Introduction
Colorectal cancer is a leading cause of mortality
worldwide, the third most common cancer and the second leading
cause of cancer-associated mortality, with increasing rates of
morbidity and mortality in recent years (1,2). In
2025 cancer statistics, colorectal cancer accounted for almost
one-half (48%) of all cancer cases in men. For women, colorectal
cancer accounts for 51% of all new diagnoses (3). Postoperative intestinal complications
are critical determinants of patient prognosis. Among these
complications, anastomotic leakage (AL) is considered the most
severe due to its association with increased morbidity and
mortality rates, adverse effects on both functional and oncological
outcomes, and substantial strain on healthcare resources (4).
Systemic opioid analgesics have long been used to
manage severe trauma-related pain; however, their use is associated
with significant risks, including respiratory depression, opioid
use disorder and potentially fatal overdose (5,6). The
higher the dose and the longer the duration of opioid use, the
greater the risk of developing both physical and psychological
dependence. In recent years, multimodal analgesia has been
increasingly advocated for various types of surgery, including
gastrointestinal procedures (7).
To minimize opioid consumption and its associated adverse effects,
multimodal analgesia incorporates alternative analgesic strategies,
such as non-steroidal anti-inflammatory drugs (NSAIDs), regional
nerve blocks and other adjuncts.
NSAIDs are recommended for the management of
perioperative pain in a variety of surgical procedures, including
colorectal surgery, to reduce the perioperative use of opioid use
and accelerate postoperative recovery (8,9).
However, the use of high doses of NSAIDs has been linked to an
elevated risk of developing intestinal complications, rendering
their use in colorectal cancer surgery a subject of ongoing debate
(10,11). Notably, a large study reported that
diclofenac treatment could increase the incidence of AL following
colorectal surgery (12).
Flurbiprofen axetil, a non-selective cyclooxygenase
(COX) inhibitor, is commonly used in clinical practice as an
antipyretic and analgesic (13).
When combined with oxycodone in patient-controlled intravenous
analgesia, flurbiprofen axetil effectively reduces pain intensity,
particularly visceral pain, and may help counteract the
immunosuppressive state during the radical resection of colorectal
cancer (14). However, despite its
widespread use, the association between flurbiprofen axetil and
intestinal complications remains poorly understood. The present
study thus aimed to investigate the association between the
perioperative use of flurbiprofen axetil and the incidence of
intestinal complications following colorectal surgery through a
retrospective analysis.
Materials and methods
Ethical considerations
This single-center retrospective study was conducted
at Peking University People's Hospital (Beijing, China) on data
retrieved from patients who underwent their first radical
colorectal surgery between January 1, 2018, and March 31, 2022. The
study was performed in accordance with the principles of the
Declaration of Helsinki and ethical approval was obtained from the
Medical Ethics Committee of Peking University People's Hospital
(approval no. 2022PHB131-001).
Inclusion and exclusion criteria
Patients aged ≥18 years who underwent elective
radical colorectal resection were included in the present study.
Inclusion criteria required an American Society of
Anesthesiologists (ASA) grade (15) of I to III and the performance of
radical colorectal surgery. Exclusion criteria included patients
who underwent a second surgery or more during the same hospital
admission, those admitted to the Intensive Care Unit (ICU) prior to
surgery, those with missing or incomplete prognostic records and
those who received intraspinal analgesia due to concerns about
interference with the administration of flurbiprofen axetil. In
addition, patients with severe liver, kidney and coagulation
dysfunction, for which the use of flurbiprofen axetil is
prohibited, were excluded from the study.
Patient characteristics
Comprehensive patient data were collected, including
age, sex, body mass index, ASA classification and medical history
(for example, hypertension, diabetes, coronary heart disease, and
pulmonary, hepatic, renal, immune and coagulation disorders).
Additionally, medication history, such as use of antiplatelet or
anticoagulant therapy, preoperative chemotherapy, anti-hypertensive
drugs and steroid hormones, was documented. Laboratory evaluations
included the assessments of preoperative albumin levels, hemoglobin
(Hb) levels and sodium levels, which were recorded from the last
preoperative laboratory report prior to surgery. The cut-off point
of the Hb level was defined as Hb <80 g/l, which was the
criterion for blood transfusion in the operating room.
In addition to the patient characteristics, detailed
data on surgical and anesthetic procedures were collected. These
data included the duration of surgery, the use of laparoscopy,
regional block techniques, intraoperative fluid infusion volume,
blood loss, blood transfusion, the use of vasopressor agents, the
administration of flurbiprofen axetil, the intraoperative
consumption of opioids, the maximum diameter of the tumor, the
presence of lymph node metastasis, detection of an intravascular
tumor thrombus and postoperative transfer to the ward or ICU.
