Introduction
With the relaxation of birth policies in China it is
possible to have a second or even a third child, and combined with
the high rates of cesarean section surgeries, the proportion of
scar pregnancies in China has significantly increased compared with
rates before this policy change (1-3).
Cesarean scar pregnancy (CSP) refers to a rare ectopic pregnancy,
where a previous pregnancy is delivered by cesarean section but
then an embryo implants in the scar site of the previous cesarean
section (4). Owing to the thin
muscle layer and poor elasticity of the scar tissue at the site of
cesarean section, various serious complications, such as uterine
perforation, blood loss and endangerment of life for the mother,
can occur as gestational age increases (5). With the widespread application of
transvaginal ultrasound examination and increasing awareness of
CSP, its diagnosis and treatment with surgery and medication have
greatly improved (6). However, in
real-world clinical settings, pregnant and postpartum women remain
frequently misdiagnosed with scarred uterine pregnancies that are
mistakenly regarded as intrauterine pregnancy or cervical
pregnancy, especially in underdeveloped areas of less developed
countries, where the misdiagnosis rate may be even higher (7). If patients are initially
misdiagnosed, they incur a particular risk of mistreatment,
hysterectomy, or even fatality due to bleeding or uterine rupture.
This risk is typically associated with both the diagnostic and
treatment capabilities of obstetricians and gynecologists, in
addition to the cognitive level of ultrasound clinicians.
Therefore, this requires the attention of clinicians and monitoring
physicians. In the present study, the clinical data of patients
with CSP who experienced misdiagnosis and mistreatment during the
initial diagnosis were retrospectively analyzed, whilst also
exploring the contributing factors and potential treatment
strategies.
Patients and methods
A retrospective review of medical record data from
January 2018 to October 2024 identified 42 cases of CSP at the
Department of Obstetrics and Gynecology, 940th Hospital of the
Joint Logistics Support Force of the Chinese People's Liberation
Army (Gansu, China).
The inclusion criteria for CSP diagnosis and
treatment (8,9) were based on a comprehensive
evaluation of clinical, imaging and laboratory examinations: i)
Clinical history, with a previous history of one or more cesarean
section (transverse incision in the lower segment of the uterus),
amenorrhea (for 5-12 weeks), presence or absence of early pregnancy
symptoms (such as vaginal bleeding and lower abdominal pain) and
the absence of serious complications (such as infection or
coagulation dysfunction); ii) transvaginal/abdominal ultrasound
features meeting at least two of the stated criteria [the absence
of gestational sac in the uterine cavity and cervical canal;
presence of the gestational sac at the scar site of the cesarean
section in the lower anterior wall of the uterus; thinning
(thickness ≤3 mm) or disruption of the muscle layer at the scar
site; and absence of uterine muscle layer between the gestational
sac and bladder is missing or presence of abundant blood flow (if
Doppler shows low impedance blood-flow signals)]; and iii)
laboratory examination showing positive blood β-human chorionic
gonadotropin, with further ultrasound imaging needed to determine
whether pregnancy had occurred.
The exclusion criteria were as follows: i) Cervical
pregnancy, where the gestational sac is located inside the cervical
canal, the cervix is enlarged and the uterine cavity is empty; ii)
intrauterine pregnancy miscarriage, where the gestational sac
detached into the cervical canal; and iii) evidence of other
ectopic pregnancy, including tubal and cornual pregnancy.
All relevant patient data were collected from the
electronic medical record system of the 940th Hospital of the Joint
Logistics Support Force of the Chinese People's Liberation Army.
The present study was approved by the Ethics Committee of the 940th
Hospital of the Joint Logistics Support Force of the Chinese
People's Liberation Army (approval no. 2024KYLL002). Written
informed consent for participation and publication was obtained
from all patients included in the present study.
Results
Of the 42 cases of CSP, 7 were identified as
misdiagnosis and mistreatment, with a misdiagnosis and mistreatment
rate of 16.67%. For the seven cases included in the present study,
the age range was 28-35 years, with a mean age of 31.86±2.67 years.
Hematological examination of the patients upon admission showed no
abnormalities (Table I). The
patients had amenorrhea for 40-94 days, whereas all had a history
of 1-2 cesarean sections. The interval between cesarean section
surgery and the current pregnancy ranged between 11-120 months. In
total, three patients had a history of 1-2 induced abortions after
cesarean section (cases 1, 5 and 7). All patients were diagnosed
with intrauterine pregnancy by ultrasound. A total of three
patients (cases 1, 4 and 6) had a small amount (<50 ml) of
irregular vaginal bleeding after 50 days of amenorrhea and were
diagnosed with threatened miscarriage (Table II).
 | Table IInitial hematological examination
indicators for the patients misdiagnosed with cesarean scar
pregnancy. |
Table I
Initial hematological examination
indicators for the patients misdiagnosed with cesarean scar
pregnancy.
