which supported the diagnosis
and informed the clinical management of MM with associated
complications, including anemia, renal insufficiency and immune
dysregulation.
Karyotype analysis results. The karyotype
analysis of the patient revealed extensive chromosomal
abnormalities consistent with MM and indicative of notable genetic
instability. The ISCN results were as follows:
‘83-85<4n>,XXY,-Y,+1,+1,dic(1;10)(p13;q26),psu idic(1)(p21),add(7)(q32),add(8)(p23) x2,?add(17)(q22)x2,-19,-19,-20,-21,-21,-22,+1-2mar,inc[cp4]/46,
XY[16]’.
The key findings in the present study included: i)
Hyperdiploidy with tetraploid cells (83-85 chromosomes), the
presence of tetraploid cells (83-85 chromosomes) indicates
hyperdiploidy, which is often associated with advanced disease. ii)
extra X chromosome (XXY), the additional X chromosome is an unusual
finding and may reflect somatic changes linked to malignancy. iii)
loss of Y chromosome (-Y), the absence of the Y chromosome is
frequently observed in hematological malignancies and is considered
a marker of disease progression in MM. iv) polysomy of chromosome 1
(+1,+1), the addition of two extra copies of chromosome 1 suggests
high-level polysomy, which is commonly associated with aggressive
forms of myeloma and may indicate a poorer prognosis. v) dicentric
chromosome [dic(1;10)(p13;q26)], the presence of a dicentric
chromosome formed by the fusion of chromosomes 1 and 10 suggests
structural chromosomal instability, which can serve a role in
tumorigenesis. vi) pseudo-isodicentric chromosome [psu
idic(1)(p21)], this abnormality in
chromosome 1, featuring duplication and inversion, reflects further
chromosomal rearrangement, indicative of genomic instability. vii)
additional material on chromosomes 7, 8, and 17: Specifically noted
in the ISCN as add(7)(q32),
add(8)(p23)x2, and ?add(17)(q22)x2, the term ‘add’ indicates the
presence of unidentified extra chromosomal material at these loci.
These changes may represent gene amplifications or rearrangements
involved in disease progression. viii) losses of chromosomes 19,
20, 21 and 22 (-19,-20,-21,-22), the deletion of these chromosomes
further supports the presence of extensive genomic instability,
often seen in advanced stages of MM. ix) marker chromosomes (mar),
the presence of 1-2 marker chromosomes signifies structurally
abnormal, unidentifiable chromosomes, which are frequently observed
in hematological malignancies and represent complex karyotypic
alterations. x) mosaicism (inc[cp4]/46,XY[16]), the karyotype
exhibits mosaicism, with four abnormal cell clones (cp4) and 16
cells with a normal male karyotype (46,XY). This mosaic pattern
suggests clonal evolution within the disease (Fig. 1).
![Karyotype analysis revealing complex
chromosomal abnormalities in advanced MM. Conventional karyotyping
revealed multiple numerical and structural abnormalities (red
arrows) characteristic of high-risk MM: i) Hyperdiploidy with
tetraploid cells (83-85<4n>,XXY), including an additional X
chromosome. ii) Loss of Y chromosome (-Y), a common finding in
hematological malignancies. iii) High-level polysomy of chromosome
1 (+1,+1), totaling four copies, indicating aggressive disease. iv)
Dicentric chromosome [dic(1;10)(p13;q26)], suggesting genomic
instability. v) Pseudo-isodicentric chromosome [psu idic(1)(p21)], reflecting complex chromosomal
rearrangement. vi) Additional material on chromosomes 7 and 8
[add(7)(q32),add(8)(p23)x2]. vii) Possible unidentified
material on chromosome 17 [?add(17)(q22)x2]. ix) Monosomy of chromosomes
19, 20, 21 and 22 (green arrows). xi) One to two marker chromosomes
(+1-2mar). xii) Mosaic karyotype: Four abnormal clones with complex
karyotype and 16 normal male karyotypes (46,XY), consistent with
clonal evolution. MM, multiple myeloma.](/article_images/etm/30/3/etm-30-03-12930-g00.jpg) | Figure 1Karyotype analysis revealing complex
chromosomal abnormalities in advanced MM. Conventional karyotyping
revealed multiple numerical and structural abnormalities (red
arrows) characteristic of high-risk MM: i) Hyperdiploidy with
tetraploid cells (83-85<4n>,XXY), including an additional X
chromosome. ii) Loss of Y chromosome (-Y), a common finding in
hematological malignancies. iii) High-level polysomy of chromosome
1 (+1,+1), totaling four copies, indicating aggressive disease. iv)
Dicentric chromosome [dic(1;10)(p13;q26)], suggesting genomic
instability. v) Pseudo-isodicentric chromosome [psu idic(1)(p21)], reflecting complex chromosomal
rearrangement. vi) Additional material on chromosomes 7 and 8
[add(7)(q32),add(8)(p23)x2]. vii) Possible unidentified
material on chromosome 17 [?add(17)(q22)x2]. ix) Monosomy of chromosomes
19, 20, 21 and 22 (green arrows). xi) One to two marker chromosomes
(+1-2mar). xii) Mosaic karyotype: Four abnormal clones with complex
karyotype and 16 normal male karyotypes (46,XY), consistent with
clonal evolution. MM, multiple myeloma. |
In summary, these findings collectively indicated a
high degree of chromosomal complexity and genetic instability,
typical of advanced MM (12). The
chromosomal abnormalities, including polysomy, structural
rearrangements, marker chromosomes and loss of specific
chromosomes, are associated with disease progression and a poor
prognosis.
IFE analysis results. SPE revealed a
prominent M-spike in the γ region, consistent with the presence of
monoclonal protein (Fig. 2A). IFE
demonstrated specific staining for IgA and λ light chains,
confirming a monoclonal IgA λ component (Fig. 2B). A second IFE analysis was
performed on a follow-up serum sample, which consistently showed
the same IgA λ monoclonal pattern (Fig. 2C). This repeat testing served as a
confirmatory step to exclude false positivity and to strengthen
diagnostic reliability. These findings provided crucial diagnostic
evidence for the presence of monoclonal gammopathy, a hallmark
feature of MM.
