Introduction
Accelerated malignant hypertension, a severe
manifestation of hypertensive disease, is characterized by markedly
elevated blood pressure (typically diastolic blood pressure
>120-130 mmHg) and progressive target organ damage. This
condition frequently leads to cardiac, renal and cerebral
complications (1,2).
Tuberculosis remains a significant global health
challenge. While Japan has seen a decline in incidence, the rate
has plateaued in recent years, particularly among older adults,
with >10,000 new cases still reported annually (3). Evidence increasingly suggests an
association between tuberculosis infection and latent and
cardiovascular risks, including hypertension (4-6).
This association is particularly pronounced in patients with
underlying conditions, such as diabetes mellitus and kidney
dysfunction, which are known to impair immune function (7,8).
Tuberculous pleuritis, the most common form of
extrapulmonary tuberculosis, represents a major cause of pleural
effusion, accounting for ~5% of all tuberculosis cases globally
(9). However, its diagnosis is
often challenging when multiple pathologies coexist. Specifically,
when organ dysfunction, for example heart or kidney failure, is
attributed to primary conditions, such as accelerated malignant
hypertension, a concurrent tuberculous infection can be easily
overlooked (10).
The present report describes a case of refractory
pleural effusion and acute kidney injury in a patient initially
diagnosed with heart failure due to accelerated malignant
hypertension. This case highlights the diagnostic challenge posed
by coexisting pathologies and underscores the importance of
considering tuberculosis in patients with treatment-refractory
symptoms, with the aim of increasing clinical awareness for similar
future cases.
Case report
A 57-year-old woman presented to Tohoku Medical and
Pharmaceutical University Hospital (Sendai, Japan) in August 2024,
