Introduction
Acute kidney injury (AKI) is a common clinical
condition that is characterized by a rapid decline in renal
function (1). AKI can lead to
incomplete renal repair, persistent chronic inflammation and
progressive fibrosis, all of which can lead to chronic kidney
disease and end-stage renal disease (2). Ischemia-reperfusion (I/R) is a major
pathogenic factor for AKI (3).
However, the mechanism of tissue damage and repair during AKI is
still unclear and the immune inflammatory response mediating tissue
damage and fibrosis has become an interest in research worldwide
(4,5).
The bioactive alkaloid tetramethylpyrazine (TMP) is
found in the Chinese herbal medicine Chuanxiong and has been proven
to possess several pharmacological properties in previous studies
(6). TMP has been shown to have
physiological properties, including antioxidative,
anti-inflammatory, anti-calcium antagonism and anti-apoptotic
effects. Furthermore TMP has been implicated in autophagy
regulation, vasodilation, angiogenesis regulation, mitochondrial
damage suppression, endothelial protection, reduction of the
proliferation and migration of vascular smooth muscle cells, and
neuroprotection (6-8).
Clinically, TMP is used to treat cardiovascular, cerebrovascular
and chronic kidney diseases due to its ability to enhance blood
flow and microcirculation (9-11);
however, its therapeutic molecular target is not clear. Previous
studies from our group have shown that TMP ameliorates AKI to a
certain extent by inducing autophagy, which can downregulate
nucleotide-binding oligomerization domain-containing protein
2-mediated inflammation; TMP may also attenuate AKI by inhibiting
nucleotide-oligomerization domain-like receptor 3 inflammasome
(12,13). Furthermore, previous studies have
demonstrated that TMP can inhibit the proliferation and
infiltration of cancer cells by blocking the Wnt/β-catenin
signaling pathway (14,15).
The Wnt/β-catenin signaling pathway is a signaling
pathway, that has been relatively well preserved throughout
evolution, which is essential for the growth and development of the
body (16). The abnormal
activation of this signaling pathway can lead to a variety of
diseases, including kidney and cardiovascular diseases and tumors
(17,18). Activation of the Wnt/β-catenin
signaling pathway has been shown to be involved in kidney injury
caused by AKI, glomerular disease, diabetic nephropathy, renal
fibrosis and cystic kidney disease (19-21).
In AKI, the activation of the Wnt/β-catenin signaling pathway has a
dual effect. Moderate activation of the Wnt/β-catenin signaling
pathway is beneficial to the regeneration of renal tubular
epithelial cells; however, excessive activation can promote the
progression of renal injury to chronic fibrosis (22,23).
The Wnt/β-catenin signaling pathway is controlled by
a number of proteins, including dickkopf-1 (DKK1) (16). Secretion of the glycoprotein DKK1
inhibits Wnt/β-catenin signaling pathway activity by binding to
cell membrane receptors, such as low-density lipoprotein
receptor-related protein 5/6 (LRP5/6) and Kremen 1, and mediates
endocytosis (24,25). Chronic kidney disease is associated
with DKK1 as it promotes accumulation of mesangial cell matrix and
renal dysfunction in response to hyperglycemia (26). In lupus nephritis, increased renal
Wnt activity is accompanied by elevated DKK1 levels, and DKK1
elevation contributes to renal injury by promoting apoptosis and
increasing the extracellular immunogenic chromatin load (27). Furthermore, DKK1 notably inhibits
fibrosis in chronic kidney injury, suggesting that this protein
might be a therapeutic target for fibrosis (28,29).
However, the role of DKK1 in AKI has not yet been discovered.
To the best of our knowledge, the present study was
the first to focus on the regulatory effect of the traditional
Chinese medicine derivative TMP on DKK1 and the Wnt/β-catenin
signaling pathway, and to investigate its effect on preventing AKI.
In the present study, a rat model was used to determine the
regulatory effect of TMP on DKK1 and the Wnt/β-catenin signaling
pathway using cell biology and molecular immunology techniques,
with the aim of revealing the mechanism of TMP in alleviating AKI
and promoting the regeneration and recovery of renal cells.
Materials and methods
Animal studies
Animal experiments were conducted in compliance with
the guidelines outlined in the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (30) and was approved by the Institutional
Animal Care and Use Committee of Binzhou Medical University
(Yantai, China; approval no. 2020-07). A total of 18 male
Sprague-Dawley (SD) rats (weight, 260-300 g; age, 8 weeks) were
purchased from the Animal Experimental Center of Shandong
University (Jinan, China). All animals were housed at a constant
temperature of 24˚C with a humidity of 55%, under a 12-h light/dark
cycle, and had unrestricted access to water and food. I/R models
were established using rats anesthetized with pentobarbital sodium
(40 mg/kg body weight) intraperitoneally (31). Subsequently, the I/R injury model
was established in SD rats by clamping the bilateral renal pedicle
with non-traumatic microvascular clamps as previously described
(12); serum creatinine (SCr),
blood urea nitrogen (BUN) levels and morphological examinations
were used to assess the success of the model establishment. The
rats were randomly divided into the following groups (n=6/group):
Sham, kidney I/R and kidney I/R with TMP treatment (I/R + TMP). The
sample sizes for the study were determined based on the resource
equation method (32). In the sham
group, the renal artery was isolated without ischemic treatment. In
the I/R + TMP group, TMP hydrochloride (40 mg/kg body weight;
Harbin Medisan Pharmaceutical Co., Ltd.) was administered by
intraperitoneal injection immediately following reperfusion, at 6 h
intervals (12,13). Following completion of the
treatment for 24 h, all rats were deeply anesthetized by
intraperitoneal injection of pentobarbital sodium (150 mg/kg) and
subsequently sacrificed by cervical dislocation.
The following criteria were used to define the
humane endpoints for the present study: i) Lack of movement or
unresponsiveness to gentle stimuli; ii) respiratory distress
(typical symptoms include drooling from the mouth or nose and/or
cyanosis); iii) diarrhea or urinary incontinence; iv) weight loss
of >20% compared with the pre-experiment body weight; v)
inability to eat or drink; vi) persistent seizures or stereotyped
behavior (in the absence of external stimuli, rats exhibited
spontaneous rotation, digging, jumping and grooming behaviors); and
vii) skin lesions covering >30% of the body or signs of purulent
infection. The surgical procedure was uneventful, with no rats
sacrificed early due to reaching humane endpoints.
Death was verified by loss of corneal reflex, a lack
of response to a firm toe pinch, and observation of cardiac and
respiratory arrest. Subsequently, kidney tissues and blood samples
from the heart were collected. Parts of the kidney tissues were
stored at -80˚C for protein detection, whereas others were fixed in
4% paraformaldehyde at 4˚C for 24 h, embedded in paraffin and
sectioned at 4-µm thickness for morphological detection. The blood
samples were used for the detection of the biochemical indicators
of renal injury.
Morphological examinations
Hematoxylin and eosin (H&E) staining was
conducted on paraffin-embedded 4-µm kidney tissue sections.
Sections were dewaxed in xylene, stained with hematoxylin for 5 min
at room temperature, and then placed in distilled water for 10-30
sec. Following this, the tissue sections were incubated in 1%
hydrochloric acid alcohol for 3 sec then washed with running water
for 10 min. Finally, the sections were stained with eosin for 10
min at room temperature then dehydrated and sealed with neutral
gum. Tubular injury of the cortex or the outer medulla was based on
epithelial necrosis, vacuolization or tubular dilatation and was
scored as follows: 0, none; 1, 1-10%; 2, 11-25%; 3, 26-45%; 4,
46-75%; 5, >75%. For each section, ≥10 fields were examined
under magnification x200. The histological scoring was performed
blindly. Visualization was performed using a Leica DM 6000 B light
microscope (Leica Microsystems, Inc.).
Renal function test
Blood was collected from the heart of the animals
after euthanasia and the samples were allowed to stand for 30 min.
Subsequently, the samples were centrifuged at 4˚C at 1,000 x g for
10 min and the serum was separated. An automatic biochemical
analyzer was used to detect SCr and BUN.
