Introduction
Stroke is a devastating neurological condition that
is one of the leading causes of long-term disability worldwide,
imposing substantial physical, emotional, and socioeconomic burdens
on survivors and their families (1). Despite significant advancements in
acute stroke care, which have contributed to reducing mortality
rates, the management of chronic stroke remains a critical and
unresolved challenge in clinical practice (1-3).
Historically, the prevailing assumption that functional recovery
plateaus within the first few months post-stroke has limited the
focus on rehabilitation efforts to the acute and subacute phases
(4). However, emerging evidence
challenges this notion by demonstrating that targeted
rehabilitation interventions can yield meaningful improvements in
functional outcomes even during the chronic phase of stroke
recovery (5,6). This potential paradigm shift
underscores the need for a deeper understanding of the mechanisms
underlying chronic stroke recovery and the development of
innovative therapeutic strategies to address the persistent unmet
needs of this patient population (7).
The triage process in stroke rehabilitation is
crucial for balancing patient outcomes and cost control (5). It involves screening patients,
establishing assessment criteria and considering stroke severity to
determine appropriate care settings (8). Although significant progress has been
made in acute stroke care, disparities remain during the later
phases of recovery (9). It has
been suggested that severely affected patients with stroke can have
the potential for benefiting from rehabilitation, albeit at a
slower pace (10). Healthcare
professionals however frequently lack awareness of the improvement
potential of patients during the chronic recovery phases (9). To address these challenges, there is
a need for improved outcome measures and carefully focused
interventions for patients with chronic stroke (10).
Neuroimaging serves a crucial role in predicting
outcomes and guiding rehabilitation strategies for patients with
stroke (11). MRI techniques,
including diffusion tensor imaging (DTI) and functional
neuroimaging, have shown promise in providing prognostic
information for individual patients during the early stages of
recovery (12). These advanced
imaging methods, when combined with clinical and neurophysiological
assessments, can be applied to tailor rehabilitation approaches
based on a patient's capacity for neural reorganization (12,13).
MRI techniques have the potential to provide comprehensive
assessments in chronic stroke, guiding treatment decisions for
optimal clinical outcomes. As neuroimaging technology continues to
advance, it is expected to serve an increasingly important role in
stroke recovery prediction and personalized rehabilitation planning
(14).
Advancements in MRI-compatible robotic devices have
enabled simultaneous neuroimaging and rehabilitation for patients
with stroke over the past decade. These devices, including soft
wearable gloves (15) and
hand-induced robotic systems (16-18),
allow for the monitoring of brain activity during rehabilitation
exercises. Previous studies have shown that training with such
devices can increase sensorimotor cortical activation, indicating
functional plasticity in patients with chronic stroke (19-21).
The combination of MRI and robotic-assisted therapy demonstrates
the potential of enhancing the understanding of brain plasticity
and improving stroke recovery outcomes (20-22).
Despite the promising advancements in neuroimaging
and robotic-assisted rehabilitation for patients with chronic
stroke, to the best of our knowledge, a comprehensive assessment
integrating functional MRI (fMRI) and DTI for triaging and
predicting motor improvement potential in this patient population
remains lacking. Current approaches are frequently ineffective at
systematically evaluating neural mechanisms underlying recovery or
identifying which patients are most likely to benefit from robotic
rehabilitation interventions. This knowledge gap limits the ability
to personalize rehabilitation strategies and optimize outcomes for
chronic stroke survivors.
Therefore, the present study aims to address this
need by leveraging a combined functional and structural brain
assessment to triage patients with chronic stroke and evaluate
their potential for motor improvement following robotic device
training. By integrating advanced neuroimaging with robotic
rehabilitation, the present study seeks to provide a deeper
understanding of brain plasticity during chronic stroke and
establish a framework for tailored, evidence-based therapeutic
interventions.
Materials and methods
Patients
Patients with chronic stroke were identified only by
using the Massachusetts General Hospital (Boston, USA) stroke
survivor registries. The patients had been admitted between July
2006 and May 2019, and the registries were accessed by the authors
between January 2019 and December 2023 for this study. Eligible
participants were right-handed [per the revised Edinburgh
Handedness Inventory EIH-8(23)]
adults who had sustained a first-ever left middle cerebral artery
ischemic stroke ≥6 months prior to enrollment and those who
exhibited persistent right-hand weakness [Medical Research Council
scale (24) <4 for ≥48 h]. To
minimize heterogeneity and ensure reliable task performance during
fMRI and robotic training, individuals with significant cognitive
or sensory impairments were excluded. These impairments were
defined as the inability to understand and comply with the fMRI
task instructions. Additional exclusion criteria comprised any
contraindications to MRI (such as metallic implants and severe
claustrophobia), a history of other neurological (for example
multiple sclerosis and Parkinson's disease) or major psychiatric
disorders (for example schizophrenia and bipolar disorder), and any
orthopedic or systemic conditions affecting motor function of the
stroke-affected hand (for example, severe arthritis and peripheral
neuropathy). All participants provided informed consent in writing,
to a study that was approved by the Central Institutional review
board serving Massachusetts General Hospital and Brigham and
Women's Hospital Institutional review board (Partners Human
Research Committee, approval no. 2005P000570).
Rehabilitation protocol
Patients underwent a supervised home-based
rehabilitation program using the third-generation Magnetic
Resonance Compatible Hand-Induced RObotic Device (MR_CHIROD), an
in-house-developed system, paired with an interactive game
(25). The training regime
consisted of 45-min sessions conducted three times per week for 10
weeks. Motor performance assessments were conducted at the
following time points: i) Before training (baseline); ii) monthly
during training; and iii) 1-month post-training. Evaluations
applied included the Fugl-Meyer assessment for upper extremity
(FMA-UE) scale for sensorimotor impairment that are comprised of
subscales for wrist, hand, coordination, sensation, passive joint
motion, joint pain and total motor function. These subscales enable
the comprehensive evaluation of upper extremity impairment
(26). Functional hand use was
assessed using the Action Research Arm Test (ARAT) (27), with subcategories for grasp, grip,
pinch, gross movement and total performance. Spasticity severity
was quantified using the modified Ashworth scale for the elbow,
wrist, fingers and thumb (28).
Hand grip strength (Force) was measured using a dynamometer (Jamar
Hydraulic Hand Dynamometer 12-0600; Fabrication Enterprises, Inc.),
whereas manual dexterity was evaluated using the Box and Blocks
Test (BBT) (29). These
assessments were selected to capture multiple dimensions of motor
impairment, functional recovery and spasticity, offering a detailed
understanding of the progress of each patient.
Imaging protocol
All imaging was performed using a 3T Siemens Skyra
scanner equipped with a 32-channel phased-array coil (Siemens
Healthineers). The imaging protocol included several sequences.
T1-weighted anatomical imaging was conducted using a sagittal
magnetization-prepared rapid gradient-echo sequence [repetition
time (TR)/echo time (TE)/inversion time (TI), 2,300/2.53/900 msec;
field of view (FOV), 256 mm; resolution, 1x1x1 mm3;
parallel acquisition technique (PAT) factor, 2; acquisition time,
5.5 min]. Field mapping utilized a double-echo fast gradient-echo
sequence (TR, 650 msec; TE1/TE2, 4.92/7.38 msec; FOV, 220 mm;
resolution, 2x2x2 mm3). fMRI data were acquired using
echo-planar imaging (TR/TE, 3,000/30 msec; FOV, 220 mm; resolution,
2x2x2 mm3; PAT factor, 2; 100 dynamics). DTI was
performed with an axial spin-echo echo-planar imaging sequence
(b-values 0/1,000 sec/mm2; TR/TE, 12,200/104 msec; 30
directions; resolution, 2x2x2 mm3). Additionally, a 3D
fluid-attenuated inversion recovery sequence was included for
clinical evaluation (TR/TE/TI, 5,000/386/1,800 msec; resolution,
0.5x0.5x0.9 mm3).
Motor paradigm
During fMRI, participants performed a motor task
using the MR_CHIROD device with their right hand in a ‘boxcar’
paradigm (30), alternating
between 21-sec rest and action blocks. During action blocks,
participants synchronized grip compression and release with a
visual metronome set at 0.52 Hz. During rest blocks, participants
fixated on a stationary cross at the center of the screen, serving
as a low-level baseline condition. In total, three fMRI scans were
conducted per imaging session, each with a resistive force
(resistive level) adjusted to 60, 40 or 20% of the pre-scanned
maximum grip strength. To minimize motion artifacts, foam padding
and straps were used, where mirror movements in the non-active hand
were closely monitored.
Image processing
fMRI data were preprocessed and analyzed using
‘SPM12’ (30) in MATLAB 9.10
(MathWorks, Inc.). Preprocessing procedures included slice timing
correction, realignment to correct head motion, co-registration of
functional to T1-weighted anatomical images, normalization to the
Montreal Neurological Institute template (30) (2x2x2 mm3 resolution) and
smoothing with an 8-mm FWHM Gaussian kernel. Intra-subject analysis
used a general linear model, with the motor task modeled as a
boxcar function convolved with the canonical hemodynamic response
function, including action/rest regressors and motion parameters as
nuisance variables. Contrasts compared action with rest, using
statistical maps thresholded at P<0.05 (family-wise
error-corrected). A region of interest analysis was conducted on
the thresholded SPM_T maps using the Human Motor Area Template
Atlas (31). For each defined
region of interest, statistical measures, including maximum
activation (max), cluster size (size) and mean activation (mean),
were calculated separately for the left (L) and right (R)
hemispheres. The analysis focused on key motor-related regions,
such as the cerebellum (Cer), primary motor cortex (M1), ventral
premotor cortex (PMv), dorsal premotor cortex (PMd), primary
somatosensory cortex (S1) and supplementary motor area (SMA).
DTI data were processed using FMRIB Software
Library, version 6.0 (Centre for Integrative Neuroimaging,
University of Oxford). Pre-processing included correction for eddy
currents and head motion, followed by brain extraction. Diffusion
tensors were fitted to generate maps of fractional anisotropy (FA)
and mean diffusivity (MD). For region-of-interest analysis, the JHU
white matter tract atlas (32) was
used to define motor-related tracts in the stroke-affected
hemisphere. Mean FA and MD values were extracted for the following
motor related tracts of the lesioned left hemisphere: Corticospinal
tract, cerebral peduncle, posterior limb of the internal capsule
and posterior corona radiata.
Statistical analysis
Generalized linear mixed models (GLMM) were employed
to analyze the repeated measures data structure arising from five
experimental sessions and three resistive levels. This modeling
approach is suited for datasets involving multiple observations
from the same participants under different conditions, since it
accounts for the non-independence of repeated measurements
(33). Both fixed effects and
random effects were included in the models. Fixed effects consisted
of session (ordinal variable with values 1,2,3,4,5), Resistive
level, sex, age and relevant clinical measures, providing estimates
of the overall influence of these variables across the entire
sample. Random intercepts were incorporated to account for
individual differences and to accurately model within-subject
variability, thereby improving the precision of effect estimates.