| Case | Medical file
number | White blood cell
count (x109/l) | Red blood cell count
(x1012/l) | Hemoglobin (g/l) | Platelet
(x109/l) | Activated partial
thromboplastin time (sec) | Prothrombin time
(sec) | Human chorionic
gonadotropin (IU/l) |
|---|
| Ref. range | - | 3.5-9.5 | 3.68-5.13 | 113-151 | 101-320 | 23-32 | 9-14 | 0-10 |
|---|
| 1 | ZX0388383 | 12.12 | 4.43 | 133 | 278 | 26.8 | 11.2 | 106.7 |
| 2 | 15069188 | 7.59 | 4.47 | 144 | 215 | 26.3 | 11.9 | 321.1 |
| 3 | 15097476 | 8.29 | 4.54 | 136 | 206 | 28.1 | 11.8 | 6,371.9 |
| 4 | 79042441 | 8.63 | 3.38 | 99 | 219 | 22.1 | 9.6 | 1,352.7 |
| 5 | ZX0493131 | 8.5 | 4.46 | 137 | 232 | 21.7 | 11.1 | 2,414.3 |
| 6 | ZX0360820 | 5.65 | 3.63 | 108 | 188 | 25.6 | 11 | 91.2 |
| 7 | ZX0400103 | 4.59 | 3.91 | 125 | 172 | 23.5 | 11.1 | 3,5470.3 |
 | Table IIBasic characteristics and treatment
status of the seven patients misdiagnosed with cesarean scar
pregnancies. |
Table II
Basic characteristics and treatment
status of the seven patients misdiagnosed with cesarean scar
pregnancies.
| Case | Case no. | Age (years) | Days of
amenorrhea | Time since last
cesarean section (months) | Misdiagnosis | Pre-treatment | Bleeding volume
(ml) | Surgery |
|---|
| 1 | ZX0388383 | 33 | 71 | 36 | Embryo arrest | Medical
abortion | 1,200 | Interventional
embolization + laparoscopic scar resection and uterine repair |
| 2 | 15069188 | 30 | 51 | 14 | Threatened
miscarriages | Ultrasound guided
curettage surgery | 300 | Interventional
surgery embolization + curettage |
| 3 | 15097476 | 30 | 40 | 11 | Threatened
miscarriages | Medical
abortion | 150 | Palace cleaning
surgery + conservative treatment with medication |
| 4 | 79042441 | 32 | 85 | 60 | Threatened
miscarriages | Ultrasound guided
curettage surgery | 600 | Interventional
embolization + curettage surgery |
| 5 | ZX0493131 | 35 | 45 | 120 | Embryo arrest | Medical
abortion | 200 | Interventional
embolization + curettage surgery |
