Introduction
Immune checkpoint inhibitors (ICIs) have markedly
improved clinical outcomes across a range of malignancies (1). By inhibiting signal transduction
pathways like programmed cell death 1 (PD-1)/programmed cell death
ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein
(CTLA)-4, ICIs enhance the T-cell-mediated anti-tumor immune
response, leading to durable remissions and survival advantages,
particularly in cancers historically considered refractory,
including metastatic melanoma and non-small cell lung cancer
(1). Despite their efficacy, ICIs
are associated with immune-related adverse events (irAEs), which
may involve nearly any organ system.
IrAEs are common, occurring in ~90% of patients
receiving anti-CTLA-4 therapy and 70% of those treated with
anti-PD-1/PD-L1 agents (2).
Although less frequent than dermatologic or gastrointestinal irAEs,
neurological irAEs, affecting ~1-3% of patients, are particularly
concerning due to their potential severity and risk of long-term
disability (3). Among these,
polyradiculoneuropathy (PRN) and myocarditis represent severe
manifestations of neurological and cardiac irAEs, respectively.
Although rare, these events can be life-threatening: Severe PRN has
been associated with mortality rates of up to 60% (4,5),
while ICI-related myocarditis has a fatality rate approaching 50%
(6). The pathogenesis of irAE has
been reported to involve complex immune dysregulation, including
autoreactive T-cell activation and cellular/humoral immune-mediated
tissue damage (3).
The management of severe or refractory irAEs remains
a significant clinical challenge. First-line treatment typically
consists of high-dose corticosteroids combined with supportive care
(1). However, second-line
therapies such as intravenous immunoglobulin (IVIG) or
plasmapheresis demonstrate variable efficacy and substantial
mortality (4,5). This highlights the urgent need for
novel therapeutic strategies targeting specific immune pathways.
Efgartigimod, a neonatal Fc receptor (FcRn) antagonist, enhances
the degradation of pathogenic IgG autoantibodies. Its established
efficacy and favorable safety profile in antibody-mediated
disorders like generalized myasthenia gravis (7) suggest its potential utility in
treating refractory, antibody-mediated irAEs (8).
The present case report describes marked
neurological and cardiac improvement in a patient with metastatic
pancreatic cancer who developed severe, refractory ICI-induced PRN
and myocarditis following treatment with efgartigimod. The findings
underscore FcRn blockade as a promising therapeutic approach for
managing life-threatening, treatment-resistant irAEs.
Case report
Clinical presentation
A 63-year-old female was diagnosed with pancreatic
ductal adenocarcinoma in December 2023 at Zhongshan Hospital, Fudan
University (Shanghai, China). The patient received first-line
chemotherapy with 1.4 g cytarabine plus 170 mg gemcitabine every 3
weeks from the end of December 2023 to mid-July 2024, completing a
total of 10 cycles. In mid-July 2024, the patient was initiated on
the PD-1 inhibitor sintilimab at a dose of 100 mg every 3 weeks.
Concurrently, the patient underwent radiotherapy targeting the
lesion from mid-July to mid-August. From mid-August, the patient
gradually developed bilateral ptosis, accompanied by generalized
fatigue, a noticeable reduction in walking speed, impaired
stair-climbing ability (limited to four flights with rest
intervals) and decreased strength in both upper limbs. Over the
following 2 weeks, the patient's symptoms progressively worsened,
culminating in bulbar dysfunction characterized by dysarthria and
dysphagia.