Immunohistochemical (IHC)
staining
IHC staining was performed on paraffin-embedded 4-µm
kidney tissue sections. Sections were dewaxed and placed in citrate
buffer (0.01 M, pH 6.0) for antigen retrieval; briefly, samples
were placed in a microwave, boiled on high heat for 4 min then
allowed to stand for 5 min, this was followed by a further 1 min at
medium heat then allowed to stand for 5 min. Subsequently, the
samples were cooled to room temperature and washed three times with
PBS (5 min each). The sections were then incubated with 3%
H2O2 at room temperature for 20 min to remove
endogenous peroxidase activity and washed three times with PBS (5
min each). The sections were blocked with 5% normal goat serum
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 1 h and incubated with the following primary
antibodies at 4˚C overnight: DKK1 (cat. no. ab109416; 1:250;
Abcam), Wnt1 (cat. no. ab15251; 1:100; Abcam), β-catenin (cat. no.
WL0962a; 1:200; Wanleibio Co., Ltd.), caspase-3 (cat. no. WL04004;
1:200; Wanleibio Co., Ltd.), Bax (cat. no. ER0907; 1:200; HUABIO)
and Bcl-2 (cat. no. ET1702-53; 1:200; HUABIO). The following day,
the slides were rewarmed for 45 min at room temperature and washed
three times with PBS (3 min each) to remove unbound primary
antibodies. The sections were then incubated with a horseradish
peroxidase (HRP)-conjugated goat anti-rabbit or mouse secondary
antibody (undiluted; cat. no. PV-6000; OriGene Technologies, Inc.)
for 2 h at room temperature, and washed three times with PBS (3 min
each) to wash off the unbound secondary antibody. Subsequently,
3,3'-diaminobenzidine staining was performed at room temperature in
accordance with the kit's instructions (cat. no. ZLI-9018; OriGene
Technologies, Inc.). Tap water was used to terminate the reaction
and hematoxylin staining was used to stain the cellular nuclei at
room temperature for 5 min followed by rinsing in tap water.
Sections were differentiated in 1% hydrochloric acid-alcohol for
1-3 sec, rinsed in tap water for 10 min to achieve bluing,
dehydrated and cleared in xylene. The sections were sealed with
neutral gum and visualized using a Leica DM 6000 B light microscope
(Leica Microsystems, Inc.). ImageJ software (version 1.8.0;
National Institutes of Health) was used for
semi-quantification.
TUNEL staining
TUNEL staining was carried out on paraffin-embedded
4-µm kidney tissue sections. According to the instructions of the
TUNEL kit (Roche Diagnostics), the sections were dewaxed, 20 µg/ml
DNase-free proteinase K was added dropwise, and sections were
incubated at 37˚C for 15-30 min, and then washed with PBS three
times. Subsequently the samples were incubated with 3%
H2O2 at room temperature for 20 min to
inactivate the endogenous peroxidase activity, followed by washing
with PBS three times. Following this, 50 µl TdT and 450 µl
fluorescein-labeled dUTP was mixed as biotin labeling solution and
added to the sections at 37˚C for 1 h in a dark box. Cellular
nuclei were stained with 4',6-diamidino-2-phenylindole (cat. no.
C1006; Beyotime Institute of Biotechnology) at room temperature for
5 min. After washing with PBS, slides were incubated with
anti-fluorescence quenching reagent to seal the slides. A
fluorescence microscope was used to observe the samples.
Western blot analysis
The renal cortex was homogenized in ice-cold
radioimmunoprecipitation lysis buffer containing 1 mM
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology). The total amount of protein extracted was
determined with the BCA Protein Assay kit (cat. no. P0010; Beyotime
Institute of Biotechnology). In each lane, 40 µg extracted protein
was loaded, separated by 8-10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis, and transferred onto
polyvinylidene difluoride membranes (MilliporeSigma). The membranes
were blocked using 5% skimmed milk at room temperature for 1 h.
Subsequently, the membranes were incubated with primary antibodies
overnight at 4˚C, washed and incubated with HRP-conjugated goat
anti-mouse IgG (cat. no. SA00001-1; 1:5,000; ProteinTech Group,
Inc.) and HRP-conjugated goat anti-rabbit IgG (cat. no. SA00001-2;
1:5,000; ProteinTech Group, Inc.) at room temperature for 2 h. The
protein bands were visualized using an enhanced chemiluminescence
detection system (Tanon Science and Technology Co., Ltd.) and
Millipore Immobilon ECL (MilliporeSigma). Western blot bands were
semi-quantified using ImageJ software (version 1.8.0; National
Institutes of Health). The following primary antibodies were used
in the present study: DKK1 (cat. no. ab109416; 1:1,000; Abcam),
Wnt1 (cat. no. ab15251; 1:1,000; Abcam), β-catenin (cat. no.
WL0962a; 1:1,000; Wanleibio Co., Ltd.), caspase-3 (cat. no.
WL04004; 1:1,000; Wanleibio Co., Ltd.), cleaved caspase-3 (cat. no.
WL01992; 1:1,000; Wanleibio Co., Ltd.), Bcl-2 (cat. no. ET1603-11;
1:1,000; HUABIO) and β-actin (cat. no. 66009-1-Ig; 1:5,000;
ProteinTech Group, Inc.).
Statistical analysis
The renal tubular pathological injury score data are
presented as the median (IQR), and were analyzed using
Kruskal-Wallis test and Dunn's post hoc test. The other data are
presented as the mean ± SEM, and the significance of differences in
among the groups was examined by one-way ANOVA followed by Duncan's
or Tukey's post hoc test. GraphPad Prism 9 (Dotmatics) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
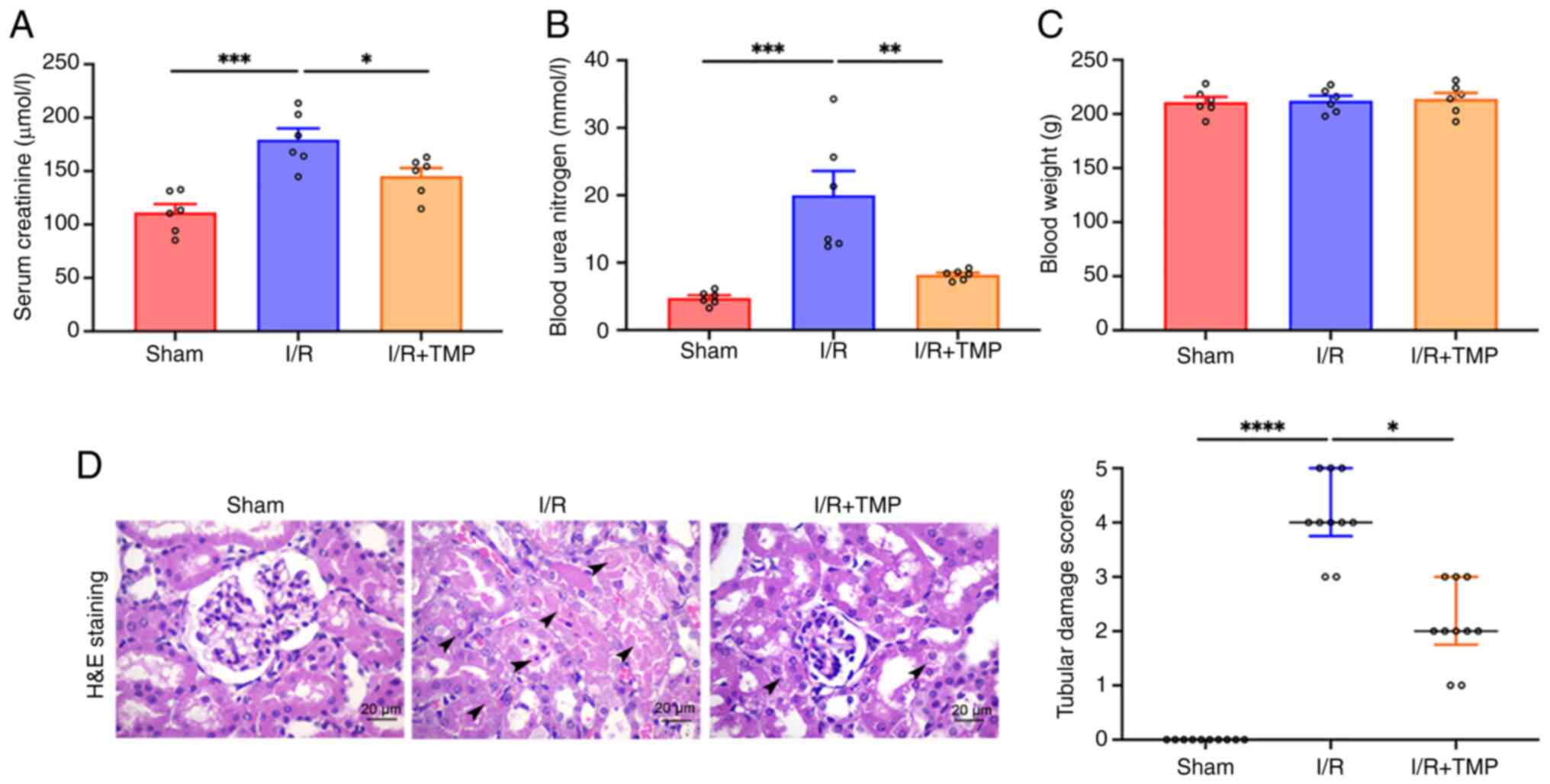
TMP protects against AKI by
alleviating renal tubular pathological injury and improving renal
function following I/R in a rat model
The results of SCr and BUN quantification indicated
that compared with in the sham group, the SCr and BUN levels of the
rats in the I/R group were significantly increased (Fig. 1A and B). By contrast, the SCr and BUN levels of
the rats in the I/R + TMP group were significantly decreased
compared with the levels in the I/R group, indicating that TMP
treatment could reduce I/R kidney injury. No significant difference
was noted in the body weight of rats in the I/R and the I/R + TMP
groups compared with that in the sham group (Fig. 1C). H&E staining indicated that
the glomeruli and renal tubules in the sham operation group were
intact without apparent morphological abnormalities, whereas
H&E staining in the I/R group indicated swelling, vacuolar
degeneration, necrosis and shedding, and cast formation in tubular
lumen (Fig. 1D). In the I/R + TMP
group, the aforementioned pathological changes were alleviated, and
the course of the disease was decelerated. Double-blind analysis of
renal tubular pathological injury scores by pathologists revealed
that TMP could significantly alleviate I/R renal injury in
rats.