| 6 | ZX0360820 | 28 | 94 | 36 | Embryo arrest | Medical
abortion | 1,400 | Interventional
embolization + laparoscopic scar resection and uterine repair |
| 7 | ZX0400103 | 35 | 45 | 36 | Embryo arrest | Medical
abortion | 100 | Palace cleaning
surgery + conservative treatment with medication |
Of the three cases (cases 2, 3 and 4) misdiagnosed
with threatened miscarriage, two cases underwent abortion under
anesthesia for a curettage procedure (cases 2 and 4) and one case
required a medical drug-induced abortion (case 3). In total, 3 days
after surgery, these three patients' ultrasound examinations
revealed an incomplete abortion, resulting in uterine curettage
being performed under ultrasound guidance in these three patients.
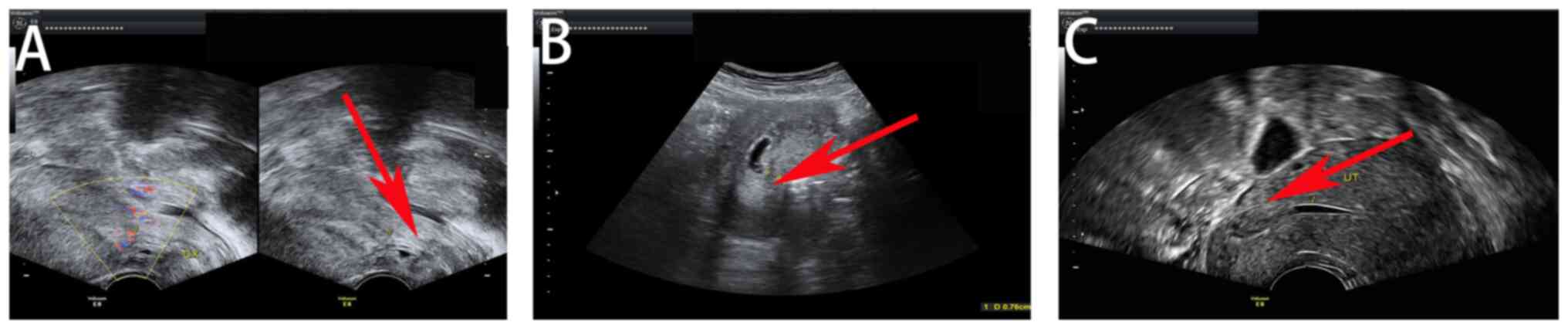
During surgery, clear endometrial lines were observed and abnormal
echoes were located at the scar site of the original cesarean
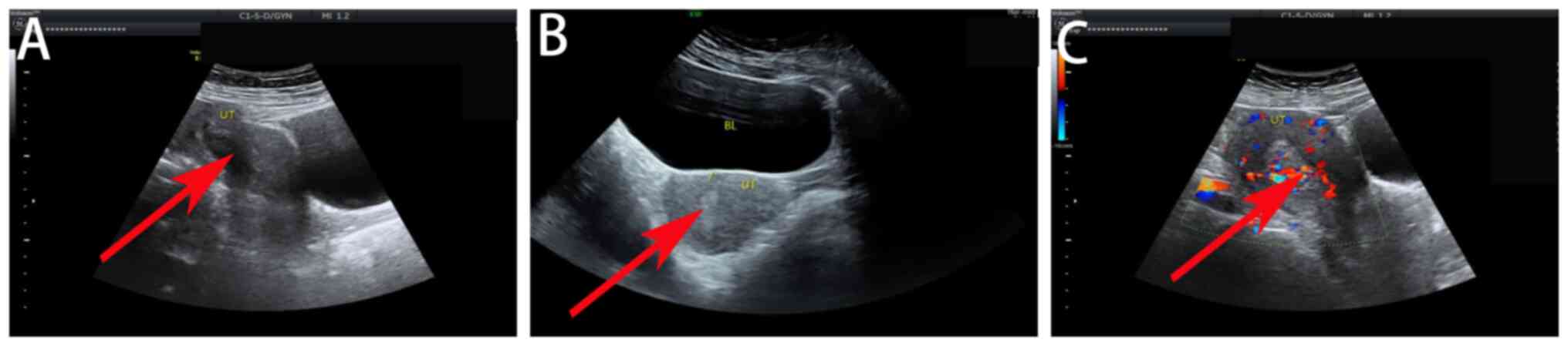
section in the lower segment of the uterus (Fig. 1). CSP was therefore considered for
the diagnosis. Two patients had minimal bleeding (<50 ml) and
were administered a single intramuscular injection of methotrexate
(50 mg/m2) and an oral administration of 50 mg
mifepristone once every 12 h for three doses (cases 2 and 3). In
one case (case 4) the intraoperative bleeding volume reached 800
ml. After emergency bilateral uterine artery embolization, uterine
bleeding ceased. For case 3 requiring a medical abortion, a mass
with a diameter of ~4 cm was observed on the right side of the
lower segment of the uterus, where the intraoperative bleeding
volume reached 600 ml. A total of 2 days after blood transfusion,
fluid replacement and anti-shock treatment, laparoscopic uterine
CSP resection and uterine repair surgery were performed, where
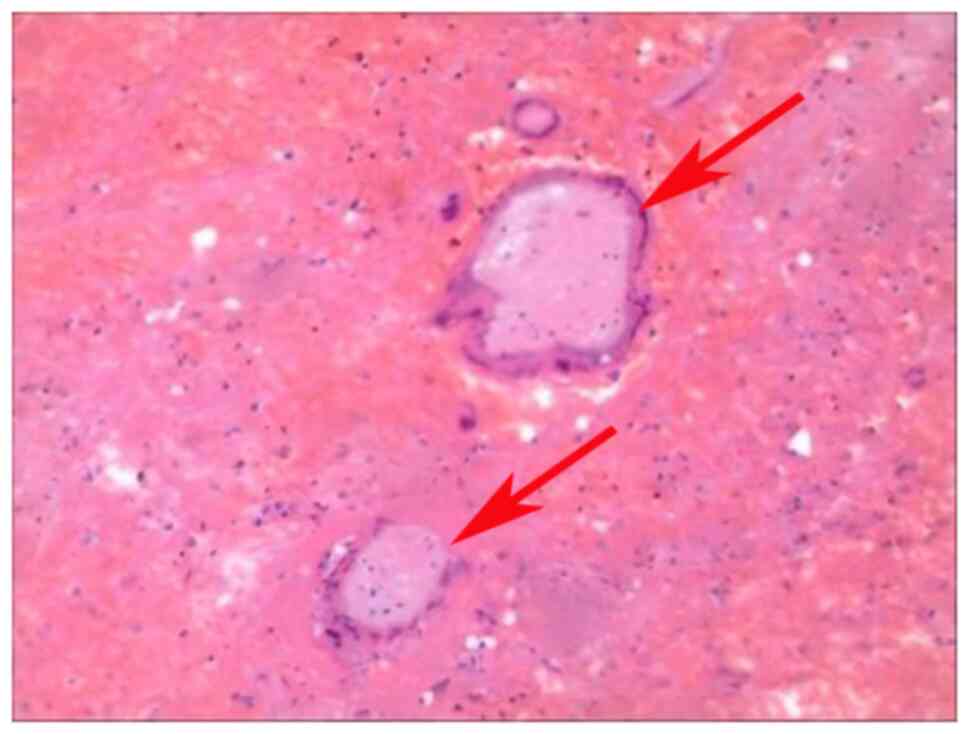
postoperative pathological specimens showed villous tissue and
trophoblast cells in case 3 (Fig.
2).