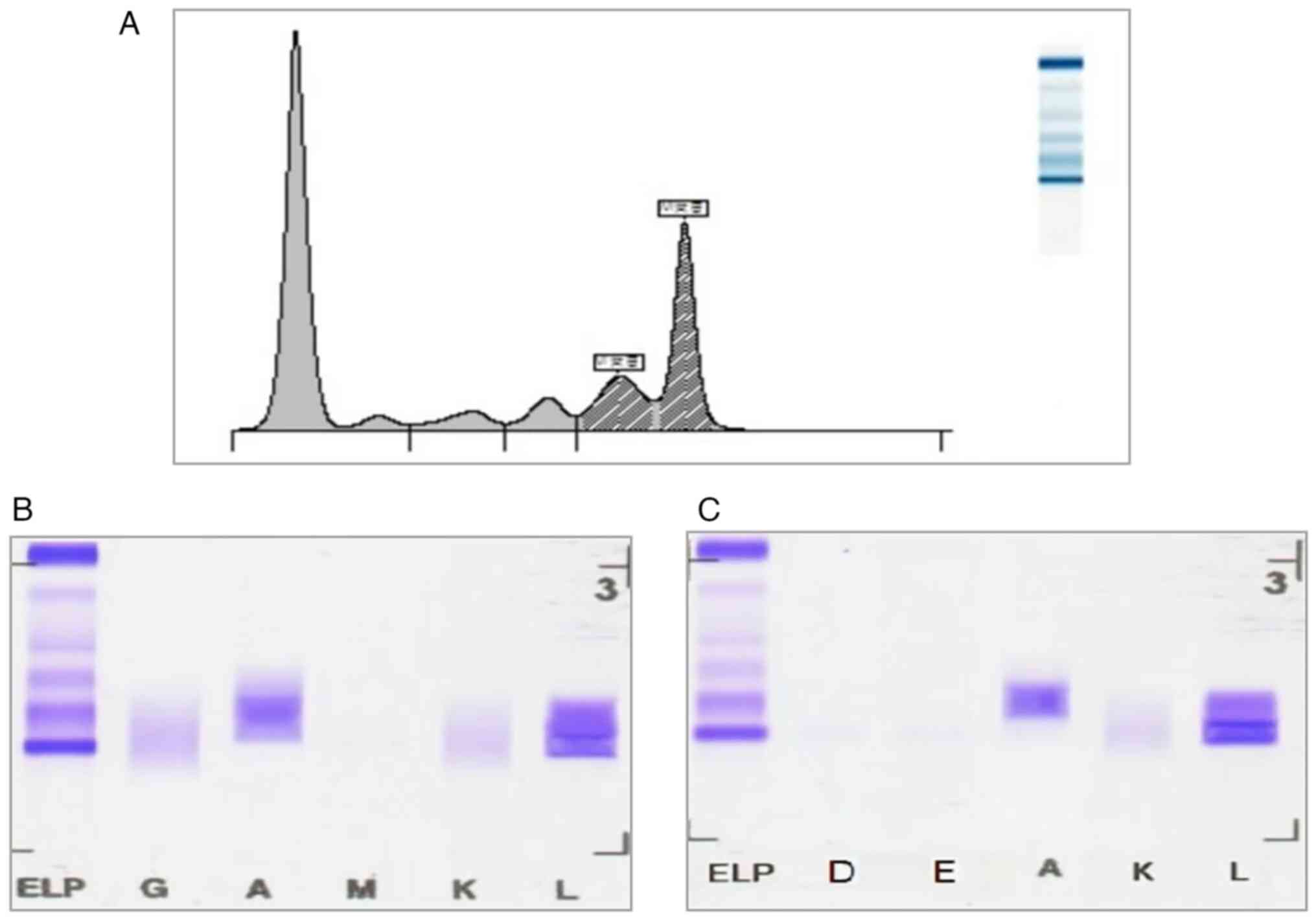 | Figure 2SPE and immunofixation confirming
monoclonal gammopathy. (A) SPE demonstrating an M-spike in the γ
region. (B) Immunofixation electrophoresis revealing a monoclonal
IgA λ pattern, confirming the presence of IgA-λ type multiple
myeloma. (C) Immunofixation electrophoresis further confirming the
monoclonal IgA λ pattern. AU, arbitrary units; SPE, serum protein
electrophoresis. ELP, electrophoresis reference; G, IgG; A, IgA; M,
IgM; K, κ light chain; L, λ light chain; D, IgD; E, IgE. |
Bone marrow biopsy results. Bone marrow
biopsy revealed markedly active hematopoietic tissue proliferation,
occupying >90% of the marrow volume, with a corresponding
reduction in adipose tissue. Plasma cell hyperplasia was evident,
characterized by medium-sized cytoplasm, abundant cytosol, nuclear
eccentricity and patchy distribution. Additionally, areas of
fibrous tissue proliferation were observed, as shown by
representative hematoxylin and eosin-stained sections of the bone
marrow biopsy.
The IHC findings are summarized in Table II, and provide critical insights
into the cellular and molecular characteristics of the bone marrow
biopsy. Weak positive staining was observed for both CD3 and CD20,
indicating limited involvement of T cells (CD3) and B cells (CD20).
For CD56, patchy positive staining was noted. For CD56, patchy and
heterogeneous positive staining was noted across the biopsy sample.
CD56, a neural cell adhesion molecule, is frequently expressed in
plasma cell dyscrasias, including MM (15). The non-uniform distribution and
limited number of CD56-positive cells suggested partial expression
in this case. For CD138, patchy positive staining was also present,
confirming the presence of plasma cells. Regarding κ and λ light
chains, κ light chain staining was negative, whereas λ light chains
exhibited fragmented positive staining. This pattern indicates a
monoclonal plasma cell population with λ light chain restriction,
consistent with the diagnosis of MM. For multiple myeloma oncogene
1 (MUM-1), fragmented positive staining was detected,
characteristic of late-stage B-cell differentiation and plasma
cells, a hallmark feature in MM cases. The immunohistochemical
profile, including the expression of CD138, CD56, MUM-1 and the λ
light chain restriction, strongly supported the diagnosis of MM
with a monoclonal λ light chain profile (16).
 | Table IIImmunohistochemistry findings for the
patient. |
Table II
Immunohistochemistry findings for the
patient.
| Variable | Result |
|---|
| CD3 | Weakly positive
staining |
| CD20 | Weakly positive
staining |
| CD56 | Patchy positive
staining |
| CD138 | Patchy positive
staining |
| κ light chain | Negative
staining |
| λ light chain | Fragmented positive
staining |
| Multiple myeloma
oncogene 1 | Fragmented positive
staining |
The bone marrow biopsy findings demonstrated notable
proliferation of monoclonal plasma cells, accounting for ~70% of
the marrow composition. These results are consistent with a
diagnosis of plasma cell myeloma (PCM) (17).