with progressive lower-extremity edema and uncontrolled
hypertension. Despite awareness of hypertension since her 40s, the
patient had received no treatment, with recent health screenings
documenting systolic blood pressure consistently exceeding 200
mmHg. The patient's medical history was unremarkable, but the
family history included hypertension in both parents, with a
paternal history of type 2 diabetes mellitus and heart failure. The
patient had a 37-pack-year smoking history.
Initial physical examination revealed a blood
pressure of 208/122 mmHg (normal value, <120/80 mmHg),
tachycardia (111 beats/min; normal range, 60-100 beats/min), a
normal body temperature (36.5˚C; normal range, 36.0-37.0˚C) and
mild hypoxemia (oxygen saturation, 93% on room air; normal value,
≥95%). Cardiovascular examination revealed a grade 3/6 systolic
murmur at the second right sternal border, jugular venous
distention and marked bilateral lower-extremity edema. Chest
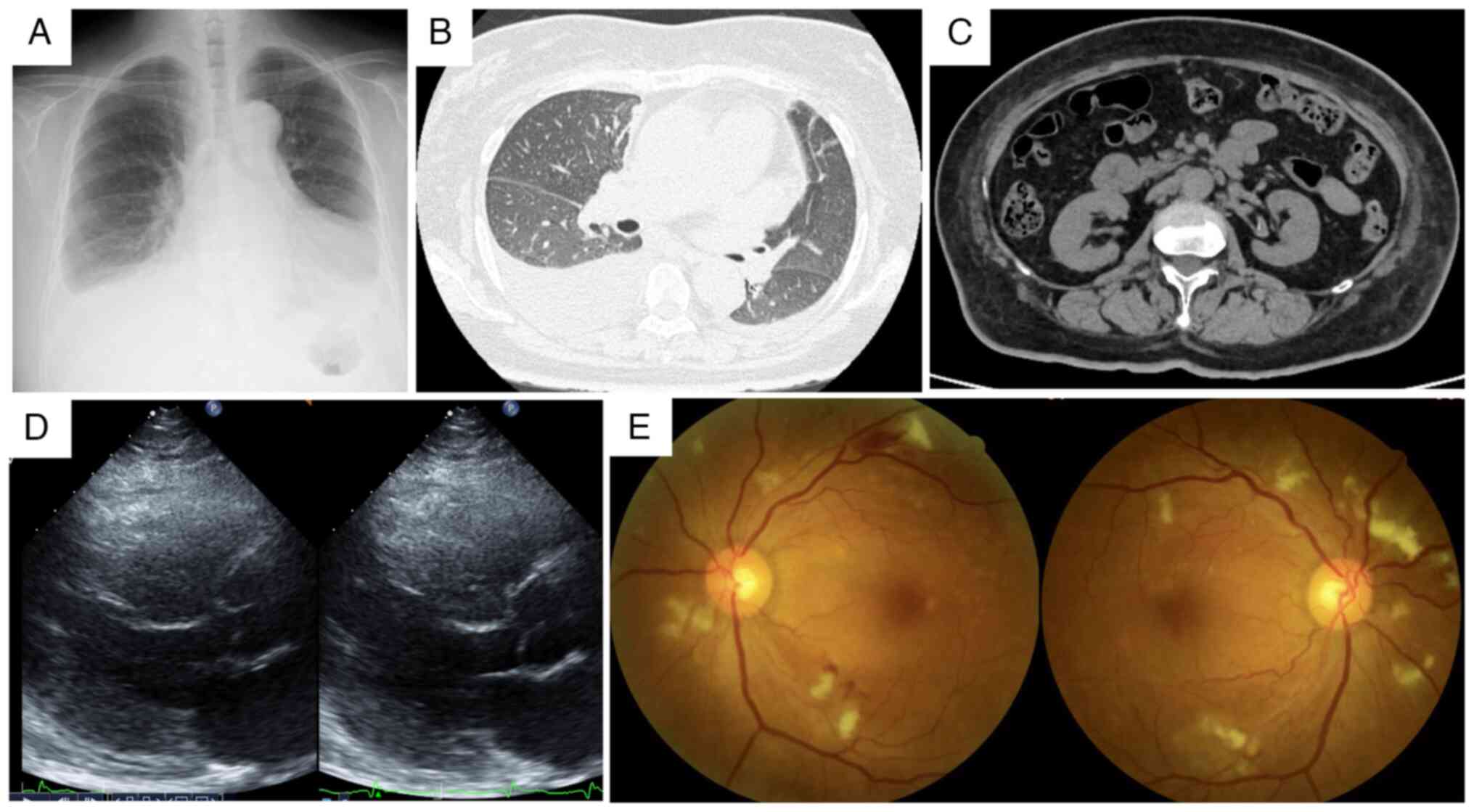
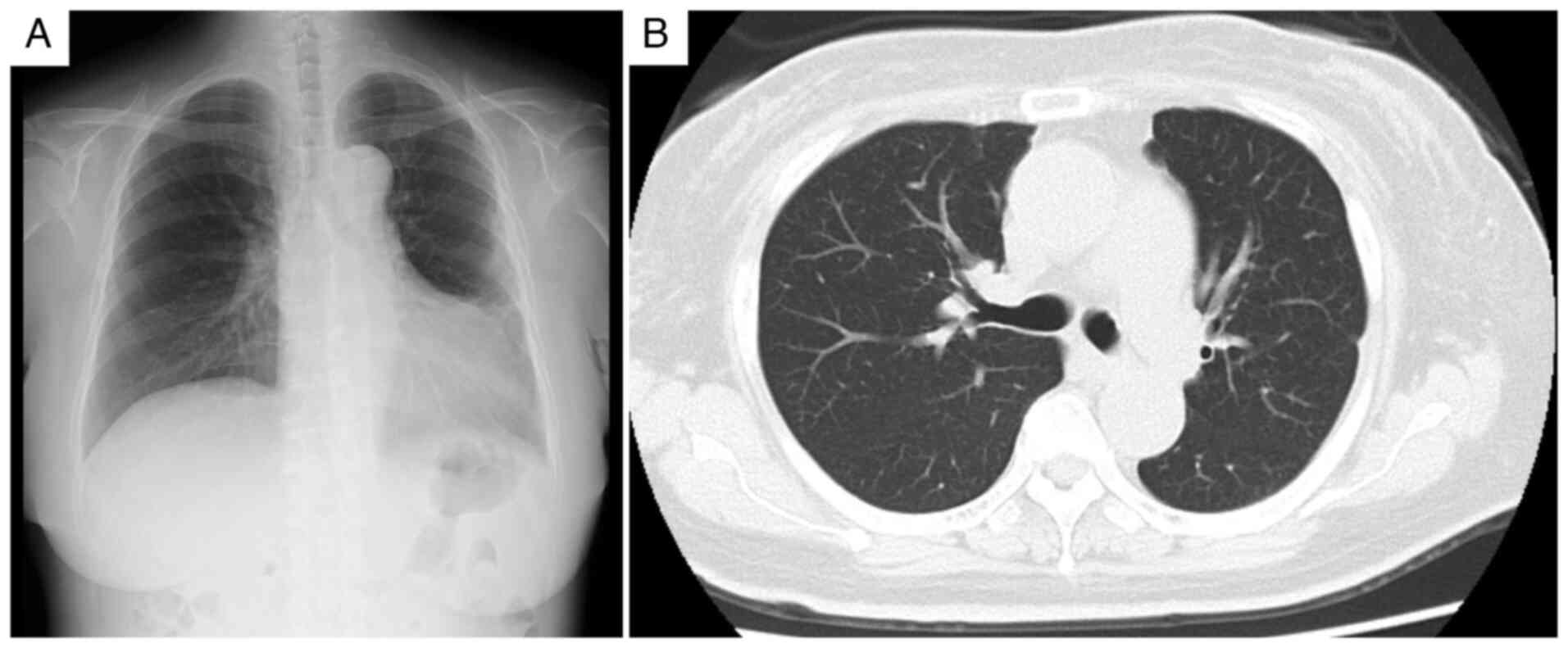
radiography and computed tomography revealed cardiomegaly,
pulmonary congestion, bilateral pleural effusions with a
right-sided predominance and bilateral renal atrophy (Fig. 1). Laboratory values demonstrated
significant renal dysfunction (serum creatinine, 4.74 mg/dl; normal
range, 0.6-1.0 mg/dl), moderate proteinuria (2.35 g/gCr; normal
value, <0.15 g/gCr) and substantially elevated brain natriuretic
peptide (3,252.6 pg/ml; normal value, <18.4 pg/ml). HIV test
results were negative. C-reactive protein levels were minimally
elevated at 0.87 mg/dl (normal value, <0.3 mg/dl) (Table I). Transthoracic echocardiography
revealed diffuse left ventricular hypokinesis with reduced ejection
fraction (42.5%; normal range, 55-70%) (Fig. 1D). Fundoscopic examination
confirmed hypertensive retinopathy with hemorrhage and exudates
(Fig. 1E).
 | Table ILaboratory data on admission. |
Table I
Laboratory data on admission.