Anesthetic procedure
All patients received general anesthesia, induced
with intravenous propofol (1.5 to 2.5 mg/kg), sufentanil (0.3
µg/kg) and rocuronium (0.6 mg/kg). Anesthesia was maintained with
the inhalation of sevoflurane or desflurane, supplemented with the
continuous infusion of propofol (100-300 µg/kg/min) and
remifentanil (0.05-0.2 µg/kg/min) to maintain a bispectral index
between 40 and 60. Flurbiprofen axetil was the only NSAID used
during the perioperative period, with its use determined by the
attending anesthesiologist and surgeon. Postoperatively, the
patients were extubated in the Post-Anesthesia Care Unit or
Operating Room or transferred to the ICU with endotracheal
intubation, if necessary. Extubation criteria included the recovery
of consciousness, respiratory and circulatory stability, muscle
strength, and patients would then be returned to the ward.
Postoperative analgesia was managed (patient-controlled analgesia)
with opioids (sufentanil patient-controlled analgesia) (3 µg each,
with locking time 15 min).
Vasopressor agents such as phenylephrine (with a
dose of 50 µg) were used empirically during surgery by the
anesthesiologist, based on the initial and intraoperative blood
pressure of the patient. Between the flurbiprofen axetil group
(FBP) group and non-flurbiprofen axetil group (N-FBP), there was no
significant difference in the proportion of colon/rectal surgeries
(P=0.601) or anastomosis method (data not shown). Surgeons
determined whether to use the end-to-end or end-to-side anastomosis
method based on surgical site, with both groups operated on by the
same surgical team, eliminating potential operator bias.
Outcomes
The primary outcome was the incidence of
postoperative intestinal complications within 30 days of surgery.
These complications included AL, ileus and celiac fistula. AL was
defined according to a modified Reisinger criterion, which includes
the extraluminal presence of contrast fluid, leakage with evidence
of bowel content extravasation, intraabdominal collection, or gas
on contrast-enhanced CT scan or radiographic enema, upon
re-laparotomy or during endoscopy, requiring reintervention or
treatment (16).
Ileus was defined as bowel occlusion or paralysis
preventing the forward passage of intestinal contents, leading to
accumulation proximal to the blockage site. The diagnosis was
determined by gastrointestinal surgeons in combination with imaging
examinations and clinical symptoms, which included bloating, cramps
and the retention of stool and flatus (17). Chylous ascites was defined as the
non-infectious extravasation of milky or creamy peritoneal fluid in
the drain tubes at a volume of ≥200 ml/day and a triglyceride level
≥110 mg/dl (18,19). All complications were diagnosed by
gastrointestinal surgeons through the comprehensive evaluation of
clinical symptoms, CT scans and other imaging techniques.
Statistical analyses
Statistical analyses were performed using SPSS 26.0
software (IBM Corp.). Continuous variables are presented as the
median (interquartile range) and compared using non-parametric
tests, specifically the Mann-Whitney U test. Categorical variables
are expressed as percentages and analyzed using the χ2
test or Fisher's exact test. In the present study, the subjects