TMP promotes the expression of DKK1 in
the renal tissues of rats following I/R
Western blot analysis revealed that the protein
expression levels of DKK1 in the I/R group were significantly lower
than those in the sham group, whereas they were significantly
increased following treatment with TMP compared with those in the
I/R group (Fig. 2A). Furthermore,
IHC analysis indicated that the expression levels of DKK1 in the
I/R group were significantly lower than those in the sham group,
whereas the expression levels of DKK1 were significantly increased
following treatment with TMP compared with the expression levels in
the I/R group (Fig. 2B). The
results of the IHC analysis were consistent with the results of the
western blot analysis. TMP significantly increased the expression
levels of DKK1 in the kidney tissues of rats following I/R.
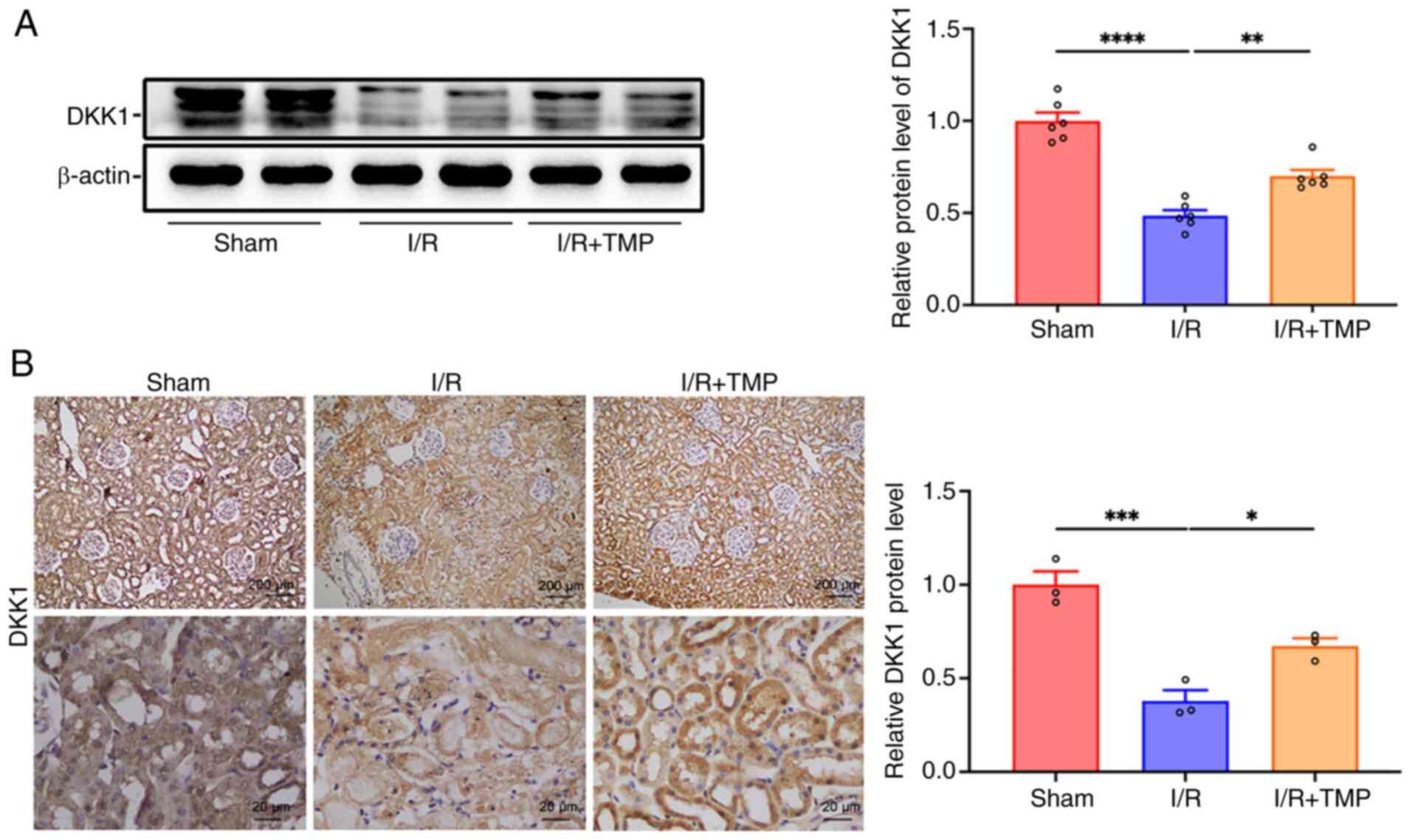 | Figure 2TMP promotes the expression of DKK1
in the renal tissue of rats following I/R. (A) Representative
western blots and semi-quantified data showing the expression of
DKK1 in the renal tissue of rats in the sham, I/R and I/R + TMP
groups. n=6. (B) Representative IHC images of DKK1 in the renal
tissue of rats in the sham, I/R and I/R + TMP groups, and
semi-quantification. n=3. IHC images: Top row, scale bars, 200 µm,
magnification, x40; bottom row, scale bars, 20 µm, magnification,
x400. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. I/R,
ischemia-reperfusion; TMP, tetramethylpyrazine; DKK1, dickkopf-1;
IHC, immunohistochemistry. |
TMP activates the Wnt/β-catenin
signaling pathway in the kidney tissues of rats following I/R
Western blot analysis of Wnt1 expression in the
renal tissues of each group indicated that compared with in the
sham group, the protein expression levels of Wnt1 were
significantly decreased in the renal tissues of the I/R group;
however, following treatment with TMP, the protein expression
levels of Wnt1 in the renal tissues of the I/R + TMP group were
significantly increased compared with those in the I/R group
(Fig. 3A). The results of the IHC
analysis indicated that the expression levels of Wnt1 in the renal
tissues were significantly decreased following renal I/R injury in
rats, whereas the expression levels of Wnt1 were significantly
increased following treatment with TMP compared with those in the
I/R group (Fig. 3B). Furthermore,
western blot analysis indicated that the protein expression levels
of β-catenin were significantly lower in the renal tissues of the
I/R group compared with those in the sham group; by contrast, the
protein expression levels of β-catenin were significantly increased
in the renal tissues following TMP treatment compared with those in
the I/R group (Fig. 3C). In
addition, IHC analysis indicated that the expression levels of
β-catenin were significantly reduced in the renal tissues following
I/R injury in rats; however, the protein expression levels of
β-catenin were significantly increased following TMP treatment
compared with those in the I/R group (Fig. 3D). Taken together, these results
suggested that TMP activated the Wnt/β-catenin signaling
pathway.