In total, four of the seven patients were
misdiagnosed as having embryonic arrest and underwent medical
abortions that included intramuscular injection of methotrexate (50
mg/m2) and an oral administration of 50 mg mifepristone
once every 12 h for three doses (cases 1, 5, 6 and 7). In these
four cases, after methotrexate and mifepristone administration,
vaginal bleeding occurred and the ‘pregnancy product’ was
discharged, which were visually confirmed by the gynecologist. The
uterus contracted well, but a moderate quantity (100-300 ml) of
blood flowed out of the uterine cavity. Bleeding gradually stopped
after local compression and intramuscular injection of oxytocin (10
U) in all four cases. A total of 10 days after surgery, one patient
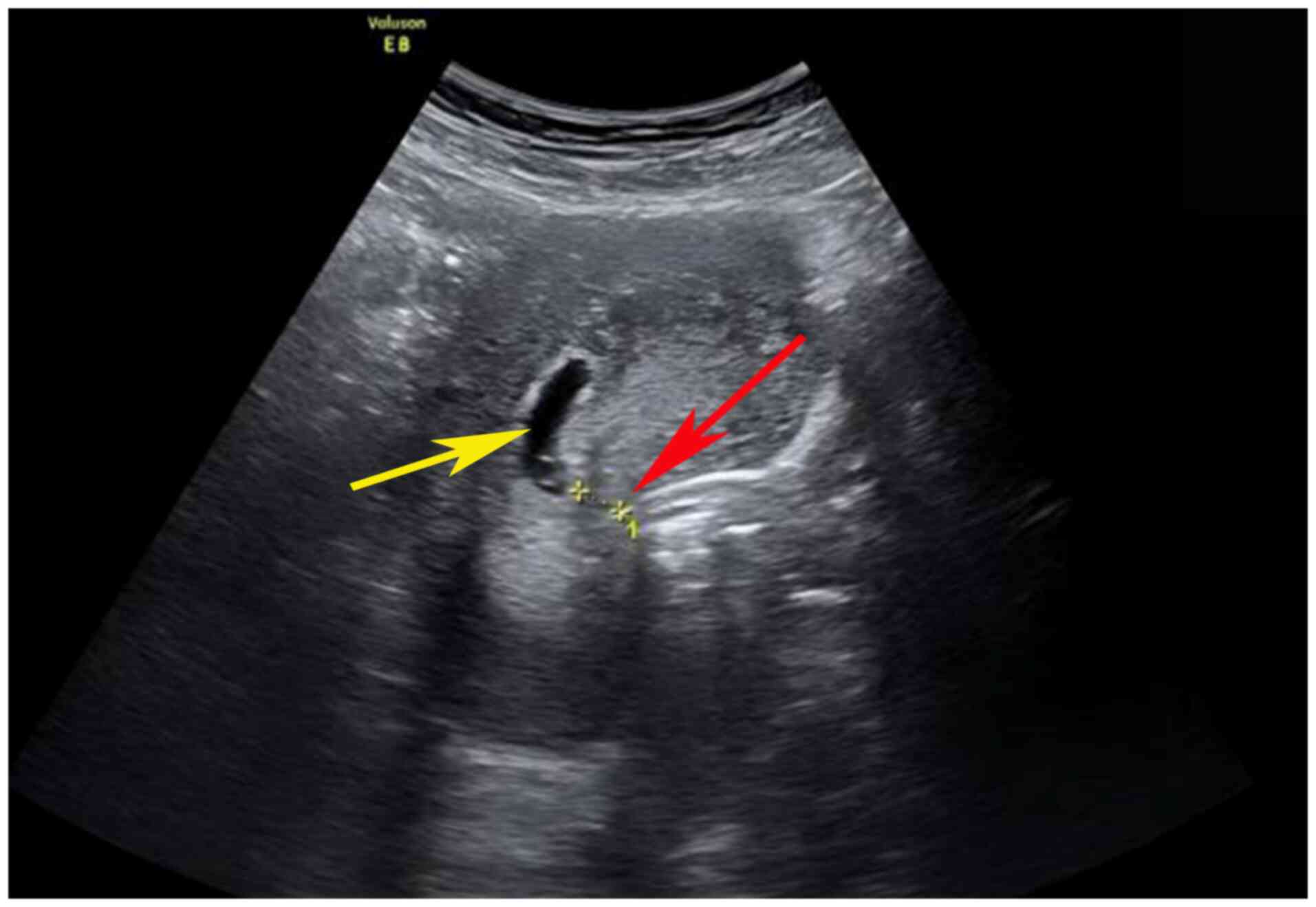
(case 6) returned to the hospital for a follow-up ultrasound
examination, where abnormal echoes in the uterine cavity were
noted. Therefore, curettage was performed under ultrasound guidance
(Fig. 3). During the procedure,
clear endometrial lines were observed and a small blood clot (15
ml) and decidual tissue were cleared. However, there was a
considerable amount of uterine bleeding, with a cumulative blood
loss of 1,400 ml during the operation. A mixed mass protruding
outward from the lower part of the anterior wall of the uterus was
noted, where the lower uterine muscle layer was thin. Therefore,
CSP was considered. Immediate uterine artery intervention
embolization was performed and bleeding ceased. Blood transfusions
were then administered. Laparoscopic scar pregnancy resection was
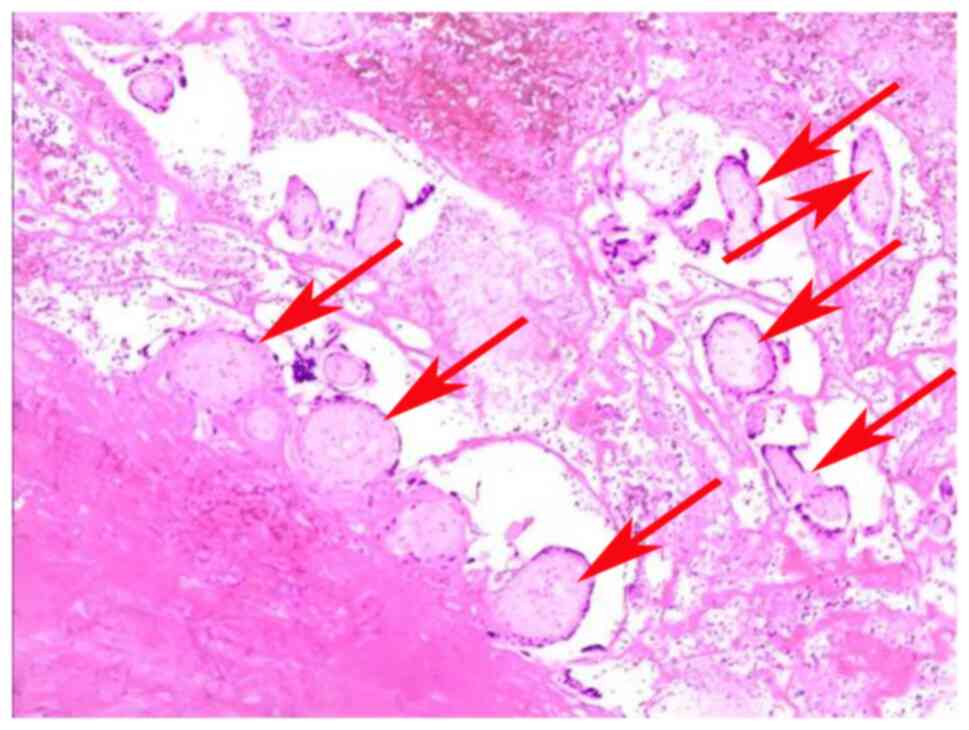
performed 3 days later, where postoperative pathological specimens
revealed chorionic tissue and trophoblast cells in case 6 (Fig. 4). The other three patients
misdiagnosed with embryonic arrest had intermittent vaginal
bleeding, and ultrasound examination 7-10 days after surgery
revealed abnormal echoes in the lower part of the uterus,
indicating CSP (Fig. 5). A single
deep intramuscular injection of methotrexate (50 mg/m2)
was administered and bilateral uterine artery embolization was
performed, followed by uterine curettage under ultrasound guidance.
All the patients recovered and were discharged after surgery.
Discussion