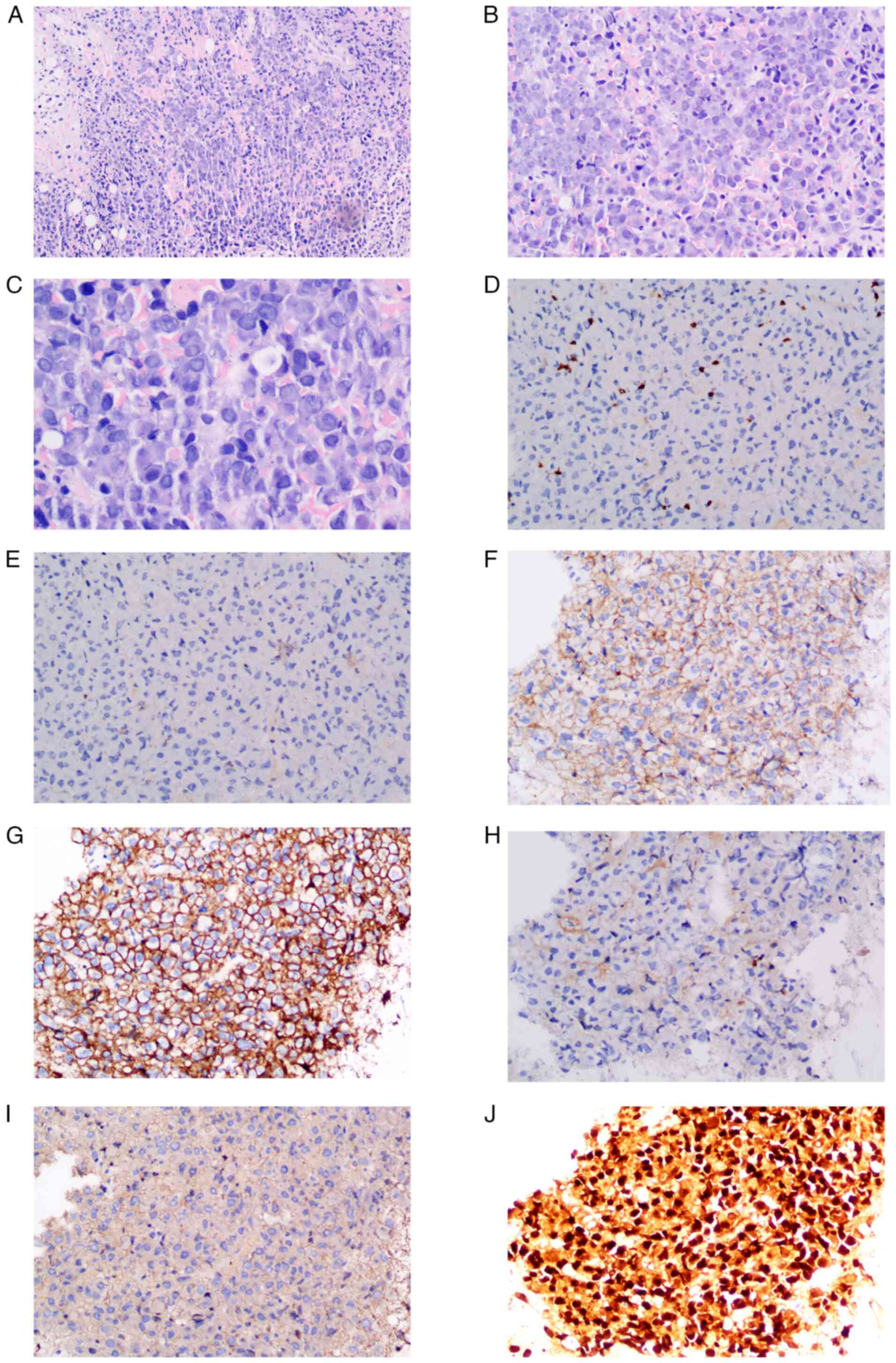
Histopathological and immunohistochemical
findings. A skin punch biopsy was obtained from the facial
keratotic papules to characterize the follicular spicules. H&E
staining revealed marked follicular plugging with compact
orthokeratosis and keratinous debris occupying dilated follicular
infundibula. The surrounding dermis exhibited mild perivascular
lymphocytic infiltration without evidence of epidermal atypia.
Congo red staining, performed on both skin and bone marrow
specimens, revealed eosinophilic amorphous deposits in the dermis
and marrow. Under polarized light, these deposits exhibited classic
apple-green birefringence, confirming amyloid deposition (Fig. 3A).
To further evaluate the bone marrow biopsy, IHC
staining was conducted to identify cellular components and assess
clonality of infiltrating plasma cells. Scattered CD3-positive T
cells and CD20-positive B cells were detected, indicating minimal
lymphoid infiltration (Fig. 3B and
C). CD56 staining showed diffuse
membranous positivity (Fig. 3D),
suggesting aberrant expression in neoplastic plasma cells or
natural killer (NK) cell involvement. CD138 was diffusely and
strongly expressed on infiltrating plasma cells (Fig. 3E), confirming their plasma cell
phenotype. Light chain restriction analysis showed complete absence
of κ light chain expression (Fig.
3F) and intense diffuse positivity for λ light chains (Fig. 3G), indicating monoclonal λ light
chain restriction. MUM-1 staining demonstrated diffuse nuclear
positivity in plasma cells (Fig.
3H), supporting their plasmacytic differentiation.
Additionally, H&E staining of the bone marrow revealed diffuse
infiltration by atypical plasma cells with irregular nuclear
morphology (Fig. 3I), consistent
with plasma cell myeloma (PCM). Positive staining for amyloid
deposits in bone marrow biopsy further confirmed amyloid
deposition, with strong eosinophilic characteristics and brown
staining (Fig. 3J).
Together, the histopathological and immunophenotypic
findings from the cutaneous tissue and bone marrow biopsy confirmed
the diagnosis of systemic amyloid light-chain amyloidosis secondary
to monoclonal PCM, presenting with rare cutaneous involvement in
the form of follicular spicules (18).
Flow cytometric immunophenotyping for hematologic
tumor classification. Multiparameter flow cytometry was
performed on fresh bone marrow aspirate samples collected in
EDTA-containing tubes to characterize the immunophenotypic profiles
of hematopoietic cell populations. Sample acquisition was conducted
on a BD FACSCanto™ II flow cytometer (BD Biosciences).
Data analysis was performed using FlowJo™ software,
version 10.8.1 (BD Biosciences). Gating strategies were applied to
total nucleated cells (NCs) using CD45 and side scatter profiles.
As shown in Fig. 4A, lymphocytes
accounted for 27.76% of total NCs. The CD4/CD8 ratio was decreased,
indicating a shift in T-cell subset balance, which may reflect
immune dysregulation associated with plasma cell disorders.
CD57+ large granular lymphocytes (LGLs) were detected
within the normal reference range (2-15% of total lymphocytes)
(Fig. 4B and C), suggesting no evidence of LGL
expansion or chronic LGL leukemia. CD19+ B lymphocytes
constituted 13.08% of the lymphocyte population, with normal
distributions of precursor and mature subsets, indicating no
disruption in B-cell maturation. Notably, a substantial expansion
of plasma cells was observed (Fig.
4D). As shown in Fig. 4E,
gating on CD38 and CD45 expression revealed a distinct
CD38bright+(CD38bri+) population, accounting
for 33.19% of total NCs. These cells exhibited low CD45 expression
and high CD38 expression, a typical immunophenotypic hallmark of
malignant plasma cells. The markedly increased proportion of
CD38bri+ cells indicated significant plasma cell
infiltration within the bone marrow.