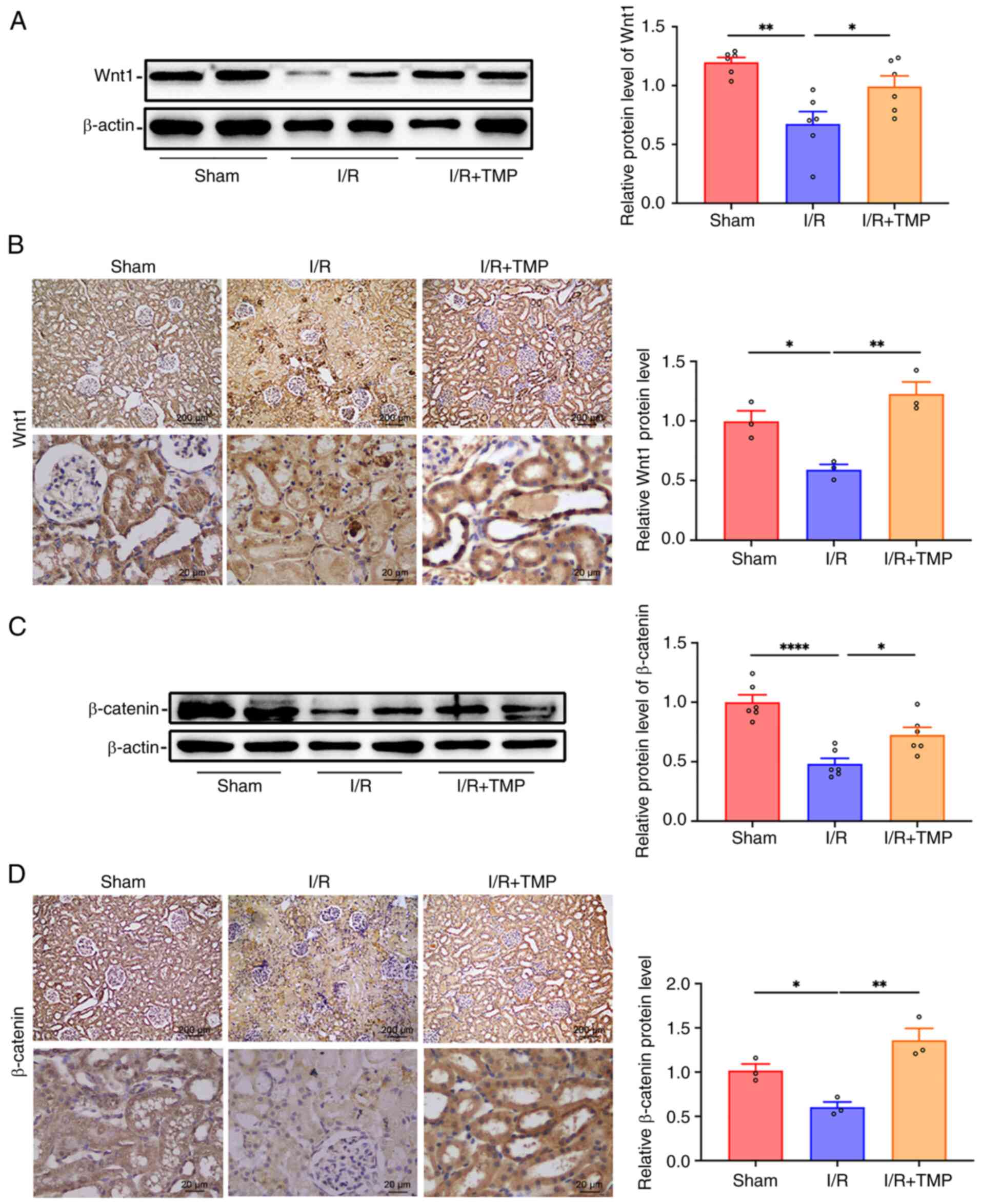 | Figure 3TMP activates the Wnt/β-catenin
signaling pathway in the kidney of rats following I/R. (A)
Representative western blots and semi-quantified protein levels
showing the expression levels of Wnt1 in the renal tissue of rats
in different groups. n=6. (B) Representative IHC images and protein
semi-quantification of Wnt1 in the renal tissue of rats in the
sham, I/R and I/R + TMP groups. n=3. (C) Representative western
blots and semi-quantified protein levels showing the expression
levels of β-catenin in the renal tissue of rats in different
groups. n=6. (D) Representative IHC images and protein
semi-quantification of β-catenin in the renal tissue of rats in the
sham, I/R and I/R + TMP groups. n=3. IHC images: Top row, scale
bars, 200 µm, magnification, x40; bottom row, scale bars, 20 µm,
magnification, x400. *P<0.05, **P<0.01,
****P<0.0001. I/R, ischemia-reperfusion; TMP,
tetramethylpyrazine; IHC, immunohistochemistry. |
TMP ameliorates renal cell apoptosis
following I/R in rats
TUNEL staining indicated that, compared with that in
the sham group, the number of apoptotic renal tubular epithelial
cells in the I/R group was significantly increased, whereas the
number of apoptotic renal tubular epithelial cells was
significantly decreased following treatment with TMP compared with
that in the I/R group (Fig. 4A).
In addition, the apoptotic proteins caspase-3 and Bax, as well as
the anti-apoptotic protein Bcl-2, were detected in the renal
tissues of rats (Fig. 4B-F). IHC
analysis indicated that rats in the I/R group expressed
significantly higher levels of the apoptotic protein Bax in renal
tissues as compared with rats in the sham group, whereas Bax
expression levels decreased significantly following treatment with
TMP compared with in the I/R group (Fig. 4B). IHC analysis also indicated that
rats in the I/R group expressed significantly higher levels of the
apoptotic protein caspase-3 in renal tissues as compared with rats
in the sham group, whereas caspase-3 expression levels decreased
significantly following treatment with TMP compared with in the I/R
group (Fig. 4C). Western blot
analysis indicated that the relative ratio of cleaved
caspase-3/caspase-3 in the renal tissues of the I/R group was
significantly higher than that in the sham group, which was
significantly decreased in the renal tissues following TMP
treatment compared with in the I/R group (Fig. 4D). IHC analysis indicated that
compared with those in the sham group, the expression levels of the
anti-apoptotic protein Bcl-2 were significantly increased in the
renal tissues of the I/R group (Fig.
4E). However, the expression levels of Bcl-2 were further
increased following treatment with TMP compared with in the I/R
group. Western blot analysis also indicated that the protein
expression levels of Bcl-2 in the renal tissues of the I/R group
were significantly higher than those in the sham group, and the
protein expression levels of Bcl-2 were further increased in the
renal tissues following TMP treatment compared with those in the
I/R group (Fig. 4F). These results
suggested that TMP could alleviate the apoptosis of renal tissue
following I/R by inhibiting the cleavage of caspase-3 and the
expression of Bax, and increasing the expression of the
anti-apoptotic protein Bcl-2.