Scar pregnancy after cesarean section is a unique
type of ectopic pregnancy, but the cause remains unclear and has
been proposed to be associated with a defect in the decidua at the
scar site (10). Trophoblasts
directly invade the uterine muscle layer, where the villi adhere
to, implant into or even penetrate the uterine wall. The early
clinical symptoms and manifestations of CSP are atypical and can be
easily misdiagnosed as threatened miscarriage (11,12).
In the present study, three patients with early pregnancy were
diagnosed with miscarriage due to a short duration of amenorrhea.
During the first pelvic ultrasound examination, only the presence
of a gestational sac in the uterus was examined, but the position
of the gestational sac was not examined. The relationship between
the location of the gestational sac and previous cesarean section
uterine scarring were not carefully examined. During the process of
medical abortion and curettage, there was marked vaginal bleeding,
which was considered an incomplete abortion. In total, four
patients had amenorrhea for >70 days and experienced minor
vaginal bleeding. Ultrasound examination revealed no original
heartbeats in cases of intrauterine pregnancy, before only
considering it as embryonic arrest and did not carefully observe
the position of the gestational sac and its relationship with
uterine scars and the bladder, leading to the incorrect original
judgment.
CSP can be classified into types I, II and III based
on the depth, growth direction and local muscle layer thickness of
the gestational sac implanted at the scar site of the cesarean
section during ultrasound examination (13,14).
The gestational sac in type I and type II CSP can grow into the
uterine cavity. If the relationship between the gestational sac
implantation site and the cesarean scar is not considered during
the ultrasound examination, it can be easily misdiagnosed as an
intrauterine pregnancy (15). As
gestational age increases, the section of the gestational sac
protruding into the uterine cavity gradually increases, such that
its relationship with the cesarean scar becomes increasingly
unclear, with indistinguishable boundaries observed by ultrasound.
Therefore, misjudgments are prone to occur during ultrasound
examinations.