| Parameter | Result | Normal range |
|---|
| Urinalysis | | |
|
Protein | (3+) | Negative |
|
Occult
blood | (1+) | Negative |
|
Red blood
cells, /HPF | <1 | <5 |
|
Protein
content, g/gCr | 2.35 | <0.15 |
|
NAG,
µg/l | 10.7 | <11.2 |
| Blood cell
counts | | |
|
White blood
cells, /µl | 9,000 | 3,300-8,600 |
|
Neutrophils,
% | 84.1 | 40.0-75.0 |
|
Hemoglobin,
g/dl | 11.9 | 13.0-16.0 |
|
Platelets,
x104/µl | 33.4 | 15.0-35.0 |
|
Hematocrit,
% | 27.9 | 38.0-48.0 |
| Blood chemistry | | |
|
Total
protein, g/dl | 6.9 | 6.7-8.3 |
|
Albumin,
g/dl | 3.4 | 4.0-5.0 |
|
Total
bilirubin, mg/dl | 0.85 | 0.3-1.2 |
|
AST,
U/l | 23 | 10-40 |
|
ALT,
U/l | 23 | 5-40 |
|
γ-GTP,
U/l | 59 | 10-70 |
|
HbA1c,
% | 7.4 | 4.6-6.0 |
|
Blood urea
nitrogen, mg/dl | 50 | 8-20 |
|
Creatinine,
mg/dl | 4.74 | 0.6-1.0 |
|
eGFR,
ml/min/1.73 m2 | 8.2 | >60 |
|
Glucose,
mg/dl | 132 | 70-109 |
|
Uric acid,
mg/dl | 12.4 | 3.5-7.0 |
|
TSH,
µU/ml | 2.9 | 0.5-5.0 |
|
Free T3,
pg/ml | 1.05 | 2.3-4.0 |
|
Free T4,
ng/dl | 1.24 | 0.9-1.7 |
|
Sodium,
mEq/l | 140 | 136-145 |
|
Potassium,
mEq/l | 4.2 | 3.5-5.0 |
|
Chloride,
mEq/l | 99 | 98-108 |
|
Calcium,
mg/dl | 8.9 | 8.5-10.2 |
|
Phosphorus,
mg/dl | 4.5 | 2.5-4.5 |
|
Iron,
µg/dl | 32 | 50-170 |
|
Ferritin,
ng/dl | 372 | 20-250 |
|
Total
cholesterol, mg/dl | 235 | 140-220 |
|
Triglycerides,
mg/dl | 150 | 30-150 |
|
LDL
cholesterol, mg/dl | 151 | 70-140 |
|
CRP,
mg/dl | 0.87 | <0.3 |
|
IgG,
mg/dl | 1,533 | 870-1,700 |
|
IgM,
mg/dl | 44 | 35-220 |
|
IgA,
mg/dl | 492 | 110-410 |
|
C3,
mg/dl | 96 | 80-160 |
|
C4,
mg/dl | 33 | 15-45 |
|
CH50,
U/ml | 59.8 | 30-45 |
|
Anti-nuclear
antibody (titer) | <1:40 | <1:40 |
|
MPO-ANCA,
U/ml | <1.0 | <3.5 |
|
PR3-ANCA,
U/ml | <1.0 | <3.5 |
|
Anti-GBM