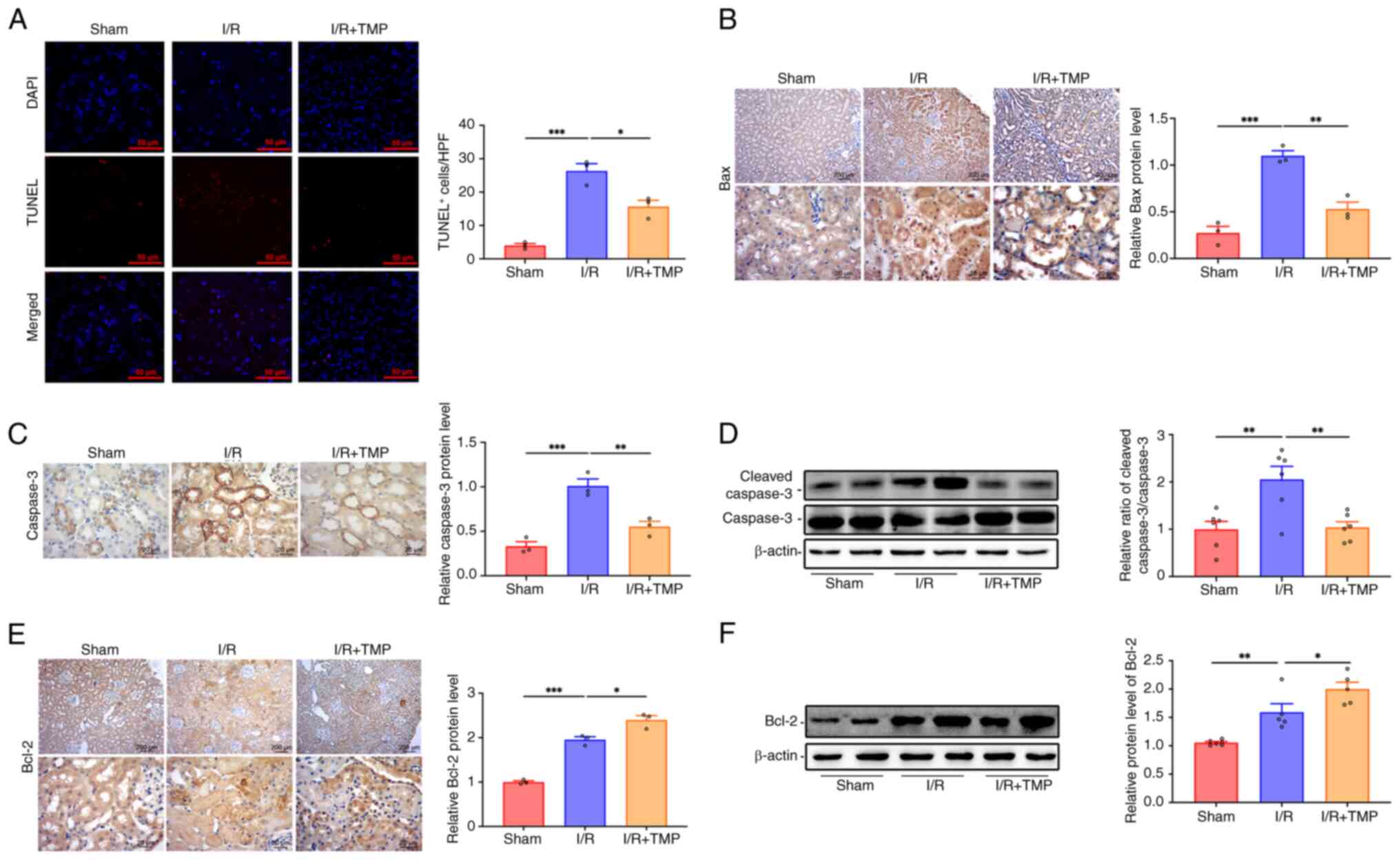 | Figure 4TMP ameliorates renal cell apoptosis
after I/R in rats. (A) TUNEL staining showing the renal cell
apoptosis levels in different groups. Scale bars, 50 µm. n=3.
Representative IHC images and protein semi-quantification of (B)
Bax and (C) caspase-3 in renal tissue of rats in different groups.
n=3. (D) Relative ratio of cleaved caspase-3/caspase-3 determined
by western blotting. n=6. (E) Representative images and protein
semi-quantification of Bcl-2 in the renal tissue of rats in
different groups. n=3. (F) Representative western blots and
semi-quantified protein levels showing the expression levels of
Bcl-2 in the renal tissue of rats in different groups. n=6. IHC
images: Top row, scale bars, 200 µm, magnification, x40; bottom
row, scale bars, 20 µm, magnification, x400. *P<0.05,
**P<0.01, ***P<0.001. I/R,
ischemia-reperfusion; TMP, tetramethylpyrazine; IHC,
immunohistochemistry. |
Discussion
The pathological mechanism of AKI is complex,
including inflammation, ischemia and nephrotoxic injury (33). To the best of our knowledge, the
present study was the first to discover the regulatory effect of
TMP on DKK1 and the Wnt/β-catenin signaling pathway in AKI renal
tissues, revealing that TMP attenuated AKI by activating the
Wnt/β-catenin signaling independent of DKK1. AKI is a group of
clinical syndromes in which renal function suddenly declines
sharply in a short period of time, and may result in chronic kidney
disease, end-stage renal disease or death (1). Notably, AKI is a common clinical
critical illness; it affects 10-15% of hospitalized patients, and
>50% of patients in the intensive care unit (34). The pathological changes of AKI are
mainly apparent in the renal tubular morphology, including loss of
renal tubular epithelial cell polarity, swelling, vacuolar
degeneration, necrosis, brush border shedding, cast formation in
the tubular lumen and inflammatory cell infiltration in the
interstitium. The main pathological mechanisms of AKI include
generation of free radicals, intracellular calcium overload
(35), inflammatory reactions and
apoptosis (36).
The Wnt/β-catenin signaling pathway is a common
developmental signaling pathway that plays a crucial role in
embryonic development, morphogenesis and organogenesis, tissue
regeneration, and the pathogenesis of various diseases (17). A number of studies have indicated
that the Wnt/β-catenin signaling pathway is involved in multiple
organ I/R injuries, such as cardiac, cerebral and renal I/R
injuries (37-40).
Studies have shown that in AKI transient activation of the
Wnt/β-catenin signaling pathway is beneficial in reducing the
development of this disease, and promoting kidney repair and
regeneration, whereas sustained activation of the Wnt/β-catenin
signaling pathway promotes the progression of AKI to chronic kidney
disease (41). It is currently
considered that the Wnt/β-catenin signaling pathway promotes renal
fibrosis in chronic kidney disease (18,42).
Inhibition of the Wnt/β-catenin signaling pathway may maintain the
integrity of podocytes, reduce proteinuria and attenuate kidney
injury (43). Wnt/β-catenin
signaling pathway serves as a regulatory network that promotes or
inhibits apoptosis/autophagy under certain circumstances (44-46).
Therefore, activation of the Wnt/β-catenin signaling pathway could
attenuate AKI caused by I/R (47).
Recent studies have demonstrated that TMP can block the
Wnt/β-catenin signaling pathway, and inhibit the proliferation and
infiltration of cancer cells (14,15).
The findings of the present study differ from those
of previous studies (19,48) and indicated that the expression
levels of Wnt1 and β-catenin were significantly decreased in rats
following I/R, while the expression levels of Wnt1 and β-catenin
were significantly increased following treatment with TMP. Western
blotting results showed that Wnt1 and β-catenin expression in the
I/R + TMP group exceeded the expression observed in the I/R group,
which was consistent with the results of IHC analysis. The IHC
analysis results showed increased protein expression in the I/R +
TMP group to a greater extent than that in the western blotting
results; however, there was no direct comparison between western
blotting and IHC analysis results. This may be due to the fact
that, compared with western blotting, IHC analysis may produce a
certain non-specific staining. In addition, following I/R, the
necrotic renal tissue had a higher background of non-specific
staining than non-necrotic tissue, which may have further
exacerbated the discrepancy between IHC analysis and western
blotting results. Therefore, there are limitations in relying on a
single experimental method to verify experimental conclusions.
Since IHC analysis has the advantages of observing morphology and
cell localization, both western blotting and IHC analysis were used
to verify the conclusions. Taken together, the results of the
present study suggested that TMP may promote the regeneration and
repair of renal cells by activating the Wnt/β-catenin signaling
pathway.
The DKK family includes the following four members:
DKK1, 2, 3 and 4. DKK1 is one of the classic inhibitors of the
Wnt/β-catenin signaling pathway, which functions by blocking the
binding of Wnt to LRP5/6 co-receptors to form dimers (49,50).