Using the sonographic reporting system for CSP in
early gestation, it is possible to reduce the misdiagnosis rate at
ultrasound examination (16). In
cases where CSP is difficult to diagnose using ultrasound,
transvaginal three-dimensional (3D) color ultrasound or MRI should
be combined to ensure timely diagnosis (17). Transvaginal ultrasonography is
considered to be the optimal imaging technique for diagnosing CSPs
due to its capabilities for soft tissue characterization, whilst
MRI can also be used to inform the decision-making process
(18). Therefore, MRI is
considered a useful complementary tool alongside transvaginal
ultrasonography for correctly diagnosing and categorizing CSPs to
inform the subsequent therapeutic approach more accurately
(19). As the gestational age
increases, not only does the difficulty of diagnosing CSP increase,
but the risk of poor treatment outcomes also increases drastically
(5,10). Termination of a midterm pregnancy
can cause severe bleeding, uterine rupture and even endanger the
life of the mother (20,21). If the patient chooses to continue
the pregnancy, the risk of serious complications, such as placental
implantation, placenta previa, uterine rupture and postpartum
hemorrhage, are increased during the middle and late stages of
pregnancy, endangering the lives of both the mother and fetus
(22,23). For patients with a history of
cesarean section who have terminated their pregnancy, a detailed
understanding of their previous pregnancy and childbirth history
should be obtained. Irregular vaginal bleeding should not be
generalized as a sign of miscarriage, whereby attention should be
paid to the source of blood supply to the gestational sac. If there
is heavy vaginal bleeding or a small amount of continuous
hemorrhage, then the operation should be suspended to avoid serious
consequences.
Performing transvaginal ultrasound examination
during early pregnancy for mothers with high-risk factors, such as
a history of pathological placenta, ectopic pregnancy and multiple
cesarean sections, can significantly improve prognosis and reduce
the occurrence of emergency surgeries (24). At present, pregnancy termination is
recommended after a clear diagnosis of scar pregnancy after
cesarean section, mainly to ensure the integrity and fertility of
the uterus, reduce the risk of major bleeding and ensure the safety
of the mother (6,10,15).
Inappropriate treatment after a misdiagnosis of CSP can lead to
hemorrhagic anemia, which can in turn render patients less tolerant
to subsequent treatment and blood coagulation (25). Mistreatment can cause CSP to evolve
into a more complex type III lump-type CSP, resulting in a more
complex and fragile local tissue structure in the cesarean scar
(25,26). When re-treated, these lump-type
masses are more likely to rupture, causing severe hemorrhage
(25,26). Therefore, remedial treatment after
misdiagnosis is frequently passive and difficult. Comprehensive
individualized considerations should be made based on the patient's
general condition, classification and previous treatment status.
Uterine artery embolization is typically the first choice of
emergency rescue treatment for hemorrhage, but caution should be
exercised regarding the possibility of disseminated intravascular
coagulation (27,28). Hysterectomy is only used as a last
resort to save the patient's life. For patients in good general
condition, such as a history of little vaginal bleeding and low
blood levels of human chorionic gonadotropin, oral mifepristone and
intramuscular injection of methotrexate can be effective (29,30).
If necessary, scar pregnancy lesion resection surgery can be
performed. However, sufficient rescue measures, such as uterine
artery embolization, surgical resection and compression hemostasis,
should be performed for heavy intraoperative bleeding.
Notably, the misdiagnosis rate of CSP varies among
different hospitals. Yu et al (31) previously reported 100 cases of CSP,
with 7 being misdiagnosed (two cases of fetal arrest, one case of
incomplete miscarriage, two cases of early pregnancy, one case of
mid-pregnancy and one case of hydatidiform mole). In another study,
Yin et al (32) reported 42
cases of CSP, amongst whom the initial symptoms varied and 14 cases
were misdiagnosed at primary hospitals (33%) before being
eventually diagnosed in Peking University First Hospital (Beijing,
China). After treatment, all patients achieved favorable
therapeutic outcomes. Therefore, in hospitals of different levels,
due to the different experience levels of obstetricians,
gynecologists and ultrasound physicians, with different symptom
manifestations, misdiagnosis rates can vary. The 940th Hospital of
the Joint Logistics Support Force of the Chinese People's
Liberation Army is located in the Gansu Province in western China,
where patients frequently face economic challenges. Amongst the 42
patients, 7 cases (16.67%) were found to be misdiagnosed. Notably,
Peking University First Hospital is a top hospital for obstetrics
and gynecology, where patients were eventually sent to this
hospital from less-specialized hospitals to confirm the diagnosis.
This highlights a gap between less-specialized and top hospitals,
implying the need for medical staff to exercise caution when making
conclusions based on lesions identified using ultrasound in this
patient population.
Misdiagnosis may be due to a number of factors. The
operator may lack experience and be unfamiliar with the ultrasound
characteristics of CSP, such as the positional relationship between
the gestational sac and scar and muscle layer thinning. These
symptoms can be confused with cervical or cornual pregnancy. This
may also be compounded by a failure to comprehensively analyze the
patient's medical history, including identifying a history of
cesarean section. In addition, low-resolution ultrasound may not be
able to clearly identify the subtle structures of gestational sacs
and scars, such as muscle layer thickness and blood flow signals,
especially during early pregnancy. Scar morphology is also
typically complex, such as scar diverticulum (defined as depression
at the incision site), which may be mistaken for a gestational sac
or conceal the true location of pregnancy. Poor scar healing,
hematoma or inflammation may further interfere with imaging.
Furthermore, excessive forward or backward bending of the uterus
may affect the probe angle and result in unclear imaging. In early
pregnancy, the gestational sac is small and has not yet been
clearly embedded in the scar tissue, which can also lead to
misdiagnosis as intrauterine pregnancy. After multiple pregnancies
or the use of assisted reproductive technology, the implantation
site of the embryo is likely to exhibit complex characteristics,
further complicating diagnosis. Additionally, it is difficult to
distinguish between cervical pregnancy and cornual pregnancy.