antibody, U/ml | <2.0 | <7.0 |
|
BNP,
pg/ml | 3,252.6 | <18.4 |
|
Plasma renin
activity, ng/ml | 6.2 | 0.3-2.9 |
|
Aldosterone,
pg/ml | 71.5 | 40-310 |
|
ACTH,
pg/ml | 49.9 | 7.2-63.3 |
|
Cortisol,
µg/dl | 20.9 | 4.5-21.1 |
|
Metanephrine,
mg/day | 0.09 | <0.5 |
|
Normetanephrine,
mg/day | 0.34 | <0.9 |
|
Adrenaline
pg/ml | 38 | <100 |
|
Noradrenaline,
pg/ml | 628 | 100-450 |
|
Dopamine,
pg/ml | 30 | <30 |
Heart and renal failure due to malignant
hypertension were diagnosed based on these findings, and treatment
with continuous nicardipine infusion (initiated at 2 µg/kg/min and
titrated to maintain systolic blood pressure <160 mmHg) was
initiated. Progressive dyspnea on minimal exertion and orthopnea
prompted the administration of intravenous carperitide (0.025
µg/kg/min) and furosemide (80 mg/day). On hospital day 2, an
altered mental status (Japan Coma Scale 3) (11) developed, characterized by
word-finding difficulty, perseveration and a right-sided tactile
extinction without apparent motor deficits (National Institutes of
Health Stroke Scale 4) (12).
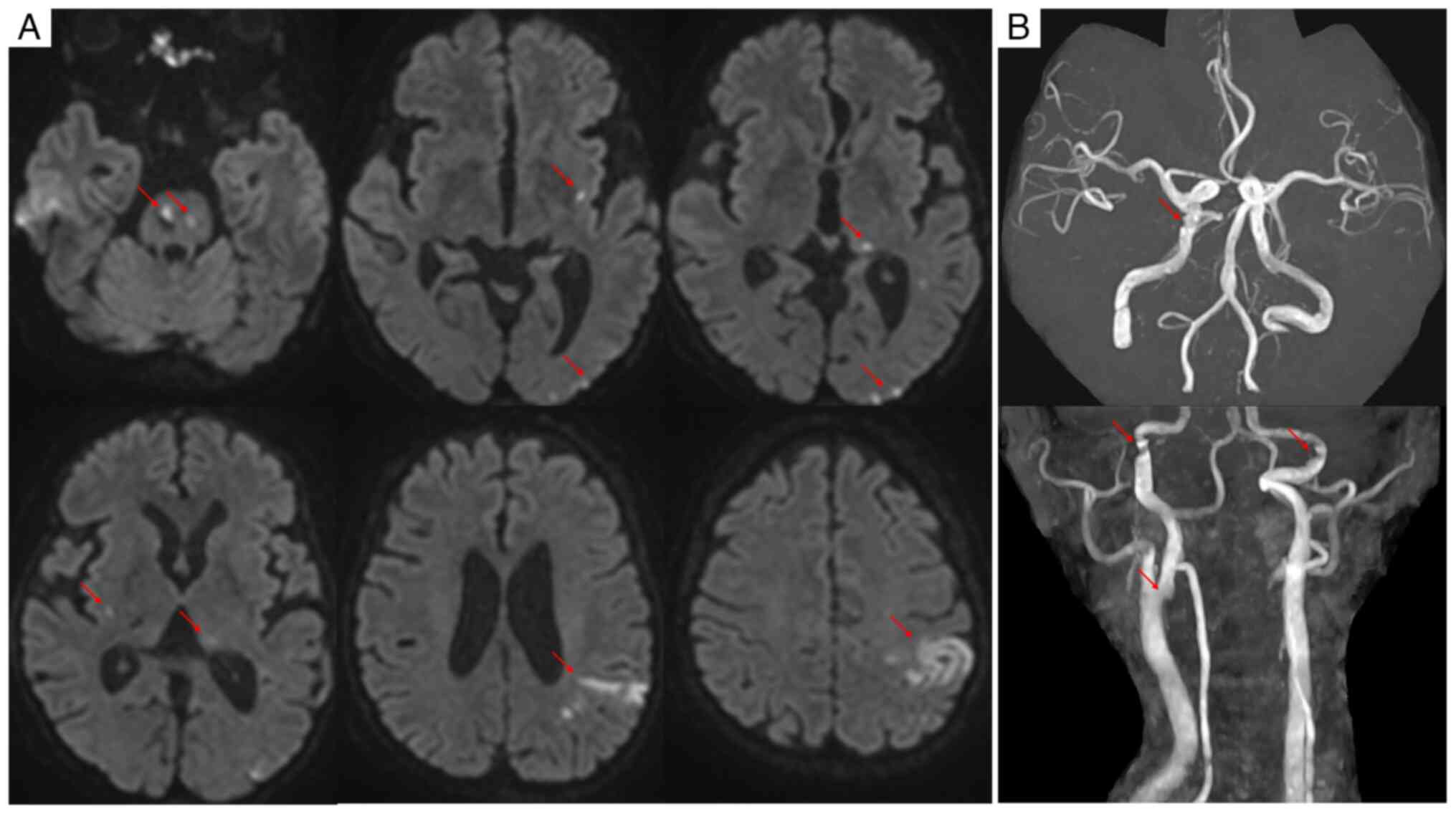
Diffusion-weighted magnetic resonance imaging revealed multiple
acute infarctions involving the pons, bilateral basal ganglia, left
thalamus and left parieto-occipital region, with hemorrhagic
transformation of the left parietal lesion (Fig. 2A). Magnetic resonance angiography
showed vessel irregularities without obvious occlusion (Fig. 2B). Despite comprehensive
evaluation, including carotid ultrasonography, Holter
electrocardiography and malignancy screening, no embolic source was
identified, leading to a diagnosis of embolic stroke of
undetermined source (ESUS). Given concurrent renal dysfunction,