Studies have shown that DKK1 is involved in the occurrence and
development of chronic kidney disease (29,51);
however, whether it promotes the progression or alleviation of
chronic kidney disease has not been clearly determined and its
effects on AKI have not been reported. The histone demethylase
inhibitor GSK-J4 has been reported to reduce the expression levels
of DKK1, thereby attenuating renal dysfunction, glomerulosclerosis,
inflammation and fibrosis in diabetic mice (52). In addition, DKK1 has been shown to
aggravate cardiac I/R injury by weakening the effect of
pifithrin-α, which alleviates acute cerebral I/R injury via the
Wnt/β-catenin pathway (53). DKK1
exacerbates ischemic heart injury mainly by inducing LRP5/6
endocytosis and degradation (54).
Although insulin-like growth factor binding protein 4 (IGFBP-4) and
DKK1 are inhibitors of the Wnt/β-catenin pathway, IGFBP-4 inhibits
β-catenin to protect the ischemic heart, whereas DKK1 aggravates
ischemic heart injury mainly by inducing LRP5/6 endocytosis and
degradation (54). Furthermore,
DKK1 can decelerate vascular calcification by promoting the
degradation of phospholipase D1, thereby reducing the high
morbidity and mortality caused by vascular calcification in
atherosclerosis, chronic kidney disease and diabetes (55). However, an accumulating number of
studies has shown that DKK1 can participate in cellular activities
independently of the Wnt/β-catenin signaling pathway. For example,
DKK1 could suppress JNK-mediated apoptosis and activate the
NF-κB-dependent cell survival mechanisms, independently of the
canonical Wnt/β-catenin signaling pathway (56). In lupus nephritis, renal Wnt
signaling activity is increased, accompanied by an increase in
renal and serum levels of DKK1(27). In the present study, it was shown
that TMP treatment increased the expression of DKK1, which is
inconsistent with the proposed function of DKK1 as an inhibitor of
Wnt/β-catenin signaling pathway. Therefore, it was considered that
the activation of the Wnt/β-catenin signaling pathway by TMP was
independent of DKK1.
Since the activation of the Wnt/β-catenin signaling
pathway by TMP was independent of DKK1, the signaling pathways or
specific molecular interactions of the Wnt/β-catenin signaling
pathway activated by TMP need to be explored. Known regulators of
the Wnt/β-catenin pathway include: R-spondins, Norrin, TSPAN12,
secreted frizzled-related protein, Wnt inhibitory factor, glycogen
synthase kinase-3β (GSK3β), axis inhibition protein 1, adenomatous
polyposis coli protein (APC), disheveled 1, sclerostin, ring finger
protein 43, zinc and ring finger 3, Cerberus, Klotho, IGFBP, Shisa,
APC down-regulated 1 and Tiki1 (57-65).
Among them, it has been reported that an analog of TMP could
inhibit the phosphorylation of GSK3β and participate in the
regulation of the Wnt/β-catenin pathway (57). Therefore, our future studies will
focus on testing whether TMP can regulate the activation of
Wnt/β-catenin pathway by regulating GSK3β phosphorylation.
Previous studies have shown that TMP has
cardiovascular protection, anti-platelet, anti-ischemic,
anti-Alzheimer's disease, neuroprotective and anticancer effects
(66). TMP has been used in the
treatment of cardiovascular, cerebrovascular, nervous, digestive
system and kidney diseases, as well as in cancer (6). For example, a previous study has
shown that TMP protects against congestive heart failure induced by
myocardial infarction by downregulating the TGF-β1/Smad signaling
pathway, suppressing the activation of the
renin-angiotensin-aldosterone system, inhibiting the synthesis of
pro-inflammatory factors and reducing oxidative stress (67). It has also been suggested that TMP
reduces cell apoptosis and inflammation caused by cerebral I/R
injury by targeting the circ_0008146/microRNA-709/Cx3cr1 axis
(68). Other studies have
suggested that TMP may alleviate diabetic nephropathy in rats
(69,70). Avila-Carrasco et al
(71) reported that TMP can
increase the expression of natural inhibitors of TGF-β, hepatocyte
growth factor, bone morphogenetic protein-7 and other natural
inhibitors, and reduce the risk of renal interstitial fibrosis.
TMP exhibits anti-apoptosis effects. Liu et
al (72) indicated that TMP
may ameliorate the apoptosis of H9C2 cardiomyoblasts by negatively
regulating hypoxia inducible factor-1α-induced Bcl-2 interacting
protein 3 expression, thereby reversing the hypoxic effects induced
by hyperglycemia. A previous study has shown that TMP can alleviate
cognitive impairment by suppressing oxidative stress,
neuroinflammation and apoptosis in type 2 diabetic rats (73). Another study has also shown that
TMP alleviates neural apoptosis in the injured spinal cord via the
downregulation of miR-214-3p (74). However, there have been limited
studies assessing the effect of TMP on apoptosis in AKI. Our
previous research showed the anti-apoptosis effect of TMP in AKI
(12,13); however, at the time there were less
data available on the effect of TMP on apoptosis-related molecules.
In the present study, multiple experiments, including detection of
the number of apoptotic cells by TUNEL staining, assessment of the
expression levels of cleaved caspase-3/caspase-3 by western
blotting, and analysis of the expression levels of
apoptosis-related molecules Bcl-2 and Bax by western blotting and
IHC staining, were performed to prove that TMP could reduce renal
cell apoptosis in AKI. The present study demonstrated that the
renal cell apoptosis in rats following I/R was significantly
reduced after TMP treatment, which was in accordance with the
results of the other study conducted by our group (13). Combined with the results that both
DKK1 upregulation and Wnt/β-catenin signaling pathway activation by
TMP could alleviate AKI renal injury, the protective effect was
most likely through the regulation of apoptosis.
The clinical value of TMP in AKI is supported by the
high incidence of AKI, its poor prognosis and the absence of
approved specific medical therapies other than general supportive
care (75). Several preclinical
studies have shown that TMP has therapeutic potential for AKI
(76,77). If possible, clinical trials
combining TMP with the current standard of care could be conducted
to further confirm the efficacy of TMP in human AKI.
The limitations of the present study include the
lack of in vitro cell experiments for mechanistic
investigation and the absence of direct molecular targets involving
TMP. To verify whether DKK1 and the Wnt/β-catenin pathway were
involved in the protective effect of TMP, the Wnt/β-catenin
signaling pathway and DKK1 expression need to be verified in in
vitro experiments. The relevant mechanistic studies that should
be conducted in future include animal experiments and cell
experiments using Wnt inhibitors, such as small interfering
(si)RNA-Wnt and/or siRNA-DKK1. Molecular docking between TMP and
regulators or key molecules of the Wnt/β-catenin pathway should
also be conducted to preliminarily screen possible direct targets
of TMP and for experimental validation. The mechanism in which DKK1
and the Wnt/β-catenin pathway affects apoptosis should also be a
point of focus. Furthermore, the sample size was not determined
through power analysis; it was determined based on the resource
equation method (32). The present
animal experiment was an exploratory study. When designing
experiments, more consideration should be given to other elements
that can be tested rather than sample size estimation to ensure the
quality of scientific research (78).
Currently, the only effective treatment strategy for
AKI is renal replacement therapy. Therefore, it is imperative to
develop more effective treatment strategies. The research results
of the in vivo rat model of AKI treated with TMP
demonstrated in the present study suggested that TMP targets DKK1
and the Wnt/β-catenin signaling pathways, and therefore could
effectively treat AKI.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Shandong Natural
Science Fund of Shandong Province (grant no. ZR2020MH080); the
Projects of Medical and Health Technology Development Program in
Shandong Province (grant nos. 202003050666 and 2019WS310); the
Projects of Technological Innovation Development Program in Yantai
City (grant no. 2022YD071); the Traditional Chinese Medicine
Science and Technology Project of Shandong Province (grant no.
M-2023017); and the Clinical +X project of Binzhou Medical
University (grant no. BY2021LCX24).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XW, XC and DY were involved in conceptualization,
conducted the experiments, and were involved in visualization and
writing of the original draft. LZ, ZG, XS and AL helped to conduct
the experiments, data analysis and draft writing. YN and PD were
involved in conceptualization, funding acquisition, project
administration, supervision, review and editing. XW and PD confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Binzhou Medical University (approval no.
2020-07; Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levey AS and James MT: Acute kidney
injury. Ann Intern Med. 167:ITC66–ITC80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Niculae A, Gherghina ME, Peride I, Tiglis
M, Nechita AM and Checherita IA: Pathway from acute kidney injury
to chronic kidney disease: Molecules involved in renal fibrosis.