To address potential violations of standard model assumptions, such
as non-normality and heteroscedasticity, robust covariance
estimates (sandwich estimators) were applied using SPSS. This
method adjusts the estimated standard errors of fixed effects to
provide valid statistical inference despite deviations from
standard assumptions (34).
Maximum likelihood estimation was used to accommodate missing data
and to handle outcome variables that did not follow a normal
distribution, ensuring that the hierarchical structure of the
dataset was appropriately modeled and that all available data were
utilized.
The statistical analysis consisted of two primary
components. The first component (motor assessment) evaluated the
association between clinical scale scores and neuroimaging
measurements across all five sessions using GLMM, thereby assessing
the relationship between brain plasticity and motor performance
throughout the intervention period. The second component (motor
prediction) focused on identifying early neuroimaging predictors of
motor improvement using GLMM by examining whether imaging
measurements from the first two sessions could predict changes in
motor performance, as measured by the difference in clinical scale
scores between the final session and the initial sessions.
Among the clinical outcome measures, particular
emphasis was placed on the ARAT Grip scores, given their direct
relevance to both the fMRI task and the rehabilitation exercises.
Additional dependent variables included the FMA_UE scale, the BBT
and Force. In all models, session, resistive level, sex and age
were included as covariates to control for potential confounding
effects. Model performance was assessed using the-2 log-likelihood
(-2LL) criterion, facilitating comparison of model fit. All
statistical analyses were conducted using SPSS version 26 (IBM
Corp.). P<0.05 was considered to indicate a significance for the
estimated regression coefficients.
Results
Baseline data
In total, 14 chronic patients with left middle
cerebral artery ischemic stroke (sex, 8 females, 6 males; age,
55.2±12.2 years) completed 210 MRI sessions (5 timepoints x3
resistive levels) during the present study. The Modified Ashworth
Scale was assessed in all patients and found to be 0, indicating
the absence of muscle spasticity, and thus spasticity was not
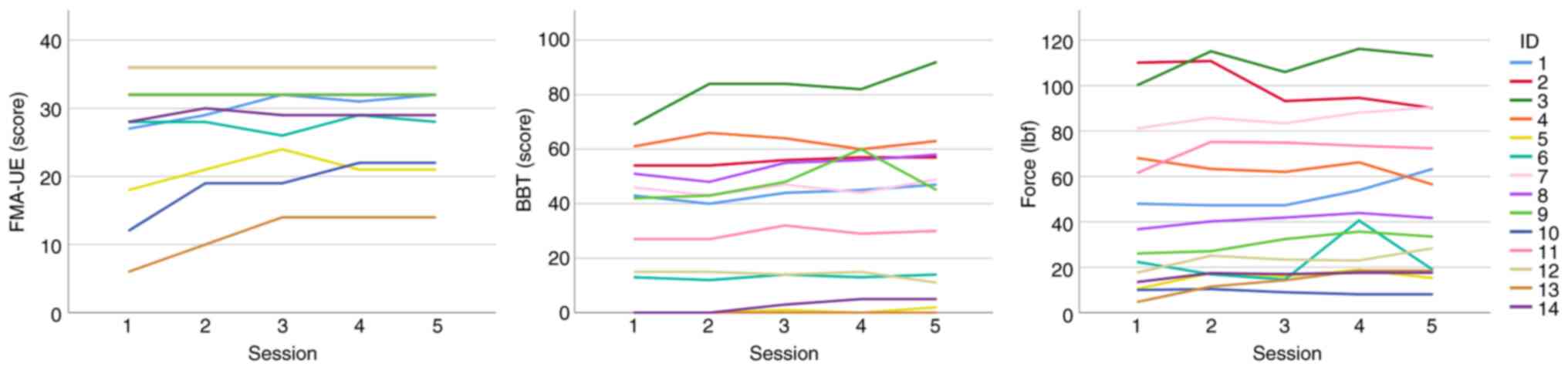
included as a variable in the statistical analyses. Table I displays their clinical
characteristics before rehabilitation, showing that according to
the FMA-UE scale, six patients presented with severe disability in
the range 0-28, and eight with moderate disability in the range
29-42(35). A total of three
patients achieved the >4-point motor improvement threshold for
clinically important difference according to the FMA-UE scale
(Fig. 1 and Table I) (36). Only two patients achieved the
corresponding improvement threshold in the BBT and seven patients
in Force scale (Table I) (37). ARAT-Grip was not analyzed, because
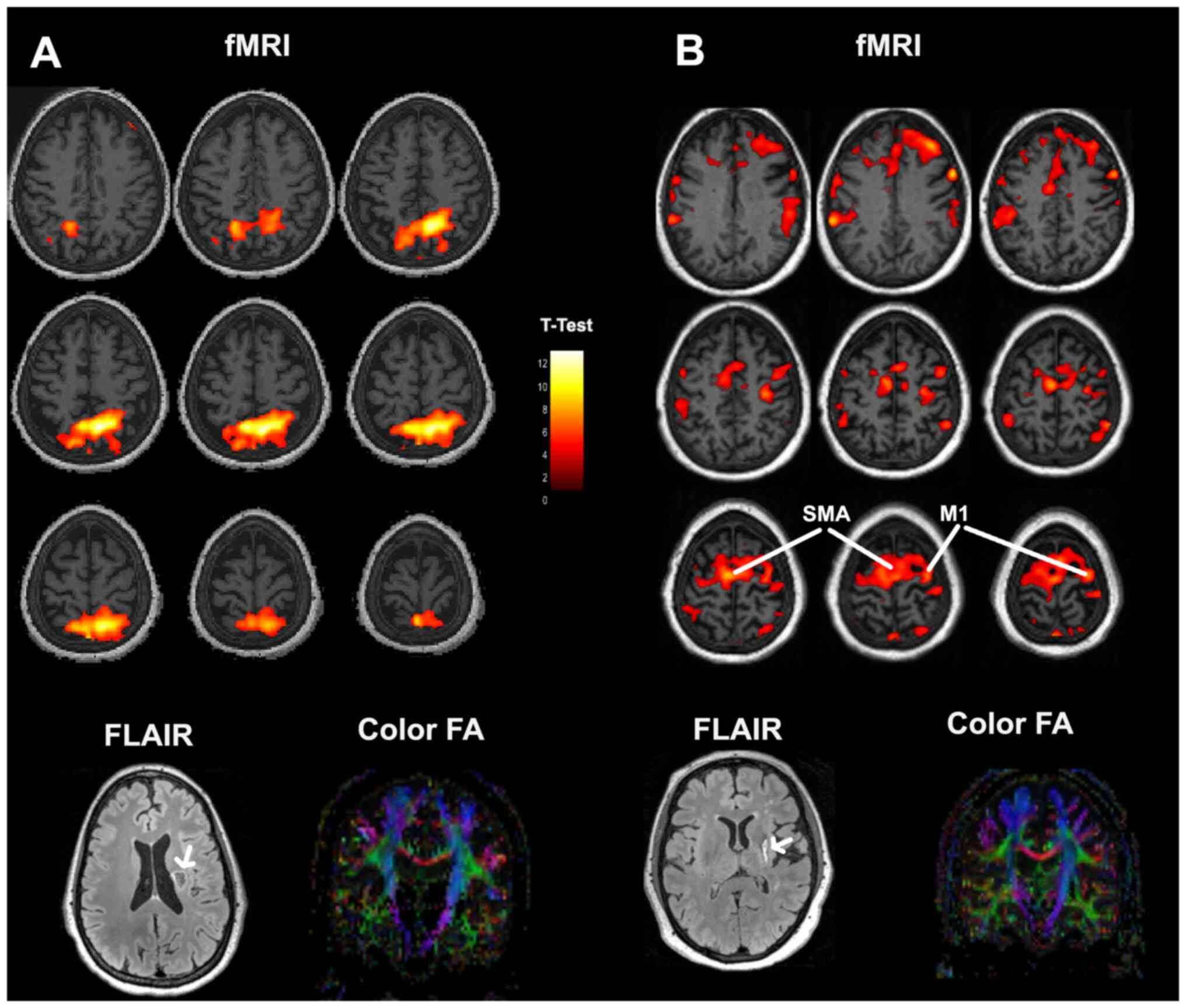
no changes were detected during the training sessions. Fig. 2 depicts a comparison between
baseline images of a patient (no. 14 in Table I) who failed to show significant
motor improvement and a patient (no. 13 in Table I) who succeeded. FLAIR images
reveal the site and extent of the stroke lesions. Colored brain
activation maps superimposed on T1 anatomical images illustrate
different activation patterns. Absence of progress is related to an
abnormal activation pattern with focal intense activation posterior
to the motor cortex. By contrast, normal left M1 and bilateral SMA
activation was observed in the patient who achieved motor gains
after rehabilitation. Coronal colored FA images indicate an intact
corticospinal tract at the lesioned hemisphere in both cases.
 | Table IClinical characteristics of patients
with chronic stroke, motor scores at baseline and differences with
the last session. |
Table I
Clinical characteristics of patients
with chronic stroke, motor scores at baseline and differences with
the last session.
| Patients no. | Sex | Age, years | FMA-UE | Δ(FMA_UE) | BBT | Δ(BBT) | Force,
pound-force | Δ(Force),
pound-force |
|---|
| 1 | F | 39.1 | 27 | 5 | 43 | 4 | 56.4 | 15.3 |
| 2 | M | 64.2 | 36 | 0 | 54 | 3 | 105.7 | -20 |
| 3 | M | 50.2 | 36 | 0 | 69 | 23 | 103.8 | 12.9 |
| 4 | F | 47.3 | 36 | 0 | 61 | 2 | 72.7 | -11.7 |
| 5 | F | 60.5 | 18 | 3 | 0 | 2 | 51.5 | 4.8 |
| 6 | F | 46.5 | 28 | 0 | 13 | 1 | 45.8 | -3.5 |
| 7 | M | 41.0 | 32 | 0 | 46 | 3 | 81.1 | 9.3 |
| 8 | F | 34.0 | 32 | 0 | 51 | 7 | 39.9 | 5 |
| 9 | F | 70.0 | 32 | 0 | 42 | 3 | 30.8 | 7.4 |
| 10 | M | 58.5 | 12 | 10 | 0 | 0 | 71.5 | -1.9 |
| 11 | M | 71.4 | 36 | 0 | 27 | 3 | 53.7 | 10.9 |
| 12 | M | 67.9 | 36 | 0 | 15 | -4 | 52.3 | 10.8 |
| 13 | F | 64.5 | 6 | 8 | 0 | 0 | 41.9 | 13.8 |
| 14 | F | 57.1 | 28 | 1 | 0 | 5 | 64.8 | 4.1 |
Motor assessment
Analysis revealed distinct patterns linking
neuroimaging markers to motor outcomes (Table II). In the ipsilesional
hemisphere, activation in M1 and SMA showed robust positive
associations with motor performance. The mean activation in left M1
is positively associated with both the BBT (B=7.57 and P<0.001)
and Force (B=8.65 and P<0.001) measures, suggesting that
stronger, more focused recruitment of the core motor areas supports
superior motor performance. By contrast, activation in left
premotor regions is characterized by negative associations.