were divided into the FBP group and N-FBP group. Univariable
analysis was performed for the initial screening of risk factors.
Factors with P<0.1 in the univariable analysis and factors
associated with outcomes in clinical experience were included in
the multivariable analysis. The results are reported as odds ratios
(ORs) with 95% confidence intervals (CIs). P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient demographic and baseline
characteristics
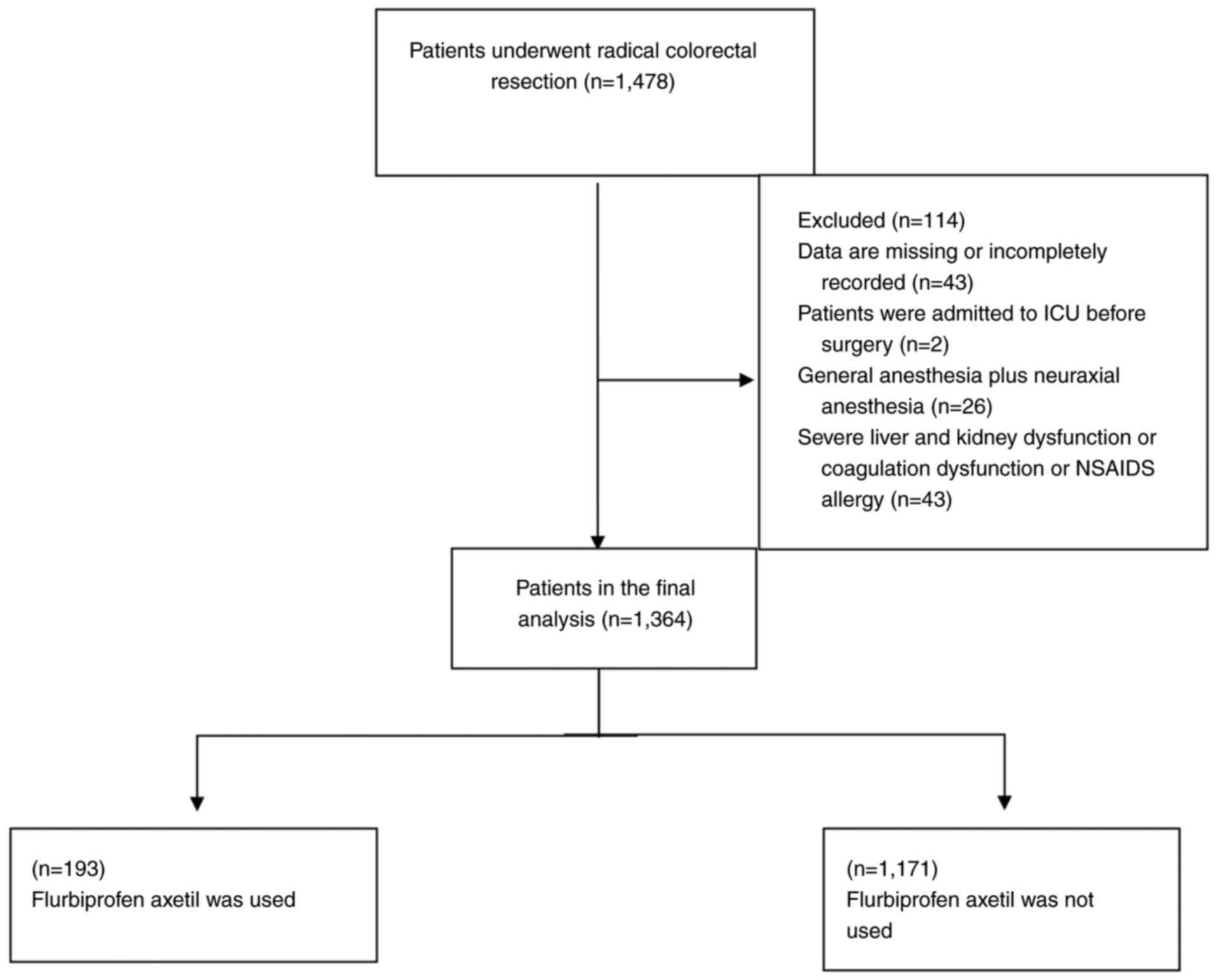
A total of 1,478 patients were included in the
study. According to the screening criteria, 114 patients were
excluded, including 43 patients with incomplete records, 2 patients
who were admitted to the ICU before the operation, 26 patients who
received general anesthesia combined with epidural anesthesia, and
43 patients with severe liver, kidney function or coagulation
dysfunction. Finally, a total of 1,364 patients were enrolled,
comprising 804 men and 560 women (Fig.
1). The patients were divided into two groups as follows: The
FBP group, consisting of 193 patients (14.1%) who received
flurbiprofen axetil perioperatively, and the N-FBP group, which
included 1,171 patients (85.9%) who did not receive the drug. Among
the 193 patients in the FBP group, 112 (58.0%) received
flurbiprofen axetil intraoperatively, 68 (35.2%) received it
postoperatively, and 13 (6.7%) were administered the drug during
both the intraoperative and postoperative periods. The administered
dose of flurbiprofen axetil was typically 50-100 mg during surgery
and/or 50 mg twice daily postoperatively. A comparison of the
baseline characteristics of the patients revealed no statistically
significant differences between the FBP and N-FBP groups, apart
from the difference in surgical method (P=0.007) (Table I).
 | Table IComparison of patient demographics and
intraoperative findings between groups. |
Table I
Comparison of patient demographics and
intraoperative findings between groups.
| Patient data | FBP (n=193) | N-FBP (n=1,171) | P-value |
|---|
| Patient
characteristics | | | |
|
Age,
yearsa | 66 (57-76) | 66 (58-74) | 0.972 |
|
Male, n
(%) | 112 (58.0) | 692 (59.1) | 0.781 |
|
BMI, n
(%) | | | 0.188 |
|
>28
kg/m2 | 27 (14.0) | 126 (10.8) | |
|
≤28
kg/m2 | 166 (86.0) | 1,045 (89.2) | |
|
ASA
classification, n (%) | | | 0.618 |
|
Grade
III | 47 (24.4) | 305 (26.0) | |
|
Less
than grade III | 146 (75.6) | 866 (74.0) | |
| Previous history, n
(%) | | | |
|
Hypertension | 77 (39.9) | 476 (40.6) | 0.844 |
|
Diabetes | 45 (23.3) | 230 (19.6) | 0.238 |
|
Coronary
heart disease | 24 (12.4) | 131 (11.2) | 0.613 |
|
Chronic lung
disease | 4 (2.1) | 18 (1.5) | 0.811 |
|
Two or more
comorbidities | 36 (18.7) | 210 (17.9) | 0.810 |
|
Albumina | 38.8
(36.2-41.6) | 39.2
(36.7-41.5) | 0.704 |
|
Hb<80
g/l | 4 (2.1) | 32 (2.7) | 0.596 |
|
Na+a | 141.2
(139.4-143) | 141.4
(139.7-143) | 0.447 |
|
Preoperative
medication, n (%) | | | |
|
Antiplatelet
or anticoagulant therapy | 24 (12.4) | 149 (12.7) | 0.911 |
|
Chemotherapy | 27 (14.0) | 175 (14.9) | 0.729 |
|
ACEIs/ARBs | 20 (10.4) | 171 (14.6) | 0.116 |
|
Statins | 22 (11.4) | 134 (11.4) | 0.986 |
|
Other
antihypertensive drugs | 49 (25.4) | 377 (32.2) | 0.059 |
|
Diuretics | 5 (2.6) | 35 (3.0) | 0.761 |
|