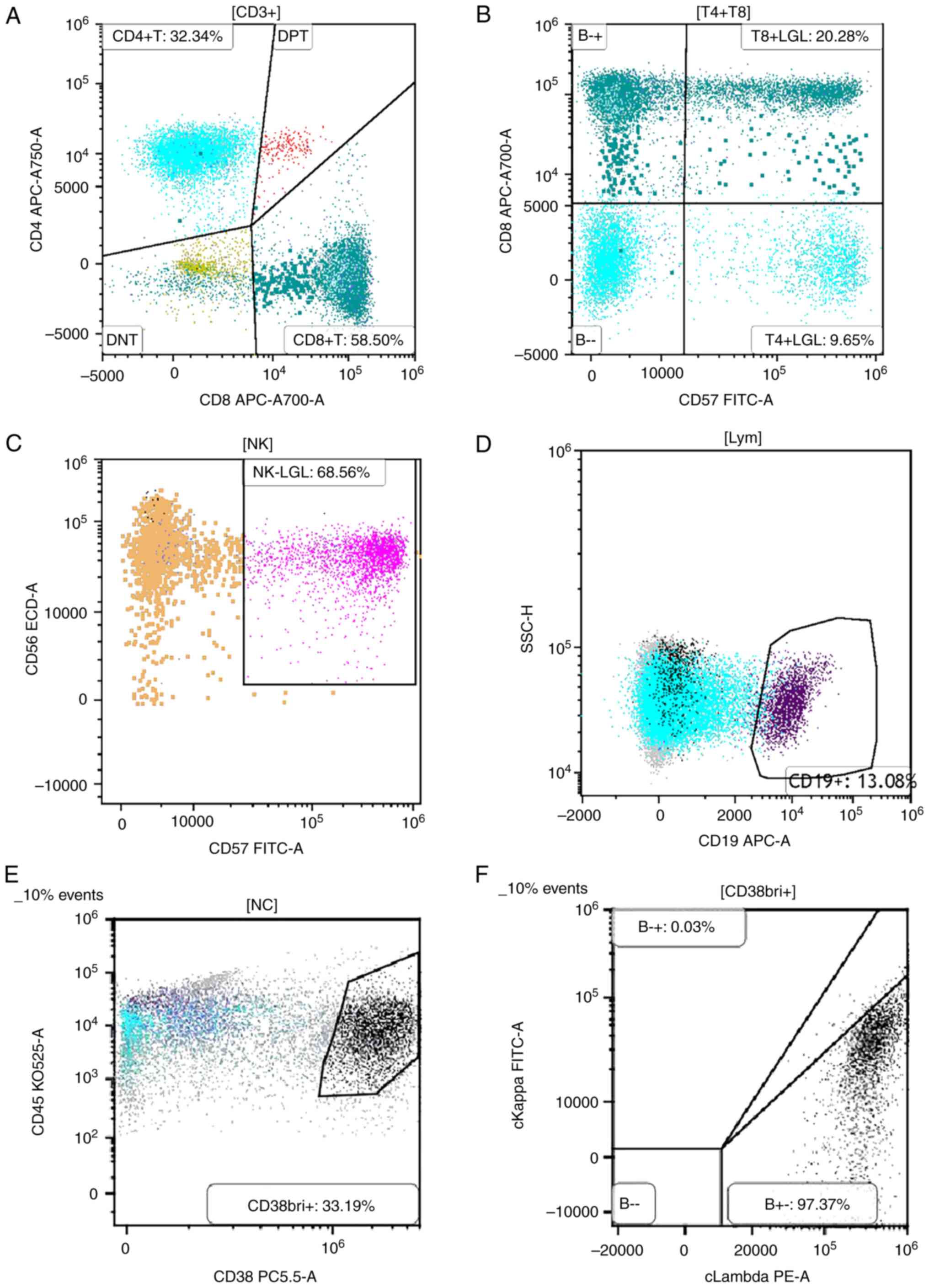 | Figure 4Flow cytometric immunophenotyping of
bone marrow cells from a patient with PCM. (A) Lymphocyte analysis
showing a decreased CD4/CD8 ratio. (B and C) Quantification of
CD57⁺ LGLs, demonstrating a normal proportion of this subset. (D)
Marked expansion of plasma cells detected within the bone marrow
aspirate. (E) Dual-parameter plot of CD38 and CD45 expression
revealing a distinct CD38bright+/CD45dim+
population, accounting for 33.19% of total nucleated cells,
consistent with malignant plasma cell infiltration. (F)
Intracellular immunoglobulin light chain staining within the
CD38bright+ population indicating prominent λ light
chain restriction (97.37%) with minimal κ expression (0.03%),
confirming clonal proliferation and supporting the diagnosis of
PCM. LGLs, large granular lymphocytes; NC, nucleated cell; PCM,
plasma cell myeloma; A, allophycocyanin-Alexa Fluor 700; APC,
allophycocyanin; CD38bri+, CD38 bright positive; DNT,
double negative T cells; DPT, dual-parameter plot; ECD,
energy-coupled dye; H, height parameter; KO525, Krome Orange 525;
Lym, lymphocytes; NK, natural killer cells; PC5.5,
phycoerythrin-cyanine 5.5; PE, phycoerythrin; SSC, side
scatter. |
Further immunophenotypic analysis of the
CD38bri+ gated population was conducted to assess light
chain restriction. As demonstrated in Fig. 4F, intracellular staining for
cytoplasmic immunoglobulin light chains revealed predominant
expression of λ light chains in 97.37% of the gated plasma cells,
with minimal κ expression (0.03%). This pronounced λ light chain
restriction confirms the presence of a clonal plasma cell
population and supports the diagnosis of PCM. Collectively, these
flow cytometry findings demonstrated substantial clonal
proliferation of λ-restricted malignant plasma cells within the
bone marrow, consistent with a diagnosis of MM.
Imaging and histopathological findings. A
thorough imaging evaluation was conducted, including CT scans of
the pelvis, cervical spine and head. The key findings are
summarized as follows: i) Cranial findings, head CT revealed no
abnormalities in the brain parenchyma. Sinus findings revealed
evidence of bilateral maxillary and ethmoid sinusitis. ii) Skeletal
lesions, multiple hypodense lesions were identified in the skull,
several vertebrae, bilateral ribs, scapulae and pelvic bones, which
are findings consistent with lytic lesions of MM. iii) Fractures
and spinal changes, bilateral pathological rib fractures,
compression of the T12 vertebral body and a suspected
intravertebral lesion in T1 were observed, along with degenerative
changes in the spine. iv) Pulmonary findings, bilateral lung
infections, pleural thickening and small bilateral pleural
effusions were evident (Fig.
5A-C). v) Renal findings, CT revealed small calculi in the
right kidney. vi) Abdominal findings, increased soft tissue density
surrounding the abdominal aorta was detected. vii) Pelvic findings,
small pelvic effusions were observed. viii) Histopathological
findings, dermatopathological analysis revealed squamous epithelium
with areas of hyperkeratosis (Fig.
5D). Furthermore, Fig. 6
presents a detailed histopathological view, showing an area of
extensive infiltration of malignant plasma cells in the dermal
layer, consistent with cutaneous involvement in MM. These findings
collectively indicated advanced MM with extensive skeletal
involvement and associated systemic complications.