Specifically, increased activity in the left PMv (max_PMv_L,
B=-0.91 and P=0.001) and PMd (mean_PMd_L, B=-1.70 and P=0.023 for
FMA-UE; B=-11.84 and P<0.001 for Force) appeared to associate
with poorer motor outcomes, which indicate that diffuse or
excessive recruitment in these secondary regions may reflect
compensatory mechanisms that are less efficient. A similar trend is
observed in the left S1 (max_S1_L, B=-0.54 and P=0.014), where
overactivation is negatively associated with motor performance.
 | Table IIGeneralized linear mixed model
results for motor function outcomes: Associations with age, sex,
session progression (ordinal variable with values 1-5) and imaging
measurements. |
Table II
Generalized linear mixed model
results for motor function outcomes: Associations with age, sex,
session progression (ordinal variable with values 1-5) and imaging
measurements.
| A, Fugl-Meyer
assessment for upper extremity |
|---|
| Parameter | B | SE | P-value |
|---|
| Session=1 | 49.85 | 35.69 | 0.166 |
| Session=2 | 49.90 | 35.89 | 0.168 |
| Session=3 | 51.55 | 35.79 | 0.153 |
| Session=4 | 51.42 | 35.76 | 0.154 |
| Session=5 | 50.89 | 35.67 | 0.157 |
| Resistive
level=60% | -0.26 | 0.29 | 0.370 |
| Resistive
level=20% | -0.40 | 0.24 | 0.102 |
| Sex=Female | 8.54 | 1.11 | <0.001 |
| Age, years | 0.09 | 0.12 | 0.452 |
| Max_Cer_L | -0.41 | 0.24 | 0.090 |
| Size_Cer_L |
-3.32x10-4 |
9.35x10-5 | 0.001 |
| Mean_Cer_L | 4.03 | 0.84 | <0.001 |
| Max_M1_L | 1.19 | 0.16 | <0.001 |
| Size_M1_L, (8
mm3) |
-1.61x10-3 |
8.47x10-4 | 0.061 |
| Mean_M1_L | -1.32 | 0.77 | 0.089 |
| Max_PMv_L | -0.91 | 0.26 | 0.001 |
| Size_PMv_L, (8
mm3) |
9.76x10-5 |
3.13x10-4 | 0.756 |
| Mean_PMv_L | 0.28 | 0.29 | 0.339 |
| Max_PMd_L | 0.07 | 0.17 | 0.670 |
| Size_PMd_L, (8
mm3) |
8.06x10-4 |
4.24x10-4 | 0.060 |
| Mean_PMd_L | -1.70 | 0.74 | 0.023 |
| Max_S1_L | -0.54 | 0.21 | 0.014 |
| Size_S1_L, (8
mm3) |
2.65x10-3 |
9.14x10-4 | 0.005 |
| Mean_S1_L | -0.76 | 0.47 | 0.108 |
| Max_SMA_L | 0.86 | 0.31 | 0.006 |
| Size_SMA_L, (8
mm3) |
-3.38x10-3 |
7.32x10-4 | <0.001 |
| Mean_SMA_L | 3.38 | 0.91 | <0.001 |
| Max_Cer_R | 0.27 | 0.26 | 0.298 |
| Size_Cer_R, (8
mm3) |
2.75x10-4 |
1.20x10-4 | 0.024 |
| Mean_Cer_R | -2.13 | 1.06 | 0.046 |
| Max_M1_R | -0.06 | 0.28 | 0.826 |
| Size_M1_R, (8
mm3) |
-3.82x10-4 |
3.02x10-4 | 0.208 |
| Mean_M1_R | 1.35 | 0.67 | 0.046 |
| Max_PMv_R | 0.75 | 0.39 | 0.062 |
| Size_PMv_R, (8
mm3) |
-8.89x10-6 |
4.90x10-4 | 0.986 |
| Mean_PMv_R | -3.27 | 1.19 | 0.007 |
| Max_PMd_R | 0.23 | 0.30 | 0.432 |
| Size_PMd_R, (8
mm3) |
-8.43x10-4 |
4.94x10-4 | 0.091 |
| Mean_PMd_R | 1.50 | 1.01 | 0.141 |
| Max_S1_R | 0.32 | 0.23 | 0.169 |
| Size_S1_R, (8
mm3) |
5.63x10-4 |
4.34x10-4 | 0.198 |
| Mean_S1_R | -0.30 | 0.45 | 0.503 |
| Max_SMA_R | -1.79 | 0.33 | <0.001 |
| Size_SMA_R, (8
mm3) |
2.50x10-3 |
6.12x10-4 | <0.001 |
| Mean_SMA_R | 0.06 | 0.52 | 0.907 |
| FA Corticospinal
tract | 34.88 | 5.50 | <0.001 |
| FA Cerebral
peduncle | 0.68 | 9.15 | 0.941 |
| FA Posterior limb
of internal capsule | 133.10 | 13.05 | <0.001 |
| FA Posterior corona
radiata | -283.94 | 37.85 | <0.001 |
| MD Corticospinal
tract (10-6 mm2/s) | -57,624.69 | 8909.14 | <0.001 |
| MD Cerebral
peduncle (10-6 mm2/s) | 1,850.91 | 20582.64 | 0.929 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | 89,988.54 | 21056.94 | <0.001 |
| MD Posterior corona
radiata (10-6 mm2/s) | -52,670.76 | 26162.98 | 0.047 |
| B, Box and block
test |
| Parameter | B | SE | P-value |
| Session=1 | 665.00 | 73.44 | <0.001 |
| Session=2 | 665.97 | 74.01 | <0.001 |
| Session=3 | 669.93 | 73.86 | <0.001 |
| Session=4 | 669.98 | 73.86 | <0.001 |
| Session=5 | 672.63 | 73.79 | <0.001 |
| Force=60% | 0.22 | 0.50 | 0.665 |
| Force=20% | 0.54 | 0.51 | 0.290 |
| Sex=Female | 29.01 | 2.26 | <0.001 |
| Age, years | -1.98 | 0.25 | <0.001 |
| Max_Cer_L | -1.17 | 0.50 | 0.021 |
| Size_Cer_L, (8
mm3) |
3.3x10-5 |
2.04x10-6 | 0.872 |
| Mean_Cer_L | 2.46 | 1.91 | 0.200 |
| Max_M1_L | 0.06 | 0.35 | 0.857 |
| Size_M1_L, (8
mm3) |
-2.15x10-3 |
1.60x10-3 | 0.183 |
| Mean_M1_L | 7.57 | 1.93 | <0.001 |
| Max_PMv_L | -0.29 | 0.53 | 0.580 |
| Size_PMv_L, (8
mm3) |
-9.77x10-4 |
8.26x10-4 | 0.240 |
| Mean_PMv_L | 0.11 | 0.73 | 0.875 |
| Max_PMd_L | 0.60 | 0.36 | 0.103 |
| Size_PMd_L, (8
mm3) |
1.04x10-3 |
1.13x10-3 | 0.358 |
| Mean_PMd_L | -8.52 | 1.48 | <0.001 |
| Max_S1_L | -0.69 | 0.46 | 0.133 |
| Size_S1_L, (8
mm3) |
2.97x10-3 |
1.89x10-3 | 0.120 |
| Mean_S1_L | -2.52 | 0.95 | 0.009 |
| Max_SMA_L | -0.51 | 0.66 | 0.447 |
| Size_SMA_L, (8
mm3) |
-4.60x10-3 |
1.69x10-3 | 0.008 |
| Mean_SMA_L | 7.16 | 1.88 | <0.001 |
| Max_Cer_R | -0.23 | 0.57 | 0.692 |
| Size_Cer_R, (8
mm3) |
-1.10x10-4 |
2.76x10-4 | 0.692 |
| Mean_Cer_R | 1.65 | 2.45 | 0.503 |
| Max_M1_R | -3.16 | 0.54 | <0.001 |
| Size_M1_R, (8
mm3) |
5.41x10-4 |
8.65x10-4 | 0.533 |
| Mean_M1_R | -2.07 | 1.72 | 0.232 |
| Max_PMv_R | -2.89 | 0.72 | <0.001 |
| Size_PMv_R, (8
mm3) |
-4.97x10-4 |
9.98x10-4 | 0.619 |
| Mean_PMv_R | -0.76 | 2.63 | 0.774 |
| Max_PMd_R | 2.66 | 0.49 | <0.001 |
| Size_PMd_R, (8
mm3) |
5.97x10-4 |
1.25x10-3 | 0.634 |
| Mean_PMd_R | -3.37 | 2.32 | 0.149 |
| Max_S1_R | 0.79 | 0.50 | 0.113 |
| Size_S1_R, (8
mm3) |
-5.65x10-4 |
1.04x10-3 | 0.589 |
| Mean_S1_R | 3.38 | 1.12 | 0.003 |
| Max_SMA_R | -0.15 | 0.68 | 0.827 |
| Size_SMA_R, (8
mm3) |
3.16x10-3 |
1.45x10-3 | 0.031 |
| Mean_SMA_R | 0.64 | 1.34 | 0.636 |
| FA Corticospinal
tract | -20.01 | 11.20 | 0.077 |
| FA Cerebral
peduncle | 203.93 | 19.31 | <0.001 |
| FA Posterior limb
of internal capsule | 493.18 | 26.26 | <0.001 |
| FA Posterior corona
radiata | -1,409.10 | 76.95 | <0.001 |
| MD Corticospinal
tract (10-6 mm2/s) | -25,075.34 | 18,703.06 | 0.183 |
| MD Cerebral
peduncle (10-6 mm2/s) | -322,732.16 | 42,539.39 | <0.001 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | 551,343.22 | 41,706.80 | <0.001 |
| MD Posterior corona
radiata (10-6 mm2/s) | -567,911.91 | 52,335.33 | <0.001 |
| C, Force |
| Parameter | B | SE | P-value |
| Session=1 | 400.67 | 110.93 | <0.001 |
| Session=2 | 405.02 | 111.68 | <0.001 |
| Session=3 | 403.23 | 111.31 | <0.001 |
| Session=4 | 407.88 | 111.42 | <0.001 |
| Session=5 | 407.23 | 111.39 | <0.001 |
| Force=60% | 0.51 | 0.54 | 0.348 |
| Force=20% | 0.42 | 0.60 | 0.484 |
| Sex=Female | -26.43 | 3.81 | <0.001 |
| Age, years | -0.88 | 0.40 | 0.031 |
| Max_Cer_L | 0.39 | 0.47 | 0.412 |
| Size_Cer_L, (8
mm3) |
-4.16x10-4 |
2.43x10-4 | 0.090 |
| Mean_Cer_L | -6.50 | 2.02 | 0.002 |
| Max_M1_L | -0.91 | 0.48 | 0.060 |
| Size_M1_L, (8
mm3) |
-4.93x10-3 |
1.73x10-3 | 0.005 |
| Mean_M1_L | 8.65 | 2.04 | <0.001 |
| Max_PMv_L | -1.14 | 0.62 | 0.068 |
| Size_PMv_L, (8
mm3) |
3.15x10-3 |
1.50x10-3 | 0.039 |
| Mean_PMv_L | 1.59 | 1.32 | 0.229 |
| Max_PMd_L | 1.93 | 0.48 | <0.001 |
| Size_PMd_L, (8
mm3) |
-3.42x10-3 |
1.58x10-3 | 0.033 |
| Mean_PMd_L | -11.84 | 2.18 | <0.001 |
| Max_S1_L | -1.64 | 0.61 | 0.009 |
| Size_S1_L, (8
mm3) |
3.16x10-3 |
2.30x10-3 | 0.172 |
| Mean_S1_L | -0.05 | 1.16 | 0.967 |
| Max_SMA_L | 0.36 | 0.97 | 0.710 |
| Size_SMA_L, (8
mm3) |
1.10x10-3 |
2.51x10-3 | 0.663 |
| Mean_SMA_L | 10.73 | 1.88 | <0.001 |
| Max_Cer_R | 0.45 | 0.85 | 0.596 |
| Size_Cer_R, (8
mm3) |