Obesity or intestinal gas interference, poor-quality transabdominal
ultrasound images and a lack of combined transvaginal ultrasound
examination can also contribute to missed diagnosis. The blood flow
signal around the gestational sac may not have been evaluated,
which is important given that CSP frequently presents with rich and
low obstruction of blood flow (6,10,15).
In particularly complex cases, 3D ultrasound or MRI should be
applied to clarify the spatial relationships between the
gestational sac and scar tissue.
In conclusion, in pregnant women with a history of
cesarean section, a transvaginal ultrasound examination should be
performed as soon as possible to exclude CSP. Difficulties in
diagnosis can be resolved using transvaginal 3D ultrasonography or
pelvic MRI. An accurate ultrasound investigation is necessary to
correctly diagnose CSP and select appropriate treatment plans. If
the diagnosis remains unclear, blind curettage should be avoided,
particularly when unexpected heavy bleeding occurs with
insufficient preparation, which may lead to irreversible outcomes.
The difficulty and risk of CSP remedial treatment are greater than
those of the initial treatment, where individualized comprehensive
selection should be made according to the specific situation of
each patient. The present study is limited by its retrospective
nature and limited sample size, which should be addressed in future
studies to enable suitable statistical analysis and promote the
generalizability of the findings.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Hexi University
14th Science and Technology Innovation Project (grant no. 164), the
Youth Research Project provided by the President's Fund of Hexi
University (grant no. QN2024024) and the Gansu Province University
Teacher Innovation Fund Project (grant no. 2025A-181).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XLX, WYC and YRH contributed to the drafting of the
manuscript and the design of the study. KLW, XP, RRM, QS and JXY
contributed substantially to the conceptualization and collected
data for patient cases. XLX aided with completion of the surgery.
QS and JXY approved the final version of the manuscript for
publication. XLX and JXY confirm the authenticity of all raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of 940th Hospital of the Joint Logistics Support Force of
the Chinese People's Liberation Army Hospital (approval no.
2024KYLL002; Lanzhou, China) and was performed in accordance with
local legislative guidelines and the 2024 Revision of the
Declaration of Helsinki. Written informed consent for participation
and publication was obtained from all patients included in the
present study.
Patient consent for publication
Written informed consent for participation and
publication was obtained from all patients included in the present
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang D, Laporte A, Gao X and Coyte PC: The
effect of the Two-child policy on cesarean section in China:
Identification using difference-in-differences techniques.
Midwifery. 107(103260)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yin S, Zhou Y, Yuan P, Wei Y, Chen L, Guo
X, Li H, Lu J, Ge L, Shi H, et al: Hospital variations in caesarean
delivery rates: An analysis of national data in China, 2016-2020. J
Glob Health. 13(04029)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Long Q, Kingdon C, Yang F, Renecle MD,
Jahanfar S, Bohren MA and Betran AP: Prevalence of and reasons for
women's, family members', and health professionals' preferences for
cesarean section in China: A mixed-methods systematic review. PLoS
Med. 15(e1002672)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Armstrong F, Mulligan K, Dermott RM,
Bartels HC, Carroll S, Robson M, Corcoran S, Parland PM, Brien DO,
Brophy D and Brennan DJ: Cesarean scar niche: An evolving concern
in clinical practice. Int J Gynaecol Obstet. 161:356–366.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang XJ and Sun SS: Comparison of maternal
and fetal complications in elective and emergency cesarean section:
A systematic review and Meta-analysis. Arch Gynecol Obstet.
296:503–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Silva B, Pinto VP and Costa MA: Cesarean
scar pregnancy: A systematic review on expectant management. Eur J
Obstet Gynecol Reprod Biol. 288:36–43. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan Y, Xing C, Chen J, Zheng Y, Li X, Liu
Y, Wang Z and Gong K: Provincial maternal and child information
system in Inner Mongolia, China: Descriptive implementation study.
JMIR Pediatr Parent. 7(e46813)2024.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Society for Maternal-Fetal Medicine (SMFM.
Electronic address: Pubs@smfm.org). Miller R, Timor-Tritsch IE and
Gyamfi-Bannerman C: Society for Maternal-fetal medicine (SMFM)
Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol.
222:B2–B14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Verberkt C, Jordans IPM, van den Bosch T,
Timmerman D, Bourne T, de Leeuw RA and Huirne JAF: How to perform
standardized sonographic examination of Cesarean scar pregnancy in
the first trimester. Ultrasound Obstet Gynecol. 64:412–418.
2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alameddine S, Lucidi A, Jurkovic D,
Timor-Tritsch I, Coutinho CM, Ranucci L, Buca D, Khalil A, Jauniaux
E, Mappa I, et al: Treatments for cesarean scar pregnancy: A
systematic review and meta-analysis. J Matern Fetal Neonatal Med.