Chemotherapy was discontinued due to the patient's
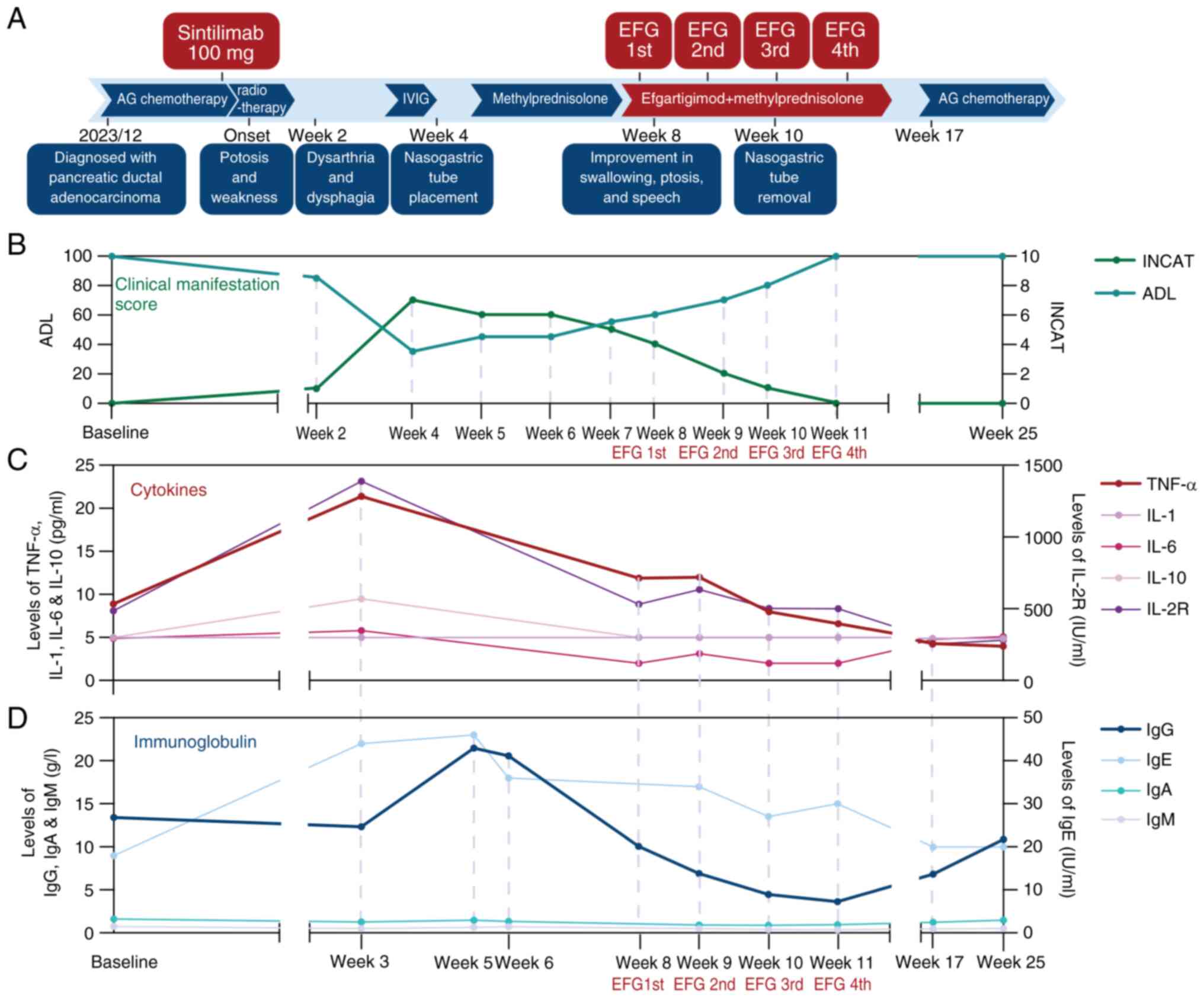
worsening clinical status 3 weeks after disease onset (Fig. 1A and B; Table
I). The patient was admitted to the Department of Neurology.
Physical examination revealed bilateral ptosis and proximal lower
limb weakness (grade IV on the Medical Research Council scale)
(9). Tendon reflexes were absent
in the upper limbs and diminished in the lower limbs. The
pharyngeal reflex was also absent. Electromyography and nerve
conduction studies demonstrated widespread denervation without a
decremental response on low-frequency repetitive nerve stimulation.