therapeutic intervention was limited to aspirin (100 mg/day), with
careful attention to blood pressure management.
Persistent pyrexia and progressive elevation of
inflammatory markers were observed. Despite adequate blood pressure
control with multiple antihypertensive agents (including 80 mg/day
nifedipine, 20 mg/day azilsartan and 10 mg/day carvedilol) and
diuretics (15 mg/day tolvaptan, 40 mg/day furosemide and 25 mg/day
spironolactone), pleural effusion and renal dysfunction persisted.
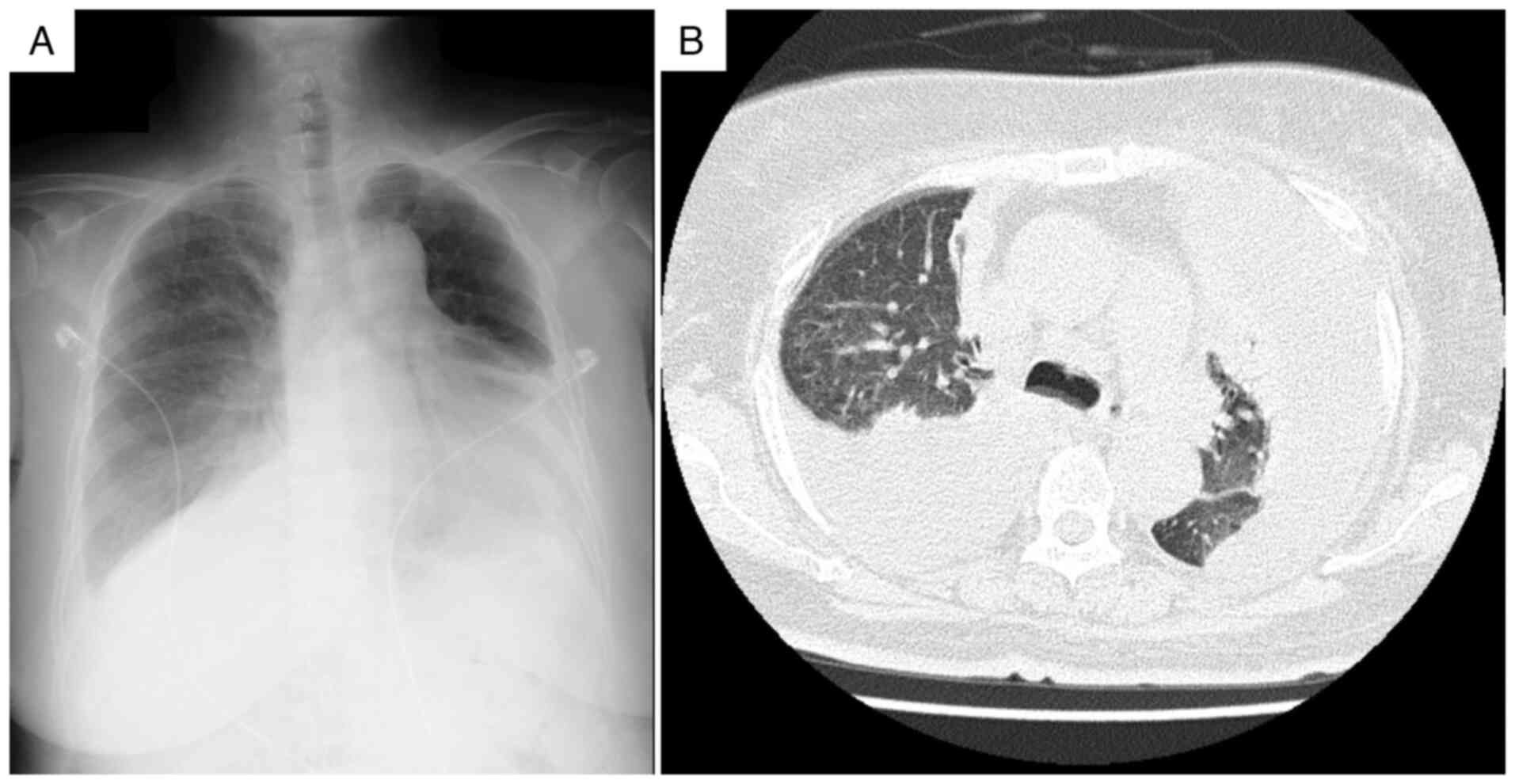
As shown in Fig. 3, chest
radiography revealed progression of the pleural effusions compared
with that recorded at admission, and computed tomography confirmed
large bilateral effusions with associated compressive atelectasis,
indicating that the effusions were refractory to standard heart
failure treatment. On hospital day 20, diagnostic thoracentesis was
performed under ultrasound guidance. Approximately 800 ml of
serous, straw-colored fluid was drained, and its analysis revealed
lymphocyte-predominant (96%) exudative pleural fluid with elevated
adenosine deaminase (ADA) levels (51.8 U/l) (Table II). A positive interferon-gamma
release assay (IGRA; QuantiFERON-TB Gold Plus; Qiagen Inc.) result
was noted, and although pleural biopsy showed no acid-fast bacilli
on Ziehl-Neelsen staining, caseating granulomas confirmed
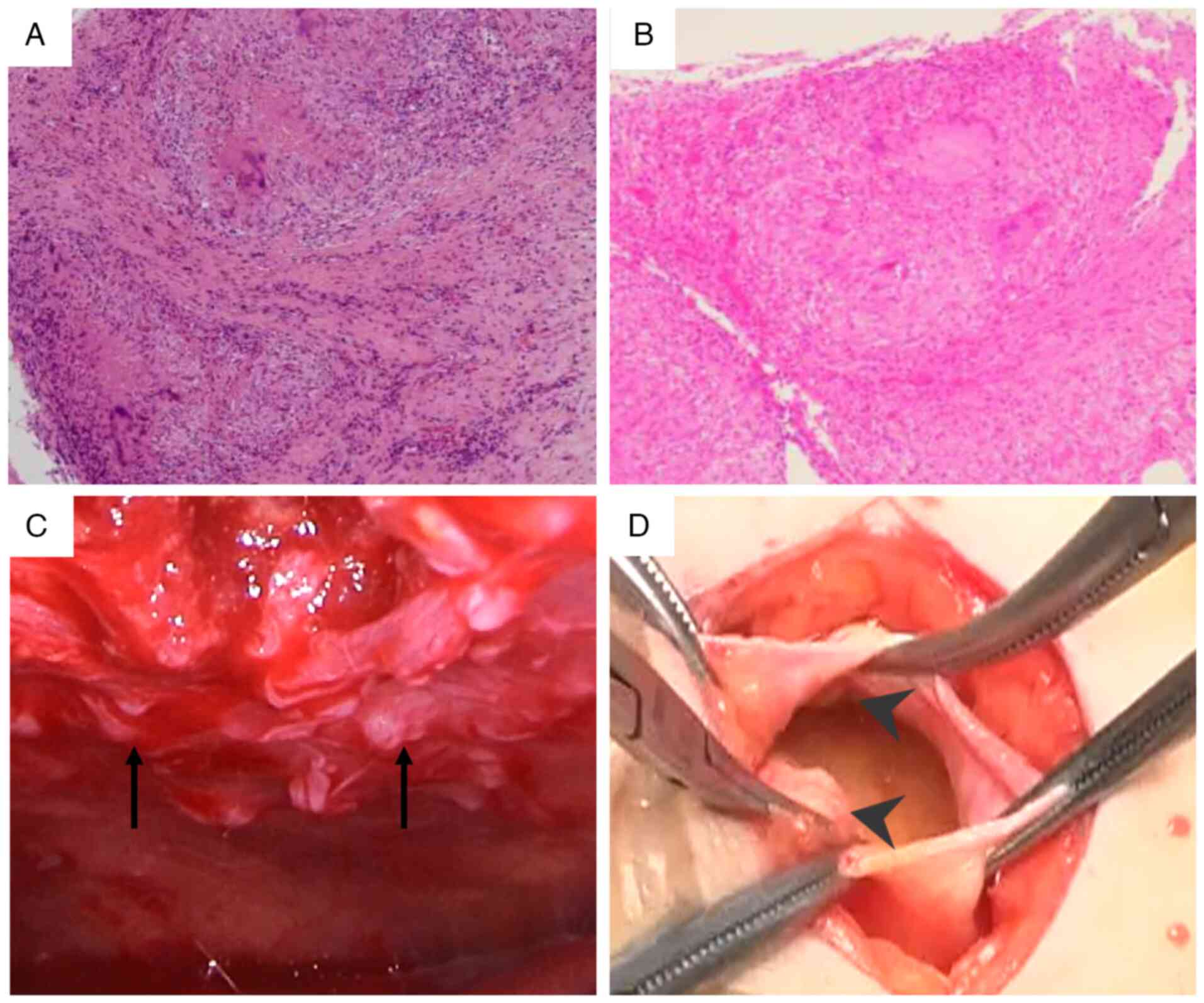
tuberculous pleuritis. To establish a definitive diagnosis, a
pleural biopsy was performed at 45 days of hospitalization. This
biopsy confirmed tuberculous pleuritis by revealing caseating
granulomas (Fig. 4A), although no
acid-fast bacilli were seen. The procedure was guided by
thoracoscopy, which had revealed multiple nodules on the pleura
(Fig. 4C).