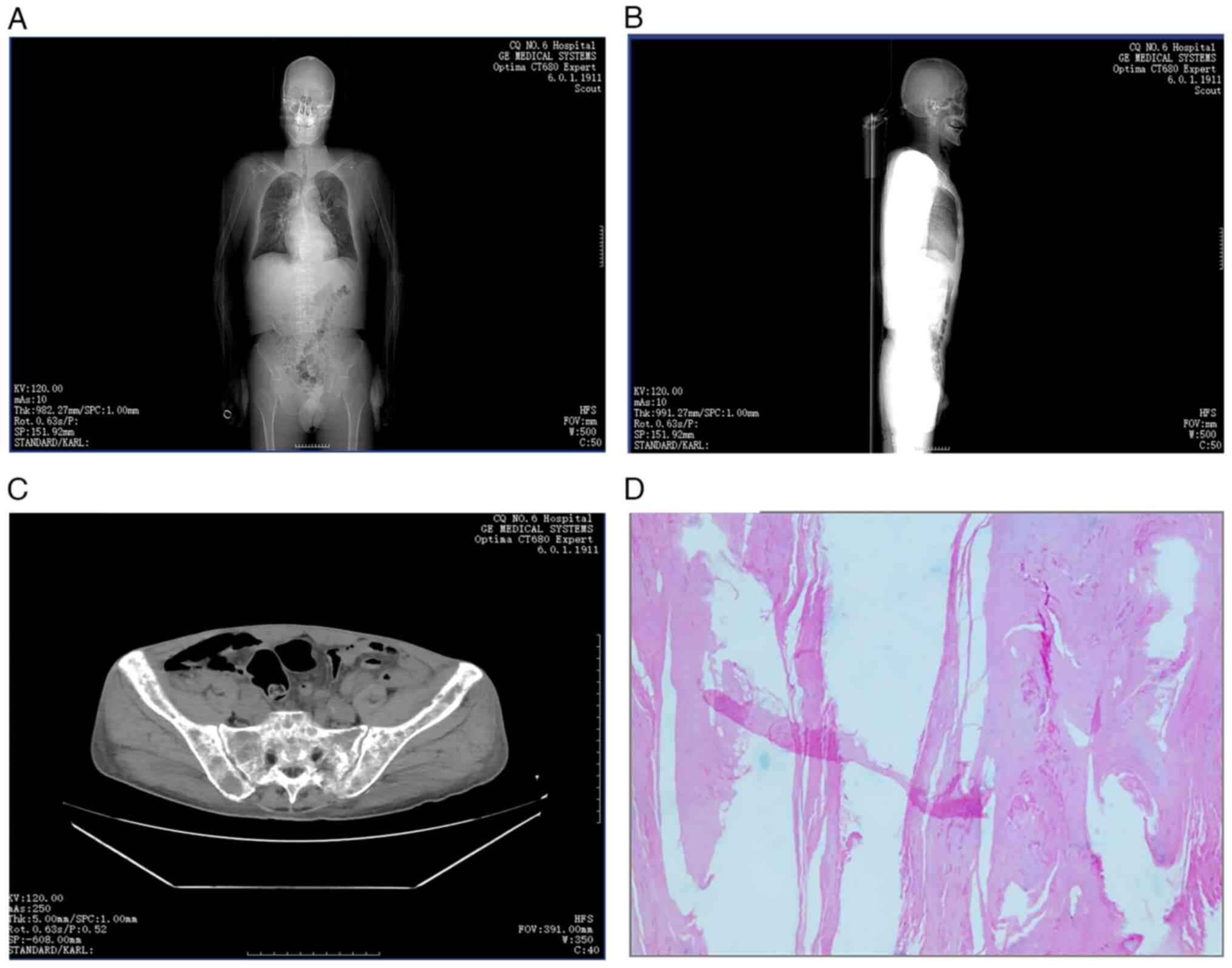 | Figure 5Comprehensive imaging and
histopathological evaluation. (A) An anterior scout image from CT
demonstrated diffuse skeletal involvement, including multiple
hypodense lesions in the skull, ribs, spine, scapulae and pelvis,
consistent with lytic lesions seen in multiple myeloma. Bilateral
pulmonary infections and pleural thickening were also visible. (B)
A lateral scout image further illustrated spinal abnormalities,
including compression of the T12 vertebral body and suspected
Hsu-Mo nodule at T1, as well as degenerative spinal changes. (C) An
axial pelvic CT revealed lytic bone lesions, bilateral pathological
rib fractures and pelvic effusion. (D) Histopathological
examination of a skin lesion showed squamous epithelium with marked
hyperkeratosis (hematoxylin and eosin staining; original
magnification, x100), supporting dermatopathological
involvement. |
Treatment timeline and clinical
course. Diagnosis
The patient was diagnosed with MM, stage III,
subtype B, IgA-λ type in February 2024. Concomitantly, the patient
had stage 5 CKD, for which maintenance hemodialysis (three sessions
per week) was initiated as supportive therapy.
Disease progression and therapeutic
interventions. The clinical course and major therapeutic
milestones are summarized in Fig.
7. The condition of the patient exhibited episodic
deterioration with corresponding escalation in supportive care.
Notably, data from March 2024 revealed critical insights into
disease progression.
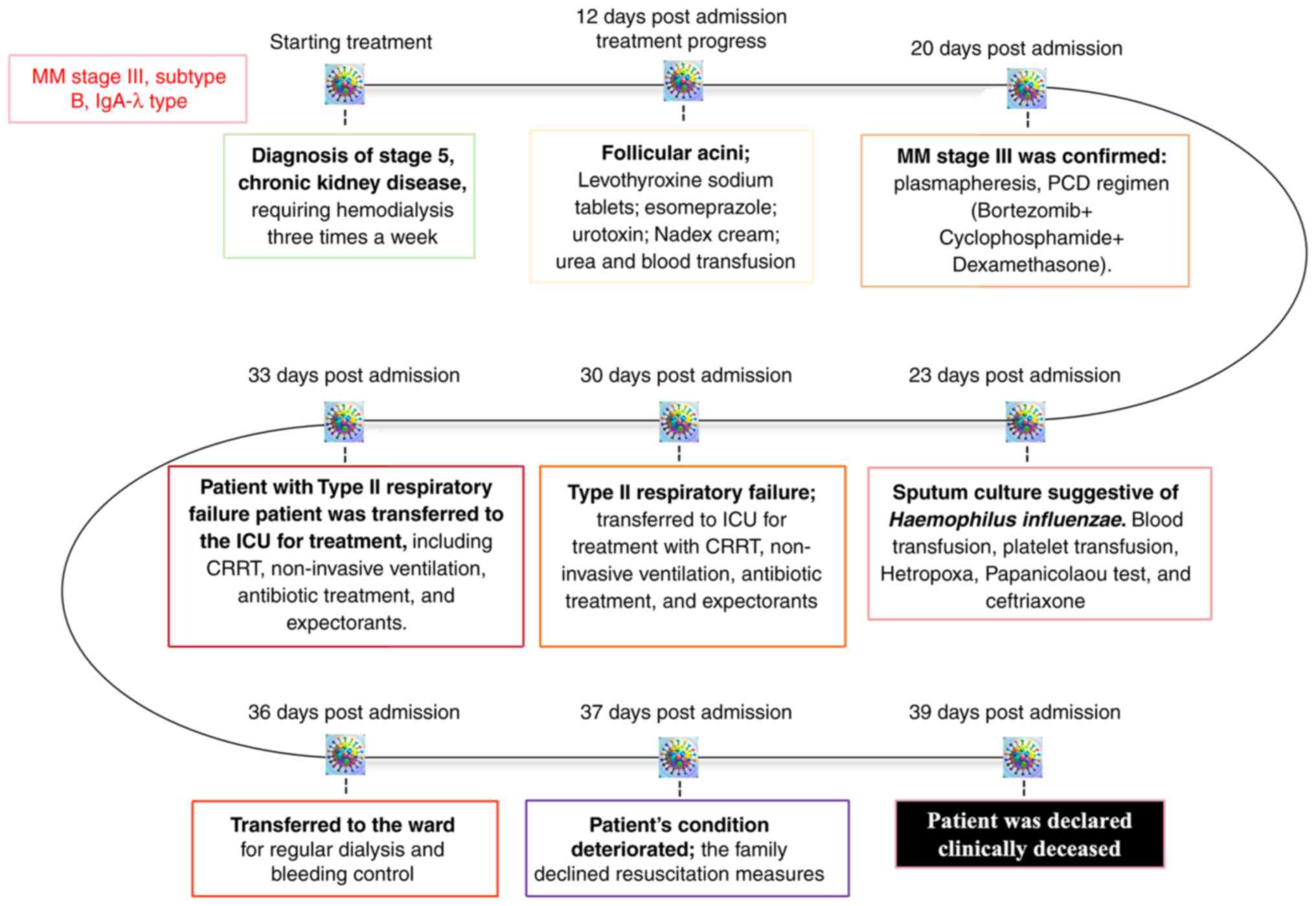 | Figure 7Timeline of clinical course and
interventions in a patient with IgA-λ type MM. The patient was
diagnosed with stage III MM (subtype B, IgA-λ type) and regular
hemodialysis was initiated. In March 2024, follicular acanthosis
was noted; treatment included levothyroxine, esomeprazole,
urotropine, urea cream, nadifloxacin cream and blood transfusion.