The neostigmine test yielded negative results. Laboratory testing
revealed elevated levels of creatine kinase at 1,633 U/l (normal
range, 26-140 U/l), creatine phosphokinase-isoenzyme-MB at 100 U/l
(normal range, 0-23 U/l) and cardiac troponin T at 0.782 ng/ml
(normal range, 0-0.014 ng/ml). Serum anti-double-stranded DNA
antibody was 186.6 IU/ml (normal range, 0-100 IU/ml) and
anti-nucleosome antibody was 20.5 RU/ml (normal range, 0-20 RU/ml).
Electrocardiography showed frequent premature ventricular
contractions, while echocardiography findings were unremarkable.
Brain MRI revealed no abnormalities (data not shown). Lumbar
puncture demonstrated normal cerebrospinal fluid pressure and
routine biochemical test results showed negative microbiological
findings, and no malignant cells were identified (data not
shown).
 | Table IClinical manifestation scores at
disease onset and during treatment. |
Table I
Clinical manifestation scores at
disease onset and during treatment.
| Scoring system | Baseline | Week 2 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 (EFG 1st) | Week 9 (EFG 2nd) | Week 10 (EFG
3rd) | Week 11 (EFG
4th) | Week 25 |
|---|
| ADL | 100 | 85 | 35 | 45 | 45 | 55 | 60 | 70 | 80 | 100 | 100 |
| INCAT | 0 | 1 | 7 | 6 | 6 | 5 | 4 | 2 | 1 | 0 | 0 |
Diagnosis and management
The patient was diagnosed with ICI-induced PRN and
cardiomyopathy. Initial treatment with IVIG at a dosage of 0.4
g/kg, administered for 5 days, yielded no clinical improvement. The
patient developed persistent chest tightness requiring supplemental
oxygen. The muscle strength in the cervical region and extremities
had markedly declined, rendering the patient bedbound. Progressive
dysphagia and dysarthria necessitated a nasogastric tube for
nutritional support.
Subsequently, corticosteroid therapy was initiated
with intravenous methylprednisolone (240 mg daily for 5 days,
followed by 120 mg daily for 3 days). This was subsequently
transitioned to oral prednisone, starting at 60 mg per day and
tapered by 5 mg per week to a maintenance dose of 30 mg. Although a
slight improvement in limb weakness was observed, ptosis and
swallowing difficulties persisted. In addition, the patient
developed tongue muscle atrophy.
In October 2024, treatment with intravenous
efgartigimod was initiated at a dose of 10 mg/kg once weekly,
leading to a notable improvement in ptosis, swallowing and speech.
Following the second dose, the patient's symptoms continued to
improve, and the patient was able to resume oral intake, although
slowly. The nasogastric tube was subsequently removed, and the
patient was discharged. During weekly hospital visits for the third
and fourth doses with concurrent clinical assessments, sustained
symptomatic improvement was observed (Fig. 1A). No adverse events were observed
during the course of treatment.
In December 2024, the patient returned for a
follow-up visit. Physical examination revealed no neurological
deficits. Blood testing showed that both muscle and cardiac enzyme
levels were within normal ranges. As a result, chemotherapy with
cytarabine plus gemcitabine was resumed. At the subsequent
follow-up in February 2025, the patient remained clinically stable
and had no complaints of discomfort.
The patient's functional status was evaluated using
the Activities of Daily Living (ADL) scale (10) and the Inflammatory Neuropathy Cause
and Treatment (INCAT) disability score (11). Rapid disease progression was noted
within one month of symptom onset, with the ADL score declining to
35 points (range, 0-100 points) and the INCAT score rising to 7
points (range, 0-10 points) following IVIG therapy. Corticosteroid
treatment led to modest improvement, with the ADL score increasing
to 45 points and the INCAT score decreasing to 6. Notably, after
initiation of efgartigimod treatment, the patient exhibited rapid
improvement in both measures. Complete recovery of activities of
daily functioning was achieved after the fourth dose of
efgartigimod and the patient remained symptom-free without relapse
during the three-month follow-up period (Fig. 1B; Table I). The patient continued cancer
treatment with regular follow-ups every 2 months, and the condition
was stable at the last follow-up.