Steroid
hormones | 18 (9.3) | 90 (7.7) | 0.434 |
| Surgery and
anesthesia | | | |
|
Duration, n
(%) | | | 0.506 |
|
>3
h | 122 (63.2) | 769 (65.7) | |
|
≤3
h | 71 (36.8) | 402 (34.3) | |
|
Surgical
method, n (%) | | | 0.007 |
|
Laparoscopy | 143 (74.1) | 964 (82.3) | |
|
Open
abdomen | 50 (25.9) | 207 (17.7) | |
|
Intraoperative
use of vasopressors, n (%) | 137 (71.0) | 786 (67.1) | 0.288 |
|
Intraoperative
blood transfusion, n (%) | 20 (10.4) | 87 (7.4) | 0.160 |
|
Intraoperative
fluid infusion volumea | 2.6 (2.1-3.4) | 2.6 (2.1-3.1) | 0.600 |
|
Intraoperative
blood loss, n (%) | | | 0.090 |
|
<100
ml | 102 (52.8) | 695 (59.4) | |
|
≥100
ml | 91 (47.2) | 476 (40.7) | |
|
Use regional
block, n (%) | 30 (15.5) | 173 (14.8) | 0.781 |
|
Postoperative
return, n (%) | | | 0.380 |
|
The
ward | 140 (72.5) | 885 (75.6) | |
|
ICU | 53 (27.5) | 287 (24.5) | |
|
Lymph node
metastasis, n (%) | 52 (26.9) | 355 (30.3) | 0.343 |
|
Tumor emboli
in blood vessels, n (%) | 35 (18.1) | 200 (17.1) | 0.719 |
|
Maximum
diameter of tumor, n (%) | | | 0.181 |
|
<5
cm | 117 (60.6) | 768 (65.6) | |
|
≥5
cm | 76 (39.4) | 403 (34.4) | |
|
Intraoperative
opioid consumptiona | 35 (30-40) | 35 (30-45) | 0.143 |
Intestinal complications
Postoperative intestinal complications were observed
in 89 patients, accounting for 6.5% of the study population. These
complications included ileus (45 patients; 3.3%), AL (40 patients;
2.9%) and celiac fistula (9 patients; 0.7%) (Table II).
 | Table IIPostoperative intestinal-related
complications. |
Table II
Postoperative intestinal-related
complications.
| Postoperative
complications | Total, n (%) | FBP, n (%) | N-FBP, n (%) |
|---|
| Ileus | 45 (3.3) | 10 (5.2) | 35 (3.0) |
| AL | 40 (2.9) | 11 (5.7) | 29 (2.5) |
| Celiac fistula | 9 (0.7) | 0 (0.0) | 9 (0.8) |
Ileus
Ileus was identified in 45 patients (3.3% of the
study population), including 10 patients (5.2%) in the FBP group
and 35 patients (3.0%) in the N-FBP group.
AL
AL was observed in 40 patients (2.9% of the study
cohort), with 11 patients in the FBP group (5.7%) and 29 patients
(2.5%) in the N-FBP group.
Celiac fistula
A total of 9 patients (0.7%) developed celiac
fistula, and none of them had received flurbiprofen axetil during
or following surgery.
Univariate analysis identified several relevant
factors, including age (P=0.030), male sex (P=0.033), preoperative
use of steroid hormones (P=0.016), perioperative use of
flurbiprofen axetil (P=0.008), duration of surgery >3 h
(P=0.003), intraoperative use of vasopressors (P=0.040), and
intraoperative opioid consumption (P=0.038). Based on the results,
a multivariate analysis was then performed. Logistic regression was
mainly used in multivariate analysis; it identified age (OR, 1.022;
95% CI, 1.002-1.042; P=0.028), perioperative use of flurbiprofen
axetil (OR, 2.072; 95% CI, 1.223-3.508; P=0.007), duration of the
surgery >3 h (OR, 2.032; 95% CI, 1.181-3.496; P=0.010) and
intraoperative consumption of opioids (OR, 1.012; 95% CI,
1.003-1.022; P=0.009) as significant risk factors for the
development of postoperative intestinal complications (Table III).
 | Table IIIUnivariate and multivariate analysis
of intestinal complications. |
Table III
Univariate and multivariate analysis
of intestinal complications.