3.19x10-4 |
3.14x10-4 | 0.312 |
| Mean_Cer_R | 6.85 | 3.11 | 0.030 |
| Max_M1_R | -5.64 | 0.74 | <0.001 |
| Size_M1_R, (8
mm3) |
2.79x10-4 |
9.01x10-4 | 0.757 |
| Mean_M1_R | -0.40 | 2.06 | 0.848 |
| Max_PMv_R | -3.05 | 0.84 | <0.001 |
| Size_PMv_R, (8
mm3) |
-2.90x10-3 |
1.20x10-3 | 0.018 |
| Mean_PMv_R | 1.99 | 3.20 | 0.536 |
| Max_PMd_R | 3.35 | 0.70 | <0.001 |
| Size_PMd_R, (8
mm3) |
1.26x10-3 |
1.49x10-3 | 0.397 |
| Mean_PMd_R | -3.81 | 3.18 | 0.234 |
| Max_S1_R | 1.79 | 0.75 | 0.018 |
| Size_S1_R, (8
mm3) |
-1.33x10-3 |
1.10x10-3 | 0.233 |
| Mean_S1_R | 3.28 | 1.23 | 0.009 |
| Max_SMA_R | -2.22 | 0.88 | 0.013 |
| Size_SMA_R, (8
mm3) | 0.01 |
1.73x10-3 | 0.004 |
| Mean_SMA_R | -0.46 | 1.10 | 0.677 |
| FA Corticospinal
tract | -55.34 | 17.05 | 0.002 |
| FA Cerebral
peduncle | -97.80 | 36.37 | 0.008 |
| FA Posterior limb
of internal capsule | 172.38 | 34.05 | <0.001 |
| FA Posterior corona
radiata | -256.99 | 122.14 | 0.038 |
| MD Corticospinal
tract (10-6 mm2/s) | 21,981.23 | 28,434.79 | 0.441 |
| MD Cerebral
peduncle (10-6 mm2/s) | 63,281.49 | 72,490.82 | 0.385 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | -24,991.11 | 74,130.48 | 0.737 |
| MD Posterior corona
radiata (10-6 mm2/s) | -284,152.48 | 81,310.86 | 0.001 |
In the contralesional hemisphere, the relationships
were found to be more nuanced. Activation in right M1 (max_M1_R,
B=-3.16 and P<0.001 for BBT; B=-5.64 and P<0.001 for Force)
and right PMv (max_PMv_R, B=-2.89 and P<0.001 for BBT) exhibited
negative associations with motor scales, implying that excessive
recruitment in these regions may be maladaptive. However, right PMd
showed a positive association (max_PMd_R: B=2.66 and P<0.001 for
BBT; B=3.35 and P<0.001 for Force), which may suggest that
selective engagement of this region can serve a compensatory
function when the ipsilesional network is compromised. In addition,
right S1 activation is positively associated with motor performance
(mean_S1_R, B=3.38 and P=0.003 for BBT; mean_S1_R, B=3.28 and
P=0.009 for Force), indicating that contralesional sensory
processing may serve a beneficial role. By contrast, right SMA
activation (max_SMA_R, B=-1.79 and P<0.001 for FMA-UE) was
negatively associated with motor function, further supporting the
notion that not all contralesional recruitment contributes equally
to recovery.
DTI metrics add a complementary perspective, by
reflecting white matter integrity. Higher FA values in the
corticospinal tract (B=34.88 and P<0.001 for FMA-UE) and
posterior limb of the internal capsule (B=133.10 and P<0.001 for
FMA-UE; B=493.18 and P<0.001 for BBT) associated positively with
motor scales, supporting the importance of intact descending
pathways for functional recovery. By contrast, increased FA in the
posterior corona radiata (B=-283.94 and P<0.001 for FMA-UE;
B=-1,409.10 and P<0.001 for BBT) was negatively associated with
motor performance. MD measurements also aligned with this
interpretation. Specifically, elevated MD in posterior corona
radiata (B=-567,911.91 and P<0.001 for BBT; B=-284,152.48 and
P=0.001 for Force) tended to associate with inferior motor
outcomes, suggesting that tissue damage and subsequent structural
disorganization adversely affect recovery.
Motor prediction
Changes in the ipsilesional cortex (left hemisphere)
demonstrate a mixed pattern of positive and negative associations
between peak or mean activation and rehabilitation-induced motor
gains (Table III). Maximum
activation in left M1 (max_M1_L, B=0.67 and P=0.002) and cluster
size in left M1 (size_M1_L, B=2.81x10-3 and P=0.001)
both show positive relationships with Δ(FMA-UE), whilst mean
activation in left M1 (mean_M1_L, B=-3.44 and P=0.001) is
negatively associated with Δ(FMA-UE). Similarly, left PMv exhibited
a positive association with Δ(FMA-UE) (mean_PMv_L, B=2.95 and
P=0.012), whereas left PMd associated negatively (mean_PMd_L,
B=-2.18 and P=0.010). In the left somatosensory cortex, activation
cluster size showed an inverse relationship with Δ(FMA-UE)
(size_S1_L: B=-2.32x10-3 and P=0.029) but a positive one
with Δ(BBT) (size_S1_L: B=0.02 and P=0.002), whereas mean
activation is positively associated with Δ(FMA-UE) (mean_S1_L,
B=2.27 and P=0.021). Finally, negative associations in left SMA
maximum (max_SMA_L, B=-1.46 and P=0.044) and mean activations
(mean_SMA_L, B=-2.84 and P=0.004) suggest that heightened
activation in this region may not necessarily translate into
improved upper-limb function.
 | Table IIIResults of generalized linear mixed
model analysis: Rehabilitation induced motor scores' changes
predicted by demographics and imaging measurements from the first
two sessions (session values 1 and 2). |
Table III
Results of generalized linear mixed
model analysis: Rehabilitation induced motor scores' changes
predicted by demographics and imaging measurements from the first
two sessions (session values 1 and 2).
| A, Δ(Fugl-Meyer
assessment for upper extremity) |
|---|
| Parameter | B | SE | P-value |
|---|
| Session=1 | -8.34 | 28.98 | 0.777 |
| Session=2 | -9.18 | 28.84 | 0.754 |
| Resistive
level=60% | -0.11 | 0.33 | 0.753 |
| Resistive
level=40% | 0.16 | 0.15 | 0.281 |
| Sex=Female | -9.71 | 2.24 | <0.001 |
| Age, years | 0.26 | 0.11 | 0.026 |
| Max_Cer_L | -0.87 | 0.3 | 0.009 |
| Size_Cer_L, (8
mm3) |
1.31x10-4 |
2.41x10-4 | 0.595 |
| Mean_Cer_L | -0.3 | 1.24 | 0.812 |
| Max_M1_L | 0.67 | 0.19 | 0.002 |
| Size_M1_L, (8
mm3) |
2.81x10-3 |
6.62x10-4 | 0.001 |
| Mean_M1_L | -3.44 | 0.84 | 0.001 |
| Max_PMv_L | 0.14 | 0.38 | 0.721 |
| Size_PMv_L, (8
mm3) |
2.89x10-4 |
3.84x10-4 | 0.462 |
| Mean_PMv_L | 2.95 | 1.05 | 0.012 |
| Max_PMd_L | -0.13 | 0.36 | 0.724 |
| Size_PMd_L, (8
mm3) |
-1.04x10-4 |
5.27x10-4 | 0.846 |
| Mean_PMd_L | -2.18 | 0.76 | 0.01 |
| Max_S1_L | -0.15 | 0.32 | 0.644 |
| Size_S1_L, (8
mm3) |
-2.32x10-3 |
9.75x10-4 | 0.029 |
| Mean_S1_L | 2.27 | 0.89 | 0.021 |
| Max_SMA_L | -1.46 | 0.67 | 0.044 |
| Size_SMA_L, (8
mm3) |
-1.02x10-3 |
5.59x10-4 | 0.086 |
| Mean_SMA_L | -2.84 | 0.86 | 0.004 |
| Max_Cer_R | 0.85 | 0.31 | 0.016 |
| Size_Cer_R, (8
mm3) |
-2.37x10-4 |
1.55x10-4 | 0.145 |
| Mean_Cer_R | 1.45 | 0.76 | 0.073 |
| Max_M1_R | 0.07 | 0.25 | 0.77 |
| Size_M1_R, (8
mm3) |
1.59x10-4 |
4.84x10-4 | 0.747 |
| Mean_M1_R | 0.22 | 0.43 | 0.611 |
| Max_PMv_R | -0.81 | 0.31 | 0.019 |
| Size_PMv_R, (8
mm3) |
9.32x10-4 |
5.03x10-4 | 0.081 |
| Mean_PMv_R | -0.66 | 1.16 | 0.579 |
| Max_PMd_R | -0.78 | 0.4 | 0.066 |
| Size_PMd_R, (8
mm3) |
-1.37x10-3 |
5.87x10-4 | 0.032 |
| Mean_PMd_R | 3.43 | 0.7 | <0.001 |
| Max_S1_R | 0.18 | 0.2 | 0.383 |
| Size_S1_R, (8
mm3) |
1.98x10-4 |
6.88x10-4 | 0.777 |
| Mean_S1_R | -0.51 | 0.76 | 0.514 |
| Max_SMA_R | 0.77 | 0.27 | 0.011 |
| Size_SMA_R, (8
mm3) |
-9.67x10-4 |
6.21x10-4 | 0.138 |
| Mean_SMA_R | 5.27 | 1.05 | <0.001 |
| FA Corticospinal
tract | 0.35 | 2.92 | 0.906 |
| FA Cerebral
peduncle | -57.84 | 13.54 | 0.001 |
| FA Posterior limb
of internal capsule | -102.86 | 24 | <0.001 |
| FA Posterior corona
radiate | 164.28 | 42.06 | 0.001 |
| MD Corticospinal
tract (10-6 mm2/s) | 5,347.84 | 6,443.77 | 0.418 |
| MD Cerebral