 | Table IIThoracentesis findings. |
Table II
Thoracentesis findings.
| Parameter | Result | Normal range |
|---|
| Total protein,
g/dl | 4.8 | <2.5 |
| Albumin, g/dl | 2.4 | >1.2
(serum-pleural fluid albumin gradient) |
| LDH, U/l | 204 | <200 |
| Cell count,
/µl | 2,936 | <1,000 |
| Neutrophils, % | 2 | <25 |
| Eosinophils, % | 0 | <10 |
| Basophils, % | 0 | <1 |
| Lymphocytes, % | 96 | 50-70 |
| Monocytes, % | 2 | 3-5 |
| ADA, U/l | 51.8 | <40 |
| CYFRA, ng/ml | 14.6 | <50 |
| CEA, ng/ml | 2.1 | <5 |
| ProGRP, pg/ml | 168 | <50 |
| NSE, ng/ml | 3.3 | <15 |
| pH | 7.4 | 7.35-7.45 |
| Glucose, mg/dl | 131 | 70-109 |
| Albumin, % | 49.2 | 50-60 |
| γ-globulin, % | 18.5 | 11-22 |
Peritoneal dialysis catheter placement was performed
due to persistent elevation of serum creatinine (6-7 mg/dl).
Although tuberculosis was suspected, a definitive diagnosis of
tuberculous pleuritis remained pending, and multiple sputum
cultures (three consecutive early morning samples) demonstrated no
mycobacterial growth. Due to the progression of uremia requiring
renal replacement therapy, a surgical procedure for peritoneal
dialysis catheter placement was performed on hospital day 51.
During the operation, visual inspection through the peritoneal
incision revealed multiple disseminated peritoneal nodules.
Subsequent histopathological examination confirmed caseating
granulomas, establishing concurrent tuberculous peritonitis
(Fig. 4B and D).
On hospital day 52, quadruple antituberculous
therapy (300 mg/day isoniazid, 450 mg/day rifampicin and 1,200 mg
pyrazinamide three times per week, and 750 mg ethambutol three
times per week) was initiated and a marked improvement in pleural
effusion was observed within 3 weeks of starting the therapy
(Fig. 5A and B). Reduction of diuretic therapy, which
involved discontinuing furosemide and reducing tolvaptan to 7.5
mg/day while spironolactone was continued at 25 mg/day, previously
administered for presumed heart failure-related effusions, led to
improved renal function, with serum creatinine decreasing to 2.61
mg/dl, and the peritoneal catheter was removed in October 2024. As
of the last follow-up in June 2025, the patient's condition has
remained stable with no recurrence of pleural effusion (Fig. 5A), with a serum creatinine level of
2.49 mg/dl. The patient continues to receive monthly follow-up care
at the Outpatient Department, with ongoing management focused on
controlling hypertension, managing chronic kidney disease and
monitoring the patient for any recurrence of pleural effusion.
Discussion
The present case illustrates a rare clinical
scenario involving the coexistence of accelerated malignant
hypertension and tuberculosis. The initial diagnosis of accelerated
malignant hypertension with organ dysfunction was well-supported by
the clinical presentation, including severe hypertension, cardiac
dysfunction, acute kidney injury and hypertensive retinopathy
(2,13). However, an inadequate response to
standard therapeutic interventions provided crucial clinical
indicators suggesting an additional underlying pathology.
Pleural effusion refractory to diuretic therapy
warrants thorough investigation and illustrated a key diagnostic
challenge in this case. Thoracentesis revealed
lymphocyte-predominant (96%) exudative fluid with elevated ADA 51.8
U/l, findings strongly suggestive of tuberculous pleuritis
(10,14). Although ADA levels >40 U/l are
considered highly indicative (sensitivity, ~92%; specificity,
~90%), elevations can occur in other conditions and confirmation is
often required (14,15). Acid-fast bacilli smear microscopy
of pleural fluid demonstrates poor sensitivity (~5-10%) (9). Although the IGRA was positive, it
cannot distinguish active disease from latent infection and has
only moderate sensitivity (~77%) and specificity (~71%) for
diagnosing tuberculous pleuritis, often necessitating additional
testing (16). In the present
case, a definitive diagnosis was established through pleural
biopsy, which demonstrated caseating granulomas (9), highlighting the importance of
pursuing invasive diagnostics when clinical suspicion remains high
and noninvasive tests are inconclusive.
The systemic inflammatory response associated with
tuberculosis likely contributed to endothelial dysfunction and
vascular injury, thereby exacerbating the pathogenesis of malignant
hypertension (4,17). Increasing evidence suggests a link
between tuberculosis infection (even latent infection) and
cardiovascular risks, such as hypertension (4-6).
Tuberculosis infection activates monocytes and macrophages,
enhancing the production of inflammatory cytokines (e.g., IL-6,
TNF-α) that impair endothelial function by reducing nitric oxide
availability and increasing vasoconstrictor production (6,18).
Immunological changes, such as increased C-X3-C motif chemokine
receptor 1 expression in activated monocytes, can promote
endothelial adhesion and infiltration, thereby amplifying local
inflammation (5). This persistent
inflammatory milieu may have worsened vascular endothelial
dysfunction and contributed to the severity of the hypertensive
phenotype observed in the patient of the present study (5,19).
Although the initial C-reactive protein level was only mildly
elevated (0.87 mg/dl), it persistently remained >3 mg/dl prior
to anti-tuberculous therapy, reflecting ongoing inflammation that
likely contributed to the refractory nature of the patient's
hypertension and organ damage. The interaction between tuberculosis
and malignant hypertension appears to affect multiple organs,
particularly the kidneys, potentially creating a vicious cycle
(8).