| | Multivariate
analysis |
|---|
| | | | 95% CI | |
|---|
| Patient data | Intestinal
complications (n=89) | No complications
(n=1,275) | Univariate
P-value | OR | Lower | Upper | P-value |
|---|
| Patient
characteristics | | | | | | | |
|
Age,
yearsa | 69 (64-76) | 66 (57-74) | 0.030 | 1.022 | 1.002 | 1.042 | 0.028 |
|
Males, n
(%) | 62 (69.7) | 742 (58.2) | 0.033 | 1.513 | 0.941 | 2.435 | 0.088 |
|
BMI, n
(%) | | | 0.733 | | | | |
|
>28
kg/m2 | 9 (10.1) | 144 (11.3) | | | | | |
|
≤28
kg/m2 | 80 (89.9) | 1,131 (88.7) | | | | | |
|
ASA, n
(%) | | | 0.796 | | | | |
|
Grade
III | 24 (27.0) | 328 (25.7) | | | | | |
|
Less
than grade III | 65 (73.0) | 947 (74.3) | | | | | |
| Previous history, n
(%) | | | | | | | |
|
Hypertension | 35 (39.3) | 518 (40.6) | 0.809 | | | | |
|
Diabetes | 18 (20.2) | 257 (20.2) | 0.988 | | | | |
|
Coronary
heart disease | 8 (9.0) | 147 (11.5) | 0.465 | | | | |
|
Chronic lung
disease | 2 (2.2) | 20 (1.6) | 0.955 | | | | |
|
Two or more
comorbidities | 15 (16.9) | 231 (18.1) | 0.764 | | | | |
|
Albumina | 39.1
(36.4-41.8) | 39.2
(36.7-41.5) | 0.776 | | | | |
|
Hb <80
g/l | 0 (0.0) | 36 (2.8) | 0.206 | | | | |
|
Na+a | 141.4
(139.2-142.8) | 141.4
(139.7-143) | 0.689 | | | | |
|
Preoperative
medication, n (%) | | | | | | | |
|
Antiplatelet
or anticoagulant therapy | 11 (12.4) | 162 (12.7) | 0.924 | | | | |
|
Chemotherapy | 18 (20.2) | 184 (14.4) | 0.137 | | | | |
|
ACEIs/ARBs | 14 (15.7) | 177 (13.9) | 0.627 | | | | |
|
Statins | 13 (14.6) | 143 (11.2) | 0.331 | | | | |
|
Other
anti-hypertensive drugs | 29 (32.6) | 397 (31.1) | 0.776 | | | | |
|
Diuretics | 4 (4.5) | 36 (2.8) | 0.563 | | | | |
|
Steroid
hormones | 13 (14.6) | 95 (7.5) | 0.016 | 1.888 | 1.000 | 3.565 | 0.050 |
| Surgery and
anesthesia, n (%) | | | | | | | |
|
Perioperative
use of flurbiprofen axetil | 21 (23.6) | 172 (13.5) | 0.008 | 2.072 | 1.223 | 3.508 | 0.007 |
|
Duration | | | 0.003 | 2.032 | 1.181 | 3.496 | 0.010 |
|
>3
h | 71 (79.8) | | | | | | |
|
≤3
h | 18 (20.2) | 455 (35.7) | | | | | |
|
Surgical
method | | | 0.829 | | | | |
|
Laparoscopy | 73 (82.0) | 1,034 (81.1) | | | | | |
|
Open
abdomen | 16 (18.0) | 241 (18.9) | | | | | |
|
Intraoperative
use of vasopressors | 69 (77.5) | 854 (67.0) | 0.040 | 1.534 | 0.907 | 2.594 | 0.111 |
|
Intraoperative
blood transfusion | 5 (5.6) | 102 (8.0) | 0.419 | | | | |
|
Intraoperative
fluid infusion volumea | 2.6 (2.3-3.1) | 2.6 (2.1-3.1) | 0.905 | | | | |
|
Intraoperative
blood loss | | | 0.657 | | | | |
|
<100
ml | 54 (60.7) | 743 (58.3) | | | | | |
|
≥100
ml | 35 (39.3) | 532 (41.7) | | | | | |
|
Use regional
block during operation | 8 (9.0) | 195 (15.3) | 0.106 | | | | |
|
Postoperative
return | | | 0.764 | | | | |
|
The
ward | 68 (76.4) | 956 (75.0) | | | | | |
|
ICU | 21 (23.6) | 319 (25.0) | | | | | |
| Surgery and
anesthesia, n (%) | | | | | | | |
|
Lymph node
metastasis | 21 (23.6) | 386 (30.3) | 0.183 | | | | |
|
Tumor emboli
in blood vessels | 17 (19.1) | 218 (17.1) | 0.629 | | | | |
|
Maximum
diameter of tumor | | | 0.328 | | | | |
|
<5
cm | 62 (69.7) | 823 (64.5) | | | | | |
|
≥5
cm | 27 (30.3) | 452 (35.5) | | | | | |
|
Intraoperative
opioid consumption | 40 (30-50) | 35 (30-45) | 0.038 | 1.012 | 1.003 | 1.022 | 0.009 |
Discussion
In the present study, all four statistically
significant factors exhibited an OR >1, indicating that an
advanced age, perioperative flurbiprofen axetil use, a prolonged
duration of surgery and increased opioid consumption were
identified as potential risk factors for postoperative intestinal
complications. The present study investigated the incidence of
intestinal complications, including AL, ileus and chylous fistula,
which affect the prognosis of patients undergoing colorectal
surgery. These findings revealed that age (P=0.028), the
perioperative use of flurbiprofen axetil (P=0.007), duration of the
surgery >3 h (P=0.010) and intraoperative opioid consumption
(P=0.009) are significant risk factors for the development of these
complications. Among these, the perioperative use of flurbiprofen
axetil is an influencing factor that has not been previously
mentioned, at least to the best of our knowledge.