Cytokine and immunoglobulin
monitoring
Cytokine levels (TNF-α, IL-6, IL-10 and IL-2
receptor) were measured by flow cytometry as previously described
(12). At disease onset, cytokine
levels were significantly elevated. Although IVIG and
corticosteroid therapy led to a reduction in cytokine levels, no
corresponding clinical improvement was observed. Subsequent
treatment with efgartigimod resulted in a further decline in
cytokine levels (Fig. 1C; Table II). Following IVIG administration,
serum IgG levels initially increased but later returned to normal.
A gradual decrease in IgG was observed after the initiation of
efgartigimod, while the levels of other immunoglobulins remained
stable (Fig. 1D; Table II).
 | Table IISerum cytokine and immunoglobulin
dynamics at disease onset and during treatment. |
Table II
Serum cytokine and immunoglobulin
dynamics at disease onset and during treatment.
| Parameter | Baseline | Week 3 | Week 5 | Week 6 | Week 8 (EFG 1st) | Week 9 (EFG 2nd) | Week 10 (EFG
3rd) | Week 11 (EFG
4th) | Week 17 | Week 25 | Normal range |
|---|
| Immunoglobulin | | | | | | | | | | | |
|
IgG,
g/l | 13.41 | 12.34 | 21.47 | 20.58 | 10.06 | 6.89 | 4.46 | 3.64 | 6.83 | 10.88 | 8.60-17.40 |
|
IgA,
g/l | 1.61 | 1.26 | 1.47 | 1.34 | NA | 0.91 | 0.9 | 0.94 | 1.25 | 1.49 | 1.00-4.20 |
|
IgM,
g/l | 0.74 | 0.5 | 0.66 | 0.71 | NA | 0.51 | 0.41 | 0.35 | 0.47 | 0.51 | 0.50-2.80 |
|
IgE,
IU/ml | 18 | 44 | 46 | 36 | NA | 34 | 27 | 30 | 20 | 20 | 0-200 |
| Cytokines | | | | | | | | | | | |
|
TNF-α,
pg/ml | 8.9 | 21.4 | NA | NA | 11.9 | 12 | 8 | 6.6 | 4.4 | 4.1 | 0-8.1 |
|
IL-1β,
pg/ml | 5 | 5 | NA | NA | 5 | 5 | <5.0 | <5.0 | <5.0 | <5.0 | 0-5.0 |
|
IL-2R,
IU/ml | 485 | 1,389 | NA | NA | 531 | 635 | 503 | 501 | 260 | 287 | 223-710 |
|
IL-6,
pg/ml | 4.9 | 5.8 | NA | NA | <2.0 | 3.1 | <2.0 | <2.0 | 4.9 | 5.2 | 0-3.4 |
|
IL-10,
pg/ml | 5 | 9.5 | NA | NA | 5 | 5 | <5.0 | <5.0 | <5.0 | <5.0 | 0-9.1 |
Discussion
ICIs can cause severe, though rare, neuroimmune
complications, such as myositis, myocarditis and PRN, typically
emerging after one to two treatment cycles (3). The incidence of ICI-induced PRN is
estimated to be between 0.1 and 0.3% (13), with clinical manifestations,
including limb weakness and bulbar symptoms. Although
corticosteroids and IVIG are considered first-line therapies, the
associated mortality rate remains high, reaching up to 60%
(4,5). In the present case, the patient
exhibited a limited clinical response to corticosteroids and IVIG.
However, substantial neurological and cardiac improvement was
achieved following administration of efgartigimod, a novel
immunosuppressive agent and first-in-class FcRn antagonist.
During the course of treatment, cytokine and
immunoglobulin levels were monitored, noting initial elevations
indicative of immune activation. Although cytokine levels decreased
following conventional treatment, no corresponding clinical
improvement was observed, suggesting that cytokine-driven pathways
may not be dominant in the pathogenesis of irAEs in the
patient.
Importantly, antibody-mediated immune processes have
been increasingly implicated in the development of irAEs. Previous
studies have reported that up to 40% of patients developed
encephalitis, 34.4% had myasthenia gravis and 22% of patients
experienced peripheral neuropathy and Guillain-Barré Syndrome and
tested positive for autoantibodies (14,15).