Int J Mol Sci. 24(14019)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Deng Y, Zeng L, Liu H, Zuo A, Zhou J, Yang
Y, You Y, Zhou X, Peng B, Lu H, et al: Silibinin attenuates
ferroptosis in acute kidney injury by targeting FTH1. Redox Biol.
77(103360)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gonçalves GM, Castoldi A, Braga TT and
Câmara NO: New roles for innate immune response in acute and
chronic kidney injuries. Scand J Immunol. 73:428–435.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou M, Tang W, Fu Y, Xu X, Wang Z, Lu Y,
Liu F, Yang X, Wei X, Zhang Y, et al: Progranulin protects against
renal ischemia/reperfusion injury in mice. Kidney Int. 87:918–929.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin J, Wang Q, Zhou S, Xu S and Yao K:
Tetramethylpyrazine: A review on its mechanisms and functions.
Biomed Pharmacother. 150(113005)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen HY, Xu DP, Tan GL, Cai W, Zhang GX,
Cui W, Wang JZ, Long C, Sun YW, Yu P, et al: A Potent
multi-functional neuroprotective derivative of tetramethylpyrazine.
J Mol Neurosci. 56:977–987. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bai XY, Wang XF, Zhang LS, Du PC, Cao Z
and Hou Y: Tetramethylpyrazine ameliorates experimental autoimmune
encephalomyelitis by modulating the inflammatory response. Biochem
Biophys Res Commun. 503:1968–1972. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang B, Ni Q, Wang X and Lin L:
Meta-analysis of the clinical effect of ligustrazine on diabetic
nephropathy. Am J Chin Med. 40:25–37. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shao H, Zhao L, Chen F, Zeng S, Liu S and
Li J: Efficacy of ligustrazine injection as adjunctive therapy for
angina pectoris: A systematic review and meta-analysis. Med Sci
Monit. 21:3704–3715. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ni X, Ni X, Liu S and Guo X: Medium- and
long-term efficacy of ligustrazine plus conventional medication on
ischemic stroke: A systematic review and meta-analysis. J Tradit
Chin Med. 33:715–720. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang G, Xin R, Yuan W, Zhang L, Meng X,
Sun W, Han H, Hou Y, Wang L and Du P: Ligustrazine ameliorates
acute kidney injury through downregulation of NOD2-mediated
inflammation. Int J Mol Med. 45:731–742. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun W, Li A, Wang Z, Sun X, Dong M, Qi F,
Wang L, Zhang Y and Du P: Tetramethylpyrazine alleviates acute
kidney injury by inhibiting NLRP3/HIF-1α and apoptosis. Mol Med
Rep. 22:2655–2664. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang M, Zhang L, Huang X and Sun Q:
Ligustrazine promotes hypoxia/reoxygenation-treated trophoblast
cell proliferation and migration by regulating the
microRNA-27a-3p/ATF3 axis. Arch Biochem Biophys.
737(109522)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jung YY, Mohan CD, Eng H, Narula AS,
Namjoshi OA, Blough BE, Rangappa KS, Sethi G, Kumar AP and Ahn KS:
2,3,5,6-Tetramethylpyrazine targets epithelial-mesenchymal
transition by abrogating manganese superoxide dismutase expression
and TGFβ-driven signaling cascades in colon cancer cells.
Biomolecules. 12(891)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Steinhart Z and Angers S: Wnt signaling in
development and tissue homeostasis. Development.
145(dev146589)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schunk SJ, Floege J, Fliser D and Speer T:
WNT-β-catenin signalling-a versatile player in kidney injury and
repair. Nat Rev Nephrol. 17:172–184. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huffstater T, Merryman WD and Gewin LS:
Wnt/β-catenin in acute kidney injury and progression to chronic
kidney disease. Semin Nephrol. 40:126–137. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kawakami T, Ren S and Duffield JS: Wnt
signalling in kidney diseases: Dual roles in renal injury and
repair. J Pathol. 229:221–231. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tan RJ, Zhou D, Zhou L and Liu Y:
Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl
(2011). 4:84–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aggarwal S, Wang Z, Rincon Fernandez
Pacheco D, Rinaldi A, Rajewski A, Callemeyn J, Van Loon E,
Lamarthée B, Covarrubias AE, Hou J, et al: SOX9 switch links
regeneration to fibrosis at the single-cell level in mammalian
kidneys. Science. 383(eadd6371)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ming WH, Luan ZL, Yao Y, Liu HC, Hu SY, Du
CX, Zhang C, Zhao YH, Huang YZ, Sun XW, et al: Pregnane X receptor
activation alleviates renal fibrosis in mice via interacting with
p53 and inhibiting the Wnt7a/β-catenin signaling. Acta Pharmacol
Sin. 44:2075–2090. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Niehrs C: Function and biological roles of
the dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ng LF, Kaur P, Bunnag N, Suresh J, Sung
ICH, Tan QH, Gruber J and Tolwinski NS: WNT signaling in disease.
Cells. 8(826)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH
and Wang FS: Dickkopf-1 promotes hyperglycemia-induced accumulation
of mesangial matrix and renal dysfunction. J Am Soc Nephrol.
21:124–135. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tveita AA and Rekvig OP: Alterations in
Wnt pathway activity in mouse serum and kidneys during lupus
development. Arthritis Rheum. 63:513–522. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Klavdianou K, Liossis SN and Daoussis D:
Dkk1: A key molecule in joint remodelling and fibrosis. Mediterr J
Rheumatol. 28:174–182. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hsu YC, Chang CC, Hsieh CC, Huang YT, Shih
YH, Chang HC, Chang PJ and Lin CL: Dickkopf-1 acts as a profibrotic
mediator in progressive chronic kidney disease. Int J Mol Sci.
24(7679)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the care and use of laboratory animals, 8th
edition. Washington (DC): National Academies Press (US), 2011.
|
|
31
|
Office of the Institutional Animal Care
and Use Committee, The University of Iowa: Anesthesia (Guideline).
https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Accessed August 21, 2025.
|
|
32
|
Charan J and Kantharia ND: How to
calculate sample size in animal studies? J Pharmacol Pharmacother.
4:303–306. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pickkers P, Darmon M, Hoste E, Joannidis
M, Legrand M, Ostermann M, Prowle JR, Schneider A and Schetz M:
Acute kidney injury in the critically ill: An updated review on
pathophysiology and management. Intensive Care Med. 47:835–850.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ostermann M, Lumlertgul N, Jeong R, See E,
Joannidis M and James M: Acute kidney injury. Lancet. 405:241–256.
2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mehrotra P, Sturek M, Neyra JA and Basile
DP: Calcium channel Orai1 promotes lymphocyte IL-17 expression and
progressive kidney injury. J Clin Invest. 129:4951–4961.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kar F, Hacioglu C, Senturk H, Donmez DB
and Kanbak G: The role of oxidative stress, renal inflammation, and
apoptosis in post ischemic reperfusion injury of kidney tissue: The
protective effect of dose-dependent boric acid administration. Biol
Trace Elem Res. 195:150–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chi F, Feng L, Li Y, Zhao S, Yuan W, Jiang
Y and Cheng L: MiR-30b-5p promotes myocardial cell apoptosis in
rats with myocardial infarction through regulating Wnt/β-catenin
signaling pathway. Minerva Med. 114:476–484. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sun JD, Li XM, Liu JL, Li J and Zhou H:
Effects of miR-150-5p on cerebral infarction rats by regulating the
Wnt signaling pathway via p53. Eur Rev Med Pharmacol Sci.
24:3882–3891. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen X, Tan H, Xu J, Tian Y, Yuan Q, Zuo
Y, Chen Q, Hong X, Fu H, Hou FF, et al: Klotho-derived peptide 6
ameliorates diabetic kidney disease by targeting Wnt/β-catenin
signaling. Kidney Int. 102:506–520. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Al-Salam S, Jagadeesh GS, Sudhadevi M and
Yasin J: Galectin-3 and autophagy in renal acute tubular necrosis.
Int J Mol Sci. 25(3604)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Meng P, Zhu M, Ling X and Zhou L: Wnt
signaling in kidney: The initiator or terminator? J Mol Med (Berl).