NSAIDs are among the most widely used medications
globally, both via prescription and over-the-counter, for treating
fever, acute or chronic pain, and inflammatory conditions such as
rheumatic diseases. It is estimated that >30 million individuals
use NSAIDs daily (20). These
drugs inhibit COXs, key enzymes involved in the synthesis of
prostaglandins from arachidonic acid. Traditional NSAIDs inhibit
both COX-1 and COX-2 isoforms, while selective NSAIDs primarily
inhibit COX-2, and low-dose aspirin specifically inhibits
COX-1(21).
In colorectal surgery, NSAIDs are often utilized for
their opioid-sparing effects. Numerous epidemiological studies have
demonstrated that the long-term use of NSAIDs is associated with a
significant reduction in cancer incidence and can delay the
progression of malignant disease. Additionally, NSAID use has been
linked to a reduced risk of cancer-related mortality and distant
metastasis (22,23). As a non-selective COX inhibitor,
flurbiprofen axetil is commonly used in postoperative analgesia.
However, studies on the impact of NSAIDs on bowel function recovery
have indicated that these drugs may not be entirely safe for
patients undergoing radical colorectal surgery (24-26).
Specifically, the early postoperative use of NSAIDs has been
significantly associated with AL in patients undergoing colorectal
cancer surgery at high-volume tertiary care centers (27). Similar findings have been reported
in a meta-analysis (28). Another
study examined the prophylactic use of flurbiprofen to prevent
mesenteric traction syndrome during abdominal surgery. It reported
an increased risk of postoperative leakage or bleeding in patients
who did not develop mesenteric traction syndrome but received
flurbiprofen axetil prophylactically (29).
The results of the present study align with these
findings, indicating that the perioperative use of flurbiprofen
axetil is associated with an increased risk of intestinal
complications following radical colorectal surgery. Flurbiprofen
axetil may impair the intestinal healing process by suppressing
COX-2 expression, thereby increasing the risk of intestinal
complications. Another proposed mechanism is that NSAID treatment
may reduce collagen production and impair angiogenesis, further
contributing to these complications (30). However, with regard to its safety
in patients, particularly in those undergoing gastrointestinal
surgery, studies have demonstrated differential results. A
retrospective cohort study investigated the short-term
postoperative use of flurbiprofen in patients undergoing elective
gastrointestinal surgery for cancer resection. The study found no
significant increase in AL rates among patients who received
flurbiprofen compared with that in patients who did not (1.62% vs.
1.46%; P=0.70) (31). However,
only postoperative use was included in the study, and flurbiprofen
axetil is often used intraoperatively, when patients have not yet
left the operating room. Moreover, AL is not the only related
complication that raises concern.
Furthermore, the present study suggests that older
patients, a prolonged surgical duration and the increased use of
opioids during the surgery may lead to an increased risk of
developing intestinal complications following colorectal surgery.
These observations are consistent with the findings of previous
studies (32,33). An advanced age has long been
recognized as an independent risk factor for postoperative
complications across various surgical procedures. Elderly patients
typically present with more preoperative comorbidities, decreased
physiological resilience to anesthesia and surgical stress, and
slower postoperative recovery compared with younger individuals. As
a result, they are more susceptible to postoperative complications,
often related to underlying health conditions or infections
(34). In the present study, the
findings suggest that advanced age may contribute to the risk of
postoperative intestinal complications. This may possibly be due to
impaired baseline bowel function and delayed gastrointestinal
recovery in older patients.
In addition to age, a prolonged surgical duration
(>3 h) and excessive perioperative opioid use were also
identified as significant contributors to postoperative intestinal
complications. Unlike NSAIDs, whose administration generally
follows a standardized protocol, opioid consumption tends to
increase with the length and complexity of surgery. Notably,
collinearity diagnostics indicated no significant multicollinearity
between operative time and opioid use, suggesting these variables
likely exert independent effects or interact with distinct
confounding factors. Therefore, the multivariate analysis performed
herein incorporated a comprehensive set of covariates to improve
the robustness and interpretability of the model.
Surgical duration plays a crucial role in
determining postoperative outcomes. Even with intraoperative fluid
management, extended operative times often indicate greater
surgical complexity and are associated with increased fluid loss,
more extensive tissue trauma and slower postoperative recovery
(35). At the same time, opioid
use contributes to gastrointestinal side-effects, such as nausea
and vomiting, and inhibits bowel motility, thereby delaying the
return of normal gastrointestinal function (36).