In April 2024, the patient developed type II expiratory failure and
was admitted to the ICU for CRRT, non-invasive ventilator support,
and symptomatic management. Despite intensive care, the patient
succumbed to disease-related complications. CRRT, continuous renal
replacement therapy; ICU, Intensive Care Unit; MM, multiple
myeloma. |
i) 12 days post-admission: The patient was
prescribed a supportive pharmacological regimen, which included the
following treatments: i) Levothyroxine sodium (50 µg, oral, daily)
to treat hypothyroidism, which was diagnosed based on thyroid
function tests; ii) esomeprazole (40 mg, oral, daily), prescribed
for gastroesophageal reflux disease and to prevent gastric ulcers,
a common side effect of other medications used in the patient's
treatment.; iii) urotoxin (5 ml, topical, applied twice daily) to
treat localized urinary tract infection symptoms, primarily urinary
discomfort; iv) Nadex cream (1% strength, topical, applied twice
daily), applied to manage dermatological symptoms related to skin
irritation and hyperkeratosis associated with the patient's
condition; v) urea preparations (10%, topical, applied as needed),
for moisturizing and treating dry, flaky skin, a complication
arising from systemic treatment and disease progression; and vi)
blood transfusions (two units of packed red blood cells,
intravenous infusion) administered for symptomatic anemia, which
was necessary due to the patient's worsening hematological
parameters. These treatments were provided in conjunction with
ongoing monitoring and adjustments to the supportive care plan
based on the patient's clinical response.
ii) 20 days post-admission: The patient presented
with cutaneous manifestations characterized by the emergence of
follicular spicules, suggestive of a dermatological response
possibly associated with the underlying hematological or renal
pathology. The patient received the same treatment as
aforementioned, namely, levothyroxine sodium tablets (for
hypothyroidism), esomeprazole (proton pump inhibitor for gastric
protection), urotoxin (for renal support), nadex cream (for
dermatological lesions) and urea cream (for skin hydration). In
addition, blood transfusion was provided to address persistent
anemia.
iii) 23 days post-admission: Microbiological testing
was conducted on a sputum sample using standard bacteriological
culture techniques. The specimen was cultured on chocolate agar and
incubated under 5% CO2 at 37˚C for 24-48 h. Bacterial
identification was performed using Gram staining and confirmed with
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry. Haemophilus influenzae was isolated,
indicating a lower respiratory tract infection. The patient was
treated with ceftriaxone (2 g/day, intravenous infusion, for 5
days) targeting the identified pathogen. Blood and platelet
transfusions were also administered to manage anemia and
thrombocytopenia. Additionally, a sputum cytology specimen was
processed and stained using Hetropoxa-Papanicolaou staining was
performed at room temperature (20-25˚C) for 15 min for cytological
evaluation. The result revealed numerous neutrophils and
degenerating epithelial cells, consistent with an active infectious
process.
iv) 30 to 33 days post-admission: The patient
developed type II respiratory failure, characterized by hypercapnia
and hypoxemia, necessitating transfer to the Intensive Care Unit.
Treatment included continuous renal replacement therapy for
metabolic stabilization, and non-invasive mechanical ventilation
(BiPAP mode, FiO2 40-60%) to support respiratory
function. Pharmacological management comprised of the following: i)
Broad-spectrum antibiotics, including piperacillin-tazobactam (4.5
g IV every 8 h) to empirically cover gram-negative and anaerobic
pathogens pending culture results; ii) moxifloxacin (400 mg IV once
daily) to provide additional coverage for atypical respiratory
organisms; and iii) mucolytic therapy, including acetylcysteine
(600 mg oral, three times daily), to reduce mucus viscosity and
improve airway clearance. Despite these interventions, the
patient's respiratory compromise persisted, with ongoing oxygen
dependence and elevated arterial CO2 levels, indicating
progressive ventilatory failure.
v) 36 days post admission: Following partial
stabilization, the patient was transferred back to the general
ward, and continued on regular hemodialysis and received care for
recurrent bleeding episodes.
vi) 37 days post admission: The clinical status of
the patient deteriorated again. After a discussion with the family,
a do-not-resuscitate order was established and aggressive
life-sustaining interventions were withheld.
vii) 39 days post admission: The patient was
pronounced clinically deceased.
Discussion
The diagnosis of FSMM necessitates the exclusion of
other dermatoses that can present with similar follicular
hyperkeratotic features. Conditions such as vitamin A deficiency
may lead to follicular hyperkeratosis, but they are often
accompanied by xerosis and ocular findings such as Bitot's spots
(19). In the present case, the
serum vitamin A levels of the patient were normal and no ocular
signs were present. HIV-associated follicular hyperkeratosis,
particularly in cases of advanced immunosuppression, can clinically
mimic FSMM; however, the patient tested negative for HIV via both
serological and molecular methods, and exhibited no signs of
immunodeficiency (20,21).
Skin manifestations related to chronic renal
failure, such as prurigo nodularis and perforating dermatoses, may
involve follicular lesions; however, these lack the distinct
histopathological characteristics of FSMM, including
intrafollicular monoclonal protein deposition (22). Additionally, disorders such as
acanthosis nigricans and lichen spinulosus can present with spiny
papules but do not exhibit the immunoglobulin deposition typical of
FSMM (23). Thus, the definitive
diagnosis in the present case was supported by the presence of
monoclonal immunoglobulin λ light chain deposition in the
follicular epithelium, along with plasma cell-rich dermal
infiltrates, consistent with the known PCM of the patient.
The pathophysiology of FSMM remains incompletely
understood. Current evidence suggests that monoclonal
immunoglobulins, particularly light chains secreted by clonal
plasma cells, are deposited within follicular structures, leading
to epithelial disruption and reactive hyperkeratosis. These
deposits may also undergo conformational changes, promoting
amyloidogenesis and contributing to the formation of follicular
spicules (24,25).
Notably, not all patients with MM develop FSMM.