The etiology of cerebral infarction in the patient
of the present study was likely multifactorial. Accelerated
malignant hypertension damages the cerebral vasculature through
endothelial dysfunction and impaired autoregulation (2,13).
However, the pattern of multiple acute infarcts across different
vascular territories prompted the consideration of additional
factors. Concurrent tuberculosis is increasingly being recognized
as an independent risk factor for ischemic stroke, likely mediated
by systemic inflammation, endothelial activation and potential
prothrombotic effects (20-22).
Although no embolic source was identified (ESUS classification), it
was hypothesized that the synergy between severe
hypertension-induced vascular injury and tuberculosis-mediated
systemic inflammation significantly increases the propensity for
these multiple cerebral events.
Progressive renal dysfunction provided additional
diagnostic challenges. While hypertensive nephrosclerosis was
initially considered, deterioration despite blood pressure control
indicated the presence of other contributing factors. Aggressive
diuretic therapy for presumed heart failure likely induced prerenal
acute kidney injury (AKI) due to volume depletion. However, the
marked improvement in renal function following anti-tuberculous
therapy (serum creatinine falling from a peak of 7.11 to 2.61
mg/dl, obviating the need for renal replacement therapy) strongly
suggested a direct contribution from the tuberculosis infection
itself (23). Tuberculosis can
impair renal function through several mechanisms, including direct
bacillary invasion and granuloma formation within the kidney (renal
tuberculosis), immune complex deposition leading to
glomerulonephritis and systemic inflammation driven by cytokines
affecting renal tissues (24,25).
This case emphasizes the importance of considering tuberculosis in
the differential diagnosis of AKI, particularly when other
explanations are incomplete or the response to standard therapy is
poor, as timely intervention can result in significant renal
recovery (8,23). Patients with concurrent
tuberculosis and severe renal impairment are at substantial risk,
highlighting the necessity of accurate diagnosis and prompt
management (8,23).
Multiorgan tuberculosis (pleuritis and peritonitis)
was diagnosed incidentally during peritoneal dialysis catheter
placement, highlighting the potential for disseminated disease,
particularly in immunocompromised patients. Risk factors, notably
uncontrolled diabetes mellitus (glycated hemoglobin A1c 7.4%) and
kidney dysfunction, are well-established contributors to increased
tuberculosis susceptibility (3,8).
Diabetes mellitus is a major risk factor for active tuberculosis
(7). These conditions likely
facilitated the development and dissemination of tuberculosis in
this patient.
In the present case, the coexistence of accelerated
malignant hypertension and tuberculosis posed unique diagnostic and
therapeutic challenges. Misattribution of pleural effusion to heart
failure led to potentially deleterious therapeutic decisions,
whereas the eventual diagnosis of tuberculosis necessitated a
fundamental revision of the management strategy.
While this case report provides valuable clinical
insights into a rare co-occurrence of diseases, it has several
limitations. First, as a single case report, the generalizability
of its findings is limited. The pathophysiological mechanisms
observed (e.g., the association between systemic inflammation due
to tuberculosis and the exacerbation of malignant hypertension)
need to be validated in larger-scale studies. Second, although the
individual patient's clinical course was meticulously examined, the
patient's long history of untreated hypertension meant that the
precise duration of hypertension and the specific details regarding
treatment adherence during that period could not be fully
ascertained in this case. Furthermore, definitively differentiating
the causal relationship between tuberculosis and hypertension is
difficult in a single case due to their complex interactions.
Future research should involve larger cohort studies
to elucidate the pathophysiological mechanisms in the co-existence
of tuberculosis infection and hypertensive diseases. In particular,
detailed molecular-level investigations are warranted regarding the
impact of chronic inflammation caused by tuberculosis on
endothelial dysfunction and its role in the progression of
multi-organ damage, including the kidneys. It is also crucial to
consider when tuberculosis screening should be recommended for
hypertensive patients, particularly those with risk factors such as
diabetes mellitus and kidney dysfunction, including a
cost-effectiveness analysis. As suggested by this case,
establishing early diagnostic and interventional strategies for
tuberculosis in patients with refractory pleural effusion and
worsening renal function in the context of malignant hypertension
could significantly contribute to improving outcomes in these
complex cases.
In conclusion, this case highlights two crucial
lessons: i) Treatment-refractory symptoms should prompt
reevaluation of the initial diagnosis even when well-supported by
clinical presentation; and ii) lymphocyte-predominant exudative
effusions warrant investigation for tuberculous pleuritis,
particularly in patients with risk factors such as diabetes
mellitus and kidney dysfunction. Early recognition and appropriate
intervention for concurrent tuberculosis can significantly improve
outcomes in these complex cases.
Acknowledgements
Not applicable.
Funding
Funding: The Division of Integrative Renal Replacement Therapy
is an endowed department supported in part by JMS Co., Ltd. (Tokyo,
Japan), Terumo Corporation (Tokyo, Japan) and Southern Tohoku
Hospital Group (Koriyama, Japan).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HI and KY were the patient's primary physicians
responsible for patient treatment. HH and KY also performed the
literature search, acquired the data and drafted the manuscript.
YS, and YT contributed to the clinical management of the patient
and interpretation of the data. FS, SS and TH were involved in the
diagnostic and therapeutic procedures. IOY and TM conceived the
case report, supervised the project and critically revised the
manuscript for important intellectual content. All authors have
read and approved the final version of the manuscript. HI and TM
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This case report was approved by the Institutional
Review Board of Tohoku Medical and Pharmaceutical University
(approval no. 2024-4-082-0000). It was conducted in accordance with
the Declaration of Helsinki of the World Medical Association.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lip GYH, Coca A, Kahan T, Boriani G,
Manolis AS, Olsen MH, Oto A, Potpara TS, Steffel J, Marín F, et al:
Hypertension and cardiac arrhythmias: Executive summary of a
consensus document from the European heart rhythm association
(EHRA) and ESC council on hypertension, endorsed by the heart
rhythm society (HRS), Asia-Pacific heart rhythm society (APHRS),
and sociedad latinoamericana de estimulación cardíaca y
electrofisiología (SOLEACE). Eur Heart J Cardiovasc Pharmacother.