peduncle (10-6 mm2/s) | 83,833.91 | 21,245.93 | 0.001 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | -132,136.83 | 26,796.45 | <0.001 |
| MD Posterior corona
radiata (10-6 mm2/s) | 67,404.04 | 20,132.5 | 0.004 |
| B, Δ(Box and block
test) |
| Parameter | B | SE | P-value |
| Session=1 | 548.91 | 132.81 | 0.001 |
| Session=2 | 547.51 | 132.31 | 0.001 |
| Force=60% | -0.2 | 1.32 | 0.879 |
| Force=40% | 0.29 | 0.96 | 0.766 |
| Sex=female | 15.45 | 6.66 | 0.033 |
| Age, years | -3.07 | 0.57 | <0.001 |
| Max_Cer_L | 0.66 | 0.81 | 0.43 |
| Size_Cer_L, (8
mm3) |
-4.33x10-4 |
6.72x10-4 | 0.528 |
| Mean_Cer_L | 0.84 | 4.14 | 0.841 |
| Max_M1_L | -1.24 | 0.71 | 0.1 |
| Size_M1_L, (8
mm3) |
-1.54x10-3 |
2.38x10-3 | 0.526 |
| Mean_M1_L | 4.82 | 2.66 | 0.087 |
| Max_PMv_L | 2.58 | 1.31 | 0.066 |
| Size_PMv_L, (8
mm3) |
-3.63x10-3 |
1.69x10-3 | 0.046 |
| Mean_PMv_L | -3.85 | 3.17 | 0.242 |
| Max_PMd_L | 1.11 | 1.35 | 0.421 |
| Size_PMd_L, (8
mm3) |
-1.53x10-3 |
1.67x10-3 | 0.373 |
| Mean_PMd_L | 0.77 | 2.43 | 0.754 |
| Max_S1_L | 0.12 | 0.92 | 0.899 |
| Size_S1_L, (8
mm3) | 0.02 |
4.47x10-3 | 0.002 |
| Mean_S1_L | -4.61 | 3.55 | 0.212 |
| Max_SMA_L | -0.99 | 1.63 | 0.552 |
| Size_SMA_L, (8
mm3) |
-9.01x10-4 |
2.14x10-3 | 0.679 |
| Mean_SMA_L | 0.81 | 4.1 | 0.846 |
| Max_Cer_R | -0.36 | 1.29 | 0.787 |
| Size_Cer_R, (8
mm3) |
3.71x10-4 |
4.98x10-4 | 0.466 |
| Mean_Cer_R | -3.68 | 2.61 | 0.176 |
| Max_M1_R | -0.17 | 0.89 | 0.849 |
| Size_M1_R, (8
mm3) |
-1.84x10-4 |
1.26x10-3 | 0.885 |
| Mean_M1_R | 2.41 | 2.65 | 0.376 |
| Max_PMv_R | 0.99 | 1.12 | 0.387 |
| Size_PMv_R, (8
mm3) |
3.96x10-3 |
1.93x10-3 | 0.056 |
| Mean_PMv_R | 2.03 | 2.61 | 0.448 |
| Max_PMd_R | -0.42 | 1.33 | 0.754 |
| Size_PMd_R, (8
mm3) |
4.39x10-3 |
1.70x10-3 | 0.019 |
| Mean_PMd_R | -1.56 | 3.02 | 0.613 |
| Max_S1_R | 1.96 | 0.51 | 0.001 |
| Size_S1_R, (8
mm3) |
-3.98x10-3 |
1.95x10-3 | 0.057 |
| Mean_S1_R | -4.82 | 2.95 | 0.121 |
| Max_SMA_R | -0.76 | 1 | 0.456 |
| Size_SMA_R, (8
mm3) |
-1.98x10-3 |
2.13x10-3 | 0.366 |
| Mean_SMA_R | -2.68 | 3.71 | 0.48 |
| FA Corticospinal
tract | -73.6 | 16.25 | <0.001 |
| FA Cerebral
peduncle | 230.56 | 42.29 | <0.001 |
| FA Posterior limb
of internal capsule | 137.27 | 60.69 | 0.037 |
| FA Posterior corona
radiate | -556.04 | 126.08 | <0.001 |
| MD Corticospinal
tract (10-6 mm2/s) | 145,126.61 | 32,423.39 | <0.001 |
| MD Cerebral
peduncle (10-6 mm2/s) | -494,726.62 | 87,353.51 | <0.001 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | 485,377.99 | 89,218.61 | <0.001 |
| MD Posterior corona
radiata (10-6 mm2/s) | -461,455.03 | 87,259.39 | <0.001 |
| C, Δ(Force) |
| Parameter | B | SE | P-value |
| Session=1 | 690.63 | 118.59 | <0.001 |
| Session=2 | 686.6 | 118.22 | <0.001 |
| Resistive
level=60% | 1.58 | 1.04 | 0.145 |
| Resistive
level=40% | 1.76 | 0.93 | 0.075 |
| Sex=female | -29.42 | 5.52 | <0.001 |
| Age, years | -2.16 | 0.55 | 0.001 |
| Max_Cer_L | 0.72 | 1.12 | 0.526 |
| Size_Cer_L, (8
mm3) |
1.15x10-3 |
7.90x10-4 | 0.164 |
| Mean_Cer_L | -23.16 | 5.08 | <0.001 |
| Max_M1_L | 1.11 | 0.96 | 0.263 |
| Size_M1_L, (8
mm3) |
4.13x10-3 |
2.51x10-3 | 0.118 |
| Mean_M1_L | -7.19 | 3.84 | 0.079 |
| Max_PMv_L | 4.61 | 1.45 | 0.006 |
| Size_PMv_L, (8
mm3) |
1.51x10-4 |
2.45x10-3 | 0.952 |
| Mean_PMv_L | 1.46 | 3.6 | 0.69 |
| Max_PMd_L | -1.47 | 1.63 | 0.381 |
| Size_PMd_L, (8
mm3) |
-3.85x10-3 |
2.45x10-3 | 0.135 |
| Mean_PMd_L | -2.6 | 2.65 | 0.339 |
| Max_S1_L | -1.44 | 1.2 | 0.246 |
| Size_S1_L, (8
mm3) |
2.96x10-3 |
4.81x10-3 | 0.546 |
| Mean_S1_L | 6.97 | 4.18 | 0.114 |
| Max_SMA_L | -3.08 | 2.39 | 0.214 |
| Size_SMA_L, (8
mm3) |
2.57x10-3 |
2.32x10-3 | 0.283 |
| Mean_SMA_L | -0.54 | 4.26 | 0.901 |
| Max_Cer_R | 1.9 | 1.61 | 0.254 |
| Size_Cer_R, (8
mm3) |
-5.92x10-4 |
6.65x10-4 | 0.386 |
| Mean_Cer_R | 10.64 | 2.86 | 0.002 |
| Max_M1_R | 0.39 | 1.17 | 0.746 |
| Size_M1_R, (8
mm3) |
-6.09x10-4 |
1.29x10-3 | 0.644 |
| Mean_M1_R | 7.67 | 2.6 | 0.009 |
| Max_PMv_R | 0.37 | 1.47 | 0.803 |
| Size_PMv_R, (8
mm3) |
5.82x10-3 |
2.52x10-3 | 0.034 |
| Mean_PMv_R | 0.81 | 4.06 | 0.845 |
| Max_PMd_R | -0.85 | 1.23 | 0.503 |
| Size_PMd_R, (8
mm3) | 0.01 |
2.00x10-3 | 0.014 |
| Mean_PMd_R | -0.69 | 3.73 | 0.855 |
| Max_S1_R | -0.56 | 0.69 | 0.428 |
| Size_S1_R, (8
mm3) |
-1.41x10-3 |
3.23x10-3 | 0.669 |
| Mean_S1_R | -8.28 | 4.18 | 0.064 |
| Max_SMA_R | 0.71 | 1.22 | 0.572 |
| Size_SMA_R, (8
mm3) |
-6.46x10-3 |
2.88x10-3 | 0.038 |
| Mean_SMA_R | 0.98 | 3.81 | 0.8 |
| FA Corticospinal
tract | 27.6 | 20.53 | 0.196 |
| FA Cerebral
peduncle | 5.48 | 61.34 | 0.93 |
| FA Posterior limb
of internal capsule | -334.48 | 58.89 | <0.001 |
| FA Posterior corona
radiata | -182.63 | 121.68 | 0.152 |
| MD Corticospinal
tract (10-6 mm2/s) | 105,826.78 | 37,490.28 | 0.012 |
| MD Cerebral
peduncle (10-6 mm2/s) | -173259.08 | 106,242.82 | 0.121 |
| MD Posterior limb
of internal capsule (10-6 mm2/s) | 34,301.53 | 110,648.92 | 0.76 |
| MD Posterior corona
radiata (10-6 mm2/s) | -279,777.8 | 84,242.02 | 0.004 |
In the contralesional hemisphere, fewer significant
patterns emerged for motor improvement. Maximum activation in right
Cer showed a positive association with Δ(FMA-UE) (max_Cer_R, B=0.85
and P=0.016), whereas maximum activation in right PMv yielded a
negative association for the same outcome (max_PMv_R, B=-0.81 and
P=0.019). Right PMd is positively associated with Δ(FMA-UE)
(mean_PMd_R, B=3.43 and P<0.001), whilst its cluster size
exhibited a negative association with Δ(FMA-UE) (size_PMd_R,
B=-1.37x10-3 and P=0.032) and a positive association for
the other two scales [Δ(BBT): Size_PMd_R, B=4.39x10-3
and P=0.019; Δ(Force): Size_PMd_R, B=0.01 and P=0.014]. Right
somatosensory cortex yielded a positive association with changes in
BBT (max_S1_R, B=1.96 and P=0.001), whereas right SMA shows
consistent positive association with Δ(FMA-UE) (mean_SMA_R, B=5.27
and P<0.001).
Diffusion metrics revealed additional insights.
Negative associations between FA in the cerebral peduncle (FA
Cerebral peduncle, B=-57.84 and P=0.001) or posterior limb of the
internal capsule (FA Posterior limb of internal capsule, B=-102.86
and P<0.001) and Δ(FMA-UE), coupled with positive associations
for BBT (FA Cerebral peduncle, B=230.56 and P<0.001; FA
Posterior limb of internal capsule, B=137.27 and P=0.037), suggest
that microstructural changes in specific descending pathways may
differentially influence upper-limb function compared with gross
dexterity. FA in the posterior corona radiata exhibited an opposite
pattern [positive for Δ(FMA-UE), B=164.28 and P=0.001; negative for
Δ(BBT), B=-556.04 and P<0.001), indicating that microstructural
integrity in more diffuse white matter regions may favor certain
motor outcomes but not others. MD metrics reinforced this
distinction, with some regions (such as the corticospinal tract)
associating positively with Δ(BBT) (MD Corticospinal tract,
B=145,126.61 and P<0.001), whereas others (such as the cerebral
peduncle) appear to associate negatively to BBT (MD Cerebral
peduncle, B=-494,726.62 and P<0.001). Taken together, these
patterns reflect a complex interplay among focal ipsilesional
reactivation, contralesional compensatory mechanisms and white
matter tract integrity in determining changes in motor
outcomes.