According to a previous study, follicular spicules associated with
FSMM occur in <1% of patients with MM, highlighting its rarity
and the likelihood that additional host or environmental factors,
such as serum paraprotein concentration, the structural properties
of light chains or the local skin microenvironment, may influence
susceptibility (26). In the
current case, skin biopsy revealed follicular hyperkeratosis with
prominent λ light chain deposition, consistent with this
hypothesized mechanism. The absence of cryoglobulinemia was
confirmed by both negative cryoglobulin screening in serum at 4˚C
and a lack of clinical symptoms typically associated with
cryoglobulinemia, indicating that cold-precipitable immunoglobulins
are not essential for FSMM development.
In the present case report, the comorbid end-stage
CKD and long-term hemodialysis introduced additional diagnostic
complexity. CKD is associated with a broad spectrum of
dermatological manifestations, including uremic pruritus,
perforating dermatoses and calciphylaxis. However, these
CKD-related dermatoses differ significantly from FSMM in both
clinical manifestations and histopathological features (27).
Calciphylaxis typically manifests with painful,
ulcerative lesions in areas of adiposity and vascular
calcification, whereas uremic pruritus lacks follicular-based
histological findings. Notably, monoclonal immunoglobulin
deposition is rarely observed in CKD-related dermatoses (28). Therefore, the presence of
immunoglobulin light chains within follicular structures in the
present case strongly supports the diagnosis of FSMM over other
CKD-related skin disorders (29).
The present case illustrates the clinical value of
recognizing FSMM as a cutaneous marker of plasma cell dyscrasia.
FSMM may precede systemic symptoms, serve as a sign of disease
relapse or reflect a poor prognosis. Recognition of its
characteristic morphology, together with timely histological and
immunofluorescence evaluation, can enable early diagnosis and guide
therapeutic decisions.
Novel learning points from the present case include:
i) FSMM may occur independently of cryoglobulinemia, challenging
earlier assumptions (30); ii)
immunoglobulin light chain deposition can be a key distinguishing
factor in patients with comorbid renal disease; and iii) FSMM
serves as a potential non-invasive biomarker for disease activity
in MM. Had the condition of the patient stabilized, long-term
management could have included systemic anti-myeloma therapy
adapted for renal impairment, topical keratolytics or retinoids for
symptom control, and regular dermatological monitoring to assess
treatment response. Nutritional support and optimization of
dialysis care may also contribute to maintaining skin integrity
(31).
In conclusion, FSMM should be considered in patients
with unexplained follicular hyperkeratosis and suspected or
confirmed plasma cell neoplasms. Dermatological manifestations in
such patients warrant thorough evaluation, including biopsy and
immunoglobulin profiling. Further research is needed to clarify the
mechanisms underlying FSMM, and to establish standardized
diagnostic and therapeutic approaches.
Acknowledgements
The authors would like to express their sincere
gratitude to the late Dr Junling Tang (Department of Occupational
Disease and Poisoning Medicine, The First Affiliated Hospital of
Chongqing Medical and Pharmaceutical College, Chongqing, China) for
their early involvement in the clinical evaluation and patient
management in this case. These contributions laid the foundation
for the development of this report. The authors would also like to
thank Mr. Zhenjun Xi and Ms. Qianqian Liu (Department of
Occupational Disease and Poisoning Medicine, The First Affiliated
Hospital of Chongqing Medical and Pharmaceutical College,
Chongqing, China) for their invaluable contributions to the data
collection and experimental process; their meticulous efforts
ensured the accuracy and reliability of our research findings.
Additionally, the authors are grateful to Mrs. Li Yan and Mr. Lvsu
Ye (Department of Occupational Disease and Poisoning Medicine, The
First Affiliated Hospital of Chongqing Medical and Pharmaceutical
College, Chongqing, China) for their assistance in data analysis
and interpretation, which greatly enhanced the depth and quality of
the research outcomes.
Funding
Funding: The authors declare that financial support was received
for the research, authorship and publication of this article. The
work was funded by the Chongqing Medical Scientific Research
Project (Joint Project of Chongqing Health Commission and Science
and Technology Bureau) (grant nos. 2023GGXM006, 2024ZDXM026 and
2024ZDXM026), the Key Research Project from Chongqing Medical and
Pharmaceutical Vocational Education Group (grant no. CQZJ202329),
the Chongqing Key Municipal Public Health Specialty Construction
Project, 2024 Scientific Research Project of Chongqing Medical and
Pharmaceutical College (grant no. ygzrc2024101), the Chongqing
Education Commission Natural Science Foundation (grant no.
KJQN202402821), the Chongqing Shapingba District Science and
Technology Bureau Project (grant no. 2024071), the 2024 Chongqing
Medical and Pharmaceutical College Innovation Research Group
Project (grant no. ygz2024401), and the Chongqing Science and
Health Joint Medical Research Project (grant no.
2024SQKWLHMS051).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XiL and XuL designed the study and coordinated the
clinical evaluation. QinL, QiaL and XuL participated in the
diagnosis and treatment of the patient. XiL, QiaL and QinL were
responsible for data collection, clinical interpretation and
histological assessments. YL, LZ and LW contributed to the analysis
and interpretation of the laboratory and imaging data, and
supervised the study. All authors participated in the writing of
the original draft and figure preparation. YL, LZ and LW critically
reviewed and revised the manuscript. XiL and YL confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of clinical details and any
accompanying images. The patient was fully informed about the
purpose of the publication and explicitly consented to the use of
facial photographs and other potentially identifiable data. The
consent included acknowledgment that the images may be published in
scientific journals and disseminated online in an academic context.
Efforts have been made to ensure patient anonymity where possible,
while recognizing that complete anonymity cannot be guaranteed in
cases involving facial features.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rudnicka L, Chrostowska S, Kamiński M,
Waśkiel-Burnat A, Michalczyk A, Rakowska A and Olszewska M: The
role of trichoscopy beyond hair and scalp diseases. A review. J Eur
Acad Dermatol Venereol: Mar 15, 2023 (Epub ahead of print).
|
|
2
|
Michael A, Fuller T, Brogan S and Murphy
MC: An intrathecal pump misadventure in two acts: An unrecognized
partial pocket fill followed by an unusual withdrawal syndrome two
months later. J Palliat Med: Jan 30, 2025 (Epub ahead of
print).
|
|
3
|
Chang YC, Peng CY, Chi KY, Song J, Chang Y
and Chiang CH, Gao W and Chiang CH: Cardiovascular outcomes and
mortality in diabetic multiple myeloma patients initiated on
proteasome inhibitors according to prior use of glucagon-like
peptide 1 agonists. Eur J Prev Cardiol: Jan 29, 2025 (Epub ahead of
print).
|
|
4
|
Jia J, Yin J, Geng C and Liu A:
Characteristics and outcomes of secondary acute lymphoblastic
leukemia (sALL) after multiple myeloma (MM): SEER data analysis in
a single-center institution. Cancer Pathog Ther. 3:76–80.