It is important to acknowledge the potential
limitations of the present single-center study. Although real-world
data were used to explore the association between flurbiprofen
axetil and intestinal complications, the design of the study may
introduce certain biases. Flurbiprofen axetil was the only NSAID
included in the present study, and it was administered
intraoperatively or postoperatively in single or intermittent doses
ranging from 50 to 100 mg. The lack of a standardized dosage
regimen for all patients complicates the interpretation of whether
dosage directly affects the incidence or severity of postoperative
complications. Therefore, further research is required to provide
more robust evidence on the association between NSAIDs and
intestinal complications.
In conclusion, the present study demonstrates that
the perioperative use of flurbiprofen axetil may increase the risk
of developing postoperative intestinal complications in patients
undergoing radical colorectal resection. Further studies are
warranted in order to establish standardized protocols for the use
of NSAIDs in this patient population to minimize these risks.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Key
Research and Development Program of China (grant no.
2018YFC2001905) and the Jie-ping Wu Fund (grant no. 2016-Z-24).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YF and XT were responsible for the overall content
as the guarantors. YF proposed the research conception and designed
the study. WD drafted the manuscript and collected the data. XT
performed the data analysis and revised the manuscript. KS
contributed to the diagnosis of the complications described in the
study. WR provided advice on the study design and statistical
analysis. All authors have read and approved the final manuscript.
WD and XT confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Peking University People's Hospital (Beijing, China;
approval no. 2022PHB131-001). All methods were performed according
to the relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kijima S, Sasaki T, Nagata K, Utano K,
Lefor AT and Sugimoto H: Preoperative evaluation of colorectal
cancer using CT colonography, MRI, and PET/CT. World J
Gastroenterol. 20:16964–16975. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chadi SA, Fingerhut A, Berho M, DeMeester
SR, Fleshman JW, Hyman NH, Margolin DA, Martz JE, McLemore EC,
Molena D, et al: Emerging trends in the etiology, prevention, and
treatment of gastrointestinal anastomotic leakage. J Gastrointest
Surg. 20:2035–2051. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cunningham DJ, LaRose M, Zhang G, Patel P,
Paniagua A, Gadsden J and Gage MJ: Regional anesthesia associated
with decreased inpatient and outpatient opioid demand in tibial
plateau fracture surgery. Anesth Analg. 134:1072–1081.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abdel Shaheed C, Hayes C, Maher CG,
Ballantyne JC, Underwood M, McLachlan AJ, Martin JH, Narayan SW and
Sidhom MA: Opioid analgesics for nociceptive cancer pain: A
comprehensive review. CA Cancer J Clin. 74:286–313. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Parfait M, Rohrs E, Joussellin V, Mayaux
J, Decavèle M, Reynolds S, Similowski T, Demoule A and Dres M: An
initial investigation of diaphragm neurostimulation in patients
with acute respiratory distress syndrome. Anesthesiology.
140:483–494. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Halvey EJ, Haslam N and Mariano ER:
Non-steroidal anti-inflammatory drugs in the perioperative period.
BJA Educ. 23:440–447. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gustafsson UO, Scott MJ, Hubner M, Nygren
J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG,
Soop M, et al: Guidelines for perioperative care in elective
colorectal surgery: Enhanced recovery after surgery
(ERAS®) society recommendations: 2018. World J Surg.
43:659–695. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rutegård J and Rutegård M: Non-steroidal
anti-inflammatory drugs in colorectal surgery: A risk factor for
anastomotic complications? World J Gastrointest Surg. 4:278–280.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang H, Liu T, Li Q, Cui R, Fan X, Tong Y,
Ma S, Liu C and Zhang J: NSAID treatment before and on the early
onset of acute kidney injury had an opposite effect on the outcome
of patients With AKI. Front Pharmacol. 13(843210)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Klein M, Gögenur I and Rosenberg J:
Postoperative use of non-steroidal anti-inflammatory drugs in
patients with anastomotic leakage requiring reoperation after
colorectal resection: Cohort study based on prospective data. BMJ.
345(e6166)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumpulainen E, Välitalo P, Kokki M,
Lehtonen M, Hooker A, Ranta VP and Kokki H: Plasma and
cerebrospinal fluid pharmacokinetics of flurbiprofen in children.
Br J Clin Pharmacol. 70:557–566. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wan Z, Chu C, Zhou R and Que B: Effects of
oxycodone combined with flurbiprofen axetil on postoperative
analgesia and immune function in patients undergoing radical
resection of colorectal cancer. Clin Pharmacol Drug Dev.
10:251–259. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Keats AS: The ASA classification of
physical status-a recapitulation. Anesthesiology. 49:233–236.