Model comparison
Model fit comparisons for the three motor
assessments are presented in Table
IV. For prediction models, the -2LL values were substantially
lower overall because they used a smaller dataset. Within each
model type (assessment vs. prediction), the FMA-UE consistently
outperformed the BBT and Force, suggesting the greater sensitivity
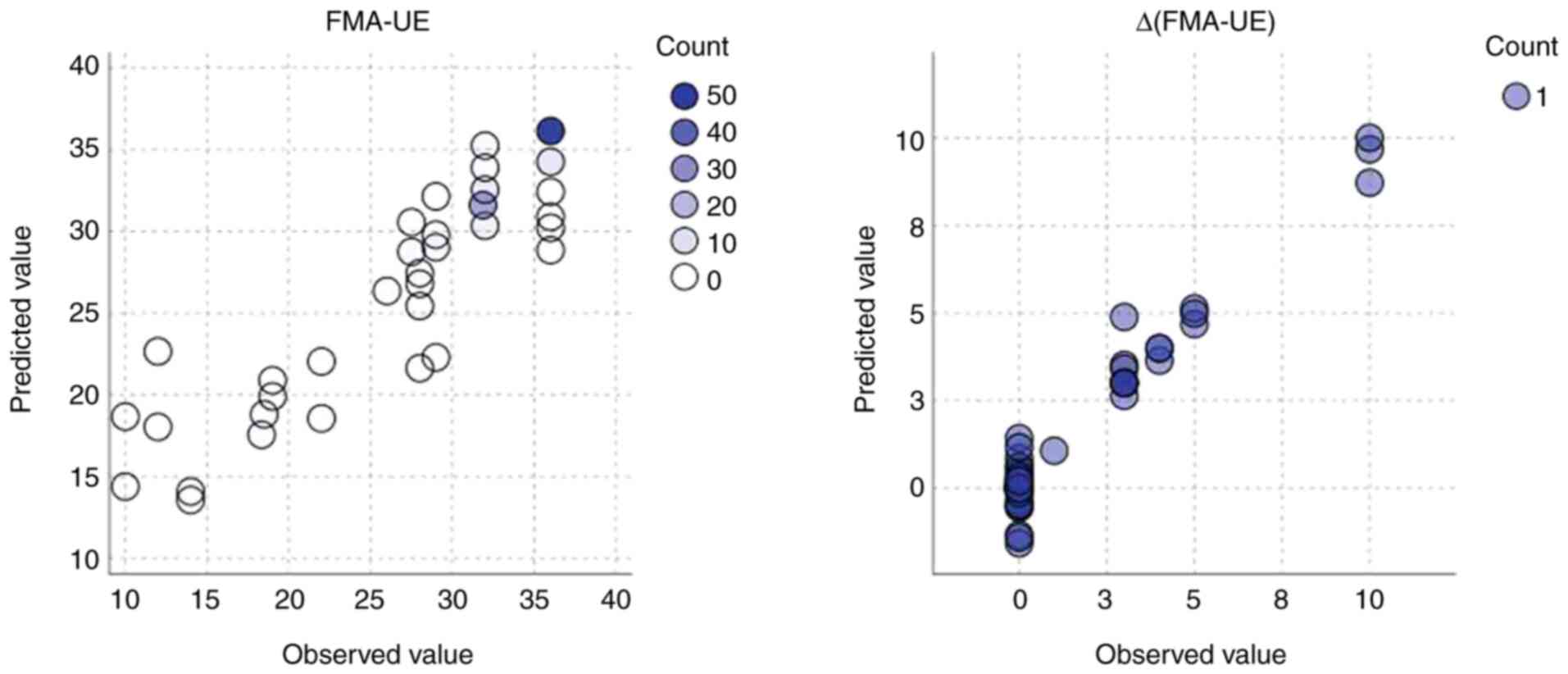
of neuroimaging data to FMA_UE changes. Fig. 3 illustrates the performance of GLMM
in predicting FMA-UE and its change [Δ(FMA-UE)] using circular
scatter plots displaying observed vs. predicted values, where
circle color indicates frequency. The clustering of the majority of
circles along the diagonal line of perfect prediction (observed
value=predicted value) in both plots indicates a strong agreement
between the model's estimates and the actual observed data. This
close alignment, observed for both absolute and change scores,
suggests that the FMA-UE is a reliable score for the assessment and
prediction of motor recovery, as evidenced by the model's ability
to accurately estimate both static and dynamic aspects of upper
extremity function.
 | Table IVComparison of model fit across motor
assessments using-2 log likelihood values. |
Table IV
Comparison of model fit across motor
assessments using-2 log likelihood values.
| Motor | Fugl-Meyer
assessment for upper extremity | Box and blocks
test | Force |
|---|
| Assessment
model | 652.998 | 790.758 | 880.792 |
| Prediction
model | 210.889 | 251.080 | 257.632 |
Discussion
The present study demonstrated that although motor
performance improvement is feasible in patients with chronic
stroke, it is not universally achievable. Among the 14
participants, only three attained clinically meaningful gains in
the FMA-UE scale, whilst two showed improvements in gross dexterity
(BBT) and seven in grip strength (Force). This variability in
outcomes aligns with prior evidence suggesting that chronic stroke
recovery is contingent on the interplay among residual brain
plasticity, lesion characteristics, and rehabilitation intensity
(4,38,39).
The motor scales used capture distinct facets of
recovery, each associated with specific neural parameters. The
FMA-UE, which evaluates sensorimotor coordination and selective
movement, primarily reflects the integrity of the ipsilesional
corticospinal tract and functional reorganization within the
primary motor and premotor cortices (40-42).
These regions are critical for precision and voluntary control. By
contrast, the BBT, assessing gross manual dexterity and speed,
mainly engages cerebellar-thalamocortical circuits and bilateral
premotor areas, which support rhythmic, goal-directed movements
(43). Finally, grip strength
(Force), a measure of maximal voluntary contraction, relies heavily
on corticospinal tract integrity and motor unit recruitment, which
is primarily modulated by the M1 and spinal cord pathways (41,44).
These theoretical distinctions highlight why the detected recovery
trajectories diverged across scales, since each metric taps into
unique neuroanatomical and functional systems.
The observed lateralization patterns in motor
assessment analysis suggest the task-specific recruitment of motor
networks during the chronic stroke phase. The preservation of
ipsilesional left M1 and cerebellar activation in FMA-UE aligns
with functional reorganization within the spared peri-lesional
circuits, a hallmark of successful motor recovery (45,46).
Conversely, the negative relationship between contralesional PMv/M1
activation and BBT or Force may reflect inefficient recruitment of
non-primary motor regions, consistent with previous studies finding
maladaptive overactivation in contralesional areas during simple
motor tasks (47-49).
The divergent positive association of PMd with both BBT and Force
may point to a region that, when appropriately engaged, supports
motor function. These results emphasize that not all contralesional
activations are equal, whereby the functional role of a region
(such as planning compared with execution) is key to understanding
its impact on motor performance.
Cluster size associations further illuminate neural
efficiency mechanisms. Smaller, focused activations in ipsilesional
SMA and M1 were found to be associated with superior FMA-UE and
Force, suggesting that functional recovery relies on streamlined,
instead of diffuse, engagement of primary motor regions. Ward et
al (50) previously
demonstrated that motor recovery was correlated with
renormalization of activity toward ipsilesional M1 and reduced
diffuse recruitment of non-primary regions, suggesting a shift from
compensatory to more efficient processing. By contrast, larger
clusters in the ipsilesional Cer and contralesional SMA were
associated with poorer performance, potentially reflecting
compensatory efforts or reduced network specificity. Consistent
with the present findings, previous studies also noted that
cerebellar overactivation may reflect attempts to compensate for
impaired corticocortical or corticostrial connectivity (50-53),
whilst contralesional SMA hyperactivity was associated with poor
motor outcomes in patients with chronic stroke (54). By contrast, the positive role of
ipsilesional SMA suggests activation across motor scales
underscores its importance in motor planning and execution,
corroborating its known involvement in internally guided movements
(55,56).
Diffusion metric findings highlight structural
underpinnings of these functional patterns. Higher FA in
corticospinal tracts and posterior limb of the internal capsule,
markers of white matter integrity, likely facilitate efficient
signal transmission, supporting motor performance (57). However, increased FA in posterior
corona radiata, a region integrating sensorimotor fibers, may
disrupt feedback loops critical for motor control or it could
reflect maladaptive tissue reorganization, such as gliosis scarring
(58). These results extend
current models, by integrating functional reorganization with
structural connectivity, proposing that both focused ipsilesional
activation and preserved white matter integrity are pivotal for
optimal motor function.
The overall pattern of associations underscores the
importance of lateralized, focused recruitment within the
ipsilesional hemisphere for achieving improvements in upper-limb
function. Positive association of left M1 with Δ(FMA-UE) suggest
that re-engagement of the primary motor area on the stroke-affected
side can facilitate fine motor control, consistent with motor
assessment evidence that normalization of ipsilesional activity is
beneficial (59). In addition, the
mixture of positive and negative associations within ipsilesional
premotor and sensory cortices indicates that excessive or diffuse
engagement of these secondary areas may not always be adaptive.
These findings collectively suggest that whilst SMA overactivation
may reflect inefficient compensation, as previously observed in
studies linking diffuse recruitment during poorly performed tasks
to maladaptive neural resource allocation (60,61),
PMv engagement represents a more targeted adaptive mechanism for
motor recovery. This aligns with its role in higher-order motor
planning and bilateral coordination, demonstrated in previous
studies where preserved PMv activity supported grasp posture
encoding and compensated for compromised primary motor pathways
(62).
Contralesional recruitment emerged as variably
supportive. Although positive association in the right PMd aligned
with prior reports of an ancillary contribution from premotor
regions in the unaffected hemisphere (63,64),
negative association in right PMv and positive ones in right SMA
suggest that each contralesional region may differentially
influence distinct facets of motor recovery. The fact that right
somatosensory cortex activation is positively associated with
changes in gross dexterity suggests that contralesional sensory
processing can aid performance when ipsilesional pathways are
compromised, but such recruitment may be less crucial for fine
motor tasks (65,66).