98:1511–1523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li SS, Sun Q, Hua MR, Suo P, Chen JR, Yu
XY and Zhao YY: Targeting the Wnt/β-catenin signaling pathway as a
potential therapeutic strategy in renal tubulointerstitial
fibrosis. Front Pharmacol. 12(719880)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou L and Liu Y: Wnt/β-catenin signalling
and podocyte dysfunction in proteinuric kidney disease. Nat Rev
Nephrol. 11:535–545. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tao H, Chen F, Liu H, Hu Y, Wang Y and Li
H: Wnt/β-catenin signaling pathway activation reverses gemcitabine
resistance by attenuating Beclin1-mediated autophagy in the MG63
human osteosarcoma cell line. Mol Med Rep. 16:1701–1706.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Menon NA, Kumar CD, Ramachandran P, Blaize
B, Gautam M, Cordani M and Kumar LD: Small-molecule inhibitors of
WNT signalling in cancer therapy and their links to autophagy and
apoptosis. Eur J Pharmacol. 986(177137)2025.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ma Q, Yu J, Zhang X, Wu X and Deng G:
Wnt/β-catenin signaling pathway-a versatile player in apoptosis and
autophagy. Biochimie. 211:57–67. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen X, Wang CC, Song SM, Wei SY, Li JS,
Zhao SL and Li B: The administration of erythropoietin attenuates
kidney injury induced by ischemia/reperfusion with increased
activation of Wnt/β-catenin signaling. J Formos Med Assoc.
114:430–437. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou
FF and Liu Y: Sustained activation of Wnt/β-catenin signaling
drives AKI to CKD progression. J Am Soc Nephrol. 27:1727–1740.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Baetta R and Banfi C: Dkk (dickkopf)
proteins. Arterioscler Thromb Vasc Biol. 39:1330–1342.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liang L, He H, Lv R, Zhang M, Huang H, An
Z and Li S: Preliminary mechanism on the methylation modification
of Dkk-1 and Dkk-3 in hepatocellular carcinoma. Tumour Biol.
36:1245–1250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li YH, Cheng YC, Wu J and Lee IT: Plasma
dickkopf-1 levels are associated with chronic kidney disease.
Metabolites. 15(300)2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hung PH, Hsu YC, Chen TH, Ho C and Lin CL:
The histone demethylase inhibitor GSK-J4 Is a therapeutic target
for the kidney fibrosis of diabetic kidney disease via DKK1
modulation. Int J Mol Sci. 23(9407)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang H, Du D, Gao X, Tian X, Xu Y, Wang
B, Yang S, Liu P and Li Z: PFT-α protects the blood-brain barrier
through the Wnt/β-catenin pathway after acute ischemic stroke.
Funct Integr Genomics. 23(314)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wo D, Peng J, Ren DN, Qiu L, Chen J, Zhu
Y, Yan Y, Yan H, Wu J, Ma E, et al: Opposing roles of Wnt
inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential
targeting of LRP5/6 and β-catenin. Circulation. 134:1991–2007.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li X, Liu XL, Li X, Zhao YC, Wang QQ,
Zhong HY, Liu DD, Yuan C, Zheng TF and Zhang M: Dickkopf1 (Dkk1)
alleviates vascular calcification by regulating the degradation of
phospholipase D1 (PLD1). J Cardiovasc Transl Res. 15:1327–1339.
2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yuan S, Hoggard NK, Kantake N, Hildreth BE
III and Rosol TJ: Effects of dickkopf-1 (DKK-1) on prostate cancer
growth and bone metastasis. Cells. 12(2695)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zou Y, Zhao D, Yan C, Ji Y, Liu J, Xu J,
Lai Y, Tian J, Zhang Y and Huang Z: Novel ligustrazine-based
analogs of piperlongumine potently suppress proliferation and
metastasis of colorectal cancer cells in vitro and in vivo. J Med
Chem. 61:1821–1832. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Albrecht LV, Tejeda-Muñoz N and De
Robertis EM: Cell biology of canonical Wnt signaling. Annu Rev Cell
Dev Biol. 37:369–389. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cruciat CM and Niehrs C: Secreted and
transmembrane wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5(a015081)2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Farnhammer F, Colozza G and Kim J: RNF43
and ZNRF3 in Wnt signaling-A master regulator at the membrane. Int
J Stem Cells. 16:376–384. 2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gao Y, Chen N, Fu Z and Zhang Q: Progress
of Wnt signaling pathway in osteoporosis. Biomolecules.
13(483)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fetisov TI, Lesovaya EA, Yakubovskaya MG,
Kirsanov KI and Belitsky GA: Alterations in WNT signaling in
leukemias. Biochemistry (Mosc). 83:1448–1458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ho HYH: The tale of capturing Norrin.
Elife. 13(e98933)2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Raslan AA and Yoon JK: R-spondins:
Multi-mode WNT signaling regulators in adult stem cells. Int J
Biochem Cell Biol. 106:26–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zou J, Gao P, Hao X, Xu H, Zhan P and Liu
X: Recent progress in the structural modification and
pharmacological activities of ligustrazine derivatives. Eur J Med
Chem. 147:150–162. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Chen Q, Zhang D, Bi Y, Zhang W, Zhang Y,
Meng Q, Li Y and Bian H: The protective effects of liguzinediol on
congestive heart failure induced by myocardial infarction and its
relative mechanism. Chin Med. 15(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Li L, Zhang D, Yao W, Wu Z, Cheng J, Ji Y,
Dong L, Zhao C and Wang H: Ligustrazine exerts neuroprotective
effects via circ_0008146/miR-709/Cx3cr1 axis to inhibit cell
apoptosis and inflammation after cerebral ischemia/reperfusion
injury. Brain Res Bull. 190:244–255. 2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Rai U, Kosuru R, Prakash S, Tiwari V and
Singh S: Tetramethylpyrazine alleviates diabetic nephropathy
through the activation of Akt signalling pathway in rats. Eur J
Pharmacol. 865(172763)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yang QH, Liang Y, Xu Q, Zhang Y, Xiao L
and Si LY: Protective effect of tetramethylpyrazine isolated from
Ligusticum chuanxiong on nephropathy in rats with
streptozotocin-induced diabetes. Phytomedicine. 18:1148–1152.
2011.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Avila-Carrasco L, Majano P, Sánchez-Toméro
JA, Selgas R, López-Cabrera M, Aguilera A and González Mateo G:
Natural plants compounds as modulators of epithelial-to-mesenchymal
transition. Front Pharmacol. 10(715)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Liu SP, Shibu MA, Tsai FJ, Hsu YM, Tsai
CH, Chung JG, Yang JS, Tang CH, Wang S, Li Q and Huang CY:
Tetramethylpyrazine reverses high-glucose induced hypoxic effects
by negatively regulating HIF-1α induced BNIP3 expression to
ameliorate H9c2 cardiomyoblast apoptosis. Nutr Metab (Lond).
17(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Dhaliwal J, Dhaliwal N, Akhtar A, Kuhad A
and Chopra K: Tetramethylpyrazine attenuates cognitive impairment
via suppressing oxidative stress, neuroinflammation, and apoptosis
in type 2 diabetic rats. Neurochem Res. 47:2431–2444.
2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Fan Y and Wu Y: Tetramethylpyrazine
alleviates neural apoptosis in injured spinal cord via the
downregulation of miR-214-3p. Biomed Pharmacother. 94:827–833.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Matuszkiewicz-Rowińska J and Małyszko J:
Acute kidney injury, its definition, and treatment in adults:
Guidelines and reality. Pol Arch Intern Med. 130:1074–1080.
2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li J and Gong X: Tetramethylpyrazine: An
active ingredient of Chinese herbal medicine with therapeutic
potential in acute kidney injury and renal fibrosis. Front
Pharmacol. 13(820071)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Li J, Li T, Li Z, Song Z and Gong X:
Potential therapeutic effects of Chinese meteria medica in
mitigating drug-induced acute kidney injury. Front Pharmacol.
14(1153297)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ko MJ and Lim CY: General considerations
for sample size estimation in animal study. Korean J Anesthesiol.
74:23–29. 2021.PubMed/NCBI View Article : Google Scholar
|