1978.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reisinger KW, Poeze M, Hulsewé KW, van
Acker BA, van Bijnen AA, Hoofwijk AG, Stoot JH and Derikx JP:
Accurate prediction of anastomotic leakage after colorectal surgery
using plasma markers for intestinal damage and inflammation. J Am
Coll Surg. 219:744–751. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vilz TO, Stoffels B, Strassburg C, Schild
HH and Kalff JC: Ileus in adults. Dtsch Arztebl Int. 114:508–518.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Besselink MG, van Rijssen LB, Bassi C,
Dervenis C, Montorsi M, Adham M, Asbun HJ, Bockhorn M, Strobel O,
Büchler MW, et al: Definition and classification of chyle leak
after pancreatic operation: A consensus statement by the
international study group on pancreatic surgery. Surgery.
161:365–372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baek SJ, Kim SH, Kwak JM and Kim J:
Incidence and risk factors of chylous ascites after colorectal
cancer surgery. Am J Surg. 206:555–559. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Singh G and Triadafilopoulos G:
Epidemiology of NSAID induced gastrointestinal complications. J
Rheumatol Suppl. 56:18–24. 1999.PubMed/NCBI
|
|
21
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: Structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gurpinar E, Grizzle WE and Piazza GA:
NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res.
20:1104–1113. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schack A, Fransgaard T, Klein MF and
Gögenur I: Perioperative use of nonsteroidal anti-inflammatory
drugs decreases the risk of recurrence of cancer after colorectal
resection: A cohort study based on prospective data. Ann Surg
Oncol. 26:3826–3837. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
García-Rayado G, Navarro M and Lanas A:
NSAID induced gastrointestinal damage and designing GI-sparing
NSAIDs. Expert Rev Clin Pharmacol. 11:1031–1043. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hakkarainen TW, Steele SR, Bastaworous A,
Dellinger EP, Farrokhi E, Farjah F, Florence M, Helton S, Horton M,
Pietro M, et al: Nonsteroidal anti-inflammatory drugs and the risk
for anastomotic failure: A report from Washington State's surgical
care and outcomes assessment program (SCOAP). JAMA Surg.
150:223–228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haddad NN, Bruns BR, Enniss TM, Turay D,
Sakran JV, Fathalizadeh A, Arnold K, Murry JS, Carrick MM,
Hernandez MC, et al: Perioperative use of nonsteroidal
anti-inflammatory drugs and the risk of anastomotic failure in
emergency general surgery. J Trauma Acute Care Surg. 83:657–661.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ju JW, Lee HJ, Kim MJ, Ryoo SB, Kim WH,
Jeong SY, Park KJ and Park JW: Postoperative NSAIDs use and the
risk of anastomotic leakage after restorative resection for
colorectal cancer. Asian J Surg. 46:4749–4754. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bhangu A, Singh P, Fitzgerald JE, Slesser
A and Tekkis P: Postoperative nonsteroidal anti-inflammatory drugs
and risk of anastomotic leak: Meta-analysis of clinical and
experimental studies. World J Surg. 38:2247–2257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Suzuki M, Shibata J, Mochizuki T and Bito
H: Contribution of prophylactic administration of flurbiprofen for
mesenteric traction syndrome to postoperative leakage or bleeding
in gastrointestinal surgery: A retrospective observational study.
Langenbecks Arch Surg. 408(337)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Klein M, Krarup PM, Kongsbak MB, Agren MS,
Gögenur I, Jorgensen LN and Rosenberg J: Effect of postoperative
diclofenac on anastomotic healing, skin wounds and subcutaneous
collagen accumulation: A randomized, blinded, placebo-controlled,
experimental study. Eur Surg Res. 48:73–78. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nie H, Hao Y, Feng X, Ma L, Ma Y, Zhang Z,
Han X, Zhang JZ, Zhang P, Zhao Q and Dong H: Postoperative
short-term use of the nonsteroidal anti-inflammatory drug
flurbiprofen did not increase the anastomotic leakage rate in
patients undergoing elective gastrointestinal surgery-a
retrospective cohort study. Perioper Med (Lond).
11(38)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu XR, Liu F, Li ZW, Liu XY, Zhang W and
Peng D: The risk of postoperative complications is higher in stage
I-III colorectal cancer patients with previous abdominal surgery: A
propensity score matching analysis. Clin Transl Oncol.
25:3471–3478. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kang T, Kim HO, Kim H, Chun HK, Han WK and
Jung KU: Age over 80 is a possible risk factor for postoperative
morbidity after a laparoscopic resection of colorectal cancer. Ann
Coloproctol. 31:228–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Johnson AG, Quinn DI and Day RO:
Non-steroidal anti-inflammatory drugs. Med J Aust. 163:155–158.
1995.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Scott CF Jr: Length of operation and
morbidity: Is there a relationships? Plast Reconstr Surg.
69:1017–1021. 1982.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Akbarali HI and Dewey WL: Gastrointestinal
motility, dysbiosis and opioid-induced tolerance: Is there a link?
Nat Rev Gastroenterol Hepatol. 16:323–324. 2019.PubMed/NCBI View Article : Google Scholar
|