White matter diffusion metrics corroborated these
aforementioned functional observations, showing that the structural
integrity of critical descending pathways (corticospinal tract,
internal capsule) may have different impact on fine compared with
gross motor recovery. Negative associations of FA in the cerebral
peduncle and posterior limb of the internal capsule with Δ(FMA-UE),
and a negative association of FA in the posterior limb of the
internal capsule with Δ(Force), but positive ones for Δ(BBT),
suggest that microstructural reorganization in these tracts may
support certain compensatory strategies (such as improved motor
speed or gross upper-limb function) at the expense of refined
upper-limb coordination (67). By
contrast, the positive association of FA in the posterior corona
radiata with Δ(FMA-UE), a measure of fine motor recovery, suggests
that diffuse connectivity changes in this region may selectively
enhance sensorimotor integration, which is critical for tasks
requiring refined coordination (including finger individuation or
selective movement) (68). This
aligns with the posterior corona radiata's role in integrating
corticopontine and associative fibers that facilitate cross-modal
connectivity between sensory feedback and motor planning (69). However, the negative associations
of posterior corona radiata FA with Δ(BBT) and Δ(Force), which are
measures of gross dexterity and strength, indicate that diffuse
reorganization here may divert resources from task-specific
corticospinal pathways, which are more directly involved in force
generation and rhythmic movements (70). Therefore, the inverse associations
of FA in the posterior corona radiata with Δ(FMA-UE) compared with
Δ(BBT) may seem contradictory. However, this likely reflects the
dual role of this region in integrating sensory and motor signals.
Higher FA in this area may facilitate fine motor coordination (such
as finger individuation), supporting improvements in FMA-UE, whilst
potentially disrupting the streamlined corticospinal output
necessary for gross dexterity tasks, such as the BBT. These
findings underscore the importance of interpreting diffusion
metrics within the specific motor domains they influence.
Taken together, these findings highlight a dynamic
interplay between lateralized, focused activation of ipsilesional
motor regions and selective recruitment of contralesional premotor
and sensory areas, all modulated by the structural state of
relevant white matter tracts. Although contralesional engagement
can provide some compensatory support, particularly in sensory
processing or force production, sustained gains in upper-limb
function appear most strongly associated with ipsilesional
reactivation. Future rehabilitation strategies may therefore focus
on facilitating the targeted activation of ipsilesional M1 and
optimizing microstructural integrity in key motor pathways, whilst
also tailoring contralesional involvement to each patient's
specific deficit profile.
The present study has limitations that warrant
consideration. The small sample size limited statistical power and
generalizability, particularly for outcomes, such as grip strength,
where only a few patients demonstrated measurable improvements.
However, the use of GLMM enhanced statistical robustness by
accounting for repeated measures and within-subject variability. By
excluding individuals with significant cognitive or sensory
impairments, where conditions that can affect ≤50% of chronic
stroke survivors and frequently co-occur with motor deficits, the
present cohort does not fully represent the broader stroke
population observed in clinical practice. Future trials should
include participants with mild to moderate sensory and cognitive
comorbidities to assess the generalizability of the present
neuroimaging biomarkers in real-world settings. The present study
also focused exclusively on patients with left middle cerebral
artery infarcts, which limits the generalizability to other stroke
subtypes and lesion locations. Additionally, the observational
design and absence of a control group constrain causal inference.
Whilst including healthy or mildly impaired controls can introduce
ceiling effects and minimal variability, future studies should
consider matched chronic stroke control groups receiving standard
care as a more appropriate comparator. Finally, neuroimaging data
were acquired at a single institution using a specific robotic and
imaging protocol, which may limit reproducibility across settings.
To address these limitations, future research should recruit larger
and more clinically heterogeneous cohorts, encompass diverse lesion
sites and functional baselines, whilst integrating peripheral
physiological markers (such as electromyography or kinematic
analysis) to refine predictive models and enhance
applicability.
The present study builds upon prior knowledge by
identifying neuroimaging signatures (such as ipsilesional M1
cluster size and posterior corona radiata FA/MD) as potential
biomarkers for predicting response to MRI-compatible robotic
training, offering a bridge between neuroplasticity theories and
device-based rehabilitation outcomes. It refines the understanding
of contralesional contributions, revealing region-specific effects
(adaptive right PMd compared with maladaptive PMv/M1 activation)
that nuance earlier dichotomous interpretations of compensatory
mechanisms (47,49,62).
By mapping motor scales, such as FMA-UE, BBT and Force, to distinct
neural circuits, the present study provides a framework for
interpreting heterogeneous recovery patterns, whilst the inverse
relationship between posterior corona radiata FA and outcomes
challenges assumptions regarding diffuse white matter
reorganization. Methodologically, the integration of real-time
neuroimaging during robotic training and a predictive GLMM
framework offered preliminary tools for patient stratification,
distinguishing efficient, focused ipsilesional reorganization from
maladaptive compensatory recruitment. Although modest in scale,
these findings highlight interactions between structural
connectivity and task-specific functional activation, advancing
precision in post-stroke rehabilitation targeting. They also
indicate that the integration of fMRI and DTI with robotic
rehabilitation provides a novel framework for identifying neural
correlates and predictors of recovery, emphasizing the potential
for personalized therapeutic strategies.
Acknowledgements
The present study was conducted at the Athinoula A.
Martinos Center for Biomedical Imaging, (Charlestown, USA). The
authors would like to sincerely thank Dr Bruce R. Rosen, Director
of the Athinoula A. Martinos Center, for providing access to the
center, and the center's staff, for their technical assistance with
scanning procedures. The authors are also especially grateful to Dr
Mark P. Ottensmeyer, Department of Radiology, Massachusetts General
Research Institute (Boston, USA) for his invaluable contributions
in developing and maintaining the robotic device throughout the
study.
Funding
Funding: The present study was supported by a grant from the
National Institute of Neurological Disorders and Stroke (grant no.
1R01NS105875-01A1) of the National Institutes of Health.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MM and LGA analyzed the data and prepared the
manuscript. SE selected and trained the patients, collected and
curated the data. AAT designed the study. SE and AAT confirm the
authenticity of all the raw data. All authors read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
Institutional review board approval of the study was
granted by the Partners Human Research Committee (approval no.
2005P000570) serving Massachusetts General Hospital (Boston, USA)
and all participants provided informed consent for participation in
the study, including data collection, anonymized data analysis, and
publication of the results.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baricich A and Carda S: Chronic stroke: An
oxymoron or a challenge for rehabilitation? Funct Neurol.
33:123–124. 2018.PubMed/NCBI
|
|
2
|
Herpich F and Rincon F: Management of
acute ischemic stroke. Crit Care Med. 48:1654–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Solomonow-Avnon D and Mawase F: The dose
and intensity matter for chronic stroke. J Neurol Neurosurg
Psychiatry. 90:1187–1188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carson RG: The physiology of stroke
neurorehabilitation. J Physiol. 603:611–615. 2025.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Teasell RW, Murie Fernandez M, McIntyre A
and Mehta S: Rethinking the continuum of stroke rehabilitation.
Arch Phys Med Rehabil. 95:595–596. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paolucci T, Agostini F, Mussomeli E,
Cazzolla S, Conti M, Sarno F, Bernetti A, Paoloni M and Mangone M:
A rehabilitative approach beyond the acute stroke event: A scoping
review about functional recovery perspectives in the chronic
hemiplegic patient. Front Neurol. 14(1234205)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Campos B, Choi H, DeMarco AT,
Seydell-Greenwald A, Hussain SJ, Joy MT, Turkeltaub PE and Zeiger
W: Rethinking remapping: Circuit mechanisms of recovery after
stroke. J Neurosci. 43:7489–7500. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Garraway WM, Akhtar AJ, Smith DL and Smith
ME: The triage of stroke rehabilitation. J Epidemiol Community
Health. 35:39–44. 1981.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miller EL, Murray L, Richards L, Zorowitz
RD, Bakas T, Clark P and Billinger SA: American Heart Association
Council on Cardiovascular Nursing and the Stroke Council.
Comprehensive overview of nursing and interdisciplinary
rehabilitation care of the stroke patient: A scientific statement
from the American Heart Association. Stroke. 41:2402–2448.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sackley CM and Gladman JRF: The evidence
for rehabilitation after severely disabling stroke. Phys Ther Rev.
3:19–29. 1998.
|
|
11
|
Włodarczyk L, Cichon N, Saluk-Bijak J,
Bijak M, Majos A and Miller E: Neuroimaging techniques as potential
tools for assessment of angiogenesis and neuroplasticity processes
after stroke and their clinical implications for rehabilitation and
stroke recovery prognosis. J Clin Med. 11(2473)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stinear CM and Ward NS: How useful is
imaging in predicting outcomes in stroke rehabilitation? Int J
Stroke. 8:33–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gale SD and Pearson CM: Neuroimaging
predictors of stroke outcome: Implications for neurorehabilitation.
NeuroRehabilitation. 31:331–344. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gaviria E and Eltayeb Hamid AH:
Neuroimaging biomarkers for predicting stroke outcomes: A
systematic review. Health Sci Rep. 7(e2221)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong KY, Kamaldin N, Jeong HL, Nasrallah
FA, Goh JCH and Chen-Hua Y: A magnetic resonance compatible soft
wearable robotic glove for hand rehabilitation and brain imaging.
IEEE Trans Neural Syst Rehabil Eng. 25:782–793. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khanicheh A, Mintzopoulos D, Weinberg B,
Tzika AA and Mavroidis C: MR_CHIROD v.2: Magnetic resonance
compatible smart hand rehabilitation device for brain imaging. IEEE
Trans Neural Syst Rehabil Eng. 16:91–98. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khanicheh A, Muto A, Triantafyllou C,
Weinberg B, Astrakas L, Tzika A and Mavroidis C: fMRI-compatible
rehabilitation hand device. J Neuroeng Rehabil.
3(24)2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharini H, Riyahi Alam N, Khabiri H,
Arabalibeik H, Hashemi H, Azimi AR and Masjoodi S: Novel
FMRI-Compatible wrist robotic device for brain activation
assessment during rehabilitation exercise. Med Eng Phys.
83:112–122. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Astrakas LG, Naqvi SHA, Kateb B and Tzika
AA: Functional MRI using robotic MRI compatible devices for
monitoring rehabilitation from chronic stroke in the molecular
medicine era (Review). Int J Mol Med. 29:963–973. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mintzopoulos D, Khanicheh A, Konstas AA,
Astrakas LG, Singhal AB, Moskowitz MA, Rosen BR and Tzika AA:
Functional MRI of rehabilitation in chronic stroke patients using
novel MR-Compatible hand robots. Open Neuroimag J. 2:94–101.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jo S, Song Y, Lee Y, Heo SH, Jang SJ, Kim
Y, Shin JH, Jeong J and Park HS: Functional MRI assessment of brain
activity during hand rehabilitation with an MR-Compatible soft
glove in chronic stroke patients: A preliminary study. IEEE Int
Conf Rehabil Robot. 2023:1–6. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mintzopoulos D, Astrakas LG, Khanicheh A,
Konstas AA, Singhal A, Moskowitz MA, Rosen BR and Tzika AA:
Connectivity alterations assessed by combining fMRI and
MR-compatible hand robots in chronic stroke. NeuroImage. 47 (Suppl
2):T90–T97. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oldfield RC: The assessment and analysis
of handedness: The Edinburgh inventory. Neuropsychologia. 9:97–113.