The most frequently detected neuro-antibodies include
anti-acetylcholine receptor antibodies, anti-Ma family member 2
antibodies and anti-Hu antibodies (15). Additionally, a range of
autoantibodies has been detected in ICI-induced myocarditis,
including those targeting myosin heavy chain, cardiac troponin I
and other cardiac antigens, such as β-1 adrenergic receptor,
muscarinic M2 receptor and Na-K-ATPase (16). Nonetheless, a significant
proportion of patients remain negative for these antibodies,
suggesting the involvement of alternative pathogenic mechanisms or
the presence of unidentified antibodies.
In the patient of the current study, the presence of
positive serum anti-double-stranded DNA antibodies, along with
significant clinical improvement and a sustained reduction in IgG
levels following efgartigimod treatment, supports the hypothesis
that antibody-mediated immune responses play a central role in the
pathogenesis of ICI-induced paraneoplastic neurological syndrome.
Efgartigimod, originally developed for the treatment of myasthenia
gravis and other autoimmune disorders, functions by binding to
FcRn, thereby preventing the recycling of IgG and promoting its
degradation (7). This mechanism
reduces circulating IgG levels without inhibiting IgG synthesis,
offering a promising therapeutic option for patients who are
unresponsive to conventional immunosuppressive therapies. Within
the therapeutic context of irAEs, efgartigimod shows potential
efficacy through the targeted clearance of pathogenic
autoantibodies, which in turn attenuates downstream inflammatory
cascades. Furthermore, a previously reported case demonstrated the
significant efficacy of efgartigimod in treating ICI-induced
myositis, myocarditis and myasthenia gravis (8), further emphasizing the therapeutic
potential of FcRn antagonists in eliminating pathogenic
autoantibodies and supporting FcRn blockade as a promising strategy
for managing severe neurological and cardiac irAEs.
While the present case demonstrates promising
results, the generalizability of these findings is inherently
limited by the single-patient design. Multicenter collaborations
and larger cohorts of patients with refractory irAE are needed to
validate the efficacy of efgartigimod. Although the patient
exhibited positive serum anti-double-stranded DNA antibodies,
anti-nucleosome antibodies and initially elevated immunoglobulin
levels, further investigation is necessary to elucidate the role of
these antibodies in the onset and progression of ICI-induced PRN.
Comprehensive serological profiling, including testing for
anti-ganglioside, onconeural, myositis-specific and myasthenia
gravis-associated antibodies, along with advanced molecular
analyses, will be essential for uncovering the underlying
mechanisms of irAE pathogenesis (3).
In light of the findings of this case study, several
clinical applications for efgartigimod may also be proposed. This
agent may offer a particular benefit to patients with ICI-induced
neuroimmune complications who are unresponsive to conventional
treatments such as corticosteroids and IVIG. Therapeutic monitoring
should include regular assessments of serum IgG levels and other
immune markers to enable individualized treatment adjustments.
Future research should focus on refining patient selection criteria
and establishing standardized protocols for the clinical use of
FcRn antagonists in the management of ICI-induced irAEs.
While this case provides compelling evidence
supporting the efficacy of efgartigimod in treating ICI-induced PRN
and cardiomyopathy, further research is required to establish its
role in broader clinical practice. The limitations of this report,
including the single-patient design and the short duration of
follow-up, should be acknowledged. Future studies should aim to
address these gaps to inform evidence-based clinical
decision-making.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD was involved in the conceptualization of the
study, data curation, formal analysis, writing the original draft,
and reviewing and editing the manuscript. ZW performed data
curation and writing of the original draft. QW contributed with
data curation, visualization, and reviewing and editing the
manuscript. ZH was involved in visualization, and reviewing and
editing the manuscript. JD and SW contributed by reviewing and
editing the manuscript, and aided study conceptualization. All
authors have read and approved the final version of the manuscript.
Two authors have checked and confirmed the authenticity of the raw
data.