3:235–250. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van den Born BH, Lip GYH, Brguljan-Hitij
J, Cremer A, Segura J, Morales E, Mahfoud F, Amraoui F, Persu A,
Kahan T, et al: ESC council on hypertension position document on
the management of hypertensive emergencies. Eur Heart J Cardiovasc
Pharmacother. 5:37–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
World Health Organization (WHO): Global
tubeculosis report 2024, Geneva, 2024. Available from:
https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024.
|
|
4
|
Huaman MA, Henson D, Ticona E, Sterling TR
and Garvy BA: Tuberculosis and cardiovascular disease: Linking the
epidemics. Trop Dis Travel Med Vaccines. 1(10)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mandieka E, Saleh D, Chokshi AK, Rivera AS
and Feinstein MJ: Latent tuberculosis infection and elevated
incidence of hypertension. J Am Heart Assoc.
9(e019144)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salindri AD, Auld SC, Gujral UP, Urbina
EM, Andrews JR, Huaman MA and Magee MJ: Tuberculosis infection and
hypertension: Prevalence estimates from the US national health and
nutrition examination survey. BMJ Open. 14(e075176)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al-Rifai RH, Pearson F, Critchley JA and
Abu-Raddad LJ: Association between diabetes mellitus and active
tuberculosis: A systematic review and meta-analysis. PLoS One.
12(e0187967)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Romanowski K, Clark EG, Levin A, Cook VJ
and Johnston JC: Tuberculosis and chronic kidney disease: An
emerging global syndemic. Kidney Int. 90:34–40. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shaw JA, Diacon AH and Koegelenberg CFN:
Tuberculous pleural effusion. Respirology. 24:962–971.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Light RW: Update on tuberculous pleural
effusion. Respirology. 15:451–458. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ohta T, Waga S, Handa W, Saito I and
Takeuchi K: New grading of level of disordered consiousness
(author's transl). No shinkei geka. 2:623–627. 1974.PubMed/NCBI(In Japanese).
|
|
12
|
Brott T, Adams HP Jr, Olinger CP, Marler
JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg
V, et al: Measurements of acute cerebral infarction: A clinical
examination scale. Stroke. 20:864–870. 1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Boulestreau R, Śpiewak M, Januszewicz A,
Kreutz R, Guzik TJ, Januszewicz M, Muiesan ML, Persu A, Sarafidis
P, Volpe M, et al: Malignant hypertension: A systemic
cardiovascular disease: JACC review topic of the week. J Am Coll
Cardiol. 83:1688–1701. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Porcel JM: Advances in the diagnosis of
tuberculous pleuritis. Ann Transl Med. 4(282)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liang QL, Shi HZ, Wang K, Qin SM and Qin
XJ: Diagnostic accuracy of adenosine deaminase in tuberculous
pleurisy: A meta-analysis. Respir Med. 102:744–754. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aggarwal AN, Agarwal R, Gupta D, Dhooria S
and Behera D: Interferon gamma release assays for diagnosis of
pleural tuberculosis: A systematic review and meta-analysis. J Clin
Microbiol. 53:2451–2459. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feria MG, Chang C, Ticona E, Moussa A,
Zhang B, Ballena I, Azañero R, Ticona C, De Cecco CN, Fichtenbaum
CJ, et al: Pro-inflammatory alterations of circulating monocytes in
latent tuberculosis infection. Open Forum Infect Dis.
9(ofac629)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clemente C, Rius C, Alonso-Herranz L,
Martín-Alonso M, Pollán Á, Camafeita E, Martínez F, Mota RA, Núñez
V, Rodríguez C, et al: MT4-MMP deficiency increases patrolling
monocyte recruitment to early lesions and accelerates
atherosclerosis. Nat Commun. 9(910)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Munisankar S, Rajamanickam A,
Balasubramanian S, Muthusamy S, Menon PA, Ahamed SF, Whalen C,
Gumne P, Kaur I, Nadimpalli V, et al: Prevalence of proximate risk
factors of active tuberculosis in latent tuberculosis infection: A
cross-sectional study from South India. Front Public Health.
10(1011388)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sheu JJ, Chiou HY, Kang JH, Chen YH and
Lin HC: Tuberculosis and the risk of ischemic stroke: A 3-year
follow-up study. Stroke. 41:244–249. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Adefuye MA, Manjunatha N, Ganduri V,
Rajasekaran K, Duraiyarasan S and Adefuye BO: Tuberculosis and
cardiovascular complications: An overview. Cureus.
14(e28268)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee HR, Yoo JE, Choi H, Han K, Jung JH,
Park J, Lee H and Shin DW: Tuberculosis and risk of ischemic
stroke: A nationwide cohort study. Stroke. 53:3401–3409.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kansiime G, Aklilu AM, Baluku JB, Yasmin
F, Kanyesigye M, Muzoora CK, Wilson FP, Bajunirwe F, Brewster U and
Kalyesubula R: Incidence of acute kidney injury and associated
mortality among individuals with drug-susceptible tuberculosis in
Uganda. Kidney360. 5:1446–1454. 2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamei W and Tao Y: Tuberculosis-associated
glomerulonephritis: A case report and literature review. J Int Med
Res. 46:2549–2557. 2018.
|
|
25
|
Domingo-Gonzalez R, Prince O, Cooper A and
Khader SA: Cytokines and chemokines in mycobacterium tuberculosis
infection. Microbiol Spectr. 4(10)2016.PubMed/NCBI View Article : Google Scholar
|