2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rapparini L, Pileri A, Robuffo S,
Agostinelli C and La Placa M: Cutaneous involvement in multiple
myeloma. A rare entity. Clin Case Rep. 13(e9476)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Růžičková T, Vlachová M, Pečinka L,
Brychtová M, Večeřa M, Radová L and Ševčíková S, Jarošová M, Havel
J, Pour L and Ševčíková S: Detection of early relapse in multiple
myeloma patients. Cell Div. 20(4)2025.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang J, Hu Q, Lu J, Wang J, Wu D, Fei X,
Wang L, Yu X and Tang Y: DRESS is not a rare complication during
the initial treatment of newly diagnosed multiple myeloma: The
experience of two medical institutes. Acta Haematol: Jan 27, 2025
(Epub ahead of print).
|
|
8
|
Wildes TM: First relapse in older adults
with multiple myeloma: Creating new pathways in uncharted
territory. Br J Haematol. 206:1523–1525. 2025.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xue W, Li Y, Ma Y and Zhang F:
GDF15-mediated enhancement of the Warburg effect sustains multiple
myeloma growth via TGFβ signaling pathway. Cancer Metab.
13(3)2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sampath VS: Isatuximab-based therapy for
multiple myeloma. N Engl J Med. 392:518–519. 2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oymanns M, Baltaci M, Bellm A and Assaf C:
Paraneoplastic filiform hyperkeratosis and
immunoglobulin-associated vasculitis in myeloma progression: A case
report. Case Rep Dermatol. 13:563–567. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Cao J, Gu W, Shi M, Lan J, Yan Z,
Jin L, Xia J, Ma S, Liu Y, et al: Long-term follow-up of
combination of B-cell maturation antigen and CD19 chimeric antigen
receptor T cells in multiple myeloma. J Clin Oncol. 40:2246–2256.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mettias S, ElSayed A, Moore J and Berenson
JR: Multiple myeloma: Improved outcomes resulting from a rapidly
expanding number of therapeutic options. Target Oncol. 20:247–267.
2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liehr T: International system for human
cytogenetic or cytogenomic nomenclature (ISCN): Some thoughts.
Cytogenet Genome Res. 161:223–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giorgetti G, Maroto-Martin E, Soncini D,
Fenoglio D, Becherini P, Benzi A, Ravera S, Traverso I, Ladisa F,
Lai F, et al: CD56 expression modulates NAD+ metabolic landscape
and predicts sensitivity to anti-CD38 therapies in multiple
myeloma. Blood Cancer J. 15(83)2025.PubMed/NCBI View Article : Google Scholar
|
|
16
|
L B Andrade C, Ferreira MV, M Alencar B, S
B Filho JL, A Guimaraes M, Porto Cruz Moraes I, S Lopes TJ, S Dos
Santos A, M Dos Santos M, C S E Silva MI, et al: PCMMD: A novel
dataset of plasma cells to support the diagnosis of multiple
myeloma. Sci Data. 12(161)2025.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Penfield JG: Multiple myeloma in end-stage
renal disease. Semin Dial. 19:329–334. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bou Zerdan M, Nasr L, Khalid F, Allam S,
Bouferraa Y, Batool S, Tayyeb M, Adroja S, Mammadii M, Anwer F, et
al: Systemic AL amyloidosis: current approach and future direction.
Oncotarget. 14:384–394. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jhaveri KD, Meena P, Bharati J and Bathini
S: Recent updates in the diagnosis and management of kidney
diseases in multiple myeloma. Indian J Nephrol. 35:8–20.
2025.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Utsu Y, Isono Y, Masuda SI, Arai H,
Shimoji S, Matsumoto R, Tsushima T, Tanaka K, Matsuo K, Kimeda C,
et al: Time-dependent recovery of renal impairment in patients with
newly diagnosed multiple myeloma. Ann Hematol. 104:573–579.
2025.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dhakal B, Hari P, Chhabra S, Szabo A, Lum
LG, Glass DD, Park JH, Donato M, Siegel DS, Felizardo TC and Fowler
DH: Rapamycin-resistant polyclonal Th1/Tc1 cell therapy (RAPA-201)
safely induces disease remissions in relapsed, refractory multiple
myeloma. J Immunother Cancer. 13(e010649)2025.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abdi J and Redegeld F: Toll-like receptor
1/2 activation reduces immunoglobulin free light chain production
by multiple myeloma cells in the context of bone marrow stromal
cells and fibronectin. PLoS One. 20(e0310395)2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martins Rodrigues F, Jasielec J, Perpich
M, Kim A, Moma L, Li Y, Storrs E, Wendl MC, Jayasinghe RG, Fiala M,
et al: Germline predisposition in multiple myeloma. iScience.
28(111620)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yoon IC, Do N, Vazquez T, Elder DE, Steele
KT and Rosenbach M: Injection site reaction to teclistamab in a
patient with multiple myeloma. JAAD Case Rep. 56:48–50.
2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jimeno Sandoval JC, Cantatore M, Meakin L,
Menghini T, Owen L, Doran I, Erskine M and Rossanese M: Treatment,
prognosis, and outcome of dogs treated for rectal plasmacytoma: A
multicentric retrospective study. J Am Vet Med Assoc. 263:1–8.
2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fotiou D and Katodritou E: From biology to
clinical practice: The bone marrow microenvironment in multiple
myeloma. J Clin Med. 14(327)2025.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Kuang L, Huang B, Liu J, Chen M, Li
X, Gu J, Yu T and Li J: Early identification of the
non-transplanted functional high-risk multiple myeloma: Insights
from a predictive nomogram. Biomedicines. 13(145)2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bahrani E, Perkins IU and North JP:
Diagnosing calciphylaxis: A review with emphasis on histopathology.
Am J Dermatopathol. 42:471–480. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou Y, Chen Y, Yin G and Xie Q:
Calciphylaxis and its co-occurrence with connective tissue
diseases. Int Wound J. 20:1316–1327. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hosler GA, Weibel L and Wang RC: The cause
of follicular spicules in multiple myeloma. JAMA Dermatol.
151:457–458. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Satta R, Casu G, Dore F, Longinotti M and
Cottoni F: Follicular spicules and multiple ulcers: Cutaneous
manifestations of multiple myeloma. J Am Acad Dermatol. 49:736–740.
2003.PubMed/NCBI View Article : Google Scholar
|















![Karyotype analysis revealing complex
chromosomal abnormalities in advanced MM. Conventional karyotyping
revealed multiple numerical and structural abnormalities (red
arrows) characteristic of high-risk MM: i) Hyperdiploidy with
tetraploid cells (83-85<4n>,XXY), including an additional X
chromosome. ii) Loss of Y chromosome (-Y), a common finding in
hematological malignancies. iii) High-level polysomy of chromosome
1 (+1,+1), totaling four copies, indicating aggressive disease. iv)
Dicentric chromosome [dic(1;10)(p13;q26)], suggesting genomic
instability. v) Pseudo-isodicentric chromosome [psu idic(1)(p21)], reflecting complex chromosomal
rearrangement. vi) Additional material on chromosomes 7 and 8
[add(7)(q32),add(8)(p23)x2]. vii) Possible unidentified
material on chromosome 17 [?add(17)(q22)x2]. ix) Monosomy of chromosomes
19, 20, 21 and 22 (green arrows). xi) One to two marker chromosomes
(+1-2mar). xii) Mosaic karyotype: Four abnormal clones with complex
karyotype and 16 normal male karyotypes (46,XY), consistent with
clonal evolution. MM, multiple myeloma.](/article_images/etm/30/3/etm-30-03-12930-g00.jpg)