37(2327569)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Timor-Tritsch IE, Horwitz G, D'antonio F,
Monteagudo A, Bornstein E, Chervenak J, Messina L, Morlando M and
Cali G: Recurrent cesarean scar pregnancy: Case series and
literature review. Ultrasound Obstet Gynecol. 58:121–126.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Timor-Tritsch IE, Monteagudo A, Calì G,
D'Antonio F and Agten AK: Cesarean scar pregnancy: Diagnosis and
pathogenesis. Obstet Gynecol Clin North Am. 46:797–811.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin Y, Xiong C, Dong C and Yu J:
Approaches in the treatment of cesarean scar pregnancy and risk
factors for intraoperative hemorrhage: A retrospective study. Front
Med (Lausanne). 8(682368)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Du Q, Liu G and Zhao W: A novel method for
typing of cesarean scar pregnancy based on size of cesarean scar
diverticulum and its significance in clinical Decision-making. J
Obstet Gynaecol Res. 46:707–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gonzalez N and Tulandi T: Cesarean scar
pregnancy: A systematic review. J Minim Invasive Gynecol.
24:731–738. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jordans IPM, Verberkt C, De Leeuw RA,
Tritsch IT, Coutinho CM, Ranucci L, Buca D, Khalil A, Jauniaux E,
Mappa I, et al: Definition and sonographic reporting system for
cesarean scar pregnancy in early gestation: Modified Delphi method.
Ultrasound Obstet Gynecol. 59:437–449. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Guo S, Wang H, Yu X, Yu Y and Wang C: The
diagnostic value of 3.0T MRI in cesarean scar pregnancy. Am J
Transl Res. 13:6229–6235. 2021.PubMed/NCBI
|
|
18
|
Han X and Zhang B: Comparison of the value
of transvaginal ultrasonography and MRI in the diagnosis of
cesarean scar pregnancy: A Meta-analysis. J Matern Fetal Neonatal
Med. 38(2445661)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Comune R, Liguori C, Tamburrini S, Arienzo
F, Gallo L, Dell'Aversana F, Pezzullo F, Tamburro F, Affinito P and
Scaglione M: MRI assessment of cesarean scar pregnancies: A Case
Series. J Clin Med. 12(7241)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tanos V and Toney ZA: Uterine scar
Rupture-prediction, prevention, diagnosis, and management. Best
Pract Res Clin Obstet Gynaecol. 59:115–131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Calì G, Timor-Tritsch IE,
Palacios-Jaraquemada J, Monteaugudo A, Buca D, Forlani F, Familiari
A, Scambia G, Acharya G and D'Antonio F: Outcome of Cesarean scar
pregnancy managed expectantly: Systematic review and Meta-analysis.
Ultrasound Obstet Gynecol. 51:169–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fu L, Luo Y and Huang J: Cesarean scar
pregnancy with expectant management. J Obstet Gynaecol Res.
48:1683–1690. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kennedy A, Debbink M, Griffith A, Kaiser J
and Woodward P: Cesarean scar ectopic pregnancy: A do-not-miss
diagnosis. RadioGraphics. 44(e230199)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi L, Huang L, Liu L, Yang X, Yao D, Chen
D, Xiong J and Duan J: Diagnostic value of transvaginal
three-dimensional ultrasound combined with color Doppler ultrasound
for early cesarean scar pregnancy. Ann Palliat Med. 10:10486–10494.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun H, Wang J, Fu P, Zhou T and Liu R:
Systematic evaluation of the efficacy of treatments for cesarean
scar pregnancy. Reprod Biol Endocrinol. 22(84)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ma R, Chen S, Xu W, Zhang R, Zheng Y, Wang
J, Zhang L and Chen R: Surgical treatment of cesarean scar
pregnancy based on the Three-category system: A retrospective
analysis. BMC Pregnancy Childbirth. 24(687)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu
KS, Qian JS, Zhou B, Li MA and Shan H: Uterine artery embolization
combined with methotrexate in the treatment of cesarean scar
pregnancy: Results of a case series and review of the literature. J
Vasc Interv Radiol. 23:1582–1588. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He Y, Wu X, Zhu Q, Wu X, Feng L, Wu X,
Zhao A and Di W: Combined laparoscopy and hysteroscopy vs. Uterine
curettage in the uterine artery embolization-based management of
cesarean scar pregnancy: A retrospective cohort study. BMC Womens
Health. 14(116)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stabile G, Vona L, Carlucci S, Zullo F,
Laganà AS, Etrusco A, Restaino S and Nappi L: Conservative
treatment of cesarean scar pregnancy with the combination of
methotrexate and mifepristone: A systematic review. Womens Health
(Lond). 20(17455057241290424)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Masten M and Alston M: Treatment of
recurrent Cesarean scar pregnancy with oral mifepristone, systemic
methotrexate, and ultrasound-guided suction dilation and curettage.
Cureus. 15(e36200)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu XL, Zhang N and Zuo WL: Cesarean scar
pregnancy: An analysis of 100 cases. Zhonghua Yi Xue Za Zhi.
91:3186–3189. 2011.PubMed/NCBI(In Chinese).
|
|
32
|
Yin L, Tao X, Zhu YC, Yu XL, Zou YH and
Yang HX: Cesarean scar pregnancy analysis of 42 cases. Zhonghua Fu
Chan Ke Za Zhi. 44:566–569. 2009.PubMed/NCBI(In Chinese).
|