1971.PubMed/NCBI View Article : Google Scholar
|
|
24
|
London Medical Research Council: Aids to
the Examination of the Peripheral Nervous System. Her Majesty's
Stationery Office, London, 1976.
|
|
25
|
Astrakas LG, De Novi G, Ottensmeyer MP,
Pusatere C, Li S, Moskowitz MA and Tzika AA: Improving motor
function after chronic stroke by interactive gaming with a
redesigned MR-compatible hand training device. Exp Ther Med.
21(245)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson
S and Steglind S: The Post-stroke hemiplegic patient. 1. a method
for evaluation of physical performance. Scand J Rehabil Med.
7:13–31. 1975.PubMed/NCBI
|
|
27
|
Lyle RC: A performance test for assessment
of upper limb function in physical rehabilitation treatment and
research. Int J Rehabil Res. 4:483–492. 1981.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bohannon RW and Smith MB: Interrater
reliability of a modified Ashworth scale of muscle spasticity. Phys
Ther. 67:206–207. 1987.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mathiowetz V, Volland G, Kashman N and
Weber K: Adult norms for the box and block test of manual
dexterity. Am J Occup Ther. 39:386–391. 1985.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mazziotta J, Toga A, Evans A, Fox P,
Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, et al: A
probabilistic atlas and reference system for the human brain:
International consortium for brain mapping (ICBM). Philos Trans R
Soc Lond B Biol Sci. 356:1293–1322. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mayka MA, Corcos DM, Leurgans SE and
Vaillancourt DE: Three-dimensional locations and boundaries of
motor and premotor cortices as defined by functional brain imaging:
A meta-analysis. Neuroimage. 31:1453–1474. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wakana MS, Nagae-Poetscher LM, van Zijl
PCM and Crain B: MRI Atlas of Human White Matter. 1st edition.
Elsevier, Amsterdam, 2005.
|
|
33
|
Bolker BM, Brooks ME, Clark CJ, Geange SW,
Poulsen JR, Stevens MHH and White J-SS: Generalized linear mixed
models: A practical guide for ecology and evolution. Trends Ecol
Evol. 24:127–135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
White H: A Heteroskedasticity-consistent
covariance matrix estimator and a direct test for
heteroskedasticity. Econometrica. 48:817–838. 1980.
|
|
35
|
Woytowicz EJ, Rietschel JC, Goodman RN,
Conroy SS, Sorkin JD, Whitall J and Waller SM: Determining levels
of upper extremity movement impairment by applying a cluster
analysis to the Fugl-Meyer Assessment of the Upper Extremity in
Chronic Stroke. Arch Phys Med Rehabil. 98:456–462. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Page SJ, Fulk GD and Boyne P: Clinically
important differences for the Upper-extremity Fugl-meyer scale in
people with minimal to moderate impairment due to chronic stroke.
Phys Ther. 92:791–798. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen HM, Chen CC, Hsueh IP, Huang SL and
Hsieh CL: Test-retest reproducibility and smallest real difference
of 5 hand function tests in patients with stroke. Neurorehabil
Neural Repair. 23:435–440. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Teasell R, Bayona N, Salter K, Hellings C
and Bitensky J: Progress in clinical neurosciences: Stroke recovery
and rehabilitation. Can J Neurol Sci. 33:357–364. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Miltner WHR: Plasticity and reorganization
in the rehabilitation of stroke. Zeitschrift für Psychologie.
224:91–101. 2016.
|
|
40
|
Fan Y, Lin K, Liu H, Chen Y and Wu C:
Changes in structural integrity are correlated with motor and
functional recovery after post-stroke rehabilitation. Restor Neurol
Neurosci. 33:835–844. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schulz R, Park CH, Boudrias MH, Gerloff C,
Hummel FC and Ward NS: Assessing the integrity of corticospinal
pathways from primary and secondary cortical motor areas after
stroke. Stroke. 43:2248–2251. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Saini M, Singh N, Kumar N, Srivastava MVP
and Mehndiratta A: A novel perspective of associativity of upper
limb motor impairment and cortical excitability in sub-acute and
chronic stroke. Front Neurosci. 16(832121)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Debaere F, Wenderoth N, Sunaert S, Van
Hecke P and Swinnen SP: Cerebellar and premotor function in
bimanual coordination: Parametric neural responses to
spatiotemporal complexity and cycling frequency. Neuroimage.
21:1416–1427. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ward NS, Newton JM, Swayne OB, Lee L,
Frackowiak RS, Thompson AJ, Greenwood RJ and Rothwell JC: The
relationship between brain activity and peak grip force is
modulated by corticospinal system integrity after subcortical
stroke. Eur J Neurosci. 25:1865–1873. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Favre I, Zeffiro TA, Detante O, Krainik A,
Hommel M and Jaillard A: Upper limb recovery after stroke is
associated with ipsilesional primary motor cortical activity.
Stroke. 45:1077–1083. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dong Y, Winstein CJ, Albistegui-DuBois R
and Dobkin BH: Evolution of FMRI activation in the perilesional
primary motor cortex and cerebellum with rehabilitation
training-related motor gains after stroke: A pilot study.
Neurorehabil Neural Repair. 21:412–428. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ward NS, Brown MM, Thompson AJ and
Frackowiak RSJ: The influence of time after stroke on brain
activations during a motor task. Ann Neurol. 55:829–834.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Calautti C, Naccarato M, Jones PS, Sharma
N, Day DD, Carpenter AT, Bullmore ET, Warburton EA and Baron JC:
The relationship between motor deficit and hemisphere activation
balance after stroke: A 3T fMRI study. Neuroimage. 34:322–331.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Braaß H, Feldheim J, Chu Y, Tinnermann A,
Finsterbusch J, Büchel C, Schulz R and Gerloff C: Association
between activity in the ventral premotor cortex and spinal cord
activation during force generation-A combined cortico-spinal fMRI
study. Hum Brain Mapp. 44:6471–6483. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ward NS, Brown MM, Thompson AJ and
Frackowiak RSJ: Neural correlates of motor recovery after stroke: A
longitudinal fMRI study. Brain. 126:2476–2496. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Calautti C and Baron JC: Functional
neuroimaging studies of motor recovery after stroke in adults: A
review. Stroke. 34:1553–1566. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Feydy A, Carlier R, Roby-Brami A, Bussel
B, Cazalis F, Pierot L, Burnod Y and Maier MA: Longitudinal study
of motor recovery after stroke: Recruitment and focusing of brain
activation. Stroke. 33:1610–1617. 2002.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Z and Wang L, Gao F, Dai Y, Liu C, Wu
J, Wang M, Yan Q, Chen Y, Wang C and Wang L: Exploring cerebellar
transcranial magnetic stimulation in Post-stroke limb dysfunction
rehabilitation: A narrative review. Front Neurosci.
19(1405637)2025.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wong WW, Chan ST, Tang KW, Meng F and Tong
KY: Neural correlates of motor impairment during motor imagery and
motor execution in Sub-cortical stroke. Brain Injury. 27:651–663.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Loubinoux I, Carel C, Pariente J,
Dechaumont S, Albucher JF, Marque P, Manelfe C and Chollet F:
Correlation between cerebral reorganization and motor recovery
after subcortical infarcts. Neuroimage. 20:2166–2180.
2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Peng X, Srivastava S, Sutton F, Zhang Y,
Badran BW and Kautz SA: Compensatory increase in ipsilesional
supplementary motor area and premotor connectivity is associated
with greater gait impairments: A personalized fMRI analysis in
chronic stroke. Front Hum Neurosci. 18(1340374)2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wen H, Alshikho MJ, Wang Y, Luo X, Zafonte
R, Herbert MR and Wang QM: Correlation of fractional anisotropy
with motor recovery in patients with stroke after postacute
rehabilitation. Arch Phys Med Rehabil. 97:1487–1495.
2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Budde MD, Janes L, Gold E, Turtzo LC and
Frank JA: The contribution of gliosis to diffusion tensor
anisotropy and tractography following traumatic brain injury:
Validation in the rat using Fourier analysis of stained tissue
sections. Brain. 134:2248–2260. 2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pelicioni MCX, Novaes MM, Peres AS, Lino
de Souza AA, Minelli C, Fabio SR, Pontes-Neto OM, Santos AC and de
Araujo DB: Functional versus nonfunctional rehabilitation in
chronic ischemic stroke: Evidences from a randomized functional MRI
study. Neural Plast. 2016(6353218)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Saleh S, Sandroff BM, Vitiello T, Owoeye
O, Hoxha A, Hake P, Goverover Y, Wylie G, Yue G and DeLuca J: The
role of premotor areas in dual tasking in healthy controls and
persons with multiple sclerosis: An fNIRS imaging study. Front
Behav Neurosci. 12(296)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pinson H, Van Lerbeirghe J, Vanhauwaert D,
Van Damme O, Hallaert G and Kalala JP: The supplementary motor area
syndrome: A neurosurgical review. Neurosurg Rev. 45:81–90.
2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Davare M, Andres M, Cosnard G, Thonnard JL
and Olivier E: Dissociating the role of ventral and dorsal premotor
cortex in precision grasping. J Neurosci. 26:2260–2268.
2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Stewart JC, Dewanjee P, Shariff U and
Cramer SC: Dorsal premotor activity and connectivity relate to
action selection performance after stroke. Hum Brain Mapp.
37:1816–1830. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Harrington RM, Chan E, Rounds AK, Wutzke
CJ, Dromerick AW, Turkeltaub PE and Harris-Love ML: Roles of
lesioned and nonlesioned hemispheres in reaching performance
post-stroke. Neurorehabil Neural Repair. 34:61–71. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
McPherson JG, Chen A, Ellis MD, Yao J,
Heckman CJ and Dewald JPA: Progressive recruitment of
contralesional cortico-reticulospinal pathways drives motor
impairment post stroke. J Physiol. 596:1211–1225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee K, Barradas V and Schweighofer N:
Self-organizing recruitment of compensatory areas maximizes
residual motor performance post-stroke bioRxiv, 2024 doi:
10.1101/2024.06.28.601213.
|
|
67
|
Lee J, Chang WH and Kim YH: Relationship
between the corticospinal and corticocerebellar tracts and their
role in upper extremity motor recovery in stroke patients. J Pers
Med. 11(1162)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jang SH, Ahn SH, Ha JS, Lee SJ, Lee J and
Ahn YH: Peri-infarct reorganization in a patient with corona
radiata infarct: A combined study of functional MRI and diffusion
tensor image tractography. Restor Neurol Neurosci. 24:65–68.
2006.PubMed/NCBI
|
|
69
|
Li C, Dang C, Liu G, Chen L, Zhang J, Li
J, Ou Z, Zhang Y and Xu A: Secondary damage in left-sided frontal
white matter detected by diffusion tensor imaging is correlated
with executive dysfunction in patients with acute infarction at the
ipsilateral posterior corona radiata. Eur J Med Res.
19(44)2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lafe CW, Liu F, Simpson TW, Moon CH,
Collinger JL, Wittenberg GF and Urbin MA: Force oscillations
underlying precision grip in humans with lesioned corticospinal
tracts. Neuroimage Clin. 38(103398)2023.PubMed/NCBI View Article : Google Scholar
|