Ethics approval and consent to
participate
The study involving human participants was approved
by the Ethics Committee of Zhongshan Hospital, Fudan University
(approval no. CR2025-001). All procedures were conducted in
accordance with the principles of the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report, including the
publication of potentially identifiable data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Larroquette M, Domblides C, Lefort F,
Lasserre M, Quivy A, Sionneau B, Bertolaso P, Gross-Goupil M,
Ravaud A and Daste A: Combining immune checkpoint inhibitors with
chemotherapy in advanced solid tumours: A review. Eur J Cancer 158:
Oct 13, 2021 (Epub ahead of print).
|
|
2
|
Lee DJ, Lee HJ Jr, Farmer JR and Reynolds
KL: Mechanisms driving immune-related adverse events in cancer
patients treated with immune checkpoint inhibitors. Curr Cardiol
Rep. 23(98)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Farina A, Villagrán-García M, Vogrig A,
Zekeridou A, Muñiz-Castrillo S, Velasco R, Guidon AC, Joubert B and
Honnorat J: Neurological adverse events of immune checkpoint
inhibitors and the development of paraneoplastic neurological
syndromes. Lancet Neurol. 23:81–94. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pathak R, Katel A, Massarelli E, Villaflor
VM, Sun V and Salgia R: Immune checkpoint inhibitor induced
myocarditis with myositis/myasthenia gravis overlap syndrome: A
systematic review of cases. Oncologist. 26:1052–1061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arora P, Talamo L, Dillon P, Gentzler RD,
Millard T, Salerno M, Slingluff CL Jr and Gaughan EM: Severe
combined cardiac and neuromuscular toxicity from immune checkpoint
blockade: An institutional case series. Cardiooncology.
6(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Raikhelkar J and Uriel N: Immune
checkpoint inhibitor myocarditis. Curr Opin Cardiol. 34:303–306.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ulrichts P, Guglietta A, Dreier T, van
Bragt T, Hanssens V, Hofman E, Vankerckhoven B, Verheesen P,
Ongenae N, Lykhopiy V, et al: Neonatal Fc receptor antagonist
efgartigimod safely and sustainably reduces IgGs in humans. J Clin
Invest. 128:4372–4386. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Campbell M, Rodriguez-Hernandez A, Rizvi
Z, Swerdloff M, Zakin E and Faktorovich S: Efgartigimod for
pembrolizumab-induced myasthenia gravis refractory to standard
therapy (P1-8.006). Neurology. 100 (Suppl 17)(S2319)2023.
|
|
9
|
Aids to the investigation of peripheral
nerve injuries. JAMA. 122(266)1943.
|
|
10
|
Katz S, Ford AB, Moskowitz RW, Jackson BA
and Jaffe MW: Studies of illness in the aged. The index of adl: A
standardized measure of biological and psychosocial function. JAMA.
185:914–919. 1963.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merkies ISJ, Schmitz PIM, Van Der Meché
FGA, Samijn JPA and Van Doorn PA: Psychometric evaluation of a new
handicap scale in immune-mediated polyneuropathies. Muscle Nerve.
25:370–377. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jin L, Xu K, Liang Y, Du P, Wan S and
Jiang C: Effect of hyaluronic acid on cytokines and immune cells
change in patients of knee osteoarthritis. BMC Musculoskelet
Disord. 23(812)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okada K, Seki M, Yaguchi H, Sakuta K,
Mukai T, Yamada S, Oki K, Nakahara J and Suzuki S:
Polyradiculoneuropathy induced by immune checkpoint inhibitors: A
case series and review of the literature. J Neurol. 268:680–688.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Salim A, Tapia Rico G, Shaikh A and Brown
MP: A systematic review of immune checkpoint inhibitor-related
neurological adverse events and association with anti-neuronal
autoantibodies. Expert Opin Biol Ther. 21:1237–1251.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seki M, Kitano S and Suzuki S:
Neurological disorders associated with immune checkpoint
inhibitors: An association with autoantibodies. Cancer Immunol
Immunother. 71:769–775. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Won T, Song EJ, Kalinoski HM, Moslehi JJ
and Čiháková D: Autoimmune myocarditis, old dogs and new tricks.
Circ Res. 134:1767–1790. 2024.PubMed/NCBI View Article : Google